Summary
Chronic airway infections with Pseudomonas aeruginosa are the major co-morbidity in most people with cystic fibrosis (CF) sustained by neutrophils as the major drivers of lung inflammation, damage, and remodeling. Phagocytosis assays were performed with clonal consortia of longitudinal P. aeruginosa airway isolates collected from people with CF since the onset of lung colonization until patient’s death or replacement by another clone. The extra- and intracellular abundance of individual strains was assessed by deep amplicon sequencing of strain-specific single nucleotide variants in the bacterial genome. The varied microevolution of the accessory genome of the P. aeruginosa clones during mild and severe courses of infection corresponded with a differential persistence of clonal progeny in the neutrophil phagosome. By simultaneously exposing the ancestor and its progeny to the same habitat, the study recapitulated the time lapse of the temporal change of the fitness of the clone to survive in neutrophils.
Subject areas: Respiratory medicine, Immunology, Microbiology
Graphical abstract

Highlights
-
•
Phagocytosis assays with Pseudomonas aeruginosa clonal consortia from cystic fibrosis lungs
-
•
Strain-specific single nucleotide variants as readouts of bacterial survival
-
•
Ancestor is more resistant to killing by neutrophils than its clonal progeny
-
•
P. aeruginosa loses fitness during severe chronic cystic fibrosis lung infections
Respiratory medicine; Immunology; Microbiology
Introduction
Cystic fibrosis (CF), the most common severe autosomal recessive trait in populations of European descent,1,2 is caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene.3 Chronic airway infections with bacterial opportunistic pathogens, namely Staphylococcus aureus and Pseudomonas aeruginosa, are the major co-morbidity in most people with CF.4 Bacterial persistence in biofilms and immigration of immune effector cells sustain a vicious cycle of chronic infection, inflammation, lung damage, and remodeling which leads to ongoing deterioration of pulmonary function and—at least prior to the introduction of highly effective CFTR modulators into the clinic—finally to respiratory failure.2,5,6 The introduction of mutation-type specific therapy by CFTR modulators that attenuate the basic defect has significantly improved the quality of life and overall prognosis within the last years.1 Although the typical CF airway pathology of mucus obstruction, wall thickening, bronchiectasis, and even consolidations has been shown to be partially reversible by treatment with CFTR modulators,7 the chronic persistence of S. aureus and P. aeruginosa seems to be rather refractory to eradication by pharmacological attenuation of the CF-specific basic defect.8,9,10
Massive neutrophil influx,11 the subsequent release of neutrophil extracellular traps (NETs), and terminal apoptosis are key events that result in the accumulation of extracellular DNA,12,13 histones, and enzymes such as elastase and myeloperoxidase causing persistent CF lung injury.5,14,15 Neutrophilic infiltration of the airways occurs secondary to the presence of cytokines and chemokines.16 Neutrophils accumulate in the lumen in intimate contact with the microcolony-embedded bacteria,17,18 mainly driving inflammation and subsequent damage and remodeling of the CF lung.5,14,15,19
P. aeruginosa is the most prevalent pathogen in the airways of CF in adolescents and adults.20 Once P. aeruginosa has successfully conquered the patient’s airways, the bacterium will chronically persist and adapt to its niches in the upper and lower airways.21,22 The microevolution of P. aeruginosa in the CF airways has been investigated by whole-genome sequencing of isolates from unique patients and major transmissible lineages and finally of serial isolates from CF patient cohorts.23,24,25,26,27,28
We have collected serial P. aeruginosa isolates from CF patients since the onset of their airway colonization for a period of now more than 40 years29 Serial isolates from twelve patients were selected for in-depth genomic and phenotypic characterization.27 Having these data at hand, we now examined the influence of the bacterial microevolution within CF lungs on the interaction between bacterium and neutrophil, the major antipseudomonal weapon of the CF host. Mixtures of an early isolate and its clonal descendants were exposed to competitive phagocytosis and killing assays with human neutrophils. Thereby the clonal lineages from very mild and fatal courses of chronic infection demonstrated a divergent profile of survival and persistence within the neutrophil. In other words, the lung environment of the individual CF host had shaped the evolution of the bacterial response to the neutrophil, the major defense mechanism of the human host.
Results
Outline of the bacterial fitness study
The intraclonal competitive fitness of P. aeruginosa was examined in phagocytosis and killing assays with human neutrophils (Figure 1). Twelve clonal lineages of P. aeruginosa were tested, each of which was represented by a temporal series of CF airway isolates that had been collected from unrelated people with CF over a median period of ten years (see Table S1 for more detail). Same colony-forming unit (CFU) aliquots of serial clonal isolates from one CF donor were concurrently exposed at a total multiplicity of infection of 50 to activated neutrophils freshly prepared from the peripheral blood of a healthy human donor. Samples were taken after 30 s, 30 min, and 60 min, and the proportions of the individual bacterial strains within the neutrophils and in the extracellular space were subsequently quantified by amplicon sequencing of multiplex PCR products carrying strain-specific sequence variants.
Figure 1.
Concept and flowchart of the competition experiments of serial P. aeruginosa isolates in human neutrophils
According to bacterial counts documented by electron micrographs taken at 5, 30, and 60 min of four phagocytosis assays, 1% of the bacteria adhered to the cell surface of the neutrophils, whereas 99% of the bacteria resided in the cytosol or the lysosomal compartment. These intracellular bacteria with still intact cell walls were clearly discerned from non-viable bacteria showing the various stages of neutrophil-mediated bactericidal activity from disrupted cell walls at the beginning to lysed bacteria and finally irregularly shaped remnants. To harvest the viable intracellular bacteria for subsequent multi-marker PCR, neutrophils were lysed with the detergent saponine, which also prevented the inclusion of damaged bacteria into the next processing steps of the protocol.
Features of the clonal P. aeruginosa CF airway isolates selected for competition experiments
P. aeruginosa isolates had been collected in half-year intervals from the 32 people with CF regularly seen at the CF clinic Hannover who had become chronically colonized with P. aeruginosa in the 1980s. With respect to antipseudomonal chemotherapy, patients administered aerosolized tobramycin and on clinical indication received 2-week intravenous courses of monotherapy with tobramycin or combination therapy with azlocillin/tobramycin or since the mid-1990s with ceftazidime/tobramycin.29
Whole-genome sequencing was performed on serial airway isolates from the six patients with the most severe courses who died of respiratory insufficiency 64–162 months after they had irreversibly acquired P. aeruginosa and from the six patients with the mildest clinical courses who had enjoyed a normal daily life for at least the first 20 years of their chronic airway colonization with P. aeruginosa.27 By 2022, five of the six females had regained a stable lung function of more than 70% FEV1 predicted after starting triple combination therapy with the CFTR modulators elexacaftor/tezacaftor/ivacaftor.30
Each of the 12 patients was harboring one persistent P. aeruginosa clone in the airways (Table S1). To perform intraclonal competition experiments, we selected isolates that covered the whole colonization time of the initially acquired clone (Figure 2A). During this time period, antipseudomonal chemotherapy was very conservative (see above).29 The clonal lineages of this study were repetitively exposed to the same antimicrobials. In other words, during the study period, there was no temporal change of the therapeutic pressure on the P. aeruginosa clones in our cohort. Each chosen strain was carrying at least one informative and technically robust single nucleotide variant (SNV) that was sensitive and specific for strain identification by multi-marker PCR within a mixture of four to nine clonally related P. aeruginosa isolates.31 When growing separately, the clone representatives covered a broad range of phenotypes in motility, secretion, and biofilm formation (Figure 2B).
Figure 2.
Clonal P. aeruginosa CF airway isolates selected for competition experiments
Mild (blue) and fatal (red) courses of infection are designated by capital letters. Serial P. aeruginosa isolates chosen for competitive fitness experiments in the presence of neutrophils are marked by green dots (early isolates), yellow triangles (midterm isolates), and gray squares (late isolates), respectively.
(A) Trajectory of CF patient’s lung function (FEV1% predicted) during colonization of the first persisting P. aeruginosa clone.
(B) Phenotype of the selected P. aeruginosa isolates in quantifiable plate assays.
Characteristics of human neutrophils
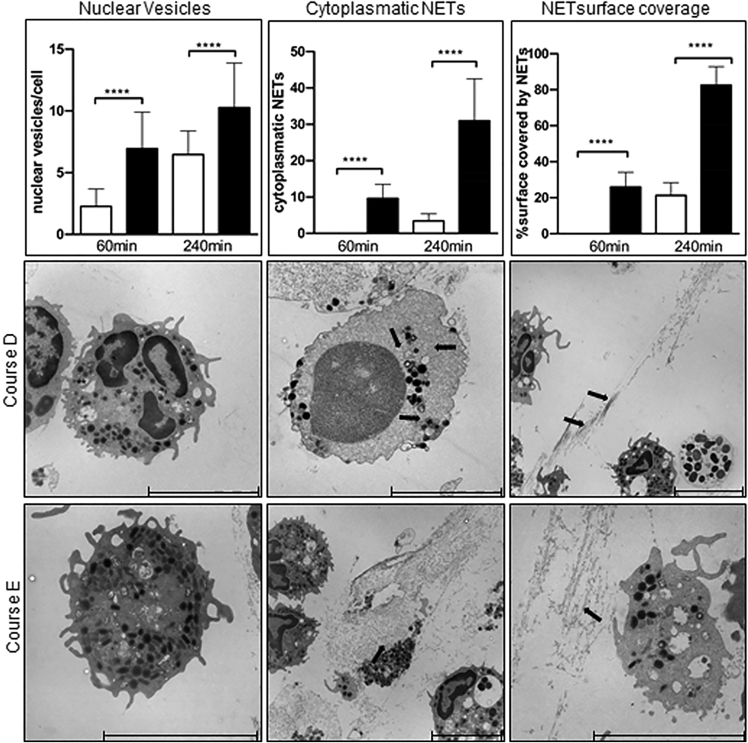
The interaction of P. aeruginosa with human neutrophils was examined by the time course of phagocytosis and killing (Table S2). Neutrophils were freshly isolated from blood of four healthy human donors by low-speed density centrifugation.32 Flow cytometry regularly demonstrated the purity and homogeneity of the neutrophil preparations (Figure 3A). Of the neutrophil markers CD184, BLT, and FPR1, only BLT1 displayed a significant increase when in contact with any P. aeruginosa isolate. Neutrophils became more damaged by exposure to serial isolates from severe courses than from mild courses, which was not visible by 1 h, but became evident by 4 h of infection (Figures 3C and 3F). Neutrophils of all donors showed similar kinetics of peptide cleavage by membrane-bound elastase (Figure 3D) indicating a comparable donor-independent bactericidal activity of the cells.33 To minimize the influence of spontaneous activation or cell death of isolated neutrophils during cultivation, we decided to confine the competition assays to the first hour as no changes in morphology of uninfected neutrophils were apparent until this time point. However, exposure to P. aeruginosa reduced the viability of granulocytes within the first hour clone-dependently by 10–30% (Figures 3C and 3E) concurrent with the emergence of damaged granulocytes in electron micrographs. By 1 h postinfection the neutrophils did not release NETs, a phenomenon associated with neutrophil death called NETosis,34 but by 4 h postinfection more than 70% of all neutrophils released NETs (Figure 3E). Kinetics of NET formation that is ascribed to three processes, i.e., nuclear vesicles, intracellular NET formation, and extracellular NET formation (Figure 4), was more strongly induced by serial P. aeruginosa isolates from clinically severe courses than by those from mild courses (cf. course E vs. D, Figure 4).
Figure 3.
Characteristics of the human donors’ neutrophils
(A) Sorting of freshly isolated viable CD16+ neutrophils by flow cytometry. Uninfected cells showed 94 9% vitality after 60 min (see Table S2).
(B) Neutrophil surface marker expression of CD184 (black), BLT1 (dark gray), and FPR1 (light gray) in the absence (circles) and presence of P. aeruginosa CF isolates of distinct competitive fitness, i.e., from a severe course (squares) or a mild course (triangles) (cf. Figure 3) immediately before and after 1 h exposure to bacterial inoculum or medium control.
(C) Boxplots of the LDH release from neutrophils during phagocytosis assays with mixtures of serial P. aeruginosa isolates from severe (n = 48 assays, red) or mild courses of infection (n = 48, blue) (∗, p < 0.05; ∗∗, p < 0.01, paired Wilcoxon rank-sum test; p < 0.0001, group comparison by Kruskal-Wallis test).
(D) Activity of membrane-bound neutrophil elastase assessed with the FRET – sensor NEmo-2.32 The kinetics of the cleavage of the intramolecular peptide substrate was not significantly different between neutrophils of 3 biological replicates each from the 4 donors.
(E) NET formation in neutrophils during phagocytosis assays. The percentage of neutrophils with NETs was quantified in uninfected (white bars) and infected samples (black bars) by confocal immunofluorescence microscopy. The data represent the mean ± SD of the analysis of randomly taken images from four independent competition assays (no. of images from uninfected samples: 0 h, n = 24; 1h, n = 20; 4h, n = 24; no. of images from infected samples: 1h, n = 24; 4h, n = 36). NET formation was significantly higher in infected samples at the 4 h time point (∗∗∗∗, Pcorr < 0.0001, two-tailed unpaired Student’s t test) but was not seen at the 1 h time point.
(F) Representative overlay images of NET components (blue = DNA, green = DNA/histone-1 complexes, red = myeloperoxidase) of uninfected and infected neutrophils (scale bar = 100 μm). Settings of the microscope were adjusted to the respective isotype control.
Figure 4.
NET detection in neutrophils infected with P. aeruginosa clinical isolates of CF patients
Shown are the three different categories of NET detection after 60 min and 240 min: nuclear vesicles, cytoplasmic NETs, and NET surface coverage for a mild (course D; white bars) and a severe course (course E; black bars). NETs are marked by arrows. Significance was calculated by Mann-Whitney test; ∗∗∗p < 0.0001. The lower two panels show electron micrographs of nuclear vesicles, cytoplasmic, and NET surface coverage after 240 min exposure to serial P. aeruginosa isolates from a mild (course D; upper panel) or severe course (course E; lower panel). NETs (identified by immunostaining of citH3 and human neutrophil elastase) are marked by arrows. All aspects of NET formation were more strongly induced by serial P. aeruginosa isolates from severe courses than by those from mild courses.
Increased persistence of early P. aeruginosa isolates from severe infections in neutrophils
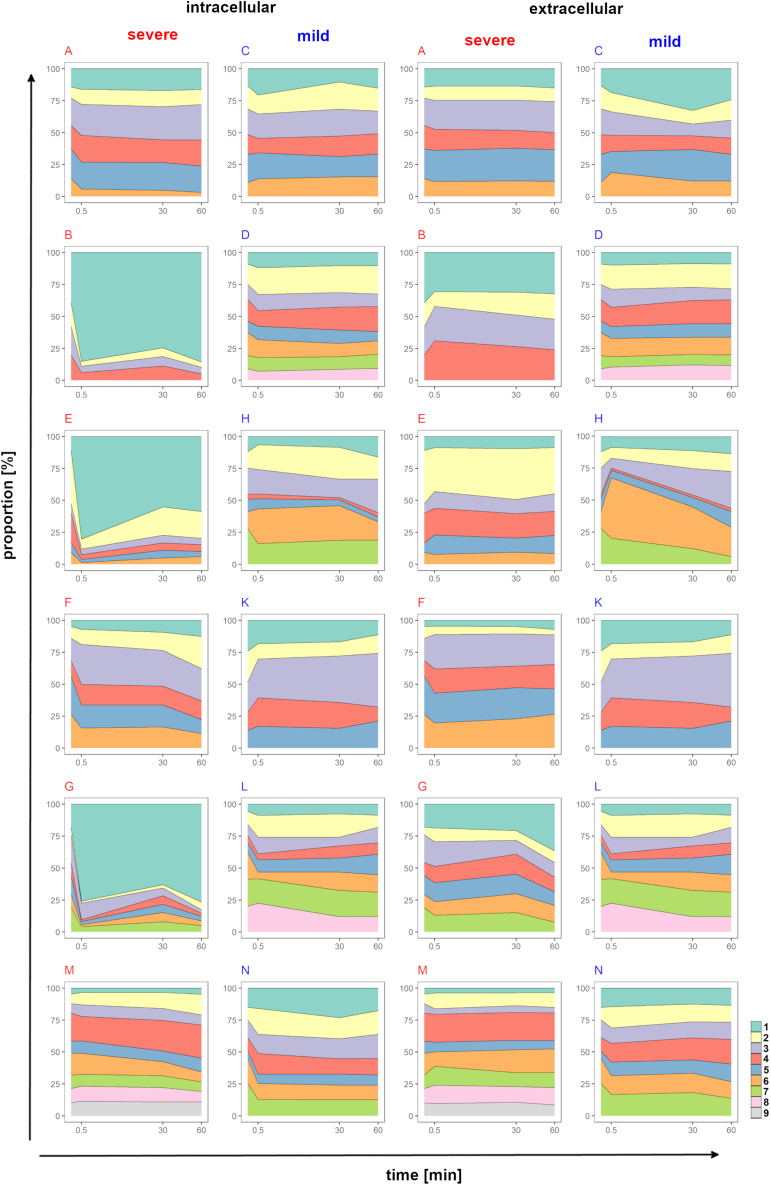
Mixtures of equal proportions of clonal P. aeruginosa CF airway isolates covering the whole colonization time of the initially acquired clone were simultaneously exposed to freshly prepared neutrophils. The extra- and intracellular abundance of individual clonal strains at time points 30 s, 30 min, and 60 min was assessed by deep amplicon sequencing of strain-specific SNVs in the bacterial genome (Tables S3 and S4). In other words, the individual strains within the clonal consortium were recognized by their signature of specific sequence variants. Figure 5 visualizes the relative distribution of serial isolates from the 12 chronic CF airway infections during the time course of the phagocytosis assays of one biological replicate (Figures S1–S3).
Figure 5.
Intra- and extracellular persistence of communities of serial P. aeruginosa CF airway isolates during phagocytosis assays with human neutrophils
Proportions of the individual clonal P. aeruginosa isolates of all 12 longitudinal patient courses (A – N) prior to and after 30 s, 30 min, and 60 min co-incubation with neutrophil granulocytes were determined by amplicon sequencing of strain-specific SNVs. The normalized values depict the average of the technical replicates of single phagocytosis assays with the neutrophils of donors 1 and 3. The outcome of the second biological replicate and the phagocytosis assays with the neutrophils of donors 2 and 4 are depicted in Figures S1–S3.
Statistical analysis of the amplicon sequence data revealed significantly different behavior of the clonal lineages of the mild and severe courses of infection (Figure 6). Bacterial strains were grouped by CF lung colonization time into early, midterm, and late isolates. In case of the mild courses, early isolates of all clones retained their initial proportion at all time points of the phagocytosis assay in both the intra- and extracellular compartments (Figure 6B). The populations of the midterm (Figure 6E) and late isolates (Figure 6F) were more diverse showing a broader distribution of the abundance of individual strains with no global shift toward depletion or overrepresentation. The early isolates slightly increased their share in the neutrophils at the 30- and 60-min time points. However, none of these trends reached statistical significance. In particular, the P. aeruginosa isolates from the mild chronic infections did not differ in their distribution in the intra- and extracellular compartments.
Figure 6.
Higher fitness of early P. aeruginosa isolates from severe infections to persist in neutrophils
The figure indicates the probability density distribution of the relative change of the proportion of individual P. aeruginosa strains in the intracellular and extracellular compartment during phagocytosis assays at time points 30 s, 30 min, and 60 min. The relative change describes the change of the proportion of individual strains compared to the initial inoculum at a given time point and is depicted in logarithmic scale. The density distributions show how often a certain relative change can be found; for example, a maximum close to 1 means that the bacterial isolates retained their initial proportion. Curves with a larger width of the half maximum reflect a higher variability of the change of the strains’ abundance. The distribution of the late isolates of severe courses (I) is shifted to the left in the direction of a relative decrease, and the distribution of the early isolates (G) shows an opposite shift to a relative increase in comparison to the other isolates. Each curve includes data of all phagocytosis assays with the neutrophils of the four healthy human blood donors. Upper Panel. Probability density distributions of all P. aeruginosa isolates from all (A), the mild (B), and the severe (C) courses of the chronic infections of CF patients’ airways. Middle/lower panel. Probability density distributions of all P. aeruginosa isolates from the mild/severe courses of infections differentiated by colonization time into early (D; G), midterm (E; H), and late (F; I) isolates (cf. Figure 1A).
Clonal isolates from the fatal chronic infections showed a different behavior in the phagocytosis assays (Figures 6C and 6G–6I). Strains maintained their initial proportions in the extracellular milieu irrespective of clonal descent and CF lung colonization time, but the early isolates dominated in the intracellular compartment whereas the late isolates were depleted. Correspondingly, the late isolates were significantly more prevalent in the extracellular space than within the neutrophils at all examined time points of the phagocytosis assays (30 s, Pcorr < 0.0001; 30 min, Pcorr < 0.01; 60 min; Pcorr < 10−6). Moreover, the clonal consortia from the severe courses were significantly more cytotoxic for neutrophils than those from the mild courses (p < 0.05, Figure 3C). In summary, the intracellular persistence of P. aeruginosa in neutrophils was not generally governed by clonal lineage and its microevolution in CF lungs but by the origin of the bacterial strains from a mild or severe CF lung infection.
Discussion
This study exposed P. aeruginosa strains to neutrophils, the major antipseudomonal weapon of the human host. Phagocytosis assays were performed with clonal consortia of longitudinal airway isolates collected from people with CF since the onset of lung colonization until patient’s death or replacement by another clone. The change of the composition of the clonal consortia was monitored for the first hour after the P. aeruginosa bacteria had been exposed to the granulocytes. According to our experience gained during the preparation phase of the study, phagocytosis is almost complete within the first minute at the chosen multiplicity of infection of 50. Hence, the time window of 1 h should have been sufficient to capture the resistance of the individual bacterial strains to killing by the lipopolysaccharide (LPS)-activated neutrophil. By limiting the observation period to 1 h, secondary events such as bacterial growth and loss of viability of the granulocyte should not have significantly influenced the relative fitness of a strain to evade killing by the neutrophil in presence of its clonal competitors. By the 4 h time point, the exposure to the P. aeruginosa bacteria grown to exponential phase at baseline, had induced NETosis in the majority of neutrophils, which generated visual aspects similar to neutrophils exposed to rhamnolipid that is produced in large amounts by P. aeruginosa during stationary growth.35,36,37
The experiments assessed the temporal change of fitness of a P. aeruginosa clone to withstand the neutrophil during the chronic colonization of the CF lung. Prima vista one would assume that the outcome of the fitness experiments was driven by the virulome and the spectrum of de novo mutations in the individual strains and lineages. However, only few targets of the core genome were identified in our mutation database of the 12 clones27 that were hit by two or more clonal lineages. We did not detect any case of a mutation that emerged in midterm or late isolates, which was continuously retained by its progeny. Some mutations were maintained within a lineage but did not spread to other concurrent lineages that had co-evolved from the same ancestor. Cul-de-sac de novo mutations happened frequently, particularly among the clinically severe courses. In other words, the dataset of de novo mutations that emerged during microevolution is too diverse to draw any general conclusion about associations between individual mutations, virulence, and fitness.
Even though the individual lineages differed by a broad range of clone-specific mutations, the group of mild clinical courses and the group of severe clinical courses of chronic infection showed a similar evolution of their fitness traits within their group but a divergent evolution between the two groups. The differential outcome of the competitive fitness experiments between mild and severe courses was not associated with clonal virulence in infection models,38 the clonal annual mutation rate of the core genome during CF lung colonization, or the intraclonal fitness gradient to grow in nutrient-poor or nutrient-rich liquid media.27,31 However, the microevolution of the accessory genome of P. aeruginosa clones was distinct.27 During mild courses, the clonal lineages acquired genes for motility, adhesion, and metabolic pathways that are underrepresented or absent in the P. aeruginosa core genome. Acquisition of these genes from the polymicrobial community by horizontal gene transfer may have led to the strain-specific modulation of persistence within neutrophils seen in the midterm and late isolates of the mild courses (Figures 5E and 5F). Co-occurrence networks of commensals co-exist with P. aeruginosa in CF lungs of patients with close-to-normal lung function27 but not in those of patients with advanced or end-stage lung disease. Correspondingly, during severe courses of infection, the horizontal gene transfer to and from the accessory genome was more confined to the gene pool of the Pseudomonas pangenome.27
All patients who died from respiratory failure suffered from an already substantially compromised lung function when P. aeruginosa conquered their lower airways (Figure 2A). Thus, from the very beginning, P. aeruginosa resided in an inflamed and structurally remodeled CF lung habitat sharing the intraluminal space with immigrated neutrophils. Neutrophils and especially their elastase drive the vicious cycle of inflammation and remodeling.14 Neutrophil elastase has been demonstrated to cleave the receptor CR1 on neutrophils and the receptor C3bibound on the surface of opsonized P. aeruginosa.39,40 This “opsonin-receptor mismatch” impairs the phagocytosis of opsonized P. aeruginosa, the stimulation of superoxide production, and finally killing of the opsonized P. aeruginosa by neutrophils. The end-stage lung disease of the six late CF patients of our cohort was characterized by monocultures of P. aeruginosa. The “opsonin-receptor mismatch” of the local neutrophils resident in the patients’ lungs could have protected the P. aeruginosa bacteria of being efficiently phagocytosed and killed. Hence, their resistance against the hostile environment within the granulocyte was not anymore essential for bacterial survival. In summary, our fitness experiments with the clonal lineages from the severe courses of infection taught us that the living conditions in the inflamed and remodeled airways shaped uniform evolutionary traits of the P. aeruginosa clones. Compared to the ancestor from the inanimate environment, the chronic intraluminal persistence in CF bronchi generated a uniform phenotype of lower metabolic proficiency,31 lower fitness to thrive in healthy airways,41 and lower resistance to killing by neutrophils (this work).
There are only a few options to study the microevolution of a bacterial pathogen during the chronic infection of a human habitat and its impact on the interaction with the host. Besides the lifelong colonization of the human stomach with Helicobacter pylori,42 the chronic CF airway infections with P. aeruginosa are another rare opportunity to trace the microevolution of a bacterial clone in a human habitat.21 The adaptation of the aquatic microorganism P. aeruginosa to the CF host and the concomitant microbiome has been extensively examined for short time periods.4 Most changes in genotype and phenotype were reported to occur during the first years of infection, but our own genome sequence data tell us that P. aeruginosa evolves within the CF host with a rather uniform strain-specific mutation rate.27 As shown for the first time in this report, strain collections like ours, which retrieved serial isolates in regular intervals from patient’s airways for now more than 40 years, should be a rich resource to resolve the impact on bacterial microevolution on host defense over the whole time period of infection. So far, microevolution has mainly examined in terms of the de novo mutations in coding sequences with particular focus on metabolic fitness, virulence, and control of gene expression.21,22,43,44 Fitness traits and evolutionary trajectories have been constructed, but major players of the fitness game are currently not accessible to analysis. Appropriate protocols and tools are still missing for a comprehensive study of epigenome and transcriptome. We have recently performed direct strand-specific RNA sequencing of P. aeruginosa clone C strains grown under standard conditions.45 An avalanche of antisense RNAs was detected that had never been identified before by conventional high-throughput cDNA sequencing.46 Distinct antisense transcripts were as frequent as sense transcripts and were present in comparable abundance. It is reasonable to assume that antisense transcripts affect the competitive fitness of clonal serial isolates. However, the protocols for direct RNA sequencing still require orders of magnitude more RNA than the amount of bacterial RNAs that currently can be recovered from a competitive fitness experiment as described in this manuscript. It will probably take many years to come until we have appropriate protocols for epigenome and direct RNA sequencing at hand, and hence by now we should avoid any general statements about bacterial microevolution in CF lungs based on current protocols and technologies that likely will not sustain the test of time. Morphology and physiology of the CF airway habitat are constantly changing, and the CF community is anxious to learn how and to what extent highly efficient modulator therapy will affect the interaction of the CF host with its most common pathogen P. aeruginosa.8,30
Limitations of the study
Our study has limitations; phagocytosis assays with neutrophils from CF donors would have been closer to the real world of CF lung infection, particularly because the basic defect is also expressed in these non-epithelial cells.47 However, recurrent blood sampling from people with CF was not reasonable. Moreover, we are confident that our results are also applicable to CF neutrophils because within experimental error occasionally performed phagocytosis assays with CF neutrophils reproduced the data obtained with non-CF neutrophils.
In our within-clone competition experiments, the relative quantities of individual P. aeruginosa strains were determined by amplicon sequencing of multiplex PCR products of strain-specific SNVs. This marker-free approach bypasses the need to modify the original strains with secondary genotypic or phenotypic tags. Initially established and validated in competition experiments with planktonic bacteria grown in liquid media,31 this study now demonstrates that the marker-free approach can be reliably applied to competitive fitness experiments within the arena of host—microbe interaction. In our case the ancestor and its progeny were simultaneously exposed to the same habitat, thus monitoring within time lapse the temporal change of the fitness of the clone during years of microevolution. However, some constraints need to be mentioned: Firstly, the large proportion of host DNA within the PCR template may limit the number of strains that can be analyzed by amplicon sequencing, and secondly, amplicon sequencing inherently cannot discriminate between viable and dead bacteria. To cope with the latter point by protocol, we lysed pre-damaged bacteria within the neutrophils with detergent prior to multiplex PCR so that only SNVs of viable intracellular bacteria with intact cell walls were quantified by amplicon sequencing.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| APC anti-human CD45 | BD Pharmingen | Cat: 555485; Isotype: Mouse IgG1, κ; RRID: AB_314400; LOT #3074786 |
| APC/Cyanine7 anti-human CD184 | Biolegend | Cat: 306528; Isotype: Mouse IgG2a, κ; RRID: AB_2565994; LOT#B321997 |
| FITC anti-human FPR1 | Biolegend | Cat: 391604; Isotype: Mouse IgG1, κ; RRID: AB_2728398; LOT#B252257 |
| PE anti-human Leukotriene B4 Receptor/BLT1 | Cayman Chemical | Cat: LS-A101254-100; Isotype: Polyclonal Rabbit; LOT#LAH0521011 |
| PE/Cyanine7 anti-human CD16 | Biolegend | Cat: 302016; Isotype: Mouse IgG1, κ; RRID: AB_314216; LOT#310460 |
| Goat anti-rabbit IgG (H+L) cross-adsorbed secondary antibody, Alexa Fluor 633 | Invitrogen | Cat: A 21070; LOT#2160429 |
| Goat anti-mouse IgG (H+L) highly cross-adsorbed secondary antibody, Alexa Fluor Plus 488 | Invitrogen | Cat: A32723; LOT#VA297822 and #VJ308481 |
| mouse anti-DNA-histone 1 monoclonal IgG2a | SigmaMilllipore | Cat: MAB3864; LOT#3560106; Isotype: Mouse IgG2a |
| Rabbit anti-human myeloperoxidase | Dako | Cat: A0398; LOT#20082378; Isotype: Rabbit IgG |
| Rabbit polyclonal anti-citrullinated-Histone H3 (Arg2/8/17) | abcam | Cat: ab5103; Isotype: Rabbit IgG; RRID:AB_304752; LOT#GR27606-1 |
| Rabbit monoclonal anti-neutrophil elastase (clone EPR7479) | abcam | Cat: ab131260; Isotype: Rabbit IgGa; RRID:AB_11155265 |
| Bacterial andvirusstrains | ||
| P. aeruginosa CF airway isolates used in this study | Hannover Medical School | See Table S1 |
| Biological samples | ||
| Donkey serum | Sigma Aldrich | Cat: D9663-10ML; LOT#SLBL4004V |
| Human AB Serum | Institute of Transfusion Medicine and Transplant Engineering (Hannover Medical School) | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| AMPure XP Beads | Beckman Coulter | Cat: A63881 |
| Albumin fraction V | Carl Roth | Cat: CP84.2; LOT#380298758 |
| Aurion BSA-c | Fisher scientific | Cat: 50-248-05; LOT#50512/3 |
| DiffQuick – Haema QuickStain | Labor + Technik Eberhard Lehmann | LT 005 |
| Dimethyl sulfoxide | Carl Roth | Cat: 4720.1; CAS 67-68-5 |
| Gelatin from cold water fish skin | Sigma Aldrich | Cat: G7041-100G; LOT#SLCC7086 |
| GelRed | Linaris Biologische Produkte | Cat: 41003 |
| Glutaraldehyde | Sigma-Aldrich | Cat: G5882; CAS 111-30-8 |
| GoTaq Long PCR Master Mix | Promega | Cat: 114021 |
| Hoechst/bisBenzimide H 33342 trihydrochloride | Sigma Aldrich | Cat: G7041-100G; LOT#BCBL5830V |
| Lead citrate | Sigma-Aldrich | Cat: 15326; CAS 6107-83-1 |
| Lipopolysaccharide (P. aeruginosa serotype 10) | Sigma Aldrich | Cat: L7018-10MG |
| NEmo-2 FRET reporter | SiChem | Cat: SC-0201; Lot# SC-0201-003_P-125_3-IV.2 |
| Osmium tetroxide | Sigma-Aldrich | Cat: O5500-250MG; CAS 20816-12-0 |
| Paraformaldehyde 16% EM grade | Science Services | Cat: E15710-250 |
| Poly-L-lysine | Sigma Aldrich | Cat: P4707; LOT#RNBJ8041 |
| Polymorphprep | Progen | Cat: 1114683; LOT#00121 |
| ProLong Gold antifade mountant | Invitrogen | Cat: P36930; LOT#2273617 |
| Saponine | Serva Electrophoresis | Cat: 34655.01; CAS: 8047-15-2 |
| Sivelestat sodium salt hydrate | Sigma Aldrich | Cat: S7198-5MG; LOT# 0000044591 |
| Sodium cacodylate trihydrate | Sigma-Aldrich | Cat: 20840; CAS 6131-99-3 |
| Sodium periodate | Sigma-Aldrich | Cat: S1878; CAS 7790-28-5 |
| Unconjugated gold colloid 5 nm | Ted Pella | Cat: 15702-20 |
| Unconjugated gold colloid 10 nm | Ted Pella | Cat: 15703-20 |
| Triton X 100 | Carl Roth | Cat: 3051.3; CAS: 9036-19-5 |
| Tween 20 | Sigma Aldrich | Cat: P1379-100 ml; LOT#SLCH7513 |
| Uranyl acetate | Ted Pella | Cat: 19481; CAS: 541-09-3 |
| Critical commercial assays | ||
| Cytotoxicity Detection Kit | Sigma Aldrich | Cat: 11644793001 |
| Epoxy Embedding Medium Kit | Sigma-Aldrich | Cat: 45359 |
| LIVE/DEAD Fixable Aqua Dead Cell Stain Kit | Invitrogen | Cat: L34957 |
| NEBNext Ultra II DNA Library Kit for Illumina | New England Biolabs | Cat: E7645L |
| NEBNext Multiplex Oligos for Illumina (Set 1) | New England Biolabs | Cat: E7335L |
| NEBNext Multiplex Oligos for Illumina (Set 2) | New England Biolabs | Cat: E6442S |
| NEBNext Multiplex Oligos for Illumina (Set 3) | New England Biolabs | Cat: E6444S |
| NextSeq 500/550 Mid Output kit v2.5 (300 cycles) | Illumina | Cat: 20024905 |
| Qubit dsDNA HS Assay Kit | ThermoFisher Scientific | Cat: Q32854 |
| Deposited data | ||
| Anonymous patient data | This work | see Table S1 |
| P. aeruginosa PA14 genome | NC_002516.2 | Database: https://www.ncbi.nlm.nih.gov/genome/?term=Pseudomonas+aeruginosa+PA14 |
| Oligonucleotides | ||
| 412 primer sequences | This work | Mendeley Data: https://data.mendeley.com/datasets/c87p4pytt9/draft?a=689a644e-37cc-448d-b1ef-0c16e6b2370d |
| Software and algorithms | ||
| FlowJo v10.6.1. | TreeStar | https://www.flowjo.com/ |
| GraphPad Prism 8 | GraphPad Software | https://www.graphpad.com/ |
| R 3.4.0. | R | https://r-project.org |
| Pipeline of evaluation of amplicon sequences (PIASneutro) | This work | Database: https://data.mendeley.com/datasets/c87p4pytt9/draft?a=689a644e-37cc-448d-b1ef-0c16e6b2370d |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Nina Cramer (cramer.nina@mh-hannover.de).
Materials availability
This study did not generate new unique reagents.
Experimental model and subject details
Study participants
People with CF selected for the study
Patients had been regularly seen at the Hannover CF clinic since the age of diagnosis or at least 12 months prior to the first detection of P. aeruginosa in throat swab or sputum. CF patients’ clinical data were collected from the medical charts that mainly had been recorded by one of the authors (BT). At each patient’s visit to the clinic, height, weight and spirometric lung function were recorded and throat swabs or sputa were collected for microbiological analysis. Patient’s CFTR mutation genotype had been determined in the authors’ laboratory during the years 1990–1993 by intragenic haplotyping, cascade screening of common CF-causing mutations and subsequent verification of all non-p.Phe508del CF mutations by Sanger sequencing of the respective exon.48,49
Of the 32 patients who became chronically P. aeruginosa – positive between 1982 and 1991, the six patients with the most severe clinical courses (4 males and 2 females, born between 1968- 1981) and the six patients with the mildest courses of lung disease (6 females, born between 1966- 1982) after acquisition of P. aeruginosa were selected. These severely affected patients had the shortest lifespan of the cohort after acquisition of P. aeruginosa and died of respiratory insufficiency. Of the 22 patients who neither died nor had received a lung transplant by 2010, the six patients were chosen who consistently exhibited the best lung function in spirometry throughout the last 40 years and who enjoyed a normal daily life for at least the first 20 years of their chronic infection with P. aeruginosa.
The study has been approved by the Ethics Committee of Hannover Medical School (study no. 3739). Written informed consent was obtained from patients and their parents.
Healthy controls
Blood was repetitively drawn from four healthy adults of European descent (ethic approval number 2701-2015) with no case of CF in their family history (21 years, female; 43 years, female; 52 years, female; 68 years, male). Subjects had no clinical signs of infection at all dates of blood collection.
Method details
Bacterial strain collection
P. aeruginosa strains were isolated from deep throat swabs or spontaneous sputa that were plated onto MacConkey, Mueller-Hinton, tryptic soy and chocolate agar supplemented with 8% erythrocytes and 2% horse serum. Isolates retrieved from every other patient visit in 6 ± 2 months intervals were incorporated into the strain collection. When P. aeruginosa had been detected for the first time, the isolates of all first three P. aeruginosa – positive samples were stored. Second subcultures of P. aeruginosa isolates were stored in 0.3 ml aliquots in micro-tubes at −80°C in Luria broth (LB) supplemented in 17% (v/v) glycerol.
P. aeruginosa strain genotyping
All P. aeruginosa strains used in the study were genotyped by a custom-made microarray following exactly the detailed protocol published previously.50 The low-resolution microarray represents the conserved core genome with 16 informative SNVs and the variable accessory genome with 42 marker genes.
WGS of serial P. aeruginosa isolates
Whole genome short-read sequencing on a SOLiD 5500XL system and the subsequent processing and bioinformatics analysis of the datasets have been extensively described in the supplement of our previous publication.27 The sequence datasets had been fully evaluated prior to the start of this project. Overall, about 500 isolates were sequenced from the six patients with the most severe course of chronic airway infection and the six patients with the mildest course of chronic airway infection (Table S1).27 For each patient course, the genome sequences were searched for SNVs that could serve as an identifier of individual strains. A SNV-spanning multiplex PCR with subsequent amplicon sequencing was established and examined in its specificity and sensitivity in competitive fitness experiments in vitro and ex vivo.31,41 Eighty solates thereof were selected to perform competition experiments in the presence of neutrophils.
P. aeruginosa growth characteristics
Competitive fitness experiments were performed with P. aeruginosa strains grown to exponential phase at baseline when similar CFU of each strain were combined for subsequent immediate exposure to the habitat of interest. The protocol for the exposure of bacteria to neutrophils is described below in the paragraph ‘Competitive fitness experiments’. Selected early, intermediate, and late isolates from three mild and three severe courses had been examined in their singular growth characteristics31 yielding similar kinetics of lag phase, exponential growth and stationary phase for all strains if they were growing separately in LB under matching conditions in vitro. To ensure that the bacteria grown by protocol were in exponential phase when they were combined for the competitive fitness experiments and did not produce any rhamnolipid taken as a surrogate to produce quorum-sensing regulated secondary metabolites, the time course of rhamnolipid production was monitored by the hemolysis of erythrocytes. The biosurfactant rhamnolipid has been classified as the heat-stable hemolysin.51,52,53
P. aeruginosa isolates of the courses B and E were grown as outlined in the paragraph ‘Competitive fitness experiments’ (see below). The kinetics of rhamnolipid production was monitored at time points 0, 30 min, 1 h, 4 h, 24 h, 96 h and 168 h. An aliquot of 3.5 ml of a 200 ml LB culture was taken, heat-inactivated for 5 min at 70 °C and then added to a suspension of 0.15 ml concentrated red cells. Lysis of erythrocytes was documented by photographs and spectrophotometry (550 nm). No hemolysis was observed at time points up to 4 h, but strong hemolysis occurred during stationary phase at the 24 h time point and later. According to these control experiments quorum-sensing regulated production of secondary metabolites did not occur during the time course of the competitive fitness experiments.
Isolation and characterization of neutrophils
Isolation of neutrophil granulocytes
A volume of 22.5 ml blood was drawn from healthy donors into three K-EDTA tubes. An aliquot of 20 ml blood was overlaid on 15 ml Polymorphprep® in a 50 ml Falcon tube. After a density centrifugation at 500 g for 35 min at room temperature and subsequent deceleration without brake, the middle fraction with the neutrophils was removed by pipetting and suspended in 50 ml RPMI medium/25 mM HEPES. The suspension was centrifuged at 500 g for 10 min, the supernatant was decanted and then the cell pellet was exposed for 30 s to 1 ml distilled H2O in order to lyse erythrocytes. Lysis was stopped by the addition of 9 ml RPMI medium/25 mM HEPES. The cell suspension was purified by two washing steps with RPMI medium/25 mM HEPES and then re-suspended in 3 ml colorless RPMI medium 1640. Granulocyte preparations were characterized in cell number by manual hemocytometry, viability by trypan blue dye exclusion and purity by microscopic examination of a Pappenheim stain.
Flow cytometry was performed with the FACS Canto instrument (BD Biosciences). Freshly isolated LPS-activated neutrophils were analyzed prior and at the end of infection experiments with P. aeruginosa CF airway isolates. Samples of 0.5 ml were pelleted by centrifugation (10 min, 300 g). After the removal of the supernatant, the pellet was re-suspended in 0.2 ml FACS buffer (PBS + 2 mM EDTA + 2% (v/v) fetal calf serum) containing a mixture of antibody conjugates of surface markers (0.28 ng phycoerythrin anti-human BLT1 antibody, 0.2 μg phycoerythrin/cyanine 7 anti-human CD16 antibody, 0.2 μg allophycocyanine anti-human CD45 antibody, 0.3 μg allophycocyanine/ cyanine 7 anti-human CD184 antibody, 0.2 μg fluorescein isothiocyanate FITC anti-human FRP1 antibody) and incubated for 30 min at 4°C in the dark. Then the suspension was centrifuged again (10 min, 300 g). The cell pellet was re-suspended in 0.2 ml 1% (v/v) LIVE/DEAD stain solution (Invitrogen) and incubated for 30 min at 4°C in the dark. Cells were washed with 0.2 ml FACS buffer and fixed with 0.2 ml 2% (w/v) paraformaldehyde/FACS buffer (1:1; v/v). Prior to flow cytometry the cellular suspension was centrifuged again (10 min, 300 g) and re-suspended in 0.1 ml FACS buffer. For each surface marker, paired samples with the marker antibody and a) all fluorophores (positive sample) or b) all fluorophores minus the specific fluorophore (negative control) were assayed with the FACS Canto (BD Biosciences). Data were evaluated with the FlowJo™ software (version 10.6.1).
Neutrophil elastase
Total elastase activity of the isolated neutrophils was measured with a commercial kit (Sigma Aldrich MAK246). Membrane-bound elastase activity was assessed by cleavage of the intramolecular peptide substrate sequence QPMAVVQSVPEE of the palmitoylated Förster resonance energy transfer – based reporter NEmo-2. This ratiometric fluorescent reporter NEmo-2 contains coumarin 343 as the donor fluorophore and rhodamine as the acceptor fluorophore.54 Two samples per donor of 106 neutrophil granulocytes each were suspended in 0.2 ml phosphate buffered saline and activated with 0.1 μg P. aeruginosa LPS serotype 10 for 30 min at 37°C (negative control: 106 A549 cells in 0.2 ml RPMI 1640 medium). Samples were centrifuged at 300 g for 10 min at room temperature. Cell pellets were re-suspended in 0.2 ml PBS supplemented with 1.8 mM CaCl2 and 0.8 mM MgSO4. One sample per donor received 4 μl of the 5 mM leukocyte elastase inhibitor Sivelestat. After an incubation for 15 min at room temperature 2 μl of 0.2 mM NEmo-2 were added under mixing. Fluorescence emission of donor (425 nm–475 nm) and acceptor fluorophore (600 nm–620 nm) was recorded at time points 2, 5, 10, 15, 30, 45, and 60 min with a LSR II flow cytometry cell analyzer (Becton Dickinson). Data were evaluated with the FlowJo™ software (version 10.6.1). Elastase activity was given by the difference of the ratio of donor to acceptor fluorescence in the absence and presence of Sivelestat.
Cytotoxicity assay
The release of lactate dehydrogenase from the neutrophils during the time course of infection experiments was monitored by sampling aliquots of 0.25 ml at time points 0 s, 30 s, 30 min and 60 min followed immediately by 10 min centrifugation at 300 g (positive control: sample with 1 % (v/v) Triton X100; negative control: RPMI medium) (see Table S2). 0.1 ml of the supernatant was incubated with 0.1 ml test reagent (Cytotoxicity Detection Kit, Sigma Aldrich) for 30 min at room temperature in the dark. The reaction was stopped with 0.2 ml 1 M HCl. The absorption of two 0.1 ml aliquots of samples was measured at 490 nm in 96-well format with an ELISA reader.
Formation of NETs
NETs were visualized by immune fluorescence microscopy. 2 x 105 neutrophils were seeded on 8 mm cover slips (Thermo Fisher) coated with 0.01 % (w/V) poly-L-lysine within a well of a 48 well-plate (Greiner Bio-one, cat.no. 677102).55 After centrifugation of the plate at 250 g for 5 min the samples were fixed with 4% (w/v) paraformaldehyde. The plates were wrapped with Para film and stored at 4°C until further staining. For staining,56,57 samples were first washed three times with PBS. After 5 min permeabilization with 0.05% (v/v) TritonX100, samples were first incubated for 20 min with blocking buffer (1% BSA, 3% donkey serum, 3% cold water fish gelatin, 0.05% Tween 20 in PBS) and then for 1 h at room temperature with mouse monoclonal antibody (IgG2a) against DNA/Histone 1 (0.55 mg/mL, diluted 1:1000 in blocking buffer) and a rabbit anti-human myeloperoxidase (3.3 mg, diluted 1:300 in blocking buffer). As isotype controls murine IgG2a (0.2 mg/mL, 1:364 diluted in blocking buffer) and rabbit IgG (1.16 mg, 1:105 diluted in blocking buffer) were used. After washing three times with PBS, the secondary antibodies goat anti-mouse Alexa 488Plus (1:500 diluted in blocking buffer) and goat anti-rabbit Alexa 633 (1:500 diluted in blocking buffer) were applied for 1 h at room temperature in the dark. After washing three times with PBS and once with distilled H2O the samples were stained for 10 min (in the dark, room temperature) with Hoechst 33342 (1:1000, stock 50 mg/mL, diluted in distilled water). The slides were washed three times with distilled water, embedded in 3–5 μL Prolong Gold and dried overnight at 4°C. All cover slips were surrounded with clear nail polish. Samples were stored at 4°C in the dark until analysis by fluorescence microscopy. Of each sample, at least six pictures were randomly taken by a Leica TCS SP5 AOBS confocal inverted-base fluorescence microscope equipped with a HCX PL APO ×40 0.75–1.25 oil immersion objective.58 The total number of neutrophils was manually counted using ImageJ software (version 1.53c). A neutrophil was classified as activated or net-releasing if an offshoot of DNA was visible or if at least two of the following criteria were fulfilled, i.e. enlarged nucleus, de-condensed nucleus or blurry rim.59,60,61,62
Transmission electron microscopy
Aliquots of infected neutrophils were directly taken from the preparations of the competition experiments (see below). Fixation of cells was performed in the same way as for IFM. Cells were then pelleted by centrifugation (5 min at 400g and room temperature), the supernatants discarded and 250 μL 2.5% (vol/vol) glutaraldehyde in 0.15 M sodium cacodylate (pH 7.2) were added to the pellets for 2 h at 4°C, followed by washing steps (3 × 0.15 M sodium cacodylate, pH 7.2). They were then post fixed with 1% osmium tetroxide (wt/vol) in 0.15 M sodium cacodylate (pH 7.2) for 1 h at 4°C, washed (3 × 0.15 M sodium cacodylate, pH 7.2), dehydrated in an ascending series of ethanol and further processed for standard Epon embedding as described previously.33 Sections were cut in 70 nm slices with an LKB ultratome and mounted on Formvar-coated copper grids. The ultrathin sections were post-stained with uranyl acetate (Laurylab, Saint Fons, France) and lead citrate (Laurylab). Immunolabeling of thin sections after antigen unmasking with sodium metaperiodate (Merck) with gold-labeled anti-Histone H3 (citrulline R2 + R8 + R17) antibody (H3cit, 5 nm gold), 1:80 dilution (ab5103, Abcam, Berlin, Germany) and anti-neutrophil elastase antibody (NE, 10 nm gold), 1:80 dilution (ab131260, Abcam, Berlin, Germany) was performed as described previously,63 with the modification that Aurion-BSA (Aurion, Wageningen, The Netherlands) was used as a blocking agent. The sections examined in a Philips/FEI CM100 BioTwin transmission electron microscope operated at a 60-kV accelerating voltage. Images were recorded with a Gatan Multiscan 791 charge-coupled device camera.
For quantitative analysis, electron micrographs of 60 and 240 min had been evaluated for a severe and a mild patient’s course each. The cellular profiles of 30 randomly selected fields on the thin sections were analyzed per sample for three different analysis points. For each analysis point, 30 different fields were assessed as follows:
NET release from the nuclei: loss of intact cytoplasmic structure with release of nuclear DNA and presence of nuclear vesicles in the surrounding containing double positive staining for NE and citH3, was counted as positive. The percentage of positive cells was calculated in relation to the total number of observed cells.
NET surface coverage: the area covered with NET structures that was found in the extracellular environment of neutrophils was measured. The percentage indicated the fraction of the area covered by NETs in relation to the total area of the respective field. Characteristic web-like fibrillary NET structures were first identified at high magnification, then further confirmed in combination with TEM/gold-labelled immunostaining of NET components. Assessment of NETs areas was analyzed by Adobe Photoshop CS5. Briefly, the Ruler Tool was used to calculate the number of pixels/square micrometer. NETs areas were then transformed to pixel number with the Magic Wand Selection Tool. Thus, the proportion of NETs area relative to the entire area of a given electron micrograph was determined.
Nuclear vesicles per neutrophil: the number of the intracellular nuclear vesicles (citH3 and neutrophil elastase positive) were counted per neutrophil in the randomly selected fields. The analyzed neutrophils show an intact cytoplasmic structure with nucleus, double nuclear membrane and intact granules.
Competitive fitness experiments
Optimization of PCR primers for multiplex PCR
Primer pairs spanning a strain-specific SNV were designed by web-based ‘Primer 3 Input’ (http://bioinfo.ut.ee/primer3) for two to four SNVs per isolate.
The analysis of marker-free competition experiments would have required thousands of single PCR reactions. To reduce the number of independent reactions, PCR conditions were iteratively optimized by systematic modification of conditions. At first, hybridization temperature and reaction time were varied. If a PCR product was not reliably generated, variable amounts of MgCl2, DMSO, and/or betaine were added to the reaction mixture. Yield and absence of by-products were controlled by agarose gel electrophoresis of PCR products of at least two unrelated DNA templates. If individual PCR reactions failed to amplify under the conditions used for the detection of other clonal isolates of the patient’s longitudinal course, new primers were designed and subsequently adapted to the PCR condition of the other longitudinal isolates.
Next, the conditions for multiplex PCR of strain-specific SNVs of clonal isolates were established. The quality of the initial multiplex PCR experiments was confirmed by observing the product pattern produced during agarose gel electrophoresis. PCR reactions were executed with either 5, 10 or all primer pairs of isolates selected from one patient course. The absence of PCR by-products was verified with combinatorial PCR of variable numbers of primer pairs. Hence, the products generated with up to 10 primer pairs were sequenced by next generation sequencing (Illumina NextSeq) in order to test whether all PCR-products were synthesized in one reaction. If the yield turned out to be comparable for the various strain-specific PCR products of one isolate, these conditions were accepted for the analysis of the bacterial genomic DNA obtained from the competition experiments. Of the 129 isolates that were found to be suitable for inclusion into the competition experiments, 86 isolates were finally selected, which evenly covered the temporal order of each chronic infection of early, mid-term and late isolates.
Conduct of competitive infection experiments
LB agar plates were inoculated in parallel with loops of frozen glycerol stocks of all selected serial P. aeruginosa isolates of the same CF patient and incubated overnight at 37°C. Two days later, a set of culture flasks (100 ml) with 25 ml LB was prepared. Each flask was inoculated with a loop of the bacterial lawn taken from the overnight plate of one isolate followed by parallel incubation of all flasks for 14 h at 37°C on the shaker platform (150 rpm). The next morning aliquots of one milliliter from each culture were added to a new set of 100 ml flasks with 25 ml LB and then cultured in parallel (150 rpm, 37°C) for another 5 h so that the culture was within the exponential growth phase. Aliquots of each culture were adjusted to an OD (600 nm) = 0.6 which corresponds to 109 CFU/ml. Equal aliquots of the adjusted cultures were mixed so that 500 μl of this mixture diluted with 9.5 ml RPMI medium 1640 corresponded to an infection dose of 5 × 107 CFU in 750 μl. Following this protocol, the infection dose consisted of viable planktonic exponentially growing bacteria but did not contain any biologically active secreted bacterial metabolite such as rhamnolipids that could interfere with the phagocytosis assay (cf. paragraph ‘P. aeruginosa growth characteristics’).
The exact CFU infection dose was retrospectively determined by serial plating of 50 μl of 10-fold dilutions of the bacterial mixture of the infection dose.
Two hours after the onset of culturing the serial isolates, neutrophil granulocytes were isolated from blood drawn from two healthy donors. The freshly isolated granulocytes were stimulated with 0.1 μg P. aeruginosa LPS serotype 10 for 30 min at 37°C. Then a suspension of 106 neutrophils in 6 ml RPMI 1640/10% (v/v) human AB serum was prepared and put on a shaking platform (300 rpm, 37°C). AB serum was used to activate the complement system. A 0.5 mL aliquot from the 10 ml suspension of the mixture of serial P. aeruginosa isolates was taken and put on ice, and immediately thereafter 0.75 ml of the bacterial mixture corresponding to 5 × 107 CFU (MOI 50) were added to the suspension of the activated granulocytes. Samples of 0.5 ml each were taken 30 s, 30 min and 60 min after the addition of the bacteria to the neutrophils. Pilot experiments taking samples after 5 s, 15 s and 30 s had shown before that the relative proportion of the serial isolates in the intra- and extracellular compartment did not significantly differ within the first minute of the phagocytosis assay. Hence, the time point of 30 s was chosen that inherently was least affected by slightly variable mixing times of bacteria and mammalian cells. During each infection experiment samples were taken in triplicate from the same single-tube granulocyte preparation (technical replicates). Infection experiments were repeated with the donor’s granulocytes isolated on another day (biological replicate).
During the infection experiment, each collected 0.5 ml sample was immediately mixed with 1 ml ice-cold PBS in a 2 ml Eppendorf tube placed on ice. The sample was then centrifuged (270 g) for 10 min at 4°C. After careful removal of the supernatant to another Eppendorf tube, the pellet was incubated with 300 μl saponine (Serva) for 3 min at 37°C to lyse the neutrophils and intracellular bacteria with pre-damaged cell walls. The reaction was stopped with 350 μl ice-cold PBS. This tube and the other tube with the supernatant were then centrifuged at 13,000 g for 10 min at 4°C to pellet the bacteria. After removal of the supernatants the pellets of extracellular and intracellular bacteria were re-suspended in 25 μl double-distilled H2O, stored at 4°C for a period of up to 2 h until all samples from the two donors had been processed and then subjected to multiplex PCR of strain-specific SNVs.
Multiplex PCR
The final protocol for multiplex PCR contained 25 cycles (60 s at 60°C, 60 s at 72°C and 90 s at 94 °C) with a final volume of 30 μl containing the mixture of primers (3 pmol per primer), 5 μl of the re-suspended pellet, 2 mM MgCl2, 3% DMSO (v/v) and 15 μl Go Taq® Long PCR Master Mix (Promega).
Fragment libraries and amplicon sequencing
For each growth experiment of clonal isolates from one CF donor, one technical replicate was combined prior to the preparation of libraries for amplicon sequencing, if SNV regions were not overlapping between clonal lineages. Thus, the number of fragment libraries that had to be prepared was reduced by 9-fold from 2,736 to 304 samples. Amounts of 0.1–1 μg of pooled multiplex PCR products were sheared in a Covaris S2 system for 14 min. Fragment libraries were prepared at the E120 scale according to the protocols adapted for the G + C-rich P. aeruginosa as described previously.64 The sequencing of the amplicon pools was performed on an Illumina NextSeq with 200 bp read length.
Sequence analysis
Using an in-house script (https://data.mendeley.com/datasets/c87p4pytt9/draft?a=689a644e-37cc-448d-b1ef-0c16e6b2370d), Illumina NextSeq paired-end reads were trimmed, paired reads were merged and aligned to the reference genome PA14.65,66 Afterwards the number of reads carrying strain-specific SNVs was extracted directly from the generated samfiles. Abundance of a strain was represented by the mean of the read percentage of the two to four strain-specific SNVs as well as by the normalized mean percentage of the six technical replicates. Outliers: If individual values exceeded the range of mean ±2 σ, they were eliminated at the levels of both the strain-specific SNVs and the bacterial strain. If the mean number of reads assigned to a strain was below 0.5% in at least three of the six technical replicates of the amplicon reads, the bacterial strain was classified as ‘detectable, but not quantifiable’ due to the overlap of correct reads with the inherent sequencing error of the Illumina platform. In this case, the reliability of SNP detection was checked by two criteria. Firstly, the number N of normalized non-canonical base counts were determined in 10 bases adjacent to the SNV position (x = N/60). If the number of strain-specific SNV reads exceeded the confidence interval of (x ± 1.96 x0.5), the strain was classified as ‘being present’. Secondly, the number of the strain-specific nucleotides had to exceed the number y of the two non-canonical nucleotides at the SNV position by (y ± 1.96 y0.5).
Quantification and statistical analysis
Characterization of neutrophils
The percentage of cells with NET formation in one sample was quantified by counting in total 200–1,800 neutrophils from six immunofluorescence microscope slides whereby maximal 300 cells should have been fixed on one slide. The primary flow cytometry data were evaluated with the FlowJo™ software (version 10.6.1). Immunofluorescence and flow cytometry data sets were analyzed with programs of the GraphPad Prism 8 package. Shapiro-Wilk- and Levene-tests were applied to prove whether the data sets followed a Gaussian distribution with variance homogeneity. If this prerequisite was fulfilled, a variance analysis followed. Otherwise, the non-parametric Kruskal-Wallis- or Friedman-tests were applied. The cytotoxicity of P. aeruginosa isolates during the time course of an infection experiment was compared by Kruskal-Wallis and Wilcoxon rank-sum test.
Competitive fitness experiments
The relative abundance of an individual P. aeruginosa strain was given as the mean of its proportion among the strain-specific SNVs of the technical replicates taken from the same preparation of granulocytes. A strain was eliminated if its contents was less than 1% of the sequence reads in the bacterial inoculum. Comparability of technical and biological replicates was evaluated by Kruskal-Wallis and Wilcoxon sign-rank tests, respectively. The logarithm of the relative change of the abundance of all strains or subgroups thereof during the time course of an infection experiment compared to its initial representation in the inoculum was cumulatively visualized by probability density functions. The P. aeruginosa serial isolates were first allocated to the individual courses and then again to the groups of all, early, midterm or late isolates from all, severe or mild courses of infection in either the intracellular or the extracellular compartment. Group comparisons between early, midterm and late isolates were performed with Wilcoxon rank-sum tests. Differences between groups were judged as being significant if the Pcorr value with Bonferroni correction was below the level of significance of α = 0.05.
Acknowledgments
The collection of strains over a 40-year period by the Institut für Medizinische Mikrobiologie und Krankenhaushygiene is gratefully acknowledged. The authors are grateful to Silke Hedtfeld, Ilona Rosenboom, and Joshua Schulte for their participation in bacterial culturing and preparation of fragment libraries; Maria Dorda and Lutz Wiehlmann for amplicon sequencing; and Sebastian Fischer and Marie-Madlen Pust for their support in data processing. AM thanks Dario Fey and Matteo Guerra (University of Heidelberg) for their practical advice in the FRET assay.
This work was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – SFB900/3–158989968 - A2 and Z1. PK received a stipend from the German Center for Infection Research (DZIF TI07.003_Kuschnerow_00). AM acknowledges personal funds from the German Center for Lung Research (DZL).
Author contributions
Pia Kuschnerow: Formal analysis, Investigation, Validation, Visualization, Writing - original draft, Writing - review & editing; Antje Munder: Conceptualization, Funding acquisition, Methodology, Supervision, Visualization, Writing - review & editing; Nicole de Buhr: Investigation, Methodology, Visualization, Writing - review & editing; Mania Ackermann: Investigation, Methodology; Adan Chari Jirmo: Investigation, Methodology, Visualization; Maren von Köckritz-Blickwede: Methodology, Writing - review & editing; Burkhard Tümmler: Conceptualization, Formal analysis, Funding acquisition, Methodology, Resources, Supervision, Writing - original draft, Writing - review & editing; Nina Cramer: Conceptualization, Data Curation, Formal analysis, Investigation, Methodology, Software, Supervision, Visualization, Writing - original draft, Writing - review & editing.
Declaration of interests
The authors declare no competing interests.
Inclusion and diveristy
We support inclusive, diverse, and equitable conduct of research.
Published: March 24, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.106475.
Supplemental information
Data and code availability
-
•
The datasets generated and/or analyzed during the current study are available as supplemental information (Tables) or have been deposited at Mendeley. All data are publicly available as of the date of publication. The link to the Mendeley data is listed in the key resources table.
-
•
All original data have been deposited at Mendeley and are publicly available as of the date of publication. The link is listed in the key resources table.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- 1.Bell S.C., Mall M.A., Gutierrez H., Macek M., Madge S., Davies J.C., Burgel P.R., Tullis E., Castaños C., Castellani C., et al. The future of cystic fibrosis care: a global perspective. Lancet Respir. Med. 2020;8:65–124. doi: 10.1016/S2213-2600(19)30337-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shteinberg M., Haq I.J., Polineni D., Davies J.C. Cystic fibrosis. Lancet. 2021;397:2195–2211. doi: 10.1016/S0140-6736(20)32542-3. [DOI] [PubMed] [Google Scholar]
- 3.Cutting G.R. Cystic fibrosis genetics: from molecular understanding to clinical application. Nat. Rev. Genet. 2015;16:45–56. doi: 10.1038/nrg3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blanchard A.C., Waters V.J. Microbiology of cystic fibrosis airway disease. Semin. Respir. Crit. Care Med. 2019;40:727–736. doi: 10.1055/s-0039-1698464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan M.A., Ali Z.S., Sweezey N., Grasemann H., Palaniyar N. Progression of cystic fibrosis lung disease from childhood to adulthood: neutrophils, neutrophil extracellular trap (NET) formation, and NET degradation. Genes. 2019;10:183. doi: 10.3390/genes10030183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stoltz D.A., Meyerholz D.K., Welsh M.J. Origins of cystic fibrosis lung disease. N. Engl. J. Med. 2015;372:351–362. doi: 10.1056/NEJMra1300109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McBennett K.A., Davis P.B., Konstan M.W. Increasing life expectancy in cystic fibrosis: advances and challenges. Pediatr. Pulmonol. 2022;57:5–12. doi: 10.1002/ppul.25733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Durfey S.L., Pipavath S., Li A., Vo A.T., Ratjen A., Carter S., Morgan S.J., Radey M.C., Grogan B., Salipante S.J., et al. Combining ivacaftor and intensive antibiotics achieves limited clearance of cystic fibrosis infections. mBio. 2021;12:e0314821. doi: 10.1128/mbio.03148-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hisert K.B., Heltshe S.L., Pope C., Jorth P., Wu X., Edwards R.M., Radey M., Accurso F.J., Wolter D.J., Cooke G., et al. Restoring cystic fibrosis transmembrane conductance regulator function reduces airway bacteria and inflammation in people with cystic fibrosis and chronic lung infections. Am. J. Respir. Crit. Care Med. 2017;195:1617–1628. doi: 10.1164/rccm.201609-1954OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jorth P., Staudinger B.J., Wu X., Hisert K.B., Hayden H., Garudathri J., Harding C.L., Radey M.C., Rezayat A., Bautista G., et al. Regional isolation drives bacterial diversification within cystic fibrosis lungs. Cell Host Microbe. 2015;18:307–319. doi: 10.1016/j.chom.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin W.C., Fessler M.B. Regulatory mechanisms of neutrophil migration from the circulation to the airspace. Cell. Mol. Life Sci. 2021;78:4095–4124. doi: 10.1007/s00018-021-03768-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D.S., Weinrauch Y., Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 13.Kim J.H., Podstawka J., Lou Y., Li L., Lee E.K.S., Divangahi M., Petri B., Jirik F.R., Kelly M.M., Yipp B.G. Aged polymorphonuclear leukocytes cause fibrotic interstitial lung disease in the absence of regulation by B cells. Nat. Immunol. 2018;19:192–201. doi: 10.1038/s41590-017-0030-x. [DOI] [PubMed] [Google Scholar]
- 14.Chmiel J.F., Berger M., Konstan M.W. The role of inflammation in the pathophysiology of CF lung disease. Clin. Rev. Allergy Immunol. 2002;23:5–27. doi: 10.1385/CRIAI:23:1:005. [DOI] [PubMed] [Google Scholar]
- 15.Konstan M.W., Hilliard K.A., Norvell T.M., Berger M. Bronchoalveolar lavage findings in cystic fibrosis patients with stable, clinically mild lung disease suggest ongoing infection and inflammation. Am. J. Respir. Crit. Care Med. 1994;150:448–454. doi: 10.1164/ajrccm.150.2.8049828. [DOI] [PubMed] [Google Scholar]
- 16.Bonfield T.L., Panuska J.R., Konstan M.W., Hilliard K.A., Hilliard J.B., Ghnaim H., Berger M. Inflammatory cytokines in cystic fibrosis lungs. Am. J. Respir. Crit. Care Med. 1995;152:2111–2118. doi: 10.1164/ajrccm.152.6.8520783. [DOI] [PubMed] [Google Scholar]
- 17.Hubeau C., Lorenzato M., Couetil J.P., Hubert D., Dusser D., Puchelle E., Gaillard D. Quantitative analysis of inflammatory cells infiltrating the cystic fibrosis airway mucosa. Clin. Exp. Immunol. 2001;124:69–76. doi: 10.1046/j.1365-2249.2001.01456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Regamey N., Tsartsali L., Hilliard T.N., Fuchs O., Tan H.L., Zhu J., Qiu Y.S., Alton E.W.F.W., Jeffery P.K., Bush A., Davies J.C. Distinct patterns of inflammation in the airway lumen and bronchial mucosa of children with cystic fibrosis. Thorax. 2012;67:164–170. doi: 10.1136/thoraxjnl-2011-200585. [DOI] [PubMed] [Google Scholar]
- 19.Thanabalasuriar A., Scott B.N.V., Peiseler M., Willson M.E., Zeng Z., Warrener P., Keller A.E., Surewaard B.G.J., Dozier E.A., Korhonen J.T., et al. Neutrophil extracellular traps confine Pseudomonas aeruginosa ocular biofilms and restrict brain invasion. Cell Host Microbe. 2019;25:526–536.e4. doi: 10.1016/j.chom.2019.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parkins M.D., Somayaji R., Waters V.J. Epidemiology, biology, and impact of clonal Pseudomonas aeruginosa infections in cystic fibrosis. Clin. Microbiol. Rev. 2018;31 doi: 10.1128/CMR.00019-18. e00019-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Camus L., Vandenesch F., Moreau K. From genotype to phenotype: adaptations of Pseudomonas aeruginosa to the cystic fibrosis environment. Microb. Genom. 2021;7 doi: 10.1099/mgen.0.000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Winstanley C., O'Brien S., Brockhurst M.A. Pseudomonas aeruginosa evolutionary adaptation and diversification in cystic fibrosis chronic lung infections. Trends Microbiol. 2016;24:327–337. doi: 10.1016/j.tim.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cramer N., Klockgether J., Wrasman K., Schmidt M., Davenport C.F., Tümmler B. Microevolution of the major common Pseudomonas aeruginosa clones C and PA14 in cystic fibrosis lungs. Environ. Microbiol. 2011;13:1690–1704. doi: 10.1111/j.1462-2920.2011.02483.x. [DOI] [PubMed] [Google Scholar]
- 24.Smith E.E., Buckley D.G., Wu Z., Saenphimmachak C., Hoffman L.R., D'Argenio D.A., Miller S.I., Ramsey B.W., Speert D.P., Moskowitz S.M., et al. Genetic adaptation by Pseudomonas aeruginosa to the airways of cystic fibrosis patients. Proc. Natl. Acad. Sci. USA. 2006;103:8487–8492. doi: 10.1073/pnas.0602138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marvig R.L., Johansen H.K., Molin S., Jelsbak L. Genome analysis of a transmissible lineage of Pseudomonas aeruginosa reveals pathoadaptive mutations and distinct evolutionary paths of hypermutators. PLoS Genet. 2013;9 doi: 10.1371/journal.pgen.1003741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moore M.P., Lamont I.L., Williams D., Paterson S., Kukavica-Ibrulj I., Tucker N.P., Kenna D.T.D., Turton J.F., Jeukens J., Freschi L., et al. Transmission, adaptation and geographical spread of the Pseudomonas aeruginosa Liverpool epidemic strain. Microb. Genom. 2021;7 doi: 10.1099/mgen.0.000511. mgen000511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klockgether J., Cramer N., Fischer S., Wiehlmann L., Tümmler B. Long-Term microevolution of Pseudomonas aeruginosa differs between mildly and severely affected cystic fibrosis lungs. Am. J. Respir. Cell Mol. Biol. 2018;59:246–256. doi: 10.1165/rcmb.2017-0356OC. [DOI] [PubMed] [Google Scholar]
- 28.Marvig R.L., Sommer L.M., Molin S., Johansen H.K. Convergent evolution and adaptation of Pseudomonas aeruginosa within patients with cystic fibrosis. Nat. Genet. 2015;47:57–64. doi: 10.1038/ng.3148. [DOI] [PubMed] [Google Scholar]
- 29.Cramer N., Wiehlmann L., Ciofu O., Tamm S., Høiby N., Tümmler B. Molecular epidemiology of chronic Pseudomonas aeruginosa airway infections in cystic fibrosis. PLoS One. 2012;7 doi: 10.1371/journal.pone.0050731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tümmler B., editor. Mutation-Specific Therapies in Cystic Fibrosis. Second edition. Uni-Med; 2022. [Google Scholar]
- 31.Cramer N., Fischer S., Hedtfeld S., Dorda M., Tümmler B. Intraclonal competitive fitness of longitudinal cystic fibrosis Pseudomonas aeruginosa airway isolates in liquid cultures. Environ. Microbiol. 2020;22:2536–2549. doi: 10.1111/1462-2920.14924. [DOI] [PubMed] [Google Scholar]
- 32.Ferrante A., Thong Y.H. Optimal conditions for simultaneous purification of mononuclear and polymorphonuclear leucocytes from human blood by the Hypaque-Ficoll method. J. Immunol. Methods. 1980;36:109–117. doi: 10.1016/0022-1759(80)90036-8. [DOI] [PubMed] [Google Scholar]
- 33.Frey D.L., Guerra M., Mall M.A., Schultz C. Monitoring neutrophil elastase and cathepsin G activity in human sputum samples. J. Vis. Exp. 2021 doi: 10.3791/62193. [DOI] [PubMed] [Google Scholar]
- 34.Fuchs T.A., Abed U., Goosmann C., Hurwitz R., Schulze I., Wahn V., Weinrauch Y., Brinkmann V., Zychlinsky A. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 2007;176:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alhede M., Alhede M., Qvortrup K., Kragh K.N., Jensen P.Ø., Stewart P.S., Bjarnsholt T. The origin of extracellular DNA in bacterial biofilm infections in vivo. Pathog. Dis. 2020;78 doi: 10.1093/femspd/ftaa018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Syldatk C., Lang S., Wagner F., Wray V., Witte L. Chemical and physical characterization of four interfacial-active rhamnolipids from Pseudomonas spec. DSM 2874 grown on n-alkanes. Z. Naturforsch. C Biosci. 1985;40:51–60. doi: 10.1515/znc-1985-1-212. [DOI] [PubMed] [Google Scholar]
- 37.Syldatk C., Lang S., Matulovic U., Wagner F. Production of four interfacial active rhamnolipids from n-alkanes or glycerol by resting cells of Pseudomonas species DSM 2874. Z. Naturforsch. C Biosci. 1985;40:61–67. doi: 10.1515/znc-1985-1-213. [DOI] [PubMed] [Google Scholar]
- 38.Hilker R., Munder A., Klockgether J., Losada P.M., Chouvarine P., Cramer N., Davenport C.F., Dethlefsen S., Fischer S., Peng H., et al. Interclonal gradient of virulence in the Pseudomonas aeruginosa pangenome from disease and environment. Environ. Microbiol. 2015;17:29–46. doi: 10.1111/1462-2920.12606. [DOI] [PubMed] [Google Scholar]
- 39.Berger M., Sorensen R.U., Tosi M.F., Dearborn D.G., Döring G. Complement receptor expression on neutrophils at an inflammatory site, the Pseudomonas-infected lung in cystic fibrosis. J. Clin. Invest. 1989;84:1302–1313. doi: 10.1172/JCI114298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tosi M.F., Zakem H., Berger M. Neutrophil elastase cleaves C3bi on opsonized pseudomonas as well as CR1 on neutrophils to create a functionally important opsonin receptor mismatch. J. Clin. Invest. 1990;86:300–308. doi: 10.1172/JCI114699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cramer N., Nawrot M.L., Wege L., Dorda M., Sommer C., Danov O., Wronski S., Braun A., Jonigk D., Fischer S., et al. Competitive fitness of Pseudomonas aeruginosa isolates in human and murine precision-cut lung slices. Front. Cell. Infect. Microbiol. 2022;12 doi: 10.3389/fcimb.2022.992214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ailloud F., Estibariz I., Suerbaum S. Evolved to vary: genome and epigenome variation in the human pathogen Helicobacter pylori. FEMS Microbiol. Rev. 2021;45 doi: 10.1093/femsre/fuaa042. [DOI] [PubMed] [Google Scholar]
- 43.Bartell J.A., Sommer L.M., Haagensen J.A.J., Loch A., Espinosa R., Molin S., Johansen H.K. Evolutionary highways to persistent bacterial infection. Nat. Commun. 2019;10:629. doi: 10.1038/s41467-019-08504-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rossi E., La Rosa R., Bartell J.A., Marvig R.L., Haagensen J.A.J., Sommer L.M., Molin S., Johansen H.K. Pseudomonas aeruginosa adaptation and evolution in patients with cystic fibrosis. Nat. Rev. Microbiol. 2021;19:331–342. doi: 10.1038/s41579-020-00477-5. [DOI] [PubMed] [Google Scholar]
- 45.Pust M.M., Davenport C.F., Wiehlmann L., Tümmler B. Direct RNA nanopore sequencing of Pseudomonas aeruginosa clone C transcriptomes. J. Bacteriol. 2022;204 doi: 10.1128/JB.00418-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thöming J.G., Häussler S. Transcriptional profiling of Pseudomonas aeruginosa infections. Adv. Exp. Med. Biol. 2022;1386:303–323. doi: 10.1007/978-3-031-08491-1_11. [DOI] [PubMed] [Google Scholar]
- 47.Averna M., Melotti P., Sorio C. Revisiting the role of leukocytes in cystic fibrosis. Cells. 2021;10 doi: 10.3390/cells10123380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dörk T., Kälin N., Stuhrmann M., Schmidtke J., Tümmler B. A termination mutation (2143delT) in the CFTR gene of German cystic fibrosis patients. Hum. Genet. 1992;90:279–284. doi: 10.1007/BF00220079. [DOI] [PubMed] [Google Scholar]
- 49.Tümmler B., Storrs T., Dziadek V., Dörk T., Meitinger T., Golla A., Bertele-Harms R.M., Harms H.K., Schröder E., Claass A., et al. Geographic distribution and origin of CFTR mutations in Germany. Hum. Genet. 1996;97:727–731. doi: 10.1007/BF02346181. [DOI] [PubMed] [Google Scholar]
- 50.Wiehlmann L., Wagner G., Cramer N., Siebert B., Gudowius P., Morales G., Köhler T., van Delden C., Weinel C., Slickers P., Tümmler B. Population structure of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA. 2007;104:8101–8106. doi: 10.1073/pnas.0609213104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hauser G., Karnovsky M.L. Rhamnose and rhamnolipide biosynthesis by Pseudomonas aeruginosa. J. Biol. Chem. 1957;224:91–105. [PubMed] [Google Scholar]
- 52.Döring G., Maier M., Müller E., Bibi Z., Tümmler B., Kharazmi A. Virulence factors of Pseudomonas aeruginosa. Antibiot. Chemother. 1987;39:136–148. doi: 10.1159/000414341. [DOI] [PubMed] [Google Scholar]
- 53.Kownatzki R., Tümmler B., Döring G. Rhamnolipid of Pseudomonas aeruginosa in sputum of cystic fibrosis patients. Lancet. 1987;1:1026–1027. doi: 10.1016/s0140-6736(87)92286-0. [DOI] [PubMed] [Google Scholar]
- 54.Gehrig S., Mall M.A., Schultz C. Spatially resolved monitoring of neutrophil elastase activity with ratiometric fluorescent reporters. Angew. Chem. Int. Ed. Engl. 2012;51:6258–6261. doi: 10.1002/anie.201109226. [DOI] [PubMed] [Google Scholar]
- 55.de Buhr N., Bonilla M.C., Pfeiffer J., Akhdar S., Schwennen C., Kahl B.C., Waldmann K.H., Valentin-Weigand P., Hennig-Pauka I., von Köckritz-Blickwede M. Degraded neutrophil extracellular traps promote the growth of Actinobacillus pleuropneumoniae. Cell Death Dis. 2019;10:657–664. doi: 10.1038/s41419-019-1895-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fingerhut L., Ohnesorge B., von Borstel M., Schumski A., Strutzberg-Minder K., Morgelin M., Deeg C.A., Haagsman H.P., Beineke A., von Köckritz-Blickwede M., et al. Neutrophil extracellular traps in the pathogenesis of equine recurrent uveitis (ERU) Cells. 2019;8 doi: 10.3390/cells8121528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Buhr N., von Köckritz-Blickwede M. Detection, visualization, and quantification of neutrophil extracellular traps (NETs) and NET markers. Methods Mol. Biol. 2020;2087:425–442. doi: 10.1007/978-1-0716-0154-9_25. [DOI] [PubMed] [Google Scholar]
- 58.de Buhr N., Neumann A., Jerjomiceva N., von Köckritz-Blickwede M., Baums C.G. Streptococcus suis DNase SsnA contributes to degradation of neutrophil extracellular traps (NETs) and evasion of NET-mediated antimicrobial activity. Microbiology (Read.) 2014;160:385–395. doi: 10.1099/mic.0.072199-0. [DOI] [PubMed] [Google Scholar]
- 59.Masuda S., Nakazawa D., Shida H., Miyoshi A., Kusunoki Y., Tomaru U., Ishizu A. NETosis markers: quest for specific, objective, and quantitative markers. Clin. Chim. Acta. 2016;459:89–93. doi: 10.1016/j.cca.2016.05.029. [DOI] [PubMed] [Google Scholar]
- 60.Xia M., Xu F., Ni H., Wang Q., Zhang R., Lou Y., Zhou J. Neutrophil activation and NETosis are the predominant drivers of airway inflammation in an OVA/CFA/LPS induced murine model. Respir. Res. 2022;23:289. doi: 10.1186/s12931-022-02209-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Melero I., Villalba-Esparza M., Recalde-Zamacona B., Jiménez-Sánchez D., Teijeira Á., Argueta A., García-Tobar L., Álvarez-Gigli L., Sainz C., Garcia-Ros D., et al. Neutrophil extracellular traps, local IL-8 expression, and cytotoxic T-lymphocyte response in the lungs of patients with fatal COVID-19. Chest. 2022;162:1006–1016. doi: 10.1016/j.chest.2022.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Calo G., Trevani A.S., Grasso E., Keitelman I.A., Ramhorst R., Leirós C.P., Sabbione F. In vitro identification and isolation of human neutrophil extracellular traps. Methods Mol. Biol. 2021;2255:97–117. doi: 10.1007/978-1-0716-1162-3_10. [DOI] [PubMed] [Google Scholar]
- 63.Roth J. Post-embedding cytochemistry with gold-labelled reagents: a review. J. Microsc. 1986;143:125–137. doi: 10.1111/j.1365-2818.1986.tb02771.x. [DOI] [PubMed] [Google Scholar]
- 64.Fischer S., Klockgether J., Morán Losada P., Chouvarine P., Cramer N., Davenport C.F., Dethlefsen S., Dorda M., Goesmann A., Hilker R., et al. Intraclonal genome diversity of the major Pseudomonas aeruginosa clones C and PA14. Environ. Microbiol. Rep. 2016;8:227–234. doi: 10.1111/1758-2229.12372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee D.G., Urbach J.M., Wu G., Liberati N.T., Feinbaum R.L., Miyata S., Diggins L.T., He J., Saucier M., Déziel E., et al. Genomic analysis reveals that Pseudomonas aeruginosa virulence is combinatorial. Genome Biol. 2006;7:R90. doi: 10.1186/gb-2006-7-10-r90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li H., Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
The datasets generated and/or analyzed during the current study are available as supplemental information (Tables) or have been deposited at Mendeley. All data are publicly available as of the date of publication. The link to the Mendeley data is listed in the key resources table.
-
•
All original data have been deposited at Mendeley and are publicly available as of the date of publication. The link is listed in the key resources table.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.