Abstract
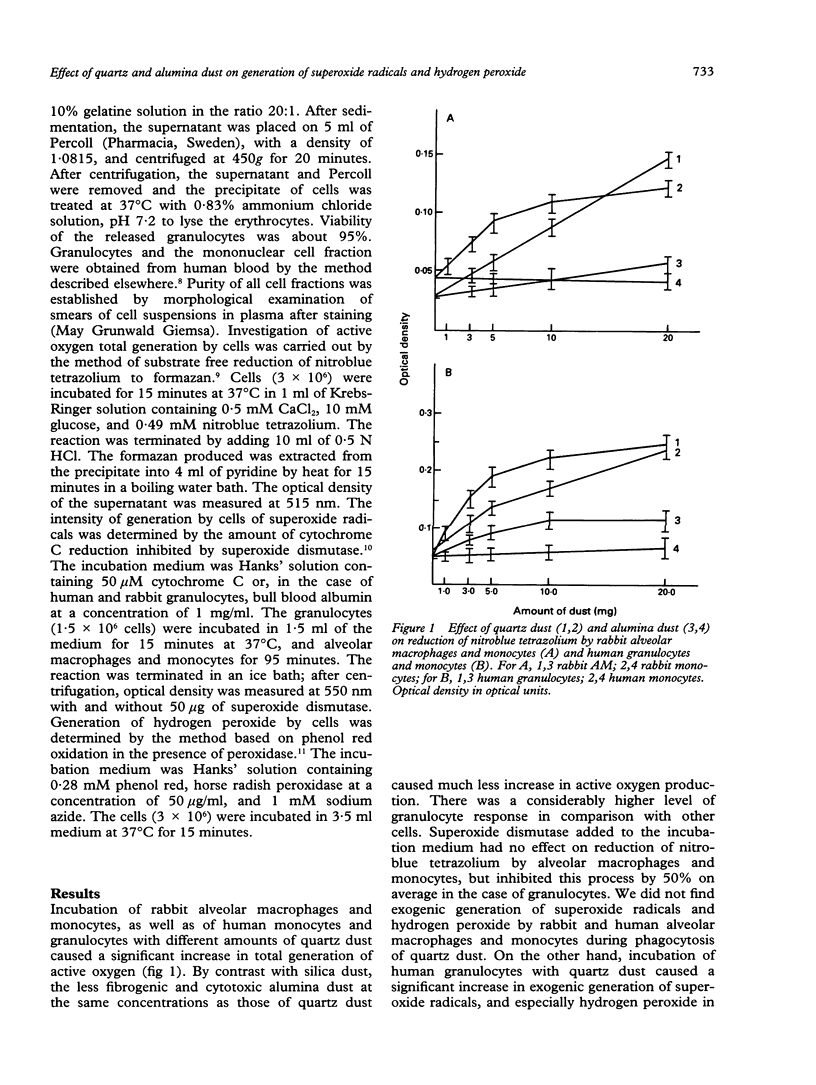
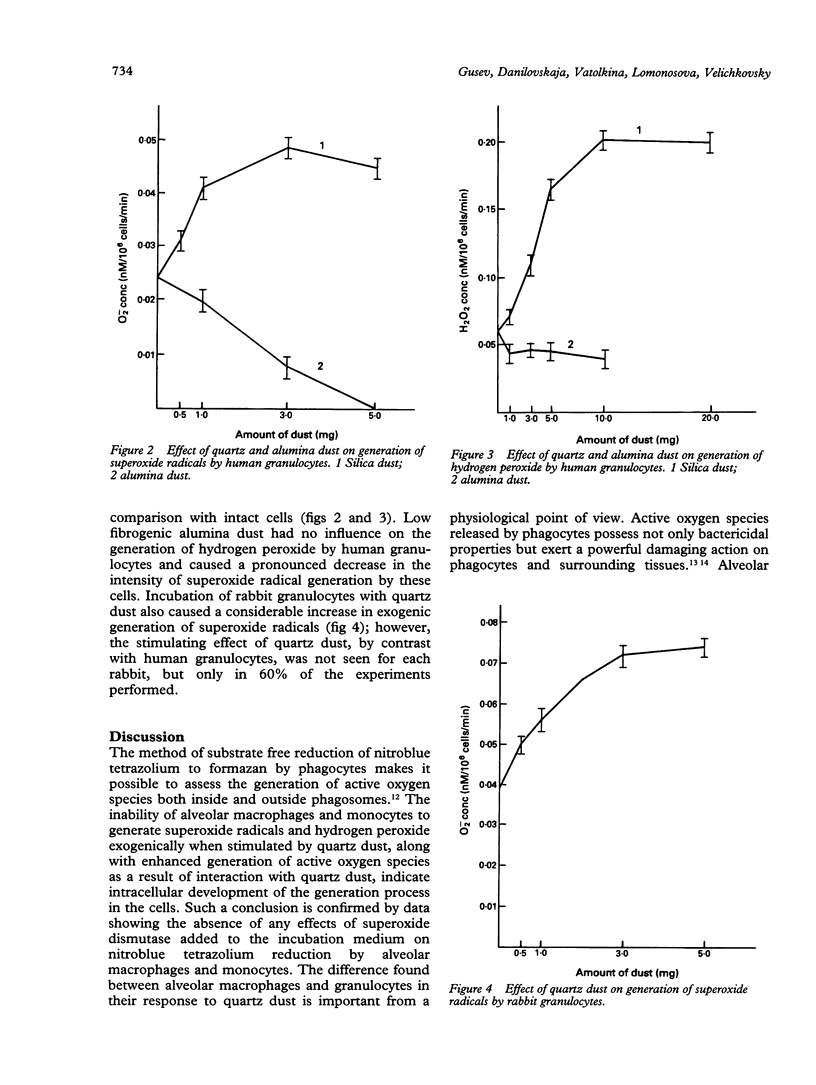
Phagocytosis of quartz particles by rabbit alveolar macrophages and monocytes and human granulocytes and monocytes was accompanied by stimulation of substrate free reduction of nitroblue tetrazolium to formazan. This reflects activation of an oxygen dependent bactericidal system of phagocytes and total (exogenic and endogenic) generation of active oxygen species. Low fibrogenic and cytotoxic alumina dust tended to increase formazan production by comparison with quartz dust. During phagocytosis of quartz dust by alveolar macrophages and monocytes there was no exogenic generation of superoxide radicals and hydrogen peroxide by these cells. By contrast, incubation of human granulocytes with quartz dust caused a significant increase in exogenic generation of superoxide radicals and hydrogen peroxide. Under such conditions, low fibrogenic alumina dust had no effect on hydrogen peroxide generation and substantially decreased the level of superoxide radical generation by human granulocytes. During incubation of rabbit granulocytes with quartz dust, an increase in the level of superoxide radical generation was also detected. It is considered that the differences between alveolar macrophages and granulocytes in their response to quartz dust are important from a physiological point of view. Alveolar macrophages are permanently present in pulmonary alveolae in large quantities; therefore their uncontrolled generation of superoxide radicals and hydrogen peroxide might immediately cause damage to pulmonary parenchyma. At the same time, destruction products from alveolar macrophages that died during phagocytosis of quartz particles contain a factor attracting granulocytes. Presence of a significant number of granulocytes in bronchopulmonary lavage fluid in cases of silicosis indicates development of a pathological process. This agrees well with the data obtained on exogenic generation of superoxide radicals and hydrogen peroxide by granulocytes, and on stimulation of this process due to phagocytosis of the quartz dust.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehner R. L., Nathan D. G. Quantitative nitroblue tetrazolium test in chronic granulomatous disease. N Engl J Med. 1968 May 2;278(18):971–976. doi: 10.1056/NEJM196805022781801. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Donaldson K., Bolton R. E., Jones A., Brown G. M., Robertson M. D., Slight J., Cowie H., Davis J. M. Kinetics of the bronchoalveolar leucocyte response in rats during exposure to equal airborne mass concentrations of quartz, chrysotile asbestos, or titanium dioxide. Thorax. 1988 Jul;43(7):525–533. doi: 10.1136/thx.43.7.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman D. L., Mogelesky T. C. Use of Histopaque for isolating mononuclear cells from rabbit blood. J Immunol Methods. 1987 Sep 24;102(2):243–249. doi: 10.1016/0022-1759(87)90083-4. [DOI] [PubMed] [Google Scholar]
- Gusev V. A., Danilovskaia E. V. Rol' aktivnykh form kisloroda v patogeneze pnevmokoniozov (obzor). Vopr Med Khim. 1987 Sep-Oct;33(5):9–15. [PubMed] [Google Scholar]
- Hartiala K. T., Langlois L., Goldstein I. M., Rosenbaum J. T. Endotoxin-induced selective dysfunction of rabbit polymorphonuclear leukocytes in response to endogenous chemotactic factors. Infect Immun. 1985 Nov;50(2):527–533. doi: 10.1128/iai.50.2.527-533.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindena J., Burkhardt H. Separation and chemiluminescence properties of human, canine and rat polymorphonuclear cells. J Immunol Methods. 1988 Nov 25;115(1):141–147. doi: 10.1016/0022-1759(88)90321-3. [DOI] [PubMed] [Google Scholar]
- MYRVIK Q., LEAKE E. S., FARISS B. Studies on pulmonary alveolar macrophages from the normal rabbit: a technique to procure them in a high state of purity. J Immunol. 1961 Feb;86:128–132. [PubMed] [Google Scholar]
- Pick E., Keisari Y. A simple colorimetric method for the measurement of hydrogen peroxide produced by cells in culture. J Immunol Methods. 1980;38(1-2):161–170. doi: 10.1016/0022-1759(80)90340-3. [DOI] [PubMed] [Google Scholar]
- Salin M. L., McCord J. M. Free radicals and inflammation. Protection of phagocytosine leukocytes by superoxide dismutase. J Clin Invest. 1975 Nov;56(5):1319–1323. doi: 10.1172/JCI108208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schopf R. E., Mattar J., Meyenburg W., Scheiner O., Hammann K. P., Lemmel E. M. Measurement of the respiratory burst in human monocytes and polymorphonuclear leukocytes by nitro blue tetrazolium reduction and chemiluminescence. J Immunol Methods. 1984 Feb 24;67(1):109–117. doi: 10.1016/0022-1759(84)90090-5. [DOI] [PubMed] [Google Scholar]
- Thommasen H. V. The role of the polymorphonuclear leukocyte in the pathogenesis of the adult respiratory distress syndrome. Clin Invest Med. 1985;8(2):185–194. [PubMed] [Google Scholar]
- Xaubet A., Rodriguez-Roisín R., Bombí J. A., Marín A., Roca J., Agustí-Vidal A. Correlation of bronchoalveolar lavage and clinical and functional findings in asbestosis. Am Rev Respir Dis. 1986 May;133(5):848–854. [PubMed] [Google Scholar]
- deShazo R. D. Current concepts about the pathogenesis of silicosis and asbestosis. J Allergy Clin Immunol. 1982 Jul;70(1):41–49. doi: 10.1016/0091-6749(82)90200-7. [DOI] [PubMed] [Google Scholar]



