Abstract
Introduction:
Hepatitis C virus (HCV) prevalence among transgender and gender diverse (TGD) individuals ranges from 1.8%−15.7% vs. 1% in the general population. Prior HCV studies inclusive of TGD individuals primarily rely on convenience-based sampling methods or are geographically restricted. The purpose of this study is to compare the prevalence of HCV diagnoses, testing, and care engagement between TGD and cisgender individuals.
Methods:
Using Optum’s de-identified Clinformatics® Data Mart Database, in 2022, the unadjusted prevalence of HCV testing among all adults and people who inject drugs (PWID) from January 2001 to December 2019 was measured. Multivariable logistic regression was used to compare the adjusted odds of HCV diagnoses and care engagement by gender subgroup.
Results:
The overall unadjusted frequency of HCV diagnoses among TGD individuals was approximately 3 times that of cisgender individuals (1.06% vs. 0.38%, p<0.001), including among PWID (6.36% vs. 2.36%, p=0.007). Compared to cisgender women, trans feminine/non-binary individuals had over 5 times the adjusted odds of an HCV diagnosis and approximately 3.5 times the odds of being tested for HCV. Additionally, compared to cisgender women, trans feminine/non-binary individuals had significantly increased odds of having an HCV-related procedure (e.g., abdominal ultrasounds, liver biopsies, Fibroscans). Cisgender men had significantly increased odds of receiving HCV medication compared to cisgender women.
Conclusions:
While testing was higher among TGD individuals, the higher overall frequency of HCV diagnoses among TGD vs. cisgender individuals signal persistent health disparities. Interventions are warranted to prevent HCV and increase ongoing testing and treatment uptake among TGD populations.
Keywords: transgender and gender diverse, hepatitis C, injection drug use, HCV testing, HCV care cascade
INTRODUCTION
Transgender and gender diverse (TGD) refers to people whose gender identities depart from cultural norms associated with their sex assigned at birth. TGD populations experience social and health disparities associated with an increased risk of Hepatitis C virus (HCV), including a higher prevalence of injection drug use, sex work, incarceration, and homelessness.1–5 Although HCV is the most common bloodborne infection in the United States,6 only a few studies have reported the HCV prevalence among TGD individuals. Across three studies, the HCV prevalence among TGD individuals ranged from 1.8%−15.7%,7–9 compared to approximately 1% of the US general population.10 Domestic epidemiological data show higher HCV prevalence among trans feminine individuals11 with a reported prevalence of HCV of 9% in Los Angeles-based sample and 24.8% in San Francisco-based sample.12–14 HCV prevalence studies with trans masculine individuals are even more limited, but domestic and global estimates range from 1–8%.9, 14, 15 Notably, these data are drawn from largely geographically-restricted, convenience samples7–9, 12, 14 limiting the understanding of the HCV burden among TGD individuals.
HCV testing is necessary to identify infection and link individuals to care, yet data on the prevalence of HCV testing among TGD individuals is limited. A study of TGD adults from an LGBTQ-friendly clinic in New York City estimated that 27% of TGD adults were tested for HCV between 2012 and 2015.7 However, national research among TGD individuals seeking care in diverse healthcare settings is needed to determine the extent to which TGD individuals are being tested for HCV. In terms of engaging in HCV care for those diagnosed, recent study describing the HCV care cascade among 951 low-income trans feminine individuals in San Francisco revealed that 56.5% of participants had initiated HCV care.12 Another study with a sample of 300 trans feminine individuals in San Francisco found that among individuals with a positive HCV viral load, 77.9% had engaged in some form of care.2 In these small, geographically-constricted studies with trans feminine individuals, “treatment” was based on self-report rather than more objective indicators such as electronic health records or insurance claims data. To the authors’ knowledge, no prior studies have used a large dataset to characterize HCV testing and care cascade engagement among a national sample of TGD and cisgender individuals.
Recent developments in algorithms for more accurately identifying TGD individuals have allowed researchers to explore health disparities by gender identity where self-identified gender information is lacking.16, 17 In this study, a national insurance claims dataset was used to estimate the prevalence of HCV diagnoses and testing (any HCV antibody or RNA test since laboratory testing sequence can vary)18 among TGD vs. cisgender adults from 2001 to 2019. Since HCV is most often spread by sharing or reuse of non-sterile injection drug use equipment,19 risk-based testing among TGD people who inject drugs (PWID) and cisgender PWID were also compared. Additionally, the prevalence of HCV care cascade engagement among all TGD and cisgender individuals with chronic HCV was assessed. Considering recent shifts in HCV guidelines and treatment options,20 identifying individuals with the greatest need for testing and treatment is vital for clinicians, and public health practitioners focused on addressing gender-related disparities.
METHODS
A retrospective cohort study using Optum’s de-identified Clinformatics® Data Mart Database was conducted in 2022. The dataset included claims from private insurance or Medicare Advantage (i.e., Part C) and insurance adhering to Medicare guidelines offered by private companies for approximately 84 million unique individuals nationwide from January 1, 2001, to December 31, 2019. Although the dataset represents individuals from all 50 states, it does not include those insured through Medicaid or who are uninsured, which omits a significant portion of TGD individuals and people who use substances and/or may be at risk for HCV.21, 22 Data included de-identified member information, medical claims, pharmacy claims, and provider data. The University of Michigan IRB determined that this study was exempt (HUM00161819).
Study Sample
Since claims databases do not uniformly nor consistently collect information on gender identity and sex assigned at birth, TGD enrollees were identified using a hierarchical algorithm of transgender-related diagnosis codes from ICD-9 and ICD-10 procedure codes.16 Although the algorithm is described elsewhere,16 briefly, individuals were categorized as “TGD” if they had some combination of 1) transgender-related diagnosis (e.g., Gender Identity Disorder), 2) transgender-conclusive procedure (e.g., vaginoplasty) not due to medical reasons such as cancer, 3) had a gender-affirming hormone prescription that was incongruent with their sex marker and a suggestive procedure (e.g., vaginoplasty), or if they 4) had an Endocrine disorder Not Otherwise Specified diagnosis along with a suggestive procedure or gender-affirming hormone prescription. Individuals were stratified as trans feminine and non-binary (TFN), trans masculine and non-binary (TMN), and TGD unclassified. In total, 38,598 unique TGD individuals were identified and a 10% random sample of 10,469,393 cisgender individuals were used due to computational limitations common when working with these data.23
Injection drug use (IDU) history was identified based on a previously validated algorithm with high sensitivity (85%) and specificity (80%).24 Individuals were required to have at least one ICD-9 or ICD-10 diagnosis code for injectable substances during the study period. There were 5,195 individuals who met the criteria for IDU.
Measures
To assess the frequency of diagnosed HCV infection, ICD-9 or ICD-10 diagnosis codes were used (Appendix Table 1). Individuals with a diagnosis from any prior year were removed from the subsequent years as new infections.
To assess the overall prevalence of HCV testing among all adults (≥18 years of age), individuals who were not continuously enrolled for the full year (continuous enrollment was defined as January 1 to December 31 of a given year), not 18 years old for the entire first year of enrollment, and had an ICD-9 or ICD-10 diagnosis code for hepatitis C were excluded. The proportion of individuals screened or tested for HCV according to CPT and HCPCS codes served as the numerator (Appendix Table 2) and the overall number of individuals enrolled for each calendar year was the denominator. For PWID, individuals under the age of 18 were included since anyone who injects drugs may be tested for HCV. Individuals were required to be enrolled for at least one year beyond the first documentation of IDU.25
HCV care cascade engagement26 was measured among individuals with chronic HCV, defined as individuals who received an HCV RNA test followed by ≥3 HCV diagnosis codes on different service dates or ≥2 HCV diagnosis codes separated by ≥60 days.27 Following the HCV RNA test date, individuals were required to be enrolled continuously for ≥1 year. Individuals with chronic HCV were assessed for their engagement in the HCV care cascade, which included calculating the proportion who had ≥1 visit(s) to a specialist (gastroenterologist, infectious disease specialist, hepatologist), were engaged in ≥1 HCV-related procedure(s) (e.g., abdominal ultrasounds, liver biopsies, Fibroscans),26 and had ≥1 pharmacological treatment based on National Drug Codes for dispensed HCV medications (Appendix Table 3).
Statistical Analysis
The testing prevalence among all adults ≥18 years old was calculated by dividing the numerator by the denominator for each calendar year. The testing prevalence among PWID was calculated as those tested for HCV divided by one year from the first occurrence of IDU. Chi-square and Fisher’s exact tests were used to calculate the unadjusted frequency of HCV diagnoses and testing prevalence for TGD vs. cisgender individuals and among gender sub-groups. To test differences in HCV infection and testing, multivariable logistic regression models were used and adjusted for gender identity, race, US census region, enrollment year, months of enrollment, and age at enrollment. For PWID, enrollment variables were replaced with age at first documentation of IDU (index date), months past the index date, and first year of the index date in the study period. Significance was set at p<0.05. For the care cascade analysis, chi-square tests were used to estimate care engagement by gender. Adjusted odds ratios from multivariable logistic regression were used to compare differences in care engagement by demographic characteristics. Analyses were conducted using Stata/MP software, version 14.2 (StataCorp LLC, College Station, TX).
RESULTS
Demographics of the overall sample (Appendix Table 4) and among PWID (Appendix Table 5) are presented. Most individuals in the study were from the South with the least being from the Northeast. A majority of the sample was identified as non-Hispanic White (52.66% overall and 67.05% among PWID) followed by unknown race/ethnicity (25.54% overall and 12.24% among PWID).
The unadjusted prevalence of HCV diagnosis was 1.06% among TGD individuals vs. 0.38% among cisgender individuals (p<0.001) and 6.36% among TGD PWID vs. 2.36% among cisgender PWID (p=0.007) (Table 1). TFN individuals had the highest prevalence of HCV diagnosis (1.86%), followed by unclassified TGD individuals (0.90%), TMN individuals (0.73%), cisgender men (0.45%), unclassified cisgender individuals (0.38%) and cisgender women (0.30%) (p<0.001). Among PWID, unclassified TGD individuals (16.67%), TFN (7.69%), and TMN (4.17%) had the highest HCV diagnosis prevalence compared to all cisgender groups (p<0.01). In adjusted analyses, TFN (aOR=5.09, 95% CI=4.39–5.90, p<0.001) and TMN individuals (aOR=2.28, 95% CI=1.93–2.71, p<0.001) had significantly increased odds of having an HCV diagnosis than cisgender women (Table 2).
Table 1.
Unadjusted Frequency of Hepatitis C virus (HCV) Diagnoses, 2001–2019
| Entire Sample (N=10,507,834) | People who inject drugs (N=5,195) | ||||||
|---|---|---|---|---|---|---|---|
| Gender | n | % | P | Gender | n | % | P |
| TGD | TGD | ||||||
| No HCV | 38,032 | 98.94 | *** | No HCV | 103 | 93.64 | ** |
| HCV | 409 | 1.06 | HCV | 7 | 6.36 | ||
| Cis | Cis | ||||||
| No HCV | 10,430,093 | 99.62 | No HCV | 4,965 | 97.64 | ||
| HCV | 39,300 | 0.38 | HCV | 120 | 2.36 | ||
| Gender Subgroups | n | % | P | Gender Subgroups | n | % | P |
| TFN | TFN | ||||||
| No HCV | 9,755 | 98.14 | *** | No HCV | 24 | 92.31 | ** |
| HCV | 185 | 1.86 | HCV | 2 | 7.69 | ||
| TMN | TMN | ||||||
| No HCV | 18,745 | 99.27 | No HCV | 69 | 95.83 | ||
| HCV | 137 | 0.73 | HCV | 3 | 4.17 | ||
| TGD Un. | TGD Unclassified | ||||||
| No HCV | 9,532 | 99.10 | No HCV | 10 | 83.33 | ||
| HCV | 87 | 0.90 | HCV | 2 | 16.67 | ||
| Cis Women | Cis Women | ||||||
| No HCV | 5,199,428 | 99.70 | No HCV | 2,587 | 98.14 | ||
| HCV | 15,830 | 0.30 | HCV | 49 | 1.86 | ||
| Cis Men | Cis Men | ||||||
| No HCV | 5,228,289 | 99.55 | No HCV | 2,377 | 97.10 | ||
| HCV | 23,461 | 0.45 | HCV | 71 | 2.90 | ||
| Cis Unclassified | Cis Unclassified | ||||||
| No HCV | 2,376 | 99.62 | No HCV | 1 | 100% | ||
| HCV | 9 | 0.38 | HCV | -- | -- | ||
Cis, Cisgender; P, P-value; TGD, Transgender and gender diverse; TFN, Trans feminine and non-binary; TMN, Trans masculine and non-binary. Note: Unadjusted frequency of hepatitis C virus (HCV) diagnoses among entire sample and people who inject drugs stratified by gender in Optum’s de-identified Clinformatics® Data Mart Database from 2001–2019. Race: Asian, Black, and White categories are non-Hispanic. P-values were derived from Fisher’s exact test for gender subgroups among people who inject drugs; χ2 tests were used for all other analyses.
Boldface indicates statistical significance (*p≤0.05, **p≤0.01, ***p≤0.001).
Table 2.
Adjusted Multivariable Models of Gender and Hepatitis C virus (HCV) Diagnoses, 2001–2019
| Entire Sample (N=10,507,834) | People who inject drugs (N=5,195) | ||||||
|---|---|---|---|---|---|---|---|
| HCV Diagnosis | HCV Diagnosis | ||||||
| Characteristic | aOR | 95% CI | P | Characteristic | aOR | 95% CI | P |
| PRIMARY PREDICTOR | PRIMARY PREDICTOR | ||||||
| Gender Subgroups | Gender Subgroups | ||||||
| Cis Women | Ref. | -- | -- | Cis Women | Ref. | -- | -- |
| Cis Men | 1.64 | 1.61–1.67 | *** | Cis Men | 1.51 | 1.04–2.19 | * |
| Cis Unclassified | 1.76 | 0.91–3.40 | 0.09 | Cis Unclassified | -- | -- | -- |
| TFN | 5.09 | 4.39–5.90 | *** | TFN | 4.37 | 0.98–19.39 | 0.05 |
| TMN | 2.28 | 1.93–2.71 | *** | TMN | 2.20 | 0.66–7.32 | 0.20 |
| TGD Unclassified | 4.19 | 3.39–5.19 | *** | TGD Unclassified | 7.83 | 1.62–37.94 | ** |
| CONTROL VARIABLES | CONTROL VARIABLES | ||||||
| Race | Race | ||||||
| White | Ref. | -- | -- | White | Ref. | -- | -- |
| Asian | 1.05 | 0.99–1.11 | 0.08 | Asian | -- | -- | -- |
| Black | 1.68 | 1.63–1.73 | *** | Black | 1.93 | 1.16–3.21 | ** |
| Hispanic | 1.17 | 1.13–1.21 | *** | Hispanic | 0.82 | 0.41–1.66 | 0.58 |
| Unknown | 0.89 | 0.87–0.91 | *** | Unknown | 1.17 | 0.68–2.01 | 0.57 |
| Region | Region | ||||||
| Midwest | Ref. | -- | -- | Midwest | Ref. | -- | -- |
| South | 1.66 | 1.62–1.71 | *** | South | 1.34 | 0.83–2.15 | 0.23 |
| West | 1.39 | 1.34–1.44 | *** | West | 1.33 | 0.73–2.43 | 0.35 |
| Northeast | 1.68 | 1.61–1.74 | *** | Northeast | 2.84 | 1.65–4.86 | *** |
| Unknown | Unknown | -- | -- | -- | |||
| Enrollment Age (years) | 1.04 | 1.04–1.04 | *** | Age of First IDU (years) | 0.99 | 0.98–1.00 | 0.03 |
| Enrollment Months | 1.01 | 1.01–1.01 | *** | Months Past IDU | 1.00 | 1.00–1.01 | 0.07 |
| Enrollment Year | 0.99 | 0.98–0.99 | *** | First Year of IDU | 1.02 | 0.98–1.06 | 0.33 |
aOR, Adjusted odds ratio; CI, Confidence interval; Cis, Cisgender; IDU, Injection drug use; P, P-value; TFN, Trans feminine and non-binary; TMN, Trans masculine and non-binary. Note: Adjusted multivariable models examining the association between gender and hepatitis C virus (HCV) diagnoses among entire sample and people who inject drugs in Optum’s de-identified Clinformatics® Data Mart Database from 2001–2019. Race: Asian, Black, and White categories are non-Hispanic. Variables with missing data indicate dropped observations from perfect predictions. Outcome: Hepatitis C virus (HCV) diagnosis (ref=no HCV diagnosis). Model adjusted for all listed demographic and enrollment characteristics.
Boldface indicates statistical significance (*p≤0.05, **p≤0.01, ***p≤0.001).
Figure 1 displays the unadjusted prevalence of HCV testing among adults by gender from 2001 to 2019. Across the study period, TGD adults had a higher prevalence of testing each year and had an overall testing prevalence of 14.83% compared to 3.73% among cisgender adults (p<0.001) (Appendix Table 6). Among PWID, TGD individuals had a testing prevalence of 13% compared to 7% among cisgender PWID (p<0.045). Adjusting for demographic and enrollment characteristics, TMN (TMN: aOR: 2.67, 95% CI: 2.56–2.78, p<0.001) and TFN (aOR: 2.63, 95% CI: 2.49–2.79, p<0.001) had significantly higher odds of being tested for HCV during the study period than cisgender women (Appendix Table 7).
Figure 1.
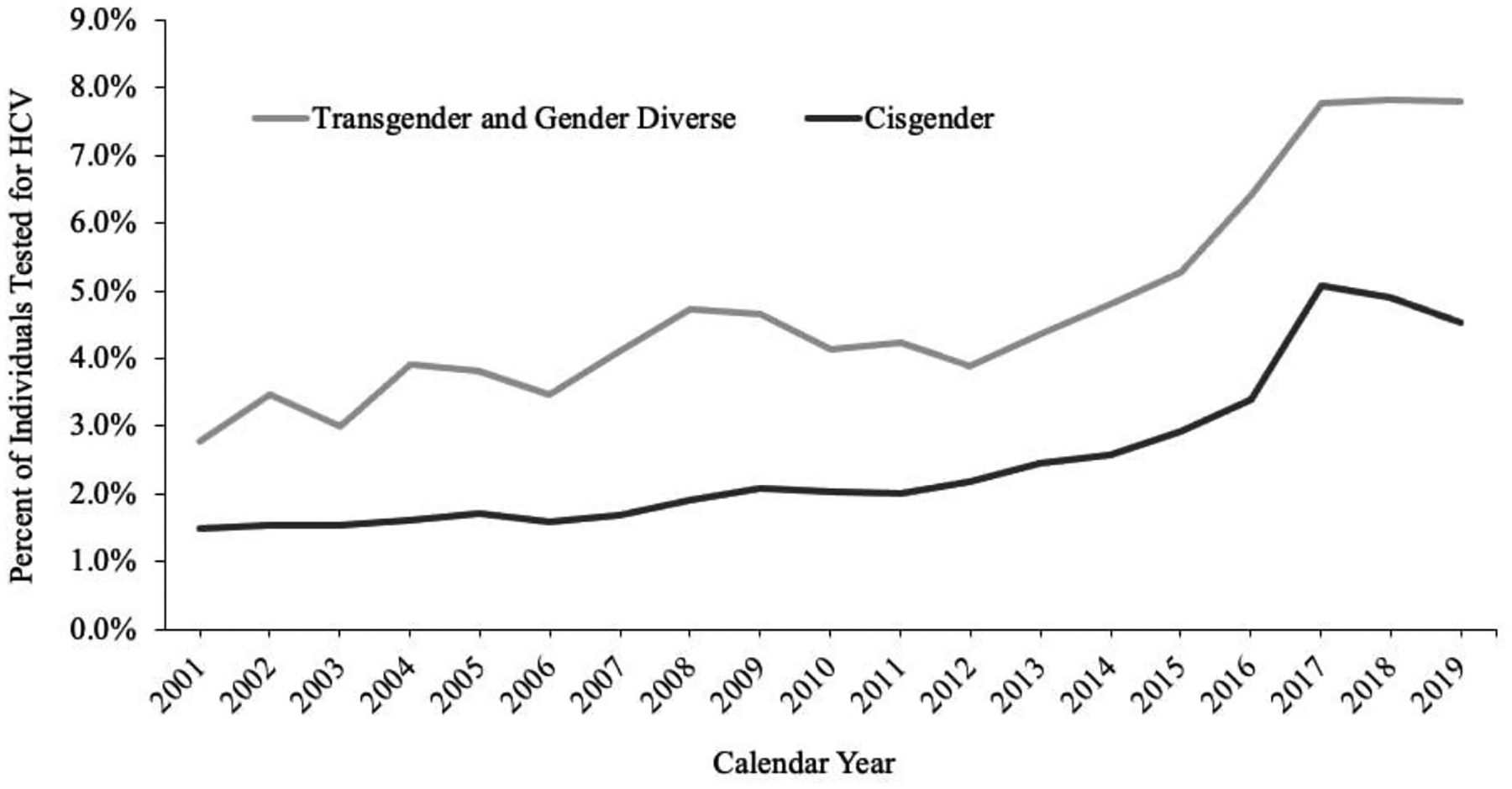
Unadjusted prevalence of hepatitis C virus (HCV) testing among all adults stratified by gender minority status in Optum’s de-identified Clinformatics® Data Mart Database from 2001–2019 (N=10,507,834)
HCV, Hepatitis C Virus. Guidelines by the Centers for Disease Control and Prevention (CDC) were updated in 2012 to recommend one-time HCV screening for individuals born between 1945–1965.42
Figure 2 displays the proportion of individuals with chronic HCV engaged in each cascade step. The prevalence of following up with a specialist was similar for TGD (77%) vs. cisgender individuals (74%; p=0.382). Overall, 95% of TGD individuals received an HCV-related procedure compared to 85% of cisgender individuals (p=.001). A higher proportion of TGD individuals (48%) received HCV medication than cisgender individuals (40%, p=0.045).
Figure 2.
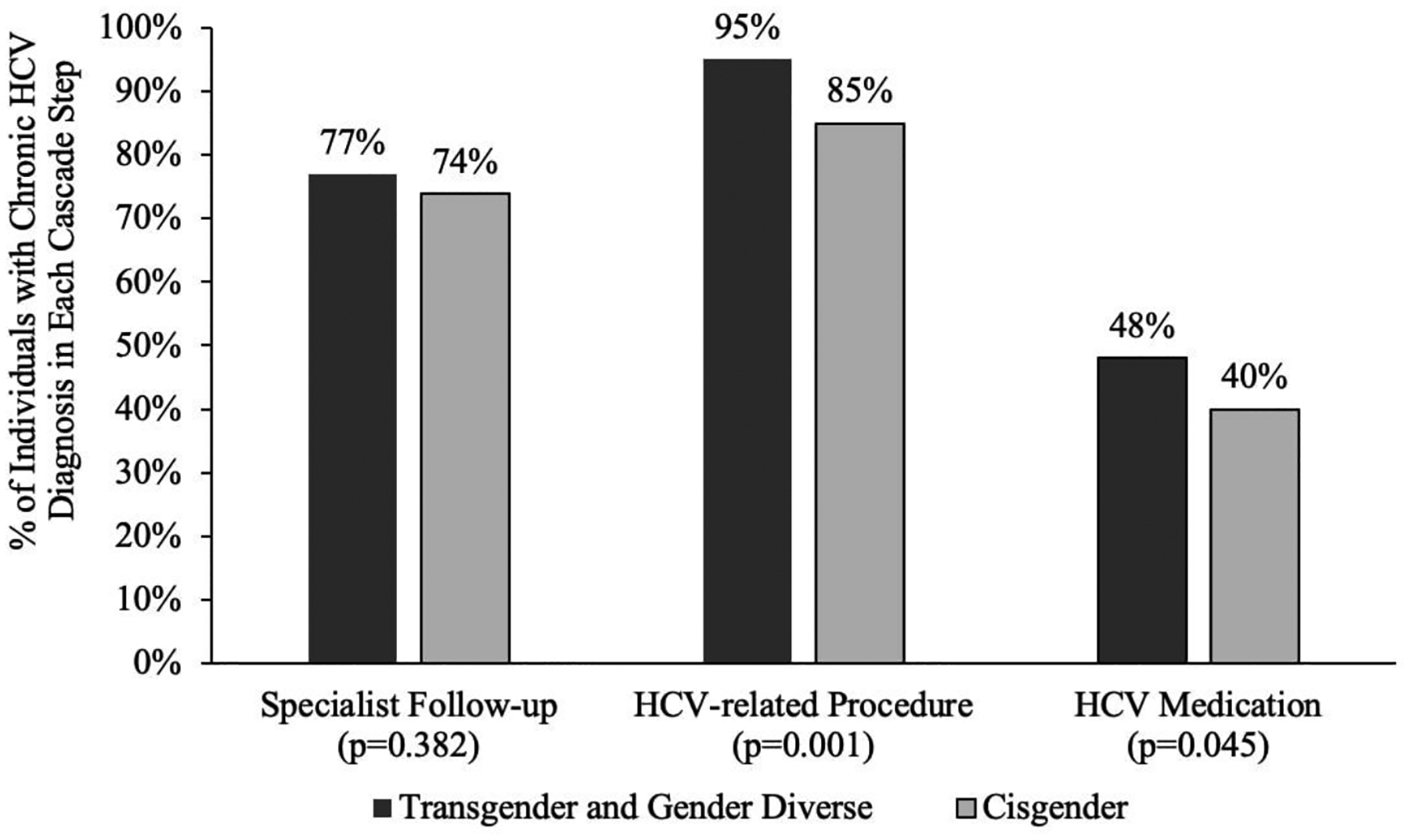
Individuals with chronic hepatitis C virus (HCV) engaged in each care cascade step stratified by gender minority status in Optum’s de-identified Clinformatics® Data Mart Database from 2001–2019 (N=13,134)
HCV, Hepatitis C Virus. P-values were derived from χ2 tests. Chronic HCV diagnosis: Transgender and gender diverse: N=149, Cisgender: N=12,985.
As shown in Appendix Table 8, in adjusted multivariable analyses, TFN individuals had significantly increased odds of having an HCV-related procedure than cisgender women (aOR: 2.69, 95% CI: 1.08–6.74, p=0.03). Additionally, being a cisgender man was significantly associated with increased odds of receiving HCV antiviral medication compared to cisgender women, although the effect size was small (aOR: 1.17, 95% CI: 1.09–1.26, p<0.001).
DISCUSSION
In this national sample of commercially insured TGD and cisgender adults, these findings confirm prior research documenting disparities in HCV among TGD vs. cisgender adults.2, 7, 11–14 Tailored interventions to prevent HCV among TGD individuals and continued efforts to ensure that all people at-risk for or living with HCV are tested and treated are warranted.
The frequency of HCV diagnosis was significantly higher among TGD individuals than cisgender individuals (1.06% versus 0.38%). Although most prior studies with TGD samples have reported a higher prevalence of HCV seropositivity among TGD individuals (0%−15.7% among trans feminine individuals),7–9, 11 most studies utilized convenience sampling to recruit potentially higher-risk samples and focused on large, urban locations that are known HCV “hotspots.”7, 9, 12–14 Although our study is based on commercially-insured individuals from across the U.S., our findings are consistent with prior survey research with high-risk, urban populations, as higher prevalence of HCV diagnoses among TFN individuals was observed relative to other groups.7, 9, 28 The higher frequency of HCV diagnoses among TFN individuals and TGD individuals overall relative to cisgender individuals could reflect a true higher prevalence, but may also be a result of increased testing among TGD individuals as well. Similar differences in HCV prevalence were also observed among the subsample of PWID. Further, even after adjusting for demographic and enrollment characteristics, TFN individuals with and without a history of injection drug use were significantly more likely to have been diagnosed with HCV than any other gender group.
Increased testing from 2012–2017, irrespective of gender, may be due to the increasing availability of direct-acting antiviral drugs and attention to United States Preventive Services Task Force (USPSTF) and Centers for Disease Control and Prevention (CDC) testing guidelines.29, 30 Since TGD individuals are at greater risk for sexually-acquired infectious and injection drug use than cisgender individuals,31–33 the higher prevalence of testing documented among TGD individuals in the sample may represent a recognition of this risk by medical providers and TGD individuals. Since claims data cannot be leveraged to identify potential avenues of infection, future research with TGD individuals that links self-reported sexual risk to medical data is needed to explore the drivers of HCV infection and testing.
To the authors’ knowledge, this is the first study that reports the prevalence of HCV diagnosis among TGD PWID, who were approximately twice as likely to be tested for HCV than cisgender PWID (13% vs. 7%). Higher overall HCV testing among PWID groups is at least partly attributable to longstanding screening guidelines for individuals who ever injected drugs.20 The finding that TGD PWID are more likely to be tested than cisgender PWID may be attributable to higher IDU, including sharing syringes for gender-affirming soft tissue fillers or hormones.34 Concentrating on intervention efforts to increase access to and utilization of harm reduction services, including syringe exchange programs and educational programs promoting protective sex, is paramount for lowering HCV prevalence in TGD populations.
Similar to testing prevalence, TGD individuals were more engaged in each step of the HCV care cascade than cisgender individuals. Prior research has found that accessing gender-affirming medical treatments may facilitate more engagement in other preventative or treatment-related healthcare services (e.g., HIV testing and treatment) as primary care providers may offer TGD individuals other forms of care when accessing gender-affirming services.35, 36 Given that the TGD individuals in the study had received a TGD-related diagnosis or related medical treatment, the acquisition of gender-affirming care may have facilitated engagement in HCV testing and treatment services. Future work should measure follow-up HCV care with primary care providers should data capabilities allow.
Although the levels of engagement in HCV testing and care were slightly higher among the TGD individuals in this sample than their cisgender counterparts, there is still room for improvement in treatment utilization among TGD individuals and cisgender individuals living with HCV. To that end, although cisgender men were significantly less likely than TFN and TMN individuals to have HCV, they had higher odds of receiving HCV medication in adjusted analyses. These results extend prior research, which found that more men than women were referred to and initiated HCV care, though the study did not account for gender minority status.37 Future mixed-methods research should examine the mechanisms underlying the differential referral to and initiation of HCV care based on gender and other key demographic attributes such as race and ethnicity.
These findings underscore the need for tailored interventions to prevent HCV acquisition, particularly among TFN subpopulations. It is essential to incorporate curriculums in medical schools, residencies, and CME programs that speak to the social and behavioral risk factors for HCV and include gender-affirming approaches to risk-based counseling and harm reduction approaches to reduce HCV risk for TGD individuals and others at risk for HCV. Further, clinicians should be trained to test and screen all individuals at risk for HCV, foster ways to reduce TGD-related stigma,38–40 and proactively link HCV-seropositive TGD and cisgender patients to treatment. Additionally, since out-of-pocket care costs and homelessness are barriers to HCV testing and treatment, particularly among TGD individuals,5, 7 future interventions should use community spaces and shelters to promote opportunities for free testing and treatment among low-income and at-risk TGD and cisgender adults.
Limitations
This study is not without limitations. Researchers have increasingly used claims datasets to measure disease diagnosis frequency and care engagement,24 but access to health insurance likely makes these subsets of individuals different from those without insurance. Other unique factors pertaining to some TGD patients that may lead to further engagement in health services, such as increased rates of HIV41 or receiving gender-affirming hormone therapy care, may lead to increased testing and treatment of HCV and other conditions. Further, it is not possible to determine if individuals engaged in HCV-related care for treatment of other health conditions (e.g., HIV co-infection, advanced liver disease). Due to data limitations, we were not able to ascertain if individuals sought HCV treatment through primary care. Considering access to specialist providers can be a barrier for TGD individuals and other subpopulations, some patients likely gained access to HCV treatment through primary care.
Misclassification bias is also possible when ascertaining TGD and IDU status via claims data proxies, in addition to excluding enrollees who are indeed TGD and/or a PWID but were not captured by the algorithms from not having the related codes. The algorithm used to identify TGD people only captures those TGD individuals who engage in a specific set of gender-affirming care practices and cannot be generalized to the overall TGD population. The algorithm does not identify those TGD people who (a) do not seek, have access to, or desire gender-affirming medical care through their insurer, (b) who engage in care but are denied gender-affirming care due to discrimination, stigma, or health conditions that preclude them from accessing gender-affirming care, or (c) do not bill for gender-affirming care through their insurance or seek it from untraditional medical sources. Additionally, individuals may have received HCV tests, such as point-of-care tests, outside of their usual healthcare and were therefore not captured by the algorithm. The avoidance of healthcare due to TGD and IDU-related stigma may have led to conservative estimates for all outcomes.16, 24
CONCLUSIONS
Leveraging insurance claims data revealed that TGD individuals had a higher frequency of HCV diagnosis and IDU prevalence than cisgender women and men and were more likely to be tested for HCV than cisgender individuals. Although fewer cisgender men had HCV than TFN and TMN individuals, cisgender men had significantly higher odds of receiving HCV medication. Future research efforts should focus on identifying the factors that facilitate testing and treatment uptake among TGD populations. Policies, guidelines, and interventions should also be developed to ensure equitable access to care for TGD and cisgender populations living with or at risk for HCV.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by the National Institute of Drug Abuse [grant number T32-DA041898 to H.L.W.]; COBRE on Opioids and Overdose funded by the National Institute of General Medical Sciences of the National Institutes of Health [grant number P20GM125507 to J.M.W.H.]; National Institute on Aging [grant number T32-AG000221 to L.D.H.]; the Eunice Kennedy Shriver National Institute of Child Health and Development [grant number T32-HD00733931 to L.D.H.]; and the Rackham Graduate School at the University of Michigan [Rackham Graduate Student Research Grant to L.D.H]. The University of Michigan Institutional Review Board determined that this study was exempt (HUM00161819).
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the funders, institutions, the Department of Veterans Affairs, National Institutes of Health, or the United States Government. The contents of this article have not been previously published nor presented elsewhere. No financial disclosures were reported by the authors of this paper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CRediT author statement
Hill L. Wolfe: Conceptualization, Methodology, Data Curation, Writing - Original Draft, Visualization; Jaclyn M.W. Hughto: Conceptualization, Methodology, Writing - Original Draft; Meg Quint: Conceptualization, Methodology, Data Curation, Writing - Original Draft; Leila Hasemi: Methodology, Writing - Original Draft; Landon D. Hughes: Methodology, Software, Formal analysis, Data Curation, Writing - Original Draft, Visualization
REFERENCES
- 1.Carobene M, Bolcic F, Farias MS, Quarleri J, Avila MM. HIV, HBV, and HCV molecular epidemiology among trans (transvestites, transsexuals, and transgender) sex workers in Argentina. J Med Virol. Jan 2014;86(1):64–70. doi: 10.1002/jmv.23805 [DOI] [PubMed] [Google Scholar]
- 2.Wilson EC, Turner C, Lin J, McFarland W, Burk K, Raymond HF. Hepatitis C seroprevalence and engagement in related care and treatment among trans women. J Viral Hepat. Jul 2019;26(7):923–925. doi: 10.1111/jvh.13089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nuttbrock L, Hwahng S, Bockting W, et al. Lifetime risk factors for HIV/sexually transmitted infections among male-to-female transgender persons. J Acquir Immune Defic Syndr. Nov 1 2009;52(3):417–21. doi: 10.1097/QAI.0b013e3181ab6ed8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poteat TC, Malik M, Beyrer C. Epidemiology of HIV, Sexually Transmitted Infections, Viral Hepatitis, and Tuberculosis Among Incarcerated Transgender People: A Case of Limited Data. Epidemiol Rev. Jun 1 2018;40(1):27–39. doi: 10.1093/epirev/mxx012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hernandez CJ, Trujillo D, Sicro S, et al. High hepatitis C virus seropositivity, viremia, and associated risk factors among trans women living in San Francisco, California. PLoS One. 2021;16(3):e0249219. doi: 10.1371/journal.pone.0249219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hofmeister MG, Rosenthal EM, Barker LK, et al. Estimating Prevalence of Hepatitis C Virus Infection in the United States, 2013–2016. Hepatology. Mar 2019;69(3):1020–1031. doi: 10.1002/hep.30297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mangla N, Mamun R, Weisberg IS. Viral hepatitis screening in transgender patients undergoing gender identity hormonal therapy. Eur J Gastroenterol Hepatol. Nov 2017;29(11):1215–1218. doi: 10.1097/MEG.0000000000000950 [DOI] [PubMed] [Google Scholar]
- 8.Porter JC, Lusk HM, Katz AR. Prevalence of HCV infection among clients in community-based health settings in Hawaii, 2002–2010: assessing risk factors. Am J Public Health. Aug 2014;104(8):1534–9. doi: 10.2105/AJPH.2013.301282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reisner SL, Vetters R, White JM, et al. Laboratory-confirmed HIV and sexually transmitted infection seropositivity and risk behavior among sexually active transgender patients at an adolescent and young adult urban community health center. AIDS Care. 2015;27(8):1031–6. doi: 10.1080/09540121.2015.1020750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.U.S. Centers for Disease Control and Prevention. Hepatitis C: By the Numbers. Accessed April 3, 2022, https://www.cdc.gov/nchhstp/newsroom/docs/factsheets/Hepatitis-c-by-the-numbers.pdf
- 11.Van Gerwen OT, Jani A, Long DM, Austin EL, Musgrove K, Muzny CA. Prevalence of Sexually Transmitted Infections and Human Immunodeficiency Virus in Transgender Persons: A Systematic Review. Transgend Health. Jun 1 2020;5(2):90–103. doi: 10.1089/trgh.2019.0053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Facente SN, Patel S, Hecht J, et al. Hepatitis C Care Cascades for 3 Populations at High Risk: Low-income Trans Women, Young People Who Inject Drugs, and Men Who Have Sex With Men and Inject Drugs. Clin Infect Dis. Sep 15 2021;73(6):e1290–e1295. doi: 10.1093/cid/ciab261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moradi G, Soheili M, Rashti R, et al. The prevalence of hepatitis C and hepatitis B in lesbian, gay, bisexual and transgender populations: a systematic review and meta-analysis. Eur J Med Res. Mar 26 2022;27(1):47. doi: 10.1186/s40001-022-00677-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shover CL, DeVost MA, Beymer MR, Gorbach PM, Flynn RP, Bolan RK. Using Sexual Orientation and Gender Identity to Monitor Disparities in HIV, Sexually Transmitted Infections, and Viral Hepatitis. Am J Public Health. Nov 2018;108(S4):S277–S283. doi: 10.2105/AJPH.2018.304751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luzzati R, Zatta M, Pavan N, et al. Prevalence of Human Immunodeficiency Virus, Hepatitis B Virus, and Hepatitis C Virus Infections Among Transgender Persons Referred to an Italian Center for Total Sex Reassignment Surgery. Sex Transm Dis. Jul 2016;43(7):407–11. doi: 10.1097/OLQ.0000000000000452 [DOI] [PubMed] [Google Scholar]
- 16.Hughto JMW, Hughes L, Yee K, et al. Improving Data-Driven Methods to Identify and Categorize Transgender Individuals by Gender in Insurance Claims Data. LGBT Health. Mar 15 2022;doi: 10.1089/lgbt.2021.0433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jasuja GK, de Groot A, Quinn EK, et al. Beyond Gender Identity Disorder Diagnoses Codes: An Examination of Additional Methods to Identify Transgender Individuals in Administrative Databases. Med Care. Oct 2020;58(10):903–911. doi: 10.1097/MLR.0000000000001362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.U.S. Centers for Disease Control and Prevention. Testing for HCV infection: An update of guidance for clinicians and laboratorians. MMWR 2013;62(18). Accessed June 21, 2022, https://www.cdc.gov/hepatitis/hcv/index.htm#:~:text=CDC%20now%20recommends%20one-time,adults%20%E2%80%93%20United%20States%2C%202020 [PMC free article] [PubMed] [Google Scholar]
- 19.U.S. Centers for Disease Control and Prevention. Hepatitis C Questions and Answers for the Public. Updated July 28, 2020. Accessed May 20, 2022, https://www.cdc.gov/hepatitis/hcv/cfaq.htm
- 20.Schillie S, Wester C, Osborne M, Wesolowski L, Ryerson AB. CDC Recommendations for Hepatitis C Screening Among Adults - United States, 2020. MMWR Recomm Rep. Apr 10 2020;69(2):1–17. doi: 10.15585/mmwr.rr6902a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Downing J, Lawley KA, McDowell A. Prevalence of Private and Public Health Insurance Among Transgender and Gender Diverse Adults. Med Care. Apr 1 2022;60(4):311–315. doi: 10.1097/MLR.0000000000001693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Orgera K, Tolbert J. Key Facts about Uninsured Adults with Opioid Use Disorder. 2019. Accessed September 25, 2022. https://www.kff.org/uninsured/issue-brief/key-facts-about-uninsured-adults-with-opioid-use-disorder/
- 23.Hughes LD, King WM, Gamarel KE, Geronimus AT, Panagiotou OA, Hughto JMW. US Black-White Differences in Mortality Risk Among Transgender and Cisgender People in Private Insurance, 2011–2019. Am J Public Health. Oct 2022;112(10):1507–1514. doi: 10.2105/AJPH.2022.306963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Janjua NZ, Islam N, Kuo M, et al. Identifying injection drug use and estimating population size of people who inject drugs using healthcare administrative datasets. Int J Drug Policy. May 2018;55:31–39. doi: 10.1016/j.drugpo.2018.02.001 [DOI] [PubMed] [Google Scholar]
- 25.Bull-Otterson L, Huang YA, Zhu W, King H, Edlin BR, Hoover KW. Human Immunodeficiency Virus and Hepatitis C Virus Infection Testing Among Commercially Insured Persons Who Inject Drugs, United States, 2010–2017. J Infect Dis. Aug 17 2020;222(6):940–947. doi: 10.1093/infdis/jiaa017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Isenhour Cheryl, Hariri S, Vellozzi C. Monitoring the Hepatitis C Care Cascade Using Administrative Claims Data. Am J Manag Care. 2018;24(5):232–238. [PMC free article] [PubMed] [Google Scholar]
- 27.Tran JN, Wong RJ, Lee JS, et al. Hepatitis C Screening Rates and Care Cascade in a Large US Insured Population, 2010–2016: Gaps to Elimination. Popul Health Manag. Apr 2021;24(2):198–206. doi: 10.1089/pop.2019.0237 [DOI] [PubMed] [Google Scholar]
- 28.Reback CJ, Fletcher JB. HIV prevalence, substance use, and sexual risk behaviors among transgender women recruited through outreach. AIDS Behav. Jul 2014;18(7):1359–67. doi: 10.1007/s10461-013-0657-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sullivan J, Soh JE, Khan MA, Thompson WW, Nelson NP. Trend analysis in hepatitis C testing, OptumLabs® Data Warehouse, 2011–2017. Online J Public Health Inform. 2019;11(1)doi: 10.5210/ojphi.v11i1.9806 [DOI] [Google Scholar]
- 30.Kardashian Ara A, Pockros JP. New Direct-Acting Antiviral Therapies for Treatment of Chronic Hepatitis C Virus Infection. Gastroenterol Hepatol (N Y). 2015;11(7):458–466. [PMC free article] [PubMed] [Google Scholar]
- 31.Denson DJ, Padgett PM, Pitts N, et al. Health Care Use and HIV-Related Behaviors of Black and Latina Transgender Women in 3 US Metropolitan Areas: Results From the Transgender HIV Behavioral Survey. J Acquir Immune Defic Syndr. Jul 1 2017;75 Suppl 3:S268–S275. doi: 10.1097/QAI.0000000000001402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pitasi MA, Kerani RP, Kohn R, et al. Chlamydia, Gonorrhea, and Human Immunodeficiency Virus Infection Among Transgender Women and Transgender Men Attending Clinics that Provide Sexually Transmitted Disease Services in Six US Cities: Results From the Sexually Transmitted Disease Surveillance Network. Sex Transm Dis. Feb 2019;46(2):112–117. doi: 10.1097/OLQ.0000000000000917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benotsch EG, Zimmerman RS, Cathers L, et al. Non-medical use of prescription drugs and HIV risk behaviour in transgender women in the Mid-Atlantic region of the United States. Int J STD AIDS. Aug 2016;27(9):776–82. doi: 10.1177/0956462415595319 [DOI] [PubMed] [Google Scholar]
- 34.Poteat T, Malik M, Scheim A, Elliott A. HIV Prevention Among Transgender Populations: Knowledge Gaps and Evidence for Action. Curr HIV/AIDS Rep. Aug 2017;14(4):141–152. doi: 10.1007/s11904-017-0360-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenberg SD, Goldberg RW, Dixon LB, et al. Assessing the STIRR model of best practices for blood-borne infections of clients with severe mental illness. Psychiatr Serv. Sep 2010;61(9):885–91. doi: 10.1176/ps.2010.61.9.885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Samuel ST, Martinez AD, Chen Y, Markatou M, Talal AH. Hepatitis C virus knowledge improves hepatitis C virus screening practices among primary care physicians. World J Hepatol. Feb 27 2018;10(2):319–328. doi: 10.4254/wjh.v10.i2.319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haley DF, Edmonds A, Ramirez C, et al. Direct-Acting Antiviral Hepatitis C Treatment Cascade and Barriers to Treatment Initiation Among US Men and Women With and Without HIV. J Infect Dis. Jun 15 2021;223(12):2136–2144. doi: 10.1093/infdis/jiaa686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.White Hughto JM, Reisner SL, Pachankis JE. Transgender stigma and health: A critical review of stigma determinants, mechanisms, and interventions. Soc Sci Med. Dec 2015;147:222–231 doi: 10.1016/j.socscimed.2015.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rodriguez A, Agardh A, Asamoah BO. Self-Reported Discrimination in Health-Care Settings Based on Recognizability as Transgender: A Cross-Sectional Study Among Transgender U.S. Citizens. Arch Sex Behav. May 2018;47(4):973–985. doi: 10.1007/s10508-017-1028-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolfe HL, Biello KB, Reisner SL, Mimiaga MJ, Cahill SR, Hughto JMW. Transgender-related discrimination and substance use, substance use disorder diagnosis and treatment history among transgender adults. Drug Alcohol Depend. Jun 1 2021;223:108711. doi: 10.1016/j.drugalcdep.2021.108711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Becasen JS, Denard CL, Mullins MM, Higa DH, Sipe TA. Estimating the Prevalence of HIV and Sexual Behaviors Among the US Transgender Population: A Systematic Review and Meta-Analysis, 2006–2017. Am J Public Health. Jan 2019;109(1):e1–e8. doi: 10.2105/AJPH.2018.304727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith BD, Morgan RL, Beckett GA, et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945–1965. MMWR Recomm Rep. 2012;61(RR-4):1–32. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


