Abstract
The NTRK genes encode the TRK proteins. NTRK fusions lead to constitutively active, ligand-independent downstream signaling. NTRK fusions are implicated in up to 1% of all solid tumors and 0.2% of NSCLC. Larotrectinib, a highly selective small molecule inhibitor of all three TRK proteins, has a response rate of 75% across a wide range of solid tumors. Mechanisms of primary resistance to larotrectinib are not well understood. We report a case of a 75-year-old male with minimal smoking history with NTRK fusion-positive metastatic squamous NSCLC with primary resistance to larotrectinib. We suggest subclonal NTRK fusion as a possible mechanism contributing to primary resistance to larotrectinib.
Keywords: Non–small cell lung cancer, NTRK fusion, Larotrectinib, SMARCA4, Case report
Introduction
NTRK fusions drive ligand-independent signaling in a subset of NSCLC. Larotrectinib, a Food and Drug Administration–approved inhibitor of the tyrosine kinases encoded by the NTRK genes (i.e., tropomyosin receptor kinases), induces marked and durable responses in NSCLCs harboring NTRK fusions. Intrinsic resistance to larotrectinib is rare. Here, we summarize the case of a patient with metastatic NSCLC harboring an NTRK1 fusion who experienced primary progression during first-line treatment with larotrectinib. This case provides potential insights into underlying factors contributing to an atypical treatment response to a highly efficacious therapy in a rare subset of NSCLC.
Case Presentation
A 75-year-old male with hypertension, hyperlipidemia, and a remote history of prostate cancer for which he had undergone prostatectomy presented with right-sided chest pain and dyspnea on exertion. The patient had minimal smoking history and had ceased smoking 50 years before presentation. On presentation, he was noted to have supraclavicular lymphadenopathy prompting referral for neck ultrasound which confirmed enlarged right supraclavicular nodes. Subsequent imaging revealed right supraclavicular lymphadenopathy (Fig. 1A), a spiculated 4.3-cm right upper lobe mass (Fig. 1B), extensive bilateral thoracic lymphadenopathy, right pleural thickening, bilateral solid pulmonary nodules, and a lytic sternal lesion. Brain magnetic resonance imaging did not reveal intracranial metastases.
Figure 1.
(A) Baseline CT neck reveals right supraclavicular lymphadenopathy and (B) a right upper lobe mass. (C) CT with increased size of the right supraclavicular lymphadenopathy after initiation of larotrectinib. (D) CT with increased size of the right upper lobe mass and nodular interlobular septal thickening after initiation of larotrectinib. CT computed tomography.
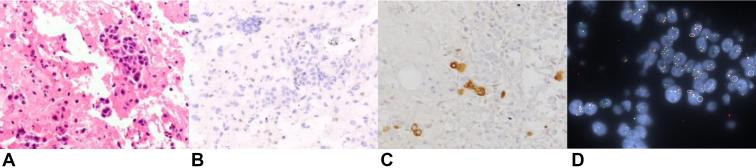
He underwent mediastinal nodal sampling by means of bronchoscopy with endobronchial ultrasound. Evaluation of right paratracheal and subcarinal lymph node stations demonstrated non–small cell carcinoma (Fig. 2A). By immunohistochemistry (IHC), the cells were diffusely positive for p40 and negative for TTF-1 and Napsin A, consistent with non–small cell carcinoma, favor squamous cell carcinoma. IHC also revealed loss of BRG1 nuclear expression in most tumor cells (Fig. 2B), whereas no membranous staining of programmed death-ligand 1 was present. DNA- and RNA-based next-generation sequencing identified a fusion juxtaposing exon 6 of the F11R gene to exon 10 of NTRK1, in addition to mutations in KEAP1 G364C, STK11 splice alteration, PIK3R1 N630T, and a deleterious SMARCA4 splice alteration.
Figure 2.
(A) A HE section revealed non–small cell carcinoma in solid clusters. Immunohistochemistry for lung lineage markers confirmed a squamous differentiation. (B) Most tumor cells had loss of BRG1 nuclear expression by immunohistochemistry. (C) Pan-TRK protein expression was observed in a minority of tumor cells by immunohistochemistry. (D) FISH using a break-apart probe to the NTRK1 locus revealed rearrangement of the NTRK1 locus visualized as split green 5' and red 3' NTRK1 signals (arrows) and isolated 5' signals (circled). The tumor cells exhibited an average of three copies of the NTRK1 locus with the predominant abnormal FISH pattern being isolated 5' only signals in 30% of cells with an additional 22% of cells with split 5' and 3' signals. FISH, fluorescence in situ hybridization; HE, hematoxylin and eosin.
On the basis of findings from molecular testing, the patient was initiated on larotrectinib 100 mg twice a day as first-line therapy for metastatic NSCLC. One month into treatment, he endorsed increased dyspnea, chest pain, unintentional weight loss, and enlarging right supraclavicular lymphadenopathy. Because of clinical concern for disease progression, expedited imaging was obtained which confirmed primary progression of disease involving the right supraclavicular region (Fig. 1C), lungs (Fig. 1D), pleura, and sternum, including increasing retrocrural lymphadenopathy. Given the unusual finding of intrinsic resistance to larotrectinib, additional studies—pan-TRK IHC and fluorescence in situ hybridization—were performed on the initial diagnostic biopsy tissue to confirm presence of the NTRK fusion (TRK IHC clone EPR17341, Abcam; TRK fluorescence in situ hybridization probe RP11-1038N13-NTRK1 3′ locus [chr1:156854507-156983651] and RP11-1047J23-NTRK1 5′ locus [chr1:156569238-156781762]). TRK protein expression was observed in a minority of tumor cells (Fig. 2C). Classic split (“break-apart”) signal was present only in 11 of 50 evaluated cells (Fig. 2D). Collectively, these findings suggested that the NTRK1 fusion was a subclonal event. Broader next-generation sequencing testing of the initial tumor biopsy using a panel encompassing more than 500 genes did not identify additional putative mediators of primary resistance to larotrectinib. At disease progression, neither plasma genotyping nor repeat tissue biopsy was performed.
He was switched from larotrectinib to carboplatin and paclitaxel. After two cycles, imaging revealed response with decrease in right upper lobe mass, right pleural metastases, interlobular septal thickening, and retrocrural lymphadenopathy. Nevertheless, after his third cycle of carboplatin and paclitaxel, imaging revealed progression of disease involving the right supraclavicular node, pleura, sternum, and a new hepatic paracapsular nodule. He was then transitioned to nivolumab.
Discussion
The NTRK genes encode the TRK proteins. NTRK fusions lead to constitutively active, ligand-independent signaling.1 NTRK fusions are identified in up to 1% of all solid tumors and 0.2% of NSCLC.1,2 In the registrational studies that supported tumor-agnostic Food and Drug Administration approval, larotrectinib induced responses in 75% of patients, with responses observed in a variety of solid malignancies. In a recent update summarizing outcomes of 15 patients with NTRK-rearranged NSCLC who participated in these studies, objective responses were observed in 73% of patients with a median progression-free survival of 35.4 months.3 Primary resistance to larotrectinib was rare. Indeed, in the phase 1 experience, only six patients (11%) had primary progression. Of these six patients, one patient was previously treated with TRK inhibitor and had a NTRK3 G623R mutation, which interferes with larotrectinib binding.1 For three of the remaining patients, central IHC testing of tumor tissue did not confirm the presence of TRK expression, raising the question of false-positive NTRK fusion or a nonfunctional fusion that was not expressed at the protein level.1
Given the rarity of NTRK fusions in NSCLC, the infrequent occurrence of intrinsic resistance to larotrectinib, and limited understanding of factors contributing to primary resistance to larotrectinib, we have summarized our patient’s unusual clinical course. Our patient’s case has several notable features, including subclonality of NTRK fusion, squamous differentiation, and co-alterations associated with aggressive tumor biology, including mutations in SMARCA4, KEAP1, and STK11. The issue of subclonality has been explored in the treatment of metastatic NSCLC with EGFR T790M mutations where clonality has been correlated with durability of response to osimertinib.4 In our patient’s case, it is possible that larotrectinib suppressed the minority NTRK fusion-positive population without exerting antiproliferative effect on the predominant population consisting of cells lacking the NTRK fusion. Of note, this situation is distinct from recent case reports in which acquired NTRK fusions emerged at resistance to RET and EGFR targeted therapies and were overcome by co-targeting the primary driver and the activated TRK protein in the subclonal-resistant population.5,6
Interestingly, our patient’s tumor harbored a concurrent SMARCA4 mutation with BRG1 deficiency. SMARCA4 mutations are found in 10% of NSCLC and are most often associated with smoking history.7 Mutations in SMARCA4 and the KEAP1 mutation noted in our patient’s tumor tissue have been linked to resistance to targeted therapy in preclinical models and retrospective clinical series.7, 8, 9 Specifically, preclinical studies suggest that KEAP1 loss alters cell metabolism and promotes survival of oncogene-driven cancer cells treated with therapies that suppress receptor tyrosine kinase and mitogen-activated pathway kinase signaling.10 Nevertheless, as these studies did not include NTRK-rearranged NSCLC, the impact of these mutations on sensitivity to TRK-directed therapies remains to be established. Finally, our patient’s tumor was of squamous histology; squamous differentiation has been implicated in primary and acquired resistance to various targeted therapies.11, 12, 13
Conclusions
In summary, we describe several tumor features that may contribute to primary resistance to larotrectinib, including clonality of the NTRK fusion. We suggest considering prioritizing genotype-agnostic therapeutic strategies over TRK inhibitors as initial therapy for NTRK-rearranged NSCLCs with these adverse molecular and histologic features.
CRediT Authorship Contribution Statement
Mary C. Boulange: Writing—original draft, Writing—review and editing.
Jennifer S. Temel: Writing—review and editing.
Mari Mino-Kenudson: Writing—review and editing, Resources.
Lauren L. Ritterhouse: Writing—review and editing, Resources.
Ibiayi Dagogo-Jack: Conceptualization, Writing—original draft, Writing—review and editing.
Acknowledgments
The authors acknowledge Valentina Nardi, MD, Jochen Lennerz, MD, PhD, and Yin P. Hung, MD, for their assistance with this patient’s care and insights during manuscript preparation. Mari Mino-Kenudson is partially supported by funding from R01-CA240317. Written informed consent was obtained for all the molecular analyses discussed previously.
Footnotes
Disclosure: Dr. Temel has received research funding from Blue Note Therapeutics to MGH. Dr. Mino-Kenudson has served as a compensated consultant for AstraZeneca, Sanofi, Bristol-Myers Squibb, and Janssen Oncology and received loyalties from Elsevier, not related to the current case. Dr. Ritterhouse has received honoraria from PeerView, Medscape, and Clinical Care Options; consulting honoraria from AbbVie, Personal Genome Diagnostics, Bristol-Myers Squibb, Loxo Oncology at Lilly, Amgen, Merck, AstraZeneca, Sanofi-Genzyme, and EMD Serono. Dr. Dagogo-Jack has received honoraria from Foundation Medicine, Creative Education Concepts, OncLive, ASCO Post, DAVA Oncology, Medscape, Total Health, Triptych Health Partners, Aptitude Health, and American Lung Association; consulting fees from AstraZeneca, Boehringer Ingelheim, Bayer, BostonGene, Bristol-Myers Squibb, Catalyst, Genentech, Janssen, Novocure, Pfizer, Sanofi-Genzyme, Syros, and Xcovery; research support from Array, Genentech, Novartis, Pfizer, and Guardant Health; and travel support from Array and Pfizer. Dr. Boulanger declares no conflict of interest.
Cite this article as: Boulanger MC, Temel JS, Mino-Kenudson M, Ritterhouse LL, Dagogo-Jack I. Primary resistance to larotrectinib in a patient with squamous NSCLC with subclonal NTRK1 fusion: case report. JTO Clin Res Rep. 2023;4:100501.
References
- 1.Drilon A., Laetsch T.W., Kummar S., et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N Engl J Med. 2018;378:731–739. doi: 10.1056/NEJMoa1714448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farago A.F., Taylor M.S., Doebele R.C., et al. Clinicopathologic features of non-small-cell lung cancer harboring an NTRK gene fusion. JCO Precis Oncol. 2018;2018 doi: 10.1200/PO.18.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drilon A., Tan D.S.W., Lassen U.N., et al. Efficacy and safety of larotrectinib in patients with tropomyosin receptor kinase fusion-positive lung cancers. JCO Precis Oncol. 2022;6 doi: 10.1200/PO.21.00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaclova T., Grazini U., Ward L., et al. Clinical impact of subclonal EGFR T790M mutations in advanced-stage EGFR-mutant non-small-cell lung cancers. Nat Commun. 2021;12:1780. doi: 10.1038/s41467-021-22057-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin G., Liu Y., Li H., Chen S., Guo Y. Emergence of NOTCH2-NTRK1 after osimertinib in a patient with lung adenocarcinoma with neuroendocrine differentiation. Clin Lung Cancer. 2021;22:e712–e715. doi: 10.1016/j.cllc.2021.01.015. [DOI] [PubMed] [Google Scholar]
- 6.Subbiah V., Shen T., Tetzlaff M., et al. Patient-driven discovery and post-clinical validation of NTRK3 fusion as an acquired resistance mechanism to selpercatinib in RET fusion-positive lung cancer. Ann Oncol. 2021;32:817–819. doi: 10.1016/j.annonc.2021.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dagogo-Jack I., Schrock A.B., Kem M., et al. Clinicopathologic characteristics of BRG1-deficient NSCLC. J Thorac Oncol. 2020;15:766–776. doi: 10.1016/j.jtho.2020.01.002. [DOI] [PubMed] [Google Scholar]
- 8.De Miguel F.J., Hu B., Cai W.L., et al. The role of SMARCA4 as an EGFR-independent mechanism of resistance to osimertinib. J Thorac Oncol. 2020;15(suppl):S35–S36. [Google Scholar]
- 9.Vokes N.I., Chambers E., Nguyen T., et al. Concurrent TP53 mutations facilitate resistance evolution in EGFR-mutant lung adenocarcinoma. J Thorac Oncol. 2022;17:779–792. doi: 10.1016/j.jtho.2022.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krall E.B., Wang B., Munoz D.M., et al. KEAP1 loss modulates sensitivity to kinase targeted therapy in lung cancer [published correction appears in Elife. 2017;6:e33173] Elife. 2017;6 doi: 10.7554/eLife.18970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schoenfeld A.J., Chan J.M., Kubota D., et al. Tumor analyses reveal squamous transformation and off-target alterations as early resistance mechanisms to first-line osimertinib in EGFR-mutant lung cancer. Clin Cancer Res. 2020;26:2654–2663. doi: 10.1158/1078-0432.CCR-19-3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewis W.E., Hong L., Mott F.E., et al. Efficacy of targeted inhibitors in metastatic lung squamous cell carcinoma with EGFR or ALK alterations. JTO Clin Res Rep. 2021;2 doi: 10.1016/j.jtocrr.2021.100237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Awad M.M., Liu S., Rybkin, et al. Acquired resistance to KRASG12C inhibition in cancer. N Engl J Med. 2021;384:2382–2393. doi: 10.1056/NEJMoa2105281. [DOI] [PMC free article] [PubMed] [Google Scholar]