Abstract
Purpose:
To determine the prevalence of fast global and central visual field (VF) progression in individuals with glaucoma under routine care.
Design:
Observational study.
Participants:
Six hundred ninety-three eyes of 461 individuals with glaucoma followed up over a median 4.5 years.
Methods:
This study included (i) patients at a private ophthalmology clinic in Melbourne, Australia, and (ii) individuals in two prospective longitudinal observational studies across three sites in the USA. All individuals had glaucoma under routine care and had performed ≥5 reliable 24–2 VF tests over a 1- to 5-year period. Ordinary least squares regression analyses were used to calculate the rate of global mean deviation (MD) change over time, and of the mean total deviation values of the 12 locations within the central 10° region (MTD10), for each eye.
Main Outcome Measures:
Prevalence of progression based on the rate of MD and the MTD10 change across various fixed cut-offs and cut-offs based on the estimated normal distribution (from the positive slopes).
Results:
Based on the MD and the MTD10, 12.5% and 11.7% of the eyes respectively exhibited a rate of change that was < -1.0dB/year (being a rate that is typically defined as “fast progression” for MD values), and 29.0% of the eyes showed a change of < -0.5dB/year on MTD10. Furthermore, 12.7% and 9.1% of the eyes exhibited a rate of change that exceeded the 1% cut-off of the estimated normal distribution MD and the MTD10 values respectively.
Conclusions:
This study found that approximately one in eight eyes with glaucoma under routine care showed fast progression based on global MD values (< -1.0dB/year), and nearly one in three eyes with < -0.5 dB/year decline centrally. These findings highlight the clinical importance of assessing progressive central visual field loss and reinforce the need for new therapies to prevent functional disability in a notable proportion of individuals who continue to exhibit fast progression.
Keywords: Glaucoma, Visual Fields, Progression
Précis:
This study observed that one in eight eyes with glaucoma under routine care exhibit fast global visual field progression, and importantly that a similar proportion also exhibit the same magnitude of central visual field progression.
INTRODUCTION
A key goal in the clinical management of glaucoma is to prevent functional disability. Visual field (VF) testing remains an essential tool to estimate the current degree and future risk of functional impairment for individuals with glaucoma.1, 2 Both fast global rates of VF loss (based on the current conventional testing of the central 30° of the visual field) and central involvement of glaucomatous VF loss (i.e., within the central 10° of the visual field, or the macular region) are strongly associated with functional disability and poorer vision-related quality of life.1, 3–8 It is thus critical to understand the prevalence of individuals with glaucoma exhibiting fast global VF progression and/or central VF progression under routine clinical care in order to appreciate what proportion of these individuals are at high risk of functional impairment.
A commonly used definition for a ‘fast progressor’ for an eye with glaucoma in one exhibiting a < -1dB/year decline in the global measure of mean deviation (MD), estimated from at least 5 VF tests.9–15 Such a rate is considered ‘fast’ as, for example, an eye a baseline MD of -3 dB (which would be considered to have early glaucoma) would develop advanced glaucoma (MD of -12 dB) within nine years. Such a rate is also considered ‘fast’ because eyes with advanced glaucoma would reach the definition of statutory blindness (MD of -22 dB, as defined by the US Social Security Administration) in 10 years or less. Based on this definition, the prevalence of fast progressors under routine clinical care has been reported to be between 5% and 12%,10–12, 15–18 although large studies of individuals from Canada (n = 2,324),10 the United Kingdom (n = 3,790)11 and North Carolina (n = 3,981)15 report a more specific prevalence of between 5% and 7.5%.
However, it remains to be determined what proportion of the population progress specifically in the central visual field, either as ‘fast progressors’ (based on a change of < -1 dB/year) in this region or at even slower rates, given the functional significance of the central visual field.1, 6–8, 19–23 With the commonly used 24–2 VF test on the Humphrey Field Analyzer (Carl Zeiss Meditec; Dublin, CA, USA), central visual field progression can be assessed by analyzing the central 12 locations of the 24–2 VF, which fall within the central 10° radius of vision.24–26 This study therefore sought to determine the prevalence of fast progressors, based on measurement of global and central VF sensitivities, across various thresholds in order to determine the proportion of glaucoma individuals under routine clinical care that are at higher risk of functional disability.
METHODS
This study included (i) a retrospective review of patients seen at a private ophthalmology clinic in Melbourne, Australia that were under routine care, and (ii) participants that were examined as part of two prospective, longitudinal observational studies of visual function and structural changes in glaucoma (the Diagnostic Innovations in Glaucoma Study [DIGS] and the African Descent and Glaucoma Evaluation Study [ADAGES]), who were also under routine care.27 DIGS and ADAGES were conducted at three sites: the Hamilton Glaucoma Center at the Viterbi Family Department of Ophthalmology, University of California, San Diego, the Edward S. Harkness Eye Institute at Columbia University Medical Center (site formerly located at New York Eye and Ear Infirmary), and the Department of Ophthalmology, University of Alabama, Birmingham. Institutional board approvals were obtained at all sites in the abovementioned studies, which were all conducted in accordance Declaration of Helsinki for research involving humans. DIGS and ADAGES were also conducted in adherence to the Health Insurance Portability and Accountability Act, and all participants provided written informed consent.
Eligibility Criteria
Individuals from Melbourne, Australia, included in this study were those under routine care within a private academic ophthalmology clinic. Patients from this clinic were eligible for inclusion if they were 18 years or older, and with a confirmed diagnosis of glaucoma in at least one eye according to a comprehensive review of their clinical record. Individuals with all types of glaucoma were included, including those with primary open angle and angle closure glaucoma, or secondary glaucoma. Eyes were excluded if they had any other ocular or systemic conditions apart from glaucoma that could significantly affect their VF test results (such as neovascular age-related macular degeneration).
In DIGS and ADAGES, all participants underwent a comprehensive ophthalmologic examination at baseline. This examination included a medical history review, visual acuity and visual field testing, slit lamp biomicroscopy and fundoscopy, stereoscopic optic disc photography, intraocular pressure measurements and gonioscopy. In DIGS and ADAGES, participants were required to be 18 years or older, have a best-corrected visual acuity of 20/40 or better, and open angles on gonioscopy at study entry. This study included eyes with glaucoma defined on the basis of: (i) evidence of glaucomatous optic neuropathy on stereophotographs (using methods described previously27, 28), and (ii) having a history of ≥3 consecutive abnormal visual field tests (based on a pattern standard deviation value of P < 0.05 or glaucoma hemifield test outside normal limits). As such, all participants from DIGS and ADAGES included in this study had primary open angle glaucoma (POAG).
Visual Field Testing
This study included individuals who underwent visual field testing using the Swedish Interactive Thresholding Algorithm Standard 24–2 strategy on the Humphrey Field Analyzer II-i (Carl Zeiss Meditec, Inc., Dublin, CA, USA). Only tests with ≤ 33% fixation losses ≤ 15% false-positive errors were considered reliable and included for the analyses. In this study, the first visual field test available from the data exported of each eye at the site was excluded to minimize the impact of a potential learning effect. Thereafter, only eyes that had ≥ 5 reliable visual field tests within a 1- to 5-year period were included in this study.
The main outcome measures from the visual field tests included: (i) the mean deviation from the entire 24–2 visual field test (MD), which is calculated on the Humphrey Field Analyzer as a weighted average of the total deviation (TD) values (the difference in visual sensitivity from age-expected values29), and (ii) the mean TD values from the 12 test locations in the central 10° region (MTD10).26, 30, 31
Statistical Analysis
The rate of MD and MTD10 change for each eye was calculated using ordinary least squares regression analyses. The prevalence of eyes exhibiting a rate of change exceeding a different range of cut-offs (less than -0.25, -0.50, -0.75 and -1.00 dB/year, and greater than 0.25, 0.50, 0.75 and 1.00 dB/year) for both MD and MTD10 were calculated using mixed-effects binary logistic regression models, to account for the correlations between two eyes of an individual.
The prevalence of eyes exhibiting a rate of change (or slopes) exceeding different probability cut-offs based on the estimated normal distribution of the slopes of MD and MTD10 was also determined to enable a more equivalent comparison between the two measures. These cut-offs included probabilities of less than 5%, 2%, 1% and 0.5%. The normal distributions were estimated by evaluating the positive slopes (rate of change >0 dB/year), which would represent half of the normal distribution of all slopes (positive or negative) in a scenario where none of the eyes were truly progressing, and when assuming that true improvements in visual field sensitivity were not occurring. Based on probability theory, the standard deviation (SD) of a normal distribution with a mean of zero can be calculated by multiplying the mean of its absolute values by. The positive slopes could thus be used to calculate the SD of the estimated normal distribution, since its distribution would match the distribution of the absolute values of all slopes in a scenario where true progression was not occurring. Linear mixed models were used to calculate this mean absolute value from the eyes exhibiting positive rates of change, to account for correlations between two eyes of an individual and between individuals within the same site. This mean absolute value was then used to calculate the SD and thus the abovementioned probability cut-offs. The percentage of eyes exhibiting a rate of change exceeding these probability cut-offs were also evaluated using mixed-effects binary logistic regression models, which also accounted for correlations at the site and individual level. Furthermore, the percentage of eyes exhibiting a rate of change based on both MD or MTD10, or based on either alone, were also calculated using mixed-effects multinomial logistic regression models to account for correlations between two eyes of an individual and individuals from the same site.
RESULTS
Participant Characteristics
This study included a total of 693 eyes from 461 individuals, who had a median of 7 visual field tests (interquartile range [IQR] = 5 to 10 tests) over a median follow-up period of 4.5 years (IQR = 4.0 to 4.8 years).
These individuals were on average 66 ± 11 years old (range, 23 to 95 years old) and 232 (50%) patients were female. A total of 627 (90%) eyes had POAG and 18 (6%) eyes had primary angle closure glaucoma. At baseline, the median 24–2 visual field MD and PSD was -4.07 dB (IQR = -9.57 to -1.50 dB) and 4.51 dB (IQR = 2.39 to 9.58 dB) respectively. The characteristics of the individuals included at each of the four sites are shown in Supplementary Table 1.
Rates of Visual Field Progression
The median rate of MD change was -0.20 dB/year (IQR = -0.54 to 0.10 dB/year), and the median rate of MTD10 was -0.19 dB/year (IQR = -0.55 to 0.10 dB/year); the distribution of the rates of change are shown in Figure 1.
Figure 1:
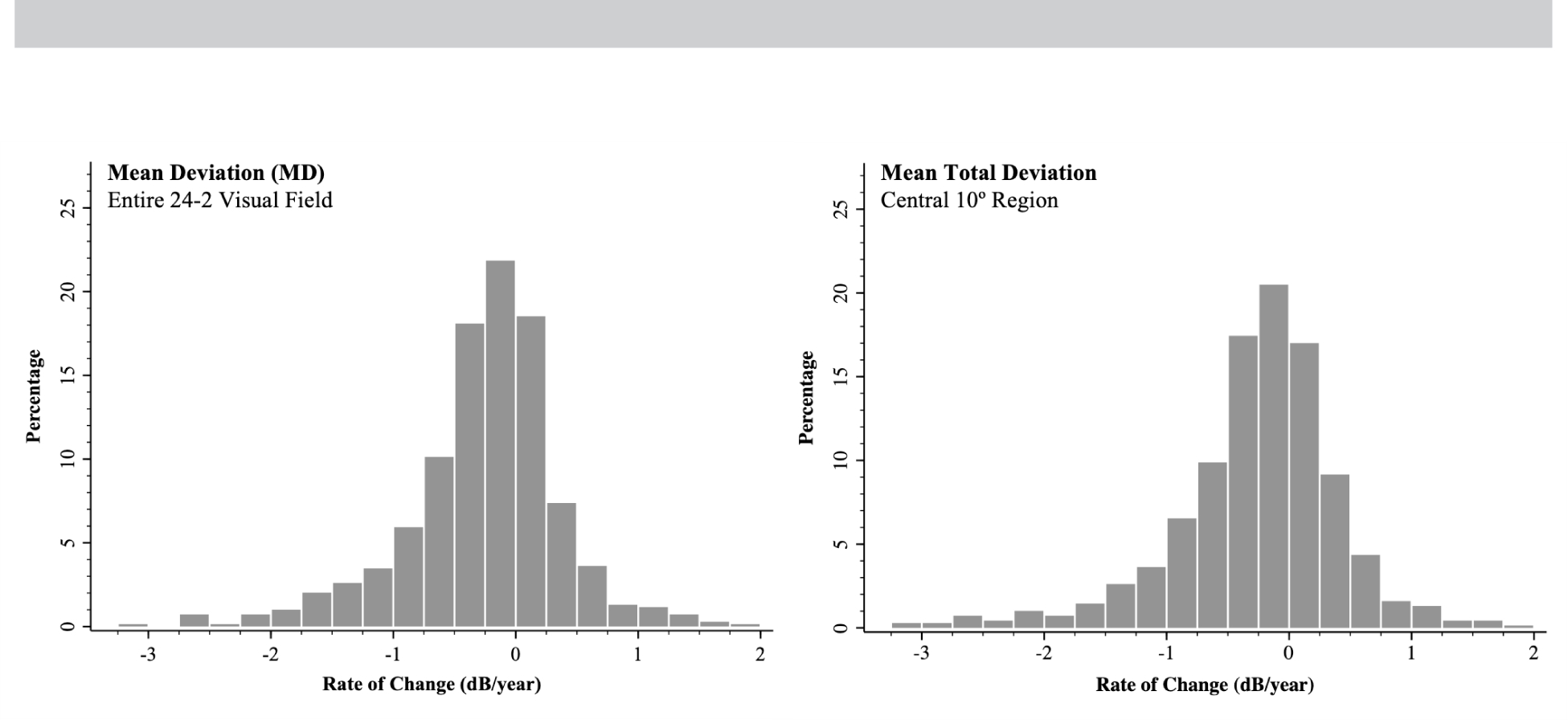
Distribution of the rates of change for the mean deviation from the entire 24–2 visual field test (left) and from the mean total deviation of the central 10° region (right).
The prevalence of eyes exhibiting a rate of visual field change exceeding a range of cut-offs are presented in Table 2. These findings show how 28.1% and 12.5% of eyes had < -0.50 and < -1.00 dB/year decline in MD in this cohort, whilst 7.9% showed a > 0.5 dB/year increase. Similarly, 29.0% and 11.7% of eyes showed a < -0.50 and < -1.00 dB/year decline in MTD10, and 8.5% of eyes showed a > 0.5 dB/year increase.
Table 2:
Prevalence (%) of glaucoma eyes with different rates of change in visual field sensitivity
| Rate of Change | MD | MTD10 |
|---|---|---|
| Negative Slopes | ||
| < −0.25 dB/year | 45.4 | 46.5 |
| < −0.50 dB/year | 28.1 | 29.0 |
| < −0.75 dB/year | 18.5 | 18.6 |
| < −1.00 dB/year | 12.5 | 11.7 |
| Positive Slopes | ||
| > 0.25 dB/year | 15.2 | 18.7 |
| > 0.50 dB/year | 7.9 | 8.5 |
| > 0.75 dB/year | 3.8 | 4.2 |
| > 1.00 dB/year | 2.4 | 3.0 |
Notes: MD = mean deviation from the entire 24–2 visual field test; MTD10 = mean total deviation from the 12 locations in the central 10° region.
The prevalence of glaucoma eyes exhibiting a rate of change in visual field sensitivity exceeding various cut-offs based on the estimated normal distribution are shown in Table 3. These findings demonstrate how 12.7% and 9.1% of eyes exhibited a rate of change for MD or MTD10 respectively that exceed the 1% cut-off of the estimated normal distribution.
Table 3:
Prevalence (%) of glaucoma eyes with visual field sensitivity decline exceeding different cut-offs based on the estimated normal.
| Cut-Off (dB/year) | Prevalence (%) | Detected Based On (%) | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| MD | MTD10 | MD | MTD10 | Both | Only MD | Only MTD10 | ||
| < 5% of estimated normal distribution | −0.69 | −0.79 | 20.2 | 17.6 | 62 | 23 | 15 | |
| < 2% of estimated normal distribution | −0.87 | −0.99 | 14.7 | 12.2 | 57 | 26 | 17 | |
| < 1% of estimated normal distribution | −0.98 | −1.12 | 12.7 | 9.1 | 50 | 33 | 17 | |
| < 0.5% of estimated normal distribution | −1.09 | −1.24 | 10.4 | 7.9 | 49 | 31 | 20 | |
Notes: MD = mean deviation from the entire 24–2 visual field test; MTD10 = mean total deviation from the 12 locations in the central 10° region.
Table 3 further examines the proportion of eyes exhibiting a decline on either MD and MTD10 exceeding various cut-offs based on the estimated normal distribution that were detected on both MD and MTD10, or based on either alone. These findings demonstrated how amongst eyes that exhibited a decline < 1% estimated normal distribution on either MD and MTD10, 50% of the eyes showed a decline on both MD and MTD10, and 33% and 17% showed a decline on only MD and only MTD10 respectively.
These findings mean that 17%, 23%, 20% and 26% of eyes that progressed by MTD10 would not have progressed by MD based on the cut-off that was < 5%, 2%, 1% and 0.5% of the estimated normal distribution respectively. Furthermore, amongst eyes deemed to have progressed based on MD, it was estimated that 74%, 69%, 60% and 61% of these eyes would also have progressed by MTD10 respectively.
DISCUSSION
In this study, we found that approximately one in eight eyes with glaucoma under routine care across four different sites exhibited fast progression, when defined by a change in MD < -1dB/year. Importantly, a similar proportion of eyes also exhibited this rate of decline in the central 10° region, and nearly one in three eyes showed a < -0.5dB/year decline in this region. These findings highlight how a notable proportion of glaucoma patients under routine care exhibit clinically important rates of central visual field progression, underscoring the importance of assessing this region in the clinical management of glaucoma when seeking to prevent functional disability.
Our finding that 12.5% of eyes in the entire cohort progressed at < -1.0dB/year, defined as ‘fast progressors’,10, 14, 32 was generally within the range of between 5% to 12% reported in previous studies with a similar design.10–12, 15–18 For example, the prevalence of fast progressors in this study was similar to the 10% prevalence seen in a cohort of eyes with clinically diagnosed glaucoma across five private ophthalmology clinics in Australia.18 However, it was higher than the prevalence reported in studies in France (11.3%; n = 223),12 the UK (7.5%; n = 3790),11 and Canada (5.7%; n =2324).10 Nonetheless, note that these studies also included eyes with ocular hypertension, suspected glaucoma, and/or unaffected eyes of individuals with unilateral glaucoma, which are expected to have a lower prevalence of fast progressors than eyes with established glaucoma.
To the best of our knowledge, this is the first study that has reported specifically on the prevalence of central VF progression in individuals with glaucoma under routine care, where we observed that 11.7% of eyes exhibited a < -1.0 dB/year change in mean TD values within the central 10° radius and 29.0% of eyes showed of decline of < -0.5 dB/year. Whilst the former rate has been described as representing “fast progression” when considering the global MD on the entire 24–2 VF test9–14, 33, an equivalent magnitude of change centrally is likely to lead to a greater degree of functional impairment. The importance of the central visual field has been reflected in a commonly used staging systems for visual field loss, whereby eyes with abnormalities in this region – irrespective of the overall MD – are assigned to a more severe category.34 As such, a decline of -0.5 dB/year could potentially be considered to represent “fast progression” if occurring in the central region, and this study observed that one in three eyes exhibited such a level decline. However, future studies are needed to understand what rate of progression in this central region would result in a similar degree of functional impairment (such as based on decline in vision-related quality of life) as fast progression (i.e., < -1.0 dB/year) based on the global MD. Note further that it may not simply be the rate, but the characteristics of the visual field loss (e.g., location of the abnormalities, and/or whether they are diffuse or focal35, 36) that is related to the degree of functional impairment.
As well as comparing VF progression against thresholds of clinical significance, we also compared our results against thresholds determined from the estimated normal distribution of truly stable eyes. This analysis was undertaken because the variability of the MTD10 is expected to be higher than MD, given that the MTD10 is derived from only 12 out of the 54 locations tested. Indeed, the SD of the residuals – being the difference between the measured and estimated sensitivity from the linear regression analysis of the longitudinal data of each eye, which provides an estimate of measurement variability – was 1.30 and 1.17 dB for MTD10 and MD respectively. This larger degree of variability for MTD10 also resulted in a higher prevalence of positive slopes based on fixed thresholds (e.g., 18.7% and 15.2% showed a rate of change > 0.25 dB/year on MTD10 and MD). To thus enable a more equivalent comparison between the two VF outcome measures, we estimated the normal distribution of the rates of change from the half-normal distribution of eyes exhibiting a positive slope for the VF outcomes, which was assumed to have occurred due to measurement variability. We observed that approximately one in five (20.2%) and one in eight (12.7%) eyes progressed at a rate exceeding the lower 5% and 1% of the estimated normal distribution for global MD respectively, and one in six (17.6%) and one in eleven (9.1%) eyes progressed at these rates respectively for MTD10.
We also observed in this study that there is a notable proportion of individuals that exhibit changes in the central visual field, but not globally. For example, when considering eyes that exhibited a rate of change exceeding the lower 0.5% of the estimated normal distribution based on MTD10, only one in four of these eyes also exhibited a change on MD exceeding the same cut-off. These findings were similar when considering other thresholds, and this highlights how there could be progressive decline in the central visual field in a notable proportion of individuals that would not be detected if only MD values are considered.
The findings of this study underscore the need for device manufacturers to consider incorporating a simple-to-derive metric of mean central visual sensitivity in their progression analysis software to aid clinical management. Until such a metric has been incorporated, these findings highlight the clinical importance of paying particular attention to the central visual field when assessing progressive changes qualitatively. The findings of this study also reinforce the notion that there is still a notable proportion of individuals with glaucoma under routine care that are fast progressors. Furthermore, note that whilst the definition of “fast progression” has often been defined as a < -1 dB/year change in global MD, a recent study showed that functional disability can already occur when the better eye has an MD of -6 dB.37 As such, even slower rates of VF progression could arguably be considered as “fast progression” when occurring in the better eye because it would, for example, only take six years for an individual with a baseline MD of -3 dB in the better eye to develop such disability if progression was occurring at a -0.5 dB/year. Finally, the findings of this study also further highlights the need to better understand the underlying disease processes driving vision loss in fast progressors, and the need to develop new therapies to complement currently available IOP-lowering strategies.38–40
Limitations of this study include the sample size of the cohort, which although is larger than several previous studies that have reported on the prevalence of fast progressors,12, 13, 18 is smaller than several previous studies that included thousands of individuals.10, 11, 15–17 However, this study included individuals under routine glaucoma care from four different sites across Australia and the USA, which we suggest aids the generalizability and clinical relevance of its findings. That said, all individuals in this study were seen at centers that were within an academic setting. A a recent study using the Intelligent Research in Sight (IRIS) Registry by the American Academy of Ophthalmology showed that individuals seen in such academic settings generally had more severe glaucoma, and also underwent diagnostic procedures such as visual field testing more frequently, than those in non-academic settings.41 The findings of this study should thus be interpreted in light of this consideration.
Furthermore, only individuals with POAG were included in DIGS/ADAGES, whilst individuals with any type of glaucoma were included at the site in Australia. However, the findings of this study remained similar even when limiting the analyses only to eyes with POAG (data not shown). Another limitation is that a quarter of the eyes in this study only had 5 VF tests available for analyses, limiting the precision of rate of change estimates when using ordinary least squares (OLS) regression analyses.42 Future studies may benefit from using Bayesian linear mixed models, which a recent study has demonstrated outperforms the OLS regression approach for predicting future MD values, especially when fewer tests are available.15 Bayesian linear mixed models may provide more precise estimates of the rate of MD change through leveraging information from the entire population to better inform its estimates. Note that a previous study observed that the prevalence of fast progressors based on the Bayesian linear mixed model with an optimal posterior distribution was slightly lower than based on the OLS estimates (4.0% and 5.0% respectively),15 meaning that the OLS-based analyses used in this study might slightly over-estimate the true prevalence of fast progressors. Nonetheless, a key strength of this study is how only individuals who met a robust set of eligibility criteria were included. Another strength of this study is our estimation of the prevalence of MD and MTD10 progression based on the estimated normal distribution, which enabled a more equivalent comparison of the two outcome measures. Note that we sought to minimize the potential impact of a learning effect – which might especially have an impact on the estimation of the normal distribution – by excluding the first visual field test available from the data exported of each eye. This was performed as we did not have data on how much prior experience each individual had with visual field testing.
Note also that whilst this study examined the center-weighted MD metric and the non-weighted metric of MTD10, the evaluation of the non-weighted metric of the mean total deviation values across the entire 24–2 visual field test showed similar findings as those based on MD (data not shown).
In conclusion, this study found that approximately one in eight eyes with glaucoma under routine care exhibited fast progression (< -1.0dB/year). Importantly, a similar proportion of eyes exhibited a rate of progression of their central visual field that also exceeded this rate, and nearly one in three eyes showed a < -0.5dB/year decline centrally. These findings underscore the importance of assessing central vision in the clinical management of glaucoma, and the need for new therapies for the notable proportion of fast progressors to prevent functional disability.
Supplementary Material
Acknowledgments
Funding Support:
This study was supported by National Health & Medical Research Council of Australia (fellowship grant no.: #2008382 [Z.W.]), the National Eye Institute, National Institutes of Health, Bethesda, Maryland (grant nos.: EY023704, P30EY022589, EY110008, EY019869, EY029885, EY027510, EY029058, and EY026574, R01 DK087914, R01 DK066358, R01 DK053591, U01 DK105556, R01 HL56266, R01 DK070941, DRC DK063491, and CTSI UL1TR001881), the EyeSight Foundation of Alabama (C.A.G., M.A.F.), and Research to Prevent Blindness, Inc., New York, New York (C.A.G., C.G.D/M., J.M.L., M.A.F., R.N.W.). CERA receives operational infrastructure support from the Victorian Government. The sponsor or funding organization had no role in the design or conduct of this research.
Footnotes
Presented in part at: Association for Research in Vision and Ophthalmology Annual Meeting, May 2022, Denver, Colorado [Virtual Presentation].
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure(s):
K.R.M. – C: Astellas, Allergan, Novartis, Quethera, Roche, Santen; F: Allergan, Bausch & Lomb, Santen; R: Allergan, Novartis, Santen; E: Ikarovec, Quethera. M.A.C. – C: Abbvie, Polyactiva; E: VividWhite. F.A.M. – C: Aerie Pharmaceuticals, Allergan, Annexon, Biogen, Carl-Zeiss Meditec, Galimedix, IDx, Novartis, Reichert, Stealth Biotherapeutics; F: Alcon Laboratories, Allergan, Bausch & Lomb, Carl Zeiss Meditec, Google, Heidelberg Engineering, Merck, National Eye Institute, Novartis, Reichert, Topcon; R: Alcon Laboratories, Allergan, Carl Zeiss Meditec, Reichert; P: NGoggle Diagnostics. C.A.G. – F: Carl Zeiss Meditec, EyeSight Foundation of Alabama, Heidelberg Engineering, Topcon Healthcare, National Eye Institute, Research to Prevent Blindness. M.A.F. – F: EyeSight Foundation of Alabama, Heidelberg Engineering, Topcon Healthcare, National Eye Institute, Research to Prevent Blindness. J.M.L. – C: Alcon, Allergan, Thea Pharmaceuticals, Genentech, Inc.; F:, Research to Prevent Blindness; R:. C.G.D.M. – C: Thea Pharmaceuticals, Carl Zeiss Meditec, Novartis, Perfuse Therapeutics, Reichert; R: Heidelberg Engineering, Topcon; E: Ora Clinical. R.N.W. – C: Abbvie, Aerie Pharmaceuticals, Alcon, Allergan, Amydis, Equinox, Eyenovia, Nicox, Topcon ; F: Bausch & Lomb, Carl Zeiss Meditec, Centervue, Heidelberg Engineering, Konan, National Eye Institute, Optovue, Topcon, Zilia. L.M.Z. – C: Abbvie, Digital Diagnostics, Topcon; F: Carl Zeiss Meditec, Heidelberg Engineering, National Eye Institute, Optovue, Topcon; P: Carl Zeiss Meditec. P: Carl Zeiss Meditec, AISight Health. A.B.J. and Z.W. report nothing to disclose.
REFERENCES
- 1.Ramulu P. Glaucoma and disability: which tasks are affected, and at what stage of disease? Curr Opin Ophthalmol 2009;20(2):92–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saunders LJ, Medeiros FA, Weinreb RN, Zangwill LM. What rates of glaucoma progression are clinically significant? Expert Rev Ophthalmol 2016;11(3):227–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baig S, Diniz-Filho A, Wu Z, et al. Association of Fast Visual Field Loss With Risk of Falling in Patients With Glaucoma. JAMA Ophthalmol 2016;134(8):880–6. [DOI] [PubMed] [Google Scholar]
- 4.Diniz-Filho A, Abe RY, Cho HJ, et al. Fast Visual Field Progression Is Associated with Depressive Symptoms in Patients with Glaucoma. Ophthalmology 2016;123(4):754–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abe RY, Diniz-Filho A, Costa VP, et al. Predicting Vision-Related Disability in Glaucoma. Ophthalmology 2018;125(1):22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abe RY, Diniz-Filho A, Costa VP, et al. The Impact of Location of Progressive Visual Field Loss on Longitudinal Changes in Quality of Life of Patients with Glaucoma. Ophthalmology 2016;123(3):552–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun Y, Lin C, Waisbourd M, et al. The Impact of Visual Field Clusters on Performance-based Measures and Vision-Related Quality of Life in Patients With Glaucoma. Am J Ophthalmol 2016;163:45–52. [DOI] [PubMed] [Google Scholar]
- 8.Blumberg DM, De Moraes CG, Prager AJ, et al. Association Between Undetected 10–2 Visual Field Damage and Vision-Related Quality of Life in Patients With Glaucoma. JAMA Ophthalmol 2017;135(7):742–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heijl A, Buchholz P, Norrgren G, Bengtsson B. Rates of visual field progression in clinical glaucoma care. Acta Ophthalmol 2013;91(5):406–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chauhan BC, Malik R, Shuba LM, et al. Rates of glaucomatous visual field change in a large clinical population. Invest Ophthalmol Vis Sci 2014;55(7):4135–43. [DOI] [PubMed] [Google Scholar]
- 11.Saunders LJ, Russell RA, Kirwan JF, et al. Examining visual field loss in patients in glaucoma clinics during their predicted remaining lifetime. Invest Ophthalmol Vis Sci 2014;55(1):102–9. [DOI] [PubMed] [Google Scholar]
- 12.Aptel F, Aryal-Charles N, Giraud JM, et al. Progression of visual field in patients with primary open-angle glaucoma - ProgF study 1. Acta Ophthalmol 2015;93(8):e615–20. [DOI] [PubMed] [Google Scholar]
- 13.Anderson AJ, Chaurasia AK, Sharma A, et al. Comparison of Rates of Fast and Catastrophic Visual Field Loss in Three Glaucoma Subtypes. Invest Ophthalmol Vis Sci 2019;60(1):161–7. [DOI] [PubMed] [Google Scholar]
- 14.Wright DM, Konstantakopoulou E, Montesano G, et al. Visual Field Outcomes from the Multicenter, Randomized Controlled Laser in Glaucoma and Ocular Hypertension Trial (LiGHT). Ophthalmology 2020;127(10):1313–21. [DOI] [PubMed] [Google Scholar]
- 15.Swaminathan SS, Berchuck SI, Jammal AA, et al. Rates of Glaucoma Progression Derived from Linear Mixed Models Using Varied Random Effect Distributions. Trans Vis Sci Tech 2022;11(2):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirwan JF, Hustler A, Bobat H, et al. Portsmouth visual field database: an audit of glaucoma progression. Eye (Lond) 2014;28(8):974–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boodhna T, Saunders LJ, Crabb DP. Are rates of vision loss in patients in English glaucoma clinics slowing down over time? Trends from a decade of data. Eye (Lond) 2015;29(12):1613–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan TCW, Bala C, Siu A, et al. Risk Factors for Rapid Glaucoma Disease Progression. Am J Ophthalmol 2017;180:151–7. [DOI] [PubMed] [Google Scholar]
- 19.Coeckelbergh TR, Cornelissen FW, Brouwer WH, Kooijman AC. The effect of visual field defects on eye movements and practical fitness to drive. Vision Res 2002;42(5):669–77. [DOI] [PubMed] [Google Scholar]
- 20.Fujita K, Yasuda N, Oda K, Yuzawa M. [Reading performance in patients with central visual field disturbance due to glaucoma]. Nippon Ganka Gakkai Zasshi 2006;110(11):914–8. [PubMed] [Google Scholar]
- 21.Murata H, Hirasawa H, Aoyama Y, et al. Identifying areas of the visual field important for quality of life in patients with glaucoma. PLoS One 2013;8(3):e58695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKean-Cowdin R, Wang Y, Wu J, et al. Impact of visual field loss on health-related quality of life in glaucoma: the Los Angeles Latino Eye Study. Ophthalmology 2008;115(6):941–8 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sawada H, Yoshino T, Fukuchi T, Abe H. Assessment of the vision-specific quality of life using clustered visual field in glaucoma patients. J Glaucoma 2014;23(2):81–7. [DOI] [PubMed] [Google Scholar]
- 24.Sullivan-Mee M, Karin Tran MT, Pensyl D, et al. Prevalence, Features, and Severity of Glaucomatous Visual Field Loss Measured With the 10–2 Achromatic Threshold Visual Field Test. Am J Ophthalmol 2016;168:40–51. [DOI] [PubMed] [Google Scholar]
- 25.Garg A, De Moraes CG, Cioffi GA, et al. Baseline 24–2 Central Visual Field Damage Is Predictive of Global Progressive Field Loss. Am J Ophthalmol 2018;187:92–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu Z, Medeiros FA, Weinreb RN, et al. Comparing 10–2 and 24–2 Visual Fields for Detecting Progressive Central Visual Loss in Glaucoma Eyes with Early Central Abnormalities. Ophthalmol Glaucoma 2019;2(2):95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sample PA, Girkin CA, Zangwill LM, et al. The African Descent and Glaucoma Evaluation Study (ADAGES): design and baseline data. Arch Ophthalmol 2009;127(9):1136–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Medeiros FA, Vizzeri G, Zangwill LM, et al. Comparison of retinal nerve fiber layer and optic disc imaging for diagnosing glaucoma in patients suspected of having the disease. Ophthalmology 2008;115(8):1340–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heijl A, Lindgren G, Olsson J. A package for the statistical analysis of visual fields. In: Greve EL, Heijl A, eds. Seventh International Visual Field Symposium, Amsterdam, September 1986 Dordrecht: Springer Netherlands, 1987. [Google Scholar]
- 30.Chakravarti T, Moghadam M, Proudfoot JA, et al. Agreement Between 10–2 and 24–2C Visual Field Test Protocols for Detecting Glaucomatous Central Visual Field Defects. J Glaucoma 2021;30(6):e285–e91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Phu J, Kalloniatis M. Comparison of 10–2 and 24–2C Test Grids for Identifying Central Visual Field Defects in Glaucoma and Suspect Patients. Ophthalmology 2021;128(10):1405–16. [DOI] [PubMed] [Google Scholar]
- 32.Jammal AA, Thompson AC, Mariottoni EB, et al. Rates of Glaucomatous Structural and Functional Change From a Large Clinical Population: The Duke Glaucoma Registry Study. Am J Ophthalmol 2020;222:238–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swaminathan SS, Berchuck SI, Jammal AA, et al. Rates of Glaucoma Progression Derived from Linear Mixed Models Using Varied Random Effect Distributions. medRxiv 2021:2021.06.01.21258173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brusini P, Johnson CA. Staging functional damage in glaucoma: review of different classification methods. Surv Ophthalmol 2007;52(2):156–79. [DOI] [PubMed] [Google Scholar]
- 35.Prager AJ, Hood DC, Liebmann JM, et al. Association of glaucoma-related, optical coherence tomography–measured macular damage with vision-related quality of life. JAMA Ophthalmol 2017;135(7):783–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blumberg DM, Liebmann JM, Hirji SH, Hood DC. Diffuse Macular Damage in Mild to Moderate Glaucoma Is Associated With Decreased Visual Function Scores Under Low Luminance Conditions. Am J Ophthalmol 2019;208:415–20. [DOI] [PubMed] [Google Scholar]
- 37.Jammal AA, Ogata NG, Daga FB, et al. What is the amount of visual field loss associated with disability in glaucoma? Am J Ophthalmol 2019;197:45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang EE, Goldberg JL. Glaucoma 2.0: neuroprotection, neuroregeneration, neuroenhancement. Ophthalmology 2012;119(5):979–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levin LA, Crowe ME, Quigley HA, et al. Neuroprotection for glaucoma: Requirements for clinical translation. Exp Eye Res 2017;157:34–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khatib TZ, Martin KR. Neuroprotection in Glaucoma: Towards Clinical Trials and Precision Medicine. Curr Eye Res 2020;45(3):327–38. [DOI] [PubMed] [Google Scholar]
- 41.Skuta GL, Ding K, Lum F, Coleman AL. An IRIS Registry-Based Assessment of Primary Open-Angle Glaucoma Practice Patterns in Academic Versus Nonacademic Settings. Am J Ophthalmol 2022;242:228–42. [DOI] [PubMed] [Google Scholar]
- 42.Jansonius NM. On the accuracy of measuring rates of visual field change in glaucoma. Br J Ophthalmol 2010;94(10):1404–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


