Summary
Introduction
Until recently, healthcare-associated E. coli bacteraemia was a neglected area of infection prevention and control (IPC), despite a 30-day mortality of 15–20%. Recently, the UK Department of Health (DH) introduced a target to reduce hospital-acquired E. coli bacteraemias by 50% over a five-year period. Following implementation of multifaceted and multidisciplinary interventions, the aim of this study was to determine its impact on achieving this target.
Methods
From April 2017 to March 2022, consecutive hospital-acquired E. coli bacteraemic inpatients within Barts Health NHS Trust were prospectively studied. Using quality improvement methodology, and implementing the plan, do, study, act (PDSA) cycle at each stage, antibiotic prophylaxis for high-risk procedures were modified and ‘good practice’ interventions around medical devices introduced. Characteristics of bacteraemic patients were analysed and trends in bacteraemic episodes recorded. Statistical analysis was undertaken in Stata SE (version 16).
Results
There were 770 patients and 797 episodes of hospital-acquired E. coli bacteraemias. Following a baseline of 134 episodes in 2017-18, this peaked at 194 in 2019-20 before dropping to 157 in 2020-21 and 159 in 2021-22. Most hospital-acquired E. coli bacteraemias occurred in those aged > 50, 551 (69.1%), with the highest proportion occurring in those age > 70, 292 (36.6%). Hospital-acquired E. coli bacteraemia occurred more commonly between October to December.
Most episodes occurred in either medicine or care of the elderly patients, 345 (43.3%), specialist surgery, 141 (17.7%), haematology/oncology, 127 (15.9%) and patients requiring critical care, 108 (13.6%). The urinary tract, 336 (42.2%), both catheter and non-catheter associated, was the commonest sites of infection. 175 (22.0%) of E. coli bacteraemic isolates were extended spectrum beta lactamase (ESB) producing. Co-amoxiclav resistance was 315 (39.5%), ciprofloxacin resistance 246 (30.9%) and gentamicin resistance 123 (15.4%). At 7 days, 77 patients (9.7%; 95% CI 7.4–12.2%) died and by 30 days this had risen to 129 (16.2%; 95% CI 13.7–19.9%).
Conclusion
Despite implementation of quality improvement (QI) interventions, it was not possible to achieve a 50% reduction from baseline although an 18% reduction was achieved from 2019-20 onwards. Our work highlights the importance of antimicrobial prophylaxis and medical device ‘good practice’. Over time, these interventions, if properly implemented, could further reduce healthcare-associated E. coli bacteraemic infection.
Keywords: Quality improvement, Hospital-acquired E. coli bacteraemia
Introduction
Until recently, healthcare-associated E. coli bacteraemia was a neglected area of infection prevention and control (IPC), despite a 30-day mortality of 15–20% [[1], [2], [3]], which is considerably higher than in infections caused by meticillin-resistant Staphylococcus aureus (MRSA), carbapenem-resistant organisms (CRO), Clostridioides difficile infection and Covid-19. Furthermore, a significant proportion of E. coli bacteraemias are preventable [4,5]. In 2011, following the introduction of mandatory reporting, hospital-acquired E. coli bacteraemia continued to rise across all UK NHS healthcare providers. In 2016, the Department of Health (DH) introduced a quality premium with the aim of reducing all E. coli bacteraemias by 10% in the first year (2016–17) and all healthcare-associated E. coli bacteraemias by 50% over a 5-year period (2017–2022) [16]. Despite the UK DH target, national trends continued to rise [7].
At hospitals in Barts Health NHS Trust, the main areas of concern, highlighted by our local data [8], were hospital-acquired urinary tract infections (UTIs) secondary to poor hydration, catheter-associated UTIs, instrumentation of the urinary or biliary tract, such as cystoscopy or endoscopic retrograde cholangiopancreatography (ERCP), prostatic biopsy, colorectal surgical site infections (SSIs) including anastomotic breakdown and haematology/oncology neutropenic sepsis.
Using quality improvement (QI) methodology, including a pareto chart for causes of hospital-acquired E. coli bacteraemia, and implementing the plan, do, study, act (PSDA) cycle at each stage [9], interventions were introduced over a 5-year period with the aim of achieving the DH target. Following implementation of several multidisciplinary and multifaceted interventions, we describe their impact on the incidence of hospital-acquired E. coli bacteraemia.
Methods
Study setting
This study was undertaken at Barts Health (BH) NHS Trust which is comprised of five hospital sites: the Royal London Hospital (RLH), Whipps Cross University Hospital (WXUH), Newham University Hospital (NUH), St Bartholomew's Hospital (SBH) and Mile End Hospital (MEH). In total there are approximately 2 500 inpatients. BH NHS Trust serves a diverse population of around 1 million patients across North-East London in the boroughs of Tower Hamlets, Waltham Forest, Newham, and City and London. At three hospital sites (RLH, NUH and WXUH) there are accident and emergency (A&E) departments, general medicine (including care of the elderly), surgery, paediatric and maternity services and high-dependency and critical care beds (HDU/ICU). At RLH, in addition to a high-level ICU, there are specialist wards for renal transplantation and haemodialysis patients and beds for neurosurgical, vascular, orthopaedic,colo-rectal and hepatobiliary surgery. At SBH, there are specialist wards for haematology and oncology patients and interventional cardiology and thoracic/cardiothoracic surgery.
Study population
From April 2017 to March 2022, consecutive hospital-acquired E. coli bacteraemic inpatients were prospectively studied.
Hospital-acquired E. coli bacteraemia cohort and definitions
Inpatients with hospital-acquired E. coli bacteraemia, age, gender, clinical specialty, site of infection, organism, susceptibility profile and mortality related outcomes were recorded. Hospital-acquired bacteraemia was defined as a bacteraemia occurring more than 48 hours after hospital admission and community onset as bacteraemia < 48 hours following admission. For hospital-acquired bacteraemia, ‘The Centres for Disease Control and Prevention’ definitions were used to define the sites of infection [10]. Bacteraemia in patients with an unknown source were classified as undefined.
Microbiology data
Blood cultures were analysed using the automated system BacT/ALERT3D® (bioMérieux, Marcy-l’Etoile, France). Isolates were identified using either the VITEK® MS system (bioMérieux, Marcy-l’Etoile, France, database v2.0) or Bruker Biotyper® (Bruker Daltonic, Leipzig, Germany, software version 3.0) MALDI-TOF MS systems according to the manufacturer's instructions and our laboratory standard operating procedures. Susceptibility testing was performed on the Microscan® WalkAway system (Siemens Healthcare Diagnostics, Deerfield, IL, US).
Clinical data ascertainment
Patients were identified via the laboratory reporting service Winpath enterprise (CliniSys, Surrey, England) and by reference to the electronic patient record in Millennium/PowerChart (Cerner Corporation, Kansas City, Missouri, USA) to determine whether bacteraemia had occurred more than 48 hours after admission. Age, gender, site of infection, clinical specialty, duration of treatment and outcomes were recorded for all patients. Data collection was in real time onto a form. Data collected were exported to Excel® (Microsoft Corporation) for analysis and cross-checked by a second independent auditor on a yearly basis.
Quality improvement interventions
PDSA cycles were implemented across the 5-year period. Guidance and leaflets on recurrent UTIs were produced in 2016. Revised antibiotic prophylaxis guidance was introduced in 2017 for urinary and biliary tract instrumentation to include a stat dose of gentamicin 2mg/kg in addition to single IV co-amoxiclav prophylaxis. In 2018, new prophylaxis guidance was also produced for prostatic biopsy. The recommendation was to give IV gentamicin 2mg/kg at induction and oral ciprofloxacin 500mg bd for 3 days post biopsy. In 2019, pre-operative rectal screens for ciprofloxacin resistant Enterobacterales was introduced and, if ciprofloxacin resistant organisms cultured, prophylaxis was adjusted. In 2019, selective digestive decontamination (neomycin 1g tds and metronidazole 400mg tds orally) for elective colorectal surgery was introduced one day prior to surgery.
In 2018, the newly-formed ‘urinary catheter quality improvement group’ produced local guidance on when urinary catheterisation was appropriate and urinary catheter pathways. In 2019, this was followed by an updated trial without catheter (TWOC) policy and greater access to bladder scanners. Nurses were empowered to TWOC post-surgical patients. In 2021, post first and second wave of the COVID-19 pandemic, the use of a standardised ‘hospital to home’ urinary catheter pack including a catheter passport was introduced in all A&E departments, then on all wards at WXUH, RLH and NUH. In 2021, at the new starter doctors' induction programmes, a card outlining the ‘HOUDINI’ principles around urinary catheterisation and appropriate catheter maintenance was distributed (Figure 1). Also in that year, Urology converted from transrectal ultrasound guided (TRUS) prostatic biopsy to transperitoneal prostatic biopsy, which is a lower risk procedure for biopsy related infection. Finally, in 2022, a urinary catheterisation video was rolled out at the doctors' induction, accompanied by key question competency testing.
Figure 1.
HOUDINI principles and urinary catheter care maintenance.
Hydration was also identified as a key area for QI. Interventions included recommendations for every patient to drink six to eight cups of fluid a day, prescribed on a drug chart where necessary and documented in the patient's electronic record. A choice of three cordials were offered to patients rather than water. The provision of dementia friendly cups on care of the elderly wards were made increasingly available.
Throughout this period, regular multidisciplinary meetings were held and hospital-acquired E. coli bacteraemia data reviewed on a yearly basis by the study group authors.
Preventability
An E. coli bacteraemia was considered preventable if appropriate antibiotic prophylaxis was not given prior to a high-risk procedure such as colorectal surgery or if an obvious lapse in compliance with care bundles around insertion, after care and removal of medical devices, had occurred, such as urinary catheters.
Statistical analysis
Characteristics of bacteraemic patients were analysed, comparing trends over the five-year period. Statistical analysis was undertaken in Stata® SE (version 16). Quantitative data were presented as numbers and percentages. As patients may present with more than one bacteraemic episode, number of patients was used as a denominator to calculate percentages for patient characteristics such as age, gender and outcomes, and number of bacteraemic episodes was used as the denominator for other infection characteristics. Median and interquartile ranges (IQRs) were calculated for length of inpatient stay post bacteraemia. Outcomes were expressed as percentages within 95% confidence intervals.
Clinical governance
The Clinical Effectiveness Unit at BH NHS Trust approved the study. As this was a QI project, ethical approval was not required.
Results
Patients, E. coli bacteraemias and hospital sites
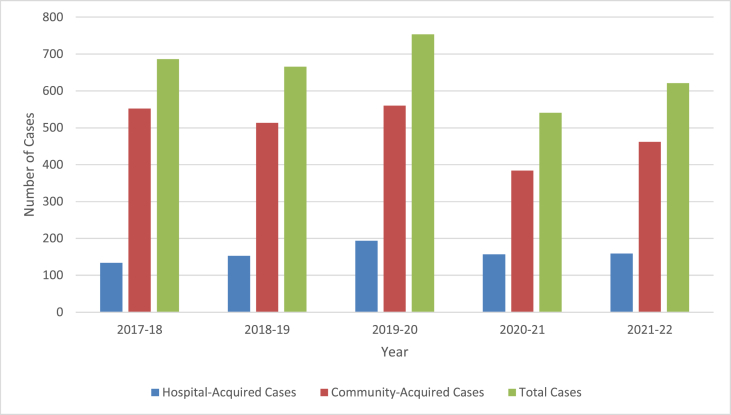
For all E. coli bacteraemias, including community-onset episodes, there were 749 E. coli bacteraemias in 2016-17, 686 in 2017-18, 666 in 2018-19, 754 in 2019-20, 541 in 2020-21 and 621 in 2021-22 which, over a 5-year period, represented a 17.1% reduction from baseline (Figure 2).
Figure 2.
Community-acquired and hospital-acquired E. coli bacteraemia episodes from 2017-2022.
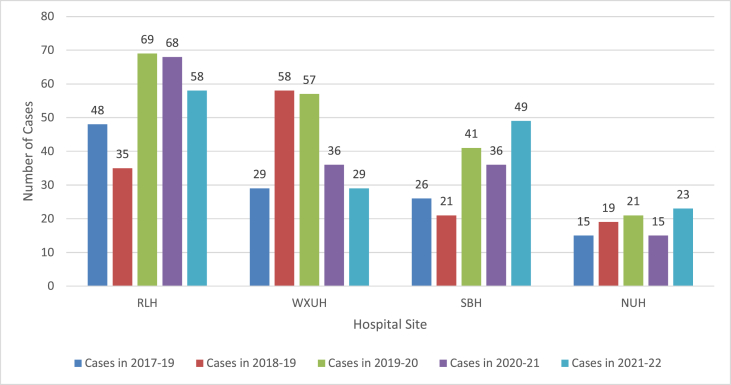
Across the five-year period, there were 770 patients and 797 episodes of hospital-acquired E. coli bacteraemia (27 recurrent cases occurred > 30 days after the first episode). Following a baseline of 134 episodes in 2017-18, this peaked at 194 in 2019-20 before dropping to 157 in 2020-21 and 159 in 2021-22 (table 1). Across the four hospital sites, 286 (35.9%) cases occurred at RLH, 221 (27.7%) at WXUH, 185 (23.2%) at SBH and 105 (13.2%) at NUH (Figure 3).
Figure 3.
Hospital-acquired E. coli bacteraemia episodes by hospital site at Barts Health NHS Trust.
Demographic data and seasonal distribution
Patient demographics are summarised in Table I. Over the 5-year period, 449 (58.3%) patients were male. Most hospital-acquired E. coli bacteraemias occurred in those aged > 50, 551 (71.6%), with the highest proportion occurring in those age > 70, 292 (37.9%). Data on nursing home residents were collected from 2018 onwards. Overall, 25 (3.2%) cases occurred in nursing home residents over a 3-year period. In 2018–19, 7 (0.9%) cases were nursing home residents, 14 (1.8%) in 2019-20, 3 (0.4%) in 2020-21, and 1 (0.1%) in 2021-22.
Table I.
Patient characteristics, clinical speciality and site of infection
| Overall | 2017-2018 | 2018-2019 | 2019-2020 | 2020-2021 | 2021-22 | |
|---|---|---|---|---|---|---|
| Total Episodes (Patients) | 797 (770) | 134 (130) | 153 (141) | 194 (189) | 157 (151) | 159 (159) |
| Age Range (%) | ||||||
| <1 month | 9 (1.2%) | 0 | 2 (1.4%) | 3 (1.6%) | 2 (1.3%) | 2 (1.3%) |
| 1 month-15 years | 15 (1.9%) | 2 (1.6%) | 3 (2.1%) | 4 (2.1%) | 2 (1.3%) | 4 (2.5%) |
| 16–30 years | 46 (6.0%) | 5 (3.8%) | 6 (4.3%) | 10 (5.3%) | 11 (7.3%) | 14 (8.8%) |
| 31–50 years | 103 (13.4%) | 23 (17.7%) | 16 (11.3%) | 18 (9.6%) | 23 (15.2%) | 23 (14.5%) |
| 51–70 years | 286 (37.1%) | 48 (36.9%) | 50 (35.5%) | 60 (31.7%) | 59 (39.1%) | 69 (43.4%) |
| >70 years | 311 (40.4%) | 52 (40.0%) | 64 (45.4%) | 94 (49.7%) | 54 (35.8%) | 47 (29.5%) |
| Gender (%) | ||||||
| Male | 449 (58.3%) | 70 (53.8%) | 84 (59.6%) | 102 (54.0%) | 98 (64.9%) | 95 (59.7%) |
| Female | 321 (41.7%) | 60 (46.2%) | 57 (40.4%) | 87 (46.0%) | 53 (35.1%) | 64 (40.3%) |
| Clinical speciality (%) | ||||||
| Medicine | 230 (28.9%) | 40 (29.9%) | 39 (25.5%) | 47 (24.2%) | 57 (36.3%) | 47 (29.6%) |
| Care of elderly | 115 (14.4%) | 8 (6.0%) | 35 (22.9%) | 34 (17.5%) | 21 (13.4%) | 17 (10.7%) |
| Haematology/Oncology | 127 (15.9%) | 21 (15.7%) | 15 (9.8%) | 28 (14.4%) | 31 (19.7%) | 32 (20.1%) |
| Surgery – Colorectal | 40 (5.0%) | 13 (9.7%) | 6 (3.9%) | 10 (5.2%) | 5 (3.2%) | 6 (3.8%) |
| Surgery – Hepatobiliary | 29 (3.6%) | 3 (2.2%) | 3 (2.0%) | 14 (7.2%) | 4 (2.5%) | 5 (3.1%_ |
| Surgery – Urology | 17 (2.1%) | 4 (3.0%) | 7 (4.6%) | 3 (1.5%) | 2 (1.3%) | 1 (0.6%) |
| Surgery – Orthopaedic | 18 (2.3%) | 1 (0.7%) | 3 (2.0%) | 6 (3.1%) | 4 (2.5%) | 4 (2.5%) |
| Surgery – Neurosurgery | 13 (1.6%) | 5 (3.7%) | 2 (1.3%) | 5 (2.6%) | 1 (0.6%) | 0 |
| Surgery– Other specialist surgery | 23 (2.9%) | 7 (5.2%) | 1 (0.7%) | 7 (3.6%) | 2 (1.3%) | 6 (3.8%) |
| HDU/ICU | 108 (13.6%) | 21 (15.7%) | 25 (16.3%) | 17 (8.8%) | 18 (11.5%) | 27 (17.0%) |
| Obstetrics & Gynaecology | 32 (4.0%) | 5 (3.7%) | 7 (4.6%) | 7 (3.6%) | 7 (4.5%) | 6 (3.8%) |
| Paediatrics | 13 (1.6%) | 1 (0.7%) | 6 (3.9%) | 4 (2.1%) | 0 | 2 (1.9%) |
| Neonatology | 12 (1.5%) | 1 (0.7%) | 0 | 3 (1.5%) | 4 (2.5%) | 4 (2.5%) |
| Other | 19 (2.4%) | 4 (3.0%) | 4 (2.6%) | 9 (4.6%) | 1 (0.6%) | 1 (0.6%) |
| Site of infection (%) | ||||||
| UTI (non-catheter associated) | 196 (24.6%) | 41 (30.6%) | 51 (33.3%) | 32 (16.5%) | 41 (26.1%) | 31 (19.5%) |
| UTI (catheter-associated) | 140 (17.6%) | 13 (9.7%) | 28 (18.3%) | 46 (23.7%) | 23 (14.6%) | 30 (18.9%) |
| Hepatobiliary Infections | 106 (13.3%) | 25 (18.7%) | 20 (13.1%) | 24 (12.4%) | 23 (14.6%) | 14 (8.8%) |
| GI surgical site infections | 48 (6.0%) | 16 (11.9%) | 12 (7.8%) | 13 (6.7%) | 1 (0.6%) | 6 (3.8%) |
| Haematology/Oncology (neutropenic sepsis) | 92 (11.5%) | 13 (9.7%) | 10 (6.5%) | 20 (10.3%) | 26 (16.6%) | 23 (14.5%) |
| Haematology/Oncology (non-neutropenic sepsis) | 7 (0.9%) | 0 | 0 | 3 (1.5%) | 2 (1.3%) | 2 (1.3%) |
| Intravascular catheters | 24 (3.0%) | 2 (1.5%) | 6 (3.9%) | 7 (3.6%) | 6 (3.8%) | 3 (1.9%) |
| Other/not defined | 171 (21.5%) | 33 (24.6%) | 38 (24.8%) | 31 (16.0%) | 32 (20.4%) | 37 (23.3%) |
| Outcomes (%; 95% CI) | ||||||
| +Death at 7 days | 77 (9.7%; 7.4–12.2%) | 19 (14.6%; 8.8–21.3%) | 14 (9.9%; 5.1–14.9%) | 11 (5.8%; 2.9–9.9%) | 22 (14.6%; 9.0–20.4%) | 11 (6.9%; 4.1–9.5%) |
| +Death at 30 days | 129 (16.2%; 13.7–19.9% | 29 (22.3%; 15.0–29.6%) | 22 (15.6%, 9.2–21.0%) | 22 (11.6%; 7.3–16.7%) | 35 (23.2%; 16.1–9.6%) | 21 (13.2%; 9.7–15.8%) |
# Patient numbers used as denominator.
+ Percentages with 95% CIs.
Data on seasonal variation was collected and stratified into three monthly periods: April-June, July-September, October-December and January-March. Hospital-acquired E. coli bacteraemias occurred mostly from October to December. This data is summarised in Figure 4.
Figure 4.
Hospital-acquired E. coli bacteraemia episodes by season.
Clinical speciality
Over a five-year period, most episodes occurred in either medicine or care of the elderly patients, 345 (43.3%), specialist surgery, 141 (17.7%), haematology/oncology, 127 (15.9%) and patients requiring critical care, 108 (13.6%). A smaller proportion of bacteraemic episodes occurred in obstetrics and gynaecology, paediatrics and neonatology, 58 (7.3%). These results are summarised in Table I.
Sites of infection
Urinary tract infection, both catheter and non-catheter associated, was the commonest site of infection, 336 (42.2%). This was followed by the hepatobiliary tract, 106 (13.3%), commonly caused by biliary outflow obstruction and associated with pancreatic cancer, cholangiocarcinoma, blocked biliary stents, gallstones, and liver abscesses. A large proportion of bacteraemic episodes, 69 (8.7%) were caused by neutropenic sepsis in haematology and oncology patients, based at a specialist unit in SBH. In 2019–2020, there was a noticeable increase in bacteraemia following the cessation of ciprofloxacin prophylaxis for neutropenic inpatients. Colorectal surgical sites infection, overall, 42 (5.3%), fell sharply over the 5-year period. Other sites of infection included the GI tract (but not related to surgery), non-GI SSIs and the genito-urinary tract following caesarean section or instrumentation. Sites of infection are summarised in Table I.
Device-related infection
Devices commonly associated with infection included urinary catheters, 37 (4.6%), ureteric stents 6 (0.8%) and nephrostomy tubes 4 (0.8%). There were 22 (2.8%) episodes associated with an intravascular catheter.
Procedure-related infection
Overall, there were 97 (12.2%) procedures associated with hospital-acquired E. coli bacteraemia, 62 (7.8%) non-surgical and 35 (4.4%) surgical. The commonest non-surgical procedure causing bacteraemia was, either ERCP, percutaneous transhepatic cholangiography (PTC), urinary catheter change or removal (Table II). Elective colorectal surgery, 13 (1.6%), was the commonest surgical procedure associated with E. coli bacteraemia.
Table II.
Bacteraemic episodes caused by non-surgical procedures
| 2017-18 | 2018-19 | 2019-20 | 2020-21 | 2021-22 | |
|---|---|---|---|---|---|
| Non-surgical procedures | 8 | 8 | 17 | 12 | 25 |
| ERCP/PTC | 5 | 5 | 6 | 5 | 6 |
| Ureteric stent insertion/manipulation | 1 | 3 | 0 | 1 | 2 |
| Nephrostomy tube insertion/aftercare | 2 | 0 | 0 | 1 | 1 |
| TWOC or catheter change | 0 | 0 | 7 | 2 | 7 |
| Other | 0 | 0 | 4 | 3 | 9 |
Estimated preventability
Out of 797 episodes of E. coli bacteraemia, 229 (28.7%) episodes were deemed preventable. This figure remained roughly static each year over the 5-year period.
E. coli susceptibility data
175 (22.0%) of E. coli bacteraemic isolates were ESBL producing. A far smaller proportion, 5 (0.6%) were carbapenem resistant. Overall, co-amoxiclav resistance was 315 (39.5%) and ciprofloxacin resistance 246 (30.9%). Gentamicin resistance was 123 (15.4%), significantly higher than amikacin resistance, 12 (1.5%). Across all categories, trends remained roughly similar over the 5-year period. Susceptibility data is summarised in Table III.
Table III.
Hospital-acquired E. coli susceptibility data by year
| 2017-18 | 2018-19 | 2019-20 | 2020-21 | 2021-22 | Total | |
|---|---|---|---|---|---|---|
| Total number of episodes | 134 | 153 | 194 | 157 | 159 | 797 |
| Co-amoxiclav resistant | 53 (39.6%) | 75 (39.9%) | 44 (22.7%) | 74 (47.1%) | 68 (42.8%) | 314 (39.4%) |
| Ciprofloxacin resistant | 43 (32.1%) | 55 (35.9%) | 53 (27.3%) | 55 (35.0%) | 39 (24.5%) | 245 (30.7%) |
| Gentamicin resistant | 15 (11.2%) | 27 (17.6%) | 37 (19.1%) | 21 (13.4%) | 24 (15.1%) | 124 (15.6%) |
| Amikacin resistant | 4 (3.0%) | 0 | 3 (1.5%) | 3 (1.9%) | 2 (1.3%) | 12 (1.5%) |
| ESBL producing enterobacterales | 30 (22.4%) | 35 (22.9%) | 42 (21.6%) | 35 (22.3%) | 33 (20.8%) | 175 (22.0%) |
| Carbapenem resistant organisms | 2 (1.5%) | 1 (0.7%) | 1 (0.5%) | 1 (0.6%) | 0 | 5 (0.6%) |
Mortality and length of inpatient stay post E. coli bacteraemia
Seven and 30-day mortality data were collected for 770 patients and is summarised in Table I. At 7 days, 77 patients (10%; 95% CI 7.4–12.2%) died and by 30 days this had risen to 129 (16.8%; 95% CI 13.7–19.9%).
The length of inpatient stay post bacteraemia was recorded for 717 patients. The median length of inpatient stay (LOIS) among all patients across all years was 14 days (IQR 6–26 days). At 30-days, the readmission rate was 54 (7.0%).
Discussion
This is the first UK study describing multifaceted and multidisciplinary interventions and their effect on hospital-acquired E. coli bacteraemia. Over 5-years, almost one third of cases were thought to be preventable. Interventions were easier to implement for small groups of healthcare workers, such as endoscopists performing ERCP and urologists undertaking prostatic biopsies but harder for patients in multiple wards across multiple sites such as inpatients with urinary catheters. The proportion of infections that were device-related did not decrease significantly over the 5-year period, the most common medical device being urinary catheters. Unsurprisingly, there was an upward trend in E. coli bacteraemia associated with the cessation of ciprofloxacin prophylaxis for haematology/oncology inpatients with neutropenic sepsis and further reductions in E. coli bacteraemia are likely to have occurred if ciprofloxacin prophylaxis had not been discontinued. Later, we identified other areas where QI interventions had not been targeted, such as post-delivery endometritis and cardiology related procedures, such as transcatheter aortic valve implantation. The trends in resistance to different antimicrobial drugs remained stable over 5 years.
This study included many E. coli bacteraemia episodes and encompassed a wide geographical area, increasing the potential generalisability of the dataset. Our work highlighted specific areas of concern and the quality interventions were, in part, novel and potentially of general interest. The delay in reduction of types of infection such as catheter-associated UTI could have been explained by the slowness of quality improvements, implemented sequentially, over the study period. Where resources to implement changes were greatest, for example in junior doctors' involvement at the WXUH site, the greatest reduction in E. coli bacteraemia was seen.
Despite 20% of all UK E. coli bacteraemias being hospital-acquired [11], there is lack of published evidence for QI interventions to reduce these bacteraemias and, therefore, few national or international papers for comparison. However, our work does share some similarities or, in the case of susceptibility profiles, differences with other cohorts. Firstly, hospital-acquired E. coli bacteraemia occurred most commonly in the elderly male population and the urinary tract was the commonest site of infection [5,8]. Secondly, urinary catheter-associated infections were the commonest preventable cause [5,[12], [13], [14]] but specific surgical and non-surgical procedures and instrumentation were also identified [13]. Also, other sites such as the GI tract, and other conditions, like neutropenic sepsis were significant causes of bacteraemia in specific patient populations [15]. Thirdly, our recurrence rate was approximately 3.5%, a percentage similar to a previous study [8]. Fourthly, estimated 30-day mortality in cohort studies range from 12-23.6% [11,14], similar to our findings and, as other studies have shown, were influenced by age, co-morbidities and multidrug resistance [13,16,17]. Fifthly, one estimate of preventability was 25% [14], similar but lower to our own estimate. Finally, testing of more than 40 000 E. coli surveillance blood culture isolates collected between 2012-14 [18] revealed resistance to ciprofloxacin (18.4%), gentamicin (9.7%) and ESBL production (10.4%), observations substantially lower than our observed rates of resistance.
A decision to stop ciprofloxacin as prophylaxis for inpatients with neutropenic sepsis led to an increase in E. coli bacteraemia at the SBH site. Although contrary to NICE guidance [19], the rational was to reduce ciprofloxacin usage and, at the same time, increase the probability of isolating an organism which could be treated with targeted therapy. This hypothesis was partially validated by a small prospective study [20]. The cessation of ciprofloxacin prophylaxis (compared to a historical control group receiving prophylaxis) did not result in an increase in patient mortality, admission to critical care (HDU/ICU) and LOIS. Although in 2020/21 three patients died following E. coli bacteraemia caused by fully susceptible isolates, a case review identified that disease rather than infection was the likely cause of death. The Business Intelligence Unit in Barts Health NHS Trust was also able to demonstrate that death due to neutropenic sepsis had not increased following this change.
One successful intervention appears to have been converting from TRUS to transperitoneal prostatic biopsies. The risk of infection is reduced as the biopsy is not taken through the rectum. This also negated the need for ciprofloxacin resistant Enterobacterales rectal swab screening and prophylactic ciprofloxacin for three days and stat amikacin. For three consecutive years, there were no E. coli bacteraemias related to prostatic biopsies.
There were limitations associated with the study. Firstly, the definition of hospital-acquired E. coli bacteraemia, onset more than 48 hours after admission, included some community-acquired infections that were slow to diagnose. Hospital-acquired episodes were also overestimated as some were community-acquired, cultured for the first time on the second day following admission. Urinary catheter QI was only targeted at secondary care level and a community focus would have stopped some patients with urinary catheters developing E. coli infection and admission to hospital. The impact of COVID-19 was a confounding limitation as, between 2019-21, there was cancellation of elective and non-urgent procedures and surgery, as well as changes in the admissions policy to critical care after repurposing for COVID-19 management. Emerging from the Covid-19 pandemic is probably the explanation for a significant rise in surgical and non-surgical procedures that caused bacteraemia in 2021-22. Unfortunately, we were unable to obtain activity data over the 5-year period, which included the 2019–2021 lock down, as medical administration staff stopped coding and were re-purposed for other activities. Also, with each PDSA cycle, we were unable to measure how well each intervention was being implemented. The judgement on preventability was subjective, although we aimed to mitigate this through independent review by a second consultant microbiologist. Finally, the 30-day follow-up was not possible for every patient and if there was no electronic record 30 days post bacteraemia, we assumed the patient had survived.
In conclusion, hospital-acquired E. coli bacteraemia is associated with significant mortality and morbidity. Despite implementation of QI interventions, it was not possible to achieve a 50% reduction. Our work highlights the importance of antimicrobial prophylaxis and medical device ‘good practice’ interventions which, over time, are likely to reduce healthcare-associated infection. Tackling E. coli bacteraemias through preventive measures, both in hospitals and in the community, can lead to improved patient safety, decreased mortality and length of hospital inpatient stay. The difficulty is not knowing what needs to be done but implementing a multifaceted QI program which is sustainable and tailored for different patients, in multiple wards across multiple sites.
Conflicts of interest
The authors have no conflicts of interest to declare.
Funding information
The authors have no sources of funding to declare.
Contributor Information
Catherine Dominic, Email: c.dominic@smd17.qmul.ac.uk.
Mark Melzer, Email: mark.melzer@nhs.net.
References
- 1.McKinnon M.C., McEwen S.A., Pearl D.L., Lyytikäinen O., Jacobsson G., Collignon P., et al. Mortality in Escherichia coli bloodstream infections: a multinational population-based cohort study. BMC Infect Dis. 2021;21(1):606. doi: 10.1186/s12879-021-06326-x. 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Melzer M., Welch C. Outcomes in UK patients with hospital-acquired bacteraemia and the risk of catheter-associated urinary tract infections. Postgrad Med J. 2013;89(1052):329–334. doi: 10.1136/postgradmedj-2012-131393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abernethy J., Guy R., Sheridan E.A., Hopkins S., Kiernan M., Wilcox M.H., et al. Epidemiology of Escherichia coli bacteraemia in England: results of an enhanced sentinel surveillance programme. J Hosp Infect. 2017;95(4):365–375. doi: 10.1016/j.jhin.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 4.Melzer M., Welch C. Is Escherichia coli bacteraemia preventable? Lancet Infect Dis. 2021;12(2):103–104. doi: 10.1016/S1473-3099(11)70356-5. [DOI] [PubMed] [Google Scholar]
- 5.Underwood J., Klein J.L., Newsholme W. Escherichia coli bacteraemia: how preventable is it? J Hosp Infect. 2011;79(4):364–365. doi: 10.1016/j.jhin.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Public Health England. Annual epidemiological commentary: gram-negative bacteraemia, MRSA bacteraemia, MSSA bacteraemia and C. difficile infections, up to and including financial year April 2020 to March 2021. Retrieved 30 November 2021, from https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1016843/Annual_epidemiology_commentary_April_2020_March_2021.pdf.
- 8.Hsu D., Melzer M. Strategy to reduce E. coli bacteraemia based on cohort data from a London teaching hospital. Postgrad Med J. 2018;94:212–215. doi: 10.1136/postgradmedj-2017-135454. [DOI] [PubMed] [Google Scholar]
- 9.Jones B., Vaux E., Olsson-Braun A. How to get started in quality improvement. BMJ. 2020;368:m865. doi: 10.1136/bmj.k5437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Healthcare Safety Network. CDC/NHSN surveillance definitions for specific types of infections. Retrieved 30 November 2021, from https://www.cdc.gov/nhsn/pdfs/pscmanual/17pscnosinfdef_current.pdf.
- 11.Bonten M., Johnson J.R., van den Biggelaar A.H.J., Georgalis L., Geurtsen J., de Palacios P.I., et al. Epidemiology of Escherichia coli Bacteremia: A Systematic Literature Review. Clin Infect Dis. 2021;72(7):1211–1219. doi: 10.1093/cid/ciaa210. [DOI] [PubMed] [Google Scholar]
- 12.Jones L.F., Meyrick J., Bath J., Dunham O., McNulty C.A.M. Effectiveness of behavioural interventions to decrease UTIs and E. coli bacteraemia for older adults across all care settings. J Hosp Infection. 2019;102(2):200–218. doi: 10.1016/j.jhin.2018.10.013. [DOI] [PubMed] [Google Scholar]
- 13.Blandy O., Honeyford K., Gharbi M., Thomas A., Ramzan F., Ellington M.J., et al. Factors that impact on the burden of E. coli bacteraemia: multivariate regression analysis of 2011-15 data from West London. J Hosp Infect. 2019;101(2):120–128. doi: 10.1016/j.jhin.2018.10.024. [DOI] [PubMed] [Google Scholar]
- 14.Lillie P.J., Johnson G., Ivan M., Barlow G.D., Moss P.J.E. coli bloodstream infection outcomes and preventability: a six month prospective observational study. J Hosp Infect. 2019;103(2):128–133. doi: 10.1016/j.jhin.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Otter J.A., Galletly T.J., Davies F., Hitchcock J., Gilchrist M.J., Dyakova E., et al. Planning to halve Gram-negative blood stream infection: getting to grips with healthcare-associated E. coli bloodstream infection sources. J Hosp Infect. 2019;101(2):129–133. doi: 10.1016/j.jhin.2018.07.033. [DOI] [PubMed] [Google Scholar]
- 16.Melzer M., Petersen I. Mortality following bacteraemic infection caused by extende spectrum beta-lactamase (ESBL) producing E. coli compared to non-ESBL producing E. coli. J Infect. 2007 Sep;55(3):254–259. doi: 10.1016/j.jinf.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 17.Baltas I., Stockdale T., Tausan M., Kashif A., Anwar J., Anvar J., et al. Long-term outcome and risk factors for late mortality in Gram-negative bacteraemia: a retrospective cohort study. J Glob Antimicrob Resist. 2021;25:187–192. doi: 10.1016/j.jgar.2021.03.019. [DOI] [PubMed] [Google Scholar]
- 18.Bou-Antoun S., Davies J., Guy R., Johnson A.P., Sheridan E.A., Hope R.J. Descriptive epidemiology of Escherichia coli bacteraemia in England, April 2012 to March 2014. Euro Surveill Bull Eur Sur Mal Transm Eur Commun Dis Bull. 2016 Sep 1;21(35) doi: 10.2807/1560-7917.ES.2016.21.35.30329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neutropenic sepsis: prevention and management in people with cancer. www.nice.org.uk/guidance/cg151 (last accessed 19/8/2022).
- 20.Caldwell L., Bapat A., Drumright L., Lambourne J., Jimenez-England F., Aries J., et al. Cessation of ciprofloxacin prophylaxis in haemto-oncology patients. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab1000. ciab1000. [DOI] [PMC free article] [PubMed] [Google Scholar]