Abstract
As society ages, the number of older adults with stable ischemic heart disease (SIHD) continues to rise. Older adults exhibit the greatest morbidity and mortality from stable angina. Furthermore, they suffer a higher burden of comorbidity and adverse events from treatment than younger patients. Given that older adults were excluded or underrepresented in most randomized controlled trials of stable ischemic heart disease, evidence for management is limited and hinges on subgroup analyses of trials and observational studies. This review aims to elucidate the current definitions of aging, assess the overall burden and clinical presentations of SIHD in older patients, weigh the available evidence for guideline-recommended treatment options including medical therapy and revascularization, and propose a framework for synthesizing complex treatment decisions in older adults with stable angina. Due to evolving goals of care in older patients, it is paramount to readdress the patient’s priorities and preferences when deciding on treatment. Ultimately, the management of stable angina in older adults will need to be informed by dedicated studies in representative populations emphasizing patient-centered endpoints and person-centered decision-making.
SUBJECT TERMS: Coronary Artery Disease, Angina, Percutaneous Coronary Intervention, Revascularization, Aging
Keywords: Stable angina, Percutaneous coronary intervention, medical therapy, chronic coronary syndrome, stable ischemic heart disease, older adults
INTRODUCTION
Stable ischemic heart disease (SIHD) is a key contributor to morbidity, mortality, and disability in older adults.1 Older adults ≥75 years old make up 30% of patients with SIHD with more than three million older Americans impacted.1,2 Multiple randomized controlled trials (RCTs) and current guideline recommendations support that most patients with SIHD and stable clinical features can be initially managed with medical therapy3–8, with revascularization indicated in patients with breakthrough symptoms and in those with high-risk anatomy. As symptom control is the primary goal of management, this presents unique challenges for clinicians owing to the higher prevalence of multimorbidity in older adults with competing conditions, polypharmacy concerns, and variable goals and priorities of care; all of which can influence both symptoms and quality of life.9–11 Given this complexity, the aim of this contemporary review is to evaluate the definition of aging for clinical and research purposes, discuss the overall burden and clinical presentation of stable angina in older adults, introduce a framework for initial medical and subsequent invasive treatment options, and delineate key future areas for investigation.
I. How Do We Define “Older” For Clinical and Research Purposes?
In less than two decades, the number of older adults is expected to overtake the number of children for the first time in US history.12 As the US and global population ages, the definition of an “older adult” has shifted. In the U.S., older adults have traditionally been defined as adults ≥65 years based on the standard retirement age, which was an arbitrary cutoff deemed acceptable for economic stability in the early 1900s. However, as medical care improved over decades, many adults aged ≥65 years have remained active and healthy, especially those <75 years old. In fact, remaining life expectancy at age 75 in 2007 was similar to life expectancy at age 65 in 195013, and hence the use of the traditional retirement age as a cutoff for older adults has become increasingly anachronistic. Given this changing landscape, older adults have been classified as age ≥75 years in recent studies of cardiovascular diseases.14–16 Future improvements in health care will continue to raise questions regarding how to optimally define this growing and diverse population.
Furthermore, clinicians and scientists are becoming increasingly aware that chronological age should be interpreted in the context of “biological” age, or the functional and physiologic changes that occur over time in an individual, which can vary greatly between people despite the same chronological age.17 There are many proposed predictors of biological age including epigenetic, telomere length, transcriptomic, proteomic, metabolomic, and composite biomarker predictors.18 Predictors of biological age may provide equal or greater prognostic ability than chronological age.19 Furthermore, geriatric syndromes, including frailty, multimorbidity, polypharmacy, and sarcopenia, may be useful surrogates for overall biological aging and serve as a reflection of the microscopic predictors of aging that may not be as readily captured or appreciated.20 Geriatric syndromes also have important implications for outcomes following invasive cardiovascular procedures including percutaneous coronary intervention (PCI) and coronary artery bypass grafting (CABG).21 Ultimately, a clear understanding of a patient’s relative biological age may inform clinical decision-making, including evaluating risks and benefits of potential therapies, while also fostering enrollment of representative aging populations in clinical trials. Figure 1 highlights the heterogeneity of biological age at a given chronological age and numerous measures of biological aging for clinicians and researchers to consider when evaluating the older adult population.19 Looking forward, using predictors of biological age alongside chronological age should consolidate understanding of the aging process and generate a more precise definition of “older” adults.
Figure 1. Contributors to the Heterogeneity of Chronological Aging.
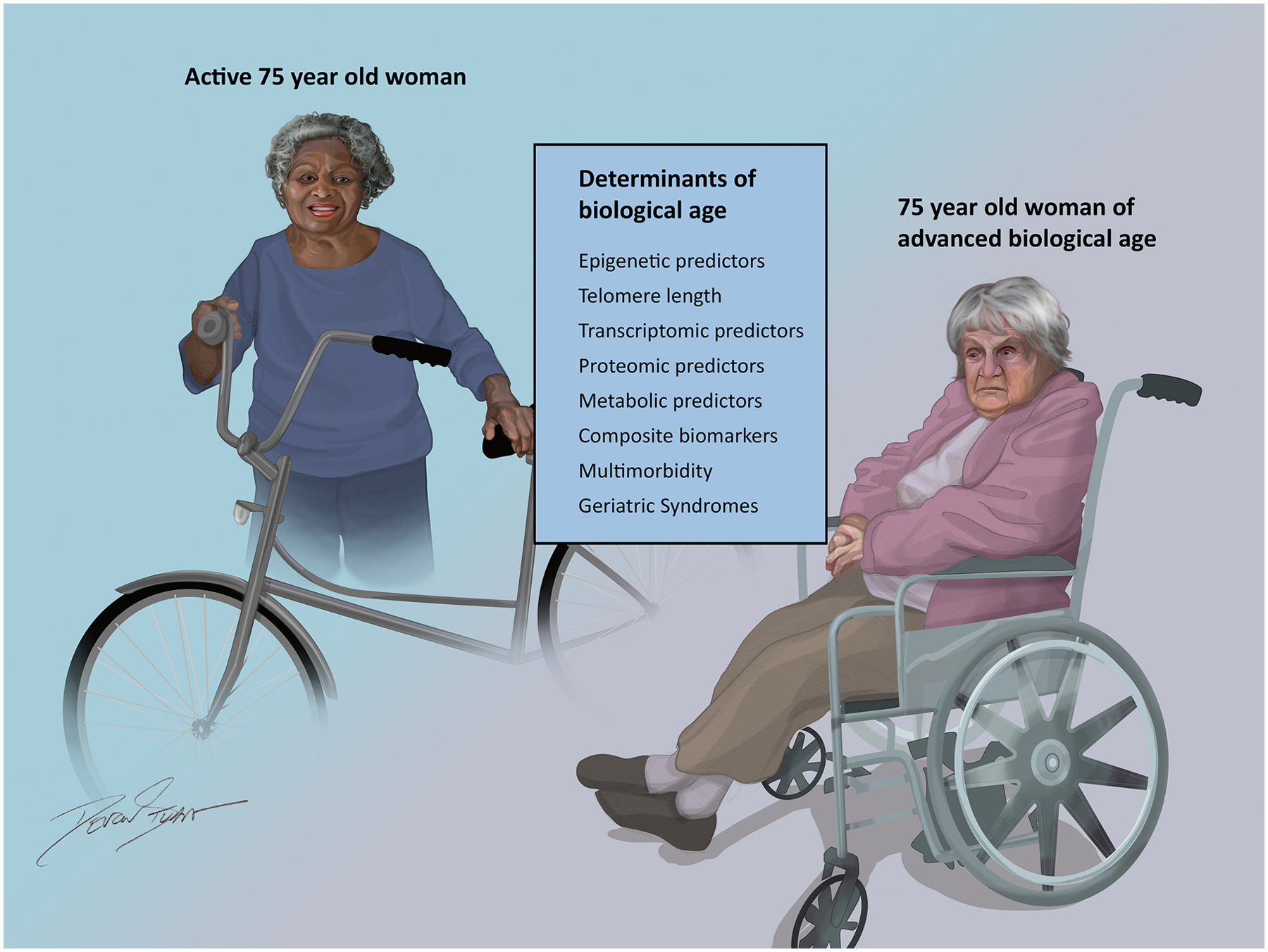
Figure 1 highlights the array of markers and measures that contribute to the heterogeneity of “biological” aging across chronological age.
II. The overall burden and clinical presentations of SIHD in older patients
Also frequently referred to as chronic coronary syndrome and stable angina, SIHD is a major contributor to morbidity, mortality, and disability among older adults.1,2 Some have estimated that nearly a quarter of individuals 75–79 years old and nearly a third of those ≥80 years old are living with coronary artery disease (CAD),22 and the number of older adults living with SIHD is only expected to rise further. This is particularly troubling given that older adults experience the highest mortality and morbidity attributable to SIHD.2 Notably, SIHD is a major contributor to disability and functional impairment, impacting mobility and management of instrumental activities of daily living,23 and serves as a more powerful predictor of future disability than acute myocardial infarction (AMI).24 Given this large disease burden, a clear understanding of the unique considerations around clinical phenotypes, potential tradeoffs of management, and goals of care in older adults with stable angina is crucial.
Clinical phenotypes
The assessment and interpretation of symptoms in older adults with SIHD can be challenging. Older adults most commonly present with chest pain or pressure as their primary anginal equivalent, but may also present with additional symptoms including dyspnea on exertion, nausea, epigastric or back discomfort, and fatigue.25 Further complicating the interpretation of symptoms in patients presenting with obstructive CAD and ischemia is that multiple chronic conditions are exceedingly common among older adults living with CAD, with CAD commonly occurring concurrently with arthritis, diabetes, malignancy, depression, renal insufficiency, stroke, and chronic lower respiratory disease, among other conditions.26 Symptoms of SIHD in older adults such as dyspnea and fatigue frequently overlap with symptoms attributable to non-cardiac conditions common in older adults, such as chronic lung disease, malignancy, anemia, frailty, depression, and chronic kidney disease.27 This has important implications for therapeutic expectations for patients and clinicians. Figure 2 presents different clinical phenotypes potentially encountered in older adults with SIHD, with examples of common symptoms of stable angina seen in older adults and both cardiac and non-cardiac clinical comorbidities that may influence both symptomatic burden and response to treatment.
Figure 2. Interpretation of Symptoms in Older Adults with Multimorbidity.
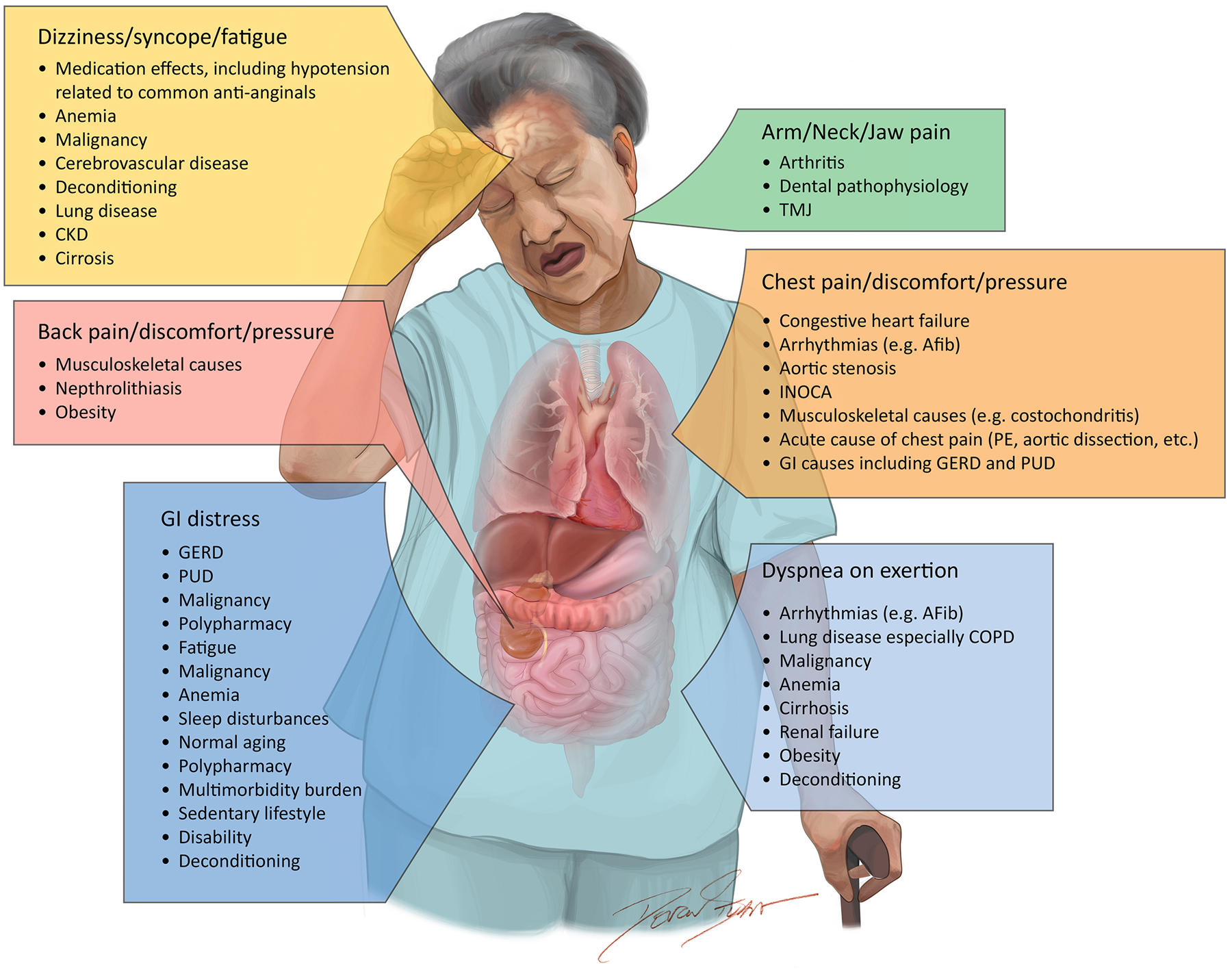
Figure 2 presents some of the multitude of symptoms that are potentially associated with stable ischemic heart disease in older adults and examples of the potential competing conditions that can contribute to the burden of that particular symptom.
III. Weighing treatment options for older adults with SIHD including medical therapy, PCI, and CABG
Medical Therapy for Symptom Control in Older Adults with SIHD
Based on randomized trial evidence, we agree with current American and European guideline recommendations, which support an initial medical therapy approach with antianginal treatment in most patients with stable angina, including older adults, with revascularization primarily reserved for patients with unacceptable angina despite medical therapy.3–8,28 Because older adults are less likely to undergo revascularization and more likely to receive incomplete revascularization,29 a firm understanding of the risks and benefits of antianginal medications in older adults is a priority.
Beta-blockers and calcium channel blockers are recommended as first- and second-line agents respectively by the current U.S. guideline and as first-line agents by the European guideline.8,30 Beta-blockers reduce ischemia by decreasing heart rate and blood pressure with activity, which can delay the onset of symptoms.31 Calcium channel blockers increase coronary blood flow by decreasing coronary vascular resistance via dilation of both the epicardial and arteriolar vasculature, which leads to improvements in angina and exercise tolerance.31 A limitation of the available evidence supporting the use of beta-blockers and calcium channel blockers is the older age of studies, many of which were nonrandomized, and the majority of which failed to include representative populations of older adults with multimorbidity, frailty, and other age-associated risks.32–35 For example, prior studies comparing beta-blockers versus calcium channel blockers enrolled younger populations (mean age of 57 years),35,36 with the largest randomized trial of beta blockers versus calcium channel blockers for stable angina with longer-term follow-up actively excluding septuagenarians and beyond.36 More recent studies assessing these agents have been non-randomized and did not specifically report outcomes stratified by age.37,38 Importantly, both agents present considerations for tolerability, adherence, and persistence, which can impact quality of life. Beta-blockers may contribute to dizziness and fatigue, bradycardia, sleep disturbances, and sexual dysfunction, with risk of cognitive and functional decline in some older patients.39 Calcium channel blockers may cause constipation, bradycardia, and lower extremity edema in older adults, with effects varying across subclasses.40 A key consideration is how symptoms possibly attributed to SIHD, such as dyspnea and fatigue, can be paradoxically exacerbated by certain antianginal therapies depending on the underlying etiology of those symptoms.
Beyond beta-blockers and calcium channel blockers, both short- and long-acting nitrates are a staple of many patients’ antianginal regimen. Since their first use reported by Thomas Lauder Brunton in the 1800s, nitrates have the longest track record of safety and efficacy for the treatment of stable angina.41 Both short- and long-acting nitrates improve exercise tolerance and delay the onset of symptoms in patients with stable angina, and older studies suggest similar efficacy to both beta-blockers and calcium channel blockers.8,35,41 Reflecting these data, long-acting nitrates are recommended as second-line agents by the current guidelines8,30 Significant limitations of nitrate treatment are headaches which can lead to non-adherence and discontinuation of therapy, as well as tachyphylaxis.42 Nitrates should not be used in certain circumstances more common in older populations, including severe aortic stenosis and in patients taking phosphodiesterase inhibitors. Importantly, many of the commonly used antianginal medications, including beta-blockers, calcium channel blockers, and nitrates, may lead to hypotension, which can result in dizziness, syncope, and potential falls. Reassuringly, the available evidence does not demonstrate an increased risk of falls with these medications, although these were limited to observational studies.43,44
Beyond the first- and second-line agents described above, additional options may be considered for the treatment of refractory angina. Ranolazine is a selective inhibitor of the late inward sodium current and reduces angina and the need for sublingual nitroglycerin. One meta-analysis of randomized controlled trials comparing the efficacy of antianginal therapies in patients with stable angina refractory to initial treatment found that ranolazine added to either a beta-blocker or calcium channel blocker demonstrated benefits in exercise tolerance, angina frequency, and nitrate use.45 However, prior studies have been limited to younger populations in their 60s with limited inclusion of older adults.46 While generally well-tolerated, ranolazine can cause dizziness, nausea, constipation, and QTc prolongation, all of which have particular relevance among older adults.30 Ivabradine is another antianginal agent that works via inhibition of the funny current, with modest evidence supporting its use for symptom control in patients with stable angina.47 Additional agents recommended by the European guidelines, such as nicorandil and trimetazidine, are not approved for use in the United States.30 Since most antianginal agents reduce blood pressure to some degree, clinicians must exercise caution when using these medications alongside other drugs which are known to lower blood pressure and cause orthostasis, a situation common in older patients with multiple comorbidities. Furthermore, cardiac rehabilitation is an excellent option for older adults with SIHD who are considering initiating an exercise regimen, with associated improvements in QoL, risk of hospitalization, and cardiovascular mortality.48 While planning is required for older adults with stable angina considering an exercise regimen, with attention to competing conditions and pharmacotherapeutics, older adults with SIHD can achieve the same health benefits from aerobic, strengthening, and stretching exercises as younger populations.49 While not often considered as directly related to symptom control, lifestyle changes and additional preventive interventions such as lipid-lowering therapies, eating a healthy diet, and smoking cessation, play a crucial role in the management of patients with SIHD.30,50–52
Percutaneous and Surgical Revascularization in Older Adults with SIHD
When compared to optimal medical therapy alone, the appropriate role for revascularization in SHID has been an area of active debate. Many SIHD trials were conducted before the widespread use of contemporary treatments and excluded patients ≥75 years of age with complex risk profiles, including those with multimorbidity, anatomic complexity, physiologic derangement, and geriatric syndromes. While trial evidence from younger and less complex cohorts suggest that revascularization may positively influence certain cardiovascular events versus medical therapy alone over time,53–56 these trials did not demonstrate a survival benefit with percutaneous revascularization when compared to medical therapy alone (Table 1).5,7,57,58
Table 1.
Results of randomized trials of revascularization strategies in patients with stable ischemic heart disease as they relate to the older adult populations ≥75 years of age.
| Trial (Author, Year) |
Study Population (Sample Size) |
Randomized Intervention | Average Age (Years) | Primary Endpoint | Secondary Endpoint (s) | Representation of Older Adults ≥75 Years* |
|---|---|---|---|---|---|---|
| ECSS (ECSSG, 1982)59 |
Men under the age of 65 with mild to moderate angina of at least 30 months with obstruction of 50% or more in at least 2 major coronary arteries (n=767) |
CABG vs. Medical Therapy |
Mean Age= 49.9 years (Age>53 years = 33%) |
Survival at 5 years:
CABG = 92.4% vs. Medical Therapy = 83.6% P<0.001 |
Men >53 years of age showed significant survival benefit compared with young patients | Older Adults ≥75 excluded |
| CASS (Passamani, 1985)60 |
Patients who were 65 years or younger with clinical and angiographic coronary disease (n=780) |
CABG vs. Medical Therapy | Mean Age = 51 years |
Survival at 8 Years:
CABG = 87% vs. Medical Therapy = 84% P =0.14 |
Patients with EF<50% did have a survival advantage at 7 years with surgery (survival 84% vs. 70%, p=0.01). Those with triple vessel disease had most survival advantage with revascularization | Older Adults ≥75 excluded |
| RITA-2 (RITA-2 trial participants 1997)61 |
Patients with at least one significant stenosis in a major epicardial artery judged to be acceptable for medical therapy or coronary angioplasty (n=1018) |
PTCA vs. Medical Therapy | Median Age = 58 years Included patients ≥70 years old (n=60) |
Death/MI at median 2.7 years follow-up:
PTCA 6.3% vs. Medical Therapy = 3.0% P =0.02 Difference due to one death and seven non-fatal myocardial infarctions related to randomized procedures |
PTCA associated with greater symptomatic improvement, especially in those with more severe angina | Results by age ≥75 years not reported No significant interaction between treatment and age Older adults are underrepresented |
| VA Cooperative Study (Peduzzi, 1998)62 |
Male patients with angina pectoris (n=686) | CABG vs. Medical Therapy | 51 |
Survival at 7 years: CABG = 77% Medical Therapy = 70% P =0.043 Survival at 22 years: CABG = 25% Medical Therapy = 20% P =0.24 |
MI Free Survival at 11 years: CABG = 49% Medical Therapy = 40% P=0.007 MI Free Survival at 22 years: CABG = 18% Medical Therapy = 11% P=0.003 |
Results by age ≥75 years not reported Older Adults are underrepresented |
| TIME (TIME Investigators, 2001)5 |
Patients who were 75 years or older with chronic angina with CCSC >2 and at least two antianginal drugs (n=305) |
Revascularization (angioplasty) vs. Medical Therapy | Mean Age = 80 years |
QoL at 6 Months (SF-36†): Revascularization = 11.4 Vs Medical Therapy = 3.8 P=0.008 |
Other Measures of QoL are improved with revascularization at 6 months MACE at 6 months Revascularization = 19% Vs. Medical Therapy = 49% However, no benefit with revascularization at 1 year. |
Older Adults were represented Multimorbidity and polypharmacy were reported at baseline but effects on outcomes were not evaluated. Frailty not reported |
| DEFER (Pijls, 2007)63 |
Patients referred for elective PCI of a single angiographically significant de novo stenosis (reference diameter>2.5mm); FFR≥0.75 (n=325) |
PCI vs. Medical Therapy | Mean Age = 61 |
Freedom from Cardiac Event at 5 years PCI = 73% vs Medical Therapy (Defer) = 79% P=0.52 |
Patients with FFR<0.75 had 5 times higher rate of cardiac death or AMI | Results by age ≥75 years not reported Older Adults are underrepresented |
| SOS (Booth, 2008)64 |
Patients with multivessel CAD (n=988) |
PCI vs CABG | Mean Age ~ 61 N=395 >65 years old |
Survival at 6 years: PCI = 10.9% vs CABG = 6.8% P=0.022 |
Death rate in diabetic sub-group: PCI = 17.6% vs CABG = 5.4% However p interaction = 0.15 for treatment effect on mortality between diabetic and non-diabetic patients |
Results by age ≥75 years not reported Older Adults are underrepresented |
| MASS-II (Hueb, 2010)65 |
Patients with multivessel CAD and documented ischemia (n=611) |
CABG vs PCI vs Medical Therapy | Mean Age = 60 |
MACE at 10-years:
CABG = 33% vs PCI = 42.4% vs Medical Therapy = 59.1% P<0.001 |
Survival at 10-years: CABG = 74.9% vs PCI = 75.1% vs Medical Therapy = 69% P=0.089 No difference by age > vs. ≤65 |
Results by age ≥75 not reported Older Adults are underrepresented |
| FAME 2 (De Bruyne, 2014)66 |
Patients with stable coronary disease with one-, two-, or three-vessel CAD suitable for PCI (n=888) |
PCI vs Medical Therapy | Mean Age = 63.5 |
MACE at mean follow-up 213–214 days (trial stopped early):
PCI = 4.3% vs Medical Therapy = 12.7% P<0.001 |
Death or MI did not differ between groups, difference in MACE was driven by difference in urgent revascularization | Results by age ≥75 years not reported Older Adults are underrepresented |
| COURAGE (Sedlis, 2015)58 |
Patients with chronic stable angina or silent ischemia and angiographic CAD >70% stenosis (n=2,287) |
PCI vs Medical Therapy | Mean Age (Extended Follow-up) = 64 |
Death at 11.9 Years:
PCI = 41% vs Medical Therapy = 42% P=0.53 |
Mortality rates were similar between PCI and medical therapy groups, in both the non-VA and VA patient sub-groups. - |
Results by age ≥75 years not reported Older Adults are underrepresented Age at 60 years did not modify outcome |
| STICH (Velazquez, 2016)67 |
Patients with CAD amenable to CABG and ejection fraction <35% (n=1,212) |
CABG vs Medical Therapy |
Mean Age ~60 Age 18–85 were included 308 patients >67 years old with median age in that group of 72 years.68 |
Death at median follow-up of 9.8 months:
CABG = 58.9% vs Medical Therapy = 66.1% P=0.02 |
Secondary outcomes including death from cardiovascular causes, HF, any cause, and other MACE favored CABG. | Results by age ≥75 years not reported Older Adults are underrepresented |
| BARI-2D (Ikeno, 2017)69 |
Patients with type 2 diabetes mellitus and evidence of ischemia (n=2,368) |
Prompt Revascularization vs Medical Therapy |
Mean Age ~ 63 Maximum age = 89.8 years. |
Death, MI or Stroke at 5 Years: Low Syntax
<22 CABG = 26.1% vs Medical Therapy = 29.9% P=0.41 Moderate to High Syntax >23 CABG = 15.3% vs Medical Therapy =30.3% P =0.02 |
Death, MI or Stroke at 5 Years: Low Syntax ≤22 PCI = 17.8% vs Medical Therapy = 19.2% P=0.84 Moderate to High Syntax ≥23 PCI = 35.6% vs Medical Therapy =26.5% P =0.12 |
Results by age ≥75 years not reported but results reported by age ≥70, n=514; also included health status outcomes.70 The effect of revascularization versus medical therapy did not differ by age for death (p interaction=0.99), major cardiovascular events, angina, or health status outcomes. Older adults underrepresented |
| ORBITA (Al-Lamee, 2018)6 |
Patients with ≥70% single vessel stenosis. (n=230) |
PCI vs Placebo Procedure | Mean Age = 66 |
Exercise Time did not improve with PCI compared with Placebo Procedure (Difference in increment between groups = 16.6 seconds, P=0.200) |
No improvement in CCSC, Seattle Angina, or EQ-5D-5L Questionnaire with PCI | Results by age ≥75 years not reported Older Adults are underrepresented |
| FREEDOM (Farkouh, 2019)71 | Patients with diabetes and multivessel CAD with diameter stenosis ≥70% in 2 or more major epicardial vessels involving with 2 separate coronary territories (n=1,900) |
CABG vs PCI |
Mean Age ~ 63 |
All-cause mortality at median follow-up 7.5 Years:
CABG = 18.3% vs PCI-DES = 24.3% P=0.01 |
Younger patients (≤63.3 years) derived preferential benefit from CABG compared with older patients (>63.3 years), P for interaction = 0.001 | Results by age ≥75 years not reported Older Adults are underrepresented |
| ISCHEMIA (Maron, 2020)7 |
Patients with stable coronary disease and moderate or severe ischemia (n=5,179)‡ |
Invasive vs Conservative Strategy | Mean Age = 64 |
MACE at median follow-up of 3.2 Years: Hazard Ratio 0.93 (95%CI 0.80 to 1.08) for invasive vs. conservative strategy. Estimated cumulative event rate at 6 months: Invasive Strategy =5.3% vs Conservative Strategy = 3.4% (difference, 1.9 percentage points; 95% CI, 0.8 to 3.0) Estimated cumulative event rate at 5 years: Invasive Strategy =16.4% vs Conservative Strategy = 18.2% (difference, −1.8 percentage points; 95% CI, −4.7 to 1.0) |
Modest improvement in angina-related health status with invasive strategy, driven by greater benefit in those with more symptomatic patients and those with moderate to severe ischemia.72 | Results by age ≥75 years not yet reported Older Adults are underrepresented |
| ISCHEMIA-CKD (Bangalore, 2020)57 |
Patients with advanced kidney disease and moderate or severe ischemia (n=777) |
Invasive vs Conservative Strategy | Median Age = 63 | Death from any cause or MI at 3 Years: Invasive Strategy = 36.4% vs Conservative Strategy = 36.7% P=0.95 |
Death from any cause, MI, Hospitalization for Angina or Heart Failure, or Resuscitated Cardiac Arrest at 3 Years Invasive Strategy = 38.5% vs Conservative Strategy = 39.7% |
Results by age ≥75 years not reported Older Adults are underrepresented |
Representation of Older Adults ≥75 years refers refer to both 1) the inclusion of individuals with chronologic age ≥75 years, as well as 2) the underrepresentation of geriatric participants including those with geriatric syndromes and reporting on those conditions
SRF-36 score 0 to 100 with higher scores indicating more favorable status.
Patients were excluded if they had eGFR<30, a recent acute coronary syndrome, unprotected left main of at least 50%, systolic dysfunction of less than 35, New York Heart Association class III or IV heart failure, and unstable angina.
Abbreviation: CCSC: Canadian Cardiovascular Society Class; QoL = Quality of Life; MACE = major adverse cardiovascular events; PCI = percutaneous coronary intervention; FFR = Fractional Flow Reserve; AMI = Acute Myocardial Infarction; CAD = coronary artery disease; MI = Myocardial Infarction; DES = Drug Eluting Stent; PTCA = Percutaneous transluminal coronary angioplasty.
In contrast, among patients with left main, complex multivessel anatomy with diabetes mellitus, and left ventricular dysfunction, surgical revascularization can provide both symptom relief and long-term survival benefits.73 However, extrapolation to the older adult population with SIHD remains problematic because participants in these trials were younger with less complex disease and lower burden of geriatric syndromes than what is seen in clinical practice.53,71–74 In older patients with higher surgical risk or with geriatric impairments, percutaneous revascularization is a reasonable alternative for left main or multivessel disease if the anatomy is suitable to provide reasonable relief of symptoms and improvement in quality of life.73 Patients potentially benefitting from percutaneous options include those who had a prior CABG with a patent left internal mammary artery, advanced physical frailty, high multimorbidity burden, cognitive or physical dysfunction, the absence of good bypass targets, poor feasibility for complete revascularization, and patient-related preferences.75 PCI in older adults is more complex than younger counterparts owing to their increased risk for procedural complications. Reasonable strategies to minimize risk include the routine use of radial artery access with up to 7F sheaths and guide-catheters, minimizing subtherapeutic or supratherapeutic anticoagulation during PCI, use of clopidogrel over more potent P2Y12 inhibitors, use of intravascular imaging for optimal stent deployment, plaque modification in the presence of severe calcific disease, and minimizing the need for large bore vascular access to introduce mechanical support devices. While mechanical support devices can assist hemodynamic stability for complex PCI, there is a higher risk of bleeding and vascular complications associated with these devices and implementation of vascular safety bundles to minimize such risks are necessary.76
The management of SIHD and revascularization strategy should take into account other therapeutic goals including reduction in angina or anginal equivalent symptoms, improvement in quality of life, and functional independence within age-related contextual factors.77 Steps to standardize the approach for the decision to proceed with the optimal revascularization strategy are critical to reduce the iatrogenic risks from both surgical or percutaneous revascularization. When discussing the approach to revascularization, clarity on each older patient’s functional capacity (physical and cognitive), frailty burden, comorbidities (including the use of the Charlson Comorbidity Index),78 personal goals, and medication profiles are needed prior to intervention.79 Non-invasive cardiac imaging may be helpful to quantify the degree of ischemia, particularly in the context of atypical symptoms, and mitigate biases of under- or over-diagnosis. Because older patients are at the greatest risk for procedural complications, a priori discussion on efforts to reduce these risks must be emphasized by the Heart Team. The optimal duration of antiplatelet therapy or other adjunctive therapies after percutaneous revascularization in older adults should also be considered to balance the tradeoffs between therapeutic benefits versus bleeding and other medication-related risks.27 This balancing act is especially pertinent in patients with atrial fibrillation, which is common in older adults, and in whom anticoagulation will also need to be considered in addition to antiplatelet agents after revascularization. The AUGUSTUS trial showed that clopidogrel and apixaban may be safer than triple therapy after PCI, and there was no significant interaction between age and the primary outcome of major or clinically relevant nonmajor bleeding (p=0.675).80 However, whether these data in patients after ACS can be extrapolated to patients with stable angina is unclear.
IV. Putting it all together
Without any mortality difference between invasive and medical therapeutic approaches, and assuming additional conditions are not present that favor one therapy over another, patients and their clinicians must prioritize strategies to optimize symptomatic improvement within the context of individualized goals for older patients. Further compounding this complexity are the potential contributions from competing chronic conditions that can influence response to therapies: depending on the primary source of the patient’s concerns, symptom burden may be improved or paradoxically exacerbated with common cardiovascular treatments. Treatment strategies must be considered within the broader context of overall quality of life and the individual patient’s priorities, preferences, and health goals. While maintenance of function and independence are a top priority for most older adults, very little is known about the impact of available treatments for SIHD on many of the outcomes that matter most to this population.81 If an older adult presents with stable angina and fatigue, will a beta-blocker improve or worsen their quality of life? If an older patient presents with dyspnea on exertion and SIHD in the context of comorbid anemia and malignancy and prioritizes maintaining their activity level, will PCI and treatment with long-term dual anti-platelet therapy provide durable symptom improvement or have the opposite effect? Clinicians and patients are frequently faced with this uncertainty and, while many of these decisions lack a clear answer, an appreciation of the multitude of potential cardiovascular and non-cardiovascular factors at play may inform a trial-and-error approach. Thus, we propose a “Consider, Listen, Decide” framework for synthesizing these multiple considerations in older adults with stable angina (Figure 3).
Figure 3. A Novel Framework for Approaching Treatment Decisions in Older Adults with Stable Angina.
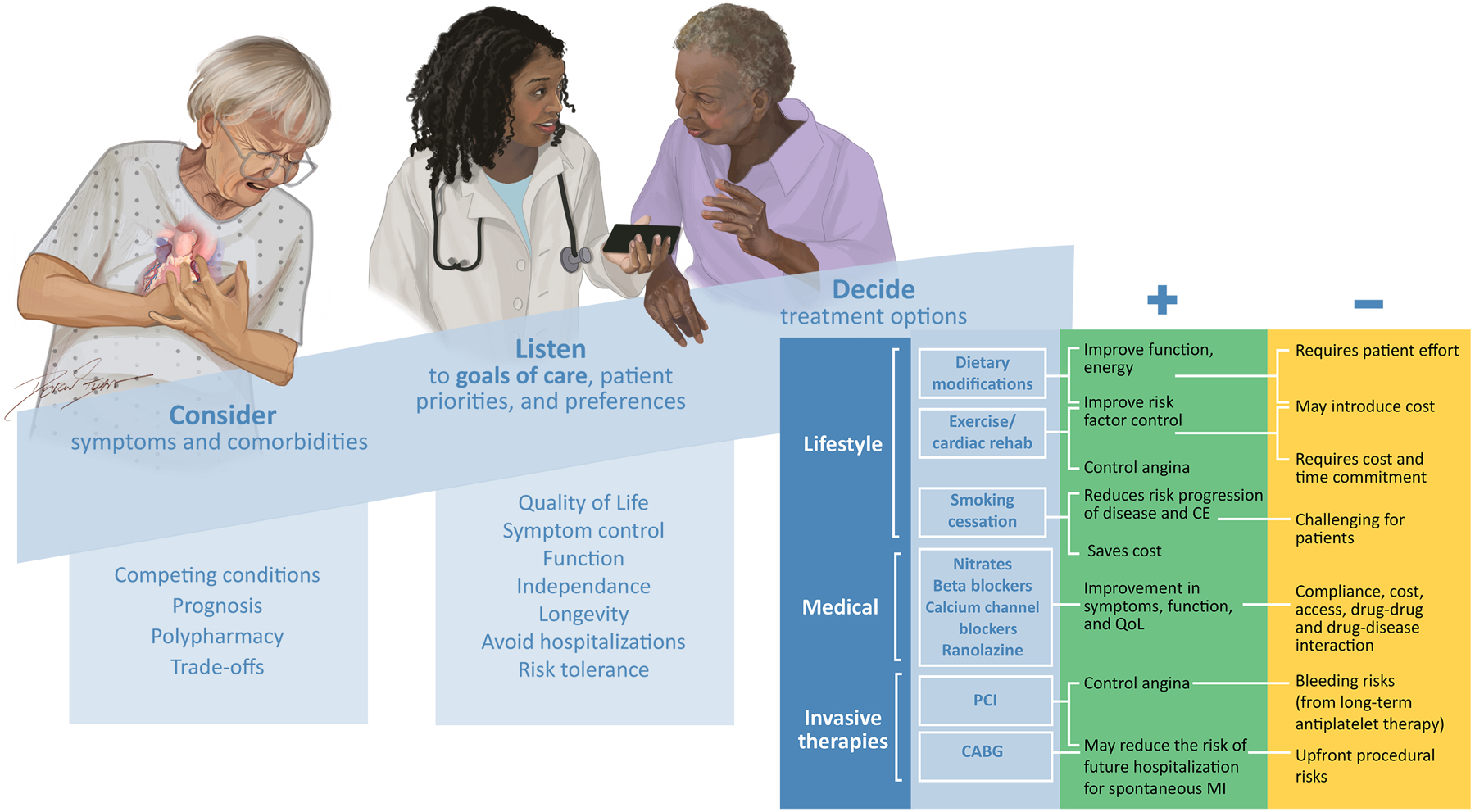
Figure 3 presents a framework for approaching complex treatment decisions in older adults with stable angina within the context of competing conditions, individual patient priorities, preferences, and goals, as well as potential benefits, tradeoffs, and harms of the available treatments, which are listed in order of priority (lifestyle, medical, and invasive therapies).
Future Directions
In response to the limited evidence around many of the available therapies for SIHD in older adults, multiple calls for inclusion of representative populations of older adults with multiple chronic conditions have occurred over the years.22,82,83 The National Institutes of Health Inclusion Across the Lifespan policy went into effect in January 2019, necessitating greater inclusion of representative populations of older adults in clinical trials. Prospective trials of available therapies on patient-centered outcomes for symptomatic SIHD in older adults are imperative to establish a firm evidence-base for treatment in this unique population. One such study is the recently PCORI funded Trial Comparing the Effectiveness and Tolerability of Medications in Older Adults with Stable Angina and Multiple Chronic Conditions: LIVE BETTER, which aims to determine the optimal first-line antianginal treatment strategy in older adults living with stable angina and multiple chronic conditions, with a focus on patient-centered outcomes such as quality of life, symptom control, and mobility. There is an urgent need for pragmatic trials that enroll older adults ≥75 years of age with SIHD to evaluate the efficacy and safety of revascularization strategies for patient-centered outcomes in older adults. While the historical clinical paradigm for treatment of stable angina has focused on the treatment of obstructive epicardial CAD, ischemia with non-obstructive coronary arteries (INOCA) has been increasingly recognized as a crucial contributor to the patient population presenting with stable angina. The literature is limited on epidemiology and management of INOCA among older adults, with the CorMicA (CORonary MICrovascular Angina) trial having a mean age of 61 years.84 The iCorMicA trial will expand upon the initial pilot study with a broader patient population and the ongoing Women’s Ischemia Trial to Reduce Events in Non-obstructive Coronary Artery Disease (WARRIOR) Trial will include approximately one-third adults ≥65 years, both of which should help inform the management of older adults with INOCA.85 The development and integration of geriatric-centric risk models and measures into clinical practice has the potential to better inform health trajectory and both patient and clinician expectations around treatment. Finally, patients with stable angina often misunderstand the potential risks and benefits of therapy, including believing that PCI will extend their lifespan.86 Thus, the development and validation of new approaches to facilitate effective communication regarding benefits and tradeoffs of potential treatments must be a priority going forward.
Acknowledgements:
We would like to thank Devon Stuart for her artistic contributions to the figures featured in this manuscript.
Disclosures:
Nanna MG: Dr. Nanna reports current research support from the American College of Cardiology Foundation supported by the George F. and Ann Harris Bellows Foundation, the Patient-Centered Outcomes Research Institute (PCORI), the Yale Claude D. Pepper Older Americans Independence Center (P30AG021342), and the National Institute on Aging/National Institutes of Health from R03AG074067 (GEMSSTAR award).
Wang SY: None
Damluji, AA: Drs. Damluji receives research funding from the Pepper Scholars Program of the Johns Hopkins University Claude D. Pepper Older Americans Independence Center funded by the National Institute on Aging P30-AG021334 and receives mentored patient-oriented research career development award from the National Heart, Lung, and Blood Institute K23-HL153771-01.
REFERENCES
- 1.Dai X, Busby-Whitehead J, Forman DE, Alexander KP. Stable ischemic heart disease in the older adults. J Geriatr Cardiol. 2016;13:109–114. doi: 10.11909/j.issn.1671-5411.2016.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsao CW, Aday AW, Almarzooq ZI, Anderson CAM, Arora P, Avery CL, Baker-Smith CM, Beaton AZ, Boehme AK, Buxton AE, et al. Heart Disease and Stroke Statistics—2023 Update: A Report From the American Heart Association. Circulation.0. doi: doi: 10.1161/CIR.0000000000001123 [DOI] [PubMed] [Google Scholar]
- 3.Pfisterer M Long-Term Outcome in Elderly Patients With Chronic Angina Managed Invasively Versus by Optimized Medical Therapy. Circulation. 2004;110:1213–1218. doi: doi: 10.1161/01.CIR.0000140983.69571.BA [DOI] [PubMed] [Google Scholar]
- 4.Pfisterer M, Buser P, Osswald S, Allemann U, Amann W, Angehrn W, Eeckhout E, Erne P, Estlinbaum W, Kuster G, et al. Outcome of elderly patients with chronic symptomatic coronary artery disease with an invasive vs optimized medical treatment strategy: one-year results of the randomized TIME trial. Jama. 2003;289:1117–1123. doi: 10.1001/jama.289.9.1117 [DOI] [PubMed] [Google Scholar]
- 5.Trial of invasive versus medical therapy in elderly patients with chronic symptomatic coronary-artery disease (TIME): a randomised trial. Lancet. 2001;358:951–957. doi: 10.1016/s0140-6736(01)06100-1 [DOI] [PubMed] [Google Scholar]
- 6.Al-Lamee R, Thompson D, Dehbi HM, Sen S, Tang K, Davies J, Keeble T, Mielewczik M, Kaprielian R, Malik IS, et al. Percutaneous coronary intervention in stable angina (ORBITA): a double-blind, randomised controlled trial. Lancet. 2018;391:31–40. doi: 10.1016/s0140-6736(17)32714-9 [DOI] [PubMed] [Google Scholar]
- 7.Maron DJ, Hochman JS, Reynolds HR, Bangalore S, O’Brien SM, Boden WE, Chaitman BR, Senior R, López-Sendón J, Alexander KP, et al. Initial Invasive or Conservative Strategy for Stable Coronary Disease. N Engl J Med. 2020;382:1395–1407. doi: 10.1056/NEJMoa1915922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, Douglas PS, Foody JM, Gerber TC, Hinderliter AL, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease. Circulation. 2012;126:e354–e471. doi: doi: 10.1161/CIR.0b013e318277d6a0 [DOI] [PubMed] [Google Scholar]
- 9.Nanna MG, Peterson ED, Wu A, Harding T, Galanos AN, Wruck L, Alexander KP. Age, knowledge, preferences, and risk tolerance for invasive cardiac care. Am Heart J. 2020;219:99–108. doi: 10.1016/j.ahj.2019.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyd C, Smith CD, Masoudi FA, Blaum CS, Dodson JA, Green AR, Kelley A, Matlock D, Ouellet J, Rich MW, et al. Decision Making for Older Adults With Multiple Chronic Conditions: Executive Summary for the American Geriatrics Society Guiding Principles on the Care of Older Adults With Multimorbidity. J Am Geriatr Soc. 2019;67:665–673. doi: 10.1111/jgs.15809 [DOI] [PubMed] [Google Scholar]
- 11.Tinetti ME, Naik AD, Dodson JA. Moving From Disease-Centered to Patient Goals-Directed Care for Patients With Multiple Chronic Conditions: Patient Value-Based Care. JAMA Cardiol. 2016;1:9–10. doi: 10.1001/jamacardio.2015.0248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vespa J The U.S. joins other countries with large aging populations [Internet]. Census.gov. 2021. [cited 2023 Feb 17];Available from: https://www.census.gov/library/stories/2018/03/graying-america.html [Google Scholar]
- 13.Life expectancy at birth, at 65 years of age, and at 75 years of age, by race and sex [Internet]. Health, United States. 2010. [cited 2023 Feb 17];Available from: https://www.cdc.gov/nchs/data/hus/2010/022.pdf [Google Scholar]
- 14.Williamson JD, Supiano MA, Applegate WB, Berlowitz DR, Campbell RC, Chertow GM, Fine LJ, Haley WE, Hawfield AT, Ix JH, et al. Intensive vs Standard Blood Pressure Control and Cardiovascular Disease Outcomes in Adults Aged ≥75 Years: A Randomized Clinical Trial. Jama. 2016;315:2673–2682. doi: 10.1001/jama.2016.7050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orkaby AR, Driver JA, Ho Y-L, Lu B, Costa L, Honerlaw J, Galloway A, Vassy JL, Forman DE, Gaziano JM, et al. Association of Statin Use With All-Cause and Cardiovascular Mortality in US Veterans 75 Years and Older. Jama. 2020;324:68–78. doi: 10.1001/jama.2020.7848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nanna MG, Hajduk AM, Krumholz HM, Murphy TE, Dreyer RP, Alexander KP, Geda M, Tsang S, Welty FK, Safdar B, et al. Sex-Based Differences in Presentation, Treatment, and Complications Among Older Adults Hospitalized for Acute Myocardial Infarction. Circ Cardiovasc Qual Outcomes. 2019;12:e005691. doi: doi: 10.1161/CIRCOUTCOMES.119.005691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamczyk Magda R, Nevado Rosa M, Barettino A, Fuster V, Andrés V. Biological Versus Chronological Aging. J Am Coll Cardiol. 2020;75:919–930. doi: 10.1016/j.jacc.2019.11.062 [DOI] [PubMed] [Google Scholar]
- 18.Jylhävä J, Pedersen NL, Hägg S. Biological Age Predictors. EBioMedicine. 2017;21:29–36. doi: 10.1016/j.ebiom.2017.03.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levine ME. Modeling the rate of senescence: can estimated biological age predict mortality more accurately than chronological age? J Gerontol A Biol Sci Med Sci. 2013;68:667–674. doi: 10.1093/gerona/gls233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ji L, Jazwinski SM, Kim S. Frailty and Biological Age. Ann Geriatr Med Res. 2021;25:141–149. doi: 10.4235/agmr.21.0080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar S, McDaniel M, Samady H, Forouzandeh F. Contemporary Revascularization Dilemmas in Older Adults. J Am Heart Assoc. 2020;9:e014477. doi: doi: 10.1161/JAHA.119.014477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Madhavan MV, Gersh BJ, Alexander KP, Granger CB, Stone GW. Coronary Artery Disease in Patients ≥80 Years of Age. J Am Coll Cardiol. 2018;71:2015–2040. doi: 10.1016/j.jacc.2017.12.068 [DOI] [PubMed] [Google Scholar]
- 23.Ahto M, Isoaho R, Puolijoki H, Laippala P, Romo M, Kivelä SL. Functional abilities of elderly coronary heart disease patients. Aging (Milano). 1998;10:127–136. doi: 10.1007/bf03339647 [DOI] [PubMed] [Google Scholar]
- 24.Pinsky JL, Jette AM, Branch LG, Kannel WB, Feinleib M. The Framingham Disability Study: relationship of various coronary heart disease manifestations to disability in older persons living in the community. Am J Public Health. 1990;80:1363–1367. doi: 10.2105/ajph.80.11.1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gulati M, Levy PD, Mukherjee D, Amsterdam E, Bhatt DL, Birtcher KK, Blankstein R, Boyd J, Bullock-Palmer RP, Conejo T, et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;144:e336–e367. doi: doi: 10.1161/CIR.0000000000001030 [DOI] [PubMed] [Google Scholar]
- 26.Weiss CO, Boyd CM, Yu Q, Wolff JL, Leff B. Patterns of Prevalent Major Chronic Disease Among Older Adults in the United States. Jama. 2007;298:1158–1162. doi: 10.1001/jama.298.10.1160-b [DOI] [PubMed] [Google Scholar]
- 27.Damluji AA, Ramireddy A, Forman DE. Management and Care of Older Cardiac Patients. In: Vasan RS, Sawyer DB, eds. Encyclopedia of Cardiovascular Research and Medicine. Oxford: Elsevier; 2018:245–265. [Google Scholar]
- 28.Fihn SD, Blankenship JC, Alexander KP, Bittl JA, Byrne JG, Fletcher BJ, Fonarow GC, Lange RA, Levine GN, Maddox TM, et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS Focused Update of the Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease. Circulation. 2014;130:1749–1767. doi: doi: 10.1161/CIR.0000000000000095 [DOI] [PubMed] [Google Scholar]
- 29.Farooq V, Serruys PW, Bourantas CV, Zhang Y, Muramatsu T, Feldman T, Holmes DR, Mack M, Morice MC, Ståhle E, et al. Quantification of Incomplete Revascularization and its Association With Five-Year Mortality in the Synergy Between Percutaneous Coronary Intervention With Taxus and Cardiac Surgery (SYNTAX) Trial Validation of the Residual SYNTAX Score. Circulation. 2013;128:141–151. doi: doi: 10.1161/CIRCULATIONAHA.113.001803 [DOI] [PubMed] [Google Scholar]
- 30.Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes: The Task Force for the diagnosis and management of chronic coronary syndromes of the European Society of Cardiology (ESC). Eur. Heart J 2019;41:407–477. doi: 10.1093/eurheartj/ehz425 [DOI] [PubMed] [Google Scholar]
- 31.Gibbons RJ, Chatterjee K, Daley J, Douglas JS, Fihn SD, Gardin JM, Grunwald MA, Levy D, Lytle BW, O’Rourke RA, et al. ACC/AHA/ACP–ASIM Guidelines for the Management of Patients With Chronic Stable Angina: Executive Summary and Recommendations. Circulation. 1999;99:2829–2848. doi: doi: 10.1161/01.CIR.99.21.2829 [DOI] [PubMed] [Google Scholar]
- 32.Maxwell CJ, Hogan DB, Ebly EM. Calcium-channel blockers and cognitive function in elderly people: results from the Canadian Study of Health and Aging. CMAJ. 1999;161:501–506. [PMC free article] [PubMed] [Google Scholar]
- 33.Rae AP, Beattie JM, Lawrie TD, Hutton I. Comparative clinical efficacy of bepridil, propranolol and placebo in patients with chronic stable angina. Br J Clin Pharmacol. 1985;19:343–352. doi: 10.1111/j.1365-2125.1985.tb02653.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ekelund LG, Orö L. Antianginal efficiency of nifedipine with and without a beta-blocker, studied with exercise test. A double-blind, randomized subacute study. Clin Cardiol. 1979;2:203–211. doi: 10.1002/clc.4960020306 [DOI] [PubMed] [Google Scholar]
- 35.Heidenreich PA, McDonald KM, Hastie T, Fadel B, Hagan V, Lee BK, Hlatky MA. Meta-analysis of trials comparing beta-blockers, calcium antagonists, and nitrates for stable angina. Jama. 1999;281:1927–1936. doi: 10.1001/jama.281.20.1927 [DOI] [PubMed] [Google Scholar]
- 36.Rehnqvist N, Hjemdahl P, Billing E, Björkander I, Eriksson SV, Forslund L, Held C, Näsman P, Wallén NH. Effects of metoprolol vs verapamil in patients with stable angina pectoris: The Angina Prognosis Study in Stockholm (APSIS). Eur Heart J. 1996;17:76–81. doi: 10.1093/oxfordjournals.eurheartj.a014695 [DOI] [PubMed] [Google Scholar]
- 37.Sorbets E, Steg PG, Young R, Danchin N, Greenlaw N, Ford I, Tendera M, Ferrari R, Merkely B, Parkhomenko A, et al. β-blockers, calcium antagonists, and mortality in stable coronary artery disease: an international cohort study. Eur Heart J. 2019;40:1399–1407. doi: 10.1093/eurheartj/ehy811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bangalore S, Steg G, Deedwania P, Crowley K, Eagle KA, Goto S, Ohman EM, Cannon CP, Smith SC, Zeymer U, et al. β-Blocker use and clinical outcomes in stable outpatients with and without coronary artery disease. Jama. 2012;308:1340–1349. doi: 10.1001/jama.2012.12559 [DOI] [PubMed] [Google Scholar]
- 39.Steinman MA, Zullo AR, Lee Y, Daiello LA, Boscardin WJ, Dore DD, Gan S, Fung K, Lee SJ, Komaiko KDR, et al. Association of β-Blockers With Functional Outcomes, Death, and Rehospitalization in Older Nursing Home Residents After Acute Myocardial Infarction. JAMA Intern Med. 2017;177:254–262. doi: 10.1001/jamainternmed.2016.7701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Benetos A, Petrovic M, Strandberg T. Hypertension Management in Older and Frail Older Patients. Circ Res. 2019;124:1045–1060. doi: doi: 10.1161/CIRCRESAHA.118.313236 [DOI] [PubMed] [Google Scholar]
- 41.Wei J, Wu T, Yang Q, Chen M, Ni J, Huang D. Nitrates for stable angina: a systematic review and meta-analysis of randomized clinical trials. Int J Cardiol. 2011;146:4–12. doi: 10.1016/j.ijcard.2010.05.019 [DOI] [PubMed] [Google Scholar]
- 42.Thadani U Challenges with nitrate therapy and nitrate tolerance: prevalence, prevention, and clinical relevance. Am J Cardiovasc Drugs. 2014;14:287–301. doi: 10.1007/s40256-014-0072-5 [DOI] [PubMed] [Google Scholar]
- 43.Lipsitz LA, Habtemariam D, Gagnon M, Iloputaife I, Sorond F, Tchalla AE, Dantoine TF, Travison TG. Reexamining the Effect of Antihypertensive Medications on Falls in Old Age. Hypertension. 2015;66:183–189. doi: 10.1161/HYPERTENSIONAHA.115.05513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Woolcott JC, Richardson KJ, Wiens MO, Patel B, Marin J, Khan KM, Marra CA. Meta-analysis of the impact of 9 medication classes on falls in elderly persons. Archives of internal medicine. 2009;169:1952–1960. doi: 10.1001/archinternmed.2009.357 [DOI] [PubMed] [Google Scholar]
- 45.Belsey J, Savelieva I, Mugelli A, Camm AJ. Relative efficacy of antianginal drugs used as add-on therapy in patients with stable angina: A systematic review and meta-analysis. Eur J Prev Cardiol. 2015;22:837–848. doi: 10.1177/2047487314533217 [DOI] [PubMed] [Google Scholar]
- 46.Kosiborod M, Arnold SV, Spertus JA, McGuire DK, Li Y, Yue P, Ben-Yehuda O, Katz A, Jones PG, Olmsted A, et al. Evaluation of ranolazine in patients with type 2 diabetes mellitus and chronic stable angina: results from the TERISA randomized clinical trial (Type 2 Diabetes Evaluation of Ranolazine in Subjects With Chronic Stable Angina). J Am Coll Cardiol. 2013;61:2038–2045. doi: 10.1016/j.jacc.2013.02.011 [DOI] [PubMed] [Google Scholar]
- 47.Tardif JC, Ponikowski P, Kahan T. Efficacy of the I(f) current inhibitor ivabradine in patients with chronic stable angina receiving beta-blocker therapy: a 4-month, randomized, placebo-controlled trial. Eur Heart J. 2009;30:540–548. doi: 10.1093/eurheartj/ehn571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dibben G, Faulkner J, Oldridge N, Rees K, Thompson DR, Zwisler AD, Taylor RS. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2021;11:Cd001800. doi: 10.1002/14651858.CD001800.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mora JC, Valencia WM. Exercise and Older Adults. Clin Geriatr Med. 2018;34:145–162. doi: 10.1016/j.cger.2017.08.007 [DOI] [PubMed] [Google Scholar]
- 50.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596–e646. doi: doi: 10.1161/CIR.0000000000000678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Bäck M, Benetos A, Biffi A, Boavida J-M, Capodanno D, et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice: Developed by the Task Force for cardiovascular disease prevention in clinical practice with representatives of the European Society of Cardiology and 12 medical societies With the special contribution of the European Association of Preventive Cardiology (EAPC). Eur Heart J. 2021;42:3227–3337. doi: 10.1093/eurheartj/ehab484 [DOI] [PubMed] [Google Scholar]
- 52.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, Ferranti Sd, Faiella-Tommasino J, Forman DE, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082–e1143. doi: doi: 10.1161/CIR.0000000000000625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chaitman BR, Hardison RM, Adler D, Gebhart S, Grogan M, Ocampo S, Sopko G, Ramires JA, Schneider D, Frye RL. The Bypass Angioplasty Revascularization Investigation 2 Diabetes randomized trial of different treatment strategies in type 2 diabetes mellitus with stable ischemic heart disease: impact of treatment strategy on cardiac mortality and myocardial infarction. Circulation. 2009;120:2529–2540. doi: 10.1161/circulationaha.109.913111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bangalore S, Maron DJ, Stone GW, Hochman JS. Routine Revascularization Versus Initial Medical Therapy for Stable Ischemic Heart Disease. Circulation. 2020;142:841–857. doi: 10.1161/CIRCULATIONAHA.120.048194 [DOI] [PubMed] [Google Scholar]
- 55.Windecker S, Stortecky S, Stefanini GG, da Costa BR, Rutjes AW, Di Nisio M, Silletta MG, Maione A, Alfonso F, Clemmensen PM, et al. Revascularisation versus medical treatment in patients with stable coronary artery disease: network meta-analysis. BMJ. 2014;348:g3859. doi: 10.1136/bmj.g3859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Navarese EP, Lansky AJ, Kereiakes DJ, Kubica J, Gurbel PA, Gorog DA, Valgimigli M, Curzen N, Kandzari DE, Bonaca MP, et al. Cardiac mortality in patients randomised to elective coronary revascularisation plus medical therapy or medical therapy alone: a systematic review and meta-analysis. Eur Heart J. 2021;42:4638–4651. doi: 10.1093/eurheartj/ehab246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bangalore S, Maron DJ, O’Brien SM, Fleg JL, Kretov EI, Briguori C, Kaul U, Reynolds HR, Mazurek T, Sidhu MS, et al. Management of Coronary Disease in Patients with Advanced Kidney Disease. N Engl J Med. 2020;382:1608–1618. doi: 10.1056/NEJMoa1915925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sedlis SP, Hartigan PM, Teo KK, Maron DJ, Spertus JA, Mancini GBJ, Kostuk W, Chaitman BR, Berman D, Lorin JD, et al. Effect of PCI on Long-Term Survival in Patients with Stable Ischemic Heart Disease. N Engl J Med. 2015;373:1937–1946. doi: 10.1056/NEJMoa1505532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Varnauskas E Twelve-year follow-up of survival in the randomized European Coronary Surgery Study. N Engl J Med. 1988;319:332–337. doi: 10.1056/nejm198808113190603 [DOI] [PubMed] [Google Scholar]
- 60.Passamani E, Davis KB, Gillespie MJ, Killip T. A Randomized Trial of Coronary Artery Bypass Surgery. N Engl J Med. 1985;312:1665–1671. doi: 10.1056/NEJM198506273122603 [DOI] [PubMed] [Google Scholar]
- 61.Coronary angioplasty versus medical therapy for angina: the second Randomised Intervention Treatment of Angina (RITA-2) trial. RITA-2 trial participants. Lancet. 1997;350:461–468. [PubMed] [Google Scholar]
- 62.Peduzzi P, Kamina A, Detre K. Twenty-Two-Year Follow-Up in the VA Cooperative Study of Coronary Artery Bypass Surgery for Stable Angina. Am J Cardiol. 1998;81:1393–1399. doi: 10.1016/S0002-9149(98)00204-5 [DOI] [PubMed] [Google Scholar]
- 63.Pijls NH, van Schaardenburgh P, Manoharan G, Boersma E, Bech JW, van’t Veer M, Bär F, Hoorntje J, Koolen J, Wijns W, et al. Percutaneous coronary intervention of functionally nonsignificant stenosis: 5-year follow-up of the DEFER Study. J Am Coll Cardiol. 2007;49:2105–2111. doi: 10.1016/j.jacc.2007.01.087 [DOI] [PubMed] [Google Scholar]
- 64.Booth J, Clayton T, Pepper J, Nugara F, Flather M, Sigwart U, Stables RH. Randomized, controlled trial of coronary artery bypass surgery versus percutaneous coronary intervention in patients with multivessel coronary artery disease: six-year follow-up from the Stent or Surgery Trial (SoS). Circulation. 2008;118:381–388. doi: 10.1161/circulationaha.107.739144 [DOI] [PubMed] [Google Scholar]
- 65.Hueb W, Lopes N, Gersh BJ, Soares PR, Ribeiro EE, Pereira AC, Favarato D, Rocha AS, Hueb AC, Ramires JA. Ten-year follow-up survival of the Medicine, Angioplasty, or Surgery Study (MASS II): a randomized controlled clinical trial of 3 therapeutic strategies for multivessel coronary artery disease. Circulation. 2010;122:949–957. doi: 10.1161/circulationaha.109.911669 [DOI] [PubMed] [Google Scholar]
- 66.De Bruyne B, Pijls NHJ, Kalesan B, Barbato E, Tonino PAL, Piroth Z, Jagic N, Möbius-Winkler S, Rioufol G, Witt N, et al. Fractional Flow Reserve–Guided PCI versus Medical Therapy in Stable Coronary Disease. N Engl J Med. 2012;367:991–1001. doi: 10.1056/NEJMoa1205361 [DOI] [PubMed] [Google Scholar]
- 67.Velazquez EJ, Lee KL, Jones RH, Al-Khalidi HR, Hill JA, Panza JA, Michler RE, Bonow RO, Doenst T, Petrie MC, et al. Coronary-Artery Bypass Surgery in Patients with Ischemic Cardiomyopathy. N Engl J Med. 2016;374:1511–1520. doi: 10.1056/NEJMoa1602001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Petrie MC, Jhund PS, She L, Adlbrecht C, Doenst T, Panza JA, Hill JA, Lee KL, Rouleau JL, Prior DL, et al. Ten-Year Outcomes After Coronary Artery Bypass Grafting According to Age in Patients With Heart Failure and Left Ventricular Systolic Dysfunction: An Analysis of the Extended Follow-Up of the STICH Trial (Surgical Treatment for Ischemic Heart Failure). Circulation. 2016;134:1314–1324. doi: 10.1161/circulationaha.116.024800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ikeno F, Brooks MM, Nakagawa K, Kim MK, Kaneda H, Mitsutake Y, Vlachos HA, Schwartz L, Frye RL, Kelsey SF, et al. SYNTAX Score and Long-Term Outcomes: The BARI-2D Trial. J Am Coll Cardiol. 2017;69:395–403. doi: 10.1016/j.jacc.2016.10.067 [DOI] [PubMed] [Google Scholar]
- 70.Chung SC, Hlatky MA, Faxon D, Ramanathan K, Adler D, Mooradian A, Rihal C, Stone RA, Bromberger JT, Kelsey SF, et al. The effect of age on clinical outcomes and health status BARI 2D (Bypass Angioplasty Revascularization Investigation in Type 2 Diabetes). J Am Coll Cardiol. 2011;58:810–819. doi: 10.1016/j.jacc.2011.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Farkouh ME, Domanski M, Dangas GD, Godoy LC, Mack MJ, Siami FS, Hamza TH, Shah B, Stefanini GG, Sidhu MS, et al. Long-Term Survival Following Multivessel Revascularization in Patients With Diabetes: The FREEDOM Follow-On Study. J Am Coll Cardiol. 2019;73:629–638. doi: 10.1016/j.jacc.2018.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Spertus JA, Jones PG, Maron DJ, O’Brien SM, Reynolds HR, Rosenberg Y, Stone GW, Harrell FE, Boden WE, Weintraub WS, et al. Health-Status Outcomes with Invasive or Conservative Care in Coronary Disease. N Engl J Med. 2020;382:1408–1419. doi: 10.1056/NEJMoa1916370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lawton JS, Tamis-Holland JE, Bangalore S, Bates ER, Beckie TM, Bischoff JM, Bittl JA, Cohen MG, DiMaio JM, Don CW, et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145:e18–e114. doi: doi: 10.1161/CIR.0000000000001038 [DOI] [PubMed] [Google Scholar]
- 74.Stone GW, Kappetein AP, Sabik JF, Pocock SJ, Morice M-C, Puskas J, Kandzari DE, Karmpaliotis D, Brown WM, Lembo NJ, et al. Five-Year Outcomes after PCI or CABG for Left Main Coronary Disease. N Engl J Med. 2019;381:1820–1830. doi: 10.1056/NEJMoa1909406 [DOI] [PubMed] [Google Scholar]
- 75.Damluji AA, Forman DE, Wang TT, Chikwe J, Kunadian V, Rich MW, Young BA, Page RL, DeVon HA, Alexander KP. Management of Acute Coronary Syndrome in the Older Adult Population: A Scientific Statement From the American Heart Association. Circulation. 2023;147:e32–e62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Damluji Abdulla A, Tehrani B, Sinha Shashank S, Samsky Marc D, Henry Timothy D, Thiele H, West Nick EJ, Senatore Fortunato F, Truesdell Alexander G, Dangas George D, et al. Position Statement on Vascular Access Safety for Percutaneous Devices in AMI Complicated by Cardiogenic Shock. JACC Cardiovasc. Interv 2022;15:2003–2019. doi: 10.1016/j.jcin.2022.08.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rich MW, Chyun DA, Skolnick AH, Alexander KP, Forman DE, Kitzman DW, Maurer MS, McClurken JB, Resnick BM, Shen WK, et al. Knowledge Gaps in Cardiovascular Care of the Older Adult Population: A Scientific Statement From the American Heart Association, American College of Cardiology, and American Geriatrics Society. Circulation. 2016;133:2103–2122. doi: 10.1161/CIR.0000000000000380 [DOI] [PubMed] [Google Scholar]
- 78.Sachdev M, Sun JL, Tsiatis AA, Nelson CL, Mark DB, Jollis JG. The prognostic importance of comorbidity for mortality in patients with stable coronary artery disease. J Am Coll Cardiol. 2004;43:576–582. doi: 10.1016/j.jacc.2003.10.031 [DOI] [PubMed] [Google Scholar]
- 79.Ijaz N, Buta B, Xue QL, Mohess DT, Bushan A, Tran H, Batchelor W, deFilippi CR, Walston JD, Bandeen-Roche K, et al. Interventions for Frailty Among Older Adults With Cardiovascular Disease: JACC State-of-the-Art Review. J Am Coll Cardiol. 2022;79:482–503. doi: 10.1016/j.jacc.2021.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lopes RD, Heizer G, Aronson R, Vora AN, Massaro T, Mehran R, Goodman SG, Windecker S, Darius H, Li J, et al. Antithrombotic Therapy after Acute Coronary Syndrome or PCI in Atrial Fibrillation. N Engl J Med. 2019;380:1509–1524. doi: 10.1056/NEJMoa1817083 [DOI] [PubMed] [Google Scholar]
- 81.Fried TR, Tinetti ME, Iannone L, O’Leary JR, Towle V, Van Ness PH. Health outcome prioritization as a tool for decision making among older persons with multiple chronic conditions. Arch Intern Med. 2011;171:1854–1856. doi: 10.1001/archinternmed.2011.424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Forman DE, Rich MW, Alexander KP, Zieman S, Maurer MS, Najjar SS, Cleveland JC, Jr., Krumholz HM, Wenger NK. Cardiac care for older adults. Time for a new paradigm. J Am Coll Cardiol. 2011;57:1801–1810. doi: 10.1016/j.jacc.2011.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rich Michael W, Chyun Deborah A, Skolnick Adam H, Alexander Karen P, Forman Daniel E, Kitzman Dalane W, Maurer Mathew S, McClurken James B, Resnick Barbara M, Shen Win K, et al. Knowledge Gaps in Cardiovascular Care of the Older Adult Population. J Am Coll Cardiol. 2016;67:2419–2440. doi: 10.1016/j.jacc.2016.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ford TJ, Stanley B, Good R, Rocchiccioli P, McEntegart M, Watkins S, Eteiba H, Shaukat A, Lindsay M, Robertson K, et al. Stratified Medical Therapy Using Invasive Coronary Function Testing in Angina: The CorMicA Trial. J Am Coll Cardiol. 2018;72:2841–2855. doi: 10.1016/j.jacc.2018.09.006 [DOI] [PubMed] [Google Scholar]
- 85.Handberg EM, Merz CNB, Cooper-Dehoff RM, Wei J, Conlon M, Lo MC, Boden W, Frayne SM, Villines T, Spertus JA, et al. Rationale and design of the Women’s Ischemia Trial to Reduce Events in Nonobstructive CAD (WARRIOR) trial. Am Heart J. 2021;237:90–103. doi: 10.1016/j.ahj.2021.03.011 [DOI] [PubMed] [Google Scholar]
- 86.Kureshi F, Jones PG, Buchanan DM, Abdallah MS, Spertus JA. Variation in patients’ perceptions of elective percutaneous coronary intervention in stable coronary artery disease: cross sectional study. BMJ. 2014;349:g5309. doi: 10.1136/bmj.g5309 [DOI] [PMC free article] [PubMed] [Google Scholar]


