Abstract
Carnitine plays multiple roles in skeletal muscle metabolism, including fatty acid transport and buffering of excess acetyl-CoA in the mitochondria. The skeletal muscle cannot synthesize carnitine; therefore, carnitine must be taken up from the blood into the cytoplasm. Carnitine metabolism, its uptake into cells, and the subsequent reactions of carnitine are accelerated by muscle contraction. Isotope tracing enables the marking of target molecules and monitoring of tissue distribution. In this study, stable isotope-labeled carnitine tracing was combined with matrix-assisted laser desorption/ionization mass spectrometry (MALDI-MS) imaging to determine carnitine distribution in mouse skeletal muscle tissues. Deuterium-labeled carnitine (d3-carnitine) was intravenously injected into the mice and diffused to the skeletal muscles for 30 and 60 min. To examine whether muscle contraction changes the distribution of carnitine and its derivatives, unilateral in situ muscle contraction was performed; 60 min muscle contraction showed increased d3-carnitine and its derivative d3-acetylcarnitine in the muscle, indicating that carnitine uptake in cells is promptly converted to acetylcarnitine, consequently, buffering accumulated acetyl-CoA. While the endogenous carnitine was localized in the slow type fibers rather than fast type, the contraction-induced distributions of d3-carnitine and acetylcarnitine were not necessarily associated with muscle fiber type. In conclusion, the combination of isotope tracing and MALDI-MS imaging can reveal carnitine flux during muscle contraction and show the significance of carnitine in skeletal muscles.
Keywords: Mass spectrometry imaging, Metabolite tracing, Acetylation
1. Introduction
Carnitine is an essential low-molecular-weight compound for skeletal muscle metabolism because it carries energy substrates across the mitochondrial membranes. The main role of carnitine is to transport acyl-CoAs derived from long-chain fatty acids into the mitochondrial matrix, where they enter the beta-oxidation pathway [1]. Long-chain acyl-CoAs are then converted to acylcarnitines by carnitine palmitoyltransferase (CPT)-1, and can be transported across the mitochondrial membrane via carnitine translocase. The acylcarnitines are re-converted to carnitine and long-chain acyl-CoAs by CPT-2, and then undergo β-oxidation. Another major role of carnitine in skeletal muscle is to buffer excess short-chain acyl-CoA (mainly acetyl-CoA). In cases where the production of acetyl-CoA through beta-oxidation and glycolysis exceeds the capacity of the tricarboxylic acid cycle, acetyl-CoA intermediates accumulate in the muscle mitochondria. This imbalance is reversed by carnitine acetyl-transferase (CrAT) that resides principally within the mitochondrial matrix where it acts selectively on short-chain acyl-CoAs. CrAT converts the accumulated short-chain acyl-CoA to acylcarnitine (mainly acetycarnitine), which then exits mitochondria and tissues [2].
Carnitine has been used as a nutritional supplement to maintain body health and improve physical performance by enhancing fat oxidation in the skeletal muscles [3]. Although several studies have reported that chronic and acute carnitine supplementation increases physical performance [4], simple carnitine supplementation does not increase skeletal muscle metabolism [5]. This is because simple carnitine supplementation does not increase the carnitine concentration in muscle cells in healthy humans [6,7]. The carnitine concentration in skeletal muscle cells is 50-to 100-fold higher than that in plasma [8] and is regulated by active transport via specific transporters. Thus, even if carnitine concentration increases in blood by supplementation, carnitine transport into muscle cells is not facilitated [9].
Exercise and muscle contractile activity enhance both glycolysis and fatty acid utilization in skeletal muscle for energy production. During muscle contraction, carnitine is converted to acetylcarnitine to buffer excess acetyl-CoA, leading to a deficiency in intracellular carnitine and potentially inhibiting fatty acid metabolism. We have been investigating the carnitine dynamics in skeletal muscle and demonstrated that carnitine uptake from the blood into skeletal muscle was enhanced by acute muscle contraction [10]. Contraction-induced carnitine uptake into the muscle cells is essential for sustained energy production. However, to conclude that carnitine uptake during muscle contraction is functionally significant, it is necessary to show that carnitine is immediately utilized as acetylcarnitine during muscle contraction.
Here, we examined whether acute muscle contraction increased carnitine uptake and its acetylation and the change in carnitine dynamics were dependent on muscle fiber types. To monitor carnitine dynamics in skeletal muscles, we used stable isotope-labeled exogenous carnitine, in which three hydrogens were replaced with deuterium (d3-carnitine). D3-carnitine was intravenously injected into mice, and carnitine distribution in skeletal muscle was analyzed by mass spectrometry imaging (MS imaging) that integrates microscopy and mass spectrometry, for label-free, ex vivo visualization of molecular localization. We showed that d3-carnitine was distributed into the skeletal muscles within 60 min, and the flux was enhanced by muscle contraction, but they were not dependent on fiber types. In contracted muscles, d3-acetylcarnitine (acetylated d3-carnitine) was also detected suggesting that carnitine that is taken up from the blood, is promptly used during muscle contraction. Our experimental model enabled the short-term monitoring of carnitine flux in short term period and revealed the role of carnitine in skeletal muscles.
2. Materials and methods
2.1. Animals
A total of 24 adult male ICR mice weighing 40–60 g were housed in cages in a temperature-controlled room at 24 °C with 12-h light-dark cycle. The mice were fasted for 16 h the day before the experiment. Mice were bred and maintained in the animal care facilities at Tokyo Metropolitan University. All animal experiments were performed according to protocols approved by the Experimental Animal Care and Use Committee of the Tokyo Metropolitan University (permit number A28–6 and A4−4).
2.2. Materials
The stable isotope l-carnitine (METHYL-D3, DLM-1871) was purchased from SI Science (Kanagawa, Japan) and 2,5-dihydroxybenzoic acid (DHB) was purchased from Bruker Daltonics (Bremen, Germany). Methanol, ethanol, and ultrapure water (Wako Pure Chemical Industries, Osaka, Japan) were used to prepare all buffers and solvents. All the chemicals used in this study were of the highest purity available. All other reagents were obtained from Sigma Chemical Co. (St. Louis, MO, USA), Wako Pure Chemical Industries, Funakoshi Co. (Tokyo, Japan), and GE Healthcare (Piscataway, NJ, USA).
2.3. In situ contraction
In situ contractions were performed as described previously [11] with some modifications. Briefly, mice were anesthetized with 0.75 mg of medetomidine hydrochloride (4 mg), and butorphanol (5 mg/kg body weight), the sciatic nerves of both legs were surgically exposed, and electrodes (Uchida Denshi, Tokyo, Japan) were attached. One leg was subjected to electrical stimulation using an electronic stimulator SEN-3401 (Nihon Kohden, Tokyo, Japan) for 30 min or 60 min (train rate, 1/s; train duration, 500 ms; pulse rate, 100 Hz; duration, 0.1 ms at 1–5 V), and the contralateral leg was considered to be the resting leg.
Prior to the experiment, the left and right jugular veins were exposed and 50 μL of blood was collected as a pre-injection sample. D3-labeled carnitine (15 mg/kg body weight) dissolved in saline was injected via the right jugular vein and time measurement subsequently began. One min after injection, blood was collected as a pre-muscle contraction sample, and 2 min after administration of d3-carnitine, in situ contraction was initiated. Blood was collected before d3-carnitine injection, and 1, 5, 10, 30, and 60 min after injection. Following contraction, the mice were sacrificed, and the tibial anterior (TA), calf muscles, and kidneys were immediately dissected, frozen in 2-methylbuthane, and stored at −80 °C without fixation. The scheme and timetable of the experiment are shown in Fig. 1 A and B.
Fig. 1.
Scheme of the experiments in this study. (A) The mice were anesthetized and placed in the supine position. Deuterium (d3)-labeled carnitine was injected into the left jugular vein at 15 mg/kg body weight body weight (BW) and blood was collected from the right jugular vein at designated time points. Both sides of the sciatic nerve were exposed, and only the left nerve was electrically stimulated. The left leg was contracted at 1 Hz for 30 or 60 min, and the tibial anterior and calf muscles from both sides were excised immediately after contraction. (B) Time course of experiments. The time point for the carnitine injection was set to 0. Blood was collected before and after injection at several time points indicated by red arrows. Muscle contraction time was measured for 30 and 60 min. (C) Chemical formulas of endogenous and d3-labeled carnitines. The molecular weight (MW) of the endogenous carnitine was 161. Because the three hydrogens of the methyl group were replaced with deuterium (highlighted in red), the MW of d3-canritine was 164. (D) Plasma concentration over time for d3-carnitine after intravenous administration in mice. Data are expressed as a ratio of d3-carnitine to endogenous carnitine. Values are presented as mean ± SEM (N = 6). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
2.4. Histochemical analyses
Sections were prepared as previously described [11]. Briefly, 10-μm sections were cut using a cryostat (CM 1850; Leica Microsystems, Wetzlar, Germany). Serial sections were mounted on coated glass slides [Matsunami Adhesive Slide (MAS), Matsunami, Osaka, Japan] for histochemical staining and indium-tin oxide (ITO)-coated glass slides (Bruker Daltonics, Germany) for MS imaging. For morphological observations, sections were stained with hematoxylin and eosin (H&E). To evaluate glycogen content, serial sections were stained with periodic acid-Schiff (PAS) stain (Millipore, MA, USA).
2.5. Immunohistochemical analysis
To determine the muscle fiber type, immunofluorescence analysis of myosin heavy chain (MHC) expression was performed. Tissue sections were blocked with 10% goat serum in PBS for 60 min at room temperature and incubated with primary antibodies against MHCI (BA-F8), MHCIIa (SC-71, 2F7), MHCIIx (6H1), MHCIIb (BF–F3) at 4 °C overnight. The sections were incubated with a mixture of 2nd antibodies including Alexa Fluor 350 IgG2b, Alexa Fluor 488 IgG1, and Alexa Fluor 555 IgM, for 2 h at room temperature. Primary antibodies were purchased from the Developmental Studies Hybridoma Bank (University of Iowa), whilst secondary antibodies were purchased from Invitrogen. The fluorescence was visualized using a fluorescence microscope (Keyence, Osaka, Japan).
2.6. Extraction of the mitochondrial fraction from skeletal muscle
The mitochondrial fraction was isolated using a mitochondria isolation kit (Thermo Fisher Scientific, MA , USA, 89801). In brief, freshly isolated TA tissues were minced on ice and incubated with trypsin in phosphate-buffered saline (PBS) for 3 min. After the removal of the trypsin solution, tissues were homogenized in BSA-containing buffer with a Polytron homogenizer (PT 2100; Kinematica, Lucerne, Switzerland) at low speed for 10 s. The homogenized samples were then centrifuged according to the manufacturer's instructions, and the pellet after the last centrifugation at 12,000×g was used as the mitochondrial fraction, whilst the supernatant was used as the cytoplasmic fraction. For western blotting, mitochondrial fractions were resuspended and homogenized using lysis buffer [12].
2.7. Western blotting
Total protein extracts were obtained from mitochondria fraction. Protein samples were separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to PVDF membranes. The membranes were blocked with Tris-buffered saline containing 5% non-fat dry milk and 0.1% Tween 20. The membranes were then incubated overnight with the following primary antibodies: Anti-CrAT (Proteintech, IL, USA, 15170-1-AP), anti-COX IV (Cell Signaling Technology, MA, USA, 4844), anti-PMP70 (Sigma-Aldrich, SAB4200181), anti-GAPDH (Cell Signaling Technology, 2118). Subsequently, the membranes were treated with rabbit horseradish peroxidase-conjugated secondary antibody (Cytiva, Tokyo, Japan). The blots were developed using ECL Plus (PerkinElmer Life Sciences) or ECL (Thermo Fisher Scientific), and analyzed using a Luminescent Image Analyzer ImageQuant800 (Cytiva). Data were then quantified using ImageJ software.
2.8. Mass spectrometry imaging
For the imaging analysis, a 10 μm thinly cryosectioned tissue is mounted on a ITO glass slide. Carnitine species were detected in positive-ion mode, where the molecules were detected as protonated molecules. For matrix-assisted laser desorption/ionization (MALDI)-MS in positive-ion mode, 50 mg/mL of DHB in methanol/water (7/3, v/v) was sprayed uniformly over the sections using a 0.2-mm nozzle caliber airbrush (Procon Boy FWA Platinum; Mr. Hobby, Tokyo, Japan). MS imaging analyses were performed using an ultraflextreme (Bruker Daltonics, Germany) for Fig. 2, Fig. 3, and TOF/TOF 5800 (AB Sciex, Framingham, MA, USA) for Fig. 4, Fig. 5. Prior to matrix-coating, the high-resolution image of the section was generated by scanning and regions of interest from the samples were selected for MS imaging. MS data are acquired by firing a laser onto the surface of the tissue, where the energy is subsequently absorbed by the matrix molecules, which are then ionized with biomolecules and measured using a mass analyzer. The laser was irradiated 100 times at each position. All imaging pixel sizes were 100 μm. Ion imaging was performed using FlexImaging software (Bruker Daltonics) and BioMap software (Novartis, Basal, Switzerland).
Fig. 2.
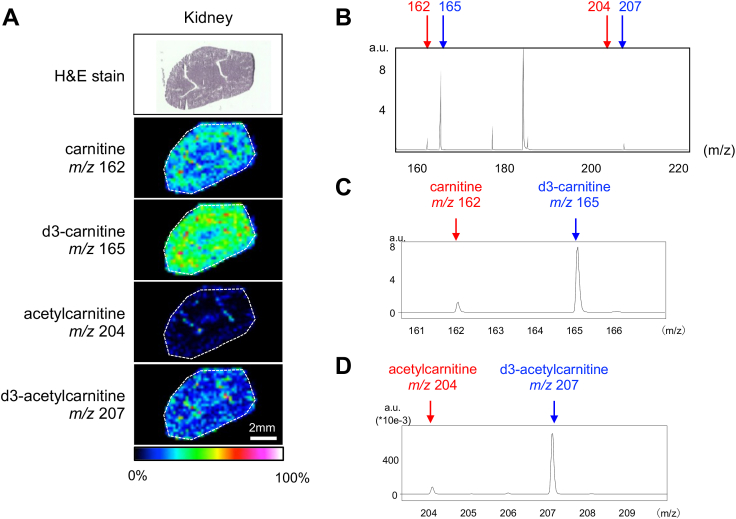
Visualization of carnitine and acetylcarnitine in kidney using MS imaging analysis after 30 min of in situ muscle contraction. (A) Hematoxylin and eosin (H&E) staining and MS imaging were performed on kidney sections. The ion intensities of carnitine (m/z 162), d3-carnitine (m/z 165), acetylcarnitine (m/z 204), and d3-acetylcarnitine (m/z 207) were visualized using color bars. The number of ions was expressed as a relative value with the highest point on each slide. (B) Comparison of the amount of carnitine species in the kidney sections. Red arrows indicate endogenous carnitine (m/z 162) and acetylcarnitine (m/z 204). Blue arrows indicate d3-carnitine (m/z 165) and d3-acetylcarnitine (m/z 207). (C) Enlarged view of the mass spectrum for carnitine and d3-carnitine. (D) Enlarged view of the mass spectrum for acetylcarnitine and d3-acetylcarnitine. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Fig. 3.
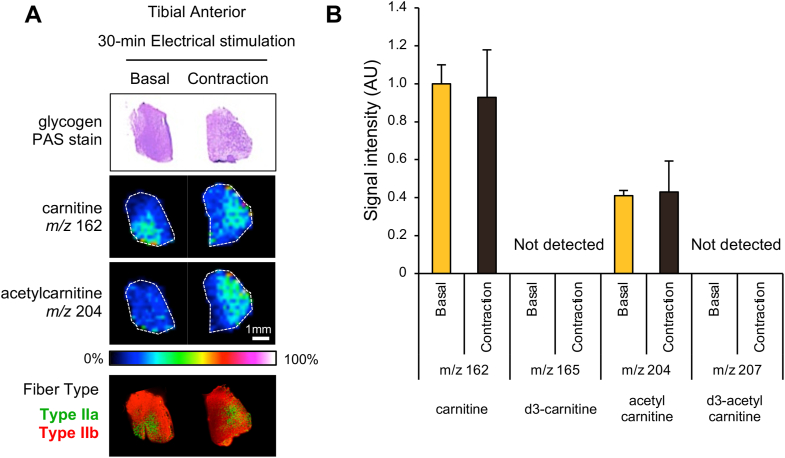
Visualization and quantification of carnitine and acetylcarnitine in tibial anterior muscles after 30 min of in situ muscle contraction. (A) Glycogen and carnitine distribution in the tibial anterior muscle with (contraction) and without (basal) 30 min muscle contraction. Bottoms are immunohistochemical images used for fiber-type identification. Serial sections were stained with antibodies against MHC IIa (green) or IIb (red). (B) Ion intensities were quantified in the whole area and expressed as a relative value to the basal value of carnitine (m/z 162). There were no significant differences between the basal and contraction groups. Values are presented as mean ± SEM (N = 4). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Fig. 4.
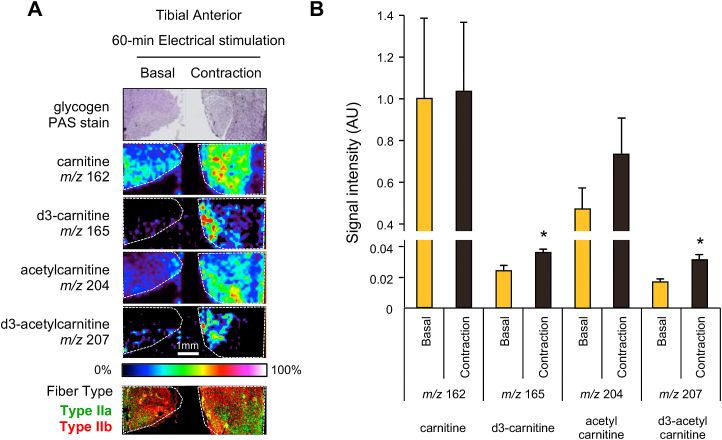
Effect of 60 min of in situ muscle contraction on carnitine distributions of tibial anterior muscles. (A) Glycogen and carnitine distribution in the tibial anterior with (contraction) and without (basal) 60 min muscle contraction. Bottoms are immunohistochemical images for the fiber type identification. Serial sections were stained with antibodies against MHC I (blue), MHC IIa (green), or IIb (red). Carnitine and acetylcarnitine were detected, and the ion intensities are visualized in a color bar. (B) The ion intensities were quantified over the entire area. The values are expressed relative to the basal sample of carnitine (m/z 162). The ion intensities of d3-carnitine (m/z 165) and d3-acetylcarnitine (m/z 207) were significantly higher in contracted muscles. *p < 0.05. Values are presented as mean ± SEM (N = 4). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Fig. 5.
Effect of 60 min of in situ muscle contraction on carnitine distributions of calf muscles. (A) Carnitine distribution in the tibial anterior with (contraction) and without (basal) 60 min of muscle contraction. Carnitine distribution in calf muscles with (contraction) and without (basal) 60 min of muscle contraction. Bottoms are immunohistochemical images used for fiber-type identification. Serial sections were stained with antibodies against MHC I (blue), MHC IIa (green), or MHC IIb (red). Carnitine and acetylcarnitine were detected, and the ion intensities are visualized in a color bar. D3-carnitine (m/z 165) was distributed in a specific region and matched with the area of type I and type IIa fibers. (B) Ion intensity was quantified over the entire study area. The values are expressed relative to the basal sample of carnitine (m/z 162). The ion intensities of d3-carnitine (m/z 165) and d3-acetylcarnitine (m/z 207) were significantly higher in contracted muscles. *p < 0.05. Values are presented as the mean ± SEM (N = 6). (C) Detection of CrAT in the mitochondrial fraction of the anterior tibial region. Mitochondrial marker protein (COX IV), cytosolic marker protein (GAPDH), peroxisome marker protein (PMP70), and CrAT in pellet and supernatant fractions after the last centrifugation of mitochondria extraction methods were determined using western blotting. The pellets were the mitochondrial fractions, as COX IV was detected and GAPDH and PMP70 were not detected. CrAT has also been detected in the mitochondrial fraction. Experiments were repeated in four mice. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
2.9. Measurement of carnitine levels in blood
Blood was withdrawn using a 26-G syringe from the left jugular vein and the serum was subsequently separated by centrifugation at 5000 g for 15 min. The supernatants were then stored at −80 °C for MS measurement. To measure the correct ratio of d3-carnitine to carnitine, serum samples were diluted 20 fold using ultra-pure water and dissolved in an equal volume with 50 mg/mL of DHB in methanol/water (1/1, v/v). The samples were then applied to a 384 plate in triplicate and allowed to dry. MS was performed using a MALDI TOF/TOF-type AB Sciex instrument. The laser was irradiated 100 times per position and the spectrum was accumulated 10 times. The ions of carnitine and d3-carnitine were detected as potassium adduct ions ([M+K]+) because of serum-derived contaminants.
2.10. Statistical analysis
All data are expressed as mean ± standard error of the mean (SEM). N in the figure legends indicates the number of mice used in the experiments. Statistical analyses were conducted using Student's t-test, and the level of significance was set at p < 0.05.
3. Results
3.1. Detection of stable isotope-labeled carnitine
To trace the exogenous carnitine distribution, stable isotope-labeled carnitine (d3-carnitine) was used, in which the three hydrogens of the methyl group were replaced with deuterium (Fig. 1C). The molecular weights of endogenous carnitine and d3-carnitine were 161 and 164, respectively. Mass spectrometry can distinguish between endogenous and extrinsic carnitine based on differences in the three mass numbers.
Blood samples were collected over time after d3-carnitine injection, alongside the changes in d3-carnitine levels across 60 min. In the crude serum samples, endogenous carnitine and d3-carnitine were detected as [M+K]+ ions at m/z 200 and 203, respectively. Fig. 1D shows the ratio of d3-carnitine to endogenous carnitine, indicating that d3-carnitine increased at 5 min after injection and plateaued at 30 min (Fig. 1D).
The kidney is one of the organs with the highest carnitine uptake and metabolism, as characterized by its high expression of carnitine transporters and enzyme [13,14]. To confirm whether labeled carnitine was distributed into tissues and detected in this system, the kidney was excised 30 min after d3-carnitine injection and analyzed for MS imaging. In the MALDI-MS imaging experiment, endogenous carnitine and d3-carnitine were detected as [M + H]+ ions at m/z 162 and 165, respectively. We have previously confirmed through tandem mass analyses that the ion at m/z 162 was carnitine, which produced a main product ion at m/z 103, as a result of the loss of the trimethyl amine group [15].
MS imaging detected these signals and revealed that the amount of d3-carnitine was higher than that of endogenous carnitine (Fig. 2A–C). This is because the kidney is not an organ for storage, but rather to immediately export carnitine into the blood, suggesting that endogenous carnitine was readily replaced by labeled carnitine for 30 min. Acetylcarnitine, the form of acetylated carnitine formed by buffering acetyl-CoA, was also detected (Fig. 2A, D). Similar to the results for carnitine, d3-acetylcarnitine was higher than acetylcarnitine, suggesting that imported carnitine was immediately acetylated in the kidneys (Fig. 2D). Carnitine and d3-carnitine levels were slightly higher on the outside of the kidney, called the renal cortex. In contrast, acetylcarnitine was distributed throughout the kidneys and was also present in the center.
3.2. Effect of in situ contraction on carnitine metabolism in TA muscle
To examine the effect of muscle contraction on carnitine distribution, we performed unilateral in situ muscle contractions by inducing electrical stimulation for 30 min in mice. Both contracted and resting control muscles were isolated and sectioned for visualization of carnitine distribution and analysis of glycogen content using PAS staining. We visualized the distribution of endogenous carnitine and acetylcarnitine, but did not detect a sufficient effect on muscle contraction because endogenous acetylcarnitine did not significantly increase in contracted muscles (Fig. 3A). In addition, d3-carnitine was not detected in contracted muscle (Fig. 3B). Since the glycogen content visualized by PAS staining did not change between the left and right muscles, the metabolic changes resulting from muscle contraction for 30 min were small, and therefore carnitine metabolism did not change significantly (Fig. 3A). The reason why d3-carnitine was detected in kidney but not in skeletal muscles was explained by the expression levels of carnitine specific transporter, novel organic transporter-2 (OCTN2). The kidney is the organ with the highest expression of OCTN2 [13], and has a high capacity of carnitine uptake.
Notably, endogenous carnitine was localized in specific regions of TA muscles. We identified muscle fiber types by staining serial sections with marker proteins. As shown in Fig. 3A, the TA muscle consisted of several types of muscle fibers, including type IIa, IIx, and IIb fibers, and the region of carnitine distribution was matched with the area where type IIa fibers were enriched. Type IIa fibers are classified as oxidative, with the most mitochondria among type II fibers [16], suggesting that carnitine content was higher in type IIa fibers than type IIb fibers.
Next, we applied in situ contraction for 60-min to extend the duration of carnitine uptake and metabolism (Fig. 1B). Muscle contraction continued for 60 min after electrical stimulation and no abnormalities were observed in the mice. Fig. 4 shows the results for the TA muscles, which are mainly composed of fast-type muscle fibers [17]. D3-carnitine was rarely detected in the basal muscle, but was detected in the contracted muscle as shown in both imaging and quantified data (Fig. 4A and B).
Although the absolute values of ion intensities for injected d3-carnitine were relatively lower than those for endogenous carnitine (Fig. 4B), the amount of d3-carnitine in contracted muscle was increased significantly compared to basal control muscle (Fig. 4B). D3-acetylcarnitine was also detected at 60 min and increased in contracted muscles (Fig. 4A and B), suggesting that carnitine transport and acetylation require at least more than 30 min of contraction.
The distribution area of d3-labeled carnitine was narrower and more restricted than that of endogenous carnitine in contracted muscle tissues. These areas did not seem to be associated with the fiber type, as identified by immunohistochemistry. In this experiment, we observed exogenous carnitine distribution over a relatively short time; therefore, d3-labeled carnitine localization was the region where carnitine was rapidly imported into TA within 60 min. It is possible that d3-labeled carnitine is distributed as endogenous carnitine over a longer period. The mismatch of d3-carnitine and endogenous carnitine localization was not a consequence of methodological artifacts, but the characterization of carnitine dynamics.
3.3. Visualization of carnitine distribution in calf muscles
Electrical stimulation of the sciatic nerve induced contraction of not only the tibial anterior, but also the calf muscles, which consist of three muscles: the soleus, gastrocnemius, and plantaris. The soleus muscle is representative of slow-type muscles because it mainly consists of type I (slow-twitch) fibers [18]. There are only a few type I fibers in the deep portion of the gastrocnemius and near the soleus, whilst the gastrocnemius and plantaris consist of type II (fast-twitch) fibers. Endogenous carnitine accumulated specifically in the type I fiber-enriched region in both the basal and contracted muscles (Fig. 5A). However, other molecules, such as d3-carnitine and acetylcarnitine, were distributed throughout the calf muscle independently of the fiber type. The signal intensities of these molecules were quantified across the entire calf muscle area and are shown in Fig. 5B. The levels of carnitine, d3-carnitine, and d3-acetylcarnitine were all significantly increased by muscle contraction (Fig. 5B).
Carnitine plays an essential role in the mitochondrial metabolism of skeletal muscle, and is also important in peroxisomal metabolism [19]. To examine whether contraction-induced acetylcarnitine production occurred in the mitochondria or peroxisomes, we extracted mitochondria and peroxisomes separately using an isolation kit. As a result, although mitochondria were purely isolated, carnitine and acetylcarnitine were not detected in the fraction by MS (data not shown). Furthermore, peroxisomes could not be isolated from TA muscles due to their low presence (data not shown). Next, to understand which organelle was the main source for acetylcarnitine production in skeletal muscles, we examined which fraction of the isolated organelles contained CrAT, the enzyme that produces acetylcarnitine from acetyl-CoA. Mitochondria were isolated as pellets after centrifugation, the last step of mitochondrial fractionation, and the supernatant was used as the cytosolic fraction, including peroxisomes. As expected, COX IV, a marker of mitochondria, was detected in the pellet fraction, whilst GAPDH and PMP70, markers of cytosol and peroxisomes, respectively, were detected in the supernatant fraction (Fig. 5C). CrAT was detected only in the pellet (mitochondrial) fraction, suggesting that it was predominantly localized in the mitochondria of the skeletal muscle. These results indicate that muscle contraction-induced carnitine acetylation occurred in mitochondria.
4. Discussion
We previously measured the radioisotope carnitine (3[H]carnitine) uptake in skeletal muscle by measuring the radioactivity in tissues [10]. Although radioisotope measurements are useful because of their high sensitivity, subsequent metabolic changes could not be observed. In this study, we used the stable isotope carnitine to monitor carnitine uptake and subsequent metabolic changes, and subsequently observed carnitine uptake into skeletal muscle cells and increase in acetylation caused by muscle contraction. Although skeletal muscle consists of heterogenous fiber types and are difficult to be characterized in terms of carnitine metabolism, we observed the fiber specific carnitine metabolism using isotope tracing combined with MS imaging in a semi-quantitative manner.
Endogenous carnitine was localized in the type I and IIa fibers rather than type IIb fibers for both TA and calf muscles (Fig. 3, Fig. 5). Since type I and IIa fibers have more mitochondria and a higher capacity for glucose and fat oxidation than IIb fibers, these data suggested that carnitine metabolism was activated in the oxidative fiber types. In contrast, contraction-induced acetylcarnitine production was not necessarily associated with muscle fiber type. Previously, we found that carnitine acetylation was activated in glycogen-depleted areas [15], but not in dependent fiber types. These observations in this manuscript supported the previous report that carnitine utilization occurred even in fast glycolytic fibers and was associated with exercise intensity [20].
Carnitine is a well-known nutritional supplement that maximizes endurance performance and reduces body weight by promoting fat oxidation in the skeletal muscles. Although the beneficial effect of carnitine supplementation has been proven by several reports [8,[21], [22], [23]], many researchers have argued that simple carnitine intake does not increase carnitine content in muscle cells [5] because the transport of carnitine into skeletal muscle cells is strictly regulated by OCTN2 [9]. Since carnitine uptake into skeletal muscle is an active transport, even if carnitine concentration in blood increases by supplementation, carnitine transport was not increased. However, in this study, we found that the levels of both endogenous and injected d3-carnitine were increased 1.5-fold by muscle contraction within 60 min. This suggests that the uptake and storage of carnitine can be enhanced by stimuli such as muscle contractile activity. Further elucidation of the mechanisms involved in carnitine dynamics in skeletal muscle is critical for advances in the fields of nutrition and exercise sciences.
The accumulation of both d3-carnitine and d3-acetylcarnitine were much higher in the kidney than in skeletal muscle, and this phenomenon was explained by the previous report that OCTN2 protein levels were much higher in the kidney than skeletal muscle [13]. The kidney plays an important role in the reabsorption of carnitine excreted in urine to maintain carnitine concentration in the blood. This study showed that skeletal muscle carnitine uptake was slower than that of the kidney but was different between muscle fiber types and was increased by muscle contraction. The comparison of carnitine dynamics between the kidney and skeletal muscle was revealed by using stable isotope labeled carnitine.
Although we analyzed the relative changes in carnitine accumulation of tissue sections in response to muscle contraction using MALDI-MS measurement, quantitative measurement was not conducted using methods such as LC-MS/MS. Quantitative measurement is necessary to show that our findings on carnitine dynamics are important for physiological conditions. In addition, this study was designed to monitor carnitine dynamics in skeletal muscle during muscle contraction rather than to explore how to maximize the effects of carnitine supplementation. Recently, it was revealed that carnitine uptake and accumulation in skeletal muscle can be enhanced by insulin and carbohydrate stimulation via the activation of OCTN2 [22,24,25]. Our experimental model was used to examine the effect of nutrients in upregulating carnitine uptake and to further identify its location.
In conclusion, we demonstrated that stable isotope-labeled carnitine enabled us to monitor carnitine uptake, localization, and subsequent metabolic reactions, when combined with MS imaging. By considering the time from d3-carnitine administration, we evaluated the speed and location of carnitine dynamics. Our data provides new insights into skeletal muscle metabolism during exercise.
Author contribution statement
Yasuro Furuichi: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Naoko Goto-Inoue: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Saki Uchida, Shun Masuda: Performed the experiments.
Yasuko Manabe, Nobuharu L. Fujii: Analyzed and interpreted the data; Wrote the paper.
Funding statement
Dr. Yasuro Furuichi was supported by Japan Society for the Promotion of Science {15K16489}, Fusion Oriented REsearch for disruptive Science and Technology {JPMJFR205K} and Tokyo Metropolitan Government Advanced Research Grant {R2-2}.
Dr. Nobuharu L. Fujii was supported by TMU strategic research fund for innovative research project.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interest's statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The monoclonal antibodies used in this study was obtained from the Developmental Studies Hybridoma Bank, created by the NICHD of the NIH and maintained at The University of Iowa, Department of Biology, Iowa City, IA 52242. This study was supported by Grants-in-Aid for Scientific Research KAKENHI (JSPS, grant no. 15K16489 to YF), the FOREST Program of the Japan Science and Technology Agency (JST, grant no. JPMJFR205K to YF), TMU strategic research fund for innovative research project to NLF; and Tokyo Metropolitan Government Advanced Research Grant [R2-2] to YF, YM, NLF. We thank Dr. Sugiyama at the University of Shizuoka for technical support, and Ms. Une and Ms. Tawara for the experiments. This work was the result of using research equipment shared in the MEXT Project to promote public utilization of advanced research infrastructure (Program for Advanced Research Equipment Platforms, Grant Number JPMXS0450200215). We would like to thank Editage (www.editage.com) for English language editing.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e15281.
Abbreviations
- COX IV
cytochrome C oxidase complex 4
- CPT
carnitine palmitoyltransferase
- CrAT
carnitine acetyl-transferase
- d3
deuterium
- DHB
2,5-dihydroxybenzoic acid
- EDL
extensor digitorum longus
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- H&E
hematoxylin and eosin
- ITO
indium-tin oxide
- MALDI-MS
matrix-assisted laser desorption/ionization mass spectrometry
- MHC
myosin heavy chain
- MS
mass spectrometry
- MW
molecular weight
- OCTN2
carnitine/organic cation transporter
- PAS
periodic acid-Schiff stain
- PMP70
peroxisomal membrane protein 70
- TA
tibial anterior
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Bremer J. Carnitine as a fatty acid carrier in intermediary metabolism. Nature. 1962;196:993–994. doi: 10.1038/196993a0. [DOI] [PubMed] [Google Scholar]
- 2.Noland R.C., Koves T.R., Seiler S.E., Lum H., Lust R.M., Ilkayeva O., Stevens R.D., Hegardt F.G., Muoio D.M. Carnitine insufficiency caused by aging and overnutrition compromises mitochondrial performance and metabolic control. J. Biol. Chem. 2009;284:22840–22852. doi: 10.1074/jbc.M109.032888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brass E.P., Hiatt W.R. The role of carnitine and carnitine supplementation during exercise in man and in individuals with special needs. J. Am. Coll. Nutr. 1998;17:207–215. doi: 10.1080/07315724.1998.10718750. [DOI] [PubMed] [Google Scholar]
- 4.Karlic H., Lohninger A. Supplementation of L-carnitine in athletes: does it make sense? Nutrition. 2004;20:709–715. doi: 10.1016/j.nut.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 5.Brass E.P. Carnitine and sports medicine: use or abuse? Ann. N. Y. Acad. Sci. 2004;1033:67–78. doi: 10.1196/annals.1320.006. [DOI] [PubMed] [Google Scholar]
- 6.Brass E.P., Hoppel C.L., Hiatt W.R. Effect of intravenous L-carnitine on carnitine homeostasis and fuel metabolism during exercise in humans. Clin. Pharmacol. Ther. 1994;55:681–692. doi: 10.1038/clpt.1994.85. [DOI] [PubMed] [Google Scholar]
- 7.Ruff L.J., Miller L.G., Brass E.P. Effect of exogenous carnitine on carnitine homeostasis in the rat. Biochim. Biophys. Acta. 1991;1073:543–549. doi: 10.1016/0304-4165(91)90228-9. [DOI] [PubMed] [Google Scholar]
- 8.Hiatt W.R., Regensteiner J.G., Wolfel E.E., Ruff L., Brass E.P. Carnitine and acylcarnitine metabolism during exercise in humans. Dependence on skeletal muscle metabolic state. J. Clin. Invest. 1989;84:1167–1173. doi: 10.1172/JCI114281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tamai I., Ohashi R., Nezu J., Yabuuchi H., Oku A., Shimane M., Sai Y., Tsuji A. Molecular and functional identification of sodium ion-dependent, high affinity human carnitine transporter OCTN2. J. Biol. Chem. 1998;273:20378–20382. doi: 10.1074/jbc.273.32.20378. [DOI] [PubMed] [Google Scholar]
- 10.Furuichi Y., Sugiura T., Kato Y., Takakura H., Hanai Y., Hashimoto T., Masuda K. Muscle contraction increases carnitine uptake via translocation of OCTN2. Biochem. Biophys. Res. Commun. 2012;418:774–779. doi: 10.1016/j.bbrc.2012.01.101. [DOI] [PubMed] [Google Scholar]
- 11.Goto-Inoue N., Manabe Y., Miyatake S., Ogino S., Morishita A., Hayasaka T., Masaki N., Setou M., Fujii N.L. Visualization of dynamic change in contraction-induced lipid composition in mouse skeletal muscle by matrix-assisted laser desorption/ionization imaging mass spectrometry. Anal. Bioanal. Chem. 2012;403:1863–1871. doi: 10.1007/s00216-012-5809-x. [DOI] [PubMed] [Google Scholar]
- 12.Manabe Y., Takagi M., Nakamura-Yamada M., Goto-Inoue N., Taoka M., Isobe T., Fujii N.L. Redox proteins are constitutively secreted by skeletal muscle. J. Physiol. Sci.: JPS. 2014;64:401–409. doi: 10.1007/s12576-014-0334-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furuichi Y., Sugiura T., Kato Y., Shimada Y., Masuda K. OCTN2 is associated with carnitine transport capacity of rat skeletal muscles. Acta Physiol. 2010;200:57–64. doi: 10.1111/j.1748-1716.2010.02101.x. [DOI] [PubMed] [Google Scholar]
- 14.Muoio D.M., Noland R.C., Kovalik J.P., Seiler S.E., Davies M.N., DeBalsi K.L., Ilkayeva O.R., Stevens R.D., Kheterpal I., Zhang J., Covington J.D., Bajpeyi S., Ravussin E., Kraus W., Koves T.R., Mynatt R.L. Muscle-specific deletion of carnitine acetyltransferase compromises glucose tolerance and metabolic flexibility. Cell Metabol. 2012;15:764–777. doi: 10.1016/j.cmet.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Furuichi Y., Goto-Inoue N., Manabe Y., Setou M., Masuda K., Fujii N.L. Imaging mass spectrometry reveals fiber-specific distribution of acetylcarnitine and contraction-induced carnitine dynamics in rat skeletal muscles. Biochim. Biophys. Acta. 2014;1837:1699–1706. doi: 10.1016/j.bbabio.2014.05.356. [DOI] [PubMed] [Google Scholar]
- 16.Schiaffino S., Reggiani C. Fiber types in mammalian skeletal muscles. Physiol. Rev. 2011;91:1447–1531. doi: 10.1152/physrev.00031.2010. [DOI] [PubMed] [Google Scholar]
- 17.Delp M.D., Duan C. Composition and size of type I, IIA, IID/X, and IIB fibers and citrate synthase activity of rat muscle. J. Appl. Physiol. 1996;80:261–270. doi: 10.1152/jappl.1996.80.1.261. [DOI] [PubMed] [Google Scholar]
- 18.Sawano S., Komiya Y., Ichitsubo R., Ohkawa Y., Nakamura M., Tatsumi R., Ikeuchi Y., Mizunoya W. A one-step immunostaining method to visualize rodent muscle fiber type within a single specimen. PLoS One. 2016;11 doi: 10.1371/journal.pone.0166080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Houten S.M., Wanders R.J.A., Ranea-Robles P. Metabolic interactions between peroxisomes and mitochondria with a special focus on acylcarnitine metabolism. Biochim. Biophys. Acta, Mol. Basis Dis. 2020;1866 doi: 10.1016/j.bbadis.2020.165720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stephens F.B., Constantin-Teodosiu D., Greenhaff P.L. New insights concerning the role of carnitine in the regulation of fuel metabolism in skeletal muscle. J. Physiol. 2007;581:431–444. doi: 10.1113/jphysiol.2006.125799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arenas J., Huertas R., Campos Y., Diaz A.E., Villalon J.M., Vilas E. Effects of L-carnitine on the pyruvate dehydrogenase complex and carnitine palmitoyl transferase activities in muscle of endurance athletes. FEBS Lett. 1994;341:91–93. doi: 10.1016/0014-5793(94)80246-7. [DOI] [PubMed] [Google Scholar]
- 22.Chee C., Shannon C.E., Burns A., Selby A.L., Wilkinson D., Smith K., Greenhaff P.L., Stephens F.B. Increasing skeletal muscle carnitine content in older individuals increases whole-body fat oxidation during moderate-intensity exercise. Aging Cell. 2021;20 doi: 10.1111/acel.13303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bruls Y.M., de Ligt M., Lindeboom L., Phielix E., Havekes B., Schaart G., Kornips E., Wildberger J.E., Hesselink M.K., Muoio D., Schrauwen P., Schrauwen-Hinderling V.B. Carnitine supplementation improves metabolic flexibility and skeletal muscle acetylcarnitine formation in volunteers with impaired glucose tolerance: a randomised controlled trial. EBioMedicine. 2019;49:318–330. doi: 10.1016/j.ebiom.2019.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stephens F.B., Constantin-Teodosiu D., Laithwaite D., Simpson E.J., Greenhaff P.L. Insulin stimulates L-carnitine accumulation in human skeletal muscle. FASEB J.: Off. Publ. Feder. Am. Soc. Exp. Biol. 2006;20:377–379. doi: 10.1096/fj.05-4985fje. [DOI] [PubMed] [Google Scholar]
- 25.Stephens F.B., Evans C.E., Constantin-Teodosiu D., Greenhaff P.L. Carbohydrate ingestion augments L-carnitine retention in humans. J. Appl. Physiol. 2007;102:1065–1070. doi: 10.1152/japplphysiol.01011.2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supplementary material/referenced in article.