Abstract
Introduction
Cancer cachexia, found in more than a third of patients with NSCLC, directly leads to functional and survival detriments. As screening and interventions for cachexia and NSCLC improve, deficits in health care access and quality among patients disadvantaged by racial-ethnic and socioeconomic factors must be addressed.
Methods
We retrospectively evaluated 957 patients diagnosed with having stage IV NSCLC between 2014 and 2020 in Dallas, Texas. Cachexia was retrospectively assessed by applying criteria for substantial unintentional weight loss in the time leading up to cancer diagnosis. Nonparametric, parametric, multivariate logistic regression, and Kaplan-Meier analyses were conducted to evaluate for variables potentially associated with cachexia incidence and survival.
Results
In multivariate analysis including age, sex, comorbidities, body mass index, risk behaviors, and tumor characteristics, Black race and Hispanic ethnicity were independently associated with more than a 70% increased risk of presenting with cachexia at the time of NSCLC diagnosis (p < 0.05). When private insurance status was included as a covariate, this association was diminished for Hispanic patients only. Black patients presented with stage IV disease at an average of approximately 3 years younger than White patients (Kruskal-Wallis p = 0.0012; t test p = 0.0002). Cachexia status at diagnosis consistently predicted for survival detriments, further highlighting the importance of addressing differential cachexia risk across racial-ethnic groups.
Conclusions
Fundamentally, our findings reveal elevated cachexia risk in Black and Hispanic patients with stage IV NSCLC with associated survival detriments. These differences are not fully accounted for by traditional determinants of health and suggest novel avenues for addressing oncologic health inequities.
Keywords: Cachexia, Health inequities, Palliative care, Lung neoplasms, Mass screening
Introduction
NSCLC is a leading cause of cancer-related mortality in the United States, as most patients present with late-stage disease and limited treatment options. It is estimated that 36% of patients with NSCLC have cachexia,1 a clinically challenging multifactorial syndrome characterized by substantial loss of muscle and adipose stores.2 Cancer cachexia is associated with decreased quality of life, reduced response to cancer therapies, and poor overall prognosis.3
Racial, ethnic, and socioeconomic status (SES) outcome disparities are prevalent in NSCLC, as higher incidence and mortality are consistently observed in marginalized groups.4 Patients with NSCLC who are Black, Hispanic, uninsured, or residing in low-income areas have been found to be less likely to receive guideline-concordant care.5
Although the importance of race, ethnicity, and SES in NSCLC is well established and encourages targeted research, the relationship is poorly defined within the context of cachexia. In this study, we reviewed a 7-year data set of patients diagnosed with having stage IV NSCLC in Dallas, Texas, to investigate associations between cachexia and clinical, demographic, and SES factors.
Materials and Methods
Cohort Characteristics and Cachexia Incidence at Diagnosis
Retrospective tumor data extraction and electronic medical record review were conducted through an institutional review board–approved study at a tertiary care center in Dallas, Texas. Demographic information, comorbid conditions, treatment information, longitudinal weight data, survival time, pertinent health behaviors, and tumor characteristics were collected for patients diagnosed with having stage IV NSCLC between 2014 and 2020.
Comorbidities were quantitatively assessed by the Charlson comorbidity index. Patients were classified as not having private insurance if they were uninsured or entirely dependent on government-associated coverage (Medicare, Medicaid, and veteran programs).
The international consensus definition2 of cachexia was applied to determine cachexia status at diagnosis. For patients with body mass index (BMI) greater than or equal to 20, unintentional weight loss exceeding 5% from baseline in the 6-month period leading to the diagnosis met the criteria for cachexia at cancer diagnosis. A cutoff of 2% was applied for patients with BMI less than 20 per consensus definition.
Statistical Evaluation
With the classification of cachexia incidence at cancer diagnosis as the binary dependent variable, multivariate logistic regression was conducted, including the following covariates: age at diagnosis, sex, pretreatment BMI, alcohol history, tobacco history, Charlson comorbidity value, tumor cell morphology, and racial-ethnic group.
Independent-sample Kruskal-Wallis testing was conducted with pairwise comparisons to determine whether any statistically significant difference existed based on age at cancer diagnosis across racial-ethnic groups of the cohort. Statistically significant comparison groups were further evaluated with independent-sample t testing.
Survival was evaluated through Kaplan-Meier estimators and Cox regression analysis for non-Hispanic (NH) White and Black patients stratified by cachexia status at diagnosis, as these groups had sufficient (n > 100) representation within the overall cohort. Survival was evaluated as time in days from cancer diagnosis date to the date of death or last contact, with appropriate censoring.
All statistical analyses, tables, and figures were created or carried out on IBM SPSS Statistics for Macintosh, Version 28.0. (Armonk, NY). Tests were conducted at the 5% significance level.
Results
Cachexia Incidence at Diagnosis
The total cohort consisted of 957 patients, with 42.95% (n = 411) meeting the criteria for cachexia at diagnosis. Table 1 displays frequencies of patient and tumor characteristics within our cohort along with cachexia incidence by category. More specific payor information for patients without private insurance can be found in Supplementary Table 1.
Table 1.
Population Characteristics and Cachexia Incidence
| Category | Characteristics | n | Cachexia Incidence (%) |
|---|---|---|---|
| Patient information | Median age at diagnosis (y), IQR | 65, 57–72 | |
| Sex | |||
| Male | 524 | 252 (48.09) | |
| Female | 432 | 158 (36.57) | |
| Race | |||
| Non-Hispanic White | 579 | 228 (39.38) | |
| Black | 216 | 118 (54.63) | |
| Asian | 72 | 23 (31.94) | |
| Hispanic | 90 | 42 (46.67) | |
| Insurance | |||
| No private insurance | 437 | 223 (51.03) | |
| Any private insurance | 475 | 175 (36.84) | |
| Risk factors | Median Charlson comorbidity score, IQR | 11, 9–13 | |
| Median pretreatment BMI (kg/m2), IQR | 24.80, 21.98–28.62 | ||
| Alcohol history | |||
| None | 575 | 250 (43.48) | |
| Current or prior | 334 | 141 (42.22) | |
| Tobacco history | |||
| None | 211 | 70 (33.18) | |
| Current or prior | 698 | 321 (45.99) | |
| Tumor characteristics | Cellular morphology | ||
| Adenocarcinoma | 705 | 283 (40.14) | |
| Squamous | 137 | 71 (51.82) | |
| Large cell | 16 | 8 (50) | |
| Mixed or NOS | 99 | 49 (49.49) | |
| Total | 957 | 411 (42.95) |
BMI, body mass index; IQR, interquartile range; NOS, not otherwise specified.
Multivariate Analyses
In multivariate analysis including private insurance as a binary covariate, Black patients had an OR of 1.558 (95% confidence interval [CI]: 1.051–2.309, p = 0.0272) toward cachexia at diagnosis. Hispanic ethnicity was not associated with an elevated risk of cachexia incidence at diagnosis (p = 0.03937). Private insurance had a protective association toward cachexia incidence, with an OR of 0.692 (95% CI: 0.496–0.964, p = 0.0298; Table 2).
Table 2.
Multivariate Analysis of Cachexia Incidence at Cancer Diagnosis
| Category | Parameter | OR (95% CI) | p value |
|---|---|---|---|
| Patient information | Age at diagnosis (y) | 1.005 (0.989–1.021) | 0.5618 |
| Female sex | 0.719 (0.520–0.994) | 0.0457 | |
| Race | |||
| Non-Hispanic White | - | 0.1575 | |
| Black | 1.558 (1.051–2.309) | 0.0272 | |
| Asian | 0.982 (0.470–2.050) | 0.9606 | |
| Hispanic | 1.285 (0.722–2.288) | 0.3937 | |
| Private insurance | 0.692 (0.496–0.964) | 0.0298 | |
| Risk factors | Charlson comorbidity score | 1.005 (0.954–1.058) | 0.8620 |
| Pretreatment BMI | 0.981 (0.954–1.008) | 0.1641 | |
| Alcohol history | 1.045 (0.751–1.453) | 0.7948 | |
| Tobacco history | 1.606 (1.027–2.510) | 0.0377 | |
| Tumor characteristics | Cell morphology | ||
| Adenocarcinoma | - | 0.4141 | |
| Squamous cell | 1.326 (0.867–2.027) | 0.1929 | |
| Large cell | 1.117 (0.373–3.345) | 0.8427 | |
| Mixed or NOS | 1.414 (0.834–2.399) | 0.1983 |
BMI, body mass index; CI, confidence interval; NOS, not otherwise specified.
Repeated analysis excluding the private insurance covariate revealed significant elevations of cachexia risk at diagnosis greater than 70% for Black (OR = 1.748, 95% CI: 1.205–2.535, p = 0.0033) and Hispanic (OR = 1.717, 95% CI: 1.005–2.932, p = 0.0477; Supplementary Table 2) patients.
Comparing Age at Diagnosis
Pairwise comparisons between racial-ethnic groups on Kruskal-Wallis testing detected a significant difference in age at diagnosis solely between NH White and Black patients (p = 0.0012; Supplementary Table 3). Independent-sample t testing between these groups again revealed a significant difference of age at diagnosis (p = 0.0002; Supplementary Table 4). NH White and Black patients presented with mean ages of 65.64 and 62.60 years at diagnosis, respectively.
Survival
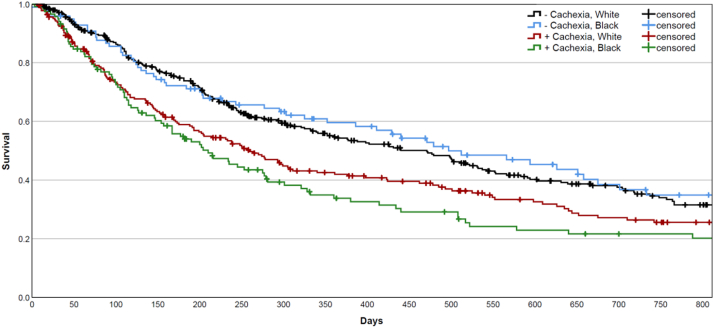
Figure 1 illustrates Kaplan-Meier curves for NH White and Black patients stratified by cachexia status at diagnosis. Within the cohort of patients who did not present with cachexia at diagnosis, there was no significant difference between survival time between NH White and Black patients detected on log-rank testing (p = 0.5121). Similar analysis within the cohort of patients with cachexia at diagnosis also found no significant difference between NH White and Black patients (p = 0.2744). Significant survival differences were found for all groups with opposing cachexia status at diagnosis (p < 0.05). Supplementary Tables 5 and 6 display median survival times and log-rank comparisons.
Figure 1.
Two-year Kaplan-Meier survival curves for non-Hispanic White and Black patients stratified by cachexia status at cancer diagnosis.
Simultaneous interpretation of survival influence of Black race—compared with NH White patients—and cachexia status was performed by means of Cox regression. Black race was associated with a hazard ratio of 1.044 (95% CI: 0.860–1.268, p = 0.6633), whereas cachexia status was associated with a hazard ratio of 1.416 (95% CI: 1.187–1.690, p = 0.0001).
Discussion
Development of cachexia is common among patients with cancer, associated with reduced function, decreased response to anticancer therapy, and increased mortality.3 Despite the prevalence and impact of cancer cachexia, weight loss in patients with cancer is rarely actively monitored.2 Although cachexia research has progressed in the past decade, our understanding of racial-ethnic disparities in cachexia is limited. One study found that an increased risk of cachexia among Black patients contributed to outcome disparities in pancreatic cancer.6 To our knowledge, this is the first study evaluating racial-ethnic disparities regarding cachexia risk in patients with NSCLC.
Health Inequity in Stage IV NSCLC Cachexia
Prior studies have revealed that Black patients with NSCLC consistently present with more advanced disease at diagnosis, undergo prolonged diagnosis-to-treatment times, and are less likely to receive recommended care.7,8 Hispanic ethnicity, lower household income, and a lack of private insurance have similarly been observed to associate with worsened outcomes and survival.9 Development of cachexia is clinically important in NSCLC care, especially in advanced stages, where pretreatment weight loss independently predicts for survival outcomes.10 In this study, we further characterize associations between cachexia with race, ethnicity, and private insurance in NSCLC.
Multivariate analysis without a private insurance covariate found a significantly increased risk of cachexia at diagnosis exceeding 70% for Black and Hispanic patients. This indicates an oncologic outcome inequity independent of underlying patient and tumor characteristics including prior comorbidities and risk behaviors—representing generally conceived determinants of health. Cachexia risk in multivariate analysis was attenuated for Hispanic patients after adjusting for access to private insurance. This suggests a contributory role of increased dependence on public insurance toward cachexia burden in Hispanic populations. Despite the consideration of this measure, Black patients had a 55.8% greater cachexia risk, revealing racial inequities persisting beyond this surrogate measure of health care access.
In survival analysis of NH White and Black patients, race was not found to significantly predict for survival within groups of equivalent cachexia status at diagnosis. Cachexia incidence, however, was consistently associated with pronounced survival detriment. This observation, in conjunction with the differential cachexia incidence we observed, suggests that the decreased survival observed in Black and Hispanic patients with NSCLC may depend on cachexia or factors contributing to the progression of cachexia. This was further substantiated in our Cox regression analysis, which found no association between Black race and survival risk when considered simultaneously with cachexia status at diagnosis.
Advanced disease is an important risk factor for development of cancer cachexia.2 The increased frequency of late-stage NSCLC presentation for Black patients has been supported to result from delayed detection and inadequate quality and consistency of screening.7,11 Although all patients in this study presented with stage IV NSCLC, the elevated incidence of cachexia in Black patients at time of diagnosis suggests a within-stage delay in cancer detection compared with NH White patients. Important factors contributing to delayed detection in Black patients are inadequacies of lung cancer screening protocols and utilization. Current U.S. Preventive Services Task Force recommendations are predominantly based on the National Lung Screening Trial,12 whose participants were disproportionately White and of higher SES compared with the general population, and the Nederlands-Leuvens Longkanker Screenings Onderzoek,13 which did not report on participant race, ethnicity, or SES. In our cohort, we found that Black patients presented significantly younger than NH White patients at diagnosis, by approximately 3 years. This finding is consistent with prior literature revealing that race-specific adjustment of age requirements in U.S. Preventive Services Task Force guidelines would result in more equitable lung cancer screening.7,14 Importantly, of the individuals eligible under current guidelines, Black patients and those with low SES are still less likely to obtain recommended screening.7 Early detection is crucial in preventing and palliating cachexia.3 This highlights the necessity for efforts in health policy to mitigate further barriers in health care access in parallel to the revision of screening criteria.
The greater cachexia risk observed in minority or socioeconomically disadvantaged groups might also depend on environmental or pathophysiological mechanisms not evaluated in this study. A key risk factor for cachexia development is poor nutrition status,2 which is well known to correlate with racial-ethnic background and SES. Our cohort received care in Texas, which has consistently revealed poor health equity measures and ranks in the bottom quartile nationally when assessed by access to, quality of, and utilization of health care services among minority populations.15
Conclusion
Marked disparities persist in cachexia risk at time of stage IV NSCLC diagnosis, even after controlling for several demographic and clinical characteristics. Hispanic patients have an increase in cachexia risk that is at least partially attributable to decreased access to private insurance. Black patients have increased cachexia risk irrespective of insurance status. As the field of cancer cachexia sees improvements in diagnostic and interventional strategy, future research must simultaneously expand to further characterize the specific mechanisms behind outcome disparities to substantiate targeted and equitable reform to health policy.
CRediT Authorship Contribution Statement
Santiago Olaechea: Conceptualization, formal analysis, investigation, methodology, visualization, writing—original draft, writing—review and editing.
Alison Liu: Writing—original draft, writing—review and editing.
Brandon Sarver: Writing—review and editing.
Linda Anne Gilmore: Data curation.
Christian Alvarez: Data curation.
Kayvon Yazdanbakhsh: Writing—review and editing.
Rodney Infante: Project administration, supervision.
Puneeth Iyengar: Data curation, project administration, supervision, writing—review and editing.
Acknowledgments
This work was supported by the National Institutes of Health (P30 CA142543); Burroughs Wellcome Fund Career Awards for Medical Scientists (1019692); American Cancer Society grant (133889-RSG-19-195-01-TBE); Cancer Prevention and Research Institute of Texas (RP200170); and the Conquer Cancer Medical Student Rotation for Underrepresented Populations Award. Any opinions, findings, and conclusions expressed in this material are those of the author(s) and do not necessarily reflect those of the American Society of Clinical Oncology or Conquer Cancer.
Footnotes
Disclosure: Mr. Olaechea has a research grant from the American Society of Clinical Oncology. Dr. Infante's laboratory has received funding from Pfizer unrelated to this work. Dr. Iyengar has worked with AstraZeneca in an advisory capacity unrelated to this work. The remaining authors declares no conflict of interest.
Cite this article as: Olaechea S, Liu A, Sarver B, et al. Racial, ethnic, and socioeconomic characteristics independently predict for cachexia risk and associated survival outcomes in stage IV NSCLC: a brief report. JTO Clin Res Rep. 2023;4:100496.
Note: To access the supplementary material accompanying this article, visit the online version of the JTO Clinical and Research Reports at www.jtocrr.org and at https://doi.org/10.1016/j.jtocrr.2023.100496.
Contributor Information
Rodney Infante, Email: Rodney.Infante@UTSouthwestern.edu.
Puneeth Iyengar, Email: Puneeth.Iyengar@UTSouthwestern.edu.
Supplementary Data
References
- 1.von Haehling S., Anker S.D. Cachexia as a major underestimated and unmet medical need: facts and numbers. J Cachexia Sarcopenia Muscle. 2010;1:1–5. doi: 10.1007/s13539-010-0002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fearon K., Strasser F., Anker S.D., et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489–495. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 3.Nishikawa H., Goto M., Fukunishi S., Asai A., Nishiguchi S., Higuchi K. Cancer cachexia: its mechanism and clinical significance. Int J Mol Sci. 2021;22:8491. doi: 10.3390/ijms22168491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tannenbaum S.L., Koru-Sengul T., Zhao W., Miao F., Byrne M.M. Survival disparities in non-small cell lung cancer by race, ethnicity, and socioeconomic status. Cancer J. 2014;20:237–245. doi: 10.1097/PPO.0000000000000058. [DOI] [PubMed] [Google Scholar]
- 5.Stein J.N., Rivera M.P., Weiner A., et al. Sociodemographic disparities in the management of advanced lung cancer: a narrative review. J Thorac Dis. 2021;13:3772–3800. doi: 10.21037/jtd-20-3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Permuth J.B., Clark Daly A., Jeong D., et al. Racial and ethnic disparities in a state-wide registry of patients with pancreatic cancer and an exploratory investigation of cancer cachexia as a contributor to observed inequities. Cancer Med. 2019;8:3314–3324. doi: 10.1002/cam4.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sosa E., D’Souza G., Akhtar A., et al. Racial and socioeconomic disparities in lung cancer screening in the United States: a systematic review. CA Cancer J Clin. 2021;71:299–314. doi: 10.3322/caac.21671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haas J.S., Earle C.C., Orav J.E., Brawarsky P., Neville B.A., Williams D.R. Racial segregation and disparities in cancer stage for seniors. J Gen Intern Med. 2008;23:699–705. doi: 10.1007/s11606-008-0545-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slatore C.G., Au D.H., Gould M.K. American Thoracic Society Disparities in Healthcare Group. An official American Thoracic Society systematic review: insurance status and disparities in lung cancer practices and outcomes. Am J Respir Crit Care Med. 2010;182:1195–1205. doi: 10.1164/rccm.2009-038ST. [DOI] [PubMed] [Google Scholar]
- 10.Ross P.J., Ashley S., Norton A., et al. Do patients with weight loss have a worse outcome when undergoing chemotherapy for lung cancers? Br J Cancer. 2004;90:1905–1911. doi: 10.1038/sj.bjc.6601781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones C.C., Mercaldo S.F., Blume J.D., et al. Racial disparities in lung cancer survival: the contribution of stage, treatment, and ancestry. J Thorac Oncol. 2018;13:1464–1473. doi: 10.1016/j.jtho.2018.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Lung Screening Trial Research Team. Aberle D.R., Adams A.M., et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Koning H.J., van der Aalst C.M., de Jong P.A., et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med. 2020;382:503–513. doi: 10.1056/NEJMoa1911793. [DOI] [PubMed] [Google Scholar]
- 14.Pasquinelli M.M., Tammemagi M.C., Kovitz K.L., et al. Brief report: risk prediction model versus United States Preventive Services Task Force Draft Lung Cancer Screening Eligibility Criteria-Reducing Race Disparities. JTO Clin Res Rep. 2020;2 doi: 10.1016/j.jtocrr.2020.100137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The Commonwealth Fund. Achieving racial and ethnic equity in U.S. health care: a scorecard of state performance. https://www.commonwealthfund.org/publications/scorecard/2021/nov/achieving-racial-ethnic-equity-us-health-care-state-performance
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.