Abstract
Purpose:
This study aims to describe the current cohort of Adolescent Medicine Trials Network for HIV/AIDS Interventions (ATN) research program participants and evaluate whether the ATN’s current 5-year cycle recruited study participants who parallel the populations most impacted by HIV in the United States (US).
Methods:
Harmonized measures across ATN studies collected at baseline were aggregated for participants aged 13–24 years. Pooled means and proportions stratified by HIV status (at risk for or living with HIV) were calculated using unweighted averages of study-specific aggregate data. Medians were estimated using a weighted median of medians method. Public use 2019 CDC surveillance data for state-level new HIV diagnoses and HIV prevalence among US youth aged 13–24 years were obtained for use as reference populations for ATN at-risk youth and youth living with HIV (YLWH), respectively.
Results:
Data from 3185 youth at-risk for HIV and 542 YLWH were pooled from 21 ATN study phases conducted across the US. Among ATN studies tailored to at-risk youth, a higher proportion of participants were White and a lower proportion were Black/African American and Hispanic/Latinx compared to youth newly diagnosed with HIV in the US in 2019. Participants in ATN studies tailored to YLWH were demographically similar to YLWH in the US.
Discussion:
The development of data harmonization guidelines for ATN research activities facilitated this cross-network pooled analysis. These findings suggest the ATN’s YLWH are representative, but that future studies of at-risk youth should prioritize recruitment strategies to enroll more participants from African American and Hispanic/Latinx populations.
Keywords: Adolescent, Adolescent Medicine Trials Network for HIV/AIDS Interventions, Data Harmonization, HIV, Youth, Gay, Bisexual, Transgender
The Adolescent Medicine Trials Network for HIV/AIDS Interventions (ATN) research program aims to contribute to the scientific evidence-base of novel strategies to defeat the rising HIV epidemic among adolescents and young adults in the United States (US). Specifically, the goals of the ATN studies are to increase awareness of HIV status among at-risk youth, bend the infection rate curve towards zero through preventive interventions, and increase the numbers of youth living with HIV (YLWH) accessing each step of the care continuum [1]. ATN research is conducted through collaborations within the network and with researchers at institutions across the US. The current cycle of ATN (ATN IV) comprises three research program projects (or U19s) and a Coordinating Center (or U24) with over 20 study protocols across the network. Protocols include interventions for prevention of HIV infection for at-risk youth as well as interventions for reducing the impact of infection among YLWH. The populations of focus for many ATN studies are typically underrepresented in research but overrepresented in the epidemic, such as people of low socioeconomic status, LGBTQ+, and youth of color [1].
To optimize cross-network analyses of ATN study protocols, the ATN Analytic Committee published guidelines for harmonizing (i.e., standardizing) measures to be collected across studies [2]. This set of harmonized measures facilitates the pooling of ATN data, allowing for the characterization of participants at risk for HIV and YLWH across ATN studies. This wealth of data describing large samples of at-risk youth and YLWH will provide guidance for future ATN recruitment efforts and help ensure that research findings are robust and applicable to the target population in the US.
The primary aim of this study was to describe the current cohort of ATN participants. The secondary aim was to evaluate whether ATN participants reflect the youth populations most impacted by HIV in the US based on key demographic characteristics by comparing the ATN youth at risk for HIV and YLWH to Centers for Disease Control and Prevention (CDC) data on US youth newly diagnosed with HIV and US YLWH, respectively.
METHODS
Study Population
Data were analyzed from all ATN IV studies with the exception of formative research activities, e.g., phase 1 studies, focus groups, etc. Each participating ATN study contributed aggregated baseline summary data for participants aged 13–24 years. The earliest data collection began in May 2017. Studies actively recruiting participants at the time of data compilation provided aggregated data for participants enrolled on or before March 31, 2021. Characteristics of participating studies (i.e., design, intervention, population) are summarized in Table A1. Among the participating studies, five recruited YLWH, 15 recruited youth at risk for HIV, and one recruited both YLWH and at-risk youth. Several studies exclusively enrolled specific populations, namely gay, bisexual, or transgender youth; young men who have sex with men; transwomen; same-sex male couples; YLWH failing antiretroviral therapy; young women at high risk for HIV; and youth experiencing homelessness. In this study, ‘at-risk youth enrolled in ATN studies’ is defined as a heterogeneous participant group pooled across studies tailored to specific at-risk populations. Eligibility criteria, geographic location of study sites, and sampling methods for each individual study protocol are summarized in Table A2 and described in detail elsewhere [3–22]. All studies were approved by the relevant institutional review boards.
CDC Surveillance Data
Public use 2019 CDC surveillance data for state-level new HIV diagnoses and YLWH among youth aged 13–24 years in the US [23] were obtained for use as reference populations for at-risk youth and YLWH participating in ATN studies, respectively. New HIV diagnoses were defined as the number of HIV infections confirmed by laboratory or clinical evidence in a calendar year and YLWH was defined using HIV prevalence data, which was the number of persons living with diagnosed HIV infection at the end of the specified year. All diagnoses reported to the CDC by the 50 states, the District of Columbia, and 6 U.S. dependent areas were included [24] (see Discussion for more details). The CDC data reported race and ethnicity categories as American Indian/Alaska Native, Asian/Pacific Islander, Black/African American, Hispanic/Latinx, White, and Multiracial. Assigned sex at birth was defined as Female and Male, and the geographic regions were defined as the US Census regions Midwest, Northeast, South, and West.
Study Measures
Key measures were collected in a uniform manner across ATN IV studies for five standard domains: demographics and socioeconomic characteristics; sexual behavior and risk; substance use and abuse; HIV-positive cascade; and HIV-negative cascade. A full listing of the ATN IV harmonized measures is defined elsewhere [2]. With the exception of HIV viral load and CD4 count data from one study, the utilized measures reflect self-reported responses to computer-assisted survey questions. In a few cases, if uncollected variables could be ascertained from a study’s inclusion criteria, then these missing values were imputed based on the relevant study inclusion criteria.
In addition to the relevant harmonized measures, data were aggregated for a derived race/ethnicity variable based on the race and ethnicity values defined in the CDC surveillance data. The CDC based race/ethnicity derived variable was defined as follows: Participants reporting Hispanic/Latinx ethnicity, regardless of the selected race, were defined as “Hispanic/Latinx”, those reporting non-Hispanic/Latinx ethnicity and a single race were defined as their reported race category, and those reporting non-Hispanic ethnicity and multiple races were defined as “Multiracial.”
Pooling Methods
Each study provided aggregated data containing the harmonized measures from the standard domains for each of the participating study phases. Study-specific aggregated data included the mean, variance, minimum, and maximum values for continuous measures. If extreme minimum or maximum values for a variable were observed, the median and interquartile range were reported in addition to the mean and variance. For categorical variables, studies provided the count of affirmative responses for each response category. The number of participants eligible to respond to each question, which was less than or equal to the sample size (e.g., due to survey logic), and the number of missing values among participants eligible to respond were reported for all measures.
For each measure, the number of participants enrolled in studies that did not collect the measure was summed and defined as “not collected by study protocol”. For measures where survey logic differed across protocols, pooled descriptive statistics were calculated using data as provided by the individual studies, and the total number ineligible to respond due to survey logic was summed and reported as “not applicable due to survey logic”. For all categorical measures, response categories containing fewer than five participants after pooling were merged with similar categories, and combined counts and proportions were reported. Responses of “decline to answer”, “refuse to answer”, “don’t know”, and “missing” were collapsed into “missing” throughout due to small cell counts.
If by design a study had not collected information for a specific variable, it was excluded from the analyses for that measure. In cases of major discrepancies in the measurement of a variable across studies (e.g., STI testing in the past 6 months vs. past 12 months), the variable definition used by the greatest number of studies was retained, and data collected according to other definitions were excluded from the analyses and categorized as “not collected by study protocol”.
Measures were omitted if reported by two or fewer studies (evaluated separately for at-risk and YLWH populations where applicable). In cases of minor discrepancies in measurement, variables were combined uniformly where possible. Particularly, responses to questions about sexual and substance use behaviors in the past 3 months and the past 4 months were combined throughout and labeled as “in the past 3 or 4 months”.
Statistical Analysis
Pooled descriptive statistics were computed for each harmonized measure. Pooled means and proportions were obtained using unweighted averages of study-specific aggregate data. For each measure, the pooled variance was estimated as the weighted average of study-specific sample variances, with weights specified as the Bessel-corrected sample sizes, plus the sample variance of the study-specific sample means. The pooled standard deviation (SD) was estimated as the square root of the pooled variance estimate. Study-specific empirical distributions for certain sexual behavior variables (number of sex partners in lifetime or past 3 to 4 months) were observed to be right skewed. For each of these measures, the median number of sex partners was estimated with a corresponding 95% confidence interval using the weighted median of medians method of [25]. All analyses were conducted in SAS version 9.4 (Cary, NC).
RESULTS
Overall, 3185 youth at risk for HIV and 542 YLWH enrolled in ATN studies between May 2017 and March 2021 were included in the pooled analysis. Demographic and socioeconomic characteristics of each group are shown in Table 1. The mean age of study participants was 21.0 years (SD=1.96 years) for at-risk youth and 21.7 years (SD=2.09 years) for YLWH. A plurality of at-risk youth were White (N=1477, 46.4%) and the majority of the YLWH were Black/African American (N=380, 70.1%). Both groups were mostly assigned male sex at birth (at-risk youth: N=2651, 83.2%; YLWH: N=440, 81.2%) and identified as gay, queer, same gender loving, or homosexual (at-risk youth: N=1877, 58.9%; YLWH: N=334, 61.6%).
Table 1.
Self-reported demographic and socioeconomic characteristics by ATN groups enrolled between May 2017 and March 2021
| Youth at risk for HIV in ATN studies | Youth Living with HIV in ATN studies | |
|---|---|---|
| Measure | N=3185 n (%)a |
N=542 n (%)a |
|
| ||
| Age, mean (SD) | 21.0 (1.96) | 21.7 (2.09) |
| 13–18 | 476 (14.9) | 52 (9.6) |
| 19–24 | 2689 (84.4) | 490 (90.4) |
| Missing | 20 (0.6) | 0 (0.0) |
| Ethnicity | ||
| Hispanic/Latinx | 777 (24.4) | 118 (21.8) |
| Non-Hispanic/Latinx | 2399 (75.3) | 416 (76.8) |
| Missingb | 9 (0.3) | 8 (1.5) |
| Ethnic ancestry c | ||
| Caribbean | 91 (27.2) | 13 (37.1) |
| Mexican | 140 (41.9) | 13 (37.1) |
| Other | 105 (31.4) | 9 (25.7) |
| Missing | 6 (1.8) | 0 (0.0) |
| Not applicabled | 1140 | 135 |
| Not collected by study protocole | 1711 | 372 |
| Race c | ||
| American Indian/Alaska Native | 162 (5.1) | 22 (4.1) |
| Asian/Pacific Islanderf | 283 (8.9) | 14 (2.6) |
| Black/African American | 1203 (37.8) | 380 (70.1) |
| White | 1477 (46.4) | 93 (17.2) |
| Other | 310 (9.7) | 60 (11.1) |
| Missingb | 98 (3.1) | 12 (2.2) |
| Region | ||
| Northeast | 529 (16.6) | 44 (8.1) |
| South | 1451 (45.6) | 284 (52.4) |
| Midwest | 275 (8.6) | 64 (11.8) |
| West | 925 (29.0) | 150 (27.7) |
| Missing | 5 (0.2) | 0 (0.0) |
| Gender identity c | ||
| Female | 269 (8.4) | 102 (18.8) |
| Male | 2443 (76.7) | 404 (74.5) |
| Transgender female or transgender woman | 126 (4.0) | 22 (4.1) |
| Transgender male or transgender man | 205 (6.4) | 1 (0.2) |
| Genderqueer or gender nonconforming | 251 (7.9) | 12 (2.2) |
| Other | 22 (0.7) | 1 (0.2) |
| Missing | 3 (0.1) | 1 (0.2) |
| Assigned sex at birth | ||
| Female | 532 (16.7) | 101 (18.6) |
| Male | 2651 (83.2) | 440 (81.2) |
| Missing | 2 (0.1) | 1 (0.2) |
| Sexual identity c | ||
| Bisexual | 738 (23.2) | 93 (17.2) |
| Gay, queer, same gender loving, or homosexual | 1877 (58.9) | 334 (61.6) |
| Straight or heterosexual | 455 (14.3) | 105 (19.4) |
| Pansexual | 81 (2.5) | 9 (1.7) |
| Anotherg | 129 (4.1) | 9 (1.7) |
| Missingb | 7 (0.2) | 3 (0.6) |
| Immediate family members aware of participant’s sexual identity h | ||
| None | 312 (11.2) | 37 (8.5) |
| Some, but less than half | 394 (14.2) | 42 (9.7) |
| About half | 176 (6.3) | 16 (3.7) |
| More than half | 661 (23.8) | 113 (26.0) |
| All | 1119 (40.3) | 215 (49.4) |
| Missingi | 113 (4.1) | 12 (2.8) |
| Not applicable | 12 | 9 |
| Not collected by study protocol | 398 | 98 |
| Peers aware of participant’s sexual identity h | ||
| None | 115 (4.1) | 19 (4.4) |
| Some, but less than half | 226 (8.1) | 40 (9.2) |
| About half | 153 (5.5) | 17 (3.9) |
| More than half | 893 (32.1) | 63 (14.4) |
| All | 1364 (49.0) | 291 (66.7) |
| Missingi | 32 (1.1) | 6 (1.4) |
| Not applicable | 4 | 8 |
| Not collected by study protocol | 398 | 98 |
| Currently enrolled in school | ||
| Yes | 1729 (54.3) | 208 (38.4) |
| No | 1449 (45.5) | 330 (60.9) |
| Missing | 7 (0.2) | 4 (0.7) |
| Current education level among those currently enrolled | ||
| 6th-12th grade | 307 (18.3) | 47 (22.6) |
| GED program | 50 (3.0) | 20 (9.6) |
| Technical or vocational school | 46 (2.7) | 13 (6.3) |
| Two-year college | 232 (13.8) | 65 (31.3) |
| Four-year college or higher | 1030 (61.4) | 60 (28.8) |
| Missing | 12 (0.7) | 3 (1.4) |
| Did not report being currently enrolled | 1403 | 334 |
| Not collected by study protocol | 105 | 0 |
| Highest education level completed among not currently enrolled | ||
| None, no formal schooling | 3 (0.2) | 1 (0.3) |
| 6th-8th grade | 20 (1.4) | 9 (2.7) |
| 9th-11th grade | 215 (15.4) | 71 (21.5) |
| High school diploma | 385 (27.6) | 110 (33.3) |
| High school certificate of completion (no diploma) | 15 (1.1) | 11 (3.3) |
| GED | 106 (7.6) | 30 (9.1) |
| Some college, technical school, or vocational school | 221 (15.8) | 60 (18.2) |
| Technical or vocational school graduate | 31 (2.2) | 14 (4.2) |
| Two-year college graduate | 93 (6.7) | 8 (2.4) |
| Four-year college graduate or higher | 298 (21.3) | 16 (4.8) |
| Missing | 10 (0.7) | 0 (0.0) |
| Did not report being currently out of school | 1683 | 212 |
| Not collected by study protocol | 105 | 0 |
| Currently employed | ||
| Yes | 1616 (53.7) | 277 (51.1) |
| No | 1357 (45.1) | 252 (46.5) |
| Missingb | 38 (1.3) | 13 (2.4) |
| Not collected by study protocol | 174 | 0 |
| Current employment type | ||
| Full-time | 694 (43.0) | 148 (53.4) |
| Part-time | 918 (56.8) | 128 (46.2) |
| Missing | 3 (0.2) | 1 (0.4) |
| Did not report being currently employed | 1396 | 265 |
| Not collected by study protocol | 174 | 0 |
| Health insurance status | ||
| Yes | 2317 (78.8) | 419 (77.3) |
| No | 453 (15.4) | 78 (14.4) |
| Missingb | 169 (5.8) | 45 (8.3) |
| Not collected by study protocol | 246 | 0 |
Except where noted otherwise
Includes responses ‘Missing’ and ‘Unknown’.
The sum of categories may exceed the column total because some studies collected only one response per participant, while other studies offered participants the option to ‘select all that apply’.
Participants in studies that collected ethnic ancestry data who self-identified as Non-Hispanic/Latinx.
443 at-risk participants in this category identified as Hispanic/Latinx; 1,261 self-identified as Non-Hispanic/Latinx; and 7 had missing or unknown values for Ethnicity. 83 YLWH in this category identified as Hispanic/Latinx; 285 identified as non-Hispanic/Latinx; and 4 had missing or unknown values for ethnicity.
One study collected Asian/Pacific Islander as a single category.
Includes responses ‘Asexual/Other’, ‘Other’, and ‘Unsure/Questioning/I don’t know’
Three studies asked this question only of participants who reported a sexual identity other than ‘Straight or heterosexual’.
Includes responses ‘Missing’ and ‘Unsure’.
Sexual behavior and risk measures for at-risk youth and YLWH participating in ATN studies are described in Table 2, respectively. The mean age at first time having sex was 16.0 years (SD=2.63 years) for at-risk youth and 15.4 years (SD=2.29 years) for YLWH. For youth at risk of HIV, 74.5% (N=1157) reported sexual intercourse without a condom in their lifetime and 42.0% (N=168) had condomless sex with a partner of unknown HIV status in the past 3 to 4 months. For YLWH, 82.2% (N=287) reported sexual intercourse without a condom in their lifetime.
Table 2.
Self-reported sexual behavior and risk by ATN groups enrolled between May 2017 and March 2021
| Youth at risk for HIV in ATN studies | Youth Living with HIV in ATN studies | |
|---|---|---|
| Measure | N=3185 n (%)a |
N=542 n (%)a |
|
| ||
| Ever had sex | ||
| Yes | 2489 (97.6) | 507 (93.5) |
| No | 52 (2.0) | 26 (4.8) |
| Missing | 8 (0.3) | 9 (1.7) |
| Not collected by study protocol | 636 | 0 |
| Age at first time sex, mean (SD) | 16.0 (2.63) | 15.4 (2.29) |
| Missingb | 90 (4.0) | 21 (6.1) |
| Did not report ever having sex | 40 | 10 |
| Not collected by study protocol | 881 | 186 |
| Who had sex with in lifetime | ||
| Females | 311 (14.5)c | 18 (5.2)d |
| Males | 1041 (48.7)e | 185 (53.5)f |
| Females and males | 667 (31.2)c,e | 126 (36.4)d,f |
| Another response | 25 (1.2)g | 3 (0.9)h |
| Missing | 95 (4.4) | 14 (4.0) |
| Did not report ever having sex | 60 | 10 |
| Not collected by study protocol | 986 | 186 |
| Female sex partners in lifetime among those with female partners ever, mean (SD) | 14.0 (56.00) | 7.2 (25.41) |
| Median (95% CI) | 5 (2,5) | 3 (2,3)i |
| Missingj | 75 (4.5) | 5 (1.7) |
| Did not report ever having sex with female partner | 525 | 54 |
| Not collected by study protocol | 986 | 186 |
| Female sex partners in past 3 to 4 months, mean (SD) k,l | 2.3 (1.90) | 0.4 (1.42) |
| Median (95% CI) | 1 (0,1) | 0 (0,1) |
| Missingj | 16 (1.4) | 3 (2.1) |
| Not applicable due to survey logic | 1325 | 225 |
| Not collected by study protocol | 701 | 173 |
| Male sex partners in lifetime among those with male partners ever, mean (SD) | 21.3 (74.46) | 42.5 (92.95) |
| Median (95% CI) | 7 (6,7) | 14 (10,23)i |
| Missingj | 122 (5.7) | 30 (8.5) |
| Did not report ever having sex with male partner | 66 | 4 |
| Not collected by study protocol | 986 | 186 |
| Male sex partners in past 3 to 4 months, mean (SD) k,l | 3.2 (7.24) | 5.1 (11.47) |
| Median (95% CI) | 2 (2,2) | 2 (2,3) |
| Missingj | 33 (1.5) | 5 (1.4) |
| Not applicable due to survey logic | 529 | 23 |
| Not collected by study protocol | 416 | 173 |
| Transgender female sex partners in lifetime among those with transgender female partners ever, mean (SD) | 1.5 (0.94) | — |
| Missing | 1 (2.7) | — |
| Did not report ever having sex with transgender female partner | 577 | — |
| Not collected by study protocol | 2571 | — |
| Transgender female sex partners in past 3 months, mean (SD) | 0.6 (1.09) | — |
| Not applicable due to survey logic | 758 | — |
| Not collected by study protocol | 2318 | — |
| Transgender male sex partners in lifetime among those with transgender male partners ever, mean (SD) | 2.0 (1.87) | — |
| Missing | 1 (1.3) | — |
| Did not report ever having sex with transgender male partner | 537 | — |
| Not collected by study protocol | 2571 | — |
| Transgender male sex partners in past 3 months, mean (SD) | 0.5 (0.89) | — |
| Missing | 1 (0.7) | — |
| Not applicable due to survey logic | 654 | — |
| Not collected by study protocol | 2397 | — |
| Substance use before last sexual intercourse k | ||
| Yes | 928 (38.7) | 162 (46.8) |
| No | 1452 (60.5) | 184 (53.2) |
| Missing | 21 (0.9) | 0 (0.0) |
| Not applicable due to survey logic | 77 | 10 |
| Not collected by study protocol | 707 | 186 |
| Sex without condom in lifetime among those who reported ever having sex | ||
| Yes | 1157 (74.5) | 287 (82.2) |
| No | 286 (18.4) | 42 (12.0) |
| Missing | 111 (7.1) | 20 (5.7) |
| Did not report ever having sex | 31 | 7 |
| Not collected by study protocol | 1600 | 186 |
| Sex without condom in past 3 to 4 months, HIV negative partner k, l | — | |
| Yes | 246 (68.3) | — |
| No | 112 (31.1) | — |
| Missing | 2 (0.6) | — |
| Not applicable due to survey logic | 66 | — |
| Not collected by study protocol | 2759 | — |
| Sex without condom in past 3 to 4 months, HIV unknown partner k, l | — | |
| Yes | 168 (42.0) | — |
| No | 227 (56.8) | — |
| Missing | 5 (1.3) | — |
| Not applicable due to survey logic | 66 | — |
| Not collected by study protocol | 2719 | — |
| Sex without condom in past 3 to 4 months, HIV Positive partner k, l | ||
| Yes | 65 (3.6) | 82 (24.8) |
| No | 1709 (95.5) | 245 (74.0) |
| Missing | 16 (0.9) | 4 (1.2) |
| Not applicable due to survey logic | 123 | 25 |
| Not collected by study protocol | 1272 | 186 |
Except where noted otherwise
Includes missing and responses less than 9. Responses less than 9 were recoded to missing, as these do not reflect age of first consensual sex.
Includes responses ‘Females’ and ‘Cisgender Women (female sex at birth)’.
Includes responses ‘Females’ and ‘Females (including nonbinary partners who have vaginas)’.
Includes responses ‘Males’ and ‘Cisgender Men (male sex at birth)’.
Includes responses ‘Males’ and ‘Males (including nonbinary partners who have penises)’.
Includes participants who did not indicate “Females”, “Cisgender Women”, “Males”, “Cisgender Men”, “Females and Males”, or “Cisgender Women and Cisgender Men”, but who did indicate “Transgender Women”, “Transgender Men”, “Transgender partners”, “Non-binary individuals”, or “People who identify their gender in some other way”.
Includes participants who did not indicate “females”, “males”, or “females and males”, but who did indicate “transgender partners”.
Three studies that focused on YLWH contributed median data. We are reporting the median (min, max) of the three observed study-specific medians.
Includes responses ‘Don’t Know’ and ‘Missing’.
Survey logic that determined whether this question was presented differed across studies.
One study (focused on at-risk youth) reported this variable for casual partners specifically.
Substance use and abuse measures for at-risk youth and YLWH participating in ATN studies are shown in Table 3. In both groups, 79.8% (at-risk youth: N=2478, YLWH: N=422) reported cannabis use at least once in their life. The most prevalent lifetime substance use reported by the at-risk youth were alcohol (N=1174, 88.0%), amphetamines (N=870, 28.0%), hallucinogens (N=758, 24.4%), cocaine (N=673, 21.7%), and inhalants (21.6%). For YLWH, these substances were alcohol (N=193, 74.8%), amphetamines (N=144, 27.2%), inhalants (N=141, 26.7%), and cocaine (N=133, 25.1%).
Table 3.
Self-reported substance use and abuse by ATN groups enrolled between May 2017 and March 2021
| Youth at risk for HIV in ATN studies | Youth Living with HIV in ATN studies | |
|---|---|---|
| Measure | N=3185 n (%)a |
N=542 n (%)a |
|
| ||
| Lifetime tobacco use | ||
| Yes | 838 (51.8) | 136 (52.7) |
| No | 769 (47.5) | 120 (46.5) |
| Missing | 12 (0.7) | 2 (0.8) |
| Not collected by study protocol | 1566 | 284 |
| Tobacco use in past 3 to 4 months b, c | ||
| Never | 1121 (51.1) | 181 (43.1) |
| Once or twice | 181 (8.3) | 28 (6.7) |
| Monthly | 60 (2.7) | 14 (3.3) |
| Weekly | 59 (2.7) | 10 (2.4) |
| Daily or almost daily | 764 (34.8) | 183 (43.6) |
| Missing | 8 (0.4) | 4 (1.0) |
| Did not report ever using tobacco | 577 | 122 |
| Not collected by study protocol | 415 | 0 |
| Lifetime alcohol use | ||
| Yes | 1174 (88.0) | 193 (74.8) |
| No | 145 (10.9) | 63 (24.4) |
| Missing | 15 (1.1) | 2 (0.8) |
| Not collected by study protocol | 1851 | 284 |
| Alcohol use in past 3 to 4 months c | ||
| Never | 448 (17.8) | 63 (13.2) |
| Once or twice | 202 (8.0) | 74 (15.5) |
| Monthly | 989 (39.2) | 184 (38.6) |
| Weekly | 656 (26.0) | 110 (23.1) |
| Daily or almost daily | 218 (8.7) | 40 (8.4) |
| Missing | 7 (0.3) | 6 (1.3) |
| Did not report ever using alcohol | 145 | 65 |
| Not collected by study protocol | 520 | 0 |
| Binge drinking c, d | ||
| Never | 886 (35.0) | 125 (40.5) |
| Once or twice | 285 (11.3) | 0 (0.0) |
| Less than monthly | 487 (19.2) | 100 (32.4) |
| Monthly | 504 (19.9) | 44 (14.2) |
| Weekly | 310 (12.2) | 30 (9.7) |
| Daily or almost daily | 43 (1.7) | 4 (1.3) |
| Missing | 16 (0.6) | 6 (1.9) |
| Not applicable due to survey logic | 654 | 60 |
| Not collected by study protocol | 0 | 173 |
| Lifetime cannabis use | ||
| Yes | 2478 (79.8) | 422 (79.8) |
| No | 607 (19.5) | 99 (18.7) |
| Missing | 21 (0.7) | 8 (1.5) |
| Not collected by study protocol | 79 | 13 |
| Cannabis use in past 3 to 4 months c | ||
| Yes | 1825 (81.4) | 383 (88.0) |
| No | 411 (18.3) | 51 (11.7) |
| Missing | 6 (0.3) | 1 (0.2) |
| Did not report ever using cannabis | 528 | 107 |
| Not collected by study protocol | 415 | 0 |
| Lifetime cocaine use | ||
| Yes | 673 (21.7) | 133 (25.1) |
| No | 2411 (77.6) | 393 (74.3) |
| Missing | 22 (0.7) | 3 (0.6) |
| Not collected by study protocol | 79 | 13 |
| Cocaine use in past 3 to 4 months c | ||
| Yes | 257 (39.0) | 68 (46.6) |
| No | 402 (61.0) | 78 (53.4) |
| Did not report ever using cocaine | 2071 | 396 |
| Not collected by study protocol | 455 | 0 |
| Lifetime amphetamine use | ||
| Yes | 870 (28.0) | 144 (27.2) |
| No | 2191 (70.5) | 381 (72.0) |
| Missing | 45 (1.4) | 4 (0.8) |
| Not collected by study protocol | 79 | 13 |
| Amphetamine use in past 3 to 4 months c | ||
| Yes | 399 (45.0) | 85 (54.1) |
| No | 488 (55.0) | 72 (45.9) |
| Did not report ever using amphetamines | 1843 | 385 |
| Not collected by study protocol | 455 | 0 |
| Lifetime inhalants use | ||
| Yes | 663 (21.6) | 141 (26.7) |
| No | 2371 (77.3) | 383 (72.4) |
| Missing | 32 (1.0) | 5 (0.9) |
| Not collected by study protocol | 119 | 13 |
| Inhalants use in past 3 to 4 months c | ||
| Yes | 373 (52.7) | 87 (56.5) |
| No | 334 (47.2) | 67 (43.5) |
| Missing | 1 (0.1) | 0 (0.0) |
| Did not report ever using inhalants | 2022 | 388 |
| Not collected by study protocol | 455 | 0 |
| Lifetime sedative use | ||
| Yes | 559 (18.2) | 95 (18.0) |
| No | 2462 (80.3) | 429 (81.1) |
| Missing | 45 (1.5) | 5 (0.9) |
| Not collected by study protocol | 119 | 13 |
| Sedative use in past 3 to 4 months c | ||
| Yes | 220 (38.1) | 51 (47.2) |
| No | 357 (61.9) | 57 (52.8) |
| Did not report ever using sedatives | 2153 | 434 |
| Not collected by study protocol | 455 | 0 |
| Lifetime hallucinogen use | ||
| Yes | 758 (24.4) | 92 (17.4) |
| No | 2318 (74.6) | 434 (82.0) |
| Missing | 30 (1.0) | 3 (0.6) |
| Not collected by study protocol | 79 | 13 |
| Hallucinogen use in past 3 to 4 months c | ||
| Yes | 283 (38.5) | 40 (38.1) |
| No | 453 (61.5) | 65 (61.9) |
| Did not report ever using hallucinogens | 1994 | 437 |
| Not collected by study protocol | 455 | 0 |
| Lifetime opioid use | ||
| Yes | 506 (16.3) | 78 (14.7) |
| No | 2579 (83.0) | 448 (84.7) |
| Missing | 21 (0.7) | 3 (0.6) |
| Not collected by study protocol | 79 | 13 |
| Opioid use in past 3 to 4 months c | ||
| Yes | 192 (35.3) | 33 (36.3) |
| No | 352 (64.7) | 58 (63.7) |
| Did not report ever using opioids | 2186 | 451 |
| Not collected by study protocol | 455 | 0 |
Except where noted otherwise
One study focused on at-risk youth and two studies focused on YLWH collected only ‘Daily’ or ‘Never’ responses. All other participants in these studies were counted under ‘Not Collected’.
Survey logic that determined whether this question was presented differed across studies.
One study defined binge drinking as 6 or more drinks; the remaining studies defined binge drinking as 4 or more drinks for female-identifying participants and 5 or more drinks for male-identifying participants.
Table 4 compares the demographic characteristics of youth at risk for HIV in ATN studies with US youth newly diagnosed with HIV in the US in 2019. Compared to youth newly diagnosed with HIV in the US, a higher proportion of ATN participants at risk for HIV were non-Hispanic/Latinx White (N=1190, 15.7% vs. N=1159, 36.4%), and a lower proportion were non-Hispanic/Latinx African American/Black (N=3923, 51.7% vs. N=987, 31.0%) or Hispanic/Latinx (N=2094, 27.6% vs. N=475, 14.9%). Assigned sex at birth was similar between at-risk youth in ATN studies and youth newly diagnosed with HIV in the US, as 83.2% (N=2651) of ATN participants and 87.6% (N=6650) of the US youth were assigned male sex at birth. The geographic distribution of ATN participants was similar to that of newly diagnosed youth in the US, except there was a relatively larger proportion of ATN participants in the West (N=925, 29.0% vs. N=1294, 17.1%). This difference is also visible in Figure 1a, which maps the locations of ATN sites against the new HIV diagnosis rate in 2019 among US youth.
Table 4.
Demographic characteristics among youth enrolled in ATN studies between May 2017 and March 2021 and US youth aged 13–24 years in 2019
| Youth at risk for HIV in ATN studies | New HIV diagnoses among youth in US | Youth living with HIV in ATN studies | Youth living with HIV in US | |
|---|---|---|---|---|
| N=3185 | N=7588 | N=542 | N=30782 | |
| Characteristic | n (%) | n (%) | n (%) | n (%) |
|
| ||||
| Race/Ethnicity | ||||
| American Indian/Alaska Native | 32 (1.0) | 46 (0.6) | 5 (0.9) | 125 (0.4) |
| Asian/Pacific Islandera | 182 (5.7) | 134 (1.8) | 7 (1.3) | 568 (1.8) |
| Black/African American | 987 (31.0) | 3923 (51.7) | 338 (62.4) | 17062 (55.4) |
| Hispanic/Latinx | 475 (14.9) | 2094 (27.6) | 104 (19.2) | 7623 (24.8) |
| White | 1159 (36.4) | 1190 (15.7) | 49 (9.0) | 4157 (13.5) |
| Multiracial | 242 (7.6) | 201 (2.6) | 29 (5.4) | 1241 (4.0) |
| Other | 56 (1.8) | N/A | 4 (0.7) | N/A |
| Missing Assigned Sex at Birth |
52 (1.6) | N/A | 6 (1.1) | 6 (0.0) |
| Female | 532 (16.7) | 938 (12.4) | 101 (18.6) | 6353 (20.6) |
| Male | 2651 (83.2) | 6650 (87.6) | 440 (81.2) | 24429 (79.4) |
| Missing Region |
2 (0.1) | N/A | 1 (0.2) | N/A |
| Northeast | 529 (16.6) | 971 (12.8) | 44 (8.1) | 5101 (16.6) |
| South | 1451 (45.6) | 4236 (55.8) | 284 (52.4) | 16158 (52.5) |
| Midwest | 275 (8.6) | 1087 (14.3) | 64 (11.8) | 4620 (15.0) |
| West | 925 (29.0) | 1294 (17.1) | 150 (27.7) | 4903 (15.9) |
| Missing | 5 (0.2) | N/A | 0 (0.0) | N/A |
One study collected Asian/Pacific Islander as a single category.
Figure 1:
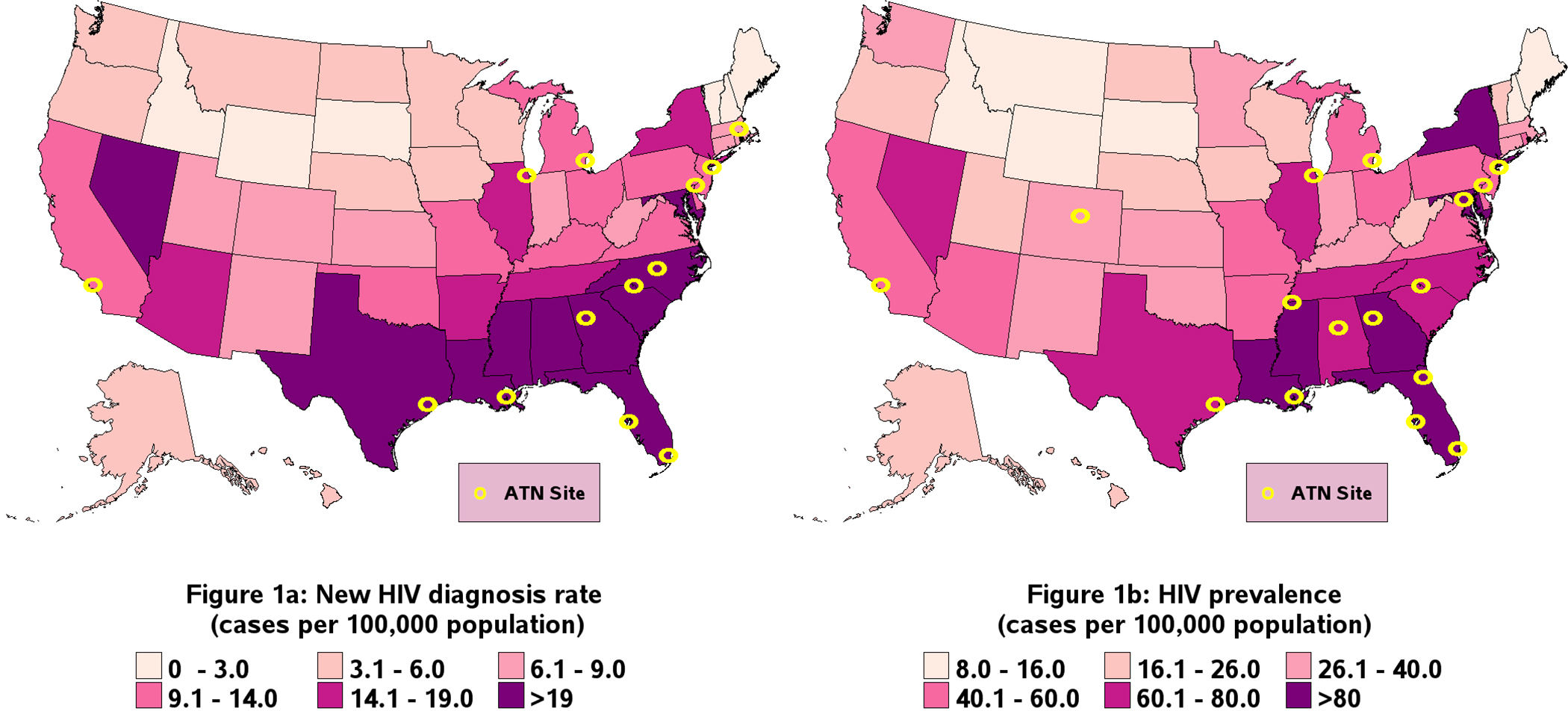
Geographic reach of ATN studiesa that enrolled youth at risk for HIV and youth living with HIV between May 2017 and March 2021 mapped alongside new and prevalent HIV cases among US youth aged 13–24 years in 2019, respectively
aFor 2 studies of at-risk youth and 1 study of youth living with HIV, study completion was virtual. Geographic locations of participants in these studies are not represented here.
Demographic characteristics of YLWH in ATN studies are compared with YLWH in the US in 2019 in Table 4. Distributions of race/ethnicity and assigned sex at birth of ATN participants were similar to YLWH in the US. The majority of both ATN participants and YLWH in the US were assigned male sex at birth (N=440, 81.2%; N=24,429, 79.4%, respectively) and a higher proportion ATN participants were non-Hispanic/Latinx Black/African American (N=338, 62.4%; N=17,062, 55.4%). Figure 1b maps the locations of ATN sites against YLWH in the US. The ATN had a greater proportion of youth in the West compared to YLWH in the US (N=150, 27.7% vs. N=4903, 15.9%, respectively) and a smaller proportion of youth in the Northeast (N=44, 8.1% vs. N=5101, 16.6%).
The HIV negative cascade characteristics among at-risk ATN participants and the HIV positive cascade characteristics among YLWH in ATN studies are summarized in Supplemental Tables A3 and A4, respectively. Among the at-risk youth, 87.4% (N=2497) reported having had an HIV test and 73.2% (N=2178) reported they had an STI test in their lifetime. The most commonly reported STI diagnoses in the past 12 months were gonorrhea (N=71, 16.4%), chlamydia (N=67, 15.5%), and syphilis (N=37, 8.5%). Most of the at-risk youth reported they had never used pre-exposure prophylaxis (PrEP) (N=1941, 76.0%) and an even greater proportion reported they had never used post-exposure prophylaxis (PEP) (N=1449, 91.5%). Among those who reported barriers to PrEP uptake, 24.9% (N=530) reported they had never heard of PrEP and 24.8% (N=528) did not think it was necessary to use. For the HIV positive cascade, participants reported being diagnosed with HIV in years ranging from 1994 to 2020 with average year of HIV diagnosis being 2014. Most YLWH (81.3%, N=430) had seen a provider for HIV in their lifetime. Only 19.5% (N=103) of YLWH had an undetectable viral load though 82.0% (N=434) of YLWH stated they were on current antiretroviral (ARV) treatment (note that almost all YLWH studies’ inclusion criteria was having a detectable viral load or being newly diagnosed). YLWH who were currently on ARV treatment missed at least one medication dose an average of 7.4 (SD=9.09) times in the past 30 days.
DISCUSSION
Overall, ATN IV studies recruited a population representative of YLWH in the US in terms of race/ethnicity, sex at birth, and geographic distribution. Among at-risk ATN participants, White youth were overrepresented in the ATN study population compared with youth newly diagnosed with HIV in the US and Black/African American youth were underrepresented. Both at-risk youth and YLWH living in the West were overrepresented in ATN studies relative to new and prevalent HIV diagnoses. Although the ATN prioritized research efforts in the South, where new diagnosis rates were markedly highest, at-risk ATN participants living in the South were underrepresented relative to new HIV diagnoses. These imbalances may be due to several factors. One study, Engaging HIV− youth (ATN 149), enrolled 1,487 participants, accounting for 46.7% of the pooled set of at-risk participants. This study enrolled 56.4% of its participants from sites in the West. While HIV prevalence in New York and Pennsylvania is relatively high (94.0 and 59.3 cases per 100,000 population, respectively), there were only two studies that recruited YLWH participants from New York and one study from Pennsylvania, likely contributing to the lower proportion of ATN YLWH in the Northeast compared to the comparator data. These population differences may also suggest that future studies focused on at-risk youth populations need to prioritize strategies to recruit underrepresented populations more efficiently, including the inclusion of more South and Northeast study sites.
This study provides data characterizing large samples of at-risk youth and YLWH in the US across important domains. The development of harmonization guidelines and the network structure of the ATN facilitated standardized collection and transfer of data, respectively, allowing for a comprehensive, detailed pooled analysis. The ATN cohort included populations that are typically underrepresented in research and overrepresented in the epidemic, such as those of low socioeconomic status, LGBTQ+, Asian Americans, African Americans, Hispanic/Latinx, and Native Americans/Alaskan Natives. In addition, published CDC data on HIV prevalence among US youth were utilized to make an appropriate comparison with ATN YLWH to evaluate how well the demographic characteristics of ATN participants align with the populations most impacted by HIV in the US.
The pooled at-risk population is diverse, and availability of published data on this age group limited our assessment of representativeness to demographic factors. Compared with the Keeping it LITE cohort study [26] of young sexual minority men and transgender individuals aged 13–34 years who reported one or more HIV risk behaviors (N=3,444, 96.7% HIV−), the proportion of ATN IV participants who were employed (53.7% ATN IV vs. 56.2% LITE) and had health insurance (78.8% ATN IV vs. 75.6% LITE) were similar, and fewer ATN IV at-risk participants attained a bachelor’s degree or higher education (41.9% ATN IV vs. 48.5% LITE). Compared with the ATN protocol 125 cohort [27] of YLWH who received care at ATN clinical sites between February 2015 and February 2016 (N=820), a higher proportion of ATN IV participants reported marijuana use in the past 3 months (70.7% ATN IV vs. 61.9% ATN 125) while a smaller proportion reported alcohol use in the past 3 months (76.4% ATN IV vs. 80.9% ATN 125). Note that these cohort comparisons should be interpreted with caution since the pooled ATN data includes studies with various recruitment methods and inclusion/exclusion criteria (Table A2).
This study does not attempt to evaluate representativeness of individual ATN studies or to make cross-study comparisons because several studies were tailored to specific populations. Rather, one aim of this analysis was to evaluate if, collectively, these tailored research efforts led to a pooled network sample that was demographically and geographically representative of the HIV epidemic among US youth. Further, the findings of this study, in part, reflect the goals/prioritizations of ATN research efforts. For example, low baseline medication adherence among YLWH in ATN IV is partly explained by the fact that ATN studies tailor interventions to support adherence and specifically recruit youth experiencing barriers to adherence. The sample size of studies included in this pooled analysis varied, particularly across ATN studies enrolling at-risk youth. While a weighted analysis could be conducted in which each study contributes equal weight, the results of such an analysis would characterize a typical ATN study, and participants in smaller studies would be over-represented relative to participants in larger studies. Instead, we used an analysis where each individual contributes equal weight, in keeping with the goals of characterizing the set of participants reached by the ATN as a whole and assessing representativeness of the pooled participants.
This study has limitations. Availability of data for some measures was limited by variation in data collection across study protocols. The ATN harmonized measures collected on YLWH did not include HIV mode of acquisition, so adolescents with perinatally acquired HIV are not identified or characterized within the YLWH group. The demographic variables presented here were categorized to align with published CDC data and do not fully reflect some participants’ identities (e.g., but not limited to, intersections of race and ethnicity, and gender and sex at birth). While CDC surveillance data are the most comprehensive source for the number and characteristics of YLWH in the US, there are limitations to this data source. Several factors impede surveillance efforts to count all HIV infections [24] including health care access, availability of testing, and differences in reporting regulations across states. It is possible that these factors lead to more pronounced undercounts of HIV infection for certain demographic or geographic groups. For this reason, CDC surveillance data reported in Table 4 should be interpreted as lower bounds for the true national burden of HIV infection. Note similar barriers may also influence participation in research. Thus, it is difficult to determine the extent to which limitations of CDC data collection procedures may impact the conclusions of this study.
Both CDC and ATN data collection could be modernized to better represent participants’ gender, racial, and ethnic identities. Specifically, changes are needed to fully represent transgender youth in data, and to represent intersections of race and ethnicity, particularly for those who identify as Latinx. It is recommended that future data harmonization efforts define race and gender contextually, e.g., by offering participants opportunities to report multiple racial, ethnic, and cultural identities and to describe their identities qualitatively [28]. It is also recommended that future ATN cycles collect information on mode of HIV acquisition across all studies of YLWH.
This study was designed to describe the cohort of ATN IV participants. The ATN network reaches large groups of youth participants across the US. Data harmonization presents a unique opportunity to address questions important to specific adolescent populations, since individual studies may not enroll many participants from a particular group of interest, and therefore only by harmonizing and pooling data across studies can we learn about these groups. Careful definition and consistent implementation of data harmonization procedures are needed to maximize this opportunity. It is recommended that future networks and ATN cycles define harmonization guidelines prior to developing individual study designs. Published harmonization guidelines for ATN IV [2] and the content of the present analysis may provide a template for and inform the development of future guidelines. Individual studies must balance the goals of collecting harmonized data, collecting study-aim-specific data, and minimizing respondent burden. Therefore, it is recommended that future research networks prioritize harmonized domains and measures that are central to network goals [2]. Support systems and resources for data harmonization are highly recommended to ensure feasibility of compliance with the harmonization efforts. Future analyses may consider leveraging the ATN IV harmonized data to expand the harmonization effort more broadly and perform additional pooled analyses.
Supplementary Material
IMPLICATIONS AND CONTRIBUTION.
This study provides characterization of large, geographically and demographically diverse samples of youth at risk for HIV and youth living with HIV in the US across important domains. Findings can be leveraged to guide future study design and recruitment efforts.
Funding and Acknowledgments
The authors thank the ATN AC, the data harmonization working group members, and the Coordinating Center administrative staff for their work in developing the data harmonization guidelines. The authors also thank the ATN EC and all research project teams who contributed to the harmonization process.
The data harmonization Substance Use and Abuse Working Group members were Matthew Psioda, Sara LeGrand, Jason Chapman, Patrick Wilson, and Sharon Nichols. The Sexual Behavior and Risk Working Group members were Eli Rosenberg, Katie Biello, Tyrel Starks, Michael Hudgens, and Richard Dunville. The HIV-Negative Cascade Working Group members were Arlene Seña, Christopher Hurt, Ken Mayer, Demetria Cain, Debra Murphy, and Micah McCumber. The HIV-Positive Cascade Working Group members were Arlene Seña, Lisa Hightow-Weidman, Tim Menza, Samiran Ghosh, Debra Murphy, and Micah McCumber. In addition, the authors thank the journal reviewers for their comments.
Coordinating Center U24 Team: The authors would like to thank the study teams for Planning4PrEP (Jessica Sales, Anandi Sheth, Maria Sanchez, Laura Renshaw, Leah Powell) and Work2Prevent (Brandon Hill, Darnell Motley, Lisa Strader, Rachel Goolsby, Betty Rupp), and Yue Shen for programming the analyses.
TERA Study: We are thankful to all participating research sites (Bronx-Lebanon Hospital Center, Emory, Johns Hopkins University, South Florida CDTC, St. Jude Children’s Research Hospital, University of Colorado Denver Children’s Hospital, University of Florida Jackson, Wayne State University), the youth advisory board members, program management staff at the University of North Carolina’s Collaborative Studies Coordinating Center, NICHD program officer, data management and analysis collaborators at Frontier Science Foundation and the Harvard T.H. Chan School of Public Health, and the intervention and project implementation and qualitative data analyses team at the University of Michigan.
CARES U19 Team: The authors would like to thank Sue Ellen Abdalian, Bonnie Ank, Robert Bolan, Yvonne Bryson, Ruth Cortado, M. Isabel Fernandez, Risa Flynn, Tara Kerin, Jeffrey Klausner, Jody Kussin, Sung-Jae Lee, Marguerita Lightfoot, Norweeta Milburn, Manuel Ocasio, Sophia Paiola, Dainna Polanco, Wilson Ramos, Cathy Reback, Adriana Romero-Espinoza, Mary Jane Rotheram-Borus, Wenze Tang, Robert E. Weiss, and Stacey Urauchi.
iTech U19 Team: The authors would like to thank K. Rivet Amico, José A. Bauermeister, Katie B. Biello, Kristi E. Gamarel, Keith Horvath, Albert Liu, Kenneth H. Mayer, Christina Psaros, Cathy J. Reback, Aaron J. Siegler, Hyman Scott, Rob Stephenson, Patrick S. Sullivan, and all contributing members of the iTech ATN U19.
Scale It Up U19 Team: The authors would like to thank Sylvie Naar; Demetria Cain; Scott Jones; the SMART protocol: Marvin Belzer, Karen MacDonell, Sitaji Gurung; the WeTest protocol: Tyrel Starks, Sarah Feldstein-Ewing, Angulique Outlaw; and the YMHP protocol: Tyrel Starks, Demetria Cain, Lawrence Friedman, Marne Castillo, and Angelique Outlaw.
The ATN is funded by the NIH through the Eunice Kennedy Shriver National Institute of Child Health and Human Development with supplemental funding from the National Institute of Mental Health, the National Institute on Drug Abuse, and the National Institute on Minority Health and Health Disparities. This research was supported by NIH grants U24HD089880, U19HD089875, U19HD089881, and 5U19HD089886. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- ATN
Adolescent Medicine Trials Network for HIV/AIDS Interventions
- CDC
Centers for Disease Control and Prevention
- NIH
National Institute of Health
- PEP
Post-exposure prophylaxis
- PrEP
Pre-exposure prophylaxis
- SD
Standard deviation
- STI
Sexually transmitted infections
- US
United States
- YLWH
Youth living with HIV
Footnotes
Conflicts of Interest
Dr. Arnold is a consultant on a project funded by Merck that is unrelated to the current project. All other authors have no potential conflicts of interest to declare.
Clinical Trial Registry Site and Number
Not applicable
Appendices
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Lee S, Kapogiannis BG, Allison S. Improving the Youth HIV Prevention and Care Continuums: The Adolescent Medicine Trials Network for HIV/AIDS Interventions. JMIR Res Protoc 2019;8:e12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].McCumber M, Cain D, LeGrand S, et al. Adolescent Medicine Trials Network for HIV/AIDS Interventions Data Harmonization: Rationale and Development of Guidelines. JMIR Res Protoc 2018;7:e11207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Horvath KJ, MacLehose RF, Martinka A, et al. Connecting Youth and Young Adults to Optimize Antiretroviral Therapy Adherence (YouTHrive): Protocol for a Randomized Controlled Trial. JMIR Research Protocols 2019;8:e11502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bauermeister JA, Golinkoff JM, Horvath KJ, et al. A Multilevel Tailored Web App-Based Intervention for Linking Young Men Who Have Sex With Men to Quality Care (Get Connected): Protocol for a Randomized Controlled Trial. JMIR Res Protoc 2018;7:e10444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Liu A, Coleman K, Bojan K, et al. Developing a Mobile App (LYNX) to Support Linkage to HIV/Sexually Transmitted Infection Testing and Pre-Exposure Prophylaxis for Young Men Who Have Sex With Men: Protocol for a Randomized Controlled Trial. JMIR Research Protocols 2019;8:e10659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Biello KB, Marrow E, Mimiaga MJ, et al. A Mobile-Based App (MyChoices) to Increase Uptake of HIV Testing and Pre-Exposure Prophylaxis by Young Men Who Have Sex With Men: Protocol for a Pilot Randomized Controlled Trial. JMIR Research Protocols 2019;8:e10694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].LeGrand S, Knudtson K, Benkeser D, et al. Testing the Efficacy of a Social Networking Gamification App to Improve Pre-Exposure Prophylaxis Adherence (P3: Prepared, Protected, emPowered): Protocol for a Randomized Controlled Trial. JMIR Research Protocols 2018;7:e10448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Belzer ME, MacDonell KK, Ghosh S, et al. Adaptive Antiretroviral Therapy Adherence Interventions for Youth Living With HIV Through Text Message and Cell Phone Support With and Without Incentives: Protocol for a Sequential Multiple Assignment Randomized Trial (SMART). JMIR Research Protocols 2018;7:e11183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Starks TJ, Ewing SWF, Lovejoy T, et al. Adolescent Male Couples-Based HIV Testing Intervention (We Test): Protocol for a Type 1, Hybrid Implementation-Effectiveness Trial. JMIR Research Protocols 2019;8:e11186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Biello KB, Psaros C, Krakower DS, et al. A Pre-Exposure Prophylaxis Adherence Intervention (LifeSteps) for Young Men Who Have Sex With Men: Protocol for a Pilot Randomized Controlled Trial. JMIR Research Protocols 2019;8:e10661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Nielsen-Saines K, Mitchell K, Kerin T, et al. Acute HIV Infection in Youth: Protocol for the Adolescent Trials Network 147 (ATN147) Comprehensive Adolescent Research and Engagement Studies (CARES) Study. JMIR Research Protocols 2019;8:e10807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rotheram MJ, Fernandez MI, Lee S-J, et al. Strategies to Treat and Prevent HIV in the United States for Adolescents and Young Adults: Protocol for a Mixed-Methods Study. JMIR Research Protocols 2019;8:e10759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Swendeman D, Arnold EM, Harris D, et al. Text-Messaging, Online Peer Support Group, and Coaching Strategies to Optimize the HIV Prevention Continuum for Youth: Protocol for a Randomized Controlled Trial. JMIR Research Protocols 2019;8:e11165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hill BJ, Motley DN, Rosentel K, et al. Work2Prevent, an Employment Intervention Program as HIV Prevention for Young Men Who Have Sex With Men and Transgender Youth of Color (Phase 3): Protocol for a Single-Arm Community-Based Trial to Assess Feasibility and Acceptability in a Real-World Setting. JMIR Research Protocols 2020;9:e18051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Amico KR, Dunlap A, Dallas R, et al. Triggered Escalating Real-Time Adherence Intervention to Promote Rapid HIV Viral Suppression Among Youth Living With HIV Failing Antiretroviral Therapy: Protocol for a Triggered Escalating Real-Time Adherence Intervention. JMIR Research Protocols 2019;8:e11416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sheth AN, Hussen SA, Escoffery C, et al. Pre-Exposure Prophylaxis Integration into Family Planning Services at Title X Clinics in the Southeastern United States: Protocol for a Mixed Methods Hybrid Type I Effectiveness Implementation Study (Phase 2 ATN 155). JMIR Research Protocols 2020;9:e18784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gamarel KE, Darbes LA, Hightow-Weidman L, et al. The Development and Testing of a Relationship Skills Intervention to Improve HIV Prevention Uptake Among Young Gay, Bisexual, and Other Men Who Have Sex With Men and Their Primary Partners (We Prevent): Protocol for a Randomized Controlled Trial. JMIR Research Protocols 2019;8:e10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Siegler AJ, Brock JB, Hurt CB, et al. An Electronic Pre-Exposure Prophylaxis Initiation and Maintenance Home Care System for Nonurban Young Men Who Have Sex With Men: Protocol for a Randomized Controlled Trial. JMIR Research Protocols 2019;8:e13982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Reback CJ, Rusow JA, Cain D, et al. Technology-Based Stepped Care to Stem Transgender Adolescent Risk Transmission: Protocol for a Randomized Controlled Trial (TechStep). JMIR Research Protocols 2020;9:e18326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Parsons JT, Starks T, Gurung S, et al. Clinic-Based Delivery of the Young Men’s Health Project (YMHP) Targeting HIV Risk Reduction and Substance Use Among Young Men Who Have Sex with Men: Protocol for a Type 2, Hybrid Implementation-Effectiveness Trial. JMIR Research Protocols 2019;8:e11184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Arnold EM, Swendeman D, Harris D, et al. The Stepped Care Intervention to Suppress Viral Load in Youth Living With HIV: Protocol for a Randomized Controlled Trial. JMIR Research Protocols 2019;8:e10791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hill BJ, Motley DN, Rosentel K, et al. An Employment Intervention Program (Work2Prevent) for Young Men Who Have Sex With Men and Transgender Youth of Color (Phase 2): Protocol for a Single-Arm Mixed Methods Pilot Test to Assess Feasibility and Acceptability. JMIR Research Protocols 2020;9:e16401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Centers for Disease Control and Prevention. NCHHSTP AtlasPlus n.d.
- [24].Centers for Disease Control and Prevention. HIV Surveillance Report, 2019; vol.32., http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html.; 2021. [accessed 22.09.22]. [Google Scholar]
- [25].McGrath S, Zhao X, Qin ZZ, et al. One-sample aggregate data meta-analysis of medians. Statistics in Medicine 2019;38:969–84. [DOI] [PubMed] [Google Scholar]
- [26].Gleason N, Serrano PA, Muñoz A, et al. Limited Interaction Targeted Epidemiology of HIV in Sexual and Gender Minority American Adolescents and Adults: Feasibility of the Keeping it LITE Study. JMIR Form Res 2021;5(11):e30761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gamarel KE, Westfall AO, Lally MA, et al. Tobacco Use and Sustained Viral Suppression in Youth Living with HIV. AIDS Behav 2018;22(6):2018–2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Taylor S, Turner K, Agola S, et al. ATN Antiracism Initiative Summary, Learnings, and Recommendations. Presented at ATN Biannual Full Network Meeting; April 28, 2022; Online. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


