Abstract
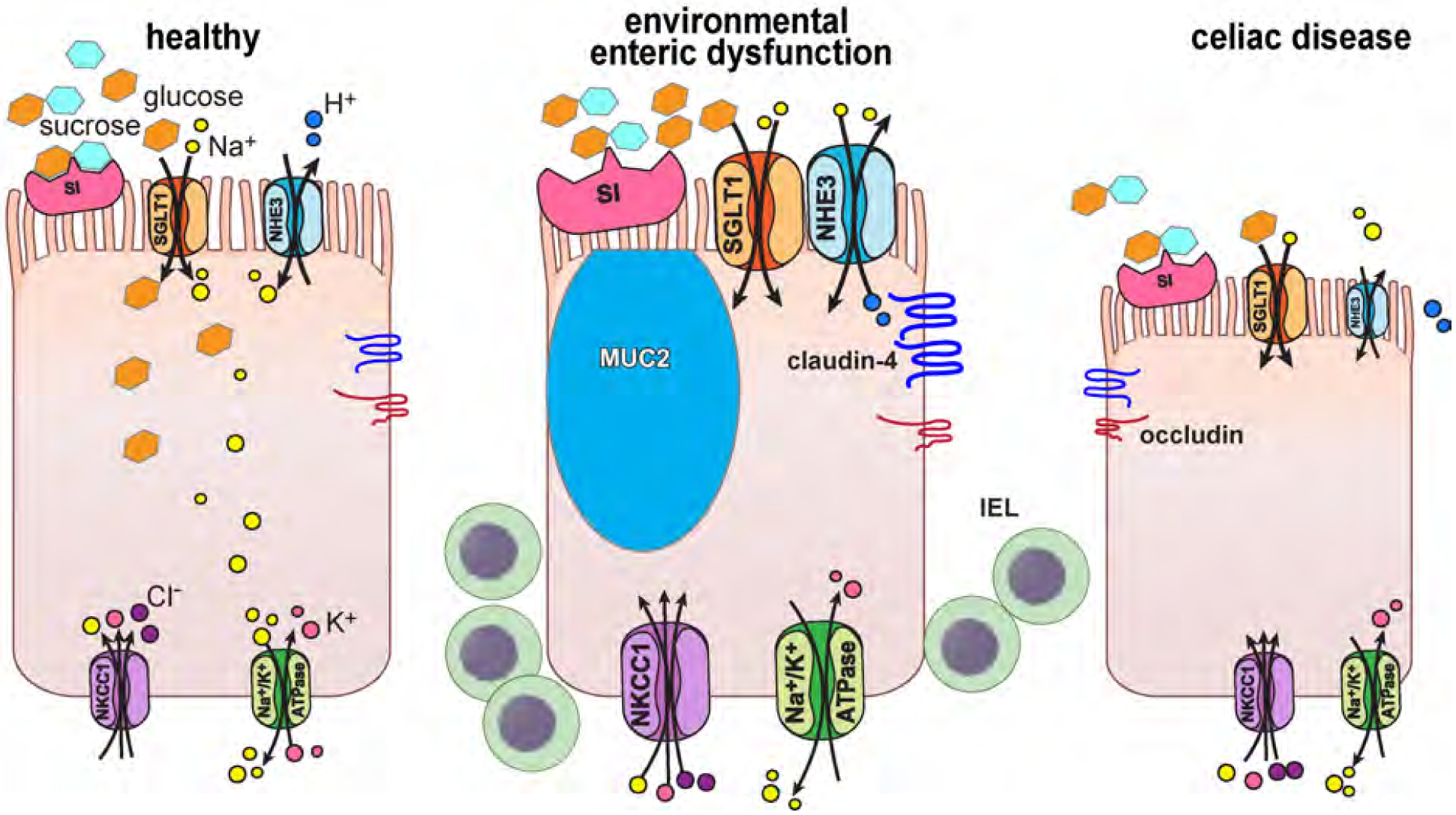
Environmental enteric dysfunction (EED) is characterized by malabsorption and diarrhea that result in irreversible deficits in physical and intellectual growth. We sought to define the expression of transport and tight junction proteins by quantitative analysis of duodenal biopsies from EED patients. Biopsies from Pakistani children with confirmed EED diagnoses were compared to those from age-matched North American healthy controls, celiac disease patients, and non-celiac patients with villous atrophy or intraepithelial lymphocytosis. Expression of brush border digestive and transport proteins, as well as paracellular (tight junction) proteins, was assessed by quantitative multiplex immunofluorescence microscopy. EED was characterized by partial villous atrophy and marked intraepithelial lymphocytosis. Epithelial proliferation and enteroendocrine, tuft, and Paneth numbers were unchanged, but there was significant goblet cell expansion in EED biopsies. Expression of proteins involved in nutrient and water absorption, as well as expression of the basolateral Cl− transport protein NKCC1 were also increased in EED. Finally, the barrier-forming tight junction protein claudin-4 was significantly upregulated in EED, particularly within villous enterocytes. In contrast, the expression of CFTR, claudin-2, claudin-15, JAM-A, occludin, ZO-1, and E-cadherin was unchanged. Upregulation of a barrier-forming tight junction protein and brush border and basolateral membrane proteins that support nutrient and water transport in EED is paradoxical, as increased expression would be expected to be correlated with increased intestinal barrier function and enhanced absorption, respectively. These data suggest that EED activates adaptive intestinal epithelial responses to enhance nutrient absorption but that these changes are insufficient to restore health.
Keywords: malabsorption, tight junction, enteropathy, tropical sprue, stunted growth
INTRODUCTION
Environmental enteric dysfunction (EED) is an early-childhood disease estimated to affect over 150 million children worldwide. EED, previously referred to as environmental enteropathy, is associated with severe malnutrition and irreversible physical and cognitive stunting. It can be particularly challenging to discriminate between severe acute malnutrition and EED, in part because there are no precise diagnostic criteria for EED. Response to hypercaloric supplementation in severe acute malnutrition, but not in EED, is one operational definition, but this can only be applied in retrospect. The pathophysiology that leads to EED remains poorly understood.
EED is most common in underresourced regions of Asia, South America, and Africa where the absence of clean drinking water and other hygienic challenges may contribute to pathogenesis.1, 2 Consistent with this, small bowel bacterial overgrowth can occur. EED has also been associated with recurrent or persistent enteric infection, intestinal barrier loss, inappropriate immune cell activation, and an altered gut microbiome.2–16 However, non-absorbable antibiotics, efforts to improve sanitation, and hyperalimentation have all failed to reverse stunting or reduce EED prevalence.1, 17 This suggests that the pathophysiology of EED may include defects in nutrient absorption or metabolism.4
Early studies of EED histopathology varied widely due to superimposition with other disorders but generally compared EED to celiac disease. EED is not, however, responsive to gluten elimination. Recent transcriptomic analyses4, 8, 18 have correlated alterations in transcription of genes associated with mucosal immune function, metabolism, and epithelial repair processes with EED,4, 18, 19 but the specific antigens or pathogens that drive mucosal immune activation in EED have not been identified. Nevertheless, the reduced oral vaccine efficacy in regions where EED is endemic suggests generalized immune dysfunction.9
Here, we analyzed the expression of ion and nutrient transport and barrier proteins within duodenal biopsies from a well-characterized patient group. Unexpectedly, the expression of absorptive proteins and a barrier-enhancing tight junction protein were increased in EED. These results suggest that EED activates adaptive intestinal epithelial responses but are ultimately insufficient to restore homeostasis.
MATERIALS AND METHODS
Subjects and biopsies
Duodenal biopsies were obtained from 10 Pakistani children with confirmed EED diagnoses from Aga Khan University, Pakistan. Clinical features of these patients and morphological analysis of their biopsies have been reported previously.20 Duodenal biopsies from age-matched North American children included healthy controls, celiac disease patients, and non-celiac patients with villous atrophy or intraepithelial lymphocytosis (Table I).
Table I.
Demographics of cases and controls
| EED | HC | CD | NCD | |
|---|---|---|---|---|
| Age (months, mean ± SD) | 16.0 ± 7.5 | 18.2 ± 5.6 | 16.7 ± 4.6 | 14.5 ± 6.0 |
| Number | 10 | 29 | 7 | 16 |
| Gender (male%) | 50% | 58% | 57% | 56% |
Environmental enteric dysfunction (EED), healthy control (HC), celiac disease (CD), and non-celiac disease controls (NCD).
Immune staining and multiplex immunofluorescence
Sections (5 μm) of formalin-fixed paraffin-embedded (FFPE) duodenal biopsies were baked overnight at 60°C. TintoDeparaffinator Citrate or EDTA (BioSB, Santa Barbara, CA) was used for heat-assisted deparaffinization, rehydration, and antigen retrieval in a pressure cooker (15 min). After rinsing with TintoDeparaffinator Hot Rinse, sections were cooled in phosphate-buffered saline (PBS). Tissue autofluorescence was minimized by two sequential incubations (1 hr each) in a bleaching buffer under broad spectrum LED light. After incubation (10 min) with Serum Free Protein Block (Agilent, Santa Clara, California), primary antibodies (Supplementary Table S1) diluted in Antibody Diluent (Agilent) were added, and slides were incubated (8 hr) at room temperature. After washing with ImmunoDNA Washer solution (BioSB), sections were incubated with secondary antibodies and Hoechst 33342 (1 μg/ml), washed, and mounted using Vectashield Plus Antifade Medium (Vector Laboratories, Newark, CA). Following imaging, coverslips were removed, fluorophores were inactivated, and slides were re-stained. After the first round of staining, only directly-conjugated antibodies were used.
Image acquisition
Each biopsy was scanned using a DM4000 microscope with 20X NA 0.7 HC PLAN APO objective (Leica, Deerfield, IL), motorized xyz stage and emission filter wheel (Ludl Electronic Products, Hawthorne, NY), multichannel dichroic and single band emission filters (Semrock, Rochester, New York), Aura light engine (Lumencor, Beaverton, OR), and ORCA-Flash 4.0 LT+ camera (Hamamatsu, Bridgewater, NJ) controlled by Metamorph 7.8 (Molecular Devices, San Jose, CA) using custom journals.21 Images of hematoxylin and eosin (H & E) stained sections were acquired similarly using a transmitted white LED light source and MicroPublisher 5.0 camera (Q Imaging, Tucson, AZ). Tiled images were stitched using Metamorph 7.8 and custom journals.
Morphometry
Villus height was measured manually using well-oriented regions within each biopsy. Quantitative immunostain analyses used CellProfiler (Broad Institute, Boston, MA); villus and crypt regions were annotated manually. After using Hoechst-labelled nuclei to identify tissue, an epithelial mask was generated based on lateral membrane labeling, e.g., E-cadherin (Fig. S1). E-cadherin staining was also used to create masks, demarcating basolateral membranes for analyses of claudin-4, NKCC1, and Na+/K+ ATPase stains. NHE3, SI, PEPT1, SGLT1, and CFTR were analyzed with apical membrane masks created using γ-actin staining (Fig. S1). Tight junction masks generated based on ZO-1 labeling were used to analyze claudin-2, claudin-15, occludin, and JAM-A expression. Preliminary validation showed that E-cadherin and γ-actin staining were consistent across biopsies, and E-cadherin was used to normalize signal intensity across biopsies (Fig. S1). Intraepithelial lymphocytes were assessed using CD3+ cells, counted as primary objects within the epithelial mask. Epithelial subpopulations were defined by POU2F3, CHGA MUC2, and LYZ expression and counted manually.
Statistical analysis
Data are presented as mean ± standard deviation (SD). Normalized data are shown for immunostains, and absolute numbers are shown for villus height and cell numbers. Comparisons between groups were performed using the Kruskal-Wallis and Dunn’s tests (Prism 9.2.0, GraphPad Software, San Diego, CA). P <0.05 was considered significant.
RESULTS
Since the first descriptions of growth stunting in very young children with repeated bouts of diarrhea, it has been clear that malabsorption is a critical component of EED. Few studies have, however, assessed the histopathology of this disorder, and none have examined transport protein expression within involved mucosa. In part, this may reflect the hesitance to obtain intestinal biopsies from affected infants and, equally importantly, control subjects. Intestinal biopsies are now being obtained from infants with malnutrition and stunting with increasing frequency, but ethical and practical concerns have precluded endoscopy and biopsy of healthy control subjects from within the same environment. The latter is critical, as mucosal histology is well known to vary as a function of geography and season. Some changes associated with EED have been described in African children, both well-nourished and malnourished, relative to Western controls.22 Although imperfect, we compared EED cases to age- and sex-matched North American healthy control subjects, celiac disease patients, and individuals with nondiagnostic histologic abnormalities, i.e., villous blunting or intraepithelial lymphocytosis, referred to as non-celiac disease subjects.
EED is characterized by villous blunting and intraepithelial lymphocytosis
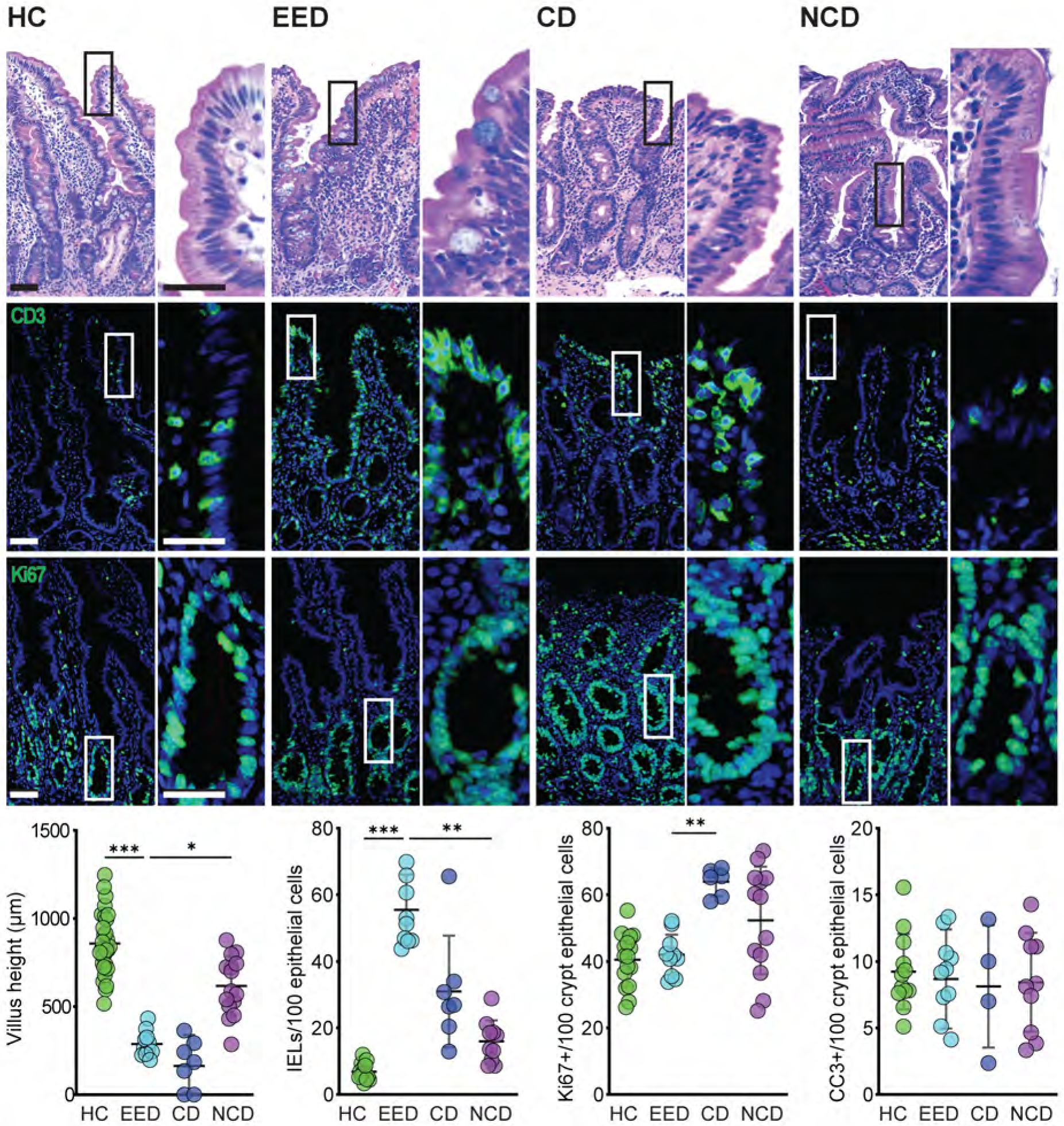
Consistent with previous reports,8, 23–25 we found that environmental enteric dysfunction is associated with partial villous atrophy but noted that this differed from the complete villous atrophy associated with pediatric celiac disease. Conversely, intraepithelial lymphocytosis in EED exceeded that of celiac disease. Moreover, despite villous blunting, epithelial proliferation, which was increased in celiac disease, was not altered in EED (Fig 1). Epithelial apoptosis, measured as cleaved caspase-3-positive epithelial cells, was not different in any group. The combination of villous blunting without reduced epithelial proliferation suggests that epithelial turnover may be accelerated in EED.
Figure 1. EED is characterized by modest villus blunting and marked intraepithelial lymphocytosis.
Villus height was significantly reduced in children with EED or celiac disease (CD) relative to healthy controls (HC) and non-celiac disease (NCD) subjects. Villous atrophy in celiac disease was significantly more severe than in EED, but numbers of CD3+ intraepithelial lymphocytes (IELs) were more prominently increased in EED. Proliferation, assessed as numbers of Ki67-positive cells, was increased in celiac disease but unchanged in EED. Numbers of cleaved caspase 3-positive cells were similar in all groups. Bars = 50 μm, 20 μm. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Goblet cell numbers are increased in EED
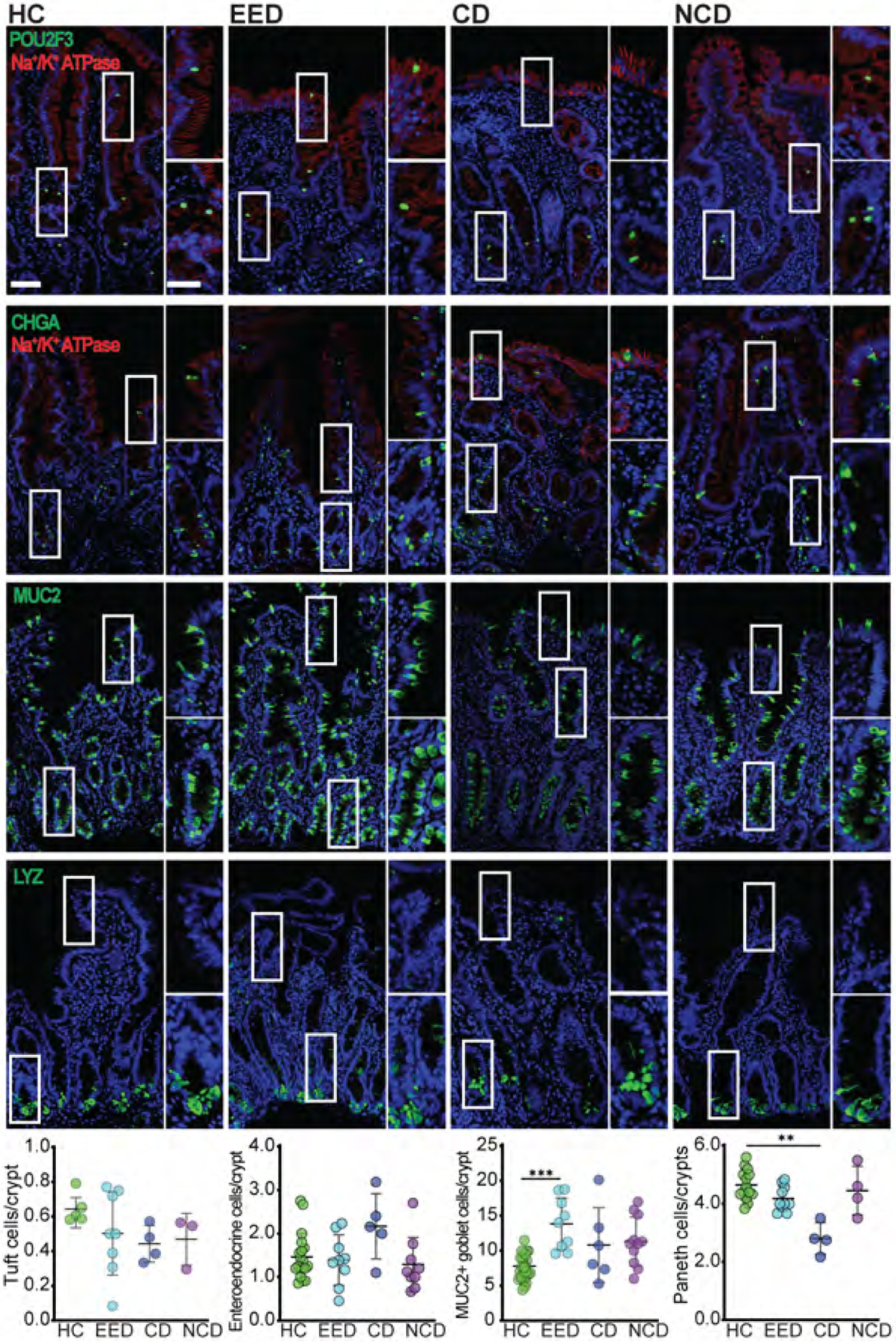
We next asked if epithelial differentiation was affected in EED. Numbers of POU2F3-positive tuft cells, chromogranin A-positive enteroendocrine cells, and lysozyme-positive Paneth cells were similar across all groups. However, MUC2-positive goblet cell numbers were significantly increased in EED, relative to healthy controls, and trended towards an increase in celiac disease (Fig 2). The number of goblet cells in non-celiac disease biopsies extended over a broad range and was not significantly different than any other group, including EED. Subset analysis of the two histologies that comprised the non-celiac group showed that the numbers of MUC2-positive cells were significantly increased, relative to normal controls, in villous atrophy but not lymphocytosis.
Figure 2. Goblet cell populations are expanded in EED.
Numbers of POU2F3-positive tuft cells and chromogranin A-positive (CHGA) enteroendocrine cells were similar in all groups. MUC2-positive goblet cell numbers were increased in EED relative to all other groups. Data shown are per crypt unit. When quantified per 100 epithelial cells, MUC2-positive goblet cells numbered 14.7±2.2; 19.8±3.4; 16.5±1.5; and 20.0±4.3 for HC, EED, CD, and NCD groups, respectively. MUC2-positive goblet cell numbers in villous atrophy and intraepithelial lymphocytosis groups were 17.0±3.8; and 23.1±2.0, respectively. Goblet cell numbers were significantly increased in EED, NCD, and villous atrophy groups relative to HC. Loss of lysozyme-positive Paneth cells was evident in celiac disease. Bars = 50 μm and 20 μm. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Na+ and nutrient absorptive processes are upregulated in EED
Although villous blunting in EED is incomplete relative to celiac disease, malnutrition is more severe in EED. We, therefore, asked if nutrient malabsorption in EED reflected the downregulation of related proteins. First, we assessed the expression of sucrase-isomaltase (SI), which is necessary for the absorption of complex carbohydrates and sucrose. SI expression on villous enterocytes was markedly upregulated in EED relative to healthy controls as well as non-celiac disease controls (Fig 3).
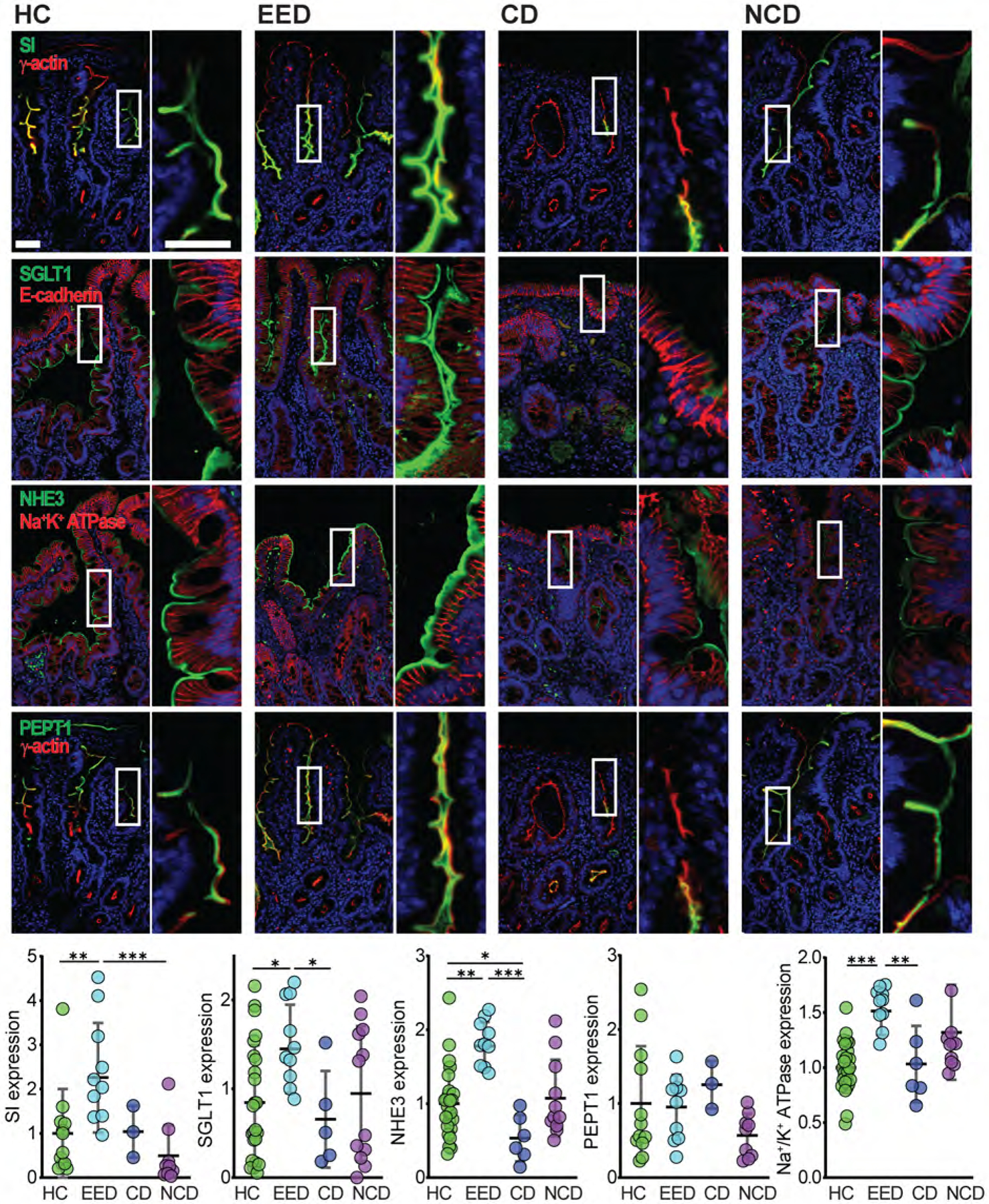
Figure 3. Brush border proteins involved in nutrient and water absorption are upregulated in EED.
Expression of the brush border proteins sucrase-isomaltase (SI), the Na+-glucose cotransporter SGLT1, and the Na+/H+ exchanger NHE3 (SLC9A3), were all increased in EED, as was Na+/K+ATPase. Conversely, NHE3 was reduced in celiac disease. PEPT1 expression was not different in any group. Bars = 50 μm and 20 μm. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Following breakdown by SI and other brush border digestive enzymes, monomeric nutrients, e.g., glucose, are primarily absorbed via Na+-coupled cotransporters. We, therefore, assessed the expression of SGLT1 (SLC5A1), the brush border Na+-glucose cotransporter. Similar to sucrase-isomaltase, SGLT1 expression was significantly increased in EED relative to healthy control and celiac disease biopsies. SGLT1 expression in non-celiac controls clustered into two groups, but unlike goblet cell numbers, these did not correlate with villous atrophy or lymphocytosis. Na+-nutrient cotransport generates a transepithelial osmotic gradient that drives fluid absorption and activates the brush border Na+-H+ exchanger NHE3 (SLC9A3), which also contributes to this gradient.26–28 NHE3 function and expression are reduced in acute and chronic diarrheal disorders.29–32 In contrast, NHE3 expression was significantly upregulated in EED relative to healthy control and celiac disease biopsies (Fig 3). The oligopeptide transporter PEPT1 (SLC15A1) expression was unchanged (Fig 3).
Ultimately, apical Na+-dependent transport, e.g., by SGLT1 and NHE3, relies on the transmembrane Na+ gradient created by the Na+/K+ ATPase. We therefore questioned if increases in SGLT1 and NHE3 expression were accompanied by changes in Na+/K+ ATPase expression. In EED, Na+/K+ ATPase expression was significantly upregulated relative to healthy control and celiac disease biopsies (Fig 3). Thus, both apical and basolateral membrane proteins required for Na+, nutrient, and water absorption are upregulated in EED. Malabsorption in EED is, therefore, not due to insufficient transport protein expression.
Cl− transport protein expression is modified in EED
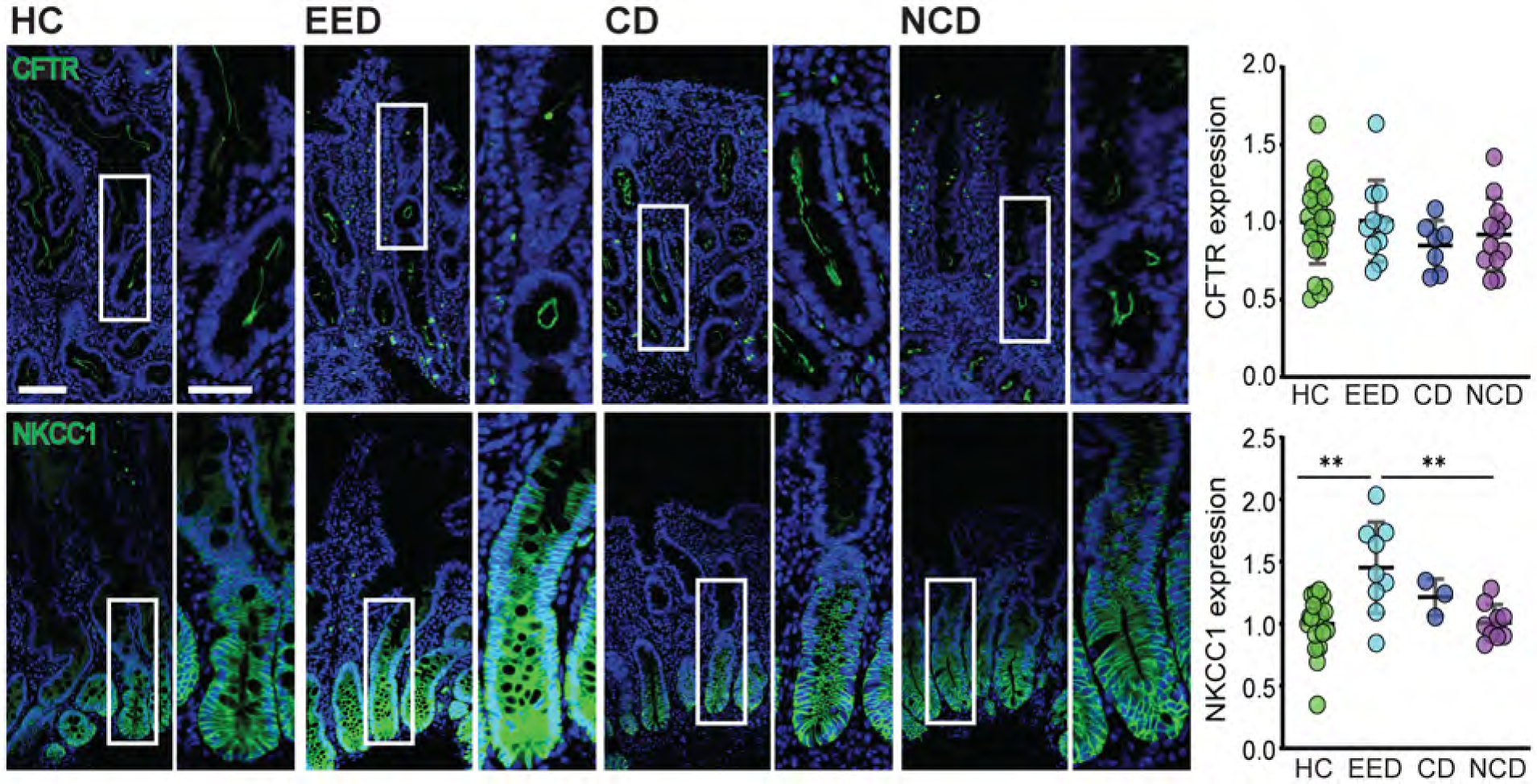
The data above indicate that proteins responsible for absorption are upregulated in EED. Nevertheless, EED patients often suffer from diarrhea, suggesting either insufficient fluid absorption or increased fluid secretion. NHE3 upregulation would be expected to amplify fluid absorption. We therefore asked if expression of CFTR (which mediates cAMP-dependent Cl− secretion across the apical membrane) or NKCC1 (SLC12A2) (which transports Cl− across the basolateral membrane) are altered in EED. CFTR expression was unchanged, but NKCC1 was upregulated in EED relative to healthy control and non-celiac biopsies (Fig 4). Together with the activation of apical Cl− channels, increased NKCC1 could potentially drive increased Cl− and, consequently, fluid secretion. Functional analyses will be required to determine if this is a mechanism of diarrhea in EED.
Figure 4. Basolateral Cl− transporter expression is upregulated in EED.
CFTR expression was unchanged in EED. In contrast, expression of the basolateral Cl− transporter NKCC1 was increased. Bars = 50 μm and 20 μm. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
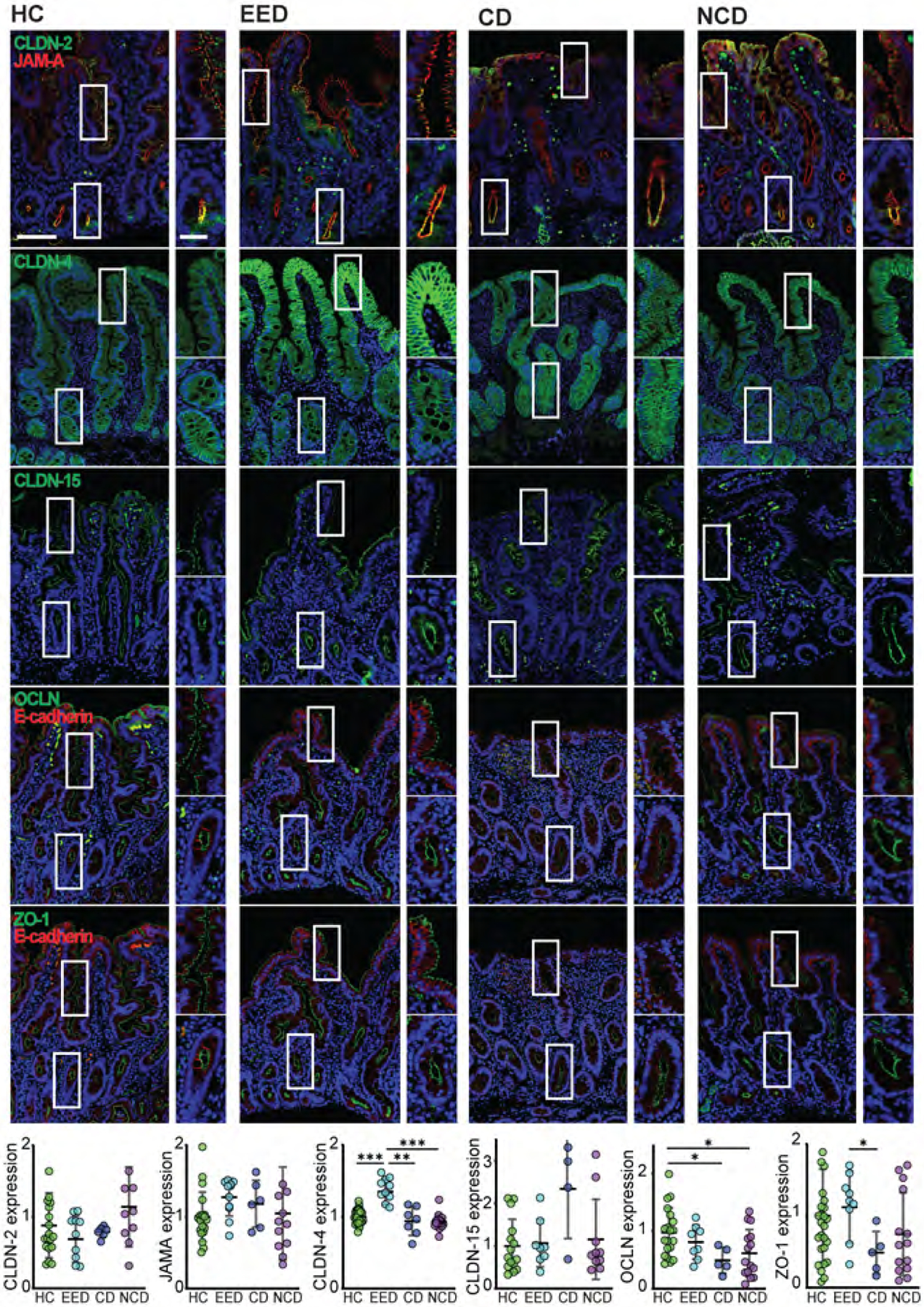
Only claudin-4 expression is modified in EED
One biochemical marker of EED is an increase in the lactulose:mannitol fractional excretion ratio, a measure of intestinal permeability. We analyzed the expression of the tight junction proteins ZO-1, occludin, JAM-A, claudins 2, 4, and 15, and the adherens junction protein E-cadherin. There were no changes in the subcellular distribution of tight junction proteins. Moreover, the funnel-like ZO-1 and occludin profiles typical of epithelial shedding were not present.33 Quantitatively, only claudin-4 expression was altered (Fig 5). This increased expression was unexpected, as claudin-4 has been characterized as a barrier-forming claudin.34 Notably, similar to previous studies,5 we found that claudin-4 expression increased at villous tips. Sites of increased claudin-4 expression were not associated with epithelial damage.5 In contrast, expression of occludin, which is necessary for intraepithelial migration of some lymphocyte subpopulations,35 was similar in EED and healthy controls but reduced, relative to healthy controls, in celiac disease and non-celiac disease biopsies.
Figure 5. Expression of barrier-forming claudin-4 increased in EED.
Among the tight junction proteins examined, only claudin-4 (CLDN4) displayed EED-associated expression changes. Increased claudin-4 expression was particularly evident in the upper half of villi. In contrast, expression of the pore-forming claudins 2 (CLDN2) and 15 (CLDN15) as well as the leak pathway regulatory proteins occludin (OCLN), ZO-1, and JAM-A were unchanged. Similarly, E-cadherin expression was unchanged in EED. Occludin expression was reduced in celiac disease. Bars = 50 μm and 20 μm. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
DISCUSSION
In recent years, numerous studies have assessed specimens from children with EED, including intestinal biopsies, feces, and urine. Some of these included quantitative analyses of histologic features visible on routine H&E stains, but in nearly all studies, histopathology and limited analyses of protein expression have been assessed subjectively. The data set analyzed here consists of 10 Pakistani children with EED defined, in part, as malnutrition, reduced weight-for-height z scores, and failure to respond to ready-to-use therapeutic food.20 Due to the absence of biopsies from healthy Pakistani children, we used three distinct cohorts of age- and sex-matched North American children: normal, celiac disease, and non-celiac disease with either villous atrophy or increased numbers of intraepithelial lymphocytes. The last group was included because villous atrophy and intraepithelial lymphocytosis are recognized features of EED. Consistent with previous reports,22 we found increased numbers of intraepithelial lymphocytes within small bowel biopsies from EED subjects. Villous blunting in EED was less severe than that in celiac disease.20 Our demonstration of enhanced expression of absorption-related transport and digestive proteins was unexpected, particularly in the context of malnutrition and villous blunting. In contrast, although expression of absorptive proteins, except NHE3 per villus epithelial cell, was not reduced in celiac disease, overall expression was decreased due to near total villous atrophy. The data suggest that, in EED, villous epithelial reprogramming leads to adaptive changes that would otherwise be expected to enhance nutrient absorption. These changes, however, fail to ameliorate malnutrition in EED.
In addition to specific proteins, we identified increased numbers of goblet cells in EED. This is consistent with a recent semi-quantitative report of a new scoring system that detected increased numbers of goblet cells in Zambian EED subjects,24 but contrasted with a study using the same histologic scoring system to assess goblet cell numbers in Pakistani EED subjects. The previous inconsistency could reflect reliance on H&E morphology while we used immunohistochemistry to detect goblet cells. We did not identify any changes in the numbers of tuft, enteroendocrine, or Paneth cells or epithelial proliferation in EED. Previous studies have described either no change or reduced numbers of Paneth cells25, 36, 37. Those analyses relied histological presence of Paneth cell granules, which can be disrupted and lost during fixation, while we relied on immunostains.
Our data suggest that EED induces adaptive responses to counteract malabsorption and barrier dysfunction. It is, therefore, surprising that these changes did not restore nutrient absorption. Importantly, our data concur with prior work showing reduced SGLT1 and NHE3 expression in celiac disease. Although previous studies have not assessed Na+/K+ATPase expression in either celiac disease or EED, it is notable that one prior study also found that methylation of both ATP1A1 and ATP1B1, which encode the primary intestinal epithelial Na+/K+ATPase, was altered in biopsies from EED subjects, relative to healthy North American controls, although transcriptional changes were not detected.4 Finally, our data demonstrate marked morphological and molecular differences between celiac disease and EED. It would be interesting for future studies to determine if the upregulation of sucrase-isomaltase, SGLT1, NHE3, and Na+/K+ATPase can serve as prognostic markers of nutritional therapy responses.
All EED cases included in this study had histories of persistent diarrhea.20 Although this could be consistent with increased expression of NKCC1 and Na+/K+ATPase, which are both required for intestinal epithelial Cl− secretion, we did not identify increased CFTR expression. While CFTR channel activation, without expression changes, could synergize with NKCC1 and Na+/K+ATPase upregulation to drive net fluid secretion, it is also possible that other apical Cl− transport pathways, e.g., Ca2+- activated Cl− channels, are involved. Unfortunately, the molecular identity of the Ca2+- activated Cl− channel has not been determined. However, some reports have suggested it may be TMEM16A/ANO1, TMEM16A transcripts, and TMEM16A protein are not present in intestinal epithelial cells.38 We cannot, therefore, rule out changes in apical Ca2+- activated Cl− channel expression or activity in EED. Nevertheless, these data suggest that, despite inflammation, diarrhea in EED is not due to reduced NHE3 expression, as this was increased in EED. Our data do not speak to the underlying mechanism, but it is possible that this represents an adaptive response to net fluid secretion.
EED and celiac disease are both characterized by epithelial barrier loss. We analyzed the expression of intestinal epithelial tight junction proteins. Importantly, expression of claudin-2, which is exquisitely sensitive to inflammatory stimuli, was unaffected. Although one report has reported increased CLDN2 transcription in EED, relative to North American controls, the observed difference in expression likely reflects the significantly younger age of EED cases, relative to controls, in that study and the well-recognized age-dependent downregulation of intestinal epithelial claudin-2 expression.4, 39 We did not detect changes in expression of the other intestinal tight junction Na+ channel-forming protein claudin-15 despite separate reports of claudin-15 protein and CLDN15 mRNA in the urine and feces of EED subjects, respectively.4, 19
In this set of Pakistani EED cases, only claudin-4 expression was increased, consistent with a previous report in Zambian EED cases.5 Claudin-4 has typically been thought of as a barrier-forming claudin, and its upregulation may therefore represent a response to barrier loss in EED. However, this requires further study, as Cldn4 knockout in mice and cell lines does not affect epithelial barrier function40–42. More importantly, increased claudin-4 expression suggests that increased tight junction permeability may not be the cause of barrier loss in EED. One alternative possibility is that EED-associated barrier loss is secondary to epithelial damage. However, the absence of increased epithelial proliferation, which is a sensitive marker of low-grade damage, does not support that hypothesis.
The major limitation of this study is the absence of healthy controls from the same environment as EED subjects. This is common to all previous studies of EED, as practical and ethical considerations have prevented the biopsy of these children without a medical indication. As a result, previous work has either relied on biopsies from non-age-matched American or UK children or adults, or in some cases, have not included any healthy controls.4, 18, 19, 22, 24, 43–45 This is insufficient, as many of the proteins studied here are regulated during development and in response to immune stimuli.18, 39, 46, 47 Nevertheless, our study only examined duodenal biopsies, and we cannot exclude the possibility that other lesions are present within the distal small intestine or colon. We also note that several study patients were infected with Giardia. Although subgroup analysis showed that changes in the expression of the proteins studied were not associated with Giardia infection, it is possible that other enteric or systemic infections may have had unrecognized effects.
As a whole, these quantitative analyses fail to explain the malabsorption and diarrhea that characterize EED. Instead, the expression data alone suggests that EED’s absorption of ions, nutrients, and water may increase. From that perspective, it seems that intestinal epithelial cell upregulation of transport and barrier protein expression may be an appropriate compensatory response but that increased expression of transport and barrier proteins is inadequate to resolve malabsorption and diarrhea. The data further suggest that descriptive studies of gene and protein expression, including this work, will not elucidate the pathophysiology of EED. Functional analyses of intestinal transport and barrier functions will be critical to the mechanistic understanding of EED.
Supplementary Material
Figure 6. Comparisons of protein expression in biopsies from healthy subjects and those with EED or celiac disease.
The cell on the left represents healthy physiology. Sucrase-isomaltase (SI) breaks sucrose into glucose and fructose. Glucose is transported across the apical brush border by the Na+-glucose cotransporter, SGLT1 (SLC5A1). Glucose is then used as an energy source by the cell or exits the cell via basolateral GLUT2 (SLC2A2, not shown). Similarly, NHE3 (SLC9A3) takes advantage of the extracellular to intracellular Na+ gradient to transport H+ across the apical membrane from the cytoplasm to the lumen. In both cases, Na+ is then pumped across the basolateral membrane by Na+/K+ATPase. NKCC1 (SLC12A2), also located on the basolateral membrane, transports Na+. K+, and Cl− into the cell, thereby providing the driving force for apical Cl− secretion. SI, SGLT1, NHE3, NKCC1, and Na+/K+ATPase are all upregulated in EED, as is the barrier-forming tight junction protein claudin-4. Increased numbers of MUC2-positive goblet cells and intraepithelial lymphocytes (IEL) are also present. Although, like EED, intraepithelial lymphocytes are increased in celiac disease, NHE3 and occludin are downregulated on a per-cell basis. The loss of absorptive epithelial cells in celiac disease due to near-total villous atrophy results in reduced overall absorptive protein expression.
Support:
This work was supported by grants from Bill and Melinda Gates Foundation (OPP1066203) and the National Institute of Diabetes, Digestive and Kidney Disease (R01DK61931, R01DK68271, and R24DK099803).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests: JRT is a founder of Thelium Therapeutics and a consultant for Entrinsic and Kallyope. No other authors have competing interests.
REFERENCES
- 1.Lin A, Ali S, Arnold BF, et al. Effects of Water, Sanitation, Handwashing, and Nutritional Interventions on Environmental Enteric Dysfunction in Young Children: A Cluster-randomized, Controlled Trial in Rural Bangladesh. Clin Infect Dis 2020; 70:738–747. [DOI] [PubMed] [Google Scholar]
- 2.Keusch GT, Denno DM, Black RE, et al. Environmental enteric dysfunction: pathogenesis, diagnosis, and clinical consequences. Clin Infect Dis 2014; 59 Suppl 4:S207–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Semba RD, Shardell M, Trehan I, et al. Metabolic alterations in children with environmental enteric dysfunction. Sci Rep 2016; 6:28009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haberman Y, Iqbal NT, Ghandikota S, et al. Mucosal Genomics Implicate Lymphocyte Activation and Lipid Metabolism in Refractory Environmental Enteric Dysfunction. Gastroenterology 2021; 160:2055–2071 e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amadi B, Besa E, Zyambo K, et al. Impaired Barrier Function and Autoantibody Generation in Malnutrition Enteropathy in Zambia. EBioMedicine 2017; 22:191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhattacharjee A, Burr AHP, Overacre-Delgoffe AE, et al. Environmental enteric dysfunction induces regulatory T cells that inhibit local CD4+ T cell responses and impair oral vaccine efficacy. Immunity 2021; 54:1745–1757 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell RK, Schulze KJ, Shaikh S, et al. Environmental enteric dysfunction and systemic inflammation predict reduced weight but not length gain in rural Bangladeshi children. Br J Nutr 2018; 119:407–414. [DOI] [PubMed] [Google Scholar]
- 8.Farras M, Chandwe K, Mayneris-Perxachs J, et al. Characterizing the metabolic phenotype of intestinal villus blunting in Zambian children with severe acute malnutrition and persistent diarrhea. PLoS One 2018; 13:e0192092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marie C, Ali A, Chandwe K, et al. Pathophysiology of environmental enteric dysfunction and its impact on oral vaccine efficacy. Mucosal Immunol 2018; 11:1290–1298. [DOI] [PubMed] [Google Scholar]
- 10.McCormick BJJ, Murray-Kolb LE, Lee GO, et al. Intestinal permeability and inflammation mediate the association between nutrient density of complementary foods and biochemical measures of micronutrient status in young children: results from the MAL-ED study. Am J Clin Nutr 2019; 110:1015–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen RY, Kung VL, Das S, et al. Duodenal Microbiota in Stunted Undernourished Children with Enteropathy. N Engl J Med 2020; 383:321–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mutasa K, Ntozini R, Mbuya MNN, et al. Biomarkers of environmental enteric dysfunction are not consistently associated with linear growth velocity in rural Zimbabwean infants. Am J Clin Nutr 2021; 113:1185–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh A, Ghosh S, Ward H, et al. Biomarkers of environmental enteric dysfunction are differently associated with recovery and growth among children with moderate acute malnutrition in Sierra Leone. Am J Clin Nutr 2021; 113:1556–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kukuruzovic RH, Brewster DR. Small bowel intestinal permeability in Australian aboriginal children. J Pediatr Gastroenterol Nutr 2002; 35:206–12. [DOI] [PubMed] [Google Scholar]
- 15.Weisz AJ, Manary MJ, Stephenson K, et al. Abnormal gut integrity is associated with reduced linear growth in rural Malawian children. J Pediatr Gastroenterol Nutr 2012; 55:747–50. [DOI] [PubMed] [Google Scholar]
- 16.Guerrant RL, Leite AM, Pinkerton R, et al. Biomarkers of Environmental Enteropathy, Inflammation, Stunting, and Impaired Growth in Children in Northeast Brazil. PLoS One 2016; 11:e0158772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rogawski McQuade ET, Platts-Mills JA, Gratz J, et al. Impact of Water Quality, Sanitation, Handwashing, and Nutritional Interventions on Enteric Infections in Rural Zimbabwe: The Sanitation Hygiene Infant Nutrition Efficacy (SHINE) Trial. J Infect Dis 2020; 221:1379–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chama M, Amadi BC, Chandwe K, et al. Transcriptomic analysis of enteropathy in Zambian children with severe acute malnutrition. EBioMedicine 2019; 45:456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu J, Ordiz MI, Stauber J, et al. Environmental Enteric Dysfunction Includes a Broad Spectrum of Inflammatory Responses and Epithelial Repair Processes. Cell Mol Gastroenterol Hepatol 2016; 2:158–174 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Syed S, Yeruva S, Herrmann J, et al. Environmental Enteropathy in Undernourished Pakistani Children: Clinical and Histomorphometric Analyses. Am J Trop Med Hyg 2018; 98:1577–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abtahi S, Gliksman NR, Heneghan JF, et al. A simple method for creating a high-content microscope for imaging multiplexed tissue microarrays. Curr Protoc 2021; 1:e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campbell DI, Murch SH, Elia M, et al. Chronic T cell-mediated enteropathy in rural west African children: relationship with nutritional status and small bowel function. Pediatr Res 2003; 54:306–11. [DOI] [PubMed] [Google Scholar]
- 23.Kelly P, Menzies I, Crane R, et al. Responses of small intestinal architecture and function over time to environmental factors in a tropical population. Am J Trop Med Hyg 2004; 70:412–9. [PubMed] [Google Scholar]
- 24.Liu TC, VanBuskirk K, Ali SA, et al. A novel histological index for evaluation of environmental enteric dysfunction identifies geographic-specific features of enteropathy among children with suboptimal growth. PLoS Negl Trop Dis 2020; 14:e0007975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mulenga C, Sviben S, Chandwe K, et al. Epithelial Abnormalities in the Small Intestine of Zambian Children With Stunting. Front Med (Lausanne) 2022; 9:849677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turner JR, Black ED. NHE3-dependent cytoplasmic alkalinization is triggered by Na(+)-glucose cotransport in intestinal epithelia. Am J Physiol Cell Physiol 2001; 281:C1533–41. [DOI] [PubMed] [Google Scholar]
- 27.Zhao H, Shiue H, Palkon S, et al. Ezrin regulates NHE3 translocation and activation after Na+-glucose cotransport. Proc Natl Acad Sci U S A 2004; 101:9485–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin R, Murtazina R, Cha B, et al. D-glucose acts via sodium/glucose cotransporter 1 to increase NHE3 in mouse jejunal brush border by a Na+/H+ exchange regulatory factor 2-dependent process. Gastroenterology 2011; 140:560–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clayburgh DR, Musch MW, Leitges M, et al. Coordinated epithelial NHE3 inhibition and barrier dysfunction are required for TNF-mediated diarrhea in vivo. J Clin Invest 2006; 116:2682–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Janecke AR, Heinz-Erian P, Yin J, et al. Reduced sodium/proton exchanger NHE3 activity causes congenital sodium diarrhea. Hum Mol Genet 2015; 24:6614–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sullivan S, Alex P, Dassopoulos T, et al. Downregulation of sodium transporters and NHERF proteins in IBD patients and mouse colitis models: potential contributors to IBD-associated diarrhea. Inflamm Bowel Dis 2009; 15:261–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yeruva S, Farkas K, Hubricht J, et al. Preserved Na(+)/H(+) exchanger isoform 3 expression and localization, but decreased NHE3 function indicate regulatory sodium transport defect in ulcerative colitis. Inflamm Bowel Dis 2010; 16:1149–61. [DOI] [PubMed] [Google Scholar]
- 33.Marchiando AM, Shen L, Graham WV, et al. The epithelial barrier is maintained by in vivo tight junction expansion during pathologic intestinal epithelial shedding. Gastroenterology 2011; 140:1208–1218 e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Itallie C, Rahner C, Anderson JM. Regulated expression of claudin-4 decreases paracellular conductance through a selective decrease in sodium permeability. J Clin Invest 2001; 107:1319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Edelblum KL, Shen L, Weber CR, et al. Dynamic migration of gammadelta intraepithelial lymphocytes requires occludin. Proc Natl Acad Sci U S A 2012; 109:7097–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kelly P, Feakins R, Domizio P, et al. Paneth cell granule depletion in the human small intestine under infective and nutritional stress. Clin Exp Immunol 2004; 135:303–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greenson JK. The biopsy pathology of non-coeliac enteropathy. Histopathology 2015; 66:29–36. [DOI] [PubMed] [Google Scholar]
- 38.Karlsson M, Zhang C, Mear L, et al. A single-cell type transcriptomics map of human tissues. Sci Adv 2021; 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ong M, Yeruva S, Sailer A, et al. Differential regulation of claudin-2 and claudin-15 expression in children and adults with malabsorptive disease. Lab Invest 2020; 100:483–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kage H, Flodby P, Gao D, et al. Claudin 4 knockout mice: normal physiological phenotype with increased susceptibility to lung injury. Am J Physiol Lung Cell Mol Physiol 2014; 307:L524–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tokuda S, Hirai T, Furuse M. Claudin-4 knockout by TALEN-mediated gene targeting in MDCK cells: Claudin-4 is dispensable for the permeability properties of tight junctions in wild-type MDCK cells. PLoS One 2017; 12:e0182521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shashikanth N, France MM, Xiao R, et al. Tight junction channel regulation by interclaudin interference. Nat Commun 2022; 13:3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kelly P Starvation and Its Effects on the Gut. Adv Nutr 2021; 12:897–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Amadi B, Zyambo K, Chandwe K, et al. Adaptation of the small intestine to microbial enteropathogens in Zambian children with stunting. Nat Microbiol 2021; 6:445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kelly P, Besa E, Zyambo K, et al. Endomicroscopic and Transcriptomic Analysis of Impaired Barrier Function and Malabsorption in Environmental Enteropathy. PLoS Negl Trop Dis 2016; 10:e0004600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raju P, Shashikanth N, Tsai PY, et al. Inactivation of paracellular cation-selective claudin-2 channels attenuates immune-mediated experimental colitis in mice. J Clin Invest 2020; 130:5197–5208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsai PY, Zhang B, He WQ, et al. IL-22 upregulates epithelial claudin-2 to drive diarrhea and enteric pathogen clearance. Cell Host Microbe 2017; 21:671–681 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


