Abstract
We examined relationships between the serotonin system and stress in major depression and suicidal behavior. Twenty-five medication-free depressed participants (13 suicide attempters) underwent same-day [11C]DASB and [11C]CUMI-101 positron emission tomography (PET) imaging. Binding potential (BPND) to the serotonin transporter (5-HTT) and serotonin 1A (5-HT1A) receptor, respectively, was quantified using the NRU 5-HT atlas, reflecting distinct spatial distributions of multiple serotonin targets. Ecological momentary assessment (EMA) measured current stress over one week proximal to imaging. EMA stress did not differ between attempters and non-attempters. In all depressed participants, 5-HTT and 5-HT1A BPND were unrelated to EMA stress. There was regionally localized lower 5-HTT BPND (p=0.002) and 5-HT1A BPND (p=0.03) in attempters vs. nonattempters. In attempters, region-specific associations between 5-HTT (p=0.03) and 5-HT1A (p=0.005) BPND and EMA stress emerged. While no post-hoc 5-HTT BPND correlations were significant, 5-HT1A BPND correlated positively in attempters with EMA stress in 9/10 regions (p-value’s<0.007), including the entire cortex except the largely occipital region 5. Brodmann-based regional analyses found diminished effects for 5-HTT and subcortically localized positive corrrelations between 5-HT1A and EMA stress, in attempters only. Given comparable depression severity and childhood and current stress between attempters and nonattempters, lower 5-HTT binding in attempters vs. nonattempters may suggest a biological risk marker. Localized lower 5-HTT and widespread higher 5-HT1A binding with stress among attempters specifically may suggest that a serotonergic phenotype might be a key determinant of risk or resiliency for suicidal behavior.
Keywords: Serotonin transporter, serotonin 1A receptor, ecological momentary assessment, current stress, major depression, suicidal behavior
INTRODUCTION
Major depressive disorder (MDD) is a leading cause of worldwide disability (Friedrich, 2017). A 31% incidence of lifetime suicide attempt is reported in MDD (Dong et al., 2019), five times higher than the general population (Nock et al., 2010). Better understanding the neurobiological basis of MDD and suicidal behavior can assist in developing better treatments and prevention (van Heeringen and Mann, 2014). The stress-diathesis model for suicidal behavior proposes that current life stressors combined with MDD can result in suicidal behavior in patients who are vulnerable because of distal factors or traits, e.g., genetics and childhood adversity (Mann and Rizk, 2020; van Heeringen and Mann, 2014).
Postmortem and in vivo studies find lower brain serotonin (5-HT) transporter (5-HTT) and higher serotonin 1A receptor (5-HT1A) binding in MDD and in suicide attempters compared with psychiatric or healthy volunteer (HV) groups, suggesting dysregulated serotonin functioning (Arango et al., 2003; Arango et al., 1995; Boldrini et al., 2008; Cannon et al., 2006; Gryglewski et al., 2014; Hesselgrave and Parsey, 2013; Joensuu et al., 2007; Kaufman et al., 2015; Lehto et al., 2006; Malison et al., 1998; Mann et al., 2000; Miller et al., 2013; Miller et al., 2008; Nye et al., 2013; Oquendo et al., 2016; Parsey et al., 2006a; Parsey et al., 2010; Parsey et al., 2006b; Reimold et al., 2008; Selvaraj et al., 2011; Staley et al., 2006; Stockmeier, 2003; Sullivan et al., 2015; Underwood et al., 2018; Willeit et al., 2000). However, some studies report no differences or opposing effects in MDD vs. HVs (Ichimiya et al., 2002; Kambeitz and Howes, 2015; Meyer et al., 2004; Meyer et al., 2001; Miller et al., 2013). Low cerebrospinal fluid 5-hydroxyindoleacetic acid has been found in suicide attempters vs. nonattempters (Åsberg et al., 1976) and predicts higher risk for suicide death in MDD (Mann et al., 2006), linking deficient serotonin release with suicidal behavior. In vivo brain positron emission tomography (PET) studies also find lower 5-HTT binding in suicide attempters vs. nonattempters or HVs (Miller et al., 2013; Nye et al., 2013), and that higher 5-HT1A binding predicts suicide attempt lethality (Oquendo et al., 2016) and positively correlates with ideation severity (Sullivan et al., 2015).
Serotonin system function can be molded in early development, moderating susceptibility for suicidal behavior. Preclinically, different impacts of early life stress on 5-HT1A expression by DNA methylation at repressor sites have been shown, with some studies reporting higher (Diamantopoulou et al., 2018; Vázquez et al., 2002) and lower 5-HT1A binding or expression (Law et al., 2009; Matsuzaki et al., 2011; Ohta et al., 2014; Spinelli et al., 2010). Early-life adversity is a suicide risk factor (Brodsky et al., 2008; van Heeringen and Mann, 2014) and is associated with lower 5-HTT and higher 5-HT1A binding in MDD (Miller et al., 2009; Yttredahl et al., 2021). Childhood sexual abuse had a greater impact on adult depressive symptoms in lower expressing allele carriers of the 5-HTTLPR polymorphism, i.e., fewer 5-HT transporters (Lesch et al., 1996), relative to higher expressing-allele carriers (Aguilera et al., 2009; Rocha et al., 2015). Additionally, lower 5-HTT and higher 5-HT1A binding postmortem in depressed suicide decedents were dependent on childhood adversity (Underwood et al., 2018).
Few in vivo studies have investigated serotonin’s role in the relationship of daily stressors to suicidal behavior. In rodents, early life stress led to long-term reductions in both motivation and 5-HT1A binding, which were both reversed with fluoxetine treatment (Leventopoulos et al., 2009). Also in rodents, higher brain 5-HT1A expression increased sensitivity to acute stress and produced stress-induced depressive phenotypes (Richardson-Jones et al., 2010). Individuals with low expressing 5-HTTLPR promotor variants had increased risk for adulthood stressors triggering new depression symptoms, MDD diagnosis, suicidal ideation, and attempt (Caspi et al., 2003), although findings have been mixed (Karg et al., 2011; Risch et al., 2009). Cortisol responses to the Trier Social Stress Test were positively correlated with brain 5-HT1A binding (Steinberg et al., 2019), supporting a model where 5-HT1A over-expression might play a role in stress hyperreactivity (Underwood et al., 2018; Yttredahl et al., 2021).
Given serotonin’s role in MDD and suicidal behavior (Mann, 1998; van Heeringen and Mann, 2014), assessing the relationship between serotonin and acute stress in individuals with a history of suicide attempt might clarify the pathophysiology of suicidal behavior. Therefore, we assessed this in 25 medication-free individuals with MDD, 13 with a previous suicide attempt, with same-day [11C]DASB (targeting 5-HTT) and [11C]CUMI-101 (targeting 5-HT1A) PET scans. Participants also underwent smartphone-administered ecological momentary assessment (EMA) of daily stressful events over one week proximal to PET scanning. Unlike retrospective reports, EMA minimizes recall bias and retrieval issues, and allows for collection of participant responses close to real-time in a naturalistic environment (Gratch et al., 2021; Shiffman et al., 2008). We used a new serotonin-specific brain atlas, the Copenhagen University Hospital Neurobiology Research Unit (NRU) 5-HT atlas, that identifies regions of uniform serotonin binding derived from in vivo human PET data for multiple serotonergic radiotracers (Beliveau et al., 2017; Beliveau et al., 2020). We tested whether suicide attempters differed from nonattempters in the relationship between EMA stress and serotonergic function. We hypothesized that attempters with the highest current stress would exhibit the lowest 5-HTT and highest 5-HT1A binding. We also compared results in the NRU 5-HT atlas, where we predicted more uniform binding within regions of interest (ROIs), to results obtained considering standard Brodmann Area-based ROIs, which exhibit more heterogeneous binding.
EXPERIMENTAL PROCEDURES
Participants
Twenty-five adults meeting DSM-IV criteria for MDD (assessed via Structured Clinical Interview for DSM-IV (SCID-I) and psychiatrist interview (First et al., 1997)) underwent same-day [11C]DASB and [11C]CUMI-101 scans, and EMA proximal to the PET scans. This study was approved by the Institutional Review Board of the New York State Psychiatric Institute. All participants provided written, informed consent.
Inclusion criteria were: 1) current major depressive episode, 2) 18–60 years-old, 3) off all drugs likely to interact with the serotonin system for at least 21 days at time of scan. Exclusion criteria included: 1) lifetime schizophrenia, schizoaffective illness, bipolar disorder, current psychotic depression, no drug or alcohol abuse in past two months, no drug or alcohol dependence in past six months, 2) first-degree family of schizophrenia if participant is <33 years-old (Loranger, 1984), 3) significant active physical illness, 4) lacking capacity to consent, 5) aggressive behavior that is a significant threat to others in the last month, such as physically assaultive behavior, assessed via clinical interview, 6) pregnancy, abortion or miscarriage within two months, or currently lactating, 7) previous head injury with evidence of cognitive impairment, 8) MRI contraindications, and 9) electroconvulsive therapy within six months.
The Columbia Suicide History Form (Oquendo et al., 2003) assessed suicide attempt history, the 17-item Hamilton Depression Rating Scale (HDRS-17) (Hamilton, 1960) and Beck Depression Inventory (BDI) (Beck et al., 1996) assessed depression severity, the Beck Hopelessness Scale (BHS) (Beck and Steer, 1988) assessed hopelessness, and the Scale for Suicidal Ideation (SSI) (Beck et al., 1979) assessed suicidal ideation. The sum across the five subscales of the Childhood Trauma Questionnaire (CTQ) (Bernstein et al., 2003) assessed childhood adversity.
Ecological Momentary Assessment (EMA) of Stress
As described, EMA was acquired via smartphone over one-week (Chaudhury et al., 2017; Gratch et al., 2021; Stanley et al., 2021). Participants were asked to provide a 12-hour window where they expected to be awake and engaged in routine daily activities. The EMA was then personalized to prompt each participant at random points within the six two-hour blocks of that 12-hour window (six sessions/day). This assessment time randomization sought to reduce anticipatory effects, and minimize consistent interruptions to participants’ fixed schedules. At each prompt, participants were asked to answer whether each of the following stressors occurred since the last prompt: (1) had a disagreement with someone, (2) experienced a loss, (3) been disappointed by someone, (4) felt neglected by someone, (5) received bad news, (6) been reminded of something painful from the past, and (7) been rejected by someone (Chaudhury et al., 2017). A summary metric, “EMA stress”, was computed as the percentage of the total number of EMA sessions with at least one endorsed stressor, which represents an aggregate of daily stress during the week. In addition to stressors, suicidal ideation and intent and other clinical data were also collected and are presented elsewhere (Chaudhury et al., 2017; Stanley et al., 2021).
PET Acquisition, Processing and Quantification
Preparation of radiotracers and scanning protocols for [11C]DASB and [11C]CUMI-101 have been described (Milak et al., 2010; Ogden et al., 2007). Briefly, a polyurethane head holder (Soule Medical, Tampa, FL, USA) was molded and used to reduce movement artifact. PET was performed on a Siemens Biograph mCT (Siemens/CTI, Knoxville, TN, USA). On each scan day, a low-dose computed tomography (CT) scan was first acquired for attenuation correction (AC), followed by an intravenous bolus of radiotracer administered over 30 seconds and 3D emission data collected for [11C]CUMI-101 (120 minutes) and [11C]DASB (100 minutes). [11C]CUMI-101 was always acquired first and time of day was held consistent.
[11C]DASB and [11C]CUMI-101 reconstruction and pre-processing were performed as described (Miller et al., 2013; Oquendo et al., 2016). Briefly, images were binned into 19 frames of increasing duration for [11C]DASB and 21 frames for [11C]CUMI-101, and reconstructed using filtered back projection to a 256×256 matrix (Ogden et al., 2007). To correct head motion, PET frames were registered to the eighth frame and coregistered to their T1-weighted MRI (DeLorenzo et al., 2009). Whole-brain, MR-space, voxel-wise BPND maps for [11C]DASB and [11C]CUMI-101 were quantified via Likelihood Estimation in Graphical Analysis using cerebellar grey matter as the reference region (Zanderigo et al., 2013).
There were 25 participants who had EMA stress and a [11C]DASB scan (13 attempters) and 24 of these participants also had a [11C]CUMI-101 scan (12 attempters). It was not possible to schedule same-day imaging for the one participant with only a [11C]DASB scan due to lack of available scanner slots and the participant and study psychiatrist agreed it was necessary to initiate medication-based treatment before a [11C]CUMI-101 scan slot became available.
NRU 5-HT atlas Processing
The NRU 5-HT atlas was created by clustering many participants’ binding maps across PET tracers targeting 5-HTT and the 1A, 1B, 2A, and 4 serotonin receptors and includes 10 brain regions that contain distinct contributions from serotonin system components (Beliveau et al., 2017; Beliveau et al., 2020). The NRU 5-HT atlas is provided as FreeSurfer overlays on the fsaverage surface (https://xtra.nru.dk/FS5ht-atlas/). To obtain correspondence between the MR-space BPND maps and the fsaverage surface, each participant’s T1-weighted MRI was run through FreeSurfer 7.1.1. The MR-space BPND maps were then grey-matter masked (SPM5, Wellcome Center for Human Neuroimaging) and resampled onto the fsaverage surface. BPND was extracted from each of the 10 regions by averaging all positive BPND values.
Statistical analysis
Participant demographics and characteristics were compared with two-tailed t-tests and Chi-square tests. To borrow strength across brain regions, accounting for intra-participant correlation among regions, we fit linear mixed-effects (LME) models to natural log-transformed BPND in all regions. Participant-specific random intercept was included and separate models were fit for [11C]DASB and [11C]CUMI-101. To test for covariates, a model was first fit with sex, age, number of days between PET and EMA, region, hemisphere, and EMA stress (log-transformed for normalization) as main effects.
We first tested for relationships with EMA stress across all participants by entering either 5-HTT or 5-HT1A BPND as model outcomes and EMA stress as a fixed effect, controlling for brain region. To test for region-specific differences in stress, additional models included an EMA stress by region effect. We then tested the impact of suicide attempt status on the relationship between EMA stress and PET. Therefore, we repeated the previous models in separate attempter and nonattempter models. Post-hoc analyses by region were conducted and standardized betas (β) reported.
All statistics were then repeated using six standard a priori Brodmann Area-based regions that have been analyzed in both 5-HTT and 5-HT1A papers from our group (anterior cingulate cortex, thalamus, amygdala, hippocampus, dorsal putamen, and midbrain) (Miller et al., 2016; Miller et al., 2009; Miller et al., 2008; Oquendo et al., 2016; Parsey et al., 2010; Sullivan et al., 2015; Zanderigo et al., 2018). They have appreciable 5-HTT and 5-HT1A binding and are defined on an in-house atlas (Miller et al., 2016; Miller et al., 2009; Miller et al., 2008; Oquendo et al., 2016; Parsey et al., 2010; Sullivan et al., 2015; Zanderigo et al., 2018).
P-values are reported unadjusted for multiple comparisons, but we also state when these p-values would survive Bonferroni correction. A Bonferroni corrected threshold for the LME main effects and interactions would be p<0.008, accounting for two tracers, two atlases, and separate attempter and non-attempter analyses. Further, Bonferroni correction on post-hoc analyses would yield p<0.005 for the ten NRU 5-HT regions and p<0.008 for the six Brodmann Area-based regions. Statistics were performed in R v4.0.3 (Bates et al., 2014; Team, 2013).
RESULTS
Participants
Table 1 displays participant characteristics. Suicide attempters and nonattempters did not differ in age, sex, race, education, radiotracer dose, depression severity, hopelessness, suicidal ideation, childhood adversity, and days between EMA and PET scan (minimum: 1 day and maximum: 36 days). [11C]DASB injected mass was greater in nonattempters than attempters (p=0.05). EMA stress over one week did not differ between attempters and nonattempters (56.1% ± 25.1% and 55.5% ± 33.0%, respectively).
Table 1.
Participant Clinical and Demographic Characteristics & Tracer Doses.
| Suicide Attempters (n=13) | Suicide Nonattempters (n=12) | p-value | |
|---|---|---|---|
| Age (years) | 30.0 ± 7.4 | 35.0 ± 10.0 | 0.10 |
| % Female | 69.2% | 33.3% | 0.11 |
| 7.7% Unknown/not reported | |||
| Total Education (years) * | 15.8 ± 2.0 | 15.3 ± 2.0 | 0.54 |
| HDRS-17 | 17.5 ± 5.6 | 17.9 ± 2.9 | 0.83 |
| BDI ** | 25.0 ± 9.0 | 27.3 ± 7.7 | 0.52 |
| BHS ** | 10.8 ± 5.8 | 13.6 ± 5.1 | 0.22 |
| [ 11 C]DASB Injected Dose (mCi) | 13.0 ± 4.9 | 11.4 ± 5.3 | 0.44 |
| [ 11 C]CUMI-101 Injected Dose (mCi) | 12.1 ± 3.9 | 10.8 ± 3.8 | 0.41 |
| [ 11 C]DASB Injected Mass (μg) | 2.0 ± 1.2 | 3.0 ± 1.1 | 0.05 |
| [ 11 C]CUMI-101 Injected Mass (μg) | 2.0 ± 1.1 | 1.7 ± 0.7 | 0.43 |
| EMA stress (proportion of prompts over one week with negative stressor endorsed) | 56.1 ± 25.1% | 55.5 ± 33.0% | 0.96 |
| Days between EMA start and PET scan (EMA – PET) | −6.6 ± 16.1 | −1.7 ± 15.5 | 0.44 |
| Childhood Trauma Questionnaire (CTQ) Total Score + | 51.8 ± 20.7 | 45.1 ± 18.9 | 0.43 |
| SSI Prior Total ++ | 8.4 ± 11.2 | 3.1 ± 3.5 | 0.26 |
| Number of prior suicide attempts +++ | 1.8 ± 1.2 | 0 ± 0 | N/A |
| Suicide attempt recency (years) ++++ | 7.6 ± 7.7 | N/A | N/A |
Abbreviations: HDRS-17 = 17-item Hamilton Depression Rating Scale, mCi = milliCuries.
Total education data missing from one nonattempter participant.
BDI and BHS missing from one attempter participant.
CTQ missing from one attempter and one nonattempter participant
SSI Prior Total missing from 6 attempter participants.
Number of prior suicide attempts missing from 1 attempter participant.
Suicide attempt recency missing from 4 attempter participants.
NRU 5-HT atlas: All Depressed Participants
In both 5-HTT and 5-HT1A receptor models, potential covariates were non-significant and were dropped from subsequent models (age: F=0.86, degrees of freedom (df)=1, 20, p=0.364 and F=0.03, df=1, 19, p=0.863; sex: F=0.57, df=1, 20, p=0.460 and F=0.08, df=1,19, p=0.782; number of days between the PET scan and EMA: F=1.24, df =1, 20, p=0.280 and F=0.29, df=1,19, p=0.599, respectively for 5-HTT and 5-HT1A).
In all participants, the main effect of EMA stress was not significant for 5-HTT or 5-HT1A BPND (F=0.80, df=1, 23, p=0.379 and F=0.36, df=1, 22, p=0.554, respectively). The relationship between EMA stress and 5-HTT or 5-HT1A BPND also did not vary by brain region (Figure 1 and 2, top; F=0.93, df=10, 150.27, p=0.510 and F=0.82, df=10, 143.46, p=0.606, respectively).
Figure 1.
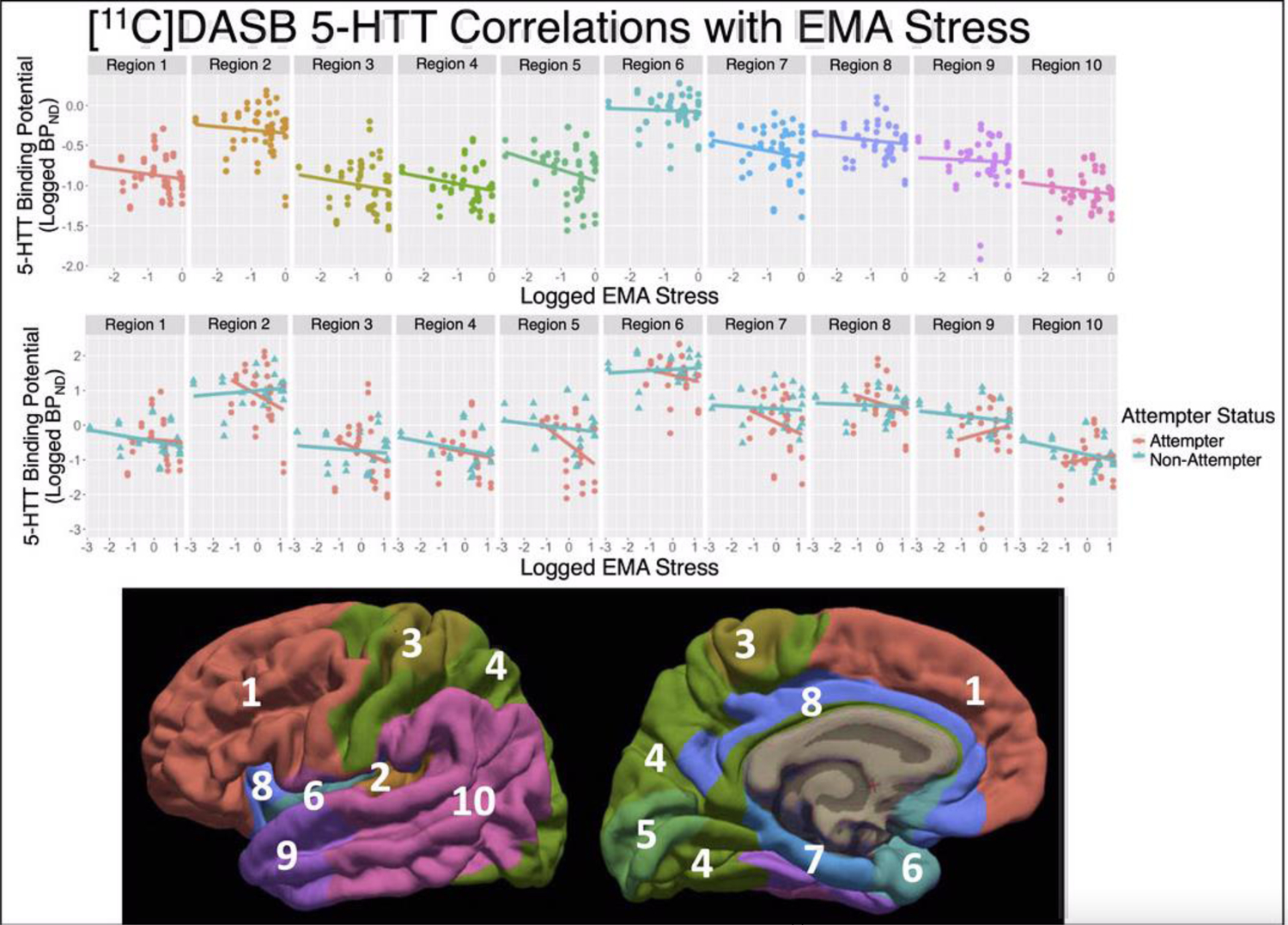
NRU 5-HT atlas Results: Scatter plots of log-transformed [11C]DASB 5-HTT BPND estimates (y-axes) versus the log-transformed EMA stress measure. Individual scan data-points are repeated in the top and center panes, but different regression lines are shown. Top: Regression line across all participants, visualizing non-significant effect of EMA stress by region (p=0.51). Middle: Regression lines for both suicide attempters (pink) and nonattempters (blue), where attempter data-points are plotted with circles and nonattempter data-points are with triangles, visualizing significant effect of EMA stress by region in attempters (p=0.031) and nonattempters (p=0.015). Bottom: The 10 regions of the NRU 5-HT atlas are shown on the lateral (left) and medial (right) fsaverage surfaces.
Figure 2.
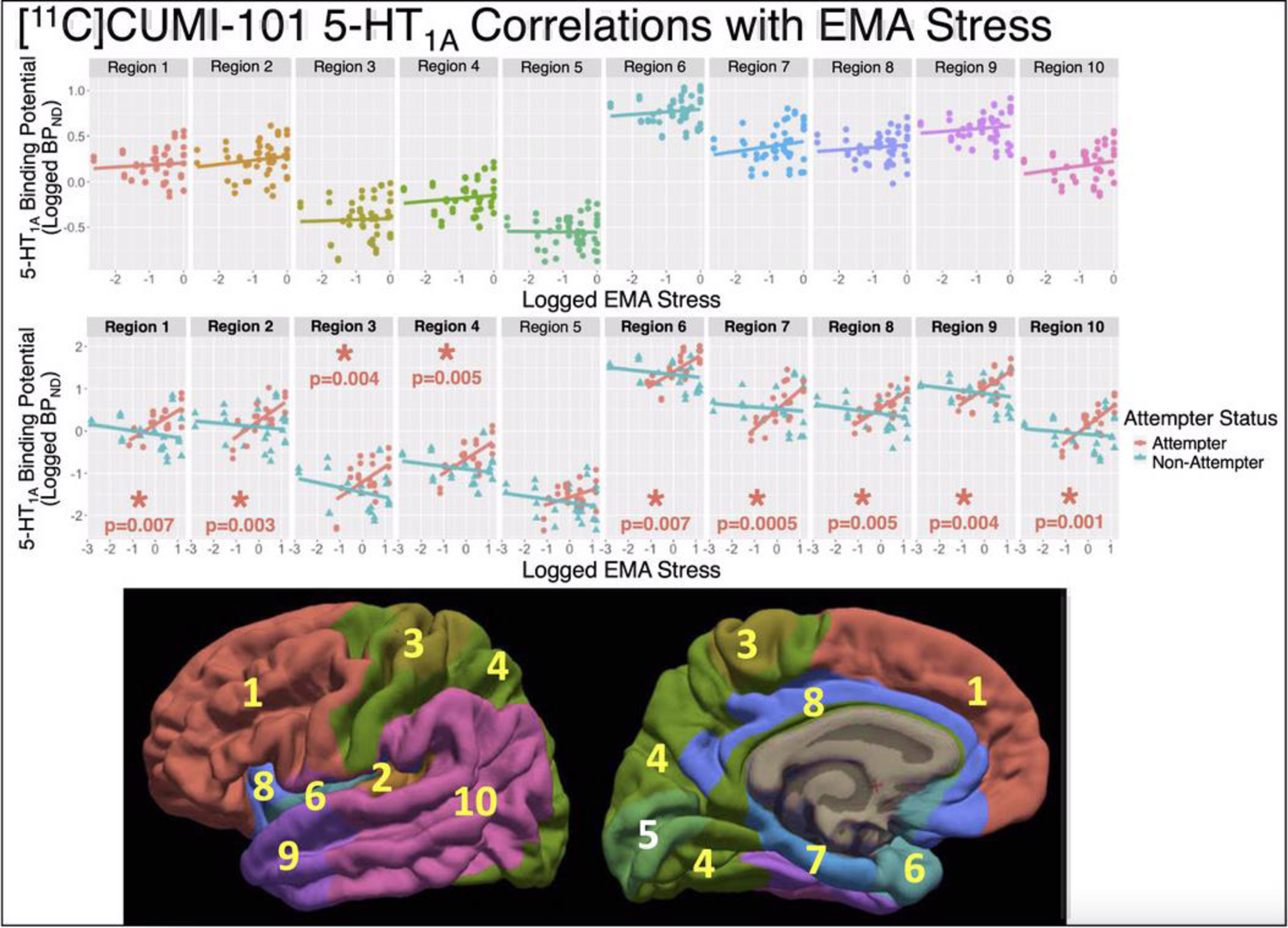
NRU 5-HT atlas Results: Scatter plots of log-transformed [11C]CUMI-101 5-HT1A BPND estimates (y-axes) versus the log-transformed EMA stress measure. Individual scan data-points are repeated in the top and center panes, but different regression lines are shown. Top: Regression line across all participants, visualizing non-significant effect of EMA stress by region (p=0.61). Middle: Regression lines for both suicide attempters (pink) and nonattempters (blue), where attempter data-points are plotted with circles and nonattempter data-points are with triangles, visualizing significant effect of EMA stress by region in attempters (p=0.005) and non-significant effect in nonattempters (p=0.95). Bottom: The 10 regions of the NRU 5-HT atlas are shown on the lateral (left) and medial (right) fsaverage surfaces, with regions revealed as p<0.05 in post-hoc testing marked with yellow font. For Regions with post-hoc p<0.05, metrics of effect size include: Region 1: β = 0.33 and r = 0.71, Region 2: β = 0.38 and r = 0.70, Region 3: β = 0.36 and r = 0.54, Region 4: β = 0.34 and r = 0.65, Region 6: β = 0.33 and r = 0.70, Region 7: β = 0.45 and r = 0.72, Region 8: β = 0.35 and r = 0.75, Region 9: β = 0.36 and r = 0.71, and Region 10: β = 0.41 and r = 0.75 (β estimates from Table 2).
NRU 5-HT atlas: Stratifying by Suicide Attempter Status
There were significant region-specific differences in brain 5-HTT and 5-HT1A BPND between attempters and nonattempters (attempter-region interaction: 5-HTT F=2.93, df=10, 150.27, p=0.002 (also survives Bonferroni correction) and 5-HT1A F=2.06, df=10, 143.46, p=0.03). Post-hoc testing of NRU 5-HT atlas brain regions revealed lower 5-HTT binding in region 5 in attempters than non-attempters (p=0.034; portions of cuneus, pericalcarine, lingual, and lateral occipital cortices), whereas no post-hoc tests were significant for 5-HT1A BPND. Given this observation of differential 5-HTT BPND as a function of suicide attempt history, we hypothesized that the two groups might have a different perception of the same level of stress because of a difference in brain biological state. We therefore examined the relationship between binding and stress while stratifying by attempt status.
Region-specific relationships were found between EMA stress and 5-HTT BPND for both attempters (F=2.16, df=10, 68.25 p=0.031) and nonattempters (F=2.46, df=10, 61.36, p=0.015). No post-hoc analyses of individual NRU 5-HT atlas regions reached significance (p-values>0.05; (Figure 1, center; Table 2). In attempters, the effect sizes for 5-HTT correlations were moderate in regions 2 (β=−0.37), 5 (β=−0.49; the same region where attempters had lower 5-HTT BPND than non-attempters), and 7 (β=−0.30), whereas the effect sizes for all correlations in nonattempters were small (β range=−0.14 to 0.06; Table 2).
Table 2.
Effect of EMA stress by region for 5-HTT and 5-HT1A in suicide attempters and nonattempters.
| Suicide Attempters: Effect of EMA stress by region | ||||
|---|---|---|---|---|
| 5-HTT BPND | 5-HT1A BPND | |||
| p=0.04* | p=0.005** | |||
| Standardized Beta (β) Estimate [95% CI] | p-value | Standardized Beta (β) Estimate [95% CI] | p-value | |
| Region 1 | −0.05 [−0.62, 0.53] | 0.87 | 0.33 [0.10, 0.55] | 0.007* |
| Region 2 | −0.37 [−0.94, 0.21] | 0.20 | 0.38 [0.15, 0.61] | 0.003** |
| Region 3 | −0.29 [−0.86, 0.29] | 0.31 | 0.36 [0.13, 0.58] | 0.004** |
| Region 4 | −0.13 [−0.71, 0.44] | 0.63 | 0.34 [0.12, 0.57] | 0.005** |
| Region 5 | −0.49 [−1.07, 0.08] | 0.09 | 0.17 [−0.05, 0.40] | 0.13 |
| Region 6 | −0.16 [−0.73, 0.42] | 0.58 | 0.33 [0.10, 0.55] | 0.007* |
| Region 7 | −0.30 [−0.88, 0.27] | 0.28 | 0.45 [0.23, 0.68] | 0.0005** |
| Region 8 | −0.20 [−0.78, 0.37] | 0.47 | 0.35 [0.12, 0.57] | 0.005** |
| Region 9 | 0.18 [−0.40, 0.75] | 0.52 | 0.36 [0.13, 0.59] | 0.004** |
| Region 10 | 0.09 [−0.49, 0.66] | 0.75 | 0.41 [0.18, 0.63] | 0.001** |
| Suicide Nonattempters: Effect of EMA stress by region | ||||
| p=0.015* | p=0.95 | |||
| Standardized Beta (β) Estimate [95% CI] | p-value | Standardized Beta (β) Estimate [95% CI] | p-value | |
| Region 1 | −0.11 [−0.36, 0.14] | 0.33 | −0.081 [−0.29, 0.13] | 0.42 |
| Region 2 | 0.06 [−0.29, 0.31] | 0.64 | −0.051 [−0.26, 0.16] | 0.61 |
| Region 3 | −0.05 [−0.30, 0.20] | 0.65 | −0.11 [−0.33, 0.10] | 0.26 |
| Region 4 | −0.12 [−0.37, 0.13] | 0.31 | −0.068 [−0.28, 0.14] | 0.50 |
| Region 5 | −0.07 [−0.33, 0.18] | 0.53 | −0.076 [−0.29, 0.14] | 0.45 |
| Region 6 | −0.04 [−0.22, 0.29] | 0.77 | −0.058 [−0.27, 0.15] | 0.56 |
| Region 7 | −0.03 [−0.29, 0.22] | 0.77 | −0.044 [−0.26, 0.17] | 0.66 |
| Region 8 | −0.03 [−0.28, 0.22] | 0.82 | −0.074 [−0.29, 0.14] | 0.46 |
| Region 9 | −0.07 [−0.32, 0.18] | 0.54 | −0.070 [−0.28, 0.14] | 0.49 |
| Region 10 | −0.14 [−0.39, 0.12] | 0.26 | −0.047 [−0.26, 0.16] | 0.64 |
Abbreviations: CI = confidence interval, 5-HTT = serotonin transporter, 5-HT1A = serotonin receptor 1A, BPND = binding potential of interest.
p<0.05,
survives Bonferroni correction thresholds: p<0.008 for LME model effects of EMA stress by region and p<0.005 for the post-hoc follow-ups in the 10 NRU 5-HT atlas regions
For the 5-HT1A receptor, a region-specific relationship was observed between EMA stress and 5-HT1A BPND in attempters (F=2.92, df=10, 61.36, p=0.005; also survives Bonferroni correction), that was absent in nonattempters (F=0.39, df=10, 61.36, p=0.95). Post-hoc, a widespread pattern of positive correlation between 5-HT1A BPND and EMA stress in attempters was found, with all NRU 5-HT atlas regions except region 5 (portions of cuneus, pericalcarine, lingual, and lateral occipital cortices) having p-values<0.05 and seven of these nine regions also surviving Bonferroni correction (Figure 2, center, Table 2). In contrast, region-wise correlations were absent in nonattempters (p’s>0.05; Figure 2, center; Table 2). The effect sizes for all nine significant 5-HT1A correlations in attempters were moderate (β range= 0.33 to 0.45), whereas the effect sizes for all correlations in nonattempters were small (β range = −0.11 to −0.04; Table 2).
Brodmann Area-Based In-House atlas
A comparison of regions in Brodmann vs. NRU 5-HT atlases is shown in Figure 3. Similar to the NRU 5-HT atlas results, in all participants, the main effect of EMA stress and the effect of EMA stress by brain region were not significant for 5-HTT BPND (F=0.022, df=1, 23, p=0.88 and F=0.48, df=6, 67.31, p=0.82, respectively) or 5-HT1A BPND (F=0.42, df=1, 22, p=0.52 and F=0.99, df=6, 64.31, p=0.44, respectively).
Figure 3.
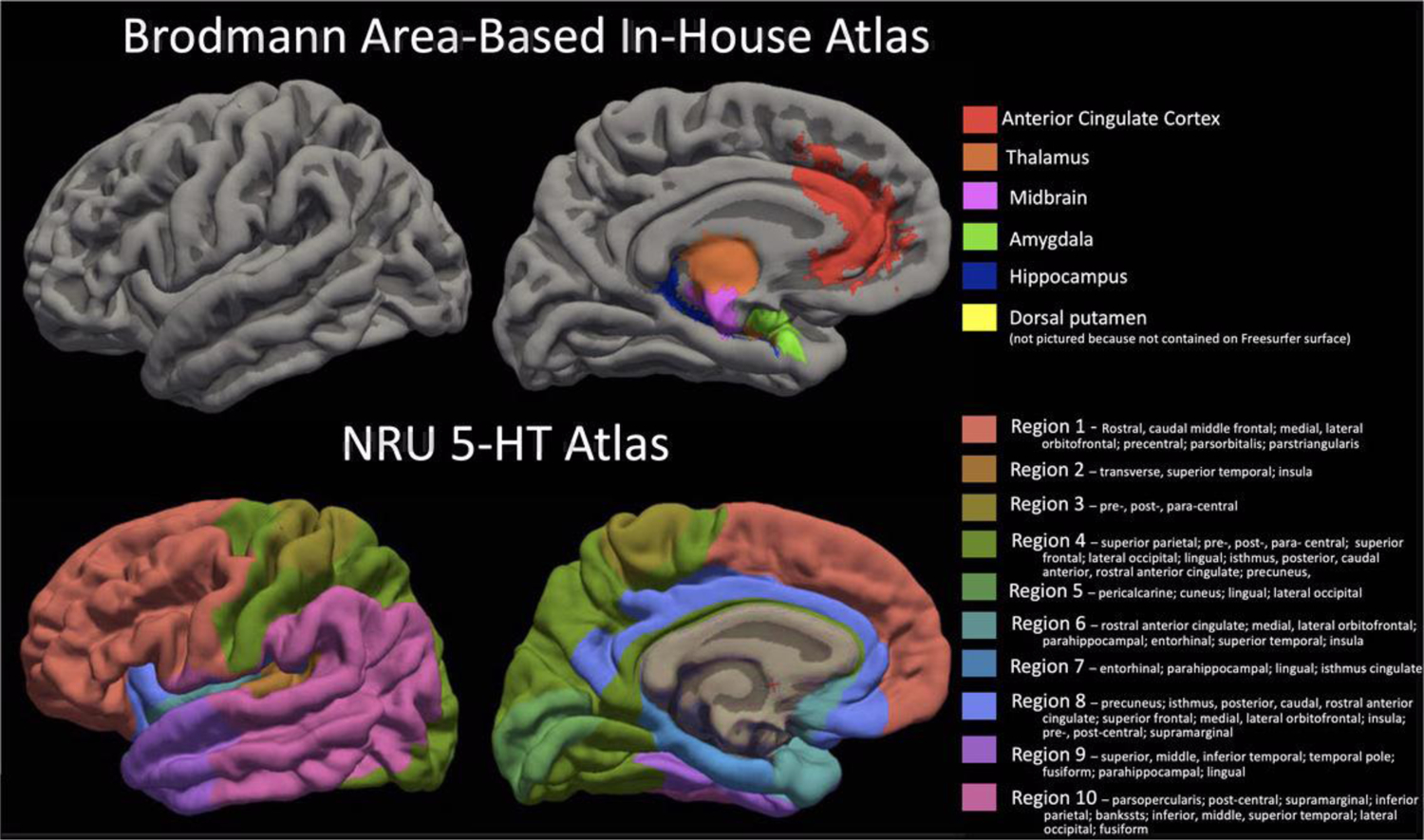
Comparison of the Brodmann Area-based in-house atlas regions of interest resampled on the Freesurfer fsaverage surface (TOP) and the NRU 5-HT atlas (BOTTOM). Regions are all shown on pial surface and NRU 5-HT atlas regions are listed with the Desikan-Killiany regions that lie within the NRU 5-HT region boundaries for reference.
When considering attempt status, Brodmann region effects were in general weaker for 5-HTT, i.e., no effect of 5-HTT BPND in attempt status group comparisons or correlations with EMA stress (attempt status by region: F=1.04, df=6, 64.31, p=0.41; EMA stress by region in attempters: F=0.67, df=6, 34.22, p=0.68, Figure 4; EMA stress by region in nonattempters: F=0.30, df=6, 25.15, p=0.93, Figure 4).
Figure 4.
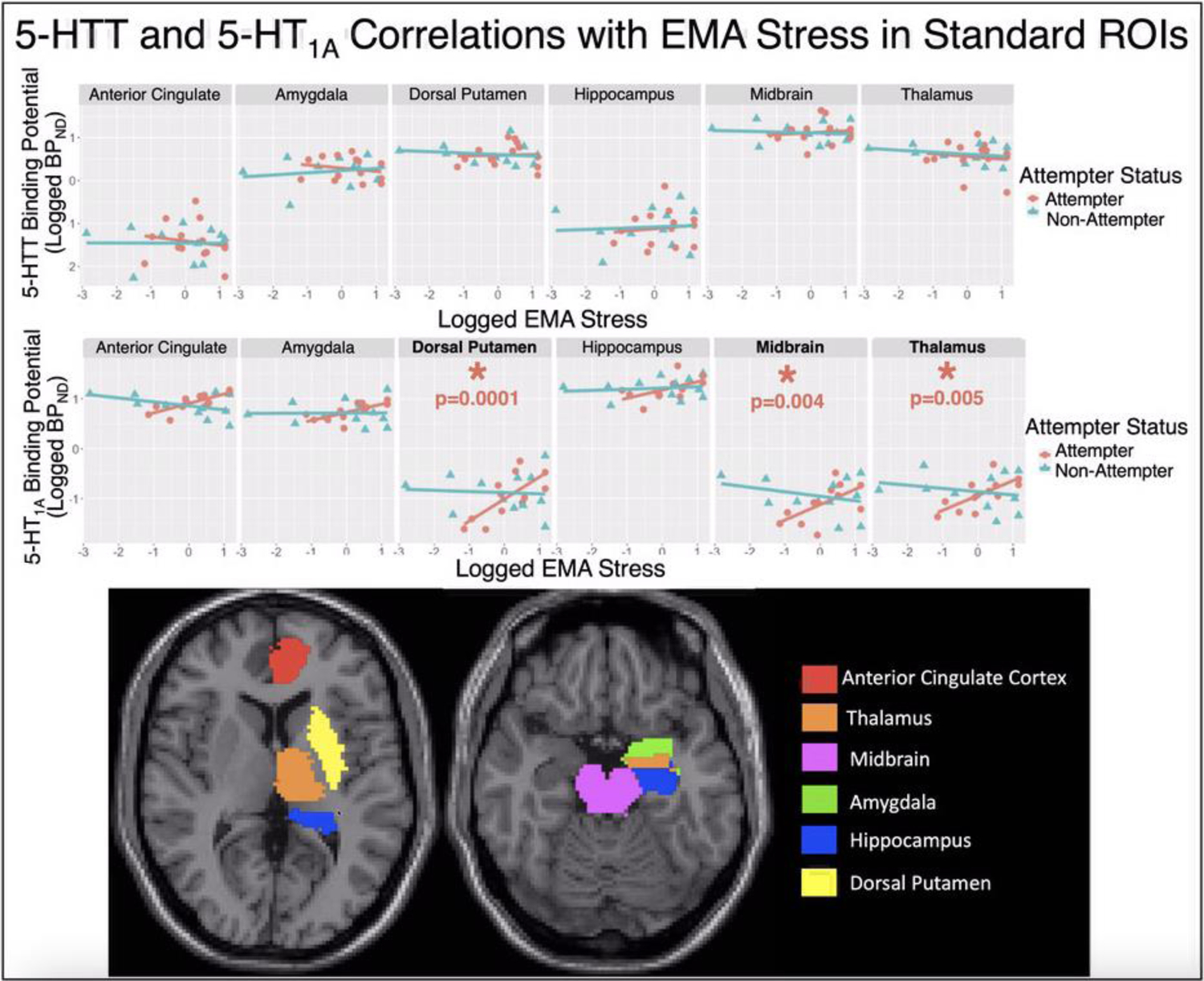
Standard Brodmann Area-Based In-House atlas Results: Scatter plots of log-transformed [11C]CUMI-101 5-HT1A BPND estimates (y-axis; TOP) and [11C]DASB 5-HTT BPND estimates (y-axis; MIDDLE) versus the log-transformed EMA stress measure (x-axes). Top and middle: Regression lines for both suicide attempters (pink) and nonattempters (blue), where attempter data-points are plotted with circles and nonattempter data-points are with triangles. Bottom: The six regions from our in-house atlas are shown on slices of a Montreal Neurological Institute (MNI) space structural MRI. For Regions with post-hoc p<0.05, metrics of effect size include: dorsal putamen: β = 0.44 and r = 0.72, thalamus: β = 0.30 and r=0.70, and midbrain: β = 0.31 and r = 0.61.
Effects were similar, but with different spatial localization of significant effects for the 5-HT1A receptor. There again was no effect of 5-HT1A BPND in attempt status group comparisons (attempt status by region: F=1.04, df=6, 64.31, p=0.41). For EMA stress, there was a significant main effect of EMA stress in attempters only (F=12.24, df=1, 10, p=0.006; also survives Bonferroni correction), with a positive correlation between 5-HT1A BPND and EMA stress across regions in attempters (β=0.26, 95% confidence interval (CI): [0.09, 0.43]).
Further, similarly to the NRU 5-HT atlas analysis, there was a region-specific association between 5-HT1A BPND and EMA stress only in attempters (F=4.40, df=6, 28.18, p=0.003; also survives Bonferroni correction; Figure 4), with 5-HT1A BPND correlating positively with EMA stress post-hoc in the dorsal putamen (β=0.44, 95% confidence interval (CI): [0.25, 0.64], p<0.001), thalamus (β=0.30, 95% CI: [0.10, 0.49], p=0.005), and midbrain (β=0.31, 95% CI: [0.11, 0.50], p=0.004; Figure 4), with the effects in all three regions surviving Bonferroni correction.
DISCUSSION
Given that neither the severity of reported stress (current and childhood) nor depression severity differed between attempters and nonattempters, the importance of stress to suicide attempts could be related to interactions between stress and brain biology. MDD suicide attempters had similar brain 5-HT1A binding, but lower 5-HTT binding specifically in portions of cuneus, pericalcarine, lingual, and lateral occipital cortices (NRU 5-HT atlas region 5). Solely in the attempters, 5-HT1A binding potentials were positively correlated with EMA stress. The positive correlation of EMA stress with 5-HT1A binding was widespread, involving nine of ten atlas regions and excluded the NRU 5-HT atlas region with significantly lower 5-HTT binding in attempters. Conversely, in currently depressed individuals without an attempt, no correlations were detected. Results using a priori Brodmann Area-based ROIs were generally weaker, except for localized positive correlations of stress with 5-HT1A binding in attempters in subcortical regions (dorsal putamen, midbrain, and thalamus) that do not overlap with the cortical NRU 5-HT atlas.
The directionality of our findings (higher 5-HT1A significantly correlating with more reported daily life stressors and significantly lower 5-HTT in attempters and lower 5-HTT tending to be correlated with more reported daily life stressors) agrees with prior work in MDD and suicidal behavior (Arango et al., 2003; Arango et al., 1995; Boldrini et al., 2008; Cannon et al., 2006; Hesselgrave and Parsey, 2013; Joensuu et al., 2007; Kaufman et al., 2015; Lehto et al., 2006; Malison et al., 1998; Mann et al., 2000; Miller et al., 2013; Miller et al., 2008; Nye et al., 2013; Oquendo et al., 2016; Parsey et al., 2006a; Parsey et al., 2010; Parsey et al., 2006b; Reimold et al., 2008; Selvaraj et al., 2011; Staley et al., 2006; Stockmeier, 2003; Sullivan et al., 2015; Underwood et al., 2018; Willeit et al., 2000). It is worth nothing that a meta-analysis by Moncrieff et al. concluded that the serotonin system is not abnormal in major depression (Moncrieff et al., 2022). Many of the measures assessed in that paper are from peripheral blood, while our study looked at the brain. Many brain studies of serotonin indices in depression, using a variety of methods, were not included in Moncrief et al. given the requirement for a minimum number of studies using the same method for meta-analysis. Because the effect found here were restricted to depressed attempters, and reported stress severity was comparable between attempters and nonattempters, this serotonergic phenotype (low 5-HTT, high 5-HT1A) may link heightened reactivity to daily life events with suicidal behavior. This model is also consistent with postmortem studies, where lower 5-HTT, higher 5-HT1A binding, and a relationship between recent stressors and a history of childhood adversity are found in depressed suicide decedents (Underwood et al., 2018).
The spatial localization of lower 5-HTT binding in attempters and a trend toward lower 5-HTT with greater EMA stress to occipital cortex areas differs from the anatomically widespread 5-HT1A binding findings. Prior work has found a relatively diffuse pattern of 5-HTT deficiency in major depression (Gryglewski et al., 2014; Kambeitz and Howes, 2015), with more localized effects in suicidal behavior (prefrontal and anterior cingulate cortices, putamen, brainstem, midbrain) (Mann et al., 2000; Miller et al., 2013; Nye et al., 2013; Underwood et al., 2018). However, in those with both the lower expressing, short-allele 5-HTT polymorphism (Lesch et al., 1996) and a history of stressful life events, heightened functional MRI activity in the occipital cortex has been shown in response to fear conditioning (Klucken et al., 2013). Taken together, this might suggest a model where pre-existing lower 5-HTT binding confers sensitivity to stressors that contributes to suicide risk.
The nine NRU 5-HT atlas regions in which 5-HT1A BPND positively correlated with EMA stress showed almost no overlap with the three Brodmann Area-based ROIs that correlated with EMA stress (Figure 3). Considering both types of regions, therefore, indicates an even more widespread pattern of link between 5-HT1A and stress cortically and subcortically than either atlas type alone. Notably, NRU 5-HT atlas region 1, covering the frontal cortex, has significant 5-HT1A binding correlations with stress, but the Brodmann Area-based anterior cingulate cortex did not show 5-HT1A binding correlations. Given postmortem and in vivo findings of elevated prefrontal and anterior cingulate 5-HT1A binding in suicide decedents, and in nonfatal suicide attempters, including correlations with suicide lethality (Oquendo et al., 2016; Sullivan et al., 2015; Underwood et al., 2018), the NRU 5-HT atlas may afford enhanced power to detect effects here over Brodmann Areas, or effects may be localized to areas of the frontal cortex not encompassing the anterior cingulate. The Brodmann-Area atlas findings should be interpreted with caution, given that the three ROIs correlating with EMA stress – midbrain, thalamus, and dorsal putamen – had notably lower 5-HT1A BPND than the non-significant regions, in agreement with previous reports in thalamus and dorsal putamen (Ito et al., 1999). While the raphe nuclei are rich with 5-HT1A, other areas of the midbrain are largely devoid of 5-HT1A receptors (Ito et al., 1999) and, therefore, the regional BPND average across the midbrain reflects low binding. It may be that the low signal decreased statistical power and inflated the Type I error rate (false-positives).
While all but one region in the NRU 5-HT atlas had significant positive relationships between EMA stress and 5-HT1A BPND in suicide attempters, the strongest effect was in region 7 (β =0.45, with CIs that excluded zero, p<0.001), which includes portions of isthmus cingulate, parahippocampal, and entorhinal cortices. These areas are associated with learning, memory and decision-making, and the diathesis for suicidal behavior (Mann and Rizk, 2020). In rodents with a chronic stress-induced depression phenotype, altered metabolic pathways were found in the entorhinal cortex (Chen et al., 2020) and stimulation of entorhinal cortex neurons yielded antidepressant-like effects (Yun et al., 2018). The isthmus cingulate, parahippocampal gyrus, and entorhinal cortex are all termini of the posterior cingulum (Weis et al., 2018), the posterior portion of the prominent axonal tract connecting subgenual cingulate to the temporal lobe, including hippocampus (Mega et al., 1997). Relationships between posterior cingulum white matter integrity and posttraumatic stress disorder have been found (Averill et al., 2018; Weis et al., 2018), further implicating this area in stress response.
The single region where 5-HTT binding was related to attempter status and tended to be related to stress, was the single region without an effect of 5-HT1A binding by stress. Perhaps 5-HTT abnormality dominates in this region without much 5-HT1A influence. Alternatively, since this region has the lowest 5-HT1A binding potential of the atlas regions (Beliveau et al., 2017; Beliveau et al., 2020), perhaps this limits statistical power to detect an effect in this location.
Strengths of this study include same-day PET imaging with multiple molecular probes and EMA in medication-free participants with MDD. Another strength is the consideration of both Brodmann-based and serotonin-based atlases for a comprehensive picture of stress interactions with 5-HTT and 5-HT1A systems. One limitation is the sample size. Although 25 participants is not a modest size for a sample being scanned with two PET tracers on the same day and with additional EMA, splitting the sample into attempter (n=13) and non-attempter (n=12) groups, yielded small sample sizes that may result in inflated false discovery rates and less reliable effect size estimation. In fact, Gryglewski et al. 2014 suggests a minimum group size of 64 for sufficient power to detect MDD vs. healthy volunteer differences from meta-analysis of molecular imaging studies of 5-HTT (Gryglewski et al., 2014) and therefore, even if the contrast of interest here is different than the one in Gryglewski et al, these findings should be interpreted with caution and treated as preliminary evidence that require follow-up in larger samples. The sample size further limited our ability to fit three-way interactions (e.g., attempter status by EMA stress by region), which could tell us if the regional EMA stress × PET relationships differed by attempter status. Because the range of EMA stress was greater in nonattempters than attempters, we repeated the nonattempter analyses excluding the participant with the lowest EMA stress of 7.1% of prompts, a potential outlier, and the non-significant findings in nonattempters were replicated.
Another limitation is that because neuroimaging and EMA were acquired after suicide attempt(s), we cannot determine if the relationships to stress were risk factors for or the result of suicide attempt(s). We hypothesize that serotonin system dysfunction may indicate a biological susceptibility to stressors, a risk factor for suicidal behavior. Mouse models are needed to determine causality, because it is possible that local levels of intrasynaptic serotonin release, which regulate 5-HTT levels through internalization and recycling, are controlled by 5-HT1A autoreceptors regulating serotonin neuron firing and release in midbrain serotonergic cell bodies (Bunin et al., 1998; Bunin and Wightman, 1998; Kittler et al., 2010; Lau et al., 2008; Montañez et al., 2003; Sotelo et al., 1990). A limitation, however, is the lack of mouse models of suicidal behavior. A large-sample, longitudinal study should test a model whereby serotonergic function mediates the risk relationship of current stress to the triggering of suicidal behavior in MDD. Such a study could also examine genetic and epigenetic effects on serotonergic function.
Individuals with active depression may or may not manifest suicidal behavior in the face of comparable stress because of differences in brain serotonergic function. 5-HT1A receptor binding potentials correlated with current stress and 5-HTT was lower in attempters than nonattempters, and these relationships involve different brain regions. Larger, longitudinal studies are needed to clarify these effects and to further understand the interplay of daily stressors and serotonin in suicide risk and resilience. One approach is to study whether treatment with medications preferentially reduces the impact of current life stressors on suicide risk in MDD with different serotonergic phenotypes.
Supplementary Material
Acknowledgement
This study was funded by the National Institute of Mental Health awards: Conte Translational Neuroscience Center P50MH090964: J John Mann, MD; and R01MH109326: Barbara Stanley, PhD and Maria Oquendo, MD, PhD. Funding was also provided in part by AFSP SRG-0-102-16: Spiro P Pantazatos, PhD. The authors acknowledge the Columbia University PET Center and all members of the Brain Imaging Lab at the Molecular Imaging and Neuropathology Area at the New York State Psychiatric Institute who contributed to this work.
Funding
This study was funded by the National Institute of Mental Health awards: Conte Translational Neuroscience Center P50MH090964: J John Mann, MD; and R01MH109326: Barbara Stanley, PhD and Maria Oquendo, MD, PhD. Funding was also provided in part by AFSP SRG-0-102-16: Spiro P Pantazatos, PhD. These funding sources had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Interest
Drs. Bartlett, Zanderigo, Stanley, Choo, Galfalvy, Pantazatos, Sublette, and Miller declares that they have no conflict of interest. Drs. Oquendo and Mann receive royalties from the Research Foundation for Mental Hygiene for the commercial use of the Columbia Suicide Severity Rating Scale. Dr. Oquendo serves as an advisor to Alkermes, Otsuka, Mind Medicine, St. George’s University and Fundacion Jimenez Diaz. Her family owns stock in Bristol Myers Squibb.
References
- Aguilera M, Arias B, Wichers M, Barrantes-Vidal N, Moya J, Villa H, Van Os J, Ibáñez MI, Ruipérez MA, Ortet G, 2009. Early adversity and 5-HTT/BDNF genes: new evidence of gene–environment interactions on depressive symptoms in a general population. Psychological medicine 39, 1425–1432. [DOI] [PubMed] [Google Scholar]
- Arango V, Huang Y. y., Underwood MD, Mann JJ, 2003. Genetics of the serotonergic system in suicidal behavior. Journal of psychiatric research 37, 375–386. [DOI] [PubMed] [Google Scholar]
- Arango V, Underwood MD, Gubbi AV, Mann JJ, 1995. Localized alterations in pre-and postsynaptic serotonin binding sites in the ventrolateral prefrontal cortex of suicide victims. Brain research 688, 121–133. [DOI] [PubMed] [Google Scholar]
- Åsberg M, Träskman L, Thorén P, 1976. 5-HIAA in the cerebrospinal fluid: A biochemical suicide predictor? Archives of general psychiatry 33, 1193–1197. [DOI] [PubMed] [Google Scholar]
- Averill CL, Averill LA, Wrocklage KM, Scott JC, Akiki TJ, Schweinsburg B, Southwick SM, Krystal JH, Abdallah CG, 2018. Altered white matter diffusivity of the cingulum angular bundle in posttraumatic stress disorder. Molecular neuropsychiatry 4, 75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Mächler M, Bolker B, Walker S, 2014. Fitting linear mixed-effects models using lme4. arXiv preprint arXiv:1406.5823. [Google Scholar]
- Beck AT, Kovacs M, Weissman A, 1979. Assessment of suicidal intention: the Scale for Suicide Ideation. Journal of consulting and clinical psychology 47, 343. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, 1988. Manual for the Beck hopelessness scale. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Beck AT, Steer RA, Brown GK, 1996. Beck depression inventory (BDI-II). Pearson. [Google Scholar]
- Beliveau V, Ganz M, Feng L, Ozenne B, Højgaard L, Fisher PM, Svarer C, Greve DN, Knudsen GM, 2017. A high-resolution in vivo atlas of the human brain’s serotonin system. Journal of Neuroscience 37, 120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beliveau V, Ozenne B, Strother S, Greve DN, Svarer C, Knudsen GM, Ganz M, 2020. The structure of the serotonin system: A PET imaging study. Neuroimage 205, 116240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, Stokes J, Handelsman L, Medrano M, Desmond D, 2003. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child abuse & neglect 27, 169–190. [DOI] [PubMed] [Google Scholar]
- Boldrini M, Underwood MD, Mann JJ, Arango V, 2008. Serotonin-1A autoreceptor binding in the dorsal raphe nucleus of depressed suicides. Journal of psychiatric research 42, 433–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky BS, Mann JJ, Stanley B, Tin A, Oquendo M, Birmaher B, Greenhill L, Kolko D, Zelazny J, Burke AK, 2008. Familial transmission of suicidal behavior: factors mediating the relationship between childhood abuse and offspring suicide attempts. Journal of Clinical Psychiatry 69, 584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunin MA, Prioleau C, Mailman R, Wightman RM, 1998. Release and uptake rates of 5-hydroxytryptamine in the dorsal raphe and substantia nigra reticulata of the rat brain. Journal of neurochemistry 70, 1077–1087. [DOI] [PubMed] [Google Scholar]
- Bunin MA, Wightman RM, 1998. Quantitative evaluation of 5-hydroxytryptamine (serotonin) neuronal release and uptake: an investigation of extrasynaptic transmission. Journal of Neuroscience 18, 4854–4860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon DM, Ichise M, Fromm SJ, Nugent AC, Rollis D, Gandhi SK, Klaver JM, Charney DS, Manji HK, Drevets WC, 2006. Serotonin transporter binding in bipolar disorder assessed using [11C] DASB and positron emission tomography. Biological psychiatry 60, 207–217. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, 2003. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 301, 386–389. [DOI] [PubMed] [Google Scholar]
- Chaudhury SR, Galfalvy H, Biggs E, Choo T-H, Mann JJ, Stanley B, 2017. Affect in response to stressors and coping strategies: an ecological momentary assessment study of borderline personality disorder. Borderline Personality Disorder and Emotion Dysregulation 4, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Lan T, Wang Y, He Y, Wu Z, Tian Y, Li Y, Bai M, Zhou W, Zhang H, 2020. Entorhinal cortex-based metabolic profiling of chronic restraint stress mice model of depression. Aging (Albany NY) 12, 3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLorenzo C, Klein A, Mikhno A, Gray N, Zanderigo F, Mann JJ, Parsey RV, 2009. A new method for assessing PET-MRI coregistration. Proceedings of SPIE - Medical Imaging 7529. [Google Scholar]
- Diamantopoulou A, Kalpachidou T, Aspiotis G, Gampierakis I, Stylianopoulou F, Stamatakis A, 2018. An early experience of mild adversity involving temporary denial of maternal contact affects the serotonergic system of adult male rats and leads to a depressive-like phenotype and inability to adapt to a chronic social stress. Physiology & behavior 184, 46–54. [DOI] [PubMed] [Google Scholar]
- Dong M, Zeng L-N, Lu L, Li X-H, Ungvari GS, Ng CH, Chow IH, Zhang L, Zhou Y, Xiang Y-T, 2019. Prevalence of suicide attempt in individuals with major depressive disorder: a meta-analysis of observational surveys. Psychological medicine 49, 1691–1704. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JB, Benjamin LS, 1997. SCID-II Personality Questionnaire. American Psychiatric Press, Washington, D.C. [Google Scholar]
- Friedrich MJ, 2017. Depression is the leading cause of disability around the world. Jama 317, 1517–1517. [DOI] [PubMed] [Google Scholar]
- Gratch I, Choo TH, Galfalvy H, Keilp JG, Itzhaky L, Mann JJ, Oquendo MA, Stanley B, 2021. Detecting suicidal thoughts: The power of ecological momentary assessment. Depression and anxiety 38, 8–16. [DOI] [PubMed] [Google Scholar]
- Gryglewski G, Lanzenberger R, Kranz GS, Cumming P, 2014. Meta-analysis of molecular imaging of serotonin transporters in major depression. Journal of Cerebral Blood Flow & Metabolism 34, 1096–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M, 1960. A rating scale for depression. J Neurol Neurosurg Psych 23, 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselgrave N, Parsey RV, 2013. Imaging the serotonin 1A receptor using [11C] WAY100635 in healthy controls and major depression. Philosophical Transactions of the Royal Society B: Biological Sciences 368, 20120004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimiya T, Suhara T, Sudo Y, Okubo Y, Nakayama K, Nankai M, Inoue M, Yasuno F, Takano A, Maeda J, 2002. Serotonin transporter binding in patients with mood disorders: a PET study with [11C](+) McN5652. Biological Psychiatry 51, 715–722. [DOI] [PubMed] [Google Scholar]
- Ito H, Halldin C, Farde L, 1999. Localization of 5-HT1A receptors in the living human brain using [carbonyl-11C] WAY-100635: PET with anatomic standardization technique. Journal of Nuclear Medicine 40, 102–109. [PubMed] [Google Scholar]
- Joensuu M, Tolmunen T, Saarinen PI, Tiihonen J, Kuikka J, Ahola P, Vanninen R, Lehtonen J, 2007. Reduced midbrain serotonin transporter availability in drug-naive patients with depression measured by SERT-specific [123I] nor-β-CIT SPECT imaging. Psychiatry Research: Neuroimaging 154, 125–131. [DOI] [PubMed] [Google Scholar]
- Kambeitz JP, Howes OD, 2015. The serotonin transporter in depression: Meta-analysis of in vivo and post mortem findings and implications for understanding and treating depression. Journal of affective disorders 186, 358–366. [DOI] [PubMed] [Google Scholar]
- Karg K, Burmeister M, Shedden K, Sen S, 2011. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: evidence of genetic moderation. Archives of general psychiatry 68, 444–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Sullivan GM, Yang J, Ogden RT, Miller JM, Oquendo MA, Mann JJ, Parsey RV, DeLorenzo C, 2015. Quantification of the serotonin 1A receptor using PET: identification of a potential biomarker of major depression in males. Neuropsychopharmacology 40, 1692–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler K, Lau T, Schloss P, 2010. Antagonists and substrates differentially regulate serotonin transporter cell surface expression in serotonergic neurons. European journal of pharmacology 629, 63–67. [DOI] [PubMed] [Google Scholar]
- Klucken T, Alexander N, Schweckendiek J, Merz CJ, Kagerer S, Osinsky R, Walter B, Vaitl D, Hennig J, Stark R, 2013. Individual differences in neural correlates of fear conditioning as a function of 5-HTTLPR and stressful life events. Social cognitive and affective neuroscience 8, 318–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau T, Horschitz S, Berger S, Bartsch D, Schloss P, 2008. Antidepressant-induced internalization of the serotonin transporter in serotonergic neurons. The FASEB Journal 22, 1702–1714. [DOI] [PubMed] [Google Scholar]
- Law AJ, Pei Q, Walker M, Gordon-Andrews H, Weickert CS, Feldon J, Pryce CR, Harrison PJ, 2009. Early parental deprivation in the marmoset monkey produces long-term changes in hippocampal expression of genes involved in synaptic plasticity and implicated in mood disorder. Neuropsychopharmacology 34, 1381–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehto S, Tolmunen T, Joensuu M, Saarinen PI, Vanninen R, Ahola P, Tiihonen J, Kuikka J, Lehtonen J, 2006. Midbrain binding of [123I] nor-β-CIT in atypical depression. Progress in Neuro-Psychopharmacology and Biological Psychiatry 30, 1251–1255. [DOI] [PubMed] [Google Scholar]
- Lesch K-P, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Müller CR, Hamer DH, Murphy DL, 1996. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 274, 1527–1531. [DOI] [PubMed] [Google Scholar]
- Leventopoulos M, Russig H, Feldon J, Pryce CR, Opacka-Juffry J, 2009. Early deprivation leads to long-term reductions in motivation for reward and 5-HT1A binding and both effects are reversed by fluoxetine. Neuropharmacology 56, 692–701. [DOI] [PubMed] [Google Scholar]
- Loranger AW, 1984. Sex difference in age at onset of schizophrenia. Arch Gen Psychiatry 41, 157–161. [DOI] [PubMed] [Google Scholar]
- Malison RT, Price LH, Berman R, Van Dyck CH, Pelton GH, Carpenter L, Sanacora G, Owens MJ, Nemeroff CB, Rajeevan N, 1998. Reduced brain serotonin transporter availability in major depression as measured by [123I]-2β-carbomethoxy-3β-(4-iodophenyl) tropane and single photon emission computed tomography. Biological psychiatry 44, 1090–1098. [DOI] [PubMed] [Google Scholar]
- Mann J, 1998. The neurobiology of suicide. Nature medicine 4, 25–30. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Currier D, Stanley B, Oquendo MA, Amsel LV, Ellis SP, 2006. Can biological tests assist prediction of suicide in mood disorders? International Journal of Neuropsychopharmacology 9, 465–474. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Huang Y. y., Underwood MD, Kassir SA, Oppenheim S, Kelly TM, Dwork AJ, Arango V, 2000. A serotonin transporter gene promoter polymorphism (5-HTTLPR) and prefrontal cortical binding in major depression and suicide. Archives of general psychiatry 57, 729–738. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Rizk MM, 2020. A brain-centric model of suicidal behavior. American journal of psychiatry 177, 902–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki H, Izumi T, Horinouchi T, Boku S, Inoue T, Yamaguchi T, Yoshida T, Matsumoto M, Togashi H, Miwa S, 2011. Juvenile stress attenuates the dorsal hippocampal postsynaptic 5-HT 1A receptor function in adult rats. Psychopharmacology 214, 329–337. [DOI] [PubMed] [Google Scholar]
- Mega MS, Cummings JL, Salloway S, Malloy P, 1997. The limbic system: an anatomic, phylogenetic, and clinical perspective. The Journal of Neuropsychiatry and Clinical Neurosciences. [DOI] [PubMed] [Google Scholar]
- Meyer JH, Houle S, Sagrati S, Carella A, Hussey DF, Ginovart N, Goulding V, Kennedy J, Wilson AA, 2004. Brain serotonin transporter binding potential measured with carbon11–labeled dasb positron emission tomography: Effects of major depressive episodes and severity of dysfunctionalattitudes. Archives of general psychiatry 61, 1271–1279. [DOI] [PubMed] [Google Scholar]
- Meyer JH, Wilson AA, Ginovart N, Goulding V, Hussey D, Hood K, Houle S, 2001. Occupancy of serotonin transporters by paroxetine and citalopram during treatment of depression: a [11C] DASB PET imaging study. American Journal of Psychiatry 158, 1843–1849. [DOI] [PubMed] [Google Scholar]
- Milak MS, DeLorenzo C, Zanderigo F, Prabhakaran J, Kumar JD, Majo VJ, Mann JJ, Parsey RV, 2010. In vivo quantification of human serotonin 1A receptor using 11C-CUMI-101, an agonist PET radiotracer. Journal of Nuclear Medicine 51, 1892–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JM, Everett BA, Oquendo MA, Ogden RT, Mann JJ, Parsey RV, 2016. Positron emission tomography quantification of serotonin transporter binding in medication-free bipolar disorder. Synapse 70, 24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JM, Hesselgrave N, Ogden RT, Sullivan GM, Oquendo MA, Mann JJ, Parsey RV, 2013. Positron emission tomography quantification of serotonin transporter in suicide attempters with major depressive disorder. Biological psychiatry 74, 287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JM, Kinnally EL, Ogden RT, Oquendo MA, Mann JJ, Parsey RV, 2009. Reported childhood abuse is associated with low serotonin transporter binding in vivo in major depressive disorder. Synapse 63, 565–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JM, Oquendo MA, Ogden RT, Mann JJ, Parsey RV, 2008. Serotonin transporter binding as a possible predictor of one-year remission in major depressive disorder. Journal of psychiatric research 42, 1137–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncrieff J, Cooper RE, Stockmann T, Amendola S, Hengartner MP, Horowitz MA, 2022. The serotonin theory of depression: a systematic umbrella review of the evidence. Molecular psychiatry, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montañez S, Daws LC, Gould GG, Frazer A, 2003. Serotonin (5-HT) transporter (SERT) function after graded destruction of serotonergic neurons. Journal of neurochemistry 87, 861–867. [DOI] [PubMed] [Google Scholar]
- Nock MK, Hwang I, Sampson NA, Kessler RC, 2010. Mental disorders, comorbidity and suicidal behavior: results from the National Comorbidity Survey Replication. Molecular psychiatry 15, 868–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nye JA, Purselle D, Plisson C, Voll RJ, Stehouwer JS, Votaw JR, Kilts CD, Goodman MM, Nemeroff CB, 2013. Decreased brainstem and putamen SERT binding potential in depressed suicide attempters using [11C]-ZIENT pet imaging. Depression and anxiety 30, 902–907. [DOI] [PubMed] [Google Scholar]
- Ogden RT, Ojha A, Erlandsson K, Oquendo MA, Mann JJ, Parsey RV, 2007. In vivo quantification of serotonin transporters using [(11)C]DASB and positron emission tomography in humans: modeling considerations. J Cereb Blood Flow Metab 27, 205–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta K. i., Miki T, Warita K, Suzuki S, Kusaka T, Yakura T, Liu J-Q, Tamai M, Takeuchi Y, 2014. Prolonged maternal separation disturbs the serotonergic system during early brain development. International Journal of Developmental Neuroscience 33, 15–21. [DOI] [PubMed] [Google Scholar]
- Oquendo MA, Galfalvy H, Sullivan GM, Miller JM, Milak MM, Sublette ME, Cisneros-Trujillo S, Burke AK, Parsey RV, Mann JJ, 2016. Positron emission tomographic imaging of the serotonergic system and prediction of risk and lethality of future suicidal behavior. JAMA psychiatry 73, 1048–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oquendo MA, Halberstam B, Mann JJ, 2003. Risk factors for suicidal behavior: the utility and limitations of research instruments, in: First MB (Ed.), Standardized Evaluation in Clinical Practice. American Psychiatric Publishing, Arlington, VA, pp. 103–130. [Google Scholar]
- Parsey RV, Hastings RS, Oquendo MA, Huang Y. y., Simpson N, Arcement J, Huang Y, Ogden RT, Van Heertum RL, Arango V, 2006a. Lower serotonin transporter binding potential in the human brain during major depressive episodes. American Journal of Psychiatry 163, 52–58. [DOI] [PubMed] [Google Scholar]
- Parsey RV, Ogden RT, Miller JM, Tin A, Hesselgrave N, Goldstein E, Mikhno A, Milak M, Zanderigo F, Sullivan GM, 2010. Higher serotonin 1A binding in a second major depression cohort: modeling and reference region considerations. Biological psychiatry 68, 170–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsey RV, Oquendo MA, Ogden RT, Olvet DM, Simpson N, Huang Y. y., Van Heertum RL, Arango V, Mann JJ, 2006b. Altered serotonin 1A binding in major depression: a [carbonyl-C-11] WAY100635 positron emission tomography study. Biological psychiatry 59, 106–113. [DOI] [PubMed] [Google Scholar]
- Reimold M, Batra A, Knobel A, Smolka M, Zimmer A, Mann K, Solbach C, Reischl G, Schwärzler F, Gründer G, 2008. Anxiety is associated with reduced central serotonin transporter availability in unmedicated patients with unipolar major depression: a [11 C] DASB PET study. Molecular psychiatry 13, 606–613. [DOI] [PubMed] [Google Scholar]
- Richardson-Jones JW, Craige CP, Guiard BP, Stephen A, Metzger KL, Kung HF, Gardier AM, Dranovsky A, David DJ, Beck SG, 2010. 5-HT1A autoreceptor levels determine vulnerability to stress and response to antidepressants. Neuron 65, 40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risch N, Herrell R, Lehner T, Liang K-Y, Eaves L, Hoh J, Griem A, Kovacs M, Ott J, Merikangas KR, 2009. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: a meta-analysis. Jama 301, 2462–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha TB-M, Hutz MH, Salatino-Oliveira A, Genro JP, Polanczyk GV, Sato JR, Wehrmeister FC, Barros FC, Menezes AM, Rohde LA, 2015. Gene-environment interaction in youth depression: replication of the 5-HTTLPR moderation in a diverse setting. American journal of psychiatry 172, 978–985. [DOI] [PubMed] [Google Scholar]
- Selvaraj S, Murthy NV, Bhagwagar Z, Bose SK, Hinz R, Grasby PM, Cowen PJ, 2011. Diminished brain 5-HT transporter binding in major depression: a positron emission tomography study with [11 C] DASB. Psychopharmacology 213, 555–562. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Stone AA, Hufford MR, 2008. Ecological momentary assessment. Annu. Rev. Clin. Psychol 4, 1–32. [DOI] [PubMed] [Google Scholar]
- Sotelo C, Cholley B, El Mestikawy S, Gozlan H, Hamon M, 1990. Direct immunohistochemical evidence of the existence of 5-HT1A autoreceptors on serotoninergic neurons in the midbrain raphe nuclei. European Journal of Neuroscience 2, 1144–1154. [DOI] [PubMed] [Google Scholar]
- Spinelli S, Chefer S, Carson RE, Jagoda E, Lang L, Heilig M, Barr CS, Suomi SJ, Higley JD, Stein EA, 2010. Effects of early-life stress on serotonin1A receptors in juvenile rhesus monkeys measured by positron emission tomography. Biological psychiatry 67, 1146–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley JK, Sanacora G, Tamagnan G, Maciejewski PK, Malison RT, Berman RM, Vythilingam M, Kugaya A, Baldwin RM, Seibyl JP, 2006. Sex differences in diencephalon serotonin transporter availability in major depression. Biological psychiatry 59, 40–47. [DOI] [PubMed] [Google Scholar]
- Stanley B, Martínez-Alés G, Gratch I, Rizk M, Galfalvy H, Choo T-H, Mann JJ, 2021. Coping strategies that reduce suicidal ideation: An ecological momentary assessment study. Journal of Psychiatric Research 133, 32–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg LJ, Rubin-Falcone H, Galfalvy HC, Kaufman J, Miller JM, Sublette ME, Cooper TB, Min E, Keilp JG, Stanley BH, 2019. Cortisol stress response and in vivo PET imaging of human brain serotonin 1A receptor binding. International Journal of Neuropsychopharmacology 22, 329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockmeier CA, 2003. Involvement of serotonin in depression: evidence from postmortem and imaging studies of serotonin receptors and the serotonin transporter. Journal of psychiatric research 37, 357–373. [DOI] [PubMed] [Google Scholar]
- Sullivan GM, Oquendo MA, Milak M, Miller JM, Burke A, Ogden RT, Parsey RV, Mann JJ, 2015. Positron emission tomography quantification of serotonin1A receptor binding in suicide attempters with major depressive disorder. JAMA psychiatry 72, 169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Team, R.C., 2013. R: A language and environment for statistical computing. Vienna, Austria. [Google Scholar]
- Underwood MD, Kassir SA, Bakalian MJ, Galfalvy H, Dwork AJ, Mann JJ, Arango V, 2018. Serotonin receptors and suicide, major depression, alcohol use disorder and reported early life adversity. Translational psychiatry 8, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heeringen K, Mann JJ, 2014. The neurobiology of suicide. The Lancet Psychiatry 1, 63–72. [DOI] [PubMed] [Google Scholar]
- Vázquez DM, Eskandari R, Zimmer CA, Levine S, López JF, 2002. Brain 5-HT receptor system in the stressed infant rat: implications for vulnerability to substance abuse. Psychoneuroendocrinology 27, 245–272. [DOI] [PubMed] [Google Scholar]
- Weis CN, Belleau EL, Pedersen WS, Miskovich TA, Larson CL, 2018. Structural connectivity of the posterior cingulum is related to reexperiencing symptoms in posttraumatic stress disorder. Chronic Stress 2, 2470547018807134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willeit M, Praschak-Rieder N, Neumeister A, Pirker W, Asenbaum S, Vitouch O, Tauscher J, Hilger E, Stastny J, Brücke T, 2000. [123I]-β-CIT SPECT imaging shows reduced brain serotonin transporter availability in drug-free depressed patients with seasonal affective disorder. Biological psychiatry 47, 482–489. [DOI] [PubMed] [Google Scholar]
- Yttredahl A, Boldrini M, Tyrer A, Bartlett E, Ananth M, Milak M, Oquendo MA, Mann JJ, Parsey R, DeLorenzo C, 2021. Higher serotonin 1A receptor hippocampal binding in major depression associated with reported childhood adversity. Under Review at JNM. [DOI] [PMC free article] [PubMed]
- Yun S, Reynolds RP, Petrof I, White A, Rivera PD, Segev A, Gibson AD, Suarez M, DeSalle MJ, Ito N, 2018. Stimulation of entorhinal cortex–dentate gyrus circuitry is antidepressive. Nature medicine 24, 658–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanderigo F, Ogden RT, Parsey RV, 2013. Reference region approaches in PET: a comparative study on multiple radioligands. Journal of Cerebral Blood Flow & Metabolism 33, 888–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanderigo F, Pantazatos S, Rubin-Falcone H, Ogden RT, Chhetry BT, Sullivan G, Oquendo M, Miller JM, Mann JJ, 2018. In vivo relationship between serotonin 1A receptor binding and gray matter volume in the healthy brain and in major depressive disorder. Brain Structure and Function 223, 2609–2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


