Abstract
Background:
The safety and efficacy of using COVID-19 positive donors in heart transplantation (HT) are increasingly relevant, but not well established. The present study evaluated the characteristics and utilization of such donors and associated post-HT outcomes.
Methods:
All adult (≥ 18 years old) potential donors and HT recipients in the United States from April 21, 2020 to March 31, 2022 were included. Donor COVID-19 status was defined by the presence (or absence) of any positive test within 21 days of organ recovery. Donor and recipient characteristics and post-HT outcomes, including a primary composite of death, graft failure, and re-transplantation, were compared by donor COVID-19 status.
Results:
Of 967 COVID-19(+) potential donors, 19.3% (n = 187) were used for HT compared to 26.7% (n = 6277) of COVID-19(−) donors (p<0.001). Transplanted COVID-19(+) vs. COVID-19(−) donors were younger, but otherwise were similar. Recipients of hearts from COVID-19+ vs. COVID-19(−) donors less frequently received pre-HT inotropes (24.1% vs. 31.7%, p = 0.023) and ventricular assist device therapy (29.7% vs. 36.8%, p = 0.040). There were no significant differences in any post-HT outcome by donor COVID-19 status, including the primary composite outcome at 90 days (5.4% vs. 5.6%, p = 0.91). Among COVID-19(+) donors, the presence of a subsequent negative test prior to transplant was not associated with post-transplant outcomes.
Conclusions:
Our results suggest that carefully selected COVID-19 positive donors may be used for HT with no difference in short-term post-transplant outcomes. Additional data regarding donor and recipient treatments and impact of vaccination should be collected to better inform our use of organs from COVID(+) donors.
Introduction:
The novel coronavirus-19 (COVID-19) pandemic has posed immense challenges for heart transplant (HT) clinicians and their patients. Recommendations and practice have evolved rapidly regarding the use of donors with COVID-19 infection,1 which anecdotal reports suggest has become increasingly common.2–5 However, there has been no systematic evaluation to date of COVID-19 (+) donor utilization for HT in the United States (US) and its variation across centers and over time.
The earliest and largest experience to-date comes from the context of abdominal organ transplant.6,7,8 In an early cohort of 10 kidney transplants using five deceased donors with COVID-19 infection, all had excellent outcomes with no cases of donor-derived infection.8 Per Organ Procurement and Transplantation Network (OPTN) data, the only three known cases of donor-derived COVID-19 infection in the United States have occurred in the context of lung transplant – non-lung recipients from the same COVID-19(+) donors were not infected.9 Less data is available in the HT context, although the largest HT case series to-date (ranging from 3–12 cases) have reported no instances of donor-derived COVID-19 infection.10–13
Less is known, however, regarding the risk of other adverse outcomes. Given the known cardiovascular manifestations of COVID-19 (e.g. myocarditis, arrhythmias, and thrombosis),10,11 an effect on donor heart function is theoretically plausible, but data in this regard are limited. In light of these knowledge gaps, our current study examined 1) utilization of hearts from COVID-19(+) donors, including its variation across centers and over time; 2) the characteristics of COVID-19(+) donors and the patients who receive their hearts for transplant; and 3) short-term post-transplant outcomes among these recipients.
Methods:
This study used the OPTN/United Network for Organ Sharing (UNOS) database and included data collected by OPTN on donor COVID-19 testing. The sample consisted of all deceased donors from whom at least one organ was recovered for transplant between April 21, 2020 and March 31, 2022. Donors were classified as “COVID(+)” if they had a positive upper or lower respiratory tract nucleic acid (NAT) or antigen test within 21 days of organ recovery.12
Donor and recipient characteristics
Adult (≥ 18 years) COVID(+) positive donors were compared with COVID(−) adult donors during the study period. Within the subset of COVID(+) donors, those used vs. not used for heart transplant (HT) were compared. Donor variables examined included donor demographics, comorbidities, cause of death, results of cardiac diagnostic tests, and other clinical characteristics. For COVID-19 positive donors, the timing (relative to recovery date) and source (upper vs. lower respiratory) of positive tests were characterized. As multiple tests were available for some patients, secondary analyses were performed to compare the COVID(+) donors with (vs. without) a subsequent negative test prior to organ recovery.
Additional analyses compared adult HT recipients by the COVID-19 status of their donor. Recipient characteristics included demographics, comorbidities, therapies administered prior to transplant, and other clinical characteristics. Variation across centers, by region, and over time in the utilization of COVID(+) donors was also characterized.
Post-Transplant Outcomes
The primary outcome was time to a composite of death, graft failure, or re-transplantation. Secondary outcomes included 1) the above composite outcome assessed at 90 days post-HT, 2) time to discharge post-HT, 3) acute rejection and 4) dialysis therapy occurring during the index hospitalization (i.e. post-HT but prior to discharge). Outcomes were compared among recipients of COVID(+) vs. COVID(−) donors. Secondary analyses compared outcomes among COVID(+) donors with (vs. without) a subsequent negative test prior to organ recovery.
Statistical Analysis
In comparisons of donor and recipient characteristics, categorical and continuous variables were compared using chi-square and two-sample t-tests, respectively. Kaplan-Meier analyses were performed to assess differences in time to the primary outcome and time to discharge by subgroup, with significance assessed using the log-rank test. While our limited sample size precluded the use of robust multivariate adjustment in our primary analysis, we performed a secondary analysis using a Cox Proportional Hazards to assess the association of donor COVID-19 status with the primary outcome after adjustment for selected donor and recipient risk factors that were chosen on the basis of clinical plausibility. Time to discharge analyses excluded patients transplanted after January 1, 2022 (i.e. those with less than 90 days of potential follow-up time); the rationale for their exclusion is further detailed in Supplemental Methods. Binary outcomes were compared using chi-squared tests. In comparisons of acute rejection and dialysis therapy, recipients who had not been discharged by the end of the study period (and thus lacked data on these outcomes) were excluded. In comparisons of the composite outcome at 90 days, those transplanted on or after January 1, 2022 were excluded due to lack of sufficient follow-up time.
As data was obtained as part of routine care and de-identified by UNOS, Institutional Review Board approval was not required. Analyses were conducted using SAS version 9.4 and Microsoft Excel 2016.
Results
Comparison of donor characteristics, by COVID status and use for HT
Out of 24,488 adult donors with at least one solid organ recovered for transplant during the study period, 967 (4.0%) tested positive for COVID-19 (Figure 1). Most COVID-19 diagnoses were based on an upper respiratory sample (n = 742, 76.7%); 131 (13.6%) were positive on both upper and lower respiratory testing and the remaining 94 (9.7%) had a positive lower respiratory test only. The average duration between a donor’s first positive test and organ recovery was 7.4 days, and 43.2% (n = 418) of COVID(+) donors had a subsequent negative test prior to organ recovery. COVID(+) donors with (vs. without) a subsequent negative test were more often of non-White race, but did not differ significantly in other demographic or clinical characteristics (Supplemental Table 1).
Figure 1. Flowchart Depicting COVID-Positive Donors and Recipients.
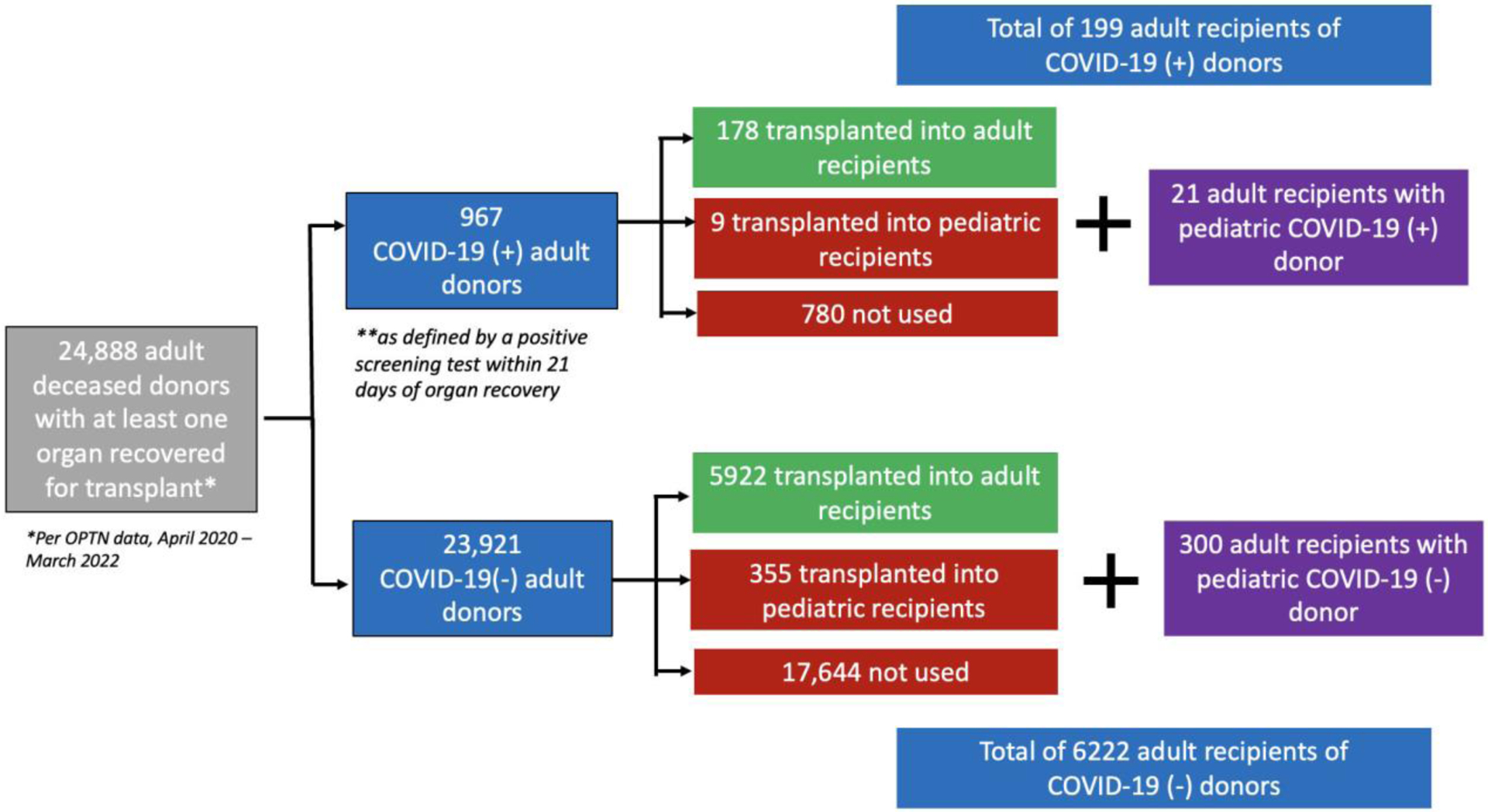
The utilization of COVID(+) and COVID (−) donors for transplantation during the study period is shown. COVID-19 = novel coronavirus-2019; HT = heart transplantation
Of the 967 COVID(+) donors, 187 (19.3%) were used for HT compared to 26.7% of COVID(−) donors (p <0.001). Among COVID(+) donors, those used (vs. not used) for HT were significantly younger (mean age 31.4 vs. 46.0 years, p <0.0001), more often male (81.8% vs 60.0%, p <0.001), and had fewer cardiovascular risk factors including hypertension and diabetes (Table 1). About two thirds (66.8%) of COVID(+) donors used for HT (vs. 37.6% of those not used for HT) had a subsequent negative COVID test before recovery. Those used for HT had a longer average duration since their first positive test (8.1 vs. 4.4 days, p <0.0001).
Table 1:
Characteristics of COVID-19 positive adult donors, by use (vs. non-use) for heart transplant (April 2020 - March 2022)
| All COVID(+) | Transplanted | Not transplanted | p-value7 | |
|---|---|---|---|---|
| Total n | 967 | 187 (19.3%) | 780 (80.7%) | |
| Demographics | ||||
| Female sex | 346 (35.8%) | 34 (18.2%) | 312 (40%) | <.0001 |
| Age (mean ± SD), years | 43.1 ± 13.4 | 31.4 ± 8.4 | 46.0 ± 12.8 | <.0001 |
| Age 18 – 34 | 283 (29.3%) | 124 (66.3%) | 159 (20.4%) | <.0001 |
| Age 35 – 49 | 347 (35.9%) | 59 (31.6%) | 288 (36.9%) | 0.17 |
| Age 50+ | 337 (34.9%) | 4 (2.1%) | 333 (42.7%) | <.0001 |
| Race | ||||
| White | 641 (66.3%) | 113 (60.4%) | 528 (67.7%) | 0.059 |
| Hispanic | 163 (16.9%) | 37 (19.8%) | 126 (16.2%) | 0.23 |
| Black | 134 (13.9%) | 32 (17.1%) | 102 (13.1%) | 0.15 |
| Asian or Other | 29 (3%) | 5 (2.7%) | 24 (3.1%) | 0.77 |
| Comorbidities | ||||
| Obese (BMI ≥ 30 kg/m2) | 444 (45.9%) | 64 (34.2%) | 380 (48.7%) | 0.0004 |
| Coronary artery disease1 | 78 (8.1%) | 1 (0.5%) | 77 (9.9%) | <.0001 |
| Smoking2 | 181 (18.7%) | 26 (13.9%) | 155 (19.9%) | 0.060 |
| Cocaine use2 | 184 (19%) | 48 (25.7%) | 136 (17.4%) | 0.01 |
| Intravenous drug use2 | 119 (12.3%) | 36 (19.3%) | 83 (10.6%) | 0.0013 |
| Diabetes mellitus | 143 (14.8%) | 9 (4.8%) | 134 (17.2%) | <.0001 |
| Hypertension | 324 (33.5%) | 25 (13.4%) | 299 (38.3%) | <.0001 |
| Cardiac diagnostic findings | ||||
| EF reported (%)3 | 580 (60%) | 187 (100%) | 393 (50.4%) | <.0001 |
| EF < 40% | 25 (8.5%) | 1 (0.5%) | 24 (12.2%) | <.0001 |
| EF 40–49% | 13 (2.5%) | 2 (1.1%) | 11 (5.6%) | 0.011 |
| EF ≥ 50% | 347 (89%) | 184 (98.4%) | 163 (82.2%) | <.0001 |
| Wall thickness reported (%)4 | 382 (39.5%) | 130 (69.5%) | 252 (32.3%) | <.0001 |
| mild LVH (1.2–1.3cm) | 65 (17%) | 25 (19.2%) | 40 (15.9%) | 0.41 |
| moderate+ LVH (≥ 1.4 cm) | 32 (8.4%) | 3 (2.3%) | 29 (11.5%) | 0.0021 |
| Other clinical characteristics | ||||
| Blood type | ||||
| A | 368 (38.1%) | 60 (32.1%) | 308 (39.5%) | 0.061 |
| B | 107 (11.1%) | 20 (10.7%) | 87 (11.2%) | 0.86 |
| AB | 32 (3.3%) | 0 (0%) | 32 (4.1%) | 0.0049 |
| O | 460 (47.6%) | 107 (57.2%) | 353 (45.3%) | 0.0033 |
| Cardiac downtime5 | 353 (39.6%) | 92 (54.8%) | 261 (36.1%) | <.0001 |
| Inotrope use | 236 (24.4%) | 47 (25.1%) | 189 (24.2%) | 0.80 |
| Acidemia (pH < 7.35) | 256 (26.5%) | 31 (16.6%) | 225 (28.9%) | 0.0006 |
| COVID test characteristics 6 | ||||
| Source of positive test | ||||
| Upper respiratory- only | 742 (76.7%) | 142 (75.9%) | 600 (76.9%) | 0.77 |
| Lower respiratory- only | 94 (9.7%) | 23 (12.3%) | 71 (9.1%) | 0.19 |
| Both upper and lower respiratory | 131 (13.6%) | 22 (11.8%) | 109 (14.0%) | 0.43 |
| Days since first positive test (mean ± SD) | 7.4 ± 10.3 | 8.1 ± 11.1 | 4.4 ± 5.3 | <.0001 |
| Subsequent negative test | 418 (43.2%) | 125 (66.8%) | 293 (37.6%) | <.0001 |
Includes either reported history of coronary artery disease or positive coronary angiogram
Includes current or prior use
Calculated prevalence of EF by strata (< 40, 40–49…) includes only donors for which EF was reported
Calculated prevalence of LVH by strata (mild, moderate+) includes only donors for which either LV posterior or septal wall thickness was reported. LVH classification was based on the higher of these two measures.
Refers to the occurrence of cardiac arrest between the time of brain death and donor organ recovery. Calculated prevalence excludes 75 donors (19 transplanted, 56 not transplanted) for which downtime was coded as “unknown”.
“Source of positive” is based only on tests performed within 21 days of donor recovery. “Days since first positive” is based on any tests performed within 60 days of donor recovery. “Subsequent negative” refers to any case where the last upper or lower respiratory test performed prior to donor recovery was negative.
Based on chi-squared test for categorical variables and t-test for continuous variables.
COVID = coronavirus-19; EF = ejection fraction; LVH = left ventricular hypertrophy
COVID(+) donors (n = 187) comprised 2.9% of the total number of adult donors (n = 6464) used for HT (Table 2). Transplanted COVID(+) [vs. COVID(−)] donors were slightly younger (mean age: 31.4 vs. 32.8 years, p = 0.0025), more often male (81.8% vs 71.6%, p = 0.0021), and less often had a cerebrovascular cause of death (8.0% vs. 13.5%, p = 0.029). There were no significant differences by donor COVID status in other clinical characteristics, including left ventricular systolic dysfunction, smoking, diabetes, and hypertension. Similarly, there were no significant differences in demographics, comorbidities, or cardiac diagnostic findings among initially COVID(+) heart transplant donors with (vs. without) a subsequent negative test result prior to organ recovery (Supplemental Table 2).
Table 2:
Characteristics of adult donors whose hearts were accepted for transplant, by COVID status (April 2020 - March 2022)
| All transplanted donors | COVID(+) | COVID(−) | p-value6 | |
|---|---|---|---|---|
| Total n | 6464 | 187 (2.9%) | 6277 (97.1%) | |
| Demographics | ||||
| Female sex | 1818 (28.1%) | 34 (18.2%) | 1784 (28.4%) | 0.0021 |
| Age (mean ± SD), years | 32.8 ± 9.6 | 31.4 ± 8.4 | 32.8 ± 9.6 | 0.025 |
| Age 18 – 34 | 3852 (59.6%) | 124 (66.3%) | 3728 (59.4%) | 0.057 |
| Age 35 – 49 | 2219 (34.3%) | 59 (31.6%) | 2160 (34.4%) | 0.42 |
| Age 50+ | 393 (6.1%) | 4 (2.1%) | 389 (6.2%) | 0.022 |
| Race | ||||
| White | 4002 (61.9%) | 113 (60.4%) | 3889 (62%) | 0.67 |
| Black | 1195 (18.5%) | 37 (19.8%) | 1158 (18.5%) | 0.64 |
| Hispanic | 1070 (16.6%) | 32 (17.1%) | 1038 (16.5%) | 0.83 |
| Asian or Other | 197 (3.1%) | 5 (2.7%) | 192 (3.1%) | 0.76 |
| Comorbidities | ||||
| Obese (BMI ≥ 30 kg/m2) | 2006 (31%) | 64 (34.2%) | 1942 (30.9%) | 0.34 |
| Coronary artery disease1 | 111 (1.7%) | 1 (0.5%) | 110 (1.8%) | 0.21 |
| Smoking2 | 773 (12%) | 26 (13.9%) | 747 (11.9%) | 0.41 |
| Cocaine use2 | 1674 (25.9%) | 48 (25.7%) | 1626 (25.9%) | 0.94 |
| Intravenous drug use2 | 1153 (17.8%) | 36 (19.3%) | 1117 (17.8%) | 0.61 |
| Diabetes mellitus | 257 (4%) | 9 (4.8%) | 248 (4%) | 0.55 |
| Hypertension | 978 (15.1%) | 25 (13.4%) | 953 (15.2%) | 0.50 |
| Cardiac diagnostic findings | ||||
| EF3 | ||||
| < 40% | 26 (0.4%) | 1 (0.5%) | 25 (0.4%) | 0.77 |
| 40–49% | 56 (0.9%) | 2 (1.1%) | 54 (0.9%) | 0.76 |
| ≥ 50% | 6376 (98.7%) | 184 (98.4%) | 6192 (98.7%) | 0.81 |
| Wall thickness reported (%)4 | 4663 (72.1%) | 130 (69.5%) | 4533 (72.2%) | 0.42 |
| any LVH (≥ 1.2 cm) | 774 (16.6%) | 28 (21.5%) | 746 (16.5%) | 0.12 |
| mild LVH (1.2–1.3cm) | 599 (12.8%) | 25 (19.2%) | 574 (12.6%) | 0.027 |
| moderate+ LVH (≥ 1.4 cm) | 175 (3.8%) | 3 (2.3%) | 172 (3.8%) | 0.38 |
| Other clinical characteristics | ||||
| Blood type | ||||
| A | 2185 (33.8%) | 60 (32.1%) | 2125 (33.9%) | 0.61 |
| B | 679 (10.5%) | 20 (10.7%) | 659 (10.5%) | 0.93 |
| AB | 105 (1.6%) | 0 (0%) | 105 (1.7%) | 0.075 |
| O | 3495 (54.1%) | 107 (57.2%) | 3388 (54%) | 0.38 |
| Cardiac downtime5 | 2861 (50.2%) | 92 (54.8%) | 2769 (50.1%) | 0.23 |
| Inotrope use | 2025 (31.3%) | 47 (25.1%) | 1978 (31.5%) | 0.064 |
| Acidemia (pH < 7.35) | 805 (12.5%) | 31 (16.6%) | 774 (12.3%) | 0.083 |
Includes either reported history of coronary artery disease or positive coronary angiogram
Includes current or prior use
Calculated prevalence of EF by strata (< 40, 40–49…) includes only donors for which EF was reported
Calculated prevalence of LVH by strata (mild, moderate+) includes only donors for which either LV posterior or septal wall thickness was reported. LVH classification was based on the higher of these two measures.
Refers to the occurrence of cardiac arrest between the time of brain death and donor organ recovery. Calculated prevalence excludes 767 donors (19 COVID(+), 748 COVID−) for which downtime was coded as “unknown”.
Based on chi-squared test for categorical variables and t-test for continuous variables.
COVID = coronavirus-19; EF = ejection fraction; LVH = left ventricular hypertrophy
Variation in COVID-19(+) donor utilization, by center and over time
Of the 130 centers that performed at least one HT during the study period, 42 centers (32.3%) performed at least two transplants with COVID(+) donors over the study period. Seventeen (13.1%) only used one COVID(+) donor and seventy-one (54.6%) centers did not use any COVID(+) donors for HT. Figure 2 shows the distribution of the number and percentage of HTs using a COVID(+) donor in the 42 centers that accepted hearts from at least 2 COVID(+) donors, which ranged from 2 to 19 HTs per center (1.2% to 17.0% of all transplants performed by each site).
Figure 2. Variation across centers in the use of COVID-positive donors for heart transplant (April 2020 – March 2022).
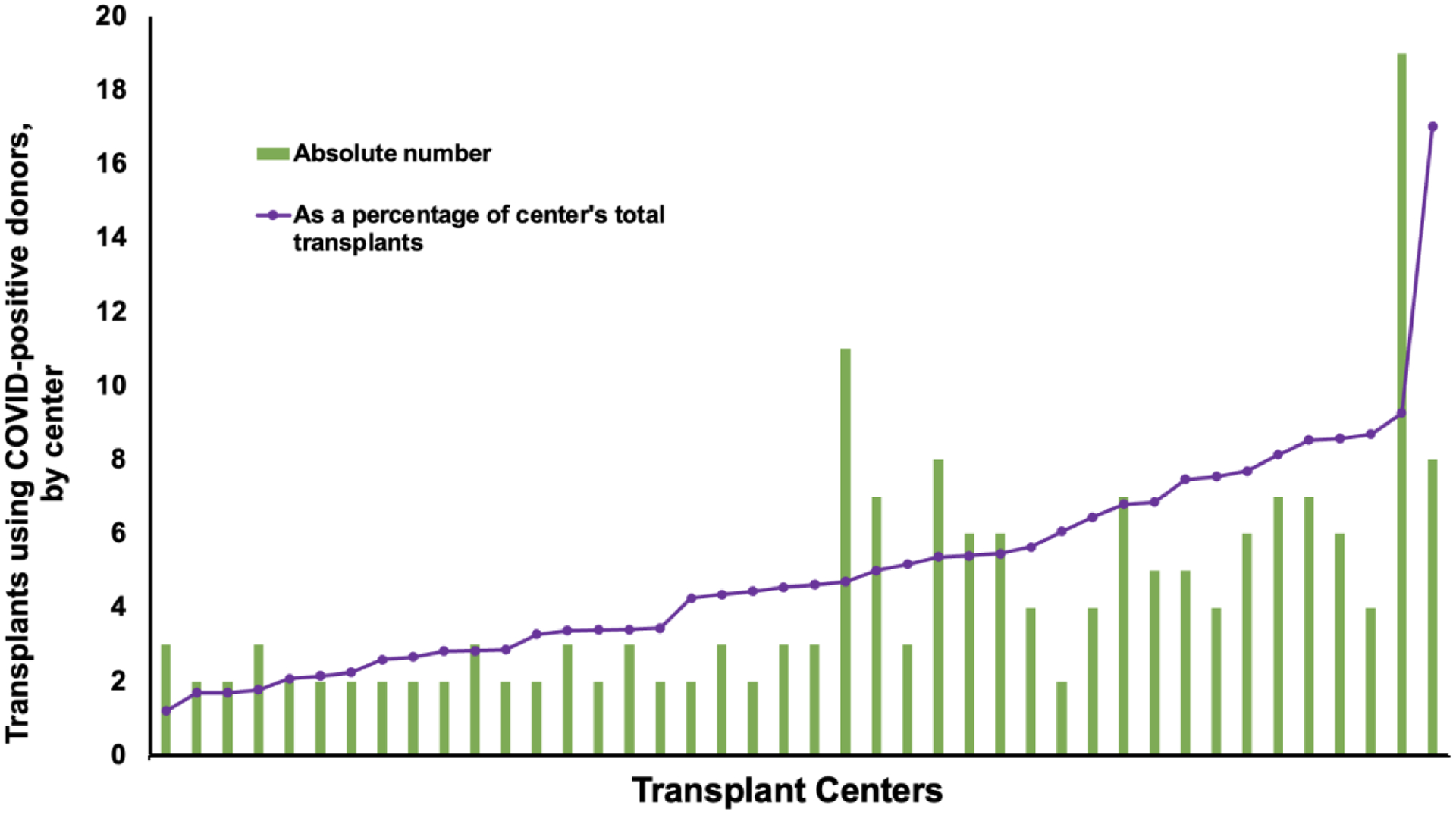
Centers that used at least 2 COVID(+) donors for HT were included. The blue bars represent the absolute number of COVID(+) donors used during the study period, whereas the orange line represents the COVID(+) donors as a percentage of the total transplants by that center during the study period.
COVID(+) donor utilization increased significantly over time (p < 0.001), as shown in Figure 3. COVID(+) donors were used in less than 1% of all HTs prior to March 2021. From March – September 2021, this percentage ranged from 1 – 3% of all HTs. Over the subsequent six months (October 2021 – March 2022), there were a total of 148 HTs using COVID(+) donors (9.1% of total HT volume). Use of COVID(+) donors varied by UNOS region with the greatest proportion of transplants with COVID(+) donors occurring in the Northeast US (UNOS regions 2 and 9) (Supplemental Figure 1).
Figure 3. Use of COVID-19 Positive Donors for HT Over Time.
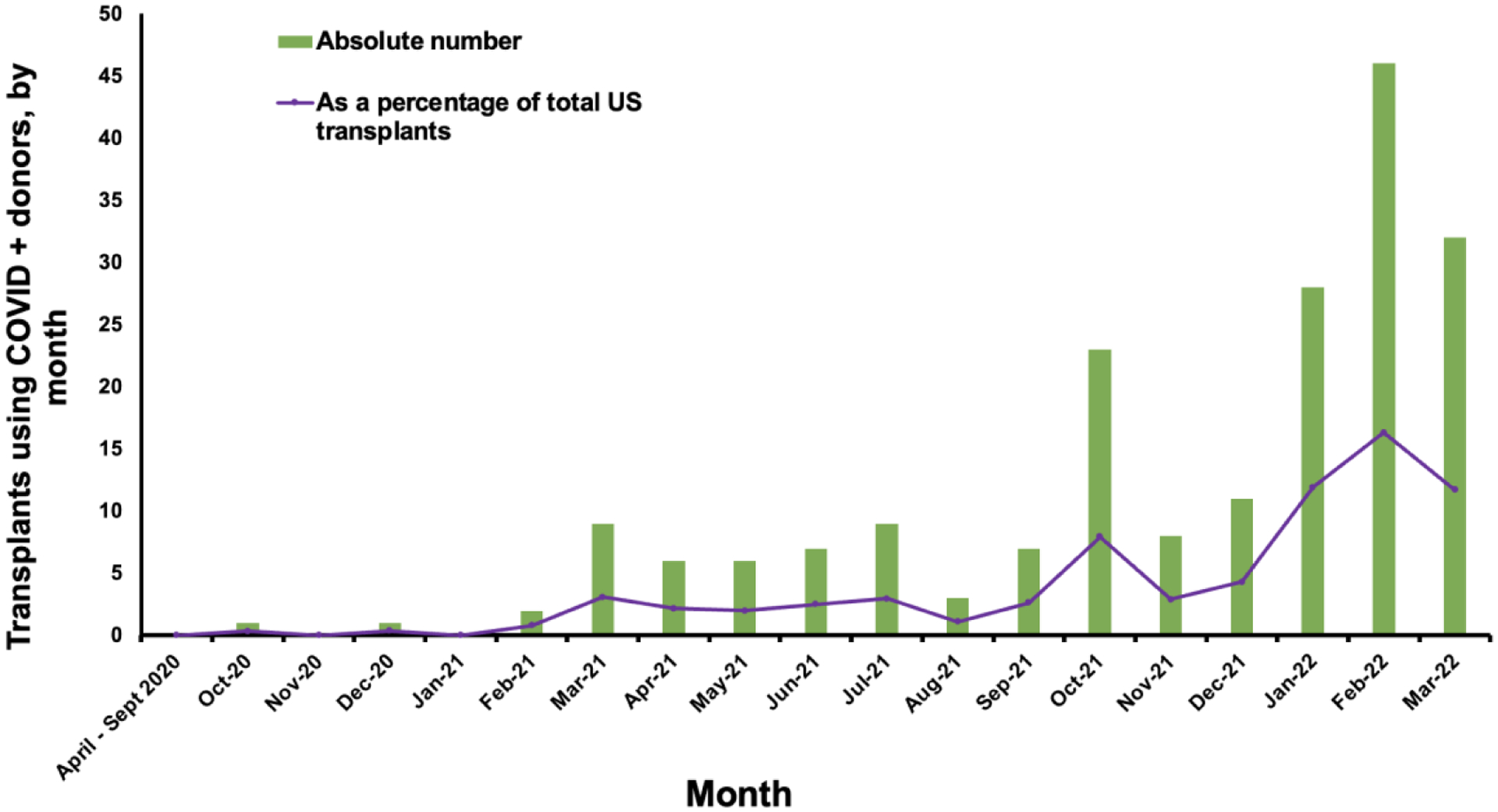
Use of COVID(+) donors for heart transplantation is shown by month from April 2020 through March 2022. The blue bars represent the absolute number of heart transplants performed using COVID(+) donors while the orange line represents the percentage of heart transplants from COVID(+) donors relative to the total number of heart transplants in the US.
Recipient characteristics, by donor COVID-19 status
During the study period, 6421 patients underwent HT. Of these, 199 adults (3.1%) received a heart from a COVID(+) donor (Table 3). There were no significant differences in recipient age, race, etiology of heart failure, or UNOS status at transplant by donor COVID-19 status. Patients who received a COVID(+) donor had lower rates of inotrope (29.7% vs. 37%, p = 0.04), durable ventricular assist device (VAD) (24.1% vs. 32%, p = 0.02), and intra-aortic balloon pump (20.6% vs 26%, p = 0.08) therapies at the time of transplant. Notably, our recipient sample includes 21 adults who received a heart from a COVID-19+ pediatric donor (with median age 15 years, range 10 – 17 years); one of these pediatric donors had hypertension, two were obese, and none had other comorbidities or cardiac diagnostic abnormalities (of those listed in Table 2).
Table 3:
Characteristics of heart transplant recipients, by donor COVID status (April 2020 - March 2022)
| All recipients | Recipients of COVID(+) donors | Recipients of COVID(−) donors | p-value† | |
|---|---|---|---|---|
| Total n* | 6421 | 199 (3.1%) | 6222 (96.9%) | |
| Demographics | ||||
| Female sex | 1685 (26.2%) | 44 (22.1%) | 1641 (26.4%) | 0.18 |
| Age (mean ± SD), years | ||||
| Age 18 – 39 | 1017 (15.8%) | 38 (19.1%) | 979 (15.7%) | 0.20 |
| Age 40 – 59 | 2846 (44.3%) | 82 (41.2%) | 2764 (44.4%) | 0.37 |
| Age 60+ | 2558 (39.8%) | 79 (39.7%) | 2479 (39.8%) | 0.97 |
| Race | ||||
| White | 3831 (59.7%) | 122 (61.3%) | 3709 (59.6%) | 0.63 |
| Hispanic | 1602 (25.0%) | 46 (23.1%) | 1556 (25%) | 0.54 |
| Black | 659 (10.3%) | 21 (10.6%) | 638 (10.3%) | 0.89 |
| Asian or Other | 329 (5.1%) | 10 (5.0%) | 319 (5.1%) | 0.95 |
| Comorbidities | ||||
| Obese (BMI ≥ 30 kg/m2) | 2105 (32.8%) | 70 (35.2%) | 2035 (32.7%) | 0.47 |
| Smoking | 2580 (40.2%) | 85 (42.7%) | 2495 (40.1%) | 0.46 |
| Diabetes mellitus | 1882 (29.3%) | 58 (29.2%) | 1824 (29.3%) | 0.96 |
| Therapies prior to transplant | ||||
| Extra-corporeal membrane oxygenation | 375 (5.8%) | 10 (5.0%) | 365 (5.9%) | 0.62 |
| Intra-aortic balloon pump | 1672 (26.0%) | 41 (20.6%) | 1631 (26.2%) | 0.076 |
| Ventricular assist device | 2021 (31.5%) | 48 (24.1%) | 1973 (31.7%) | 0.023 |
| Inotropes | 2346 (36.5%) | 59 (29.7%) | 2287 (36.8%) | 0.040 |
| Dialysis | 397 (6.2%) | 9 (4.5%) | 388 (6.2%) | 0.30 |
| Prior heart transplant | 125 (1.9%) | 0 (0.0%) | 125 (2.0%) | 0.53 |
| Prior cardiac surgery (non-transplant) | 2267 (35.3%) | 68 (34.2%) | 2199 (35.3%) | 0.73 |
| Other clinical characteristics | ||||
| Etiology of heart failure | ||||
| Ischemic | 1764 (27.5%) | 55 (27.6%) | 1709 (27.5%) | 0.96 |
| Congenital | 300 (4.7%) | 11 (5.5%) | 289 (4.6%) | 0.56 |
| Hypertrophic/restrictive | 503 (7.8%) | 11 (5.5%) | 492 (7.9%) | 0.22 |
| Non-ischemic or other | 3854 (60.0%) | 122 (61.3%) | 3732 (60.0%) | 0.71 |
| Multi-organ transplant | 722 (11.2%) | 18 (9.1%) | 704 (11.3%) | 0.32 |
| UNOS status at transplant | ||||
| 1 | 639 (10.0%) | 18 (9.1%) | 621 (10.0%) | 0.66 |
| 2 | 3107 (48.4%) | 94 (47.2%) | 3013 (48.4%) | 0.74 |
| 3 | 977 (15.2%) | 31 (15.6%) | 946 (15.2%) | 0.89 |
| 4 | 1295 (20.2%) | 45 (22.6%) | 1250 (20.1%) | 0.38 |
| 5 | 58 (0.9%) | 2 (1.0%) | 56 (0.9%) | 0.88 |
| 6 | 345 (5.4%) | 9 (4.5%) | 336 (5.4%) | 0.59 |
Post-Transplant Outcomes
Among the 199 adult recipients of COVID(+) donor hearts, median follow-up time was 35 days (IQR 15–166 days) and 131 (65.8%) had discharge outcomes available (Table 4). The prevalence of acute rejection prior to discharge was numerically lower among recipients of COVID(+) [vs. COVID(−)] donors, but did not meet statistical significance (11.4% vs. 17.4%, p = 0.077). The prevalence of the composite outcome (death, graft failure or re-transplantation at 90 days post-HT) was similar among recipients of COVID(+) [vs. COVID(−)] donors [5.4% (n = 5) vs. 5.6% (n = 312), respectively; p = 0.91]. Kaplan-Meier analyses comparing recipients of COVID(+) and COVID(−) donors (Supplemental Figure 2) showed no difference in time to discharge (p = 0.23) or time to the composite outcome (p = 0.27). Whether or not a COVID(+) donor had a subsequent negative test prior to organ recovery had no association with the primary composite or any secondary outcome (Supplemental Table 3). Donor COVID-19 status was not associated with the primary composite outcome after multivariate adjustment (HR 0.69, 95% confidence interval 0.38 – 1.26; Supplemental Table 4).
Table 4:
Selected outcomes among heart transplant recipients, by donor COVID status (April 2020 - March 2022)
| All recipients | COVID(+) donors | COVID(−) donors | p-value† | |
|---|---|---|---|---|
| Total (n) with discharge outcomes available | 5735 | 131 | 5604 | |
| Days from transplant to discharge (mean ± SD) | 23.4 ± 23.1 | 22.3 ± 20.1 | 23.4 ± 23.2 | 0.58 |
| Acute rejection prior to discharge | 988 (17.2%) | 15 (11.4%) | 973 (17.4%) | 0.077 |
| Dialysis prior to discharge | 883 (15.4%) | 23 (17.6%) | 860 (15.4%) | 0.49 |
| Total (n) with at least 90 days of potential follow-up* | 5627 | 93 | 5534 | |
| Composite outcome (death, graft failure, or re-transplant) at 90 days | 317 (5.6%) | 5 (5.4%) | 312 (5.6%) | 0.91 |
i.e. date of transplant before 1/1/22
Based on chi-squared test for categorical variables and t-test for continuous variables.
Ten recipients of hearts from COVID(+) donors experienced the composite outcome at any time after transplant, at a mean of 55.7 days after transplant (range 0 – 222 days); these cases are further detailed in Supplemental Table 5, and include two recipients with graft failure resulting in re-transplantation, three with graft failure resulting in death, and five with death from another cause.
Discussion
Our study sought to examine the utility and safety of using hearts from COVID(+) donors for transplantation. Our major findings are the following: 1) carefully selected COVID(+) donors comprise an increasing minority of heart transplants; 2) COVID(+) donors were more likely to be younger and male, but otherwise had similar characteristics compared to COVID(−) donors whose hearts used for transplant; 3) recipients of hearts from COVID(+) donors are similar to those who received hearts from COVID(−) donors, with comparable short-term outcomes; 4) there were no significant differences in clinical characteristics or post-transplant outcomes between those COVID(+) donors with a subsequent negative test and those without. Taken together, these findings suggest that COVID(+) donors, even in the absence of a subsequent negative test, can be safely used for heart transplantation.
While the use of COVID(+) donors for HT has increased over time, they remain underutilized compared with COVID(−) donors. Moreover, we find that less than one third of US centers have used more than one COVID(+) donor for HT. This finding is consistent with a recent survey of heart transplant providers and medical directors of heart transplant centers, most of whom report that they would not consider accepting a donor with a positive COVID-19 nasopharyngeal PCR (even with a negative bronchoalveolar lavage).13 Our study’s findings suggest that this majority view should be reconsidered - especially as (despite our best public health efforts) COVID(+) donors are likely to comprise a significant proportion of the donor pool for the foreseeable future.
Herein we describe the largest cohort of recipients of hearts from COVID(+) donors. Although the sample size is small and more data are needed, short-term graft outcomes appear acceptable. This is consistent with other recent reports. In two multi-organ analyses of OPTN data by Schold et al and Bock et al, 62 and 18 COVID-19 heart transplants were included, respectively.1,6 They similarly found that COVID-19 positive donor hearts were significantly less likely to be recovered despite similar outcomes. Notably, since the end of the Bock study period in August 2021, there have been an additional 137 heart transplants using COVID(+) donors included in this analysis. In addition to sample size, strengths of our analysis include more detailed characterization of donor and recipient characteristics and of center- and region-level variation.
There are limitations to our analysis that should be acknowledged. Given limited follow-up time, further studies are necessary to assess longer-term outcomes in this cohort. Markers of the severity of donor infection including symptoms and cycle threshold were unavailable; it remains possible that the subset of donors with severe infection confers additional risk. Additionally, data regarding specific COVID-19 variants, vaccination status of recipients, as well as use of antiviral therapies including monoclonal antibodies post-HT were not available. Furthermore, repeat test results were available for only a subset of the donors included. The donors without a subsequent negative test may not necessarily have been viremia at the time of recovery or transplant.
However, data regarding the role of vaccination and COVID-related therapies can be gleaned from single center reports detailing their successful experience with HT. Westchester Medical Center described their experience with organs from 12 COVID(+) donors who were transplanted into 14 recipients, including 3 hearts.2 None of the three HT recipients were vaccinated prior to transplantation. One was on extracorporeal membrane oxygenation at the time of transplant, one was on an intra-aortic balloon pump, and the third was Status 2 but not on mechanical circulatory support. There was no clinical or molecular evidence of transmission of SARS-CoV2 in any recipients.2 At one month follow-up, all had excellent graft function. Another analysis from the United Kingdom assessed 24 COVID(+) donors whose organs were used for 64 transplants, including 3 heart transplants.3 There was only one case of donor-derived infection, in a lung recipient who tested positive 5 days after transplantation. The largest single center report describes 12 HT with donors who tested positive for COVID-19 on any test.4 Ten of the 12 recipients had received at least 2 doses of mRNA vaccine against COVID-19 prior to transplant. No recipients developed signs or symptoms of COVID-19 infection. One recipient received pre-exposure prophylaxis with tixagevimab and cilgavimab but none required treatment for COVID-19.4 A similar experience with 8 HT using COVID(+) donors had no evidence of viral transmission. All patients received pre-exposure prophylaxis with tixagevimab and cilgavimab prior to hospital discharge5.
In summary, carefully selected COVID(+) donors can be used for HT with no difference in early post-transplant outcomes. These data suggest that donor COVID status should not be an isolated factor contributing to donor turndown, particularly given a limited donor pool. Data regarding longer-term outcomes will be needed.
Supplementary Material
Acknowledgements:
We would like to acknowledge Denise Tripp at the United Network for Organ Sharing for her assistance with the dataset used for this manuscript.
Disclosures/Sources of Funding:
Dr. Wayda receives support from the NIH/NHLBI F32 fellowship award titled “Narrowing the gap between supply and demand in heart transplantation (Award #: 1F32HL154750-01A1)
Dr. Khush receives support from the NIH grant R01 HL125303 entitled “Evidence Based Evaluation and Acceptance of Donor Hearts for Transplantation.”
Dr. Lala is on the Editorial Board of the Journal of Cardiac Failure, on the Advisory Board for BioVentrix and Merck, the DSMB for Sequana Medical, and has received speaker honoraria from Abbott and Zoll.
Abbreviations
- COVID-19
coronavirus-19
- HT
heart transplantation
- OPTN
Organ Procurement and Transplantation Network
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- UNOS
United Network of Organ Sharing
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Schold JD, Koval CE, Wee A, Eltemamy M, Poggio ED. Utilization and Outcomes of Deceased Donor SARS-CoV-2 Positive Organs for Solid Organ Transplantation in the United States. American J Transplantation. 2022;ajt.17126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dhand A, Gass A, John D, Kai M, Wolf D, Bodin R, Okumura K, Veillette G, Nag R, Ohira S, Diflo T, Wolfe K, Spielvogel D, Lansman S, Nishida S. Long-term and Short-term Outcomes of Solid Organ Transplantation From Donors With a Positive SARS-CoV-2 test. Transplantation. 2022; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ushiro-Lumb I, Callaghan CJ, Pettigrew GJ, Madden S, Mumford L, Currie I, Gardiner D, Thorburn D, Forsythe JLR, Manas DM, NHSBT Organ and Tissue Donation and Transplantation Clinical Team. Transplantation of Organs From SARS-CoV-2 RNA Positive Deceased Donors: The UK Experience So Far. Transplantation. 2022; [DOI] [PubMed] [Google Scholar]
- 4.Eichenberger EM, Coniglio AC, Milano C, Schroder J, Bryner BS, Spencer PJ, Haney JC, Klapper J, Glass C, Pavlisko E, Dibernardo L, Patel CB, DeVore AD, Reynolds J, Wolfe CR. Transplanting thoracic COVID-19 positive donors: An institutional protocol and report of the first 14 cases. The Journal of Heart and Lung Transplantation. 2022;S1053249822019970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Madgula AS, Nestasie M, Link C, Lander MM, Jandhyala D, Lee C, Kanwar MK. Tackling the Paradox of Orthotropic Heart Transplantation from SARS-CoV-2 positive donors: A Single Center Experience. The Journal of Heart and Lung Transplantation. 2022;S1053249822020642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bock MJ, Vaughn GR, Chau P, Berumen JA, Nigro JJ, Ingulli EG. Organ transplantation using COVID-19-positive deceased donors. Am J Transplant. 2022; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jayasekera CR, Vikram HR, Rifat Z, Wagler J, Okubo K, Braaksma BR, Harbell JW, Jadlowiec CC, Katariya NN, Mathur AK, Moss A, Reddy KS, Singer A, Orenstein R, Saling CF, Seville MT, Mour GK, Vargas HE, Byrne TJ, Hewitt WR, Aqel BA. Solid Organ Transplantation From SARS-CoV-2-infected Donors to Uninfected Recipients: A Single-center Experience. Transplant Direct. 2022;8:e1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koval CE, Poggio ED, Lin Y, Kerr H, Eltemamy M, Wee A. Early success transplanting kidneys from donors with new SARS-CoV-2 RNA positivity: A report of 10 cases. American J Transplantation. 2021;21:3743–3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Summary of Current Evidence and Information– Donor SARS-CoV-2 Testing & Organ Recovery from Donors with a History of COVID-19 [Internet]. Organ Procurement and Transplantation Network. 2022;Available from: https://optn.transplant.hrsa.gov/media/kkhnlwah/sars-cov-2-summary-of-evidence.pdf [Google Scholar]
- 10.Bhatt AS, Adler ED, Albert NM, Anyanwu A, Bhadelia N, Cooper LT, Correa A, Defilippis EM, Joyce E, Sauer AJ, Solomon SD, Vardeny O, Yancy C, Lala A. Coronavirus Disease-2019 and Heart Failure: A Scientific Statement From the Heart Failure Society of America. J Card Fail. 2022;28:93–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clerkin KJ, Fried JA, Raikhelkar J, Sayer G, Griffin JM, Masoumi A, Jain SS, Burkhoff D, Kumaraiah D, Rabbani L, Schwartz A, Uriel N. COVID-19 and Cardiovascular Disease. Circulation. 2020;141:1648–1655. [DOI] [PubMed] [Google Scholar]
- 12.OPTN Approach to Estimating the Volume of COVID-19 Positive Deceased Donors. Organ Procurement and Transplantation Network; 2022. [Google Scholar]
- 13.DeFilippis EM, Allen LA, Bhatt AS, Joseph S, Kittleson M, Vardeny O, Drazner MH, Lala A. Vaccines, Antibodies, and Donors: Varying Attitudes and Policies Surrounding COVID-19 and Heart Transplantation. Journal of Cardiac Failure. 2022;S1071916422005371. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


