Abstract
Background
The aim of this study is to evaluate the diagnostic efficiency of magnetic resonance imaging (MRI) of single parameters, unimodality, and bimodality in distinguishing glioblastoma (GBM) from atypical primary central nervous system lymphoma (PCNSL) based on diffusion-weighted imaging (DWI), dynamic susceptibility contrast (DSC) enhancement, diffusion tensor imaging (DTI), and proton magnetic resonance spectroscopy (1H-MRS) findings.
Methods
The cohort included 108 patients pathologically diagnosed with GBM and 54 patients pathologically diagnosed with PCNSL. Pretreatment morphological MRI, DWI, DSC, DTI and MRS were all performed on each patient. The quantitative parameters of multimodal MRI were measured and compared between the patients in the GBM and atypical PCNSL groups, and those parameters showing a significant difference (p < 0.05) between patients in the GBM and atypical PCNSL groups were used to develop one-parameters, unimodality, and bimodality models. We evaluated the efficiency of different models in distinguishing GBM from atypical PCNSL by performing receiver operating characteristic analysis (ROC).
Results
Atypical PCNSL had lower minimum apparent diffusion coefficient (ADCmin), mean ADC (ADCmean), relative ADC (rADC), mean relative cerebral blood volume (rCBVmean), maximum rCBV (rCBVmax), fractional anisotropy (FA), axial diffusion coefficient (DA) and radial diffusion coefficient (DR) values and higher choline/creatine (Cho/Cr) and lipid/creatine (Lip/Cr) ratios than GBM (all p < 0.05). The rCBVmax, DTI and DSC + DTI data were optimal models of single-parameter, unimodality and bimodality for differentiation of GBM from atypical PCNSL, yielding areas under the curves (AUCs) of 0.905, 0.954, and 0.992, respectively.
Conclusions
Models of single-parameter, unimodality and bimodality based on muti multiparameter functional MRI may help to discriminate GBM from atypical PCNSL.
Keywords: Glioblastoma, Atypical primary central nervous system lymphomas, Multiparametric MRI, Advanced MR Imaging, Diffusion tensor imaging
1. Introduction
The two most common primary brain tumors are glioblastoma (GBM), which accounts for approximately half (49%) [1], and primary central nervous system lymphoma (PCNSL), which accounts for 2–7% [2]. Total resection followed by temozolomide chemotherapy and radiotherapy is the standard treatment for GBM. Stereotactic biopsy is usually performed on PCNSL patients, followed by high-dose chemotherapy based on methotrexate or whole-brain radiation therapy [3]. A differential diagnosis must be established before PCNSL and GBM treatment can begin.
Standard magnetic resonance imaging (MRI) sequences can be an effective tool for differential diagnosis because immunocompetent patients suffering from PCNSL usually present with single or multiple relatively homogeneously enhanced lesions on enhanced images, whereas typically GBM shows a ring or ring-like area of contrast enhancement with a central hypointensity of necrosis [4,5]. When PCNSL exhibits an atypical presentation, its radiological characteristics may be similar to those of GBM, making the conditions difficult to distinguish on morphological MRI [6,7]. Immunocompromised patients with PCNSL typically exhibit irregular rim-like enhancement because of tumor necrosis; this occurrence is frequently observed in patients suffering from acquired immunodeficiency syndrome (AIDS) [8]. These atypical imaging characteristics of PCNSL without AIDS can generally imitate GBM. Therefore, similar to AIDS patients who exhibit these imaging findings, the differential diagnosis between GBM and PCNSL in non-AIDS patients is challenging [9].
Several advanced MR techniques, namely, diffusion-weighted imaging (DWI), dynamic susceptibility contrast enhancement (DSC), diffusion tensor imaging (DTI), and proton magnetic resonance spectroscopy (1H-MRS), have been confirmed as effective tool in differentiating between GBM and PCNSL. Notably, DWI, a noncontrast-enhanced MR modality, reflects tissue diffusivity as b values increase [10]. DSC based on T2-star (T2*) can noninvasively measure microvascular angiogenesis [11]. Water movement in the direction of the brain can be assessed using DTI to provide additional information on the microstructure of the brain [12]. MRS provides a noninvasive reflection of metabolic changes in vivo, including choline (Cho), creatine (Cr), N-acetylaspartate (NAA), lactate (Lac), and lipids (Lip), by comparing the ratio of different metabolites [13]. Some quantitative parameters derived from the aforementioned functional imaging techniques, such as the apparent diffusion coefficient (ADC) [10], fractional anisotropy (FA) [14], relative cerebral blood volume (rCBV) [4], and Cho/Cr [15], can be used for the differentiation of PCNSL from GBM. However, whether these quantitative parameters can distinguish between GBM and atypical PCNSL and whether the combination of different functional MRI techniques can improve the accuracy of differential diagnosis require further confirmation.
Thus, the principal purpose of our work was to comprehensively observe the utility of different single-parameter, unimodality and bimodality models in differentiating patients with GBM from patients with atypical PCNSL by using DWI, DSC, DTI and MRS findings as well as to compare the diagnostic efficiency of the different models.
2. Materials and methods
2.1. Study participants
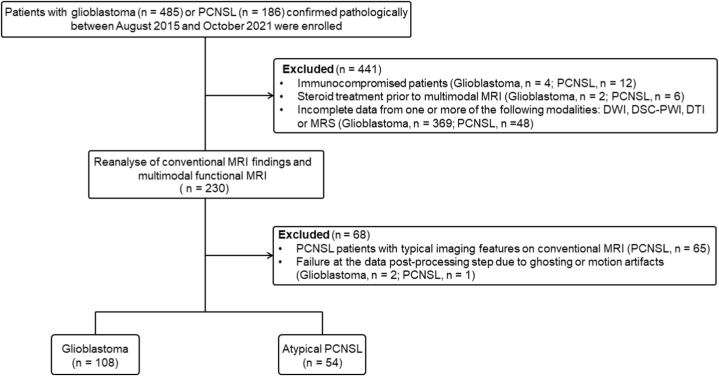
This retrospective study (KY-2023-051) was approved by the Institutional Review Board (IRB) of the First Affiliated Hospital of Jinan University and formal written consent was acquired from all subjects on the basis of the Declaration of Helsinki. Six hundred and seventy-one consecutive subjects identified in our institutional database with pathologically diagnosed GBM (n = 485) or PCNSL (n = 186) were enrolled from August 2015 to December 2021. Several reasons led to the exclusion of 509 subjects from this study. The following criteria were used for inclusion: (i) PCNSL or GBM was diagnosed pathologically in patients; (ii) the subjects were immunocompetent without iatrogenic immunosuppression, AIDS or other iatrogenic or disease-related immunodeficiencies; (iii) the patients had not received any treatment, including corticosteroid administration and invasive operation prior to the acquisition of multimodal MRI; (iv) DWI, DSC, DTI and MRS were performed in addition to conventional MRI; (v) the patients were pathologically confirmed to have PCNSL after official radiological reports had shown two different diagnoses, including GBM and PCNSL due to atypical findings on conventional morphological MRI [7]; and (vi) the quality of the multimodal images met the criteria for successful postprocessing (no ghosting or motion artifacts). Finally, 162 subjects (GBM = 108 and PCNSL = 54) met the inclusion criteria and were enrolled. The subject enrollment process is summarized in Fig. 1.
Fig. 1.
Flowchart of the study population. PCNSL, primary central nervous system lymphoma; MRI, magnetic resonance imaging; DWI, diffusion-weighted imaging; DSC, dynamic susceptibility contrast enhancement-perfusion weighted imaging; DTI, diffusion tensor imaging; MRS, magnetic resonance spectroscopy.
2.2. Imaging acquisition
A 3.0 T MRI system with eight-channel coils (Magnetom, Verio, Siemens Medical Solutions, Erlangen, Germany) MRI data were acquired. Our institution's dedicated MR protocol for subjects with brain tumors included the following: conventional morphological MRI (T1-weighted, T2-weighted, fluid-attenuated inversion recovery images and three-dimensional enhanced magnetization-prepared rapid gradient echo) as well as functional imaging (DWI, DSC, DTI, and MRS). Further details regarding the parameters of the MR protocol are provided in Appendix 1 (Supplementary Material).
2.3. Image postprocessing and analysis
Data postprocessing was implemented by two radiology specialists blinded to the histopathological data who had more than 10 years of background in neuroradiology. To improve visualization, correlation and accuracy, coregistration between 3D MPRAGE and other MRI modalities, such as ADC, rCBV, or FA maps, was performed. The regions of interest (ROIs) delineated on the functional map of three modalities (DWI, DTI and DSC) exhibited consistent characteristics, including size, form, and slices. Fig. 2, Fig. 3 present example images from this study. The detailed procedure for image postprocessing and analysis of DWI, DTI, DSC and MRS is provided in Appendix 2 (Supplementary Material).
Fig. 2.
Images acquired from a 50-year-old female pathologically diagnosed with GBM. (a and b) Axial and sagittal contrast-enhanced T1WI present relatively strong peripheral enhancement of the lesion in the right temporal lobe. (c) Axial T2WI demonstrating that a substantial area of the lesion has an equal/slightly increased signal and that the cystic and necrotic regions exhibit hyperintensity; hyperintense edema associated with the mass can be seen around the lesion. (d) Axial FLAIR showing that a substantial area of the lesion is slightly hyperintense and that the cystic and necrotic regions exhibit hypointensity; hyperintense edema associated with the mass can be seen around the lesion. (e) DWI demonstrating a hyperintense tumor mass of substantial size, central hypointense tumor necrosis, and isointense peritumoral edema. (f) ADC map with mild hypointensity of the lesion to measure ADCmean values of 0.938 × 10−3 mm2/s at substantial areas. (g) rCBV map showing hyperperfusion in a substantial area with an rCBVmean value of 6.06 at the region of the lesion; (h) FA map showing FA values of 0.22 at the area of the lesion; (i) color-coded map generated based on DTI data; (j) spectroscopy analysis revealed choline (Cho)/N-acetylaspartate (NAA), Cho/creatine (Cr), NAA/Cr, lipid (Lip)/Cr and lactate (Lac)/Cr ratios of 1.76, 1.48, 0.83, 3.12 and 1.28, respectively. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Fig. 3.
Images acquired from a 48-year-old female pathologically diagnosed with PCNSL. (a and b) Axial and coronal contrast-enhanced T1WI presents a relatively strong enhanced lesion in the right basal ganglia. (c) Axial T2WI shows that a substantial area of the lesion has an equal/slightly increased signal but no obvious cystic or necrotic regions; hyperintense edema associated with the mass can be seen around the lesion. (d) Axial FLAIR demonstrates that a substantial area of the lesion presents as slightly hyperintense, and hyperintense edema associated with the mass can be seen. (e) DWI shows a hyperintense tumor mass in a substantial region as well as isointense peritumoral edema. (f) ADC map with mild hypointensity/hypointensity of the lesion and the ADCmean values of a substantial region of 0.632 × 10−3 mm2/s (g) rCBV map showing scattered areas with substantial hypoperfusion and an rCBVmean value of 2.94; (h) FA map showing FA values of 0.14 at the region of the lesion; (i) color-coded map to be generated based on DTI data; (j) spectroscopy analysis revealed ratios of choline (Cho)/N-acetylaspartate (NAA), Cho/creatine (Cr), NAA/Cr, lipids (Lip)/Cr and lactate (Lac)/Cr of 2.14, 1.60, 0.74, 5.44 and 1.39, respectively. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
2.4. Statistical analysis
GraphPad Prism (version 9.5.1.733, La Jolla, California, USA) and SPSS (version 20.0, IBM Corp, Armonk, NY) were used to perform the statistical analysis. The means ± the standard deviation (SD) were used to describe results. The normal distributions of continuous variables were evaluated using the Kolmogorov–Smirnov test. Two-group t tests or Mann–Whitney U tests were conducted to compare between GBM and atypical PCNSL groups based on a normal distribution of the data. P < α = 0.05 indicated statistical significance, and box scatter plots were generated. Then, binary logistic regression in variables with P< α = 0.05 was used to determine whether certain combined models (unimodal or bimodal) would improve the diagnostic ability of GBM and atypical PCNSL. Based on the selected parameters and the outputs of the logistic regression model, models for single-parameter, unimodality and bimodality were constructed to differentiate GBM from atypical PCNSL, and receiver operating characteristic (ROC) curves were drawn to express the performance of different models. The differences in the area under the curve (AUC) values between different models of single-parameters, unimodalities and bimodalities for discriminating GBM and PCNSL were compared using the DeLong test [16].
3. Results
3.1. Clinical characteristics of patients in the GBM and atypical PCNSL groups
Statistical analysis of clinical characteristics showed that tumor size significantly differed between patients in the GBM and atypical PCNSL groups (p < 0.05), but no statistically significant were identified in age, sex, and tumor location between the two groups (all p > 0.05). The baseline data of subjects in the GBM and atypical PCNSL groups are presented in Table 1.
Table 1.
The baseline features for subjects in the GBM and atypical PCNSL groups.
| GBM group (n = 108) | Atypical PCNSL group (n = 54) | P | |
|---|---|---|---|
| Age (median, years) | 59 | 61 | 0.102 |
| Sex (n, %) | 0.727 | ||
| Male | 39 (36.1%) | 18 (33.3%) | |
| Female | 69 (63.9%) | 36 (66.7%) | |
| Tumor size (mean ± SD, cm) | 5.17 ± 0.79 | 4.41 ± 0.81 | < 0.001 |
| Tumor location (n, %) | 0.261 | ||
| Frontal | 35 (32.4%) | 17 (31.5%) | |
| Parietal | 22 (20.4%) | 9 (16.7%) | |
| Temporal | 28 (25.9%) | 10 (18.5) | |
| Occipital | 8 (7.4%) | 3 (5.6%) | |
| Basal ganglia | 11 (10.2%) | 8 (14.8) | |
| Corpus callosum | 4 (3.7) | 7 (13.0%) | |
Abbreviations: GBM, glioblastoma; atypical PCNSL, atypical primary central nervous system lymphoma.
3.2. Comparison of multimodal MRI parameters between the GBM and atypical PCNSL groups
Comparing multimodal MRI parameters of patients between GBM and atypical PCNSL groups revealed that minimum ADC (ADCmin), mean ADC (ADCmean) and relative ADC (rADC) values of patients with atypical PCNSL were significantly lower than those of patients with GBM (all p < 0.05). However, there was no significant difference in maximum ADC (ADCmax) values between the GBM and atypical PCNSL groups (p > 0.05). The mean rCBV (rCBVmean) and maximum rCBV (rCBVmax) values were significantly higher in patients diagnosed with GBM than in patients diagnosed with atypical PCNSL (all p < 0.05), but there was no significant difference in the minimum rCBV (rCBVmin) between the two groups (p > 0.05). The FA, axial diffusion coefficient (DA) and radial diffusion coefficient (DR) values were all significantly lower among patients diagnosed with atypical PCNSL than among patients diagnosed with GBM (all p < 0.05). The ratios of Cho/Cr and Lip/Cr were significantly lower in subjects diagnosed with GBM than in those diagnosed with atypical PCNSL (all p < 0.05), while there was no significant difference in Cho/NAA, NAA/Cr or Lac/Cr between the two groups (all p > 0.05). The detailed results for multimodal MRI parameters between the two groups are presented in Table 2 and Fig. 4 (a - j).
Table 2.
Differences in the MRI parameters between patients in the GBM and atypical PCNSL groups (mean ± SD).
| Parameters | GBM group (n = 108) | Atypical PCNSL group (n = 54) | P |
|---|---|---|---|
| ADCmin ( × 10−3 mm2/s) | 0.77 ± 0.07 | 0.68 ± 0.10 | < 0.001 |
| ADCmean ( × 10−3 mm2/s) | 0.91 ± 0.08 | 0.80 ± 0.08 | < 0.001 |
| ADCmax ( × 10−3 mm2/s) | 1.23 ± 0.16 | 1.19 ± 0.10 | 0.208 |
| rADC | 1.32 ± 0.19 | 1.13 ± 0.12 | < 0.001 |
| rCBVmin | 2.38 ± 0.62 | 2.17 ± 0.67 | 0.101 |
| rCBVmean | 4.03 ± 1.07 | 2.69 ± 0.81 | < 0.001 |
| rCBVmax | 7.31 ± 2.29 | 4.24 ± 0.80 | < 0.001 |
| FA | 0.19 ± 0.03 | 0.16 ± 0.02 | < 0.001 |
| DA | 1.24 ± 0.22 | 0.98 ± 0.18 | < 0.001 |
| DR | 0.98 ± 0.11 | 0.83 ± 0.10 | < 0.001 |
| Cho/NAA | 2.17 ± 0.80 | 2.06 ± 0.70 | 0.441 |
| Cho/Cr | 1.03 ± 0.17 | 1.34 ± 0.29 | < 0.001 |
| NAA/Cr | 1.09 ± 0.23 | 1.15 ± 0.27 | 0.111 |
| Lip/Cr | 4.03 ± 0.9 | 5.48 ± 1.27 | < 0.001 |
| Lac/Cr | 1.29 ± 0.65 | 1.40 ± 0.55 | 0.203 |
Abbreviations: GBM, glioblastoma; atypical PCNSL, atypical primary central nervous system lymphoma; ADCmin, minimum apparent diffusion coefficient values; ADCmax, maximum ADC values; rADC, relative ADC; rCBVmean, mean relative cerebral blood volume; rCBVmax, maximum relative cerebral blood volume; FA, fractional anisotropy; DA, axial diffusion coefficient; DR, radial diffusion coefficient; Cho, choline; Cr, creatine; Lip, lipids; DWI, diffusion-weighted imaging; DSC, dynamic susceptibility contrast enhancement; DTI, diffusion tensor imaging; MRS, magnetic resonance spectroscopy.
Fig. 4.
Box scatter plots of ADCmin (a), ADCmean (b), rADC (c), rCBVmean (d), rCBVmax (e), FA (f), DA (g), DR (h), Cho/Cr (i), and Lip/Cr (j). Abbreviations: ADCmin, minimum apparent diffusion coefficient values; ADCmax, maximum ADC values; rADC, relative ADC; rCBVmean, mean relative cerebral blood volume; rCBVmax, maximum relative cerebral blood volume; FA, fractional anisotropy; DA, axial diffusion coefficient; DR, radial diffusion coefficient; Cho, choline; Cr, creatine; Lip, lipids.
3.3. Differentiating GBM from atypical PCNSL based on single-parameter, unimodality and bimodality models
The optimal single-parameter models for distinguishing GBM from atypical PCNSL were based on rCBVmax values, and the AUC, sensitivity, and specificity were 0.905 (0.849 to 0.946), 67.5%, and 98.1%, respectively. The unimodal model showing the optimal efficiency in differentiating GBM from atypical PCNSL was DTI, which combined the FA, DA, and DR parameters; the AUC, sensitivity, and specificity were 0.958 (0.914 to 0.983), 86.1%, and 92.5%, respectively. Bimodal models showing optimal efficiency in distinguishing GBM from atypical PCNSL were constructed by using a multiparametric combination of DSC and DTI, and their AUC, sensitivity, and specificity were 0.992 (0.962 to 1.000), 93.5%, and 98.1%, respectively. The performances of the different models for distinguishing GBM from atypical PCNSL are presented in Table 3 and Fig. 5 (a - f).
Table 3.
Diagnostic performance for discriminating GBM from atypical PCNSL based on different models.
| Models | AUC (95% CI) | Sensitivity (%) | Specificity (%) | Cutoff |
|---|---|---|---|---|
| ADCmin ( × 10−3 mm2/s) | 0.746 (0.672 to 0.811) | 91.6 | 53.7 | >0.692 |
| ADCmean ( × 10−3 mm2/s) | 0.826 (0.759 to 0.881) | 63.8 | 88.8 | >0.871 |
| rADC | 0.792 (0.721 to 0.851) | 89.8 | 59.3 | >1.136 |
| rCBVmean | 0.862 (0.799 to 0.911) | 98.1 | 59.2 | >2.719 |
| rCBVmax | 0.905 (0.849 to 0.946) | 67.5 | 98.1 | >5.830 |
| FA | 0.817 (0.749 to 0.874) | 53.7 | 98.1 | >0.188 |
| DA | 0.815 (0.746 to 0.871) | 97.2 | 46.3 | >0.911 |
| DR | 0.820 (0.752 to 0.876) | 70.3 | 75.9 | >0.902 |
| Cho/Cr | 0.811 (0.742 to 0.868) | 94.4 | 64.8 | ≤1.287 |
| Lip/Cr | 0.799 (0.729 to 0.858) | 57.4 | 90.7 | >4.950 |
| DWI | 0.900 (0.843 to 0.941) | 64.8 | 98.1 | / |
| DSC | 0.931 (0.881 to 0.965) | 93.5 | 75.9 | / |
| DTI | 0.958 (0.914 to 0.983) | 86.1 | 92.5 | / |
| MRS | 0.884 (0.825 to 0.929) | 65.7 | 94.4 | / |
| DWI + DSC | 0.975 (0.938 to 0.993) | 93.5 | 94.4 | / |
| DWI + DTI | 0.980 (0.944 to 0.995) | 93.5 | 94.4 | / |
| DWI + MRS | 0.974 (0.936 to 0.993) | 89.8 | 94.4 | / |
| DSC + DTI | 0.992 (0.962 to 1.000) | 93.5 | 98.1 | / |
| DSC + MRS | 0.984 (0.951 to 0.997) | 96.3 | 92.5 | / |
| DTI + MRS | 0.985 (0.952 to 0.998) | 94.4 | 92.5 | / |
Abbreviations: AUC, area under the curve; 95% CI, 95% confidence interval.
Notes: DWI includes the parameters ADCmin, ADCmean, and rADC; DSC comprises the metrics rCBVmean and rCBVmax; DTI includes FA, DA and DR; MRS comprises the ratios Cho/Cr and Lip/Cr.
Fig. 5.
The ROC curves based on the single-parameter (a–d), unimodality (e) and bimodality (f) models. Abbreviations: ADCmin, minimum apparent diffusion coefficient values; ADCmax, maximum ADC values; rADC, relative ADC; rCBVmean, mean relative cerebral blood volume; rCBVmax, maximum relative cerebral blood volume; FA, fractional anisotropy; DA, axial diffusion coefficient; DR, radial diffusion coefficient; Cho, choline; Cr, creatine; Lip, lipids; DWI, diffusion-weighted imaging; DSC, dynamic susceptibility contrast enhancement; DTI, diffusion tensor imaging; MRS, magnetic resonance spectroscopy. Notes: DWI comprises the metrics ADCmin, ADCmean and rADC; DSC includes the metrics rCBVmean and rCBVmax; DTI comprises the parameters FA, DA and DR; and MRS contains ratios of Cho/Cr and Lip/Cr.
3.4. The performance differences between single-parameter, unimodality and bimodality models using the DeLong Test
The DeLong test showed that the AUCs of the single-parameter model for rCBVmax were significantly higher than those of the single-parameter models for ADCmin, ADCmean, rADC, FA, DA, DR, Cho/Cr and Lip/Cr (all p < 0.05). The AUCs of single-parameter models of ADCmin, ADCmean, rADC, FA, DA, DR and Lip/Cr were significantly lower than those of unimodal models constructed by DWI (all p < 0.05). The AUCs of all single-parameter models were significantly lower than those of the unimodal models based on DSC (all p < 0.05). The AUCs of the unimodal models based on DTI showed significant differences compared with all of the single-parameter models and the unimodal models of DWI as well as MRS (all p < 0.05). There were significant differences between the AUCs of the unimodal models based on MRS data and those of the single-parameter models based on ADCmin, rADC, Cho/Cr and Lip/Cr (all p < 0.05). The bimodal models developed by DWI + DTI, DSC + DTI and DTI + MRS had significantly better AUCs than any models of single-parameter or unimodality (all p < 0.05). Bimodal models based on DWI + DSC and DSC + MRS have significantly higher AUCs than those of all the single-parameter models and the unimodal models of DWI, DSC and MRS (all p < 0.05). Bimodal models of DWI + MRS have significantly higher AUCs than those of all the single-parameter models and the unimodal models of DWI and MRS (all p < 0.05). Comparisons of the AUCs for different models of single-parameter, unimodality and bimodality are displayed in Table 4.
Table 4.
Differences in the model AUCs for differentiating GBM from atypical PCNSL.
| ADCmin ( × 10−3 mm2/s) | ADCmean ( × 10−3 mm2/s) | rADC | rCBVmean | rCBVmax | FA | DA | DR | Cho/Cr | Lip/Cr | DWI | DSC | DTI | MRS | DWI + DSC | DWI + DTI | DWI + MRS | DSC + DTI | DSC + MRS | DTI + MRS | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ADCmin ( × 10−3 mm2/s) | 0.054 | 0.421 | 0.052 | 0.001 | 0.158 | 0.215 | 0.171 | 0.290 | 0.313 | <0.001 | <0.001 | <0.001 | 0.008 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| ADCmean ( × 10−3 mm2/s) | 0.475 | 0.438 | 0.036 | 0.825 | 0.771 | 0.881 | 0.772 | 0.492 | 0.002 | 0.003 | <0.001 | 0.159 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||
| rADC | 0.127 | 0.010 | 0.594 | 0.651 | 0.596 | 0.739 | 0.896 | <0.001 | <0.001 | <0.001 | 0.046 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |||
| rCBVmean | 0.171 | 0.308 | 0.308 | 0.405 | 0.368 | 0.223 | 0.309 | 0.001 | 0.006 | 0.616 | <0.001 | <0.001 | 0.001 | <0.001 | <0.001 | <0.001 | ||||
| rCBVmax | 0.019 | 0.031 | 0.039 | 0.049 | 0.013 | 0.173 | 0.028 | 0.038 | 0.563 | <0.001 | 0.002 | 0.007 | <0.001 | <0.001 | <0.001 | |||||
| FA | 0.952 | 0.965 | 0.898 | 0.707 | 0.023 | 0.001 | <0.001 | 0.130 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||||||
| DA | 0.908 | 0.940 | 0.721 | 0.031 | 0.002 | <0.001 | 0.068 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |||||||
| DR | 0.869 | 0.625 | 0.047 | 0.006 | <0.001 | 0.086 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||||||||
| Cho/Cr | 0.816 | 0.075 | 0.011 | <0.001 | 0.008 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |||||||||
| Lip/Cr | 0.013 | 0.001 | <0.001 | 0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||||||||||
| DWI | 0.280 | 0.016 | 0.674 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |||||||||||
| DSC | 0.249 | 0.178 | 0.001 | 0.020 | 0.056 | <0.001 | 0.001 | 0.010 | ||||||||||||
| DTI | 0.008 | 0.300 | 0.010 | 0.215 | 0.002 | 0.081 | 0.006 | |||||||||||||
| MRS | 0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||||||||||||||
| DWI + DSC | 0.689 | 0.948 | 0.094 | 0.370 | 0.416 | |||||||||||||||
| DWI + DTI | 0.535 | 0.149 | 0.693 | 0.521 | ||||||||||||||||
| DWI + MRS | 0.083 | 0.375 | 0.184 | |||||||||||||||||
| DSC + DTI | 0.323 | 0.359 | ||||||||||||||||||
| DSC + MRS | 0.942 | |||||||||||||||||||
| DTI + MRS |
Notes: Significant differences (p values < 0.05) in the AUCs of the models are labeled in bold.
4. Discussion
DWI is a noninvasive tool that quantifies physiological changes in the water diffusion of microscopic structural alterations, and these changes are reflected by the ADC. ADC is inversely related to tumor cell density, and patients who have PCNSL, a condition that exhibits high cellularity, tend to exhibit a low ADC [17]. As demonstrated in this study, the ADCmin, ADCmean, and rADC values in atypical PCNSL were significantly lower than those in GBM, in agreement with previous studies [4]. However, some studies also reported no significant differences between patients in the GBM and PCNSL groups, although patients with PCNSL had lower ADCs than patients with GBM [18,19]. The reason for this discrepancy may be associated with the method of ROI construction. A recent study [20] indicated that in both GBM and PCNSL, the selective minimum ADC values based on the ROI being placed accurately in the enhanced region with the highest CBV values while keeping away from necrotic, vessels or cystic regions were significantly higher than the nonselective minimum ADC based on the ROI being placed over the entire enhancement area, including necrotic as well as cystic regions, regardless of CBV values. Ahn et al. [21] compared three different ROI methods for calculating ADCs to discriminate GBM from PCNSL: inclusion of ROIs for whole tumor volume, inclusion of 5 circular ROIs (area = 10 mm2) in the enhanced area, and inclusion of only a single circular ROI (area = 20 mm2) in any enhanced region. Their results suggested that the ADCmean based on ROIs of the whole tumor volume and the ADCmin based on 5 circular ROIs exhibited only a slight significant difference, although the ADC values of PCNSL patients acquired with other ROI methods were lower than those of GBM patients.
DSC based on the T2* effects of contrast agents, which is the most frequently used perfusion method, can be applied to noninvasively assess tumor neovascularization. The rCBV from the analysis of signal-time course data reflects microvessel density and could be useful for discriminating GBM from PCNSL based on the presence of neovascularization of GBM, which is absent in PCNSL [22]. This notion was confirmed in our study, consistent with previous reports [9,17]. We found that rCBVmean and rCBVmax values for subjects with GBM were significantly higher than those for subjects with atypical PCNSL. However, CBV may not be applied to differentiate hypervascular PCNSL from GBM. A previous study [23] revealed no significant difference between hypervascular PCNSL and GBM in terms of either normalized CBV map (nCBVref) or leakage-corrected CBV (CBVres) values; instead, patients with hypervascular PCNSL showed significantly higher relative cerebral blood flow (rCBF) than those with GBM. This finding may explain why the rCBVmin was not significantly different between GBM and atypical PCNSL in the present study. Dynamic contrast-enhanced (DCE) methods based on a dynamic increase in T1-related signals after contrast medium injection can reflect the microvascular permeability of tumors [7]. In particular, a distinct difference in vascular permeability is noted between patients with PCNSL and those with GBM. Subjects diagnosed with GBM had lower contrast agent transfer constant (Ktrans) and flux rate constant (Kep) values than subjects diagnosed with PCNSL [24]. Histopathologically speaking, GBM exhibits intact vasculature despite endothelial proliferation, whereas the vasculature in PCNSL is damaged. These findings are reflected by the differences in the DCE parameters between PCNSL and GBM [23].
DTI can be used to study microstructural tissue alterations in entire tumors because in vivo, it can quantify microscopic features of water diffusion along different orientations. Moreover, FA can measure the degree of diffusion anisotropy and directionality [25]. Our results show that the FA of GBM was significantly higher than that of atypical PCNSL, which has higher cellularity, indicating that the relationship between FA and cellularity is inverse. Previous studies [14] using FA for the differentiation of GBM and PCNSL have reported results that are concordant with those presented in our study. Although the exact mechanism of FA reduction in brain tumors is not clarified, one study [26] suggested that this decrease is associated with an increase in the extracellular space which was results from neuronal and fiber tract destruction. Another study [27] found that low FA was associated with a reduction in the extracellular space because of tumor infiltration. This finding has been supported by the observed negative relationship between in FA and tumor cellularity. Our opinion regarding the reduction in FA in PCNSL is that attenuated tumor growth results in a reduction in the extracellular space. Our results also suggest that atypical PCNSL has significantly lower DA and DR values than GBM. The result of demyelination, axonal damage, and a tumor mass occurring in the cranial compartment may be associated with decreased DA and DR measurements [28]. Notably, DA, which has been linked to axonal damage [29], represents the diffusion properties along a particular direction (the primary axis), while DR, which is related to both demyelination and axonal damage [30], represents the diffusion properties in the other two directions perpendicular to the primary direction [25]. These findings have also been confirmed in glioma grading. Server et al. [28] reported an inverse relationship between glioma grades II–IV and DA and DR values.
MRS can be used to detect brain tissue metabolism in vivo and to depict the unique biochemical features of brain tumors. Our results suggest that the Cho/Cr ratio in subjects diagnosed with atypical PCNSL was significantly higher than that in subjects diagnosed with GBM, which is consistent with previous studies [31,32]. The reason for this finding may be correlated with the increased Cho and lower Cr contents confirmed in subjects diagnosed with atypical PCNSL relative to patients diagnosed with GBM. The increased Cho peak in subjects diagnosed with PCNSL was led by the combined effect of increased cellularity as well as rapid membrane turnover [33]. Although GBM tumors also exhibit elevated Cho levels, the interior of these tumors often exhibits necrosis, so the extent of the increase in Cho is limited. The decrease in Cr is associated with energy depletion and ischemia. PCNSL lesions have a higher cellular density (indicating that the energy metabolism of these cells is increased) and are hypovascular tumors, whereas GBM tumors have a large number of new blood vessels and an abundant blood supply. Therefore, the decrease in Cr in a PCNSL lesion may be more obvious than that in a GBM lesion. Another finding from the MRS data in our study is that the Lip/Cr ratio was significantly higher in patients diagnosed with atypical PCNSL than in patients diagnosed with the GBM. The lipid peak of patients diagnosed with PCNSL is probably due to lipid synthesis or secretion during cell differentiation or turnover. On the other hand, leukocytes and lymphocytes contain high lipid signals, especially in these cells activating or transforming because the lipid signal can reflect the triglycerides within the cell membrane [34]. Tumor necrosis may be correlated with the lipid peak in subjects diagnosed with GBM. The quantity of mobile lipids was considered to be related to the degree with microscopic cellular necrosis of the region around high-grade glioma tumors [35]. Although a lipid peak was present in both patients with PCNSL and GBM, the former had a higher peak than the latter, as confirmed in a previous study [36].
ROC curve analysis results suggested that the optimal single-parameter models of DWI, DSC, DTI and MRS for discriminating GBM from atypical PCNSL were based on ADCmean, rCBVmax, DR and Cho/Cr with AUCs of 0.826, 0.905, 0.820 and 0.811, respectively. Suh et al. [9] reported that the single-parameter models of intravoxel incoherent motion (IVIM)-derived maximum perfusion fraction (fmax) yielded AUCs of 0.962 (Reader 1) and 0.949 (Reader 2), the best performance in the differential diagnosis of atypical PCNSL and GBM. Considering the limited performance and inferior specificity and sensitivity of the single-parameter model in our study, we constructed multiparametric models using logistic regression based on the model for unimodality and bimodality to discriminate GBM from atypical PCNSL. The best models for unimodality and bimodality MRI for the differential diagnosis of GBM and atypical PCNSL were built by DTI and a combination of DTI and DSC, and the AUCs were 0.958 and 0.992, respectively. Clearly, multiparametric models can significantly improve the performance in discriminating GBM from atypical PCNSL. Notably, this phenomenon has also been observed in other advanced imaging technologies. Lu et al. [37] reported that, compared with single-parameter models of Ktrans (AUC = 0.852) and rADC (AUC = 0.858) alone for differentiating PCNSL from GBM, the diagnostic ability was significantly improved when both features were combined (AUC = 0.930). A study about histogram analysis of the nCBV and the ADC performed by Bao et al. [38] suggested single-parameter models with the best efficiency for differential diagnosis of PCNSL and GBM, constructed by average nCBV and 25th percentile of ADC, achieved AUCs of 0.869 and 0.838, respectively, whereas the AUC increased to 0.969 when the two features were combined. Although these studies used single and multiple parameters to construct models in the differentiation of PCNSL and GBM, few of them compared the efficiency of different models.
When comparisons were made among the AUCs of different models for distinguishing GBM from atypical PCNSL, these comparisons revealed significant differences in the AUCs of the optimal one-parameter models and other most single-parameter models (all p < 0.05) as well as among the AUCs of the best unimodal models and unimodal models based on DWI and MRS (all p < 0.05). In addition, significant differences (all P < 0.05) were noted among the AUCs of models for single-parameters and those for unimodality, among the AUCs of models for unimodality and those for bimodality, as well as among all AUCs of model for single-parameters and those for bimodality. These results suggest, in most cases, the performance of bimodal models in distinguishing GBM from atypical PCNSL was better than that of unimodal models, which was subsequently better than that of single-parameter models. From the point of view of clinical practice, these results may have potential value, as clinicians could select the methods with the highest cost-effectiveness to discriminate GBM from atypical PCNSL. According to our comparison of different models, DTI-based models seem to be superior models for distinguishing GBM from atypical PCNSL, demonstrating significantly better performance than any of the single-parameter and partial unimodal models; their performance can even be comparable to that of bimodal models. Compared with the suboptimal models based on DSC, models based on DTI also have another advantage, namely, the lack of a need for a dynamic contrast agent. Of course, because DTI is not currently available at all institutes, one or more of the models based on DWI, DSC and MRS can also be considered when DTI is not available.
Our study has some limitations that should be noted. First, this study was retrospective in nature and based on small samples from a single center. Second, the DWI, DSC, DTI and MRS modalities were compared in the present study due to time and economic constraints. Third, we merely analyzed parenchymal regions of the tumors because these areas were generally considered representative regions of the tumors. However, the tumor infiltration and edema, extra information based on T2 hyperintense regions, were also used to evaluate tumor heterogeneity. Prospective multicenter analyses based on a larger number of patients, more MRI modalities and different tumor regions are needed in the future.
In conclusion, our results indicate that models of single-parameter, unimodality and bimodality from DWI, DSC, DTI and MRS data may serve as imaging biomarkers to distinguish GBM from atypical PCNSL and that a unimodal model based on DTI is a cost-effective method for differentiating GBM and atypical PCNSL in clinical practice. Moreover, clinicians can select one or more MRI protocols to differentiate GBM and atypical PCNSL based on the efficiency from different models built by one-parameter, unimodal as well as bimodal MRI.
Author contribution statement
Aozi Feng: Performed the experiments; analyzed and interpreted the data; contributed reagents, materials, analysis tools or data; and wrote the paper.
Li Li: Analyzed and interpreted the data; contributed reagents, materials, analysis tools or data.
Tao Huang: Acquisition of data; analysis and interpretation of data
Shuna Li: Performed the experiments and analyzed and interpreted the data.
Ningxia He: Performed the experiments; contributed reagents, materials, analysis tools or data.
Liying Huang: Performed the experiments; contributed reagents, materials, analysis tools or data.
Mengnan Zeng; Jun Lyu: Conceived and designed the experiments; contributed reagents, materials, analysis tools or data; wrote the paper.
Funding statement
This study was supported by The Fundamental Research Funds for the Central Universities [21622314] and The Guangdong Provincial Key Laboratory of Traditional Chinese Medicine Informatization [2021B1212040007].
Data availability statement
The authors do not have permission to share data.
Declaration of interest’s statement
The authors declare no competing interests.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e15150.
Contributor Information
Mengnan Zeng, Email: zengmengnan2022@126.com.
Jun Lyu, Email: lyujun2020@jnu.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Miller K.D., Ostrom Q.T., Kruchko C., Patil N., Tihan T., Cioffi G., Fuchs H.E., Waite K.A., Jemal A., Siegel R.L., Barnholtz-Sloan J.S. Brain and other central nervous system tumor statistics. CA Canc. J. Clin. 2021;71(5):381–406. doi: 10.3322/caac.21693. [DOI] [PubMed] [Google Scholar]
- 2.Zheng X., Li P., Dong Q., Duan Y., Yang S., Cai Z., Chen F., Li W. MicroRNAs as diagnostic biomarkers in primary central nervous system lymphoma: a systematic Review and meta-analysis. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.743542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stupp R., Mason W.P., van den Bent M.J., Weller M., Fisher B., Taphoorn M.J., Belanger K., Brandes A.A., Marosi C., Bogdahn U., Curschmann J., Janzer R.C., Ludwin S.K., Gorlia T., Allgeier A., Lacombe D., Cairncross J.G., Eisenhauer E., Mirimanoff R.O. European organisation for research, treatment of cancer brain tumor, radiotherapy groups, national cancer institute of Canada clinical trials group, radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 4.Kickingereder P., Wiestler B., Sahm F., Heiland S., Roethke M., Schlemmer H.P., Wick W., Bendszus M., Radbruch A. Primary central nervous system lymphoma and atypical glioblastoma: multiparametric differentiation by using diffusion-, perfusion-, and susceptibility-weighted MR imaging. Radiology. 2014;272(3):843–850. doi: 10.1148/radiol.14132740. [DOI] [PubMed] [Google Scholar]
- 5.Xiao X., Liu X., Liang W., Han L.Y., Li X.D., Guo L.J., He W.L., Liu X.M., Zhou J., Cai Q., Xu Y.K., Tan X.L., Wu Y.K. Conventional MRI features of central nervous system embryonal tumor, not otherwise specified in adults: comparison with glioblastoma. Acad. Radiol. 2022;29(Suppl 3):S44–S51. doi: 10.1016/j.acra.2021.01.010. [DOI] [PubMed] [Google Scholar]
- 6.Chen C., Zheng A., Ou X., Wang J., Ma X. Comparison of radiomics-based machine-learning models in diagnosis of glioblastoma from primary central nervous system lymphoma. Front. Oncol. 2020;10:1151. doi: 10.3389/fonc.2020.01151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kang D., Park J.E., Kim Y.H., Kim J.H., Oh J.Y., Kim J., Kim Y., Kim S.T., Kim H.S. Diffusion radiomics as a diagnostic model for atypical manifestation of primary central nervous system lymphoma: development and multicenter external validation. Neuro Oncol. 2018;20(9):1251–1261. doi: 10.1093/neuonc/noy021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thurnher M.M., Rieger A., Kleibl-Popov C., Settinek U., Henk C., Haberler C., Schindler E. Primary central nervous system lymphoma in AIDS: a wider spectrum of CT and MRI findings. Neuroradiology. 2001;43(1):29–35. doi: 10.1007/s002340000480. [DOI] [PubMed] [Google Scholar]
- 9.Suh C.H., Kim H.S., Lee S.S., Kim N., Yoon H.M., Choi C.G., Kim S.J. Atypical imaging features of primary central nervous system lymphoma that mimics glioblastoma: utility of intravoxel incoherent motion MR imaging. Radiology. 2014;272(2):504–513. doi: 10.1148/radiol.14131895. [DOI] [PubMed] [Google Scholar]
- 10.Shim W.H., Kim H.S., Choi C.G., Kim S.J. Comparison of apparent diffusion coefficient and intravoxel incoherent motion for differentiating among glioblastoma, metastasis, and lymphoma focusing on diffusion-related parameter. PLoS One. 2015;10(7) doi: 10.1371/journal.pone.0134761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee J.Y., Bjornerud A., Park J.E., Lee B.E., Kim J.H., Kim H.S. Permeability measurement using dynamic susceptibility contrast magnetic resonance imaging enhances differential diagnosis of primary central nervous system lymphoma from glioblastoma. Eur. Radiol. 2019;29(10):5539–5548. doi: 10.1007/s00330-019-06097-9. [DOI] [PubMed] [Google Scholar]
- 12.Shrot S., Salhov M., Dvorski N., Konen E., Averbuch A., Hoffmann C. Application of MR morphologic, diffusion tensor, and perfusion imaging in the classification of brain tumors using machine learning scheme. Neuroradiology. 2019;61(7):757–765. doi: 10.1007/s00234-019-02195-z. [DOI] [PubMed] [Google Scholar]
- 13.Amin A., Moustafa H., Ahmed E., El-Toukhy M. Glioma residual or recurrence versus radiation necrosis: accuracy of pentavalent technetium-99m-dimercaptosuccinic acid [Tc-99m (V) DMSA] brain SPECT compared to proton magnetic resonance spectroscopy (1H-MRS): initial results. J. Neuro Oncol. 2012;106(3):579–587. doi: 10.1007/s11060-011-0694-2. [DOI] [PubMed] [Google Scholar]
- 14.Toh C.H., Castillo M., Wong A.M., Wei K.C., Wong H.F., Ng S.H., Wan Y.L. Primary cerebral lymphoma and glioblastoma multiforme: differences in diffusion characteristics evaluated with diffusion tensor imaging. AJNR Am. J. Neuroradiol. 2008;29(3):471–475. doi: 10.3174/ajnr.A0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chawla S., Zhang Y., Wang S., Chaudhary S., Chou C., O'Rourke D.M., Vossough A., Melhem E.R., Poptani H. Proton magnetic resonance spectroscopy in differentiating glioblastomas from primary cerebral lymphomas and brain metastases. J. Comput. Assist. Tomogr. 2010;34(6):836–841. doi: 10.1097/RCT.0b013e3181ec554e. [DOI] [PubMed] [Google Scholar]
- 16.DeLong E.R., DeLong D.M., Clarke-Pearson D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–845. [PubMed] [Google Scholar]
- 17.Nakajima S., Okada T., Yamamoto A., Kanagaki M., Fushimi Y., Okada T., Arakawa Y., Takagi Y., Miyamoto S., Togashi K. Primary central nervous system lymphoma and glioblastoma: differentiation using dynamic susceptibility-contrast perfusion-weighted imaging, diffusion-weighted imaging, and (18)F-fluorodeoxyglucose positron emission tomography. Clin. Imag. 2015;39(3):390–395. doi: 10.1016/j.clinimag.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 18.Matsushima N., Maeda M., Umino M., Suzawa N., Yamada T., Takeda K. Relation between FDG uptake and apparent diffusion coefficients in glioma and malignant lymphoma. Ann. Nucl. Med. 2012;26(3):262–271. doi: 10.1007/s12149-012-0570-y. [DOI] [PubMed] [Google Scholar]
- 19.Makino K., Hirai T., Nakamura H., Murakami R., Kitajima M., Shigematsu Y., Nakashima R., Shiraishi S., Uetani H., Iwashita K., Akter M., Yamashita Y., Kuratsu J. Does adding FDG-PET to MRI improve the differentiation between primary cerebral lymphoma and glioblastoma? Observer performance study. Ann. Nucl. Med. 2011;25(6):432–438. doi: 10.1007/s12149-011-0483-1. [DOI] [PubMed] [Google Scholar]
- 20.Eisenhut F., Schmidt M.A., Putz F., Lettmaier S., Frohlich K., Arinrad S., Coras R., Luecking H., Lang S., Fietkau R., Doerfler A. Classification of primary cerebral lymphoma and glioblastoma featuring dynamic susceptibility contrast and apparent diffusion coefficient. Brain Sci. 2020;10(11):886. doi: 10.3390/brainsci10110886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahn S.J., Shin H.J., Chang J.H., Lee S.K. Differentiation between primary cerebral lymphoma and glioblastoma using the apparent diffusion coefficient: comparison of three different ROI methods. PLoS One. 2014;9(11) doi: 10.1371/journal.pone.0112948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakajima S., Okada T., Yamamoto A., Kanagaki M., Fushimi Y., Okada T., Arakawa Y., Takagi Y., Miyamoto S., Togashi K. Differentiation between primary central nervous system lymphoma and glioblastoma: a comparative study of parameters derived from dynamic susceptibility contrast-enhanced perfusion-weighted MRI. Clin. Radiol. 2015;70(12):1393–1399. doi: 10.1016/j.crad.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 23.Lee B., Park J.E., Bjornerud A., Kim J.H., Lee J.Y., Kim H.S. Clinical value of vascular permeability estimates using dynamic susceptibility contrast MRI: improved diagnostic performance in distinguishing hypervascular primary CNS lymphoma from glioblastoma. AJNR Am. J. Neuroradiol. 2018;39(8):1415–1422. doi: 10.3174/ajnr.A5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kickingereder P., Sahm F., Wiestler B., Roethke M., Heiland S., Schlemmer H.P., Wick W., von Deimling A., Bendszus M., Radbruch A. Evaluation of microvascular permeability with dynamic contrast-enhanced MRI for the differentiation of primary CNS lymphoma and glioblastoma: radiologic-pathologic correlation. AJNR Am. J. Neuroradiol. 2014;35(8):1503–1508. doi: 10.3174/ajnr.A3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin Y., Huang C., Daianu M., Zhan L., Dennis E.L., Reid R.I., Jack C.R., Jr., Zhu H., Thompson P.M. Alzheimer's Disease Neuroimaging Initiative, 3D tract-specific local and global analysis of white matter integrity in Alzheimer's disease. Hum. Brain Mapp. 2017;38(3):1191–1207. doi: 10.1002/hbm.23448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sinha S., Bastin M.E., Whittle I.R., Wardlaw J.M. Diffusion tensor MR imaging of high-grade cerebral gliomas. AJNR Am. J. Neuroradiol. 2002;23(4):520–527. [PMC free article] [PubMed] [Google Scholar]
- 27.Stadlbauer A., Ganslandt O., Buslei R., Hammen T., Gruber S., Moser E., Buchfelder M., Salomonowitz E., Nimsky C. Gliomas: histopathologic evaluation of changes in directionality and magnitude of water diffusion at diffusion-tensor MR imaging. Radiology. 2006;240(3):803–810. doi: 10.1148/radiol.2403050937. [DOI] [PubMed] [Google Scholar]
- 28.Server A., Graff B.A., Josefsen R., Orheim T.E., Schellhorn T., Nordhoy W., Nakstad P.H. Analysis of diffusion tensor imaging metrics for gliomas grading at 3 T. Eur. J. Radiol. 2014;83(3):e156–e165. doi: 10.1016/j.ejrad.2013.12.023. [DOI] [PubMed] [Google Scholar]
- 29.Budde M.D., Xie M., Cross A.H., Song S.K. Axial diffusivity is the primary correlate of axonal injury in the experimental autoimmune encephalomyelitis spinal cord: a quantitative pixelwise analysis. J. Neurosci. 2009;29(9):2805–2813. doi: 10.1523/JNEUROSCI.4605-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klawiter E.C., Schmidt R.E., Trinkaus K., Liang H.F., Budde M.D., Naismith R.T., Song S.K., Cross A.H., Benzinger T.L. Radial diffusivity predicts demyelination in ex vivo multiple sclerosis spinal cords. Neuroimage. 2011;55(4):1454–1460. doi: 10.1016/j.neuroimage.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aburano H., Ueda F., Yoshie Y., Matsui O., Nakada M., Hayashi Y., Gabata T. Differences between glioblastomas and primary central nervous system lymphomas in 1H-magnetic resonance spectroscopy. Jpn. J. Radiol. 2015;33(7):392–403. doi: 10.1007/s11604-015-0430-5. [DOI] [PubMed] [Google Scholar]
- 32.Harting I., Hartmann M., Jost G., Sommer C., Ahmadi R., Heiland S., Sartor K. Differentiating primary central nervous system lymphoma from glioma in humans using localised proton magnetic resonance spectroscopy. Neurosci. Lett. 2003;342(3):163–166. doi: 10.1016/s0304-3940(03)00272-6. [DOI] [PubMed] [Google Scholar]
- 33.Poptani H., Gupta R.K., Roy R., Pandey R., Jain V.K., Chhabra D.K. Characterization of intracranial mass lesions with in vivo proton MR spectroscopy. AJNR Am. J. Neuroradiol. 1995;16(8):1593–1603. [PMC free article] [PubMed] [Google Scholar]
- 34.Raizer J.J., Koutcher J.A., Abrey L.E., Panageas K.S., DeAngelis L.M., Lis E., Xu S., Zakian K.L. Proton magnetic resonance spectroscopy in immunocompetent patients with primary central nervous system lymphoma. J. Neuro Oncol. 2005;71(2):173–180. doi: 10.1007/s11060-004-1360-8. [DOI] [PubMed] [Google Scholar]
- 35.Kuesel A.C., Briere K.M., Halliday W.C., Sutherland G.R., Donnelly S.M., Smith I.C. Mobile lipid accumulation in necrotic tissue of high grade astrocytomas. Anticancer Res. 1996;16(3B):1485–1489. [PubMed] [Google Scholar]
- 36.Yamasaki F., Takayasu T., Nosaka R., Amatya V.J., Doskaliyev A., Akiyama Y., Tominaga A., Takeshima Y., Sugiyama K., Kurisu K. Magnetic resonance spectroscopy detection of high lipid levels in intraaxial tumors without central necrosis: a characteristic of malignant lymphoma. J. Neurosurg. 2015;122(6):1370–1379. doi: 10.3171/2014.9.JNS14106. [DOI] [PubMed] [Google Scholar]
- 37.Lu S., Wang S., Gao Q., Zhou M., Li Y., Cao P., Hong X., Shi H. Quantitative evaluation of diffusion and dynamic contrast-enhanced magnetic resonance imaging for differentiation between primary central nervous system lymphoma and glioblastoma. J. Comput. Assist. Tomogr. 2017;41(6):898–903. doi: 10.1097/RCT.0000000000000622. [DOI] [PubMed] [Google Scholar]
- 38.Bao S., Watanabe Y., Takahashi H., Tanaka H., Arisawa A., Matsuo C., Wu R., Fujimoto Y., Tomiyama N. Differentiating between glioblastoma and primary CNS lymphoma using combined whole-tumor histogram analysis of the normalized cerebral blood volume and the apparent diffusion coefficient. Magn. Reson. Med. Sci. 2019;18(1):53–61. doi: 10.2463/mrms.mp.2017-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors do not have permission to share data.