Abstract
Hepatic innate immune function plays an important role in the pathogenesis of many diseases. Importantly, a growing body of literature has firmly established the spatial heterogeneity of hepatocyte metabolic function, however, whether innate immune function is zonated remains unknown. To test this question, we exposed adult C57BL/6 mice to endotoxemia and hepatic tissue was assessed for the acute phase response (APR). The zone-specific APR was evaluated in periportal and pericentral/centrilobular hepatocytes isolated using digitonin perfusion and on hepatic tissue using RNAscope and immunohistochemistry (IHC). Western blot, electrophoretic mobility shift assay, chromatin immunoprecipitation and IHC was used to determine the role of the transcription factor NFκB in mediating hepatic CRP expression. Finally, the ability of mice lacking the NFκB subunit p50 (p50−/−) to raise a hepatic APR was evaluated. We found that endotoxemia induces a hepatocyte transcriptional APR in both male and female mice, with Crp, Apcs, Fga, Hp, and Lbp expression being enriched in pericentral/centrilobular hepatocytes. Focusing our work on CRP expression, we determined that NFκB transcription factor subunit p50 binds to consensus sequence elements present in the murine CRP promoter. Furthermore, pericentral/centrilobular hepatocyte p50 nuclear translocation is temporally associated with zone-specific APR during endotoxemia. Lastly, the APR and CRP expression is blunted in endotoxemic p50−/− mice. These results demonstrate that the murine hepatocyte innate immune response to endotoxemia includes zone specific activation of transcription factors and target gene expression. These results support further study of zone-specific hepatocyte innate immunity and its role in the development of various disease states.
Keywords: hepatic zonation, acute phase response, NFκB
Introduction
The liver is increasingly recognized as an immunologic organ, and hepatic innate immune function plays an important role in protecting against infection and sepsis.(1–4) Located between the gut and the systemic circulation, the liver provides an important line of defense against the spread of bacteria. Many cell types contribute to this effort: natural killer T cells (NKT) cells, CD4 and CD8 T cells, neutrophils, monocytes, Kupffer cells, and hepatocytes.(5) The hepatocyte plays a central role in the innate immune response to various stimuli by producing the proteins that compose the acute phase response (APR).(3, 6–8) This response is considered a central component of hepatic innate immunity,(9) and impaired production of APR proteins predicts worse outcomes with advanced liver disease.(3)
Importantly, a growing body of literature has firmly established the spatial heterogeneity of hepatic function.(10–13) Approximately 50% of all hepatocyte genes are expressed in a zone dependent manner.(14) Most of what is known about this heterogeneity comes from studies of metabolic function. Hepatocyte metabolic function is not uniform, exhibiting distinct activity across the lobule from the periportal to the pericentral/centrilobular zone. Nutrient-rich blood from the gut arrives directly via the portal vein, allowing periportal hepatocytes to shape the metabolic input of downstream hepatocytes.(10) Metabolic function, from mitochondrial content to the handling of carbohydrates, protein, lipids and toxins is zone specific. This distribution of labor allows the liver to maintain systemic physiological homeostasis in the setting of dynamic nutrient input from the gut.
In contrast to what is known about hepatocyte metabolic zonation, whether the response to innate immune stimuli is zonated is unknown. In addition to being nutrient-rich, the blood arriving to the liver via the portal vein is highly enriched with various innate immune stimuli. This includes but is not limited to bacteria, bacterial byproducts, and endotoxin.(3) Given the variable yet continuous exposure to innate immune stimuli, it would be reasonable to expect that similar to the zonation of metabolic function, hepatocytes would exhibit zonation of innate immune function. Early reviews of hepatic zonation demonstrated that baseline hepatic expression of some APR proteins was zonated, but noted discrepancies between mRNA expression and protein.(15) A recent review noted that “it has not been clarified whether there are specific hepatocyte populations that particularly express APP (acute phase proteins) or whether the expression of individual APP varies locally.”(9)
Various inflammatory and infectious stimuli have been used in pre-clinical studies to study the hepatic APR. Among these, experimental endotoxemia has been used to study the APR across multiple species.(16–19) Here, we used an experimental model of sublethal endotoxemia to induce the hepatic APR in adult C57BL/6 mice. We found that the hepatic APR was induced in both male and female mice. We found that hepatic expression of CRP mRNA and protein increased within 5 hours of initiation of endotoxemia. Digitonin isolation of periportal and pericentral hepatocytes confirmed that LPS-induced CRP expression was enriched in the pericentral hepatocytes. Immunohistochemical staining showed that the increase in CRP expression was enhanced in pericentral hepatocytes. Importantly, with endotoxemia, we document hepatic NFκB activation, with a temporal relationship between p50 subunit nuclear translocation and CRP expression. Using electrophoretic mobility shift assay (EMSA) and chromatin immunoprecipitation (ChIP), we demonstrate p50 binding to a NFκB specific consensus sequence within the murine CRP promoter. Immunohistochemical staining localizes p50 nuclear translocation in pericentral hepatocytes at 5 hours of endotoxemia. Finally, using p50−/−, we demonstrate significant attenuation of the hepatic acute phase transcriptional response and CRP expression. Together, these data provide evidence that the APR induced by endotoxemia is zonated, with the significant contribution of CRP production coming from p50-regulated transcription in the pericentral hepatocytes. We speculate that innate immune function of hepatocytes is zone specific, and more work must be done to determine the mechanisms underlying these findings.
Materials and Methods
Murine model of endotoxemia (LPS) exposure
All procedures were approved by the Institutional Animal Care and Use Committee at the University of Colorado (Aurora, Colo., USA), and care and handling of the animals was in accord with the National Institutes of Health guidelines for ethical animal treatment. Adult (8–10 weeks) C57BL/6 and p50−/− (B6.Cg-Nfkb1tm1Bal/J, The Jackson Laboratory) male and female mice were exposed to a sublethal dose of intraperitoneal (IP) LPS (5 mg/kg; L2630, Sigma-Aldrich), or volume matched sterile PBS, and tissues were collected and preserved as described previously.(20) Throughout, this manuscript the terms “control” and “unexposed control” refer to the PBS group. All control and exposed mice were housed in the same environment.
Isolation of mRNA, cDNA synthesis and analysis of relative mRNA levels by RT-qPCR
Hepatic mRNA was isolated and cDNA synthesized as previously described.(21) Relative mRNA levels were assessed via quantitative real-time PCR using TaqMan primers (Table 1) and StepOnePlus Real-Time PCR System (Applied Biosystems) by normalizing to the endogenous control 18S using the cycle threshold (ΔΔCt) method.
Table 1.
List of genes and primers used for qPCR analysis
| Target | AssayID |
|---|---|
| Crp | Mm00432680_g1 |
| Saa-1 | Mm00656927_g1 |
| Apcs | Mm0048099_g1 |
| Lbp | Mm00493136_g1 |
| Fga | Mm00802584_m1 |
| Hp | Mm01239994-g1 |
| Alb | Mm00802090_m1 |
| Ass-1 | Mm00711256_m1 |
| Cyp2e1 | Mm00491127_m1 |
| Glul | Mm00725701_s1 |
| Cyp2f2 | Mm00484087_m1 |
| Il6 | Mm00446190_m1 |
| Il1b | Mm01336189_m1 |
| Tnf | Mm00443258_m1 |
| 18s | Mm03928990_g1 |
Histologic Evaluation of LPS-induced hepatic injury
Tissue was processed and histopathological scoring of the liver tissue was performed by a trained histologist blinded to the animal genotype and treatments as previously described.(22) Briefly, the liver injury scoring system relies on the following criteria: 1) extent of liver cell injury and death including: ballooning, acidophilic bodies (apoptosis), necrotic cells, megamitochondria, large abscesses, 2) inflammation of all kinds including inflammatory cell foci, lipogranulomas, portal inflammation, Langhans giant cells, pigmented macrophages, foamy macrophages, and inflammatory cells in the sinusoidal vasculature, 3) reactive changes including: Mallory’s hyaline, glycogenated nuclei, ductal reaction, mitotic figures, normoblast clusters, hyalinized and thickened portal vein and hepatic artery, giant cell transformation, hepatocyte polyploidization, and inclusion bodies, and 4) steatosis, including macrovesicular, and microvesicular. A total, cumulative injury score is arrived at based on the above criteria.
Preparation of whole liver lysate, cytosolic/nuclear extracts and Western Blot
Frozen pulmonary and hepatic tissue was homogenized using the Bullet Blender (NextAdvance). Cytosolic and nuclear extracts were collected in NE-PER (ThermoFisher Scientific). Hepatic whole cell lysates were collected in T-PER (ThermoFisher Scientific). Samples were electrophoresed on a 4–12% polyacrylamide gel (Invitrogen) and transferred to an Immobilon membrane (Millipore) and blotted with antibodies (Table 2). Blots were imaged using the LiCor Odyssey imaging system and densitometric analysis was performed using ImageStudio (LiCor). In the figures, cropped images grouped together are from the same gel. No images have been spliced together and no images from separate blots have been grouped together. For densitometric analysis, cytosolic levels were normalized to total protein stain. Nuclear levels were normalized to HDAC1, and the presence of cytosolic contamination evaluated with GAPDH.
Table 2.
List of antibodies used
| Target | Concentration (WB/IHC/EMSA) | Catalogue number | Company |
|---|---|---|---|
| Western Blot | |||
| CRP | 1:1000 | ab259862 | Abcam |
| PEPCK | 1:1000 | ab70358 | Abcam |
| Glutamine Synthetase | 1:1000 | ab49873 | Abcam |
| Cyp2e1 | 1:1000 | ab28146 | Abcam |
| CEBP/β | 1:1000 | ab32358 | Abcam |
| p50 | 1:1000 | ab32360 | Abcam |
| p65 | 1:1000 | 9242 | Cell Signaling |
| IHC | |||
| Glutamine Synthetase | 1:250 | #367–005 | Synpatic Systems |
| CRP | 1:20 | #710269 | ThermoFisher |
| p50 | 1:250 | ab32360 | Abcam |
| EMSA | |||
| p50 | 2 μL | #13586 | Cell Signaling |
In situ hybridization for Crp expression
RNAScope detection was used to perform in situ hybridization according to the manufacturer’s protocol (Advanced Cell Diagnostics, Hayward, CA). Briefly, formalin-fixed paraffin embedded mouse livers were cut into 5 μm thick tissue sections. Slides were deparaffinized in xylene, followed by rehydration in a series of ethanol washes. Following citrate buffer (Advanced Cell Diagnostics) antigen retrieval, slides were rinsed in deionized water, and immediately treated with protease (Advanced Cell Diagnostics) at 40° C for 30 min in a HybEZ hybridization oven (Advanced Cell Diagnostics). Probe directed against Crp mRNA was applied at 40°C in the following order: target probes, preamplifier, amplifier; and label probe for 10 minutes. After each hybridization step, slides were washed two times in a washing buffer (Advanced Cell Diagnostics) at room temperature. Chromogenic detection was performed followed by counterstaining with hematoxylin QS (Vector Labs, Burlingame, CA). Staining was visualized using an Aperio CS2 whole slide scanner (Leica Biosystems, Buffalo Grove, IL). Images were reviewed using Imagescope software (Leica).
Isolation of primary hepatocyte, periportal hepatocyte and pericentral/centrilobular hepatocyte
Primary mouse hepatocytes were isolated using Liberase perfusion protocol as previously described.(23) Briefly, 8–12 weeks old C57/BL/6 mice were euthanized (pentobarbital sodium). Mouse liver was perfused from the inferior vena cava with HBSS perfusion media (Ca2+, Mg2+, and phenol red free Hank’s balanced salt solution containing 1 mM EDTA and 25mM HEPES) at the flow rate of 3ml per minute at 42°C until the blood was completely washed out from liver. An incision was cut in the portal vein to let blood out of the liver. The liver was then perfused with 10 ml HBSS digestion media (HBSS with Ca2+, Mg2+, and phenol red containing 25mM HEPES), adding 250ug liberase™ Research grade (Roche) at the flow rate of 3 ml per minute at 42°C. After liberase perfusion was finished, liver was gently dissected out and placed in 10ml cold HBSS digestion media on ice for cell purification. The liver sack was fractured and the resulting cell suspension was filtered through a 70 um cell strainer washing with DMEM media. The resulting suspension was centrifuged at 100 × g for 2 min, and the pellet was resuspended in 20ml DMEM media. The suspension was mixed with an equal volume of 90% Percoll and centrifuged at 200 × g for 10 min. The pellet was resuspended in 20ml DMEM media and centrifuged (100 × g for 2 min). This hepatocyte pellet was processed for assays described herein.
The pericentral (PC) and periportal (PP) regions were isolated using the digitonin-collagenase perfusion method as previously described.(24, 25) For isolation of pericentral hepatocytes, 24G i.v. catheters (BD Angiocath) were placed in the portal vein and inferior vena cava. Blood was removed by infusion HBSS perfusion media from the portal vein at the flow rate of 3ml per minute at 42°C until the blood was completely flushed from the liver. Digitonin media (2ml, 4 mg/ml in HBSS perfusion media) was infused through the portal vein. Digitonin was removed by retrograde perfusion with 20–25ml HBSS perfusion media from the inferior vena cava. Then liberase buffer was infused from the inferior vena cava and steps from the described from primary hepatocytes isolation repeated at this point. The final pellet contains live purified hepatocytes from the centrilobular or pericentral (PC) region. For isolation of periportal hepatocytes, 24G i.v. catheters (BD Angiocath) were set into the portal vein and inferior vena cava. Blood was removed by infusion HBSS perfusion media from the inferior vena cava until the blood was completely flushed from the liver. Digitonin buffer (2 ml, 4 mg/ml in HBSS perfusion media) was infused from the inferior vena cava. Digitonin was removed by retrograde perfusion with 20–25ml HBSS perfusion buffer from portal vein. Then liberase buffer was infused from the portal vein and the hepatocytes were similarly isolated using the above describe method. The final pellet contains live purified hepatocytes from the periportal (PP) region.
Immunohistochemical staining of C-reactive protein (CRP), glutamine synthetase, and p50
Mouse livers were collected and fixed with 4% buffered paraformaldehyde overnight followed by 70% ethanol until embedded with paraffin. Samples were then cut into 5 μm sections and deparaffinized and rehydrated with xylene and ethanol. After antigen retrieval (antigen unmasking solution, H-3301, Vector Laboratories, Burlingame, CA, USA), tissue sections were permeabilized with 0.5% Triton X, quenched with 100 mM glycine, 0.5% Chicago Sky Blue 6B (Sigma-Aldrich, C8679–25G), and blocked with Sea Block (Thermo Scientific, #37527) and Fc receptor block (Innovex Biosciences, NB309–15). Sections were then immunostained with anti-CRP (1:200, Invitrogen # 710269) or anti-p50 (1:250, Abcam, 32360), anti-glutamine synthetase (1:250, Synaptic systems, 367005), at 4°C overnight, followed by a secondary antibody incubation in donkey-anti-rabbit Alexa Fluor 647 (1–200, Life Technologies, A31573,), donkey-anti-guinea pig 555 (1–200, Millipore, SAB4600297) for 1 hour at room temperature. 0.1% Sudan Black B (Sigma-Aldrich, 199664–25G) in 70% ethanol was used to minimize autofluorescence of liver sections in a ten-minute incubation step. Finally, nuclei of liver cells were stained with DAPI, mounted with ProLong Gold antifade reagent (Invitrogen, P36934) Sections stained for CRP and glutamine synthetase were imaged with an IX83 microscope and DP80 camera using CellSens software (Olympus Life Science, Waltham, MA, USA), while sections stained for p50 and glutamine synthetase were imaged with a BC43 Benchtop Confocal Microscope (Andor Technology, Belfast, UK) using ImarisViewer software (Oxford Instruments, Abingdon, UK).
Evaluation of nuclear NF-κB binding by EMSA
IRDye 700 phosphoramidite-labeled oligonucleotides with the consensus sequence for NFκB (5’-AGTTGAGGGGACTTTCCCAGGC-3’) (829–07924, Li-cor, Lincoln, NE, USA) and custom designed p50-CRP (5’-AGTTGAAATTTCCCATAGGC-3’) were used as a probe to evaluate nuclear NFκB binding. In order to identify the NFκB subunit proteins in the binding complex (supershift), p50 (#13586, Cell Signaling, Danvers, MA, USA) subunit antibodies were incubated with nuclear proteins for 25 min at 4°C prior to the addition of the labeled probe.
Identification of p50 binding sites in the murine CRP promoter
Using the UCSC genome browser (https://genome.ucsc.edu), we queried the Mouse Assembly GRCm39/mm39 gene sequence identified as “CRP”. We searched the 600 bases upstream of the transcription start site for the presence of p50 binding sites using LASAGNA-Search (https://biogrid-lasagna.engr.uconn.edu/lasagna_search/). (26)
Chromatin Immunoprecipitation
Chromatin was prepared using the Magna ChIP G tissue kit (Millipore) per protocol with the notable modification of sonicating tissues in nuclear lysis buffer (Millipore). Sonication was performed using the Diagenode Bioruptor Plus for three sets of ten 30 second cycles. Proper sonication of chromatin (200–900 base pairs) was verified for reverse cross-linked DNA via electrophoresis in a 1.2% agarose gel. Chromatin was quantified using a spectrophotometer. 25 μg of chromatin was diluted to a total volume of 500 μL in dilution buffer. Antibodies used for immunoprecipitation included Rabbit IgG (Millipore), and anti-p50 (Abcam, 32360). Immunoprecipitations were incubated at 4°C overnight. Reverse cross-linking of immunoprecipitated chromatin was accomplished with a two-hour incubation at 62°C. Immunoprecipitated and purified DNA quality was assessed using a spectrophotometer. Enrichment of the CRP promoter was assessed by real-time qPCR using SYBR green reagent (Qiagen) and primers designed to span a section of the promoter region located 150 base pairs upstream and downstream of the p50 consensus sequence. Results were expressed as percent input.
Statistical Analysis
Statistical analysis was conducted with GraphPad Prism 9 software (GraphPad, San Diego, CA, USA). We used the null hypothesis that no difference existed between control and endotoxemia exposed. We evaluated data using two-way ANOVA for multiple groups with potentially interacting variables (time, sex/genotype). All groups contained at least 3 animals who were exposed across at least 3 experimental replicates. Statistical significance between and within groups determined by means of Tukey’s method of multiple comparisons. Statistics were evaluated using Prism (GraphPad Software, Inc). Statistical significance was defined as p<0.05. In all figures, each data point represents a unique biologic sample.
Results
Sublethal endotoxemia induces the hepatic acute phase response in male and female mice
First, we sought to determine whether endotoxemia induced by an intraperitoneal injection of LPS (5 mg/kg, IP) induced the acute phase response in adult male and female C57BL/6 mice. For this study, we used Crp (c-reactive protein), Saa-1 (serum amyloid A1), Apcs (amyloid p component, serum), Fga (fibrinogen alpha chain), Hp (haptoglobin) and Lbp (lipopolysaccharide binding protein), to test for presence of the hepatic APR.(9) We found increased hepatic expression of these six genes in both male and female mice within 5 hours of exposure to endotoxemia (Fig. 1A–F). Of note, although hepatic expression of Crp, Apcs, and Fga significantly increased in endotoxemic female mice, induction was lower than what was observed in similarly exposed male mice (Fig. 1A, C, D). Western blot performed on hepatic tissue from endotoxemic male and female mice demonstrated significant protein accumulation at 5 hours of exposure (Fig. 1G and H). These results confirm that this model of sub-lethal endotoxemia induces the hepatic acute phase response and hepatic CRP expression in adult male and female mice.
Figure 1. Sublethal endotoxemia induces hepatic expression of APR factors.
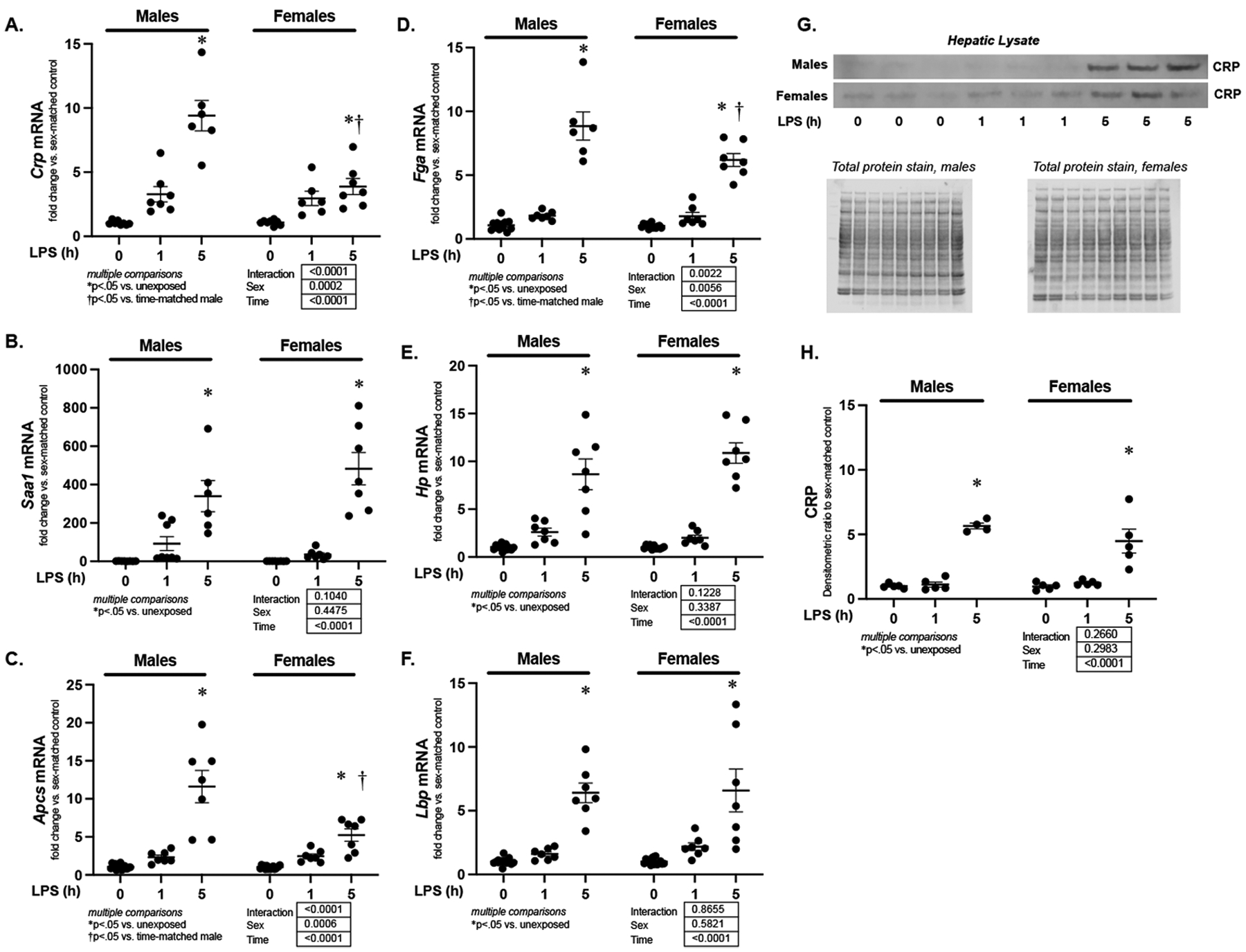
Fold change in hepatic mRNA expression of (A) Crp, (B) Saa-1, (C) Apcs, (D) Fga (E) Hp and (F) Lbp in endotoxemic (LPS 5 mg/kg, IP; 1 and 5 hours) male and female mice. Data expressed as mean ± SEM (N = 5–7 per sex per exposure). Results of 2way ANOVA for interaction, sex and time provided. Results of multiple comparisons given, with *p<.05 vs. unexposed sex- matched control. †p<.05 vs. time-matched similarly exposed male. (G) Representative Western blot of CRP on whole liver lysate isolated from endotoxemic (LPS 5 mg/kg, IP; 1 and 5 hours) male and female mice. Total protein stain as loading control. (H) Densitometric analysis of whole liver lysate CRP. Data normalized to total protein and expressed as mean ± SEM (N = 4–6 per sex per exposure). *p < 0.05 vs. unexposed control. h = hours.
Sublethal endotoxemia induces transcription of acute phase response genes in hepatocytes
Next, we sought to determine the hepatocyte specific response to endotoxemia. First, we performed blinded histopathological analysis of hepatic tissue isolated from endotoxemic mice. At 5 hours of exposure, both male and female mice had histopathological evidence of a hepatic response to endotoxemia, included elevated scores of hepatocyte injury and hepatic inflammation (Fig. 2A–D). Next whether the expression of these APR markers was increased specifically in hepatocytes isolated from endotoxemic mice. We found increased expression of Crp, Saa-1, Apcs, Fga, and Hp in hepatocytes isolated from endotoxemic mice within 1 hour and through 5 hours of exposure, while Lbp did not reach significance until 5 hours (Fig. 2E–J). These results confirm that in this model of sub-lethal endotoxemia, expression of acute phase response genes increases in hepatocytes.
Figure 2. Sublethal endotoxemia induces hepatocyte inflammation & injury, expression of APR factors.
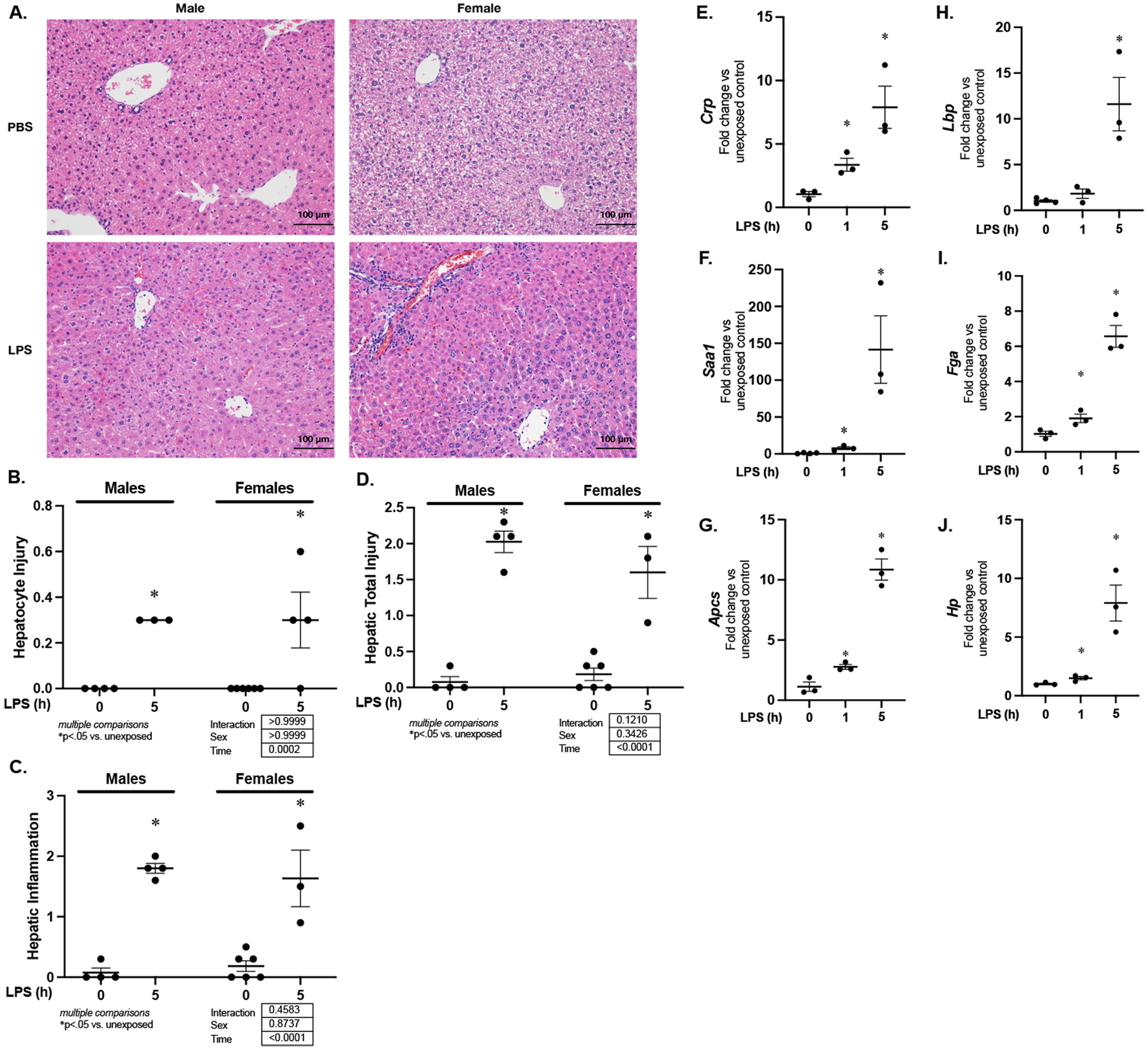
(A) Representative H&E stained hepatic sections, (B) hepatic injury scores, (C) hepatic inflammation scores, and (D) total injury scores for liver sections from endotoxemic (LPS 5 mg/kg, IP; 5 hours) male and female mice (E-J) Fold change in mRNA expression of (E) Crp, (F) Saa-1, (G) Apcs, (H) Lbp (I) Fga and (J) Hp following in hepatocytes isolated from endotoxemic (LPS 5 mg/kg, IP; 1 and 5 hours) mice. Data expressed as mean ± SEM (N = 3 per time point). *p < 0.05 vs. unexposed control. h = hours.
Sublethal endotoxemia induced APR is zone specific
Having confirmed hepatocyte expression of Crp, Saa-1, Apcs, Fga, Hp and Lbp increased during endotoxemia, we next sought to determine if this expression was zone specific. We use used digitonin-collagenase prefusion to separate periportal (PP) and pericentral (PC) hepatocytes from endotoxemic mice.(25, 27, 28) First, to confirm enrichment of PP and PC hepatocytes, we assessed expression of PP markers (Alb, albumin; Ass1, argininosuccinate synthase 1; Cyp2f2, cytochrome P450 family 2 subfamily f polypeptide 2) and PC markers (Cyp2e1, cytochrome P450 family 2 subfamily E member 1; Glul, glutamate-ammonia ligase or glutamine synthetase) in the PP and PC isolations. We found that the expression of the PP markers Alb, Ass1 and Cyp2f2 were significantly enriched in the PP isolation (Fig. 3A–C). Furthermore, we found that the PC markers Cyp2e1 and Glul were significantly increased in the PC isolation (Fig. 3D and E). To confirm that our isolations were appropriately enriched with the cells from the expected zone, we assessed the expression of the PP marker PEPCK (phosphoenolpyruvate carboxykinase) and the PC markers glutamine synthetase and CYP2E1 using Western blot. Consistent with successful enrichment of these respective fractions, we found that PEPCK was enhanced in the PP fraction, while glutamine synthetase and CYP2E1 were enriched in the PC fraction (Fig. 3F).
Figure 3. Endotoxemia-induced expression of APR factors is hepatic zone specific.
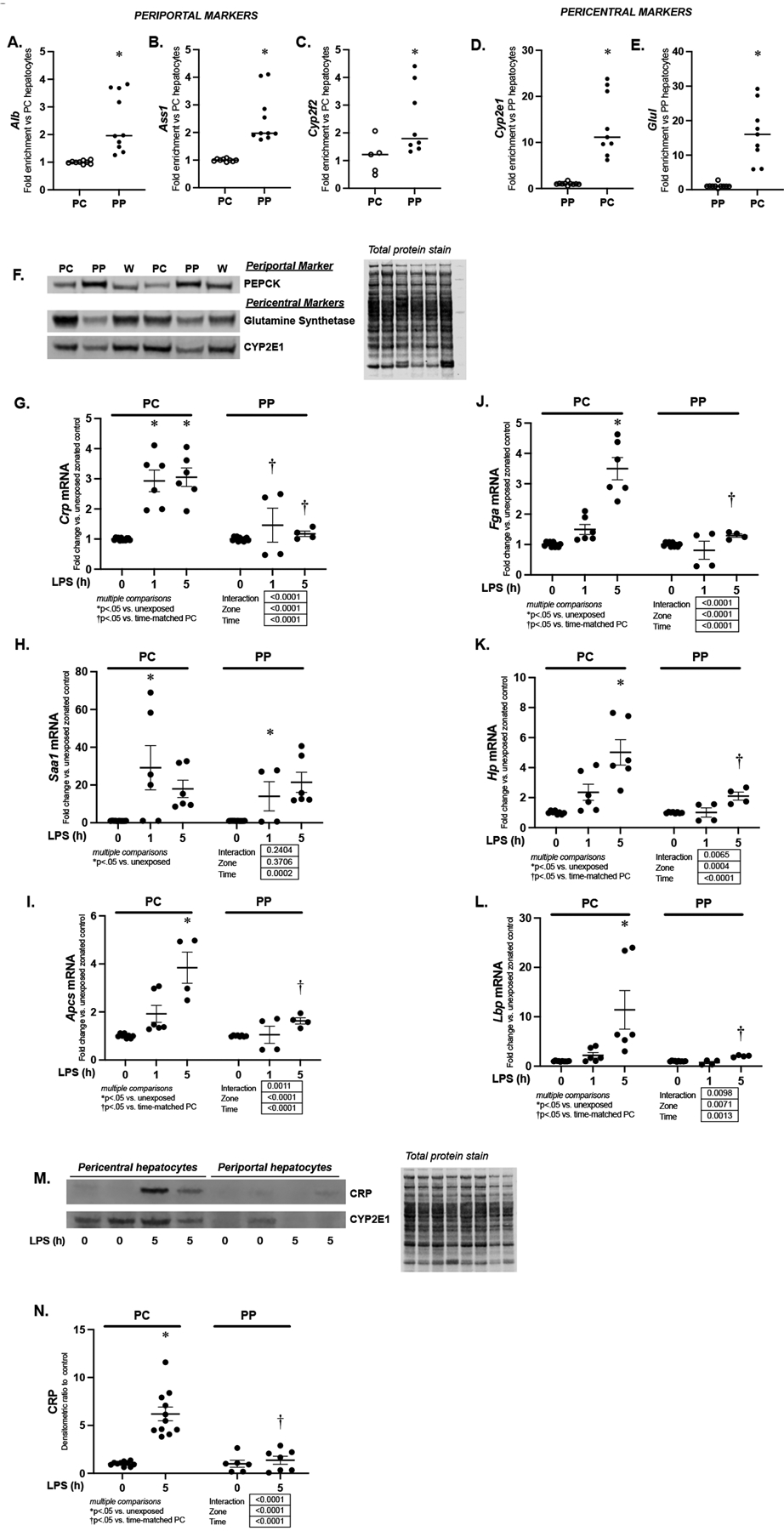
(A-C) Fold enrichment of mRNA expression of periportal markers (A) Alb, (B) Ass1 and (C) Cyp2f2 n periportal hepatocytes isolated using digitonin-collagenase perfusion. Data were normalized to pericentral expression and expressed as mean ± SEM (N = 8–10 per isolation). *p < 0.05 vs. PC expression (D-E) Fold enrichment of mRNA expression of pericentral markers (C) Cyp2e1, and (D) Glul in pericentral (PC) hepatocytes isolated using digitonin-collagenase perfusion. Data were normalized to periportal expression and expressed as mean ± SEM (N = 8–10 per isolation). *p < 0.05 vs. PP expression (F) Representative Western blot of PEPCK, glutamine synthetase, and CYP2E1 on pericentral (PC), periportal (PP) and whole liver (W) lysates. On this representative image, isolations from six separate animals are shown (Two each for PC, PP and W). Total protein stain provided as loading control. (G-L) Fold change in mRNA expression of (F) Crp, (G) Saa-1, (H) Apcs, (I) Fga (J) Hp and (K) Lbp in pericentral (PC) and periportal (PP) hepatocytes isolated from endotoxemic (LPS 5 mg/kg, IP) mice. Data expressed as mean ± SEM (N = 4–6 per time point). Results of 2way ANOVA for interaction, zone and time provided. Results of multiple comparisons given. *p < 0.05 vs. unexposed zone-matched control. †p < 0.05 vs. PC hepatocytes isolated from similarly exposed animals. h = hours. (M) Representative Western blot for CRP and Cyp2e1 on pericentral (PC) and periportal (PP) cytosolic protein samples from endotoxemic (LPS 5 mg/kg, IP; 5 hours) mice. In this image, isolations from eight separate animals are shown (Four each for PC and PP). (N) Densitometric analysis of pericentral (PC) and periportal (PP) cytosolic CRP from mice exposed to sublethal endotoxemia (LPS 5 mg/kg, IP; 5 hours). Data normalized to total protein and expressed as mean ± SEM (N = 6–11 per time point). Results of 2way ANOVA for interaction, zone and time provided. Results of multiple comparisons given, with *p<.05 vs. unexposed zone-matched controls. †p<.05 vs. time-matched similarly exposed PC hepatocytes isolated from similarly exposed animals.
Next, using these isolated cell fractions, we assessed the zone-specific expression of endotoxemia-induced APR genes. At 5 hours of exposure, expression of Crp, Apcs, Lbp, Fga and Hp were significantly elevated in only the pericentral isolates, and this expression was significantly higher compared to periportal isolates (Fig. 3G, I, J, K, L). Of note, Saa1 expression was significantly and similarly increased in both pericentral and periportal isolates at 1 hour of exposure (Fig. 3H). Having demonstrated that endotoxemia-induced hepatic expression of Crp mRNA was zone-specific, we next asked whether protein translation was zone-specific. Western blot confirmed endotoxemia-induced CRP expression was concentrated in pericentral hepatocytes (Fig. 3M and N).
Endotoxemia induced Crp localizes to pericentral hepatocytes
To assess the hepatic distribution and cell locality of Crp expression, we performed RNAScope on hepatic tissue obtained from endotoxemic mice (Fig. 4A). Following 5 hours of endotoxemia, Crp expression was enhanced in pericentral hepatocytes (Fig. 4A; left inset, black arrows pointing to hepatocytes expressing Crp mRNA) when compared to periportal hepatocytes (Fig. 4A; right inset).
Figure 4. Endotoxemia induces CRP protein expression in pericentral hepatocytes.
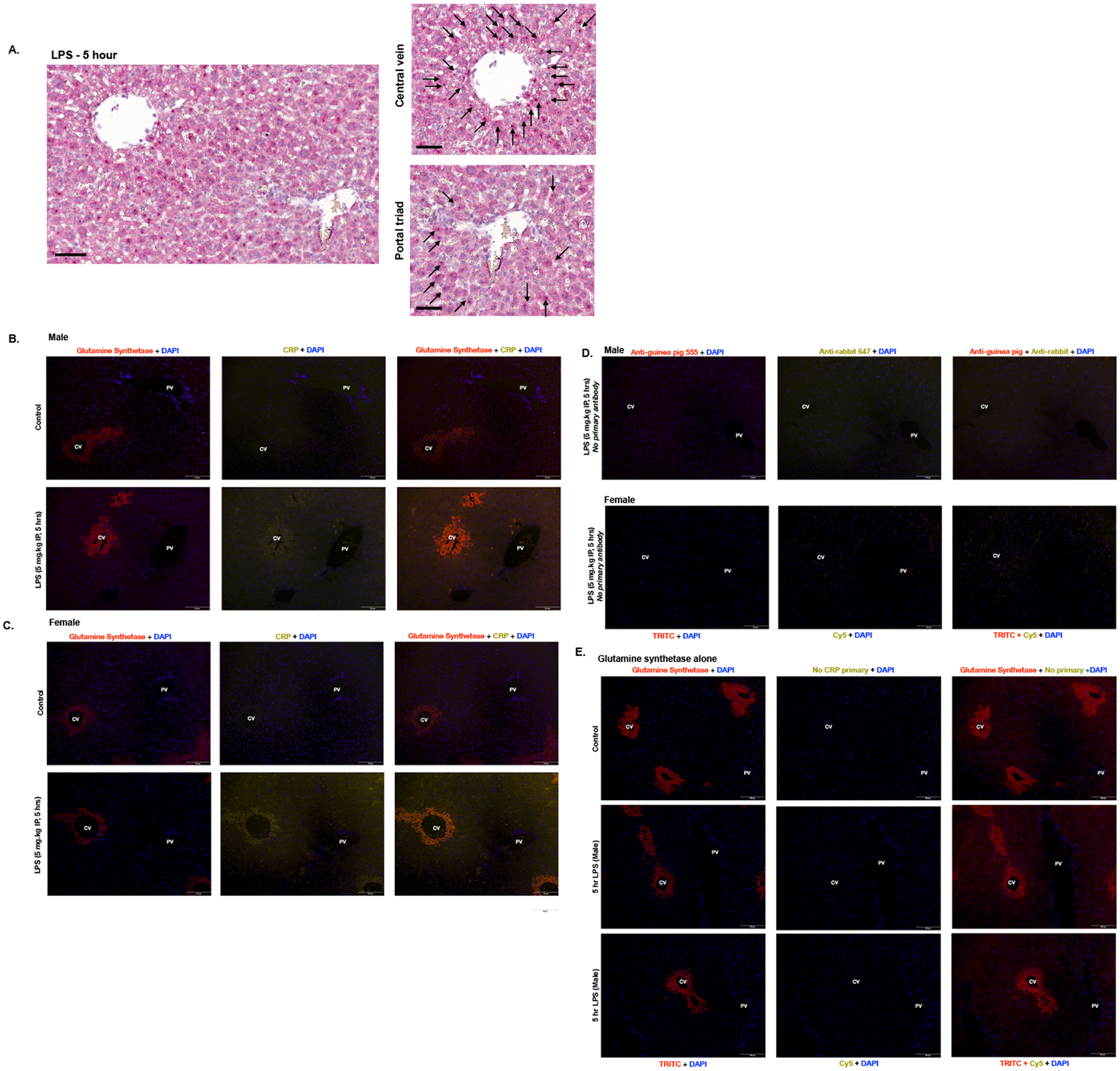
(A) In situ hybridization RNAScope with Crp (red) of hepatic specimens from endotoxemic (LPS 5 mg/kg, IP; 5 hours) mice. Black arrowheads depict positive staining cells. First image includes a portal trial and central vein, followed by zoomed in images of the portal triad and central vein. The black bar measures 60μm. (B-C) Immunofluorescent staining of hepatic tissue from (B) male and (C) female mice with glutamine synthetase (red) + DAPI nuclear stain (blue) in the Column 1; CRP (yellow) + DAPI (blue) in the Column 2; and an overlay of all three channels in the Column 3. Row 1: Hepatic tissue from PBS exposed mice. Row 2: Hepatic tissue from endotoxemic (LPS 5 mg/kg IP, 5 hours) mice. Central veins (CV) are located on the left and portal veins (PV) are on the right of each image. Scale bar is 100 μm. (D) Hepatic tissue from male (Row 1) and female (Row 2) endotoxemic (LPS 5 mg/kg IP, 5 hours) mice stained with DAPI and secondary antibody but without primary antibodies. (D) Immunofluorescent staining of hepatic tissue from control male (Row 1), endotoxemic (LPS 5 mg/kg IP, 5 hours) male (Row 2 and 3), with glutamine synthetase (red) + DAPI nuclear stain (blue) in Column 1; No CRP primary (yellow) + DAPI (blue) in the Column 2, and an overlay of all three channels in Column 3. Central veins (CV) are labeled on the left and portal veins (PV) are on the right of each image. Scale bar is 100 μm.
Next, we sought to corroborate these findings using immunohistochemical (IHC) staining. We used glutamine synthetase staining to identify the central vein which would be marked by a ring of pericentral hepatocytes expressing glutamine synthetase (Fig 4B and C). We found increased CRP expression in the hepatocytes surrounding the central vein in both male (Fig. 4B) and female (Fig. 4C) mice at 5 hours of endotoxemia. Staining of livers with just secondary antibody (Fig. 4D) or glutamine synthetase in the absence of CRP antibody (Fig. 4E) confirmed that positive signal in CRP-stained samples was not due to non-specific staining in the setting of endotoxemia.
Sublethal endotoxemia induces hepatic NFκB and CEBP/β nuclear translocation
In human hepatocytes, the transcription factors NFκB and CEBP/β regulate the increase in CRP expression observed with the APR.(29–33) However, this mechanism has not been interrogated in the mouse, or in the context of zone-specific response to endotoxemia. Therefore, we first assessed hepatic nuclear extracts from male and female mice for evidence of NFκB and CEBP/β activation. First, we performed Western blot and probed for the NFκB subunits p65 and p50, as well as CEBP/β (Fig. 5A–H). We found significant increases of p65 (1 hour, Fig. 5A, B, E, F), p50 (1 and 5 hours, Fig. 6A, C, E, G) and CEBP/β (5 hours, Fig. 6A, D, E, H). Having observed nuclear translocation of these transcription factors, we assessed for the presence of NFκB binding to oligonucleotides containing the classic consensus sequence (5’-AGTTGAGGGGACTTTCCCAGGC-3’). Consistent with nuclear accumulation of p65 and p50 at 1 hour (Fig 5A–C, E–G), consensus sequence binding increased at 1 hour (Fig. 5I). Furthermore, binding returned to baseline by 5 hours of exposure (Fig. 5J), consistent with the pattern observed for the nuclear presence of p65 (Fig. 5A, B, E, F). However, it is important to note that at this later time point, both p50 and CEBP/β remain in the nucleus at levels that exceed control (Fig. 5A, C, D, E, G, H).
Figure 5. Sublethal endotoxemia induces hepatic NFκB and CEBP/β nuclear translocation.
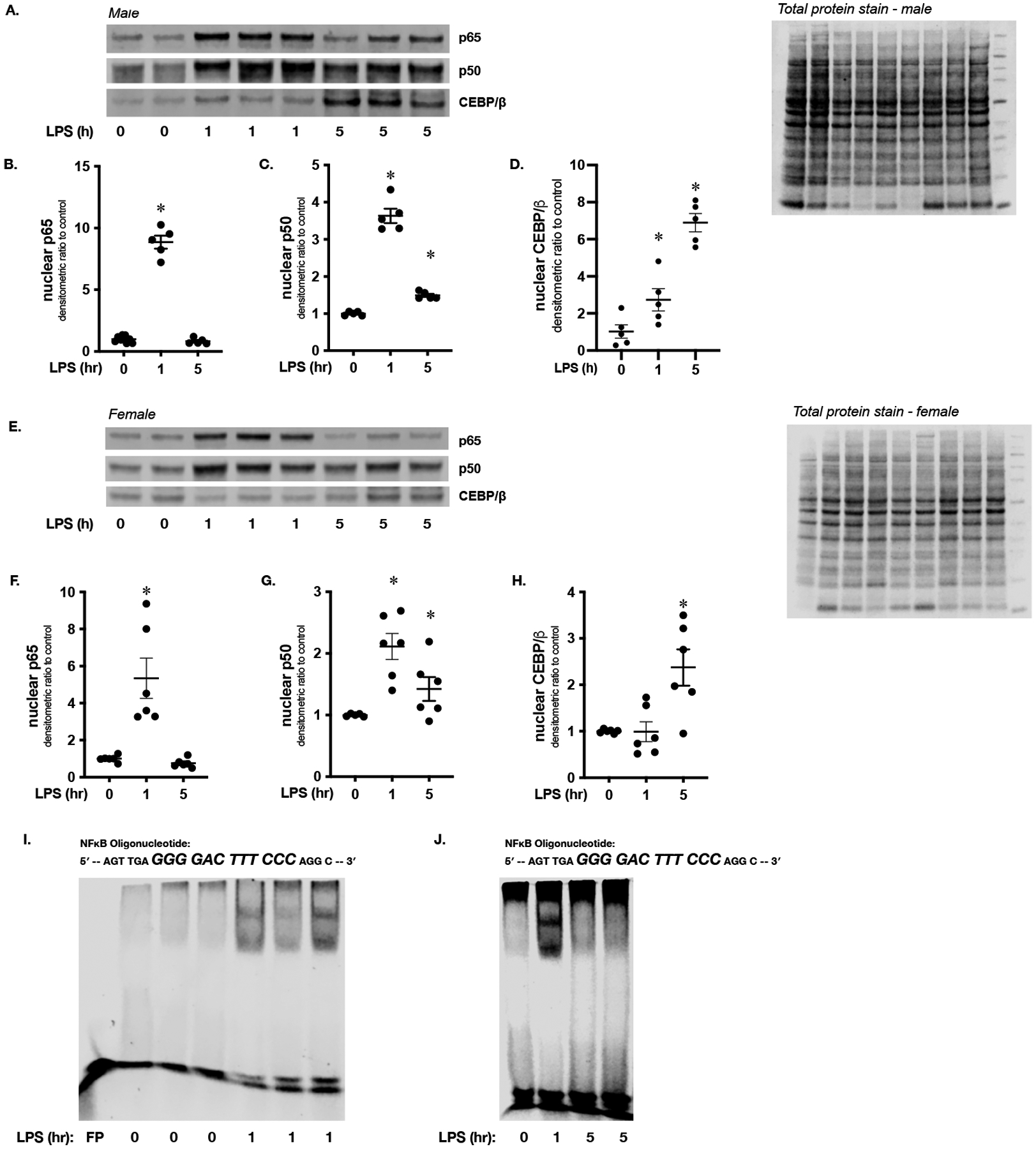
(A) Representative Western blot of p65, p50 and CEBP/β on hepatic nuclear extracts isolated from endotoxemic (LPS 5 mg/kg, IP; 1 and 5 hours) male mice. Total protein stain as loading control. (B-D) Densitometric analysis of nuclear (B) p65, (C) p50 and (D) CEBP/β. Data normalized to total protein and expressed as mean ± SEM (N = 4–5 per time point). *p < 0.05 vs. unexposed control. (E) Representative Western blot of p65, p50 and CEBP/β on hepatic nuclear extracts isolated from female mice exposed to endotoxemia (LPS 5 mg/kg, IP; 1 and 5 hours). Total protein stain as loading control. (F-H) Densitometric analysis of nuclear (F) p65, (G) p50 and (H) CEBP/β. Data normalized to total protein and expressed as mean ± SEM (N = 4–5 per time point). *p < 0.05 vs. unexposed control. (I) Representative EMSA using an IR labeled oligonucleotide containing the NFκB consensus sequence (5’-AGTTGAGGGGACTTTCCCAGGC-3’) and hepatic nuclear extracts isolated from mice exposed to endotoxemia (LPS 5 mg/kg, IP; 1 hour). FP = free probe (J) Representative EMSA using an IR labeled oligonucleotide containing the NFκB consensus sequence (5’-AGTTGAGGGGACTTTCCCAGGC-3’) and hepatic nuclear extracts isolated from endotoxemic (LPS 5 mg/kg, IP; 1 and 5 hours) mice.
Figure 6. Sublethal endotoxemia induces NFκB subunit p50 binding to consensus sequence in the murine CPR promoter.
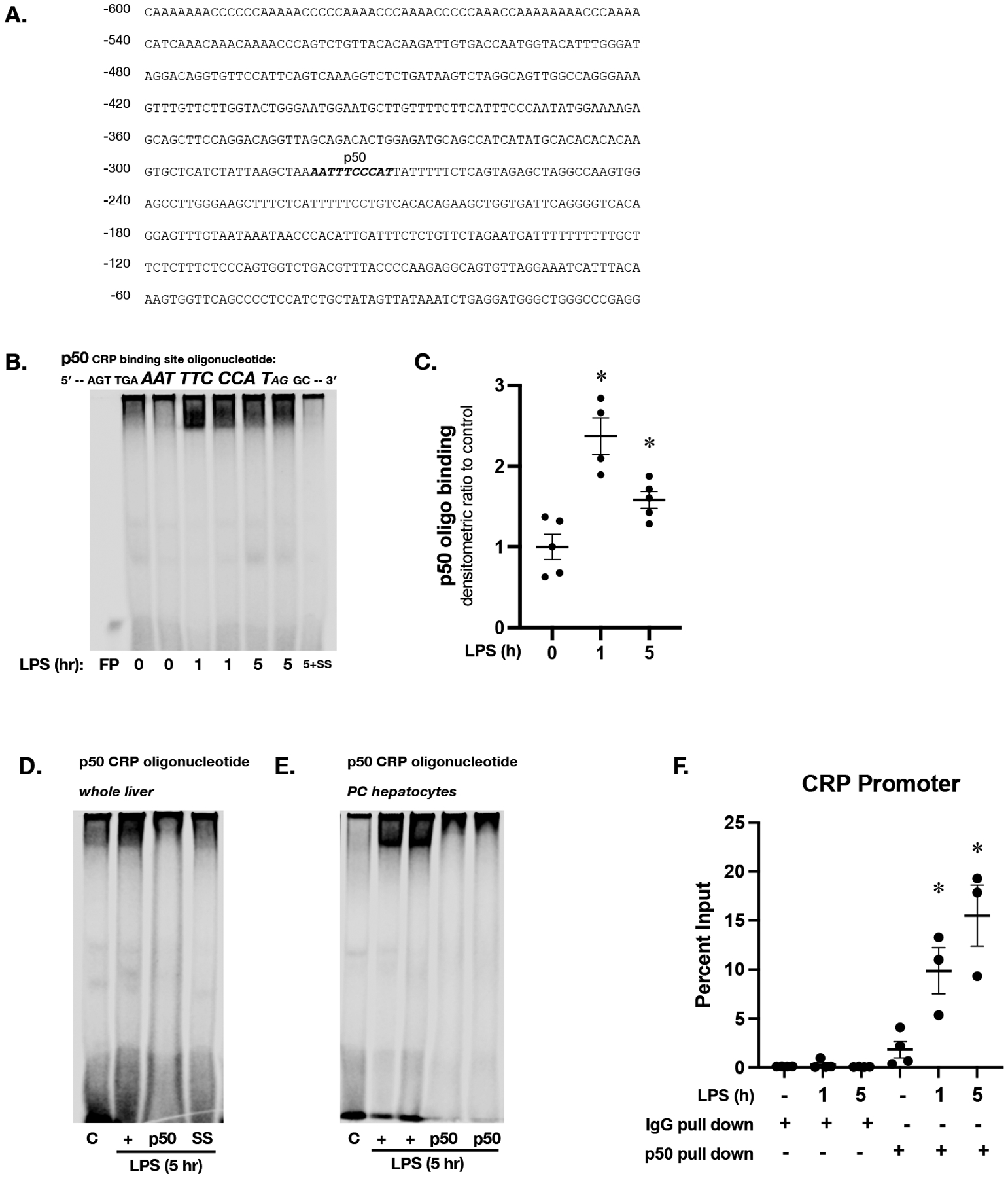
(A) Sequence of the most proximal 600 bases in the promoter of the murine CRP promoter, with putative p50 binding site labeled and in bold italics (B) Representative EMSA using an IR labeled oligonucleotide containing the p50 consensus sequence found in the murine CRP promoter (5’-AGT TGA AAT TTC CCA TAG GC-3’) and hepatic nuclear extracts isolated from mice exposed to endotoxemia (LPS 5 mg/kg, IP; 1 and 5 hours). FP = free probe; SS = salmon sperm (C) Densitometric analysis of p50 consensus sequence binding. Data expressed as mean ± SEM (N = 3 per time point). *p < 0.05 vs. unexposed control. (D) Representative EMSA using an IR labeled oligonucleotide containing the p50 consensus sequence found in the murine CRP promoter (5’-AGT TGA AAT TTC CCA TAG GC-3’) and hepatic nuclear extracts. C = unexposed control; + = LPS exposed (5 mg/kg, IP; 5 hours); p50 = LPS exposed + p50 antibody; SS = salmon sperm. (E) Representative EMSA using an IR labeled oligonucleotide containing the p50 consensus sequence found in the murine CRP promoter (5’-AGT TGA AAT TTC CCA TAG GC-3’) and pericentral hepatocyte nuclear extracts. C = control; + = LPS exposed (5 mg/kg, IP; 5 hours); p50 = LPS exposed (5 mg/kg, IP; 5 hours) + p50 antibody. (F) ChIP qPCR results with primers specific to the p50 binding site of the CRP promoter and hepatic chromatin from endotoxemic (LPS 5 mg/kg, IP; 1 and 5 hours) mice pulled down with either IgG or p50 antibody. Data expressed as mean ± SEM (N = 3 per time point). *p < 0.05 vs. control.
Sublethal endotoxemia induces NFκB and CEBP/β binding to consensus sequences present in the murine CRP promoter
Noting that by 5 hours of endotoxemia hepatic NFκB consensus sequence had returned to baseline despite the nuclear accumulation of p50 and CEBP/β, we searched the murine CRP promoter for additional potential binding sites. Importantly, the murine CRP promoter contains a p50 specific consensus sequence (−280 to −271) (Fig. 6A). Therefore, we performed EMSA using custom designed p50-CRP (5’-AGT TGA AAT TTC CCA TAG GC-3’) specific oligonucleotide. We found increased binding to the p50-CRP (Fig. 6B and 6C) oligonucleotide at 1 and 5 hours of endotoxemia. To confirm the presence of p50 at the consensus sequence binding site, we performed super-shift assays. Incubating samples with antibody directed against p50 (Fig. 6D, lane 3) obliterated binding to the p50-CRP consensus sequence observed in samples from endotoxemic mice (Fig. 6D, lane 2).
To corroborate these findings, we performed EMSA using the p50-CRP oligonucleotide with nuclear extracts from pericentral hepatocyte isolations (Fig. 6E). These results demonstrate increased binding to the p50-CRP oligonucleotide in PC hepatocytes after 5 hours of endotoxemia. Similar to what was observed using whole hepatic nuclear extracts, incubating samples with antibody directed against p50 (Fig. 6E, lanes 4 and 5) obliterated binding to the p50-CRP consensus sequence observed in pericentral samples from endotoxemic mice (Fig. 6D, lanes 2 and 3). Finally, we performed chromatin immunoprecipitation using antibody directed against p50 and primers designed specifically to capture the p50 binding site identified in the murine CRP promoter (Fig. 6F). Taken together, this set of experiments demonstrate that with endotoxemia, the NFκB subunit p50 is available in the nucleus of pericentral hepatocytes and able to bind to consensus sequences found in the murine CRP promoter.
Sublethal endotoxemia induced p50 nuclear translocation occurs in pericentral hepatocytes
Next, we used fluorescent immunostaining to determine whether p50 as present in the nuclei of pericentral hepatocytes of endotoxemic mice. In control mice, pericentral hepatocytes again identified with glutamine synthetase staining (Fig. 7A, column 1) demonstrated low levels of nuclear p50 staining (Fig. 7A, column 2). By 5 hours of endotoxemia, nuclear 50 was clearly identified in the nuclei of pericentral hepatocytes (Fig. 7B, column 2).
Figure 7. Sublethal endotoxemia induced p50 nuclear translocation occurs in pericentral hepatocytes.
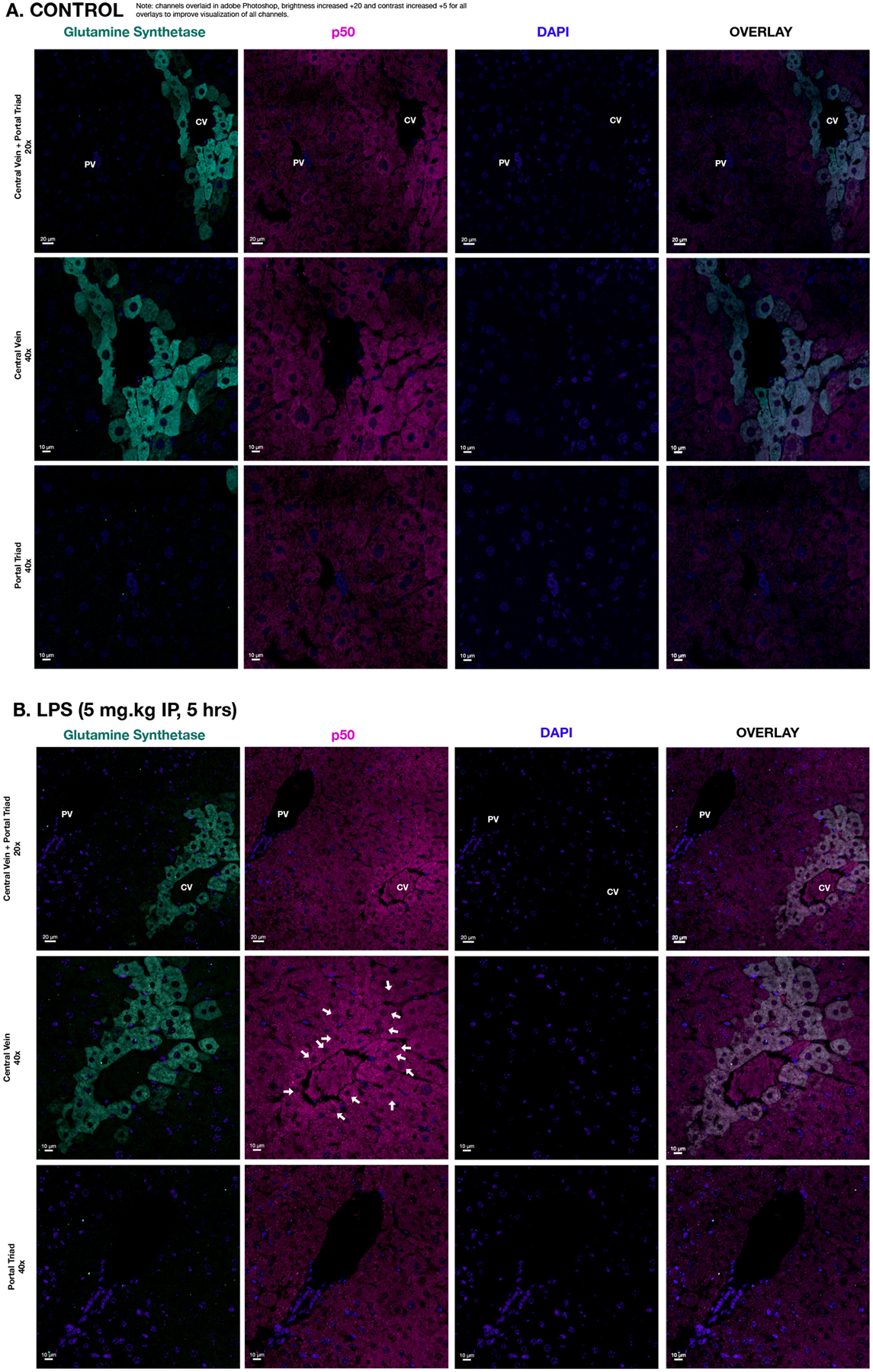
Representative immunofluorescence staining of the hepatic tissue from (A) control and (B) endotoxemic (LPS 5 mg/kg IP, 5 hrs) mice with glutamine synthetase as a central vein marker (cyan; Column 1); p50 (magenta; Column 2); DAPI (blue; Column 3); and overlay (Column 4). Row 1 contains images of a portal triad and central vein (scale bar 20 μm), while the Row 2 and 3 include images of the central vein and portal triad, respectively, at higher magnification (scale bar 10 μm). White arrows indicate nuclear p50 staining.
Sublethal endotoxemia induced APR is attenuated in p50−/− mice
Lastly, we interrogated the hepatic APR induced by endotoxemia in p50−/− mice. Of note, these mice have intact p65 nuclear translocation at 1 hour of exposure (Fig. 8A and B) and CEBP/β at 5 hours of exposure (Fig. 8A and B), indicating an intact response to endotoxemia. Furthermore, it is well recognized that IL-6, IL-1β and TNF-α are simultaneously NFkB target genes and key regulators of the APR.(9) Thus, we determined the hepatic expression of these key factors in WT and p50−/− mice (Fig. 8C). We found that the hepatic expression of Il6, Il1b and Tnf were not attenuated in endotoxemic male and female the p50−/− mice (Fig. 8C). In contrast, in both male and female mice at 5 hours of exposure, hepatic expression of Crp, Apcs, Fga, Hp and Lbp was either not induced or significantly attenuated in p50−/− mice (Fig. 8C and D). In contrast to endotoxemic WT mice, expression of Crp was not increased in pericentral hepatocytes isolated from p50−/− mice at 5 hours of exposure (Fig. 8E). Lastly, hepatic CRP protein expression was significantly attenuated in endotoxemic p50−/− male and female mice when compared to similarly exposed WT (Fig. 8F and G). These results confirm a key mechanistic role played by the NFκB subunit p50 in the transcriptional regulation of Crp in the setting of endotoxemia.
Figure 8. Endotoxemia induced APR is attenuated in p50−/− mice.
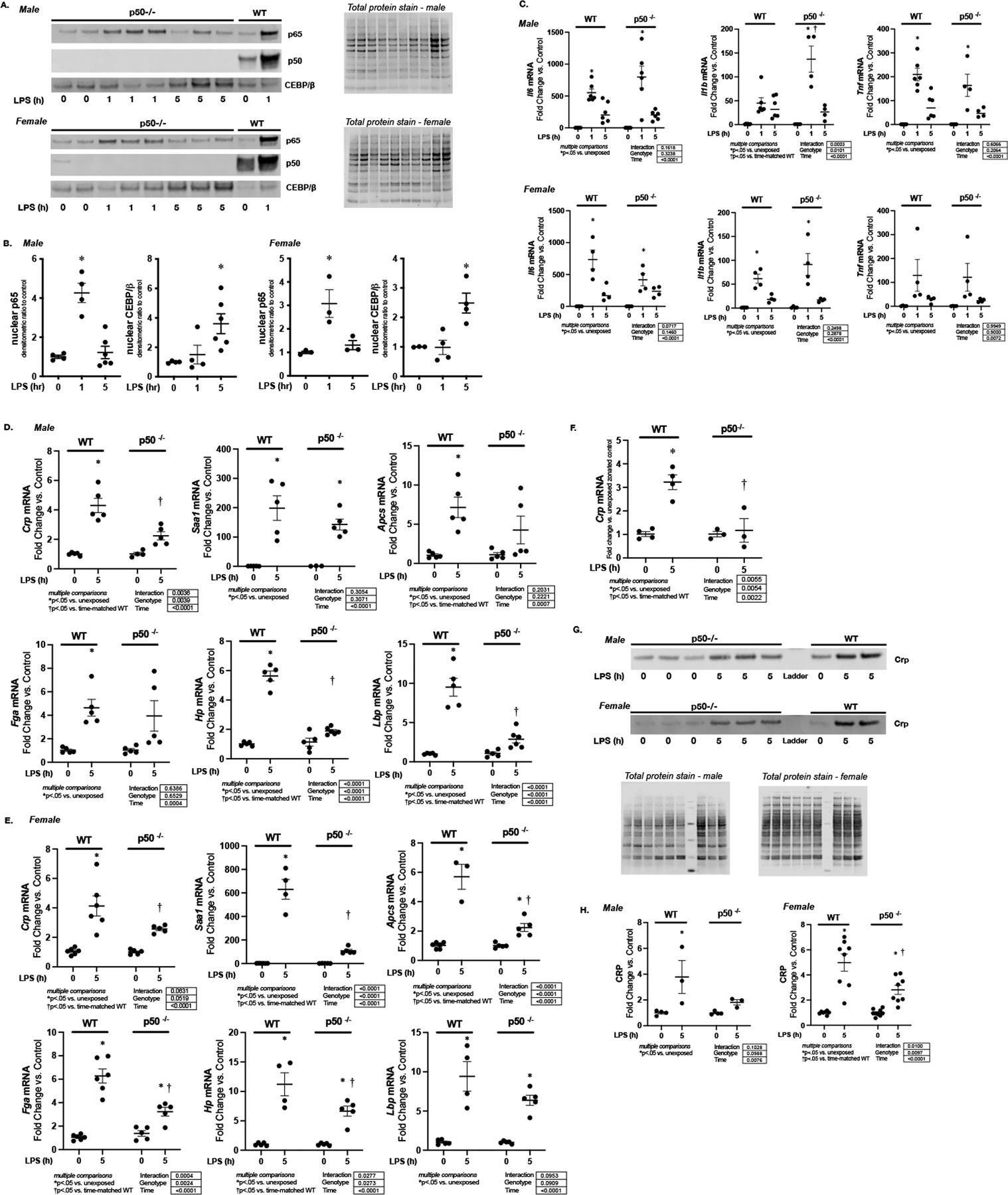
Representative Western blot of p65, p50 and CEBP/β on hepatic nuclear extracts isolated from endotoxemic (LPS 5 mg/kg, IP; 1 and 5 hours) male and female p50−/− mice. Total protein stain as loading control. Samples from WT control and endotoxemic (LPS 5 mg/kg, IP; 1 hour) male mice used as a positive control. (B) Densitometric analysis of nuclear p65, p50 and CEBP/β. Data normalized to total protein and expressed as mean ± SEM (N = 3–5 per time point). *p < 0.05 vs. unexposed control. (C) Fold change in liver mRNA expression of Il6, Il1b, and Tnf, isolated from endotoxemic (LPS 5 mg/kg, IP; 5 hours) male and female wildtype and p50−/− mice. Data expressed as mean ± SEM (N = 4–6 per time point). Results of 2way ANOVA for interaction, genotype and time provided. Results of multiple comparisons given, with *p<.05 vs. genotype-matched unexposed control. †p<.05 vs. time-matched similarly exposed WT. h = hours. (D-E) Fold change in liver mRNA expression of Crp, Saa-1, Apcs, Fga, Hp, and Lbp, isolated from endotoxemic (LPS 5 mg/kg, IP; 5 hours) (C) male and (D) female wildtype and p50−/− mice. Data expressed as mean ± SEM (N = 4–6 per time point). Results of 2way ANOVA for interaction, genotype and time provided. Results of multiple comparisons given, with *p<.05 vs. genotype-matched unexposed control. †p<.05 vs. time-matched similarly exposed WT. h = hours. (F) Fold change in Crp mRNA in pericentral (PC) hepatocytes isolated from endotoxemic (LPS 5 mg/kg, IP; 5 hours) WT and p50−/− mice. Data expressed as mean ± SEM (N = 3–4 per time point). Results of 2way ANOVA for interaction, genotype and time provided. Results of multiple comparisons given. *p < 0.05 vs. unexposed zone-matched control. †p < 0.05 vs. PC hepatocytes isolated from similarly exposed WT animals. h = hours. (G) Representative Western blot of CRP of hepatic cytoplasmic protein extracts isolated from endotoxemic (LPS 5 mg/kg, IP; 1 and 5 hours) male and female p50−/− mice. Total protein stain as loading control. (H) Densitometric analysis of cytoplasmic CRP. Data normalized to total protein and expressed as mean ± SEM (N = 3–5 per time point). Results of 2way ANOVA for interaction, genotype and time provided. Results of multiple comparisons given, with *p<.05 vs. unexposed †p<.05 vs. time-matched similarly exposed WT animals. h = hours.
Discussion
Here, we report that sublethal endotoxemia induces the hepatic APR in both male and female adult mice. Endotoxemia increases hepatocyte expression of Crp, Saa1, Apcs, Fga, Hp, and Lbp. We provide evidence that the endotoxemia-induced expression of Crp, Apcs, Fga, Hp, and Lbp is enriched in pericentral hepatocytes. We confirm that this transcriptional increase is mirrored by an increase in pericentral CRP protein expression. By focusing our work on the regulation of LPS-induced CRP expression, we determined that the p50 subunit of the NFκB transcription factor binds to a consensus sequence element found in the murine CRP promoter. These results are consistent with the results of ChIP which confirmed p50 binding to the CRP promoter in endotoxemic mice. Both RNAScope and immunostaining support the conclusion that with endotoxemia, hepatic CRP production is concentrated in pericentral hepatocytes. Importantly, these findings are temporally related to nuclear translocation of p50 in pericentral hepatocytes. Finally, induction of the APR and pericentral hepatocyte Crp expression is attenuated in p50−/− mice. These results demonstrate that the murine APR to endotoxemia is characterized by a zone-specific induction of transcription factors and CRP expression. Based on the time course of p50 nuclear translocation and target gene expression, presence of promoter region consensus sequence binding, immunohistochemistry, and the attenuated response in knockout mice, it is likely that the p50 subunit of the NFκB transcription factor is responsible for this zonal pattern of expression. These results support the hypothesis that the hepatocyte response to innate immune stimuli is zone specific, and the implications of these findings deserve further study.
Whether CRP is an acute phase protein (APP) in mice is controversial. Some reviews state it is not an APP,(34, 35) while others are more balanced and cite the conflicting literature.(36) The conclusion that CRP is not an APP in mice is based on older literature.(37, 38) These studies, published in the 1960s and 1970s, demonstrated that endotoxemia induced CRP expression, albeit at low levels.(37, 38) Since that time, more sensitive approaches have been used to show that endotoxemia induces CRP expression in mice,(19) including C57BL/6 mice exposed to similar levels of LPS used in this study.(39) Furthermore, LPS exposure increases CRP expression in cultured primary hepatocytes.(40) Other methods to induce the APR in mice also increase CRP expression.(41) Given the lack of clarity on this subject, we used multiple approaches to confirm endotoxemia-induced CRP expression in the current study. We use RT-qPCR and Western blot to evaluate hepatic CRP expression in endotoxemic mice. We demonstrate zonated CRP pericentral expression to hepatocytes by analyzing mRNA from isolated cells and by performing both RNAScope and immunostaining on hepatic tissue. These series of experiments are a comprehensive evaluation of endotoxemia-induced hepatic CRP expression. These data support the conclusion that zonated CRP expression is part of the murine APR to endotoxemia.
Our findings add to a growing body of literature demonstrating that immune function is not uniform across the hepatic zones. Neither Kupffer cells and natural killer cells are uniformly distributed across hepatic zones.(42, 43) Furthermore, single-cell RNA sequencing has allowed investigators to compare gene expression from cells isolated from different hepatic zones.(11) Various resident hepatic immune active cells have been shown to have zone dependent gene expression, including stellate cells(44), liver sinusoidal endothelial cells(45), and Kupffer cells.(43, 46) The innate immune function of the hepatocyte is well-described(7, 8), and these cells are the major source of the APR. (9) Despite this well-known role in coordinating the APR, zonation of hepatocyte innate immune function has not been described. This is surprising given well accepted paradigm of zonated hepatocyte metabolic function.(10–13) Our data add hepatocytes to the growing list of cells with zonated immune function.
Induction of the APR and activation of the transcription factors NFκB(6, 9, 17) and CEBP/β(9, 17) are closely linked. Multiple studies performed in human hepatocyte cell lines have identified a role played by both CEBP/β and NFκB in regulating CRP expression in the setting of inflammatory stimuli. These studies, performed in human hepatocyte cell lines, have demonstrated that CEBP/β and NFκB,(31, 47) and specifically the p50 subunit,(29, 30, 32, 47, 48) act synergistically to increase CRP expression. While these critical studies have identified a role played by p50 in regulating hepatocyte CRP expression in response to inflammatory stimuli, they did not determine whether these mechanisms are relevant in vivo. These cell culture studies would not be able to determine zone-specific transcriptional regulation. Furthermore, in response to systemic inflammatory insult, the milieu of factors stimulating hepatocyte innate immune activation is likely to be much more complex than those used in cell culture systems. The current study adds to this body of literature by identifying zone-specific CRP expression and transcription factor activation.
Our work highlights the importance of the p50 subunit in coordinating the systemic acute phase response. Previous work has demonstrated that recombinant p50 can bind to the consensus sequence sites in the CRP promoter in vitro.(32, 49) Furthermore, p50 overexpression in hepatocytes is enough to drive CRP expression and synergistically increases cytokine-induced CRP expression.(29, 47) Our work adds important mechanistic details to this body of literature, specifically as it relates to p50/NFκB signaling in vivo and in response to the local inflammatory milieu. Firstly, using an in vivo model we demonstrate that pericentral hepatocytes express CRP and that this is associated with p50 nuclear translocation and CRP promoter consensus sequence binding. Importantly, the APR, and specifically hepatic CRP expression, is significantly attenuated in endotoxemic p50−/− mice. Interestingly, our results show intact hepatic p65 nuclear translocation as well as induction of key regulators of the APR (Il6, Il1b, and Tnf) in endotoxemic p50−/− mice (Fig 8A–C). This finding highlights the mechanistic role played by p50 in the expression of select APP in vivo. Previous reports have demonstrated that p65/p50 heterodimers are not able to drive CRP expression.(30) This is consistent with findings in cultured human hepatocytes demonstrating that p65 binds a canonical consensus sequence, but not the NFκB sequence in the CRP promoter.(29) Of note, other NFκB subunits, including cRel have been implicated in hepatocyte CRP expression.(31, 48, 49) We conclude that even in the presence of a robust acute innate immune response, optimal CRP expression in the pericentral hepatocytes is p50 dependent. In addition, this result provides further evidence of the relationship between the unique transcriptional mechanism driving the acute innate immune response, and those that coordinate the systemic APR. Our findings highlight the importance of interrogating the spatial and cell-type specific aspects of NFκB transcriptional selectivity to better understand the hepatic innate immune response to systemic inflammatory stress.
We speculate that our findings help explain previous findings reported in studies of p50−/− mice. The Nfkb1 gene encodes a full-length transcript for the p105 protein, from which p50 is derived.(50) The subunit p50 has been found to bind to DNA in the unstimulated state, and the p50 homodimer acts as a transcriptional repressor.(50) However, in the right setting and in the presence of co-activators such as Bcl3, p50 homodimers can act as transcriptional activators.(50) Additionally, NFκB dimers pairing p50 with either cRel or p65 are transcriptionally active.(50) The earliest description of Nfkb1 (p50−/−) mice demonstrated increased susceptibility to L. monocytogenes and S. Pneumoniae infection, however the hepatic specific response was not interrogated.(51) Additionally, p50−/− mice are highly susceptible to E.coli pneumonia and endotoxemic shock, however the hepatic role in these findings was not interrogated.(52, 53) These reported findings are of interest, as the protective effect of the hepatic APR in the setting of pneumonia is well-characterized.(54–58) Many of these studies have demonstrated that absence of hepatocyte p65/NFκB signaling and an associated blunting of the hepatic APR exacerbates pulmonary injury with sepsis and pneumonia.(54–58) Our study contributes to our understanding of the relationship between systemic inflammatory exposure, hepatic NFκB activity and the APR. With endotoxemia, we demonstrate robust hepatic p65 activation (Fig. 5A, B, E, F, I). These results corroborate the importance of p65 in the hepatic response to systemic inflammatory stress. However, at later time points, we clearly demonstrate sustained hepatic p50 activation (Fig. 5 A, C, E, G; Fig. 6). This timing is temporally related to the acceleration of the hepatic APR (Fig. 1). Here we show that in p50−/− mice the acute hepatic p65 response is intact (Fig. 8), however the APR is blunted (Fig. 8). Thus, we speculate that an intact hepatic response requires acute activation of p65, followed by maturation of this response into pericentral specific p50 signaling.
Whether the findings reported here are reproducible in other APR murine models remains to be tested. Various preclinical models have been used to study the hepatic APR, including pneumonia, polymicrobial sepsis, and experimental endotoxemia.(9, 16–19) However, whether hepatocyte derived APP expression is zone-specific remains largely untested in animal models.(9) Early studies revealed that the hepatic APR to turpentine-induced liver injury is zonated.(59) However, the APR to turpentine-induced cellular injury does not predict the coordinated response to innate immune challenge.(18) The findings reported here add to the body of literature supporting a the hypothesis that the hepatocyte-derived APR is zonated and deserves study in other pre-clinical models.
Our study has multiple limitations. Firstly, we used the previously validated method of digitonin-collagenase perfusion method.(24, 25) Our results confirm enrichment of these cell subpopulations (Fig. 3), but these isolates are not free of contamination. To combat this, we have provided both immunostaining and RNAscope to supplement the studies performed on these cell isolations. Furthermore, we have performed a rather limited transcriptomic analysis of previously identified APP. A more thorough, single-cell RNA sequencing analysis of murine hepatocytes isolated from endotoxemic mice has recently been published.(60) These data demonstrate hepatocytes undergo a transcriptional switch, and the ability to identify zone-specific hepatocytes is blurred during endotoxemia. Spatial transcriptomic approaches are necessary to build on this work and the findings reported here. Also, previous studies linking p50 and CEBP/β activation to CRP expression have used cytokine exposed hepatocytes as the model system.(29–32, 47, 48) We used an in vivo endotoxemia model, and it is unclear whether pericentral hepatocytes are directly responding to the exposure (ie, LPS), or cytokines circulating systemically or produced locally by cells in the liver. Finally, STAT-3 has been implicated in APR-associated CRP expression, but those mechanisms were not interrogated here.(47, 61)
Zonation of hepatocyte metabolic function has been recognized for over a century. In 1923, the histopathologist Robert Noel characterized the periportal zone as one of “fonctionnement permanent” (permanent operation) and the pericentral zone as one of “repose permanent” (permanent rest).(62) Recent data demonstrate that these early observations extend beyond hepatocyte metabolic function. Multiple immune active cells, including stellate cells(44), liver sinusoidal endothelial cells(45), and Kupffer cells(43, 46) have zone dependent gene expression. Here, we provide evidence that endotoxemia-induced transcription factor activation and the resulting transcriptome is zonated in hepatocytes. We propose that the pericentral hepatocyte of “repose permanent” is fundamentally altered in the setting of innate immune challenge.
There are potential clinical implications of the observations reported here. Patients with chronic liver disease have an increased incidence of sepsis and higher sepsis-related mortality rates.(63, 64) While the precise mechanisms underlying this observation have yet to be elucidated, the diseased liver’s impaired acute phase response may contribute to these findings. Both preclinical and clinical data demonstrate that liver disease attenuates the hepatic response to innate immune stimulus.(65–69) Despite the APR’s largely protective role in the setting of acute infection, the mechanisms underlying the impairment of the APR among those with chronic liver disease is not completely understood. Furthermore, whether liver diseases that affect hepatocytes in a zone-dependent manner differentially impact the APR is unknown. Finally, the unique transcriptional mechanisms underlying expression of the APPs is incompletely understood. These gaps in knowledge have prevented therapeutic approaches targeting the APR to reduce the burden of infectious disease among those with existing liver pathologies. Out work illuminating zone-specific characteristics of the APR provides a foundation for an improved understanding of zone-dependent and hepatocyte-specific innate immunity. Further studies are necessary to determine the clinical implications of these observations.
Key Points:
Metabolic zonation exists, but whether innate immunity is zonated remains unclear.
The murine hepatocyte response to endotoxin shows zone-specific characteristics.
Pericentral hepatocyte p50 activation drives CRP induction.
Grant Support:
This work was supported by NIH grant R01HL132941 and R01HD107700 to CJW and a K12 Child Health Research grant from the University of Colorado Department of Pediatrics to LGS.
Footnotes
Disclosures: The authors declare no potential financial or ethical conflicts of interest.
Data Transparency: All data, analytic methods and study materials will be made available to other researchers upon request.
References
- 1.Gao B, Jeong WI, Tian Z. Liver: An organ with predominant innate immunity. Hepatology. 2008;47(2):729–36. [DOI] [PubMed] [Google Scholar]
- 2.Racanelli V, Rehermann B. The liver as an immunological organ. Hepatology. 2006;43(2 Suppl 1):S54–62. [DOI] [PubMed] [Google Scholar]
- 3.Strnad P, Tacke F, Koch A, Trautwein C. Liver - guardian, modifier and target of sepsis. Nat Rev Gastroenterol Hepatol. 2017;14(1):55–66. [DOI] [PubMed] [Google Scholar]
- 4.Yan J, Li S, Li S. The role of the liver in sepsis. Int Rev Immunol. 2014;33(6):498–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kubes P, Jenne C. Immune Responses in the Liver. Annu Rev Immunol. 2018;36:247–77. [DOI] [PubMed] [Google Scholar]
- 6.Bode JG, Albrecht U, Häussinger D, Heinrich PC, Schaper F. Hepatic acute phase proteins--regulation by IL-6- and IL-1-type cytokines involving STAT3 and its crosstalk with NF-κB-dependent signaling. Eur J Cell Biol. 2012;91(6–7):496–505. [DOI] [PubMed] [Google Scholar]
- 7.Crispe IN. Hepatocytes as Immunological Agents. J Immunol. 2016;196(1):17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou Z, Xu MJ, Gao B. Hepatocytes: a key cell type for innate immunity. Cell Mol Immunol. 2016;13(3):301–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ehlting C, Wolf SD, Bode JG. Acute-phase protein synthesis: a key feature of innate immune functions of the liver. Biol Chem. 2021;402(9):1129–45. [DOI] [PubMed] [Google Scholar]
- 10.Ben-Moshe S, Itzkovitz S. Spatial heterogeneity in the mammalian liver. Nat Rev Gastroenterol Hepatol. 2019;16(7):395–410. [DOI] [PubMed] [Google Scholar]
- 11.Cunningham RP, Porat-Shliom N. Liver Zonation - Revisiting Old Questions With New Technologies. Front Physiol. 2021;12:732929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kietzmann T Metabolic zonation of the liver: The oxygen gradient revisited. Redox Biol. 2017;11:622–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramachandran P, Matchett KP, Dobie R, Wilson-Kanamori JR, Henderson NC. Single-cell technologies in hepatology: new insights into liver biology and disease pathogenesis. Nat Rev Gastroenterol Hepatol. 2020;17(8):457–72. [DOI] [PubMed] [Google Scholar]
- 14.Halpern KB, Shenhav R, Matcovitch-Natan O, Toth B, Lemze D, Golan M, et al. Single-cell spatial reconstruction reveals global division of labour in the mammalian liver. Nature. 2017;542(7641):352–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feldmann G, Scoazec JY, Racine L, Bernuau D. Functional hepatocellular heterogeneity for the production of plasma proteins. Enzyme. 1992;46(1–3):139–54. [DOI] [PubMed] [Google Scholar]
- 16.Wyns H, Plessers E, De Backer P, Meyer E, Croubels S. In vivo porcine lipopolysaccharide inflammation models to study immunomodulation of drugs. Vet Immunol Immunopathol. 2015;166(3–4):58–69. [DOI] [PubMed] [Google Scholar]
- 17.Koj A Initiation of acute phase response and synthesis of cytokines. Biochim Biophys Acta. 1996;1317(2):84–94. [DOI] [PubMed] [Google Scholar]
- 18.Ramadori G, Christ B. Cytokines and the hepatic acute-phase response. Semin Liver Dis. 1999;19(2):141–55. [DOI] [PubMed] [Google Scholar]
- 19.Yoo JY, Desiderio S. Innate and acquired immunity intersect in a global view of the acute-phase response. Proc Natl Acad Sci U S A. 2003;100(3):1157–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nguyen L, Sandoval J, De Dios R, Yihdego E, Zarate M, Castro O, et al. The hepatic innate immune response is lobe-specific in a murine model endotoxemia. Innate Immun. 2019;25(2):144–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sherlock LG, Balasubramaniyan D, Zheng L, Grayck M, McCarthy WC, De Dios RC, et al. APAP-Induced IkappaBbeta/NFkappaB Signaling Drives Hepatic Il6 Expression and Associated Sinusoidal Dilation. Toxicol Sci. 2022;185(2):158–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lanaspa MA, Andres-Hernando A, Orlicky DJ, Cicerchi C, Jang C, Li N, et al. Ketohexokinase C blockade ameliorates fructose-induced metabolic dysfunction in fructose-sensitive mice. J Clin Invest. 2018;128(6):2226–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Charni-Natan M, Goldstein I. Protocol for Primary Mouse Hepatocyte Isolation. STAR Protoc. 2020;1(2):100086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin H, Huang YS, Fustin JM, Doi M, Chen H, Lai HH, et al. Hyperpolyploidization of hepatocyte initiates preneoplastic lesion formation in the liver. Nat Commun. 2021;12(1):645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lindros KO, Penttila KE. Digitonin-collagenase perfusion for efficient separation of periportal or perivenous hepatocytes. Biochem J. 1985;228(3):757–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee C, Huang CH. LASAGNA-Search: an integrated web tool for transcription factor binding site search and visualization. Biotechniques. 2013;54(3):141–53. [DOI] [PubMed] [Google Scholar]
- 27.Quistorff B, Dich J, Grunnet N. Periportal and perivenous hepatocytes retain their zonal characteristics in primary culture. Biochem Biophys Res Commun. 1986;139(3):1055–61. [DOI] [PubMed] [Google Scholar]
- 28.Quistorff B Preparation of isolated periportal or perivenous hepatocytes from rat liver. Methods Mol Biol. 1990;5:177–87. [DOI] [PubMed] [Google Scholar]
- 29.Cha-Molstad H, Agrawal A, Zhang D, Samols D, Kushner I. The Rel family member P50 mediates cytokine-induced C-reactive protein expression by a novel mechanism. J Immunol. 2000;165(8):4592–7. [DOI] [PubMed] [Google Scholar]
- 30.Agrawal A, Cha-Molstad H, Samols D, Kushner I. Transactivation of C-reactive protein by IL-6 requires synergistic interaction of CCAAT/enhancer binding protein beta (C/EBP beta) and Rel p50. J Immunol. 2001;166(4):2378–84. [DOI] [PubMed] [Google Scholar]
- 31.Agrawal A, Samols D, Kushner I. Transcription factor c-Rel enhances C-reactive protein expression by facilitating the binding of C/EBPbeta to the promoter. Mol Immunol. 2003;40(6):373–80. [DOI] [PubMed] [Google Scholar]
- 32.Voleti B, Agrawal A. Regulation of basal and induced expression of C-reactive protein through an overlapping element for OCT-1 and NF-kappaB on the proximal promoter. J Immunol. 2005;175(5):3386–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi YS, Hur J, Jeong S. Beta-catenin binds to the downstream region and regulates the expression C-reactive protein gene. Nucleic Acids Res. 2007;35(16):5511–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pathak A, Agrawal A. Evolution of C-Reactive Protein. Front Immunol. 2019;10:943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zemlin M, Schelonka RL, Bauer K, Schroeder HW. Regulation and chance in the ontogeny of B and T cell antigen receptor repertoires. Immunol Res. 2002;26(1–3):265–78. [DOI] [PubMed] [Google Scholar]
- 36.Torzewski M, Waqar AB, Fan J. Animal models of C-reactive protein. Mediators Inflamm. 2014;2014:683598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patterson LT, Higginbotham RD. Mouse C-reactive protein and endotoxin-induced resistance. J Bacteriol. 1965;90(6):1520–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pepys MB, Baltz M, Gomer K, Davies AJ, Doenhoff M. Serum amyloid P-component is an acute-phase reactant in the mouse. Nature. 1979;278(5701):259–61. [DOI] [PubMed] [Google Scholar]
- 39.Siboo R, Kulisek E. A fluorescent immunoassay for the quantification of C-reactive protein. J Immunol Methods. 1978;23(1–2):59–67. [DOI] [PubMed] [Google Scholar]
- 40.Goldstein I, Paakinaho V, Baek S, Sung MH, Hager GL. Synergistic gene expression during the acute phase response is characterized by transcription factor assisted loading. Nat Commun. 2017;8(1):1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simons JP, Loeffler JM, Al-Shawi R, Ellmerich S, Hutchinson WL, Tennent GA, et al. C-reactive protein is essential for innate resistance to pneumococcal infection. Immunology. 2014;142(3):414–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Geissmann F, Cameron TO, Sidobre S, Manlongat N, Kronenberg M, Briskin MJ, et al. Intravascular immune surveillance by CXCR6+ NKT cells patrolling liver sinusoids. PLoS Biol. 2005;3(4):e113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gola A, Dorrington MG, Speranza E, Sala C, Shih RM, Radtke AJ, et al. Commensal-driven immune zonation of the liver promotes host defence. Nature. 2021;589(7840):131–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Payen VL, Lavergne A, Alevra Sarika N, Colonval M, Karim L, Deckers M, et al. Single-cell RNA sequencing of human liver reveals hepatic stellate cell heterogeneity. JHEP Rep. 2021;3(3):100278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Halpern KB, Shenhav R, Massalha H, Toth B, Egozi A, Massasa EE, et al. Paired-cell sequencing enables spatial gene expression mapping of liver endothelial cells. Nat Biotechnol. 2018;36(10):962–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.MacParland SA, Liu JC, Ma XZ, Innes BT, Bartczak AM, Gage BK, et al. Single cell RNA sequencing of human liver reveals distinct intrahepatic macrophage populations. Nat Commun. 2018;9(1):4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Agrawal A, Cha-Molstad H, Samols D, Kushner I. Overexpressed nuclear factor-kappaB can participate in endogenous C-reactive protein induction, and enhances the effects of C/EBPbeta and signal transducer and activator of transcription-3. Immunology. 2003;108(4):539–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Young DP, Kushner I, Samols D. Binding of C/EBPbeta to the C-reactive protein (CRP) promoter in Hep3B cells is associated with transcription of CRP mRNA. J Immunol. 2008;181(4):2420–7. [DOI] [PubMed] [Google Scholar]
- 49.Cha-Molstad H, Young DP, Kushner I, Samols D. The interaction of C-Rel with C/EBPbeta enhances C/EBPbeta binding to the C-reactive protein gene promoter. Mol Immunol. 2007;44(11):2933–42. [DOI] [PubMed] [Google Scholar]
- 50.Pereira SG, Oakley F. Nuclear factor-kappaB1: regulation and function. Int J Biochem Cell Biol. 2008;40(8):1425–30. [DOI] [PubMed] [Google Scholar]
- 51.Sha WC, Liou HC, Tuomanen EI, Baltimore D. Targeted disruption of the p50 subunit of NF-kappa B leads to multifocal defects in immune responses. Cell. 1995;80(2):321–30. [DOI] [PubMed] [Google Scholar]
- 52.Mizgerd JP, Lupa MM, Kogan MS, Warren HB, Kobzik L, Topulos GP. Nuclear factor-kappaB p50 limits inflammation and prevents lung injury during Escherichia coli pneumonia. Am J Respir Crit Care Med. 2003;168(7):810–7. [DOI] [PubMed] [Google Scholar]
- 53.Gadjeva M, Tomczak MF, Zhang M, Wang YY, Dull K, Rogers AB, et al. A role for NF-kappa B subunits p50 and p65 in the inhibition of lipopolysaccharide-induced shock. J Immunol. 2004;173(9):5786–93. [DOI] [PubMed] [Google Scholar]
- 54.Quinton LJ, Jones MR, Robson BE, Mizgerd JP. Mechanisms of the hepatic acute-phase response during bacterial pneumonia. Infect Immun. 2009;77(6):2417–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Quinton LJ, Blahna MT, Jones MR, Allen E, Ferrari JD, Hilliard KL, et al. Hepatocyte-specific mutation of both NF-kappaB RelA and STAT3 abrogates the acute phase response in mice. J Clin Invest. 2012;122(5):1758–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Odom CV, Kim Y, Burgess CL, Baird LA, Korkmaz FT, Na E, et al. Liver-Dependent Lung Remodeling during Systemic Inflammation Shapes Responses to Secondary Infection. J Immunol. 2021;207(7):1891–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hilliard KL, Allen E, Traber KE, Yamamoto K, Stauffer NM, Wasserman GA, et al. The Lung-Liver Axis: A Requirement for Maximal Innate Immunity and Hepatoprotection during Pneumonia. Am J Respir Cell Mol Biol. 2015;53(3):378–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hilliard KL, Allen E, Traber KE, Kim Y, Wasserman GA, Jones MR, et al. Activation of Hepatic STAT3 Maintains Pulmonary Defense during Endotoxemia. Infect Immun. 2015;83(10):4015–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bernuau D, Moreau A, Tournier I, Legres L, Feldmann G. Activation of nuclear protooncogenes and alpha-fetoprotein gene in rat liver during the acute inflammatory reaction. Liver. 1993;13(2):102–9. [DOI] [PubMed] [Google Scholar]
- 60.Sun X, Wu J, Liu L, Chen Y, Tang Y, Liu S, et al. Transcriptional switch of hepatocytes initiates macrophage recruitment and T-cell suppression in endotoxemia. J Hepatol. 2022;77(2):436–52. [DOI] [PubMed] [Google Scholar]
- 61.Zhang D, Sun M, Samols D, Kushner I. STAT3 participates in transcriptional activation of the C-reactive protein gene by interleukin-6. J Biol Chem. 1996;271(16):9503–9. [DOI] [PubMed] [Google Scholar]
- 62.Nöel R Recherches histo-physiologiques sur la cellule hépatique des mammifères. Archives Anat microsc. 1923;19:1–158. [Google Scholar]
- 63.Irvine KM, Ratnasekera I, Powell EE, Hume DA. Causes and Consequences of Innate Immune Dysfunction in Cirrhosis. Front Immunol. 2019;10:293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Foreman MG, Mannino DM, Moss M. Cirrhosis as a risk factor for sepsis and death: analysis of the National Hospital Discharge Survey. Chest. 2003;124(3):1016–20. [DOI] [PubMed] [Google Scholar]
- 65.Thomsen KL, Hebbard L, Glavind E, Clouston A, Vilstrup H, George J, et al. Non-alcoholic steatohepatitis weakens the acute phase response to endotoxin in rats. Liver Int. 2014;34(10):1584–92. [DOI] [PubMed] [Google Scholar]
- 66.Pieri G, Agarwal B, Burroughs AK. C-reactive protein and bacterial infection in cirrhosis. Ann Gastroenterol. 2014;27(2):113–20. [PMC free article] [PubMed] [Google Scholar]
- 67.Perdigoto DN, Figueiredo PN, Tome LF. Clarifying the role of C-reactive protein as a bacterial infection predictor in decompensated cirrhosis. Eur J Gastroenterol Hepatol. 2018;30(6):645–51. [DOI] [PubMed] [Google Scholar]
- 68.Park WB, Lee KD, Lee CS, Jang HC, Kim HB, Lee HS, et al. Production of C-reactive protein in Escherichia coli-infected patients with liver dysfunction due to liver cirrhosis. Diagn Microbiol Infect Dis. 2005;51(4):227–30. [DOI] [PubMed] [Google Scholar]
- 69.Mackenzie I, Woodhouse J. C-reactive protein concentrations during bacteraemia: A comparison between patients with and without liver dysfunction. Intensive Care Med. 2006;32(9):1344–51. [DOI] [PubMed] [Google Scholar]


