Abstract
Intracerebroventricular infusion of resolvin E1 (RvE1), a bioactive metabolite derived from eicosapentaenoic acid, exerts antidepressant-like effects in a mouse model of lipopolysaccharide (LPS)-induced depression; these effects are blocked by systemic injection of rapamycin, a mechanistic target of rapamycin complex 1 (mTORC1) inhibitor. Additionally, local infusion of RvE1 into the medial prefrontal cortex (mPFC) or dorsal hippocampal dentate gyrus (DG) produces antidepressant-like effects. To evaluate the potential of RvE1 for clinical use, the present study examined whether treatment with RvE1 via intranasal (i.n.) route, a non-invasive route for effective drug delivery to the brain, produces antidepressant-like effects in LPS-challenged mice using tail suspension and forced swim tests. Intranasal administration of RvE1 significantly attenuated LPS-induced immobility, and these antidepressant-like effects were completely blocked by an AMPA receptor antagonist or L-type voltage-dependent Ca2+ channel blocker. The antidepressant-like effects of both i.n. and intra-mPFC administrations of RvE1 were blocked by intra-mPFC infusion of a neutralizing antibody (nAb) for brain-derived neurotrophic factor (BDNF) or vascular endothelial growth factor (VEGF). Intra-mPFC infusion of rapamycin completely blocked the antidepressant-like effects of both i.n. and intra-mPFC administrations of RvE1 as well as those of intra-mPFC infusion of BDNF and VEGF. Moreover, i.n. RvE1 produced antidepressant-like effects via mTORC1 activation in the mPFC of a mouse model of repeated prednisolone-induced depression. Intra-dorsal DG infusion of BDNF and VEGF nAbs, but not rapamycin, blocked the antidepressant-like effects of i.n. RvE1. These findings suggest that i.n. administration of RvE1 produces antidepressant-like effects through activity-dependent BDNF/VEGF release in the mPFC and dorsal DG, and mTORC1 activation in the mPFC, but not in the dorsal DG. Thus, RvE1 can be a promising candidate for a novel rapid-acting antidepressant.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13311-022-01337-1.
Keywords: BDNF, Depression, mTORC1, ω-3 polyunsaturated fatty acid, Specialized pro-resolving lipid mediator, VEGF-A
Introduction
Depression is one of the most widespread debilitating psychiatric illnesses that imposes enormous personal and socioeconomic burdens [1]. Conventional monoamine-based antidepressants, notably selective serotonin reuptake inhibitors, have significant limitations, including delayed onset of therapeutic response and low efficacy (approximately one-third of individuals with depression are considered treatment-resistant) [2]. In the 2000s, a single subanesthetic dose of ketamine, an N-methyl-d-aspartate receptor (NMDAR) antagonist, was reported to exert rapid and sustained antidepressant effects even in patients with treatment-resistant depression [3, 4]. (S)-ketamine, an enantiomer of ketamine, was approved in the form of nasal spray for treatment-resistant depression in the USA and Europe in 2019 [5, 6]. However, racemic ketamine and (S)-ketamine are associated with undesirable psychotomimetic/dissociative side effects and the potential for abuse [7–9]. Thus, there is an urgent need to develop novel, rapid, and efficacious antidepressants with fewer side effects.
Unraveling of the cellular and molecular mechanisms underlying the rapid antidepressant actions of ketamine is key for the identification and characterization of novel targets for developing safer rapid-acting antidepressants [10–12]. Brain-derived neurotrophic factor (BDNF) and the downstream mechanistic target of rapamycin complex 1 (mTORC1) signaling in the medial prefrontal cortex (mPFC) are known to be essential for the antidepressant-like effects of ketamine [10–14]. Ketamine-induced BDNF release depends on the activation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs) and L-type voltage-dependent Ca2+ channels (L-VDCCs) [10–12, 14]. Activation of BDNF-mTORC1 signaling in the mPFC is also required for the antidepressant-like effects of other rapid-acting agents, including scopolamine (a non-selective muscarinic acetylcholine receptor antagonist) and rapastinel (an NMDAR positive allosteric modulator) [10–12, 15–19]. Our recent studies demonstrate that neuronal vascular endothelial growth factor (VEGF) signaling in the mPFC also contributes to the antidepressant-like actions of ketamine [20] and a heterologous interplay between BDNF and VEGF signaling in the mPFC plays a key role in the rapid antidepressant-like responses to these neurotrophic factors [21]. BDNF and VEGF signaling in the hippocampal dentate gyrus (DG) are also involved in the antidepressant-like effects of ketamine [22, 23]. Moreover, VEGF reportedly activates the mTORC1 signaling pathway [24]. These findings suggest that compounds that can rapidly promote BDNF/VEGF release and/or activate the mTORC1 signaling are promising candidates for novel rapid-acting antidepressants.
Resolvin E1 (RvE1) is a bioactive lipid mediator derived from eicosapentaenoic acid (EPA) [25–28]. We recently found that intracerebroventricular (i.c.v.) infusion of RvE1 reverses depression-like behaviors induced by lipopolysaccharide (LPS), chronic pain, and repeated prednisolone (PSL), likely via the chemerin receptor ChemR23 [29–31]. The antidepressant-like effects of RvE1 are completely blocked by systemic injection of the mTORC1 inhibitor rapamycin [29]. We have also shown that infusion of RvE1 into the mPFC or dorsal DG produces antidepressant-like effects in mouse models of LPS- and repeated PSL-induced depression [29, 31]. These findings suggest that mTORC1 signaling mediates the antidepressant-like effects of RvE1 and that the mPFC and dorsal DG play a role in the behavioral effects of RvE1. However, the precise underlying mechanisms remain unclear.
RvE1 was administered directly into the brain in our previous studies [29–31], because RvE1 is highly unstable, when compared with its precursor EPA, and degrades rapidly in vivo [32, 33]. However, this compound should be delivered effectively to the brain via a non-invasive route for clinical use. The intranasal (i.n.) route is one of the most promising delivery routes for drugs to the brain because it bypasses the blood–brain barrier and hepatic first-pass metabolism [34]. Thus, we first examined whether i.n. administration of RvE1 produced antidepressant-like effects in a mouse model of LPS-induced depression. We then addressed the roles of AMPARs, L-VDCCs, BDNF/VEGF, and mTORC1 signaling in the mPFC and dorsal DG in the antidepressant-like effects of i.n. administration of RvE1. Moreover, we examined whether i.n. RvE1 produced antidepressant-like effects via mTORC1 activation in the mPFC in a mouse model of repeated PSL-induced depression resistant to acute treatment with a conventional antidepressant [31].
Materials and Methods
Animals
Male C57BL/6 J (6–11 weeks, n = 374) and ICR (6–8 weeks, n = 110) mice were used as models of LPS-induced and repeated PSL-induced depression, respectively. Female ICR mice (8 weeks, n = 20) were also used. They were either purchased from Japan SLC (Hamamatsu, Japan) or bred in the animal facilities of Kanazawa University, group-housed, and maintained at a constant ambient temperature (22 ± 2 °C) under a 12-h light/dark cycle with food and water available ad libitum. Animal use and procedures were approved by the Institutional Animal Care and Use Committee of Kanazawa University, and all efforts were made to minimize the suffering of mice. Mice were randomly allocated to each treatment group, and the investigators were not blinded to the group allocation.
Reagents
RvE1 was synthesized as previously described [35], dissolved in 100% ethanol, and stored at – 80 °C. These solutions (200 µg/mL for i.n. administration and 100 µg/mL for intra-mPFC/DG infusion) were diluted with sterile phosphate-buffered saline (PBS) immediately before use (final concentrations of ethanol were 0.5% for i.n. administration and 0.25% for intra-mPFC/DG infusion). LPS (serotype 0127:B8; Sigma, St. Louis, MO), NBQX (an AMPAR antagonist; Enzo Life Sciences, Farmingdale, NY), and verapamil (an L-VDCC blocker; Tokyo Chemical Industry, Tokyo, Japan) were dissolved in sterile saline. Sheep anti-BDNF neutralizing antibody (nAb) (Cat No. AB1513P, Millipore, Temecula, CA), goat anti-VEGF nAb (Cat No. AF-493-NA, R&D Systems, Minneapolis, MN), normal sheep IgG (R&D Systems), and normal goat IgG (R&D Systems) were reconstituted according to the manufacturer’s instructions, and diluted with sterile PBS or PBS containing ethanol. Recombinant BDNF (Sumitomo Pharma, Osaka, Japan) and mouse VEGF164 (the predominant VEGF isoform; Wako, Osaka, Japan) were dissolved in 0.1% bovine serum albumin (BSA; Wako)/PBS or 10% dimethyl sulfoxide (DMSO)/0.09% BSA/PBS. Rapamycin (LC Laboratories, Woburn, MA) was initially dissolved in DMSO and then diluted with sterile PBS, PBS containing BSA, or PBS containing ethanol. Water-soluble PSL (Shionogi, Osaka, Japan) was reconstituted in saline.
Surgery and Drug Treatments
Stereotaxic surgery, intra-mPFC (1.8 mm rostral, ± 0.4 mm lateral, 2.8 mm ventral to bregma) [36] and intra-dorsal DG (2.1 mm caudal, ± 1.5 mm lateral, 2.7 mm ventral to bregma) [36] infusions were performed as previously described [20, 21, 29, 31, 37, 38]. Each mouse was bilaterally infused into the mPFC or dorsal DG with RvE1 (50 pg/side), BDNF nAb (200 ng/side), normal sheep IgG (200 ng/side), VEGF nAb (80 ng/side), normal goat IgG (80 ng/side), rapamycin (0.01 nmol/side), BDNF (100 ng/side), VEGF (5 ng/side), or appropriate vehicle in a volume of 0.2 µL/side. Some mice received an intraperitoneal (i.p.) injection of NBQX (10 mg/kg), verapamil (10 mg/kg), or saline 30 min before i.n. administration of RvE1. For i.n. administration, mice were held in the supine position and administered either vehicle (0.5% ethanol/PBS; 10 µL/mouse) or RvE1 [10 ng (in 0.5% ethanol/PBS)/mouse] by slowly pipetting 5 µL into each nostril. These doses were determined based on previous studies [15, 20, 21, 29, 31, 37–40].
Lipopolysaccharide-Induced Depression Model
Lipopolysaccharide (LPS) (0.8 mg/kg) or saline was i.p. injected into C57BL/6 J mice at 10 mL/kg, as previously described [29, 38, 40, 41]. Intranasal or intra-mPFC administration of RvE1 (or vehicle) was performed 23 h after the LPS challenge, and the locomotor activity (LMA) test, tail suspension test (TST), and forced swim test (FST) were performed 1, 3, and 5 h after the administration of RvE1 (or vehicle), respectively.
Repeated Prednisolone-Induced Depression Model
Repeated administration of prednisolone (PSL) in male ICR mice induced depression-like behavior without affecting locomotor activity [31], while repeated injection of PSL to C57BL/6 mice not only induced depression-like behavior, but also reduced locomotor activity [42]. Thus, in the present study, PSL (50 mg/kg per day) or saline was injected subcutaneously (s.c.) into ICR mice for 5–6 days, as previously described [31]. Intranasal administration of RvE1 (or vehicle) was performed 23 h after the fifth injection of PSL (or saline), followed by the open field test (OFT) 1 h later. Thirty minutes after completion of the OFT, the sixth injection of PSL (or saline) was administered, and 24 h later, the mice underwent the forced swim test (FST).
Behavioral Testing
The locomotor activity (LMA) test was performed as previously described [37, 38]. Each mouse was placed in a testing chamber (38 × 26 × 24 cm) and allowed to explore freely for 10 min. During this period, the total distance traveled and time spent in the center area of the chamber (19 × 13 cm) were measured automatically using the Smart 3.0 software (PanLab Harvard Apparatus, Holliston, MA). The open field test (OFT) was performed as previously described [31, 43]. Each mouse was placed in an open field (42 × 42 × 42 cm) and allowed to explore freely for 10 min. The total distance traveled and time spent in the center area (21 × 21 cm) were analyzed using the Smart 3.0 software. The tail suspension test (TST) was performed as previously described [20, 38]. Each mouse was suspended, with its tail fixed to a hook using a small piece of adhesive tape. The duration of immobility was measured for 6 min in a blinded manner. Mice that climbed their tails during the test period were excluded from the analyses (n = 12). The forced swim test (FST) was conducted as previously described [20, 21, 29, 31, 37, 38]. Each mouse was placed in a 4-L beaker containing water (24 ± 1 °C; 15-cm depth) and forced to swim for 6 min. The duration of immobility was scored during the last 4 min in a blinded manner.
Histology
After the behavioral tests, mice were euthanized by cervical dislocation, and their brains were harvested and frozen in powdered dry ice. Coronal sections (50 µm) were prepared using a cryostat, thaw-mounted on slides, and stained with thionin to confirm the infusion sites. Mice with incorrect infusion placements were excluded from the analyses (C57BL/6 J, n = 33; ICR, n = 23).
Statistical Analyses
Sample sizes were determined based on similar studies which are sufficient to obtain statistical significances, although no statistical methods were used to predetermine the sample sizes. Data are presented as mean ± SEM and analyzed by the Student’s t-test, one-way or two-way analysis of variance (ANOVA) followed by the Holm-Sidak’s post hoc test using GraphPad Prism 6 or 9 (GraphPad Software, La Jolla, CA). Differences were considered statistically significant at p < 0.05.
Results
Intranasal Administration of RvE1 Produces Antidepressant-Like Effects via Activation of AMPARs and L-VDCCs
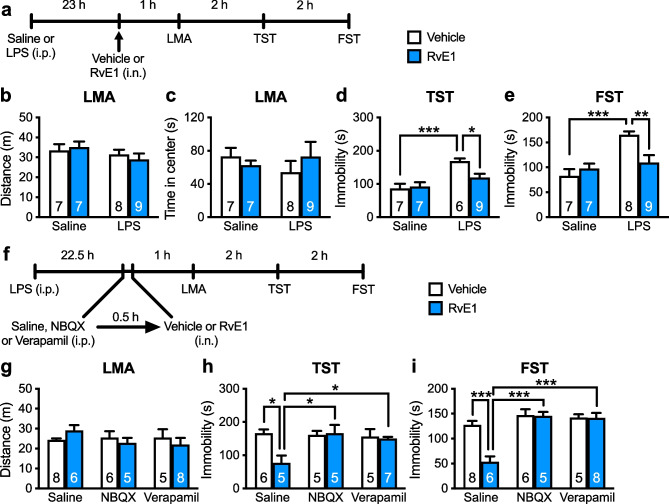
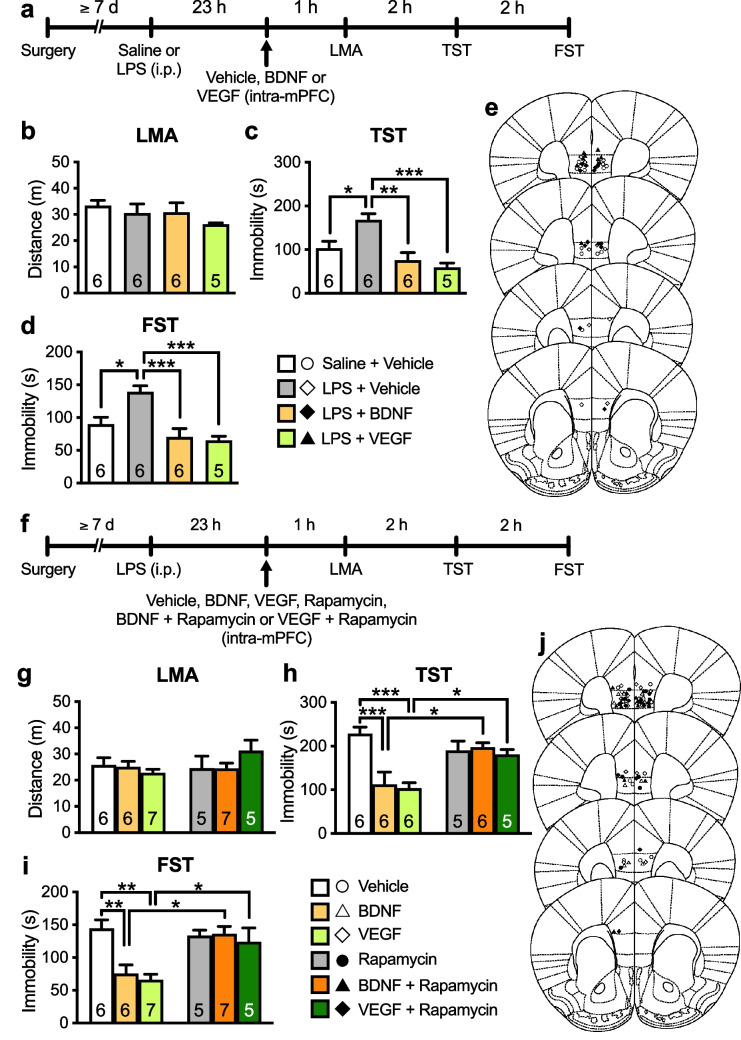
Our previous study demonstrated that i.c.v. infusion of RvE1 (1 ng/mouse) exerted antidepressant-like effects in LPS-induced depression model mice [29]. Here, we examined the antidepressant-like effects of i.n. administration of RvE1 in LPS-challenged mice by administering of RvE1 (10 ng/mouse) or vehicle (0.5% ethanol/PBS) intranasally 23 h after injection of LPS or saline and subjecting the mice to the LMA test, TST, and FST 1, 3, and 5 h after i.n. administration, respectively (Fig. 1a). LPS challenge and i.n. administration of RvE1 did not affect total distance traveled (Fig. 1b) and the time spent in the center area of the chamber (an indicator of anxiety-like behaviors; Fig. 1c) in the LMA test. LPS challenge significantly increased immobility in vehicle-treated mice in the TST (Fig. 1d) and FST (Fig. 1e), and i.n. administration of RvE1 alleviated these depression-like behaviors. RvE1 did not affect immobility in saline-injected control mice, as per the TST (Fig. 1d) and FST (Fig. 1e). These findings indicate that i.n. administration of RvE1 produces antidepressant-like effects.
Fig. 1.
Intranasal administration of RvE1 produces antidepressant-like actions via activation of AMPARs and L-VDCCs in LPS-induced depression model mice. a Experimental timeline for LPS challenge (0.8 mg/kg, i.p.), i.n. administration of either vehicle (0.5% ethanol/PBS) or RvE1 (10 ng/mouse), and behavioral testing. b, c Effects of LPS and RvE1 on total distance traveled (b, interaction, F1,27 = 0.546, p = 0.466; LPS, F1,27 = 2.13, p = 0.156; RvE1, F1,27 = 0.0235, p = 0.8794) and time in center (c, interaction, F1,27 = 1.131, p = 0.297; LPS, F1,27 = 0.0994, p = 0.755; RvE1, F1,27 = 0.0849, p = 0.773) in the LMA test. d, e Effects of LPS and RvE1 on immobility time in the TST (d, interaction, F1,25 = 5.12, p = 0.0326; two LPS + vehicle-treated mice were excluded due to tail-climbing) and FST (e, interaction, F1,27 = 8.49, p = 0.0071). f Experimental timeline for LPS challenge (0.8 mg/kg, i.p.), i.p. injection of saline, NBQX (10 mg/kg) or verapamil (10 mg/kg), i.n. administration of either vehicle (0.5% ethanol/PBS) or RvE1 (10 ng/mouse), and behavioral testing. g Effects of NBQX, verapamil and RvE1 on LMA in LPS-challenged mice (interaction, F2,32 = 1.29, p = 0.290; inhibitors, F2,32 = 0.628, p = 0.540; RvE1, F2,32 = 0.0391, p = 0.845). h, i Effects of NBQX and verapamil on the antidepressant-like actions of i.n. administration of RvE1 in the TST (h, interaction, F2,27 = 4.71, p = 0.0175; two saline + vehicle-, one saline + RvE1-, one NBQX + vehicle-, and one verapamil + RvE1-treated mice were excluded due to tail-climbing) and FST (i, interaction, F2,32 = 9.392, p = 0.0006). Data are expressed as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001 (two-way ANOVA followed by Holm-Sidak’s post hoc test)
The antidepressant-like effects of ketamine and other rapid-acting agents, require the activation of AMPARs and L-VDCCs [10–16, 19, 44]. We examined the involvement of AMPARs and L-VDCCs in the antidepressant-like effects of i.n. administration of RvE1: LPS-challenged mice were i.p. injected with NBQX (10 mg/kg), verapamil (10 mg/kg), or saline 30 min before i.n. administration of RvE1 (Fig. 1f). LMA was not significantly affected by RvE1, NBQX, or verapamil (Fig. 1g). RvE1 significantly decreased immobility in saline-pretreated mice in the TST (Fig. 1h) and FST (Fig. 1i), and these antidepressant-like effects were blocked by pretreatment with NBQX or verapamil. Neither NBQX nor verapamil altered immobility in both the TST (Fig. 1h) and FST (Fig. 1i). These results indicate that the activation of AMPARs and L-VDCCs is required for the antidepressant-like effects of i.n. RvE1.
Antidepressant-Like Effects of i.n. Administration of RvE1 Require BDNF Release in the mPFC
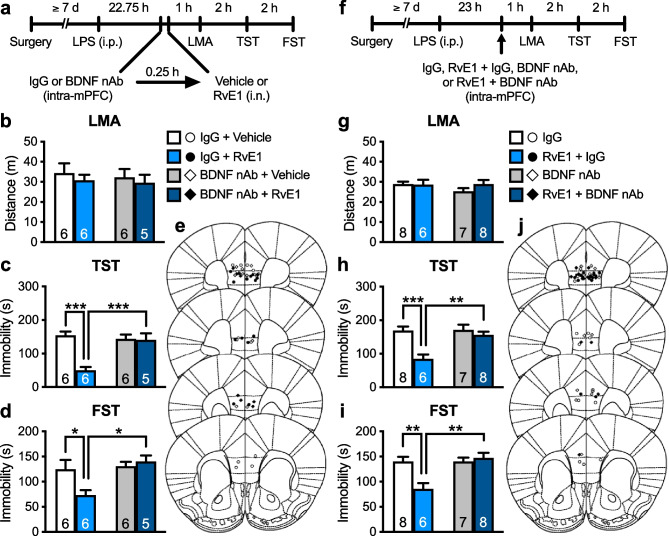
Rapid-acting antidepressants, including ketamine, stimulate BDNF release in the mPFC through activating AMPARs and L-VDCCs [10–12, 14–16, 19]. We tested whether BDNF release in the mPFC plays a role in the antidepressant-like effects of i.n. administration of RvE1. We infused BDNF nAb (200 ng/side), which would bind and sequester BDNF in the extracellular space, or control IgG (200 ng/side) into the mPFC of LPS-challenged mice 15 min before i.n. administration of RvE1 (Fig. 2a). These treatments did not significantly influence LMA (Fig. 2b). In control IgG-infused mice, i.n. administration of RvE1 decreased immobility in the TST (Fig. 2c) and FST (Fig. 2d). The antidepressant-like effects of RvE1 were completely blocked in BDNF nAb-infused mice. Intra-mPFC infusion of BDNF nAb alone did not significantly alter immobility in the TST (Fig. 2c) and FST (Fig. 2d). These results suggest that BDNF release in the mPFC is required for the antidepressant-like effects of i.n. RvE1.
Fig. 2.
Intranasal and intra-mPFC injections of RvE1 produce antidepressant-like actions via BDNF release in the mPFC in LPS-induced depression model mice. a Experimental timeline for LPS challenge (0.8 mg/kg, i.p.), intra-mPFC infusion of either control IgG (200 ng/side) or BDNF nAb (200 ng/side), i.n. administration of either vehicle (0.5% ethanol/PBS) or RvE1 (10 ng/mouse), and behavioral testing. b Effects of intra-mPFC infusion of BDNF nAb and i.n. administration of RvE1 on LMA in LPS-challenged mice (interaction, F1,19 = 0.00995, p = 0.922; BDNF nAb, F1,19 = 0.177, p = 0.679; RvE1, F1,19 = 0.642, p = 0.433). c, d Effects of intra-mPFC infusion of BDNF nAb on the antidepressant-like actions of i.n. administration of RvE1 in the TST (c, interaction, F1,19 = 13.9, p = 0.0014) and FST (d, interaction, F1,19 = 5.50, p = 0.0300) in LPS-challenged mice. e Schematic representation of the mPFC infusion sites. Plates are from ref. 36. f Experimental timeline for LPS challenge, intra-mPFC infusion of control IgG (200 ng/side), RvE1 (50 pg/side) + control IgG, BDNF nAb (200 ng/side) or RvE1 + BDNF nAb, and behavioral testing. g–i Effects of intra-mPFC infusion of RvE1 with or without BDNF nAb on LMA (g, interaction, F1,25 = 1.07, p = 0.311; BDNF nAb, F1,25 = 0.811, p = 0.376; RvE1, F1,25 = 0.821, p = 0.374) and immobility time in the TST (h, interaction, F1,25 = 7.57, p = 0.0109) and FST (i, interaction, F1,25 = 9.92, p = 0.0042) in LPS-challenged mice. j Schematic representation of the mPFC infusion sites. Plates are from ref. 36. Data are expressed as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001 (two-way ANOVA followed by Holm-Sidak’s post hoc test)
Previously, we found that intra-mPFC infusion of RvE1 (50 pg/side) exerted antidepressant-like effects in LPS-induced depression model mice [29]. To examine whether RvE1 locally induces BDNF release in the mPFC to produce the antidepressant-like effects, LPS-challenged mice received an intra-mPFC co-infusion of RvE1 and BDNF nAb (Fig. 2f). LMA was not significantly affected by intra-mPFC infusion of RvE1, BDNF nAb, or a combination of RvE1 and BDNF nAb (Fig. 2g). Intra-mPFC infusion of RvE1 significantly decreased immobility in the TST (Fig. 2h) and FST (Fig. 2i) in control IgG-co-infused mice, and these antidepressant-like effects of RvE1 were blocked by co-infusion of BDNF nAb. These results suggest that RvE1 acts locally in the mPFC to induce BDNF release and the resulting antidepressant-like effects.
Antidepressant-Like Effects of i.n. Administration of RvE1 Require VEGF Release in the mPFC
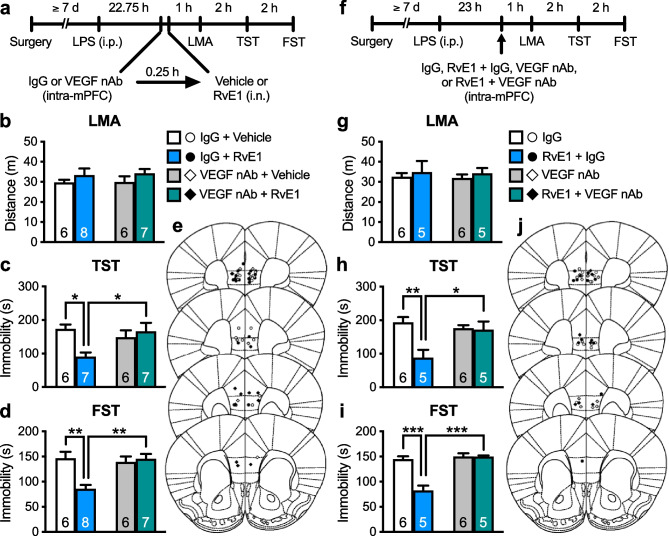
We recently found that ketamine rapidly and transiently increased VEGF release in the mPFC, which was necessary for the rapid antidepressant-like actions of this drug [20]. We investigated whether VEGF release in the mPFC plays a role in the antidepressant-like effects of i.n. administration of RvE1. LPS-challenged mice were infused with VEGF nAb (80 ng/side) or control IgG (80 ng/side) into the mPFC 15 min before i.n. administration of RvE1 (Fig. 3a). These treatments did not affect LMA (Fig. 3b). In control IgG-infused mice, i.n. administration of RvE1 decreased immobility in the TST (Fig. 3c) and FST (Fig. 3d), and these effects were completely blocked by VEGF nAb infusion. VEGF nAb alone did not significantly change immobility in both the TST (Fig. 3c) and FST (Fig. 3d). These results suggest that VEGF, as well as BDNF, release in the mPFC is required for the antidepressant-like effects of i.n. RvE1.
Fig. 3.
Intranasal and intra-mPFC injections of RvE1 produce antidepressant-like actions via VEGF release in the mPFC in LPS-induced depression model mice. a Experimental timeline for LPS challenge (0.8 mg/kg, i.p.), intra-mPFC infusion of either control IgG (80 ng/side) or VEGF nAb (80 ng/side), i.n. administration of either vehicle (0.5% ethanol/PBS) or RvE1 (10 ng/mouse), and behavioral testing. b Effects of intra-mPFC infusion of VEGF nAb and i.n. administration of RvE1 on LMA in LPS-challenged mice (interaction, F1,23 = 0.0125, p = 0.912; VEGF nAb, F1,23 = 0.0571, p = 0.813; RvE1, F1,23 = 2.39, p = 0.136). c, d Effects of intra-mPFC infusion of VEGF nAb on the antidepressant-like actions of i.n. administration of RvE1 in the TST (c, interaction, F1,22 = 6.98, p = 0.0149; one IgG + RvE1-treated mouse was excluded due to tail-climbing) and FST (d, interaction, F1,23 = 11.1, p = 0.0029) in LPS-challenged mice. e Schematic representation of the mPFC infusion sites. Plates are from ref. 36. f Experimental timeline for LPS challenge, intra-mPFC infusion of control IgG (80 ng/side), RvE1 (50 pg/side) + control IgG, VEGF nAb (80 ng/side) or RvE1 + BDNF nAb, and behavioral testing. g–i Effects of intra-mPFC infusion of RvE1 with or without VEGF nAb on LMA (g, interaction, F1,18 = 0.000105, p = 0.992; VEGF nAb, F1,18 = 0.0319, p = 0.860; RvE1, F1,18 = 0.573, p = 0.459) and immobility time in the TST (h, interaction, F1,18 = 7.55, p = 0.0133) and FST (i, interaction, F1,18 = 22.0, p = 0.0002) in LPS-challenged mice. j Schematic representation of the mPFC infusion sites. Plates are from ref. 36. Data are expressed as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001 (two-way ANOVA followed by Holm-Sidak’s post hoc test)
To determine whether RvE1 locally induces VEGF release in the mPFC to exert antidepressant-like effects, LPS-challenged mice received an intra-mPFC co-infusion of RvE1 and VEGF nAb (Fig. 3f). LMA was not significantly changed following intra-mPFC infusion of RvE1, VEGF nAb, or a combination of RvE1 and VEGF nAb (Fig. 3g). Intra-mPFC infusion of RvE1 significantly decreased immobility in the TST (Fig. 3h) and FST (Fig. 3i) in control-IgG-co-infused mice, and these antidepressant-like effects of RvE1 were blocked by co-infusion of VEGF nAb. These results suggest that RvE1 acts locally in the mPFC to induce VEGF release and the resulting antidepressant-like effects.
Antidepressant-Like Effects of i.n. Administration of RvE1 Require mTORC1 Activation in the mPFC
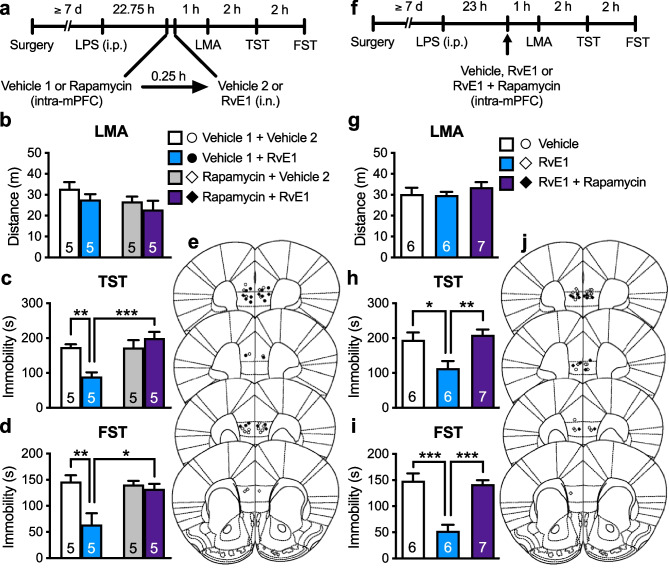
We previously showed that the antidepressant-like effect of i.c.v. infusion of RvE1 was blocked by systemic injection of rapamycin in LPS-induced depression model mice [29]. Here, to investigate the role of mTORC1 in the mPFC in the antidepressant-like actions of i.n. administration of RvE1, LPS-challenged mice were infused with rapamycin (0.01 nmol/side) or vehicle (10% DMSO/PBS, vehicle 1) into the mPFC 15 min before i.n. administration of either RvE1 or vehicle (0.5% ethanol/PBS, vehicle 2) (Fig. 4a). There was no significant difference in LMA among the groups (Fig. 4b). In vehicle 1-infused mice, i.n. administration of RvE1 significantly decreased immobility in the TST (Fig. 4c) and FST (Fig. 4d), and these antidepressant-like effects of i.n. RvE1 were completely blocked by intra-mPFC infusion of rapamycin. Rapamycin alone did not affect immobility in the TST (Fig. 4c) and FST (Fig. 4d). These results indicate that mTORC1 activation in the mPFC is required for the antidepressant-like effects of i.n. administration of RvE1.
Fig. 4.
Intranasal and intra-mPFC injections of RvE1 produce antidepressant-like actions via mTORC1 activation in the mPFC in LPS-induced depression model mice. a Experimental timeline for LPS challenge (0.8 mg/kg, i.p.), intra-mPFC infusion of either vehicle 1 (10% DMSO/PBS) or rapamycin (0.01 nmol/side), i.n. administration of either vehicle 2 (0.5% ethanol/PBS) or RvE1 (10 ng/mouse), and behavioral testing. b Effects of intra-mPFC infusion of rapamycin and i.n. administration of RvE1 on LMA in LPS-challenged mice (interaction, F1,16 = 0.0379, p = 0.848; rapamycin, F1,16 = 2.87, p = 0.110; RvE1, F1,16 = 2.09, p = 0.167). c, d Effects of intra-mPFC infusion of rapamycin on the antidepressant-like actions of i.n. administration of RvE1 in the TST (c, interaction, F1,16 = 12.8, p = 0.0025) and FST (d, interaction, F1,16 = 7.16, p = 0.0165) in LPS-challenged mice. e Schematic representation of the mPFC infusion sites. Plates are from ref. 36. f Experimental timeline for LPS challenge, intra-mPFC infusion of vehicle (10% DMSO/0.25% ethanol/PBS), RvE1 (50 pg/side) or RvE1 + rapamycin (0.01 nmol/side), and behavioral testing. g–i Effects of intra-mPFC infusion of RvE1 with or without rapamycin on LMA (g, one-way ANOVA, F2,16 = 0.761, p = 0.483) and immobility time in the TST (h, one-way ANOVA, F2,16 = 7.33, p = 0.0055) and FST (i, one-way ANOVA, F2,16 = 22.2, p < 0.0001) in LPS-challenged mice. j Schematic representation of the mPFC infusion sites. Plates are from ref. 36. Data are expressed as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001 [two-way ANOVA (b–d) and one-way ANOVA (g–i) followed by Holm-Sidak’s post hoc test]
To determine whether RvE1 stimulates mTORC1 locally in the mPFC to produce antidepressant-like effects, LPS-challenged mice were co-infused with RvE1 and rapamycin into the mPFC (Fig. 4f). There was no significant difference in LMA among the groups (Fig. 4g). The antidepressant-like effects of intra-mPFC infusion of RvE1 were completely blocked by co-infusion of rapamycin in the TST (Fig. 4h) and FST (Fig. 4i). These results suggest that RvE1 acts locally in the mPFC to produce antidepressant-like effects via mTORC1 activation.
Antidepressant-Like Effects of Intra-mPFC Infusions of BDNF and VEGF Require mTORC1 Activation in the mPFC
We previously demonstrated that intra-mPFC infusion of BDNF (100 ng/side) or VEGF (5 ng/side) produced ketamine-like antidepressant-like effects in naïve animals [15, 20, 21, 37, 38]. Here, we tested the antidepressant-like actions of BDNF and VEGF in LPS-induced depression model mice (Fig. 5a). Intra-mPFC infusion of BDNF or VEGF completely reversed LPS-induced depression-like behaviors in the TST (Fig. 5c) and FST (Fig. 5d) without affecting LMA (Fig. 5b). These results indicate that intra-mPFC infusion of BDNF/VEGF produces antidepressant-like effects in LPS-challenged mice.
Fig. 5.
Intra-mPFC infusion of BDNF or VEGF produces antidepressant-like effects via mTORC1 activation in LPS-induced depression model mice. a The experimental timeline for LPS challenge (0.8 mg/kg, i.p.), intra-mPFC infusion of vehicle (0.1% BSA/PBS), BDNF (100 ng/side) or VEGF (5 ng/side), and behavioral testing. b–d Effects of intra-mPFC infusion of BDNF or VEGF on LMA (b, one-way ANOVA, F3,19 = 1.01, p = 0.411) and immobility time in the TST (c, one-way ANOVA, F3,19 = 10.1, p = 0.0003) and FST (d, one-way ANOVA, F3,19 = 10.7, p = 0.0002) in LPS-challenged mice. e Schematic representation of the mPFC infusion sites. Plates are from ref. 36. f The experimental timeline for LPS challenge (0.8 mg/kg, i.p.), intra-mPFC infusion of vehicle (10% DMSO/0.09% BSA/PBS), BDNF (100 ng/side), VEGF (5 ng/side), rapamycin [0.01 nmol/side; a mechanistic target of rapamycin complex 1 (mTORC1) inhibitor], BDNF + rapamycin or VEGF + rapamycin, and behavioral testing. g–h Effects of intra-mPFC infusion of BDNF or VEGF with or without rapamycin on LMA (g, interaction, F2,30 = 1.86, p = 0.171; rapamycin, F1,30 = 0.975, p = 0.331; BDNF and VEGF, F2,30 = 0.377, p = 0.689) and immobility time in the TST (h, interaction, F2,28 = 7.47, p = 0.0025; one VEGF- and one BDNF + rapamycin-treated mice were excluded due to tail-climbing) and FST (i, interaction, F2,30 = 4.86, p = 0.0149) in LPS-challenged mice. j Schematic representation of the mPFC infusion sites. Plates are from ref. 36. Data are expressed as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001 (b–d, one-way ANOVA; g, h, two-way ANOVA followed by Holm-Sidak’s post hoc test)
Although previous studies showed that both BDNF and VEGF can activate mTORC1 [24, 45], to our knowledge, there is no direct behavioral evidence of whether the intra-mPFC infusion of BDNF or VEGF exerts the antidepressant-like effects via mTORC1 activation. Hence, we examined the effects of co-infusion of the mTORC1 inhibitor rapamycin (0.01 nmol/side) on the antidepressant-like effects of intra-mPFC infusion of these neurotrophic factors in mice with LPS-induced depression (Fig. 5f). There was no difference in LMA among the groups (Fig. 5g). The antidepressant-like effects of intra-mPFC infusion of BDNF or VEGF were significantly blocked by co-infusion of rapamycin in the TST (Fig. 5h) and FST (Fig. 5i). Intra-mPFC infusion of rapamycin alone did not alter immobility in both the TST (Fig. 5h) and FST (Fig. 5i). These results indicate that intra-mPFC infusion of BDNF/VEGF produces antidepressant-like effects in an mTORC1-dependent manner.
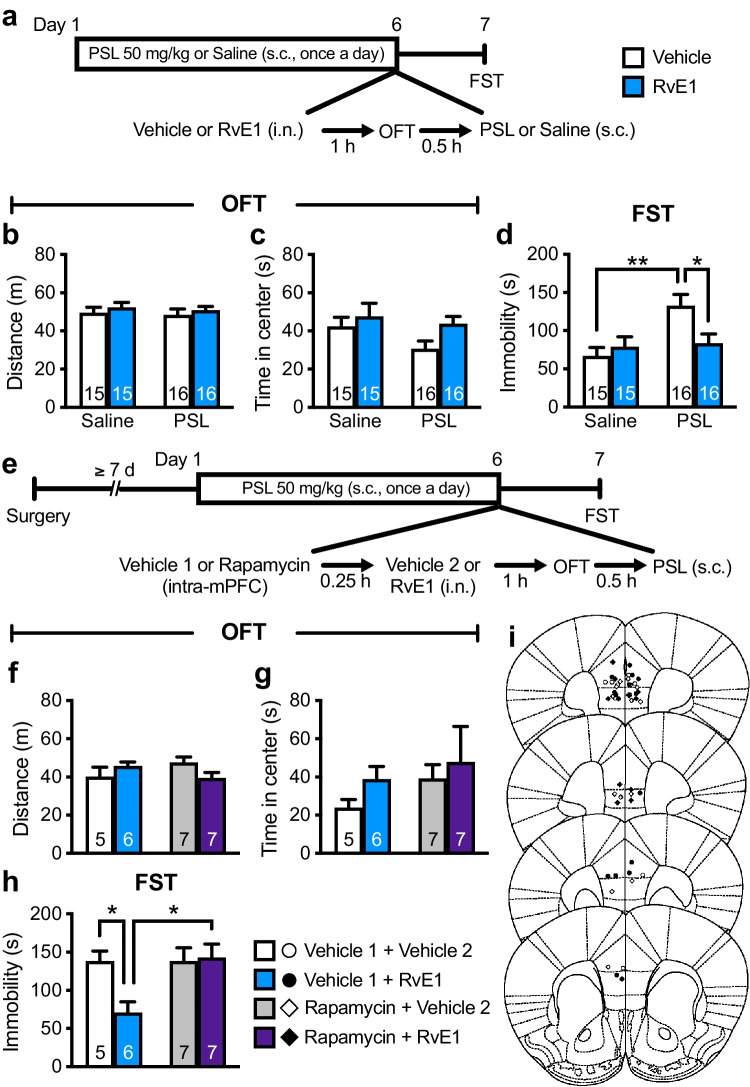
Intranasal Administration of RvE1 Attenuates Repeated PSL-Induced Depression-Like Behavior via mTORC1 Activation in the mPFC
To further confirm the role of prefrontal mTORC1 activation in the antidepressant-like actions of i.n. administration of RvE1, we examined whether these effects required mTORC1 activation in the mPFC using a mouse model of repeated PSL-induced depression in which the conventional antidepressant desipramine fails to decrease immobility as per the FST [31]. Male ICR mice were injected s.c. with PSL (50 mg/kg) or saline daily, administered i.n. with either RvE1 (10 ng/mouse) or vehicle 23 h after the fifth s.c. injection, and subjected to the OFT 1 h later (Fig. 6a). Thirty minutes after the OFT, the mice received an additional s.c. injection with either PSL or saline, and 24 h later, they underwent the FST (Fig. 6a). Repeated PSL and i.n. RvE1 administrations did not affect the total distance traveled (Fig. 6b) or time spent in the center area (Fig. 6c) in the OFT, suggesting that these treatments did not significantly influence LMA and anxiety levels. Repeated PSL injections significantly increased immobility in the FST (Fig. 6d) in vehicle-treated mice, and i.n. administration of RvE1 alleviated this depression-like behavior. RvE1 did not affect immobility in saline-injected control mice (Fig. 6d). These findings indicate that i.n. administration of RvE1 produces antidepressant-like effects in the mouse models of repeated PSL-induced, as well as LPS-induced, depression-like behaviors. PSL-treated male mice were then infused with rapamycin (0.01 nmol/side) or vehicle 1 into the mPFC 15 min before i.n. administration of either RvE1 or vehicle 2 (Fig. 6e). There were no significant differences in the distance traveled (Fig. 6f) or time spent in the center area (Fig. 6g) in the OFT among the groups. In vehicle 1-infused mice, i.n. administration of RvE1 significantly decreased immobility in the FST (Fig. 6h), and the antidepressant-like effects of i.n. RvE1 were significantly blocked by intra-mPFC infusion of rapamycin. These findings confirm the antidepressant-like potential of i.n. administration of RvE1.
Fig. 6.
Intranasal administration of RvE1 produces antidepressant-like actions via mTORC1 activation in the mPFC in repeated PSL-induced depression model mice. a Experimental timeline for PSL treatment (50 mg/kg, s.c., once a day for 6 days), i.n. administration of either vehicle (0.5% ethanol/PBS) or RvE1 (10 ng/mouse), and behavioral testing. b, c Effects of PSL and RvE1 on total distance traveled (b, interaction, F1,56 = 0.0793, p = 0.779; PSL, F1,56 = 0.829, p = 0.366; RvE1, F1,56 = 0.326, p = 0.570) and time in center (c, interaction, F1,56 = 0.0605, p = 0.807; PSL, F1,56 = 1.38, p = 0.245; RvE1, F1,56 = 4.37, p = 0.0411) in the OFT. d Effects of PSL and RvE1 on immobility time in the FST (interaction, F1,56 = 6.36, p = 0.0146). e Experimental timeline for PSL treatment (50 mg/kg, s.c., once a day for 6 days), intra-mPFC infusion of either vehicle 1 (10% DMSO/PBS) or rapamycin (0.01 nmol/side), i.n. administration of either vehicle 2 (0.5% ethanol/PBS) or RvE1 (10 ng/mouse), and behavioral testing (f, g) Effects of intra-mPFC infusion of rapamycin and i.n. administration of RvE1 on total distance traveled (f, interaction, F1,21 = 4.85, p = 0.039) and time in center (g, interaction, F1,21 = 0.0710, p = 0.793; rapamycin, F1,21 = 1.05, p = 0.318; RvE1, F1,21 = 0.978, p = 0.334) in the OFT in PSL-treated mice. h Effects of intra-mPFC infusion of rapamycin on the antidepressant-like actions of i.n. administration of RvE1 in the FST (interaction, F1,21 = 4.76, p = 0.0407). i Schematic representation of the mPFC infusion sites. Plates are from ref. 36. Data are expressed as mean ± SEM. *p < 0.05, **p < 0.01 (two-way ANOVA followed by Holm-Sidak’s post hoc test)
We also examined whether repeated administration of PSL in female ICR mice was able to induce depression-like behavior (Supplemental Fig. 1a). Repeated PSL significantly decreased time spent in the center area (Supplemental Fig. 1c) without affecting total distance traveled in the OFT (Supplemental Fig. 1b), but failed to increase immobility in the FST (Supplemental Fig. 1d). These findings suggest that repeated injection of PSL induces anxiety-like, but not depression-like, behavior in female mice. Thus, we were unable to evaluate the antidepressant-like effects of i.n. RvE1 in PSL-treated females.
Antidepressant-Like Effects of i.n. Administration of RvE1 Require BDNF/VEGF Release in the Dorsal DG
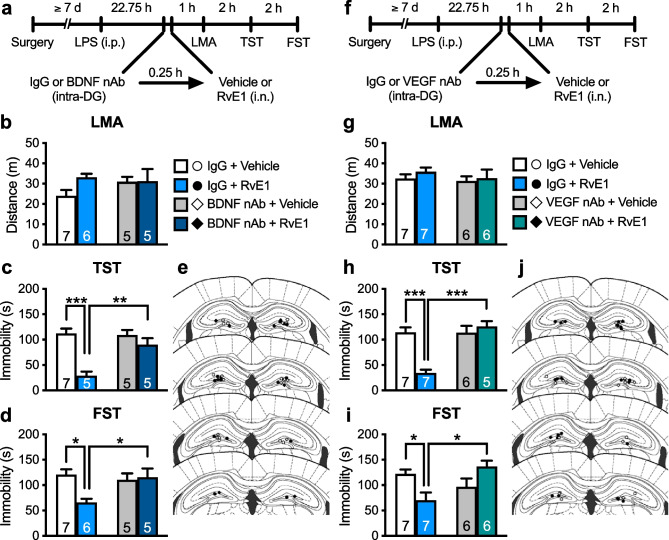
We previously demonstrated that infusion of RvE1 into the dorsal DG exerted antidepressant-like effects in LPS-challenged mice [29]. Thus, we examined whether BDNF release in the dorsal DG mediates the antidepressant-like effects of i.n. RvE1. We infused BDNF nAb (200 ng/side) or control IgG (200 ng/side) into the dorsal DG of LPS-challenged mice 15 min before i.n. administration of RvE1 (Fig. 7a). These treatments did not significantly affect LMA (Fig. 7b). In control IgG-infused mice, i.n. RvE1 decreased immobility in the TST (Fig. 7c) and FST (Fig. 7d), and these antidepressant-like actions were significantly blocked by BDNF nAb infusion. BDNF nAb alone did not significantly change immobility in the TST (Fig. 7c) and FST (Fig. 7d). These results suggest that BDNF release in the dorsal DG mediates the antidepressant-like effects of i.n. RvE1.
Fig. 7.
Intranasal administration of RvE1 produces antidepressant-like actions via BDNF/VEGF release in the dorsal DG in LPS-induced depression model mice. a Experimental timeline for LPS challenge (0.8 mg/kg, i.p.), intra-DG infusion of either control IgG (200 ng/side) or BDNF nAb (200 ng/side), i.n. administration of either vehicle (0.5% ethanol/PBS) or RvE1 (10 ng/mouse), and behavioral testing. b Effects of intra-DG infusion of BDNF nAb and i.n. administration of RvE1 on LMA in LPS-challenged mice (interaction, F1,19 = 1.66, p = 0.213; BDNF nAb, F1,19 = 0.539, p = 0.472; RvE1, F1,19 = 1.88, p = 0.186). c, d Effects of intra-DG infusion of BDNF nAb on the antidepressant-like actions of i.n. administration of RvE1 in the TST (c, interaction, F1,18 = 9.79, p = 0.0058; one IgG + RvE1-treated mouse was excluded due to tail-climbing) and FST (d, interaction, F1,19 = 6.22, p = 0.0221) in LPS-challenged mice. e Schematic representation of the DG infusion sites. Plates are from ref. 36. f Experimental timeline for LPS challenge, intra-DG infusion of either control IgG (80 ng/side) or VEGF nAb (80 ng/side), i.n. administration of either vehicle or RvE1, and behavioral testing. g Effects of intra-DG infusion of VEGF nAb and i.n. administration of RvE1 on LMA in LPS-challenged mice (interaction, F1,22 = 0.121, p = 0.731; VEGF nAb, F1,22 = 0.633, p = 0.435; RvE1, F1,22 = 0.736, p = 0.400). h, i Effects of intra-DG infusion of VEGF nAb on the antidepressant-like actions of i.n. administration of RvE1 in the TST (h, interaction, F1,21 = 20.5, p = 0.0002; one VEGF nAb + RvE1-treated mouse was excluded due to tail-climbing) and FST (i, interaction, F1,22 = 11.7, p = 0.0025) in LPS-challenged mice. j Schematic representation of the DG infusion sites. Plates are from ref. 36. Data are expressed as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001 (two-way ANOVA followed by Holm-Sidak’s post hoc test)
Next, we investigated whether VEGF release in the dorsal DG is required for the antidepressant-like actions of i.n. RvE1. LPS-challenged mice were infused with VEGF nAb (80 ng/side) or control IgG (80 ng/side) into the dorsal DG 15 min before i.n. administration of RvE1 (Fig. 7f). These treatments did not significantly influence LMA (Fig. 7g). In control IgG-infused mice, i.n. RvE1 decreased immobility in the TST (Fig. 7h) and FST (Fig. 7i), and these antidepressant-like actions were significantly blocked by VEGF nAb infusion. VEGF nAb alone did not significantly change immobility in the TST (Fig. 7h) and FST (Fig. 7i). These results suggest that VEGF release in the dorsal DG mediates the antidepressant-like effects of i.n. RvE1.
Antidepressant-Like Effects of i.n. Administration of RvE1 Are Independent of mTORC1 in the Dorsal DG
We also tested the role of mTORC1 in the dorsal DG in the antidepressant-like effects of i.n. RvE1. LPS-challenged mice were infused with rapamycin (0.01 nmol/side) or vehicle (10% DMSO/PBS, vehicle 1) into the dorsal DG 15 min before i.n. administration of either RvE1 or vehicle (0.5% ethanol/PBS, vehicle 2) (Fig. 8a). There was no significant difference in LMA among the groups (Fig. 8b). In vehicle 1-infused mice, i.n. RvE1 significantly decreased immobility in the TST (Fig. 8c) and FST (Fig. 8d), and unexpectedly, these antidepressant-like effects were not blocked by intra-DG infusion of rapamycin. Rapamycin alone did not significantly change immobility in the TST (Fig. 8c) and FST (Fig. 8d). These results suggest that mTORC1 in the dorsal DG is not involved in the antidepressant-like effects of i.n. RvE1.
Fig. 8.
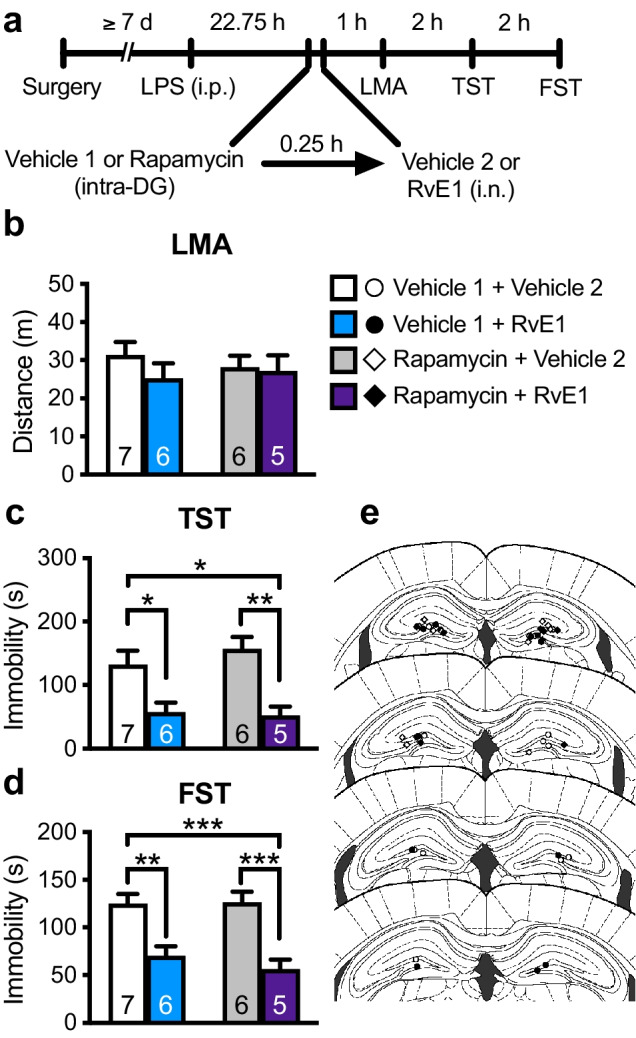
Intra-DG infusion of mTORC1 inhibitor failed to block the antidepressant-like effects of i.n. RvE1 in LPS-induced depression model mice. a Experimental timeline for LPS challenge (0.8 mg/kg, i.p.), intra-DG infusion of either vehicle 1 (10% DMSO/PBS) or rapamycin (0.01 nmol/side), i.n. administration of either vehicle 2 (0.5% ethanol/PBS) or RvE1 (10 ng/mouse), and behavioral testing. b Effects of intra-DG infusion of rapamycin and i.n. administration of RvE1 on LMA in LPS-challenged mice (interaction, F1,20 = 0.509, p = 0.484; rapamycin, F1,20 = 0.0309, p = 0.862; RvE1, F1,20 = 0.970, p = 0.336). c, d Effects of intra-DG infusion of rapamycin on the antidepressant-like actions of i.n. administration of RvE1 in the TST (c, interaction, F1,20 = 0.588, p = 0.452; rapamycin, F1,20 = 0.268, p = 0.611; RvE1, F1,20 = 22.0, p = 0.0001) and FST (d, interaction, F1,20 = 0.536, p = 0.473; rapamycin, F1,20 = 0.364, p = 0.553; RvE1, F1,20 = 35.9, p < 0.0001) in LPS-challenged mice. e Schematic representation of the DG infusion sites. Plates are from ref. 36. Data are expressed as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001 (two-way ANOVA followed by Holm-Sidak’s post hoc test)
Discussion
Our findings demonstrate that (1) i.n. administration of an extremely low dose of RvE1 (10 ng/mouse) exerts antidepressant-like effects in mouse models of LPS- and repeated PSL-induced depression-like behaviors, consistent with our previous findings that i.c.v. (1 ng/mouse), intra-mPFC (50 pg/side), and intra-dorsal DG (50 pg/side) infusions of RvE1 produce antidepressant-like effects [29–31]; (2) the antidepressant-like effects of i.n. administration of RvE1 require activation of AMPARs and L-VDCCs; (3) the antidepressant-like effects of both i.n. and intra-mPFC administrations of RvE1 require BDNF/VEGF release and subsequent activation of mTORC1 in the mPFC; (4) the antidepressant-like effects of i.n. RvE1 require BDNF/VEGF release, but not mTORC1 activation, in the dorsal DG. These results provide the first evidence that i.n. administration of RvE1 produces therapeutic effects in mouse models of depression through mechanisms similar to those underlying the rapid antidepressant actions of ketamine [10–12]. Furthermore, our findings suggest that i.n. application is an attractive option for the clinical use of unstable resolvins. RvE1 is an endogenous lipid mediator important for homeostasis [26] and is safer than ketamine. Thus, RvE1 and its receptor ChemR23 may be promising targets for developing novel rapid-acting antidepressants.
Since the dose of i.n. RvE1 (10 ng/mouse) was extremely low, it was technically difficult to measure RvE1 levels in the brain. However, given that i.n. administered peptides, steroids, and liposomal drugs are reportedly distributed throughout the whole brain [46–50], RvE1, which has a low molecular weight (350.4), can be distributed to the whole brain after i.n. administration. The present study showed that the antidepressant-like effects of i.n. RvE1 were blocked by local infusion of BDNF and VEGF nAbs in the mPFC or dorsal DG in LPS-challenged mice. These findings suggest that RvE1 can act on the mPFC and dorsal DG to produce antidepressant-like actions, which is consistent with our previous findings that local infusion of RvE1 into the mPFC and dorsal DG produces antidepressant-like effects [29, 31]. However, further investigations are required to quantify the amount of RvE1 in each brain region after i.n. administration.
As LPS-challenged mice are an inflammation-based model of depression [51], it is possible that i.n. RvE1 may specifically ameliorate LPS-induced depression-like behaviors via anti-inflammatory and pro-resolving actions. Additionally, in LPS-challenged mice, the antidepressant-like effects of RvE1 can be evaluated only during a limited period, since sickness behaviors appear with the peak at 2–6 h after the LPS challenge, and depression-like behaviors peak 1 day post-challenge and disappear within 3 days [52, 53]. Furthermore, the effects of i.n. RvE1 should be evaluated in female models of depression because depression is more prevalent in women than in men [54]. However, LPS-challenged female mice did not display depression-like behaviors [55, 56]. Thus, the antidepressant-like effects of i.n. RvE1 should be confirmed in other animal models of depression. In the present study, we used repeated PSL-treated male mice [29]. This model is based on clinical observations that patients treated with synthetic glucocorticoids have a higher prevalence of depression than untreated patients [57]. Repeated PSL-induced depression-like behavior was attenuated by RvE1 24 h after i.n. administration, suggesting that the antidepressant-like effects of i.n. RvE1 lasted for at least 24 h. Considering that RvE1 appears to be rapidly inactivated [32, 33], the current findings raise the possibility that a single i.n. administration of RvE1 could cause rapid and sustained plastic changes in brain regions, including the mPFC and DG, resulting in rapid and sustained antidepressant-like effects. This possibility should be examined further in future studies. In addition, the present study was unable to evaluate the antidepressant-like effects of RvE1 in repeated PSL-treated female mice because repeated PSL induced anxiety-like behavior instead of depression-like behavior in females. Further studies are required to evaluate the antidepressant-like effects of RvE1 in other female models of depression.
Ketamine and scopolamine rapidly increase extracellular glutamate levels in the mPFC [18, 58] via the blockade of NMDARs and M1 muscarinic acetylcholine receptors, respectively, on γ-aminobutyric acidergic (GABAergic) interneurons, resulting in disinhibition of glutamatergic neurotransmission [10–12, 59, 60]. Ketamine/scopolamine-induced glutamate burst activates postsynaptic AMPARs [18, 61]. AMPAR stimulation activates L-VDCCs, which leads to BDNF release and activation of mTORC1 signaling [62]. In addition to BDNF, neuronal VEGF signaling in the mPFC plays an essential role in the rapid and sustained antidepressant-like effects of ketamine [20], and VEGF stimulates mTORC1 signaling [24]. Consistent with these findings, the current study demonstrated that the antidepressant-like effects of BDNF and VEGF are completely blocked by intra-mPFC co-infusion of rapamycin. To our knowledge, this is the first direct behavioral evidence suggesting that both BDNF and VEGF in the mPFC require the activation of downstream mTORC1 signaling to produce antidepressant-like effects. Together, these findings confirm that BDNF/VEGF release and subsequent mTORC1 signaling in the mPFC mediate the antidepressant-like actions of ketamine and scopolamine. However, some studies raise questions about the role of mTORC1 in the antidepressant actions of ketamine. Autry et al. [63] demonstrated that ketamine did not activate mTORC1 signaling in the mPFC and hippocampus and that systemic injection of rapamycin did not reduce the antidepressant-like effects of ketamine. Moreover, Abdallah et al. [64] reported that a single oral pretreatment with rapamycin unexpectedly prolonged, rather than blocked, the antidepressant effects of ketamine in patients with depression. In contrast, NV-5138, an mTORC1 activator, produces rapid antidepressant-like effects in rodents [65]. Phase I clinical trials show that a single dose of NV-5138 produces rapid antidepressant effects with a favorable safety profile in patients with depression [66]. However, the efficacy of this drug requires confirmation in larger clinical trials (a randomized, double-blind, placebo-controlled phase II trial is currently underway; Clinicaltrials.gov Identifier: NCT05066672). Overall, although the role of mTORC1 in the antidepressant effects of ketamine remains controversial, agents that induce BDNF/VEGF release and subsequent activation of mTORC1 in the mPFC are promising candidates for a new class of rapid-acting antidepressants.
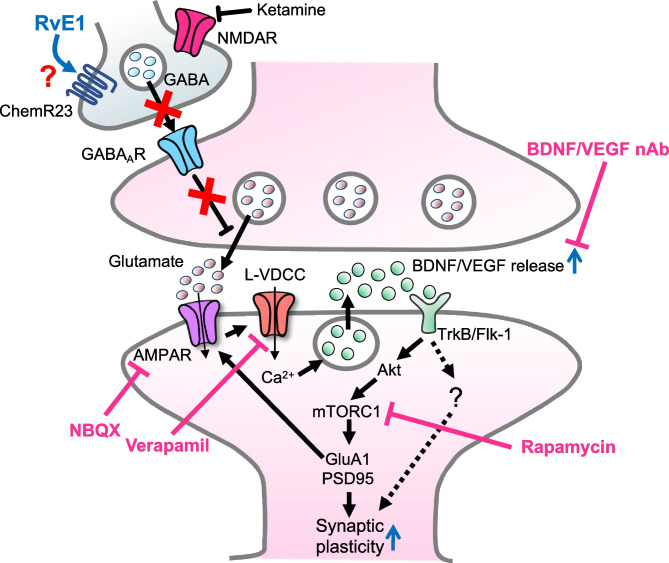
In the current study, using a mouse model of LPS-induced depression-like behaviors, we demonstrated that the antidepressant-like actions of i.n. administration of RvE1 were blocked by pretreatment with an AMPAR antagonist and L-VDCC inhibitor and that the antidepressant-like effects of i.n. and intra-mPFC administrations of RvE1 were blocked by intra-mPFC infusion of BDNF nAb, VEGF nAb, and rapamycin. Moreover, i.n. administration of RvE1 produced antidepressant-like effects via intra-mPFC mTORC1 activation in repeated PSL-treated male mice, which were resistant to a conventional antidepressant [31]. Although these findings suggest that i.n. RvE1 produces rapid antidepressant-like effects via ketamine-like mechanisms, the initial cellular target leading to the glutamate burst and AMPAR activation remains unknown. The present results, coupled with the fact that ChemR23 is an inhibitory G-protein-coupled receptor [67], raise the possibility that RvE1 initially stimulates ChemR23 in GABAergic interneurons, resulting in disinhibition and rapid glutamate burst in the mPFC. This may activate L-VDCCs and cause Ca2+ influx, leading to BDNF and VEGF release and subsequent mTORC1 activation in the mPFC, which is associated with the rapid antidepressant-like actions of RvE1 (Fig. 9). However, to our knowledge, it remains unknown whether ChemR23 is expressed by GABAergic interneurons, although this receptor is reportedly detected in neurons and glia in several human brain regions, including a part of the prefrontal cortex (Brodmann area 46) and the hippocampus [68]. Further studies are needed to test this hypothesis.
Fig. 9.
Proposed model for the cellular mechanisms underlying the rapid antidepressant-like effects of ketamine and RvE1. Ketamine blockade of NMDARs expressed on GABAergic interneurons results in disinhibition and rapid glutamate burst that activates AMPARs in the mPFC. Similarly, we hypothesize that RvE1 activates AMPARs via stimulation of the Gi-coupled ChemR23 on GABAergic interneurons, although it is still unclear whether ChemR23 is expressed by these neurons. The activation of AMPARs causes depolarization and activation of L-VDCCs, which stimulates BDNF and VEGF release. BDNF and VEGF stimulate TrkB and Flk-1, respectively, resulting in activation of mTORC1 signaling pathway. The mTORC1 pathway controls the translation and synthesis of synaptic proteins, such as GluA1 and PSD95, that are required for increases in synaptogenesis and spine maturation. These cellular events in the mPFC could be associated with the rapid antidepressant-like actions of ketamine and RvE1. BDNF and VEGF release, but not mTORC1 activation, in the dorsal DG also mediate the antidepressant-like effects of RvE1, suggesting the involvement of mTORC1-independent downstream mediators of BDNF/VEGF in the dorsal DG. AMPAR α-amino-3-hydroxy-5-methyl-4-isoxasole propionic acid receptor, Flk-1 fetal liver kinase, GABA γ-aminobutyric acid, GABAAR GABAA receptor, L-VDCC L-type voltage-dependent Ca2+ channel, mTORC1 mechanistic target of rapamycin complex 1, nAb neutralizing antibody, PSD95 postsynaptic density protein 95, NMDAR N-methyl-d-aspartate receptor, TrkB tropomyosin-related kinase B
BDNF and VEGF signaling in the DG also play critical roles in the antidepressant-like effects of ketamine [22, 23]. Similarly, the present study demonstrated that BDNF/VEGF release in the dorsal DG is required for the behavioral effects of i.n. RvE1. However, infusion of rapamycin into the dorsal DG failed to block the antidepressant-like effects of i.n. RvE1, suggesting that mTORC1 in this brain region is not involved in the behavioral effects of i.n. RvE1. These results are consistent with previous findings that ketamine does not activate mTORC1 in the dorsal hippocampus [69] and that BDNF, but not mTORC1, signaling in the hippocampus is required for the antidepressant-like actions of ketamine [63]. Therefore, it is important in future studies to determine the mTORC1-independent downstream mediators of BDNF/VEGF in the dorsal DG involved in the antidepressant-like effects of RvE1. Further molecular studies are required to confirm the impact of RvE1 on the mTORC1 signaling pathway in the dorsal DG and mPFC.
Previous studies have demonstrated that LPS (approximately 0.8 mg/kg, i.p.) modulates the mTORC1 pathway in the mouse brain; however, the results have been mixed. Francija et al. [70] reported that LPS (0.83 mg/kg, i.p.) suppressed mTORC1 signaling in the prefrontal cortex and hippocampus at 28 h post-injection; this was associated with depression-like behaviors. Genetic deletion of the NMDAR GluN2A subunit abolished LPS-induced depression-like behaviors by sustaining mTORC1 signaling activity [70]. Conversely, Zhao et al. [71] showed that LPS (0.83 mg/kg, i.p.) increased mTORC1 signaling in the forebrain 24 h after injection and concluded that LPS might induce depression-like behaviors via the activation of mTORC1 signaling. However, the causal relationship between increased mTORC1 signaling and depression-like phenotypes remains unclear, and the possibility that increased mTORC1 activity may be a compensatory response cannot be ruled out. Moreover, consistent with our previous finding that systemic injection of rapamycin did not affect LPS-induced depression-like behaviors [29, 40], the present study demonstrated that a single infusion of rapamycin into the mPFC or dorsal DG 22.75 h after the LPS challenge did not affect LPS-induced depression-like behaviors. These findings suggest that mTORC1 activation is not involved in the induction of depression-like behaviors.
In conclusion, this study demonstrates that activity-dependent BDNF/VEGF release and subsequent activation of mTORC1 in the mPFC play an essential role in the rapid antidepressant-like effects of i.n. administration of RvE1. Moreover, BDNF/VEGF release, but not mTORC1 activation, in the dorsal DG mediates the antidepressant-like actions of i.n. RvE1. However, the present study did not examine the role of BDNF/VEGF in the sustained antidepressant-like actions of i.n. RvE1 using repeated PSL-treated male mice, which should be examined in future studies. Nevertheless, the present findings suggest that i.n. administration is a viable and promising route for the delivery of unstable RvE1 to the brain and can pave the way for the application of RvE1 and other resolvins in the treatment of psychiatric and neurological disorders, including depression.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Editage (www.editage.com) for English language editing.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Author Contribution
S.D. designed the study. H.F. and S.S. synthesized RvE1. S.D., S.A., and R.S. conducted experiments and analyzed the data. S.D., M.M., and K.K. interpreted the results and wrote the manuscript. All authors reviewed and approved the final manuscript.
Funding
This study was supported by JSPS KAKENHI Grant Number JP19K07120 (S.D.), JP17K08360 (H.F.) and JP19H01014 (S.S.), the Mochida Memorial Foundation for Medical and Pharmaceutical Research (S.D.), Takeda Science Foundation (S.D.), the Uehara Memorial Foundation (S.D.), YOKOYAMA Foundation for Clinical Pharmacology (Grant Number YRY-1903; S.D.), Hokkoku Cancer Foundation (S.D.), and Japan Agency for Medical Research and Development (AMED) under Grant Number 21gm0910012h0004 (M.M.).
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Conflict of Interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Satoshi Deyama, Email: deyama@p.kanazawa-u.ac.jp.
Katsuyuki Kaneda, Email: k-kaneda@p.kanazawa-u.ac.jp.
References
- 1.Greenberg PE, Fournier AA, Sisitsky T, Pike CT, Kessler RC. The economic burden of adults with major depressive disorder in the United States (2005 and 2010) J Clin Psychiatry. 2015;76:155–162. doi: 10.4088/JCP.14m09298. [DOI] [PubMed] [Google Scholar]
- 2.Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 3.Berman RM, Cappiello A, Anand A, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/S0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 4.Zarate CA, Jr, Singh JB, Carlson PJ, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 5.Kim J, Farchione T, Potter A, Chen Q, Temple R. Esketamine for treatment-resistant depression—first FDA-approved antidepressant in a new class. N Engl J Med. 2019;381:1–4. doi: 10.1056/NEJMp1903305. [DOI] [PubMed] [Google Scholar]
- 6.Mahase E. Esketamine is approved in Europe for treating resistant major depressive disorder. BMJ. 2019;367:l7069. doi: 10.1136/bmj.l7069. [DOI] [PubMed] [Google Scholar]
- 7.Singh JB, Fedgchin M, Daly E, et al. Intravenous esketamine in adult treatment-resistant depression: a double-blind, double-randomization, placebo-controlled study. Biol Psychiatry. 2016;80:424–431. doi: 10.1016/j.biopsych.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 8.Short B, Fong J, Galvez V, Shelker W, Loo CK. Side-effects associated with ketamine use in depression: a systematic review. Lancet Psychiatry. 2018;5:65–78. doi: 10.1016/S2215-0366(17)30272-9. [DOI] [PubMed] [Google Scholar]
- 9.Yang C, Shirayama Y, Zhang JC, et al. R-ketamine: a rapid-onset and sustained antidepressant without psychotomimetic side effects. Transl Psychiatry. 2015;5:e632. doi: 10.1038/tp.2015.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deyama S, Duman RS. Neurotrophic mechanisms underlying the rapid and sustained antidepressant actions of ketamine. Pharmacol Biochem Behav. 2020;188:172837. doi: 10.1016/j.pbb.2019.172837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duman RS, Deyama S, Fogaca MV. Role of BDNF in the pathophysiology and treatment of depression: activity-dependent effects distinguish rapid-acting antidepressants. Eur J Neurosci. 2021;53:126–139. doi: 10.1111/ejn.14630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deyama S, Kaneda K. Role of neurotrophic and growth factors in the rapid and sustained antidepressant actions of ketamine. Neuropharmacology. 2023;224:109335. doi: 10.1016/j.neuropharm.2022.109335. [DOI] [PubMed] [Google Scholar]
- 13.Li N, Lee B, Liu RJ, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lepack AE, Fuchikami M, Dwyer JM, Banasr M, Duman RS. BDNF release is required for the behavioral actions of ketamine. Int J Neuropsychopharmacol. 2014;18:pyu033. doi: 10.1093/ijnp/pyu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kato T, Fogaca MV, Deyama S, Li XY, Fukumoto K, Duman RS. BDNF release and signaling are required for the antidepressant actions of GLYX-13. Mol Psychiatry. 2018;23:2007–2017. doi: 10.1038/mp.2017.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghosal S, Bang E, Yue W, et al. Activity-dependent brain-derived neurotrophic factor release is required for the rapid antidepressant actions of scopolamine. Biol Psychiatry. 2018;83:29–37. doi: 10.1016/j.biopsych.2017.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu RJ, Duman C, Kato T, et al. GLYX-13 produces rapid antidepressant responses with key synaptic and behavioral effects distinct from ketamine. Neuropsychopharmacology. 2017;42:1231–1242. doi: 10.1038/npp.2016.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Voleti B, Navarria A, Liu RJ, et al. Scopolamine rapidly increases mammalian target of rapamycin complex 1 signaling, synaptogenesis, and antidepressant behavioral responses. Biol Psychiatry. 2013;74:742–749. doi: 10.1016/j.biopsych.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lepack AE, Bang E, Lee B, Dwyer JM, Duman RS. Fast-acting antidepressants rapidly stimulate ERK signaling and BDNF release in primary neuronal cultures. Neuropharmacology. 2016;111:242–252. doi: 10.1016/j.neuropharm.2016.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deyama S, Bang E, Wohleb ES, et al. Role of neuronal VEGF signaling in the prefrontal cortex in the rapid antidepressant effects of ketamine. Am J Psychiatry. 2019;176:388–400. doi: 10.1176/appi.ajp.2018.17121368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deyama S, Bang E, Kato T, Li XY, Duman RS. Neurotrophic and antidepressant actions of brain-derived neurotrophic factor require vascular endothelial growth factor. Biol Psychiatry. 2019;86:143–152. doi: 10.1016/j.biopsych.2018.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi M, Lee SH, Chang HL, Son H. Hippocampal VEGF is necessary for antidepressant-like behaviors but not sufficient for antidepressant-like effects of ketamine in rats. Biochim Biophys Acta. 2016;1862:1247–1254. doi: 10.1016/j.bbadis.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 23.Ma Z, Zang T, Birnbaum SG, et al. TrkB dependent adult hippocampal progenitor differentiation mediates sustained ketamine antidepressant response. Nat Commun. 2017;8:1668. doi: 10.1038/s41467-017-01709-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim BW, Choi M, Kim YS, et al. Vascular endothelial growth factor (VEGF) signaling regulates hippocampal neurons by elevation of intracellular calcium and activation of calcium/calmodulin protein kinase II and mammalian target of rapamycin. Cell Signal. 2008;20:714–725. doi: 10.1016/j.cellsig.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 25.Dalli J, Serhan CN. Identification and structure elucidation of the pro-resolving mediators provides novel leads for resolution pharmacology. Br J Pharmacol. 2019;176:1024–1037. doi: 10.1111/bph.14336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deyama S, Minami M, Kaneda K. Resolvins as potential candidates for the treatment of major depressive disorder. J Pharmacol Sci. 2021;147:33–39. doi: 10.1016/j.jphs.2021.05.002. [DOI] [PubMed] [Google Scholar]
- 28.Deyama S, Kaneda K, Minami M. Resolution of depression: antidepressant actions of resolvins. Neurosci Res. in press. 10.1016/j.neures.2022.10.006. [DOI] [PubMed]
- 29.Deyama S, Shimoda K, Suzuki H, et al. Resolvin E1/E2 ameliorate lipopolysaccharide-induced depression-like behaviors via ChemR23. Psychopharmacology. 2018;235:329–336. doi: 10.1007/s00213-017-4774-7. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki H, Otsuka T, Hitora-Imamura N, et al. Resolvin E1 attenuates chronic pain-induced depression-like behavior in mice: possible involvement of chemerin receptor ChemR23. Biol Pharm Bull. 2021;44:1548–1550. doi: 10.1248/bpb.b21-00461. [DOI] [PubMed] [Google Scholar]
- 31.Aoki S, Deyama S, Sugie R, et al. The antidepressant effect of resolvin E1 in repeated prednisolone-induced depression model mice. Behav Brain Res. 2022;418:113676. doi: 10.1016/j.bbr.2021.113676. [DOI] [PubMed] [Google Scholar]
- 32.Arita M, Oh SF, Chonan T, et al. Metabolic inactivation of resolvin E1 and stabilization of its anti-inflammatory actions. J Biol Chem. 2006;281:22847–22854. doi: 10.1074/jbc.M603766200. [DOI] [PubMed] [Google Scholar]
- 33.Yoo S, Lim JY, Hwang SW. Resolvins: Endogenously-generated potent painkilling substances and their therapeutic perspectives. Curr Neuropharmacol. 2013;11:664–676. doi: 10.2174/1570159X11311060009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mittal D, Ali A, Md S, Baboota S, Sahni JK, Ali J. Insights into direct nose to brain delivery: current status and future perspective. Drug Deliv. 2014;21:75–86. doi: 10.3109/10717544.2013.838713. [DOI] [PubMed] [Google Scholar]
- 35.Ishimura K, Fukuda H, Fujiwara K, et al. Synthesis of resolvin E1 and its conformationally restricted cyclopropane congeners with potent anti-inflammatory effect. ACS Med Chem Lett. 2021;12:256–261. doi: 10.1021/acsmedchemlett.0c00639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. 3. Burlington, MA: Elsevier; 2007. [Google Scholar]
- 37.Deyama S, Kaneda K. The duration of the antidepressant-like effects of a single infusion of brain-derived neurotrophic factor into the medial prefrontal cortex in mice. Behav Brain Res. 2020;394:112844. doi: 10.1016/j.bbr.2020.112844. [DOI] [PubMed] [Google Scholar]
- 38.Deyama S, Kondo M, Shimada S, Kaneda K. IGF-1 release in the medial prefrontal cortex mediates the rapid and sustained antidepressant-like actions of ketamine. Transl Psychiatry. 2022;12:178. doi: 10.1038/s41398-022-01943-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fukumoto K, Iijima M, Funakoshi T, Chaki S. 5-HT1A receptor stimulation in the medial prefrontal cortex mediates the antidepressant effects of mGlu2/3 receptor antagonist in mice. Neuropharmacology. 2018;137:96–103. doi: 10.1016/j.neuropharm.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 40.Deyama S, Ishikawa Y, Yoshikawa K, et al. Resolvin D1 and D2 reverse lipopolysaccharide-induced depression-like behaviors through the mTORC1 signaling pathway. Int J Neuropsychopharmacol. 2017;20:575–584. doi: 10.1093/ijnp/pyx023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deyama S, Shimoda K, Ikeda H, Fukuda H, Shuto S, Minami M. Resolvin E3 attenuates lipopolysaccharide-induced depression-like behavior in mice. J Pharmacol Sci. 2018;138:86–88. doi: 10.1016/j.jphs.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 42.Kajiyama Y, Iijima S, Chiba M, et al. Prednisolone causes anxiety- and depression-like behaviors and altered expression of apoptotic genes in mice hippocampus. Prog Neuropsychopharmacol Biol Psychiatry. 2014;54:243–248. doi: 10.1016/j.pnpbp.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 43.Ito S, Deyama S, Domoto M, et al. Effects of the synthetic cannabinoid 5F-AMB on anxiety and recognition memory in mice. Psychopharmacology. 2019;236:2235–2242. doi: 10.1007/s00213-019-05222-2. [DOI] [PubMed] [Google Scholar]
- 44.Burgdorf J, Zhang XL, Nicholson KL, et al. GLYX-13, a NMDA receptor glycine-site functional partial agonist, induces antidepressant-like effects without ketamine-like side effects. Neuropsychopharmacology. 2013;38:729–742. doi: 10.1038/npp.2012.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith ED, Prieto GA, Tong L, et al. Rapamycin and interleukin-1beta impair brain-derived neurotrophic factor-dependent neuron survival by modulating autophagy. J Biol Chem. 2014;289:20615–20629. doi: 10.1074/jbc.M114.568659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Banks WA, During MJ, Niehoff ML. Brain uptake of the glucagon-like peptide-1 antagonist exendin(9–39) after intranasal administraion. J Pharmacol Exp Ther. 2004;309:469–475. doi: 10.1124/jpet.103.063222. [DOI] [PubMed] [Google Scholar]
- 47.Ducharme N, Banks WA, Morley JE, et al. Brain distribution and behavioral effects of progesterone and pregnenolone after intranasal or intravenous administration. Eur J Pharmacol. 2010;641:128–134. doi: 10.1016/j.ejphar.2010.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang ZZ, Zhang YQ, Wang ZZ, et al. Enhanced brain distribution and pharmacodynamics of rivastigmine by liposomes following intranasal administration. Int J Pharm. 2013;452:344–354. doi: 10.1016/j.ijpharm.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 49.Sasaki-Hamada S, Nakamura R, Nakao Y, et al. Antidepressant-like effects exerted by the intranasal administration of a glucagon-like peptide-2 derivative containing cell-penetrating peptides and a penetration-accelerating sequence in mice. Peptides. 2017;87:64–70. doi: 10.1016/j.peptides.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 50.Dhaliwal HK, Fan Y, Kim J, Amiji MM. Intranasal delivery and transfection of mRNA therapeutics in the brain using cationic liposomes. Mol Pharm. 2020;17:1996–2005. doi: 10.1021/acs.molpharmaceut.0c00170. [DOI] [PubMed] [Google Scholar]
- 51.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Frenois F, Moreau M, O'Connor J, et al. Lipopolysaccharide induces delayed FosB/DeltaFosB immunostaining within the mouse extended amygdala, hippocampus and hypothalamus, that parallel the expression of depressive-like behavior. Psychoneuroendocrinology. 2007;32:516–531. doi: 10.1016/j.psyneuen.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Laumet G, Edralin JD, Chiang AC, Dantzer R, Heijnen CJ, Kavelaars A. Resolution of inflammation-induced depression requires T lymphocytes and endogenous brain interleukin-10 signaling. Neuropsychopharmacology. 2018;43:2597–2605. doi: 10.1038/s41386-018-0154-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.World Health Organization. Depression fact sheets. 2021. https://www.who.int/news-room/fact-sheets/detail/depression/. Accessed 25 Apr 2022.
- 55.Millett CE, Phillips BE, Saunders EFH. The sex-specific effects of LPS on depressive-like behavior and oxidative stress in the hippocampus of the mouse. Neuroscience. 2019;399:77–88. doi: 10.1016/j.neuroscience.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 56.Jiang X, Chen Z, Yu X, et al. Lipopolysaccharide-induced depression is associated with estrogen receptor-alpha/SIRT1/NF-kappaB signaling pathway in old female mice. Neurochem Int. 2021;148:105097. doi: 10.1016/j.neuint.2021.105097. [DOI] [PubMed] [Google Scholar]
- 57.Patten SB. Exogenous corticosteroids and major depression in the general population. J Psychosom Res. 2000;49:447–449. doi: 10.1016/S0022-3999(00)00187-2. [DOI] [PubMed] [Google Scholar]
- 58.Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wohleb ES, Wu M, Gerhard DM, et al. GABA interneurons mediate the rapid antidepressant-like effects of scopolamine. J Clin Invest. 2016;126:2482–2494. doi: 10.1172/JCI85033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gerhard DM, Pothula S, Liu RJ, et al. GABA interneurons are the cellular trigger for ketamine's rapid antidepressant actions. J Clin Invest. 2020;130:1336–1349. doi: 10.1172/JCI130808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maeng S, Zarate CA, Jr, Du J, et al. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 62.Jourdi H, Hsu YT, Zhou M, Qin Q, Bi X, Baudry M. Positive AMPA receptor modulation rapidly stimulates BDNF release and increases dendritic mRNA translation. J Neurosci. 2009;29:8688–8697. doi: 10.1523/JNEUROSCI.6078-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Autry AE, Adachi M, Nosyreva E, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Abdallah CG, Averill LA, Gueorguieva R, et al. Modulation of the antidepressant effects of ketamine by the mTORC1 inhibitor rapamycin. Neuropsychopharmacology. 2020;45:990–997. doi: 10.1038/s41386-020-0644-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kato T, Pothula S, Liu RJ, et al. Sestrin modulator NV-5138 produces rapid antidepressant effects via direct mTORC1 activation. J Clin Invest. 2019;129:2542–2554. doi: 10.1172/JCI126859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Navitor. Navitor’s three phase 1 studies for NV-5138 show antidepressant effects and biomarker impact, supporting further development of direct activator of mTORC1 in depression. 2019. http://www.navitorpharma.com/navitors-three-phase-1-studies-for-nv-5138-show-antidepressant-effects-and-biomarker-impact-supporting-further-development-of-direct-activator-of-mtorc1-in-depression/. Accessed 25 Apr 2022.
- 67.Kennedy AJ, Davenport AP. International union of basic and clinical pharmacology CIII: chemerin receptors CMKLR1 (Chemerin1) and GPR1 (Chemerin2) nomenclature, pharmacology, and function. Pharmacol Rev. 2018;70:174–196. doi: 10.1124/pr.116.013177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Emre C, Hjorth E, Bharani K, et al. Receptors for pro-resolving mediators are increased in Alzheimer’s disease brain. Brain Pathol. 2020;30:614–640. doi: 10.1111/bpa.12812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yamada J, Jinno S. Potential link between antidepressant-like effects of ketamine and promotion of adult neurogenesis in the ventral hippocampus of mice. Neuropharmacology. 2019;158:107710. doi: 10.1016/j.neuropharm.2019.107710. [DOI] [PubMed] [Google Scholar]
- 70.Francija E, Lukic I, Petrovic Z, et al. GluN2A-ERK-mTOR pathway confers a vulnerability to LPS-induced depression-like behaviour. Behav Brain Res. 2022;417:113625. doi: 10.1016/j.bbr.2021.113625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhao J, Lao L, Cui W, Rong J. Potential link between the RagA-mTOR-p70S6K axis and depressive-behaviors during bacterial liposaccharide challenge. J Neuroinflammation. 2019;16:211. doi: 10.1186/s12974-019-1610-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.