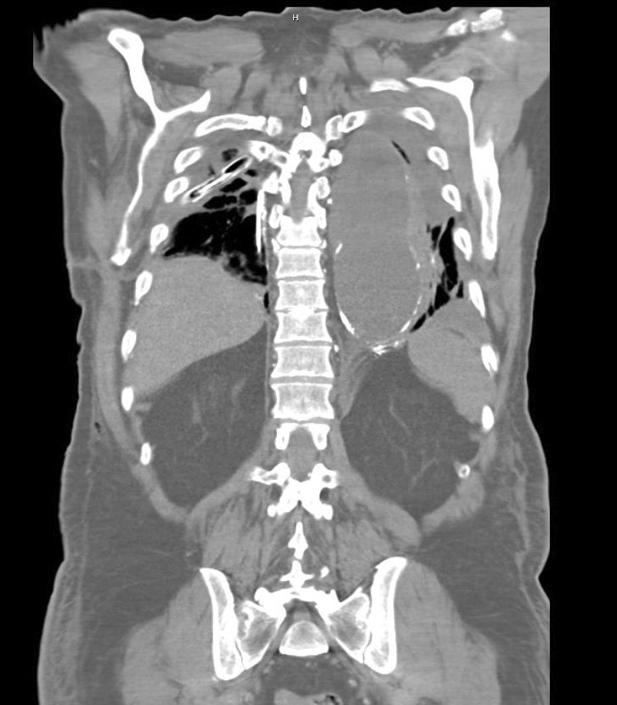
Lipiodol extravasation into left pleural space after unsuccessful embolization.
Central Message.
Microwave ablation (MWA) may serve as a potential therapeutic strategy for refractory chylothorax. Hence, further research is warranted.
Postoperative chylothorax after esophagectomy is a rare but potentially life-threatening complication.1 Without treatment, postoperative chylothorax is associated with a 5-fold increase in mortality.1 Although both conservative and surgical strategies exist, reoperation and surgical thoracic duct ligation may be necessary if conservative measures fail. Overall rates of successful resolution of chylothorax are highly variable and limited by technical difficulty.1
Interventional radiology (IR) approaches such as embolization have emerged as therapeutic methods for chylothorax. However, ablative techniques such as radiofrequency ablation or microwave ablation (MWA) have not yet been employed clinically for the treatment of chyle leak.2, 3, 4 Here, we report a case of refractory chylothorax after esophagectomy despite numerous interventions resulting from anatomic aberrancy with successful resolution following a combination of approaches, including MWA.
Case Report
A 66-year-old man with recurrent esophageal stricture requiring frequent dilations was referred for consideration of esophagectomy. After discussion with the patient, surgical resection was elected, and the patient underwent an Ivor Lewis esophagectomy. Intraoperatively, the thoracic duct was identified just above the diaphragm and mass ligated using heavy silk suture. A jejunal feeding tube and right pleural drain were placed. On postoperative day 3, jejunal trickle feeds were started with subsequent increased milky output from the patient's drain, which had elevated triglyceride levels. Enteral feeds were discontinued, and the patient underwent attempted thoracic duct embolization by IR, but the cisterna chyli was not visualized despite antegrade and retrograde approaches. Parenteral nutrition, fluid replacement, and octreotide were initiated, but high-output drainage (>1000 mL) persisted.
A second attempt at IR embolization was performed, which demonstrated numerous lymphatic collaterals along the vertebral column and a diminutive cisterna chyli but no identifiable targets for embolization. However, lipiodol extravasation into the left pleural space was noted (Figure 1). Thus, a left chest tube was placed. The patient was taken back to the operating room for left video-assisted thoracoscopic surgery for mechanical and talc pleurodesis. However, the chyle leak persisted. Sodium tetradecyl sulfate sclerosis and N-butyl cyanoacrylate glue embolization of the thoracic duct was performed by IR, during which multiple collateral channels were visualized supplying the chylous leak. Due to continued high chest tube output, the patient was taken to the operating room for exploratory laparotomy and supradiaphragmatic mass ligation of the thoracic duct performed using a 0 Ethibond suture (Ethicon), 2 to 3 cm inferior to the previous ligation site, and reinforced with large endoclips. A right-sided transhiatal chest tube was also placed. Chest tube output dropped initially, but left chest tube output rose to nearly 1 L the next day.
Figure 1.
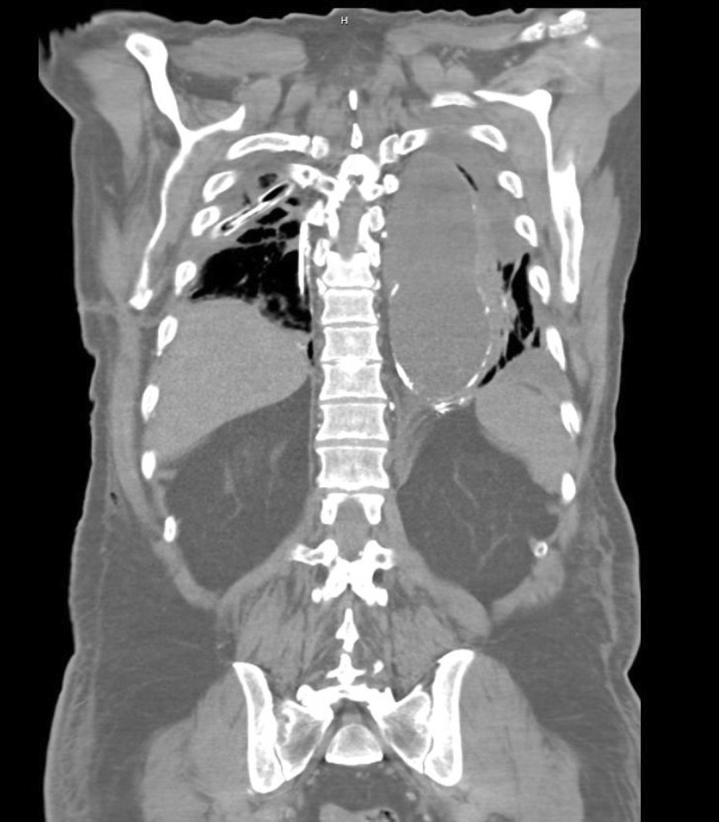
Computed tomography of the chest showing lipiodol extravasation into the left pleural space after unsuccessful interventional radiology embolization.
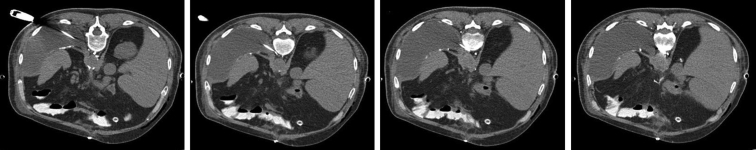
Because of concern for ongoing chyle leak, the patient underwent MWA of the thoracic duct. MWA was performed under computed tomography fluoroscopic guidance with the patient in the prone position. A NeuWave PR 17-guage needle (NeuWave Medical) was used for percutaneous retroperitoneal access, and 3 discrete areas of residual lipiodol from the previous lymphangiogram were targeted between levels T10 and T12 (Figure 2). MWA was performed at 65 watts for 1 to 2 minutes at each site, followed by slow needle retraction under cautery mode to ensure complete tract ablation. Immediately following the procedure, chest tube output ceased. The patient was slowly transitioned to oral intake with subsequent chest tube removal and discharged home after a prolonged stay.
Figure 2.
Serial images of microwave ablation performed under computed tomography fluoroscopic guidance targeting areas of previous lipiodol extravasation via the left retroperitoneal access.
Institutional review board approval was not required; the patient provided written informed consent.
Discussion
Variations in lymphatic anatomy, such as the highly collateralized branches described herein, are common and can lead to postoperative chylothorax despite prophylactic thoracic duct ligation.1 While chylothorax following other thoracic procedures may result from disruption of small lymphatic vessels, high-volume chyle leaks after esophagectomy often result from thoracic duct disruption and seldom resolve with conservative measures alone.1 Therefore, early aggressive interventions should be considered in these patients.
After confirming a chylothorax, lymphangiography is often favored over standard cross-sectional imaging because of the ability to accurately identify the site of lymphatic disruption. Expeditious IR intervention by an experienced IR attending is paramount. Although ablative IR techniques are frequently used for other conditions, reports using lymphatic ablation are limited.2,3 In this case, the decision to use MWA instead of radiofrequency ablation was based on its more predictable ablation zone to avoid injury to adjacent structures and ability to treat multiple lymphatic channels simultaneously.2,4
There are several learning points highlighted in this case. Although prophylactic thoracic duct ligation is often performed, the efficacy and potential consequences of this maneuver remain uncertain.5 Although the patient's persistent high output drainage immediately ceased following MWA, there is a possibility that our patient's chyle leak would have diminished and resolved over time without MWA intervention. Thus, further studies are needed to confirm the utility of this approach.
Footnotes
Disclosures: The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
References
- 1.Shah R.D., Luketich J.D., Schuchert M.J., Christie N.A., Pennathur A., Landreneau R.J., et al. Postesophagectomy chylothorax: incidence, risk factors, and outcomes. Ann Thorac Surg. 2012;93:897–903. doi: 10.1016/j.athoracsur.2011.10.060. discussion 903-894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGregor H., Weise L., Brunson C., Struycken L., Woodhead G., Celdran D. Percutaneous radiofrequency ablation to occlude the thoracic duct: preclinical studies in swine for a potential alternative to embolization. J Vasc Interv Radiol. 2022;33:1192–1198. doi: 10.1016/j.jvir.2022.05.007. [DOI] [PubMed] [Google Scholar]
- 3.Kim S.W., Kavanagh K., Orbach D.B., Alomari A.I., Mulliken J.B., Rahbar R. Long-term outcome of radiofrequency ablation for intraoral microcystic lymphatic malformation. Arch Otolaryngol Head Neck Surg. 2011;137:1247–1250. doi: 10.1001/archoto.2011.199. [DOI] [PubMed] [Google Scholar]
- 4.Brace C.L. Microwave tissue ablation: biophysics, technology, and applications. Crit Rev Biomed Eng. 2010;38:65–78. doi: 10.1615/critrevbiomedeng.v38.i1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crucitti P., Mangiameli G., Petitti T., Condoluci A., Rocco R., Gallo I.F., et al. Does prophylactic ligation of the thoracic duct reduce chylothorax rates in patients undergoing oesophagectomy? A systematic review and meta-analysis. Eur J Cardio Thorac Surg. 2016;50:1019–1024. doi: 10.1093/ejcts/ezw125. [DOI] [PubMed] [Google Scholar]