Abstract
Background:
Metabolic syndrome (MS) is rapidly growing as risk factor for HCC. Liver resection for HCC in patients with MS is associated with increased postoperative risks. There are no data on factors associated with postoperative complications.
Aims:
The aim was to identify risk factors and develop and validate a model for postoperative major morbidity after liver resection for HCC in patients with MS, using a large multicentric Western cohort.
Materials and Methods:
The univariable logistic regression analysis was applied to select predictive factors for 90 days major morbidity. The model was built on the multivariable regression and presented as a nomogram. Performance was evaluated by internal validation through the bootstrap method. The predictive discrimination was assessed through the concordance index.
Results:
A total of 1087 patients were gathered from 24 centers between 2001 and 2021. Four hundred and eighty-four patients (45.2%) were obese. Most liver resections were performed using an open approach (59.1%), and 743 (68.3%) underwent minor hepatectomies. Three hundred and seventy-six patients (34.6%) developed postoperative complications, with 13.8% major morbidity and 2.9% mortality rates. Seven hundred and thirteen patients had complete data and were included in the prediction model. The model identified obesity, diabetes, ischemic heart disease, portal hypertension, open approach, major hepatectomy, and changes in the nontumoral parenchyma as risk factors for major morbidity. The model demonstrated an AUC of 72.8% (95% CI: 67.2%–78.2%) (https://childb.shinyapps.io/NomogramMajorMorbidity90days/).
Conclusions:
Patients undergoing liver resection for HCC and MS are at high risk of postoperative major complications and death. Careful patient selection, considering baseline characteristics, liver function, and type of surgery, is key to achieving optimal outcomes.
INTRODUCTION
Metabolic syndrome (MS) is a cluster of inter-related risk factors, including abdominal obesity, dyslipidemia, hypertension, and insulin resistance. The prevalence of MS is increasing worldwide and currently represents one of the major health issues in Western countries, reaching rates of 25% in Europe and 43% among adults more than 60 years old in the United States.[1,2] NAFLD is the hepatic manifestation of MS, and ranges from simple steatosis to steatohepatitis and ultimately to fibrosis and cirrhosis.[3] Patients affected by MS have a 2–4 fold higher risk of developing HCC than the general population.[4] Surgical treatments such as liver resection and liver transplantation are the best available options for patients with HCC as they offer long-term survival and are considered potentially curative.[5] Though mortality and morbidity rates have significantly decreased in high volume centers over the last 2 decades, liver resection for HCC in patients with MS remains associated with a 3-fold increased risk of mortality and a 2-fold increased risk of postoperative morbidity, depending on the severity of patients’ comorbidities and parenchymal changes.[6–9] In addition to obesity, these patients also have multiple comorbidities such as type-2 diabetes, atherosclerosis, cardiovascular disease, chronic kidney disease, polycystic ovarian syndrome, obstructive apnea syndrome, and extrahepatic malignancies.[10] As a result, patients are at higher risk of liver-related, cardiovascular, and all-cause mortality and morbidity. Selecting appropriate patients with MS and HCC who need surgery is necessary to avoid unfavorable postoperative outcomes. There are, however, no data on which factors should be considered to select these patients.
This study aimed to review a large multicenter Western database of liver resections for HCC in patients with MS and evaluate the postoperative outcomes focusing on complications and death. Furthermore, we aimed to investigate predictive factors of morbidity after surgery and develop and validate a prediction model.
MATERIALS AND METHODS
Between January 2001 and January 2021, data from 24 institutions (12 European and 12 North American) experienced in the treatment of hepatobiliary malignancies were collected. Patients’ demographics, disease presentation, surgical approach, type of resection performed, intraoperative data, short-term outcomes, pathology report, and oncological outcomes were reviewed.
Patients were included only if fulfilling the following inclusion criteria: (1) receipt of pure laparoscopic, hand-assisted, robot-assisted, or open liver resection for histologically proven hepatocellular carcinoma; (2) a preoperative diagnosis of MS, defined by 3 out of 5 of the following criteria[11,12]: (a) abdominal obesity [body mass index (BMI) ≥ 30 kg/m2 or waist circumference > 102 cm in men and > 88 cm in women][13]; (b) triglycerides > 150 mg/dl; (c) high-density lipoprotein cholesterol <40 mg/dl in men and <50 mg/dl in women; (d) type-2 diabetes or glucose intolerance (fasting glucose > 110 mg/dl); (e) hypertension (blood pressure > 130/85 mm Hg); (3) older than 18; (4) anatomical and non-anatomical hepatectomies; and (5) up to one additional liver ablation. The following exclusion criteria were applied: (1) resections of HCC on viral, alcoholic (> 40 g/d, > 21 drinks per week for men and > 14 drinks per week for women),[14] or autoimmune diseases, as well as hemochromatosis and Wilson’s disease; (2) fibrolamellar HCC or mixed hepatocellular-cholangiocellular carcinoma; (3) extrahepatic metastases; (4) exploratory laparoscopy/laparotomy without liver resection; (5) main portal vein, hepatic artery, biliary duct, or inferior vena cava invasion requiring major reconstructions.
The primary endpoint was to build predictive models for postoperative major morbidity and death. As secondary endpoints, the short-term outcomes focusing on overall morbidity and mortality within 90 days of surgery were investigated. As a sensitivity analysis, outcomes according to type of hepatectomy were explored.
Institutional Review Board (IRB) approval was obtained from the coordinating center (no. 16–801, approved December 7, 2020); data transfer agreement and IRB approval were included and requested for all participating institutions. According to the centers’ policies, every case was discussed in a multidisciplinary setting, and informed consent for surgery was obtained from each patient.
Definitions
Minimally invasive liver resections were considered laparoscopic or robotic-assisted procedures, including conversion to open, according to an intention-to-treat principle. Portal hypertension was defined as the radiological presence of significant splenomegaly, umbilical vein recanalization, portosystemic shunts, and preoperative platelet count <100,000/mm3.[15] Whenever HVPG was available, a 10 mm Hg cutoff was considered as significant portal hypertension.[16] Patients’ comorbidities were graded using the Charlson Comorbidity Index.[17] Major liver resections were defined as the resection of 3 segments or more. Morbidity was graded according to the Clavien-Dindo classification and the Comprehensive Complication Index.[18,19] Major morbidity was defined as a grade 3 or more complication according to Clavien-Dindo classification.[19] Postoperative ascites was defined as a drainage output of more than 10/mL/kg/24 h.[20] Posthepatectomy liver failure and bile leakage were graded according to the International Study Group on Liver Surgery definition.[21,22] A margin of <1 mm was considered an R1 resection. Data on the nontumoral liver tissue were collected: specifically, degree of fibrosis, steatosis, lobular inflammation, and hepatocellular ballooning were graded according to the NAFLD Activity Score (NAS).[23] Furthermore, the European Association for the Study of the Liver (EASL) definition was used to categorize the nontumoral liver parenchyma as follows[24]: (1) NAFL when steatosis alone plus one of lobular or portal inflammation or ballooning was present; (2) NASH, when steatosis was associated with lobular or portal inflammation and ballooning; (3) cirrhosis, when F4 fibrosis was diagnosed; (4) normal parenchyma, when none of the above-mentioned conditions was satisfied.
Statistical analysis
Continuous data were expressed as the mean ± SD or, when appropriate, as median (interquartile ranges) for nonparametric distribution. Categorical data were expressed as numbers and percentages. The distribution of variables was analyzed using the Kolmogorov-Smirnov test.
Logistic regression was used to build a predictive model for 90 days major morbidity. Patients with missing data were excluded. Univariable logistic regression analyses were performed to evaluate the unadjusted association of patients’ and disease’s characteristics (gender, age, BMI, comorbidities, previous surgery, portal hypertension, MELD score, nontumoral liver parenchyma) and surgery (type of approach and type of hepatectomy) with 90-day major morbidity. A prediction model was then built considering all the variables with a P value <0.200 at univariable analyses. Results were presented as OR with the corresponding 95% CI and robust standard errors estimation was performed to take into account centers’ clustering. The prediction model was then built based on the multivariable logistic regression. Based on the multivariable model, a nomogram was constructed. This nomogram provides a graphical representation of the risk factors associated with 90 days major morbidity and enables calculating the risk of postoperative complications for individual patients. The model’s performance was evaluated by internal validation through the bootstrap method choosing n = 1000 resamplings. Internal validation was chosen over splitting the sample to reduce the chance of generating models with suboptimal performance (ie, models with unstable and same performance as obtained with half the sample size).[25]
The predictive discrimination of the model was assessed through the AUC, which is equivalent to the concordance index (c-index), and the values were interpreted according to Hosmer and Lemeshow.[26] The calibration curve and the Hosmer-Lemeshow test were used to assess the model’s goodness of fit. In addition, the Brier score was reported: lower values indicate a higher accuracy of the model. Univariable logistic regression analysis was performed to analyze risk factors for mortality at 90 days. A P value <0.05 was considered statistically significant. Statistical analyses were performed using the R version 4.1.1. The multivariable logistic regression analysis, the nomogram construction, and the calibration plots were performed using the “rms” package (version 5.1–3.1; https://cran.r-project.org/web/packages/rms/). Hosmer-Lemeshow test were performed with the “Hltest.R.”
RESULTS
One thousand eighty-seven patients with a mean age of 68.7 ± 9.3 were collected and reviewed (Table 1). Four hundred eighty-four patients (45.2%) were obese. Most patients presented with hypertension (78.1%), diabetes (56.4%), and dyslipidemia (54.3%). The majority (94.6%) of patients were classified as Child-Pugh A and the median MELD score of the study population was 8 (interquartile range: 6–9).
TABLE 1.
Patients’ characteristics
| n = 1087, n (%) | Missing data | |
|---|---|---|
| Age (y) | 68.7 (± 9.3) | 0 |
| Gender, female/male | 305/782 | 0 |
| Geographic area | 0 | |
| Europe | 667 (61.4) | |
| North America | 420 (38.6) | |
| BMI (kg/m2) | 29.4 (± 5.32) | 22 |
| Obesity (BMI ≥ 30 kg/m2) | 484 (45.2) | 17 |
| ASA score | 3 (2–3) | 48 |
| Charlson comorbidity index | 6 (5–7) | 390 |
| Hypertension | 844 (78.1) | 6 |
| Diabetes | 610 (56.4) | 6 |
| Ischemic heart disease | 217 (20.1) | 9 |
| Congestive cardiac failure | 61 (5.7) | 12 |
| Respiratory disease | 172 (16) | 11 |
| Dyslipidemia | 587 (54.3) | 7 |
| Child-Pugh score | 199 | |
| A | 840 (94.6) | |
| B | 47 (5.3) | |
| C | 1 (0.1) | |
| MELD score | 8 (6–9) | 74 |
| ALBI score | 649 | |
| 1 | 255 (58.2) | |
| 2 | 174 (39.7) | |
| 3 | 9 (2.0) | |
| Portal hypertension | 97 (10.4) | 156 |
| Preoperative ascites | 27 (2.7) | 97 |
| Preoperative varices | 77 (8) | 123 |
| Previous treatment | 2 | |
| Locoregional (TACE-TARE-RFA) | 122 (11.2) | |
| Liver resection | 25 (2.3) | |
| Previous supramesocolic surgery | 189 (17.7) | 18 |
| Preoperative hemoglobin (g/dL) | 13.4 (± 1.88) | 66 |
| Preoperative AST (μ/L) | 36 (25–55) | 75 |
| Preoperative ALT (μ/L) | 34 (23–51) | 74 |
| Preoperative GGT (μ/L) | 74 (42–138) | 386 |
| Preoperative bilirubin (mg/dL) | 0.7 (0.5–1) | 30 |
| Preoperative INR | 1.1 (1–1.19) | 28 |
| Preoperative creatinine (mg/dL) | 0.9 (0.77–1.16) | 25 |
| Preoperative albumin (g/L) | 4 (3.6–4.3) | 343 |
| Preoperative platelets (103/mm3) | 202 (153–266) | 20 |
| Preoperative platelets <100,000/mm3 | 80 (7.5) | |
| Preoperative AFP (ng/mL) | 9.4 (3.6–63) | 144 |
| Preoperative AFP > 200 ng/mL | 144 (15.3) | |
| Number of lesions | 1 (1–1) | 12 |
| Size of lesions (mm) | 48 (31 −75) | 12 |
Note: Continuous data were expressed as mean ± SD or median (25th–75th percentile).
Abbreviations: AFP, alfafetoprotein; ALBI, albumin-bilirubin; ALT, alanine transaminase; ASA, American Society of Anesthesiology; AST, aspartate transaminase; BMI, body mass index; GGT, gamma-glutamyl transferase; INR, international normalized ratio; MELD, Model for End Stage Liver Disease; RFA, radiofrequency ablation; TACE, transarterial chemoebolization; TARE, transarterial radioembolization.
Most liver resections were approached by open technique (59.1%), and 743 patients (68.3%) underwent a minor hepatectomy (Table 2). Thirty-one patients (2.9%) died within 90 days from surgery, and 376 (34.6%) developed postoperative complications (Tables 3, 4). One hundred and fifty patients (13.8%) developed major complications. Ascites and posthepatectomy liver failure were diagnosed in 9.8% and 3.2% of patients, respectively. The median hospital stay was 6 (interquartile range: 5–9) days, and 8.5% of patients were readmitted after discharge. Pathology reports showed an R0 resection rate of 91.2%. Nontumoral liver parenchyma evaluation demonstrated normal parenchyma in 337 (38.7%) patients while NAFL, NASH and cirrhosis was diagnosed in 91 (10.4%), 160 (18.3%), and 284 (32.6%) respectively.
TABLE 2.
Intraoperative data
| n = 1087, n (%) | Missing data | |
|---|---|---|
| Year of operation | 0 | |
| 2001–2011 | 273 (25.1) | |
| 2012–2015 | 328 (30.2) | |
| 2016–2018 | 293 (26.9) | |
| 2019–2021 | 193 (17.8) | |
| Approach | 0 | |
| Open | 642 (59.1) | |
| Laparoscopic | 391 (36.0) | |
| Robotic assisted | 54 (5.0) | |
| Type of hepatectomy | 0 | |
| Minor | 743 (68.3) | |
| Major | 344 (31.7) | |
| Type of resection | 0 | |
| Wedge | 291 (26.8) | |
| Segmentectomy | 198 (18.2) | |
| Bisegmentectomya | 118 (10.9) | |
| Left lateral sectionectomy | 79 (7.3) | |
| Left medial sectionectomy | 8 (0.7) | |
| Right anterior sectionectomy | 6 (0.6) | |
| Central hepatectomy | 12 (1.1) | |
| Left hepatectomy | 82 (7.6) | |
| Right hepatectomy | 193 (17.7) | |
| Left extended hepatectomy | 11 (1) | |
| Right extended hepatectomy | 42 (3.9) | |
| Pringle | 607 (56.4) | 10 |
| Total pringle time (min) | 35 (20–55) | 49 |
| Blood loss (mL) | 300 (100–600) | 139 |
| Blood transfusions | 130 (12.4) | 38 |
| Operative time (min) | 236 (170–304) | 120 |
Note: Continuous data were expressed as median (25th–75th percentile).
Bisegmentectomy other than the ones listed in the table.
TABLE 3.
Postoperative data
| n = 1087, n (%) | Missing data | |
|---|---|---|
| 90 d mortality | 31 (2.9) | 0 |
| 90 d morbidity | 376 (34.6) | 0 |
| Major morbidity (Clavien-Dindo ≥ III) | 150 (13.8) | 2 |
| Comprehensive complication index | 22.6 (20.9–37.1) | 5 |
| Postoperative ascites | 107 (9.8) | 0 |
| Liver failure | 35 (3.2) | 0 |
| Bile leak | 55 (5.0) | 0 |
| Sepsis | 29 (2.6) | 0 |
| Superficial surgical site infection | 23 (2.3) | 0 |
| Pleural effusion | 57 (5.2) | 0 |
| Pneumonia | 43 (4.0) | 0 |
| Deep vein thrombosis/pulmonary embolism | 14 (1.3) | 0 |
| Myocardial infarction | 10 (0.9) | 0 |
| Atrial fibrillation | 31 (2.9) | 0 |
| Urinary tract infection | 21 (1.9) | 0 |
| Acute kidney injury | 31 (2.9) | 0 |
| Hemorrhage | 24 (2.2) | 0 |
| Other | 114 (10.5) | 0 |
| POD 1 bilirubin (mg/dL) | 1.1 (0.7–1.58) | 448 |
| POD 1 INR | 1.2 (1.12–1.36) | 418 |
| POD 1 platelets (103/mm3) | 173 (133–223) | 329 |
| POD 3 bilirubin (mg/dL) | 1.2 (0.8–1.75) | 460 |
| POD 3 INR | 1.2 (1.1–1.36) | 474 |
| POD 3 platelets (103/mm3) | 161 (121–207) | 372 |
| POD 5 bilirubin (mg/dl) | 1 (0.7–1.6) | 652 |
| POD 5 INR | 1.2 (1.1–1.3) | 687 |
| POD 5 platelets (103/mm3) | 184 (141–243) | 552 |
| Hospital stay (d) | 6 (5–9) | 5 |
| Readmission | 81 (8.5) | 136 |
| R1 resection | 95 (8.8) | 12 |
Note: Continuous data were expressed as median (25th–75th percentile). Abbreviations: INR, international normalized ratio; POD, postoperative day.
TABLE 4.
Logistic regression analysis for 90-day major morbidity
| 90 D major morbidity (%, n/N) | Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|---|
| 13.4 (95/713) | OR | 95% CI | P | OR | 95% CI | P | |
| Gender | |||||||
| Female | 11.7 (25/214) | Reference | |||||
| Male | 14.1 (70/499) | 1.23 | 0.83–1.84 | 0.303 | |||
| Age | |||||||
| Years | 1.00 | 0.98–1.03 | 0.895 | ||||
| Obesity (BMI ≥ 30) | |||||||
| No | 10.7 (44/413) | Reference | Reference | ||||
| Yes | 17.0 (51/300) | 1.72 | 1.07–2.76 | 0.025 | 1.29 | 0.75–2.23 | 0.355 |
| Hypertension | |||||||
| No | 11.6 (18/158) | Reference | |||||
| Yes | 13.8 (77/556) | 1.22 | 0.84–1.78 | 0.304 | |||
| Diabetes | |||||||
| No | 9.9 (31/312) | Reference | Reference | ||||
| Yes | 16.0 (64/401) | 1.72 | 1.109–2.69 | 0.017 | 1.40 | 0.91–2.16 | 0.128 |
| Respiratory disease | |||||||
| No | 12.8 (77/601) | Reference | |||||
| Yes | 16.1 (18/112) | 1.30 | 0.75–2.26 | 0.344 | |||
| Ischemic heart disease | |||||||
| No | 12.3 (71/579) | Reference | Reference | ||||
| Yes | 17.9 (24/134) | 1.56 | 0.99–2.46 | 0.054 | 1.62 | 0.94–2.78 | 0.082 |
| Dyslipidemia | |||||||
| No | 13.0 (39/299) | Reference | |||||
| Yes | 13.5 (56/414) | 1.04 | 0.60–1.80 | 0.880 | |||
| Previous surgery | |||||||
| No | 12.9 (77/599) | Reference | |||||
| Yes | 15.8 (18/114) | 1.27 | 0.50–3.25 | 0.617 | |||
| Portal hypertension | |||||||
| No | 12.3 (79/640) | Reference | Reference | ||||
| Yes | 21.9 (16/73) | 1.99 | 1.11–3.59 | 0.021 | 2.65 | 1.36–5.16 | 0.004 |
| MELD score | |||||||
| < 9 | 12.0 (58/485) | Reference | |||||
| ≥ 9 | 16.2 (37/228) | 1.43 | 0.74–2.75 | 0.289 | |||
| Approach | |||||||
| Open | 17.9 (68/380) | Reference | Reference | ||||
| Minimally invasive | 8.1 (27/333) | 0.4 | 0.22–0.73 | 0.003 | 0.56 | 0.33–0.93 | 0.026 |
| Type hepatectomy | |||||||
| Minor | 8.3 (40/483) | Reference | Reference | ||||
| Major | 23.9 (55/230) | 3.48 | 1.97–6.16 | < 0.001 | 4.56 | 2.55–8.15 | < 0.001 |
| Nontumoral parenchyma | |||||||
| Normal parenchyma NAFL | 10.5 (31/294) | Reference | Reference | ||||
| 7.3 (6/82) | 0.67 | 0.24–1.90 | 0.451 | 0.72 | 0.28–1.89 | 0.507 | |
| NASH | 14.7 (19/129) | 1.47 | 0.70–3.06 | 0.309 | 1.79 | 1.00–3.19 | 0.048 |
| Cirrhosis | 18.8 (39/208) | 1.96 | 1.27–3.02 | 0.002 | 2.64 | 1.36–5.14 | 0.004 |
Abbreviations: BMI, body mass index; MELD, Model for End Stage Liver Disease.
Nomogram to predict 90 days major morbidity
Among 1087 patients, 713 (65%) had complete data and were included in the prediction models. No multi-collinearity was observed. The model identified open approach (P = 0.026), major hepatectomy (P < 0.001), and portal hypertension (P = 0.004) as statistically significant risk factors for postoperative major morbidity. Concerning nontumoral parenchyma, cirrhosis was associated with 2.64 times higher odds of major morbidity compared to normal parenchyma (OR = 2.64, 95% CI: 1.36–5.14; P = 0.004). Furthermore, NASH patients had higher odds of having major morbidity at 90 days compared to normal parenchyma patients (OR = 1.79, 95% CI: 1.00–3.19; P = 0.048). The pair-wise comparisons showed a significant difference between cirrhosis compared to NAFL patients (OR = 3.66, 95% CI: 1.21–11.06; P = 0.021) or compared to NASH patients (OR = 2.63, 95% CI: 1.07–6.48; P = 0.036). The discrimination power was 75.1% (95% CI: 69.8%–80.4%) and at internal validation, after bootstrapping, the model showed a corrected AUC of 72.8% (95% CI: 67.2%–78.2%). The model is graphically presented as nomogram in Figure 1. An online calculator is available at https://childb.shinyapps.io/NomogramMajorMorbidity90days/.
FIGURE 1.
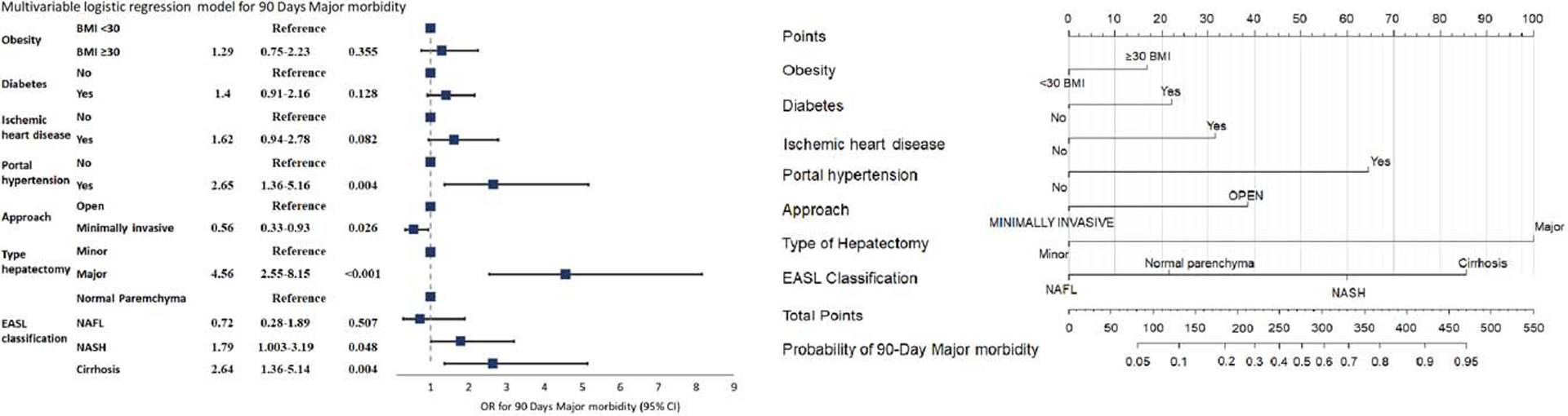
Multivariable model and nomogram to predict 90 days major morbidity following surgery for hepatocellular carcinoma on metabolic syndrome. The nomogram maps the predicted probability of 90 days postoperative major morbidity in a scale of 0–550. For each covariate, please draw a vertical line upwards and note down the corresponding points (ie, major hepatectomy = 100 points). This is repeated for each covariate ending with a total score that corresponds to a predicted probability of morbidity at the bottom of the nomogram. Please visit https://childb.shinyapps.io/NomogramMajorMorbidity90days/. Model equation on logarithmic scale was equal to: −3.2+0.26*Obesity(BMI ≥ 30)+0.34*Diabetes +0.48*Ischemic heart disease+0.98*Portal Hypertension–0.58*Approach(minimally invasive)+1.52*type of hepatectomy(major)+(if EASL classification = NAFL the coefficient was −0.32; if EASL classification = NASH the coefficient was +0.58; if EASL classification = Cirrhosis the coefficient was +0.97). Lower and upper confidence limit of the constant: −4.04 to −2.55. Abbreviations: BMI, body mass index; EASL, European Association for the Study of the Liver.
Calibration was evaluated by plotting the predicted probability of morbidity and the actual outcomes (Figure 2). The model was less accurate in estimating high probabilities, but the calibration curve showed good concordance between the predicted probability and the actual probability. The Hosmer-Lemeshow test yielded a nonsignificant statistic (P = 0.226) and the Brier score was equal to 0.102.
FIGURE 2.
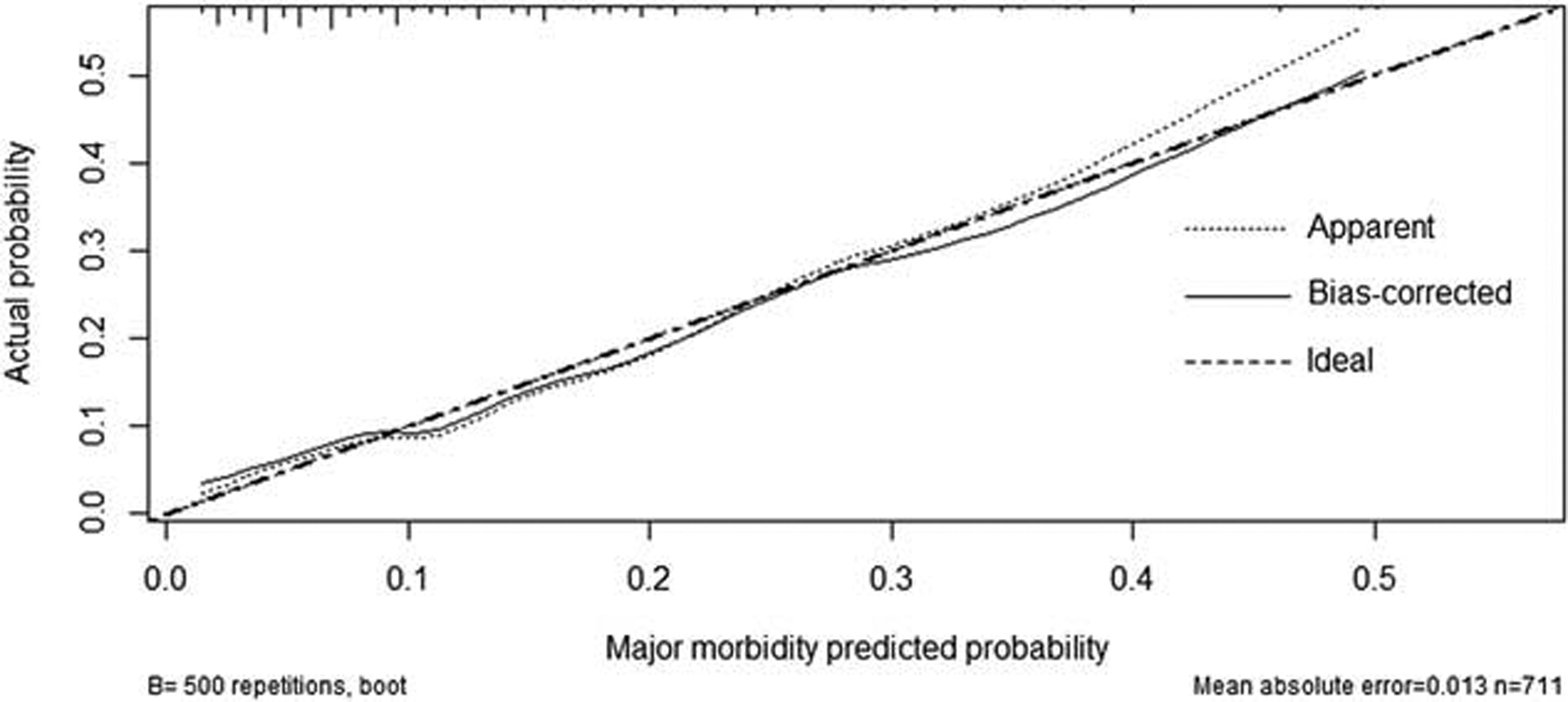
Calibration plot of the nomogram. Ideal line estimated probabilities correspond to the actual observed; apparent line, prediction capability of the model obtained after data analysis; bias-corrected line, prediction capability of the model obtained after bootstrap correction. Vertical lines at the top of the figure represent number of patients.
Only univariable analysis was performed for 90-day mortality due to the small number of events (n = 22/713, 3.1%) precluding the construction of reliable multivariable model. Portal hypertension (OR = 3.49, 95% CI: 1.22–8.81; P = 0.012), MELD score ≥ 9 (OR = 3.2, 95% CI: 1.36–7.86; P = 0.008) as well as major hepatectomies (OR = 3.84, 95% CI: 1.62–9.76; P = 0.003) were associated with postoperative mortality (Table 5).
TABLE 5.
Logistic regression analysis for 90-day mortality
| 90 D mortality (%. n/N) | Univariable analysis | |||
|---|---|---|---|---|
| 3.1 (22/713) | OR | 95% CI | P | |
| Gender | ||||
| Female | 29 (62/214) | Reference | ||
| Male | 36.5 (182/499) | 1.47 | 0.57–4.53 | 0.451 |
| Age | ||||
| Years | 1.01 | 0.96–1.06 | 0.812 | |
| Obesity (BMI ≥ 30) | ||||
| No | 2.2 (9/413) | Reference | ||
| Yes | 4.3 (13/300) | 2.03 | 0.87–4.99 | 0.107 |
| Hypertension | ||||
| No | 3.9 (6/155) | Reference | ||
| Yes | 2.9 (16/558) | 0.73 | 0.29–2.07 | 0.524 |
| Diabetes | ||||
| No | 1.6 (5/312) | Reference | ||
| Yes | 4.2 (17/401) | 2.71 | 0.99–7.45 | 0.052 |
| Respiratory disease | ||||
| No | 3 (18/601) | Reference | ||
| Yes | 3.6 (4/112) | 1.2 | 0.34–3.29 | 0.746 |
| Ischemic heart disease | ||||
| No | 2.9 (17/579) | Reference | ||
| Yes | 3.7 (5/134) | 1.28 | 0.41–3.31 | 0.632 |
| Dyslipidemia | ||||
| No | 3.3 (10/299) | Reference | ||
| Yes | 2.9 (12/414) | 0.86 | 0.37–2.07 | 0.734 |
| Previous surgery | ||||
| No | 3.3 (20/599) | Reference | ||
| Yes | 1.7 (2/114) | 0.52 | 0.08–1.80 | 0.378 |
| Portal hypertension | ||||
| No | 2.5 (16/640) | Reference | ||
| Yes | 8.2 (6/73) | 3.49 | 1.22–8.81 | 0.012 |
| MELD score | ||||
| < 9 | 1.9 (9/485) | Reference | ||
| ≥ 9 | 5.7 (13/228) | 3.2 | 1.36–7.86 | 0.008 |
| Approach | ||||
| Open | 3.4 (13/380) | Reference | ||
| Minimally invasive | 2.7 (9/333) | 0.78 | 0.32–1.84 | 0.581 |
| Type hepatectomy | ||||
| Minor | 1.7 (8/483) | Reference | ||
| Major | 6.1 (14/230) | 3.84 | 1.62–9.76 | 0.003 |
| Nontumoral parenchyma | ||||
| Normal parenchyma | 3.1 (9/294) | Reference | ||
| NAFL | 0 (0/82) | — | — | — |
| NASH | 3.1 (4/129) | 1.01 | 0.27–3.17 | 0.983 |
| Cirrhosis | 4.3 (9/208) | 1.43 | 0.55–3.73 | 0.455 |
Abbreviations: BMI, body mass index; MELD, Model for End Stage Liver Disease.
The type of hepatectomy was associated with post-operative outcomes (Supplemental Table http://links.lww.com/HEP/A52). Patients undergoing major hepatectomies had significantly higher morbidity (48.7% vs. 27.3%; P < 0.001) and mortality rates (6.1% vs. 1.7%; P = 0.001), as well as a higher chance of developing postoperative liver failure (7.4% vs. 1.0%; P = 0.002) and bile leaks (9.6% vs. 2.3%; P = 0.011) as compared to patients undergoing minor resections. Hospital stay was also longer (7 vs. 6 d; P < 0.001) and a greater proportion of patients were readmitted after discharge (13.7% vs. 7.3%; P = 0.013).
DISCUSSION
Liver resection for HCC in patients with MS is associated with high rates of postoperative morbidity and mortality.[6] A nomogram to improve patients’ selection for surgery may help decrease complications and should therefore be implemented in hepatobiliary centers managing these patients. Indeed, preoperative knowledge of factors associated with postoperative major morbidity could help surgeons identify individuals at high risk for surgery, address modifiable variables, and evaluate and discuss with the patient potential alternatives, risks, benefits, and expectations of treatment.
As viral hepatitis is significantly decreasing in recent years due to the efficacy of new generation drugs and vaccinations, NAFLD has become the leading cause of chronic liver diseases in Western countries.[27] The evolving parenchymal changes induced by NAFLD (steatosis, fibrosis, cirrhosis) lead to the development of precancerous lesions and a yearly incidence of HCC as high as 2.6%.[28] Furthermore, both MS and NAFLD promote the development of primary liver malignancies regardless of fibrosis or cirrhosis, given the pathological proinflammatory environment, the altered endocrine and immunological signaling, and the metabolic and oxidative stress.[29,30] Recent advancements in surgical techniques and technology, as well as the improvements in preoperative evaluation of patients and liver function, have resulted in a decline in the perioperative mortality and morbidity of liver surgery, which currently represents one of the potentially curative treatment options for patients with HCC.[7] Despite this, patients with MS and early-stage HCC amenable to surgical resection have increased risks of postoperative complications, representing a unique category of patients and a surgical challenge. In 2012, a large population study based on the National Surgical Quality Improvement Program (NSQIP) database from the United States showed that patients with MS undergoing hepatectomy for both benign and malignant diseases developed postoperative complications in 29% of cases, and 9% of them died within 30 days.[31] More recently, Paro et al[32] confirmed that patients with MS have higher odds of postoperative morbidity and mortality and, in turn, lower odds of achieving textbook outcomes following hepatectomy. Furthermore, patients were at higher risks of being readmitted to the hospital after discharge. Cauchy et al[6] in a study with 62 patients undergoing surgery showed a 11% mortality rate and a 58% morbidity rate. In a relatively larger study comparing 152 NAFLD and 844 non-NAFLD patients, Koh et al[8] corroborated these results disclosing a 54.6% versus 30.8% morbidity rate in NAFLD and non-NAFLD patients, respectively, with a major morbidity rate of 16.2% versus 8.1%. Compared to nonmetabolic related liver diseases, patients with NAFLD have significantly worse postoperative outcomes. Wakai and colleagues compared 17 patients with HCC on NAFLD, 61 with underlying hepatitis B disease, and 147 with underlying hepatitis C. Patients with NAFLD had a 59% morbidity rate as compared to 28% in hepatitis C and 31% in hepatitis B patients. Furthermore, mortality was also higher with a rate of 12% in NAFLD patients as compared to 0.7% in hepatitis C and 3.3% in hepatitis B.[9] In our cohort from Western tertiary-referral centers, we observed a 34.6% morbidity and 2.9% mortality at 90 days, with 13.8% of patients experiencing major complications. These results are promising, especially considering that all of our patients, as opposed to those of the above-mentioned study, were diagnosed with HCC and that 17.5% and 32.6% had significant fibrosis or cirrhosis, respectively, therefore harboring increased postoperative risks. Notwithstanding, morbidity and mortality are still high and might be ameliorated by preoperatively selecting patients at the highest risk for surgery.
Comorbidities in MS play an essential role in increasing the chance of postoperative complications. Diabetes, obesity, dyslipidemia, cardiovascular, and respiratory diseases frequently coexist in this syndrome, framing the patient as a high-risk individual from a surgical standpoint.[33,34] Indeed, MS has been recognized as a predictor of adverse postoperative outcomes in bariatric, colorectal, pancreatic, and endocrine surgery.[35–37] A high number of patients were overweight or obese in our study, with an overall high mean BMI, diabetes, hypertension, and dyslipidemia. This further highlights the complexity of managing patients with HCC on MS: indeed, in addition to the tumor itself and the decreased liver function, these individuals have the additional negative predictive factor of presenting with multiple comorbidities. In our model, diabetes and obesity were associated with postoperative major complications. Appropriate diet, exercise, and pharmacotherapy should not only be employed to prevent NAFLD and the development of malignancies but also to improve the outcomes when surgery is considered.[38] Previous studies have shown that preoperative exercise and diet alteration of proteins intake without the concomitant increase of the lipids intake are effective tools to improve short-term and long-term outcomes in cancer patients with MS.[39,40] In this setting, clinicians should consider preoperative nutrition consult and rehabilitation support to eventually improve outcomes.
For patients with early-stage HCC undergoing surgery, advanced liver cirrhosis and portal hypertension are adverse prognostic factors for both short-term and long-term outcomes.[41,42] In our study, we confirmed that the presence of clinically significant portal hypertension was associated with increased major morbidity and mortality and should therefore be preoperatively assessed in patients with MS undergoing surgery. Preoperative liver function is mostly assessed with the MELD score and Child-Pugh classifications. These scores were originally developed in patients with cirrhosis. Because our study includes both patients with and without cirrhosis, the ALBI score would be more appropriate. Unfortunately, this was available only for 438 out of the 1087 patients. This is probably related to the fact that the ALBI is currently adopted in few of the centers included in our study. Indeed, despite being developed on a Western series of patients,[43] this score has been mainly validated and used in the East.[44–47]
There is a significant association between postoperative complications in NAFLD and the extent of liver resections. A systematic review and meta-analysis in 2010 reported that patients with at least 30% steatosis had significantly increased risks of morbidity and mortality following major hepatic resections.[7] A more recent study including both benign and malignant liver tumors showed that following major resections, patients with MS had a 37% and 32% chance of postoperative morbidity and serious morbidity, respectively, and a 2.7% chance of mortality.[48] We confirmed that major hepatectomies were associated with high rates of complications and death and were among the strongest predictors of poor surgical outcomes. Indeed, posthepatectomy liver decompensation is a significant issue for patients with HCC that is further worsened in cases of major resections; depending on the preoperative clinical assessment and the estimation of the future liver remnant and its regeneration capacity, the extent of resections should be weighed against the potential drawbacks that major surgery implies. A parenchymal sparing approach should be preferred whenever possible and certainly in patients with impaired liver function and comorbidities, still maintaining oncological adequacy.
Another potentially modifiable risk factor in the hands of the surgeon is the type of surgical approach. Since its introduction, minimally invasive liver surgery has been associated with improved postoperative outcomes, including morbidity and hospital stay, especially in the setting of HCC and liver cirrhosis.[49–51] In our study, we identified that surgical approach, either laparoscopic or robotic-assisted, was associated with decreased major morbidity. Patients with MS potentially benefit from a minimally invasive approach but are frequently obese and have pulmonary or cardiovascular comorbidities, limiting the application of laparoscopy or robotics. In our opinion, a minimally invasive approach should strongly be considered in these patients as the benefits may outweigh the risks of conversion. In this setting, referral to tertiary centers where laparoscopy or robotics have been implemented should be considered.
MS and NAFLD are associated with various histopathological changes, ranging from normal parenchyma to significant fibrosis and cirrhosis. These modifications are potentially related to different rates of complications that are currently under investigation worldwide. Of note, previous studies have shown that in patients with noncirrhotic NAFLD livers, morbidity following liver resection is similar to patients with liver cirrhosis.[30,52,53] However, no correlation with histopathological data has been reported so far. To our knowledge, this is the first study demonstrating the association between nontumoral liver parenchyma changes and morbidity following surgery in patients with MS. The collaboration between experienced centers allowed gathering precise pathological data to address important clinical questions. First, cirrhotic livers were associated with almost a 3-fold increase in major complications compared to patients with normal parenchyma, similarly to what has been already reported in the literature.[42,54–56] Second, even initial parenchymal changes such as steatosis and NASH showed to have a detrimental effect on major complications following hepatectomy. This result, although intuitive, it is the first of its kind given the data and represents an important message in the field. As 75% of patients with MS showed some sort of parenchymal disease and 32.6% had cirrhosis, the preoperative knowledge of such changes might be useful in selecting patients undergoing liver resection, especially if a major operation is planned. Despite this, pathological information of nontumoral liver parenchyma requires a preoperative biopsy which is neither recommended by international guidelines nor performed in most centers worldwide. In this setting, we suggest implementing noninvasive diagnostic measurements of liver steatosis and fibrosis (serum biomarkers, MRI, fibroscan) to stratify the surgical risk for each patient, eventually improving the selection of surgical candidates and allowing for a clear patient-clinician discussion and informed consent. Indeed, the main application of the model proposed in the current study is to support a clear and thorough discussion with the patient on risks and benefits of the procedure, to clarify expectations and to guide clinicians addressing the informed consent properly.
This is a retrospective study, and selection bias might limit the data quality and results. Variables such as comorbidities can be hard to retrieve and categorize, especially in a multicenter study involving 24 centers. We only allowed the inclusion of patients satisfying at least 3 of the 5 diagnostic criteria for MS, therefore improving the homogeneity of our population. Recently, a new definition of metabolic-associated fatty liver disease was proposed:[57,58] collection of data for the present study was ongoing when this new definition was proposed. Metabolic-associated fatty liver disease should be further investigated and validated to standardize terminology in the literature. Our study is the largest available on the topic, collecting patients from 24 institutions from Western countries where MS represents a major healthcare problem. Furthermore, this is the first study reporting risk factors for postoperative major morbidity of this high-risk subset of patients. The reproducibility of our results is limited to Western countries as Asian populations have both different body compositions and different surgical policies regarding HCC. MS is, however, not only a Western disease as it is rapidly growing also in Eastern countries. Results of surgery in this setting are warranted. Weight and BMI are limited measures to truly assess the outcomes of patients undergoing surgery. Sarcopenia and body composition are more powerful predictors of outcomes and are currently assessed in different surgical settings.[38] However, sarcopenia was not considered in the current study as few centers have employed this marker in clinical practice. The prediction model presented in this study excluded patients with missing variables rather than using multiple imputations and used internal validation with the bootstrapping method. Missing data were considered to be missing completely at random in the present study, therefore being noninformative to our primary aim. We acknowledge that missing completely at random is rare and that significant information might be hidden in data that were not available in our database. For this reason, external validation of our model is required, preferably in a prospective fashion and in large populations.
CONCLUSIONS
MS is a rising disease in Western countries and will eventually represent the major cause of HCC. Patients with HCC on MS have multiple comorbidities and different degrees of liver function. In this setting, liver resection is at high risk for postoperative complications and death, and the selection of patients is mandatory to improve the outcomes. Patients’ characteristics such as, BMI, diabetes, ischemic heart disease the presence of portal hypertension, and the status of nontumoral liver parenchyma should be carefully considered before surgery. Minor resections and minimally invasive approaches should be preferred whenever possible to decrease the chance of postoperative complications.
Supplementary Material
CONFLICT OF INTEREST
C.C. consults for FujiFilm, Integra, and Stryker. G.S. consults for Integra, AstraZeneca, Roche, and Eisai; he also received grants from Roche. This work was supported by Memorial Sloan Kettering Cancer Center P30 Cancer Center Support Grant (P30 CA008748)
REFERENCES
- 1.Agrawal S, Daruwala C. Metabolic syndrome and hepatic resection: improving outcome. HPB (Oxford). 2011;13:846–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cauchy F, Belghiti J. A clinical perspective of the link between metabolic syndrome and hepatocellular carcinoma. J Hepatocell Carcinoma. 2015;2:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agopian VG, Kaldas FM, Hong JC, et al. Liver transplantation for nonalcoholic steatohepatitis: the new epidemic. Ann Surg. 2012; 256:624–33. [DOI] [PubMed] [Google Scholar]
- 4.Turati F, Talamini R, Pelucchi C, et al. Metabolic syndrome and hepatocellular carcinoma risk. Br J Cancer. 2013;108:222–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. [DOI] [PubMed] [Google Scholar]
- 6.Cauchy F, Zalinski S, Dokmak S, et al. Surgical treatment of hepatocellular carcinoma associated with the metabolic syn-drome. Br J Surg. 2013;100:113–21. [DOI] [PubMed] [Google Scholar]
- 7.de Meijer VE, Kalish BT, Puder M, et al. Systematic review and meta-analysis of steatosis as a risk factor in major hepatic resection. Br J Surg. 2010;97:1331–9. [DOI] [PubMed] [Google Scholar]
- 8.Koh YX, Tan HJ, Liew YX, et al. Liver resection for nonalcoholic fatty liver disease-associated hepatocellular carcinoma. J Am Coll Surg. 2019;229:467–78 e1. [DOI] [PubMed] [Google Scholar]
- 9.Wakai T, Shirai Y, Sakata J, et al. Surgical outcomes for hepatocellular carcinoma in nonalcoholic fatty liver disease. J Gastrointest Surg. 2011;15:1450–8. [DOI] [PubMed] [Google Scholar]
- 10.Kumar R, Priyadarshi RN, Anand U. Non-alcoholic fatty liver disease: growing burden, adverse outcomes and associations. J Clin Transl Hepatol. 2020;8:76–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–5. [DOI] [PubMed] [Google Scholar]
- 12.Eckel RH, Alberti KG, Grundy SM, et al. The metabolic syndrome. Lancet. 2010;375:181–3. [DOI] [PubMed] [Google Scholar]
- 13.Ascha MS, Hanouneh IA, Lopez R, et al. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010;51:1972–8. [DOI] [PubMed] [Google Scholar]
- 14.Sanyal AJ, Brunt EM, Kleiner DE, et al. Endpoints and clinical trial design for nonalcoholic steatohepatitis. Hepatology. 2011; 54:344–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Santambrogio R, Kluger MD, Costa M, et al. Hepatic resection for hepatocellular carcinoma in patients with Child-Pugh’s A cirrhosis: is clinical evidence of portal hypertension a contra-indication? HPB (Oxford). 2013;15:78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bosch J, Garcia-Pagan JC. Complications of cirrhosis. I. Portal hypertension. J Hepatol. 2000;32(suppl):141–56. [DOI] [PubMed] [Google Scholar]
- 17.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. [DOI] [PubMed] [Google Scholar]
- 18.Slankamenac K, Graf R, Barkun J, et al. The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg. 2013;258:1–7. [DOI] [PubMed] [Google Scholar]
- 19.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishizawa T, Hasegawa K, Kokudo N, et al. Risk factors and management of ascites after liver resection to treat hepatocellular carcinoma. Arch Surg. 2009;144:46–51. [DOI] [PubMed] [Google Scholar]
- 21.Koch M, Garden OJ, Padbury R, et al. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery. 2011;149:680–8. [DOI] [PubMed] [Google Scholar]
- 22.Rahbari NN, Garden OJ, Padbury R, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery. 2011; 149:713–24. [DOI] [PubMed] [Google Scholar]
- 23.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–21. [DOI] [PubMed] [Google Scholar]
- 24.European Association for the Study of the L, European Association for the Study of D, European Association for the Study of O. EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388–402. [DOI] [PubMed] [Google Scholar]
- 25.Steyerberg EW, Harrell FE Jr. Prediction models need appropriate internal, internal-external, and external validation. J Clin Epidemiol. 2016;69:245–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hosmer DW, Hosmer T, Le Cessie S, et al. A comparison of goodness-of-fit tests for the logistic regression model. Stat Med. 1997;16:965–80. [DOI] [PubMed] [Google Scholar]
- 27.Fierbinteanu-Braticevici C, Negreanu L, Tarantino G. Is fatty liver always benign and should not consequently be treated? J Physiol Pharmacol. 2013;64:3–9. [PubMed] [Google Scholar]
- 28.Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology. 2010;51:1820–32. [DOI] [PubMed] [Google Scholar]
- 29.Welzel TM, Graubard BI, Zeuzem S, et al. Metabolic syndrome increases the risk of primary liver cancer in the United States: a study in the SEER-Medicare database. Hepatology. 2011;54: 463–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. [DOI] [PubMed] [Google Scholar]
- 31.Bhayani NH, Hyder O, Frederick W, et al. Effect of metabolic syndrome on perioperative outcomes after liver surgery: A National Surgical Quality Improvement Program (NSQIP) analysis. Surgery. 2012;152:218–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paro A, Tsilimigras DI, Dalmacy D, et al. Impact of metabolic syndrome on postoperative outcomes among medicare beneficiaries undergoing hepatectomy. J Gastrointest Surg. 2021;25: 2545–52. [DOI] [PubMed] [Google Scholar]
- 33.Little SA, Jarnagin WR, DeMatteo RP, et al. Diabetes is associated with increased perioperative mortality but equivalent long-term outcome after hepatic resection for colorectal cancer. J Gastrointest Surg. 2002;6:88–94. [DOI] [PubMed] [Google Scholar]
- 34.Grundy SM. Metabolic syndrome: connecting and reconciling cardiovascular and diabetes worlds. J Am Coll Cardiol. 2006;47: 1093–0. [DOI] [PubMed] [Google Scholar]
- 35.Lak KL, Helm MC, Kindel TL, et al. Metabolic syndrome is a significant predictor of postoperative morbidity and mortality following bariatric surgery. J Gastrointest Surg. 2019;23: 739–44. [DOI] [PubMed] [Google Scholar]
- 36.Shariq OA, Hanson KT, McKenna NP, et al. Does metabolic syndrome increase the risk of postoperative complications in patients undergoing colorectal cancer surgery? Dis Colon Rectum. 2019;62:849–58. [DOI] [PubMed] [Google Scholar]
- 37.Tee MC, Ubl DS, Habermann EB, et al. Metabolic syndrome is associated with increased postoperative morbidity and hospital resource utilization in patients undergoing elective pancreatectomy. J Gastrointest Surg. 2016;20:189–98; discussion 198. [DOI] [PubMed] [Google Scholar]
- 38.Berardi G, Antonelli G, Colasanti M, et al. Association of sarcopenia and body composition with short-term outcomes after liver resection for malignant tumors. JAMA Surg. 2020;155: e203336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Horowitz M, Neeman E, Sharon E, et al. Exploiting the critical perioperative period to improve long-term cancer outcomes. Nat Rev Clin Oncol. 2015;12:213–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ghitea TC, Vlad S, Birle D, et al. The influence of diet therapeutic intervention on the sarcopenic index of patients with metabolic syndrome. Acta Endocrinol (Buchar). 2020;16:470–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Azoulay D, Ramos E, Casellas-Robert M, et al. Liver resection for hepatocellular carcinoma in patients with clinically significant portal hypertension. JHEP Rep. 2021;3:100190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berardi G, Morise Z, Sposito C, et al. Development of a nomogram to predict outcome after liver resection for hepatocellular carcinoma in child-pugh b cirrhosis. J Hepatol. 2020; 72:75–84. [DOI] [PubMed] [Google Scholar]
- 43.Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015; 33:550–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen B, Lin S. Albumin-bilirubin (ALBI) score at admission predicts possible outcomes in patients with acute-on-chronic liver failure. Medicine (Baltimore). 2017;96:e7142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hiraoka A, Kumada T, Kudo M, et al. Albumin-Bilirubin (ALBI) Grade as Part of the Evidence-Based Clinical Practice Guideline for HCC of the Japan Society of Hepatology: A Comparison with the Liver Damage and Child-Pugh Classifications. Liver Cancer. 2017;6:204–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hiraoka A, Kumada T, Michitaka K, et al. Usefulness of albumin-bilirubin grade for evaluation of prognosis of 2584 Japanese patients with hepatocellular carcinoma. J Gastroenterol Hepatol. 2016;31:1031–6. [DOI] [PubMed] [Google Scholar]
- 47.Lee PC, Chen YT, Chao Y, et al. Validation of the albumin-bilirubin grade-based integrated model as a predictor for sorafenib-failed hepatocellular carcinoma. Liver Int. 2018;38:321–0. [DOI] [PubMed] [Google Scholar]
- 48.Fagenson AM, Pitt HA, Moten AS, et al. Fatty liver: the metabolic syndrome increases major hepatectomy mortality. Surgery. 2021;169:1054–60. [DOI] [PubMed] [Google Scholar]
- 49.Berardi G, Van Cleven S, Fretland AA, et al. Evolution of laparoscopic liver surgery from innovation to implementation to mastery: perioperative and oncologic outcomes of 2,238 patients from 4 European Specialized Centers. J Am Coll Surg. 2017;225: 639–49. [DOI] [PubMed] [Google Scholar]
- 50.Ciria R, Cherqui D, Geller DA, et al. Comparative short-term benefits of laparoscopic liver resection: 9000 cases and climbing. Ann Surg. 2016;263:761–7. [DOI] [PubMed] [Google Scholar]
- 51.Troisi RI, Berardi G, Morise Z, et al. Laparoscopic and open liver resection for hepatocellular carcinoma with Child-Pugh B cirrhosis: multicentre propensity score-matched study. Br J Surg. 2021;108:196–204. [DOI] [PubMed] [Google Scholar]
- 52.Piscaglia F, Svegliati-Baroni G, Barchetti A, et al. Clinical patterns of hepatocellular carcinoma in nonalcoholic fatty liver disease: a multicenter prospective study. Hepatology. 2016;63: 827–38. [DOI] [PubMed] [Google Scholar]
- 53.Vigano L, Conci S, Cescon M, et al. Liver resection for hepatocellular carcinoma in patients with metabolic syndrome: a multicenter matched analysis with HCV-related HCC. J Hepatol. 2015;63:93–101. [DOI] [PubMed] [Google Scholar]
- 54.Cai X, Liang X, Yu T, et al. Liver cirrhosis grading Child-Pugh class B: a Goliath to challenge in laparoscopic liver resection? Prior experience and matched comparisons. Hepatobiliary Surg Nutr. 2015;4:391–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Harimoto N, Yoshizumi T, Fujimoto Y, et al. Surgery for hepatocellular carcinoma in patients with Child-Pugh B cirrhosis: hepatic resection versus living donor liver transplantation. World J Surg. 2018;42:2606–16. [DOI] [PubMed] [Google Scholar]
- 56.Morise Z, Sugioka A, Kawabe N, et al. Pure laparoscopic hepatectomy for hepatocellular carcinoma patients with severe liver cirrhosis. Asian J Endosc Surg. 2011;4:143–6. [DOI] [PubMed] [Google Scholar]
- 57.Eslam M, Newsome PN, Sarin SK, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73:202–9. [DOI] [PubMed] [Google Scholar]
- 58.Conci S, Cipriani F, Donadon M, et al. Hepatectomy for Metabolic Associated Fatty Liver Disease (MAFLD) related HCC: propensity case-matched analysis with viral- and alcohol-related HCC. Eur J Surg Oncol. 2022;48:103–12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


