Abstract
Antagonists of the serotonin receptor 2B (5-HT2B) have shown great promise as therapeutics for the treatment of pulmonary arterial hypertension, valvular heart disease, and related cardiopathies. Herein, we describe a high-throughput screen campaign that led to the identification of highly potent and selective 5-HT2B antagonists. Furthermore, selected compounds were profiled for their predicted ability to cross the blood–brain barrier. Two exemplary compounds, VU0530244 and VU0631019, were predicted to have very limited potential for brain penetration in human subjects, a critical profile for the development of 5-HT2B antagonists devoid of centrally-mediated adverse effects.
Keywords: serotonin; 5-HT2B, pulmonary arterial hypertension; high-throughput screen
INTRODUCTION
The serotonin receptor 2B (5-HT2B) has long been considered an “antitarget” in medicinal chemistry1; compounds that activate this G protein-coupled receptor are known to cause serious and sometimes fatal side effects in animal models and human subjects alike.2–4 Consequently, a routine practice in medicinal chemistry programs is to screen compounds of interest against 5-HT2B, and molecules displaying any activity at the receptor are often rapidly deprioritized.5,6
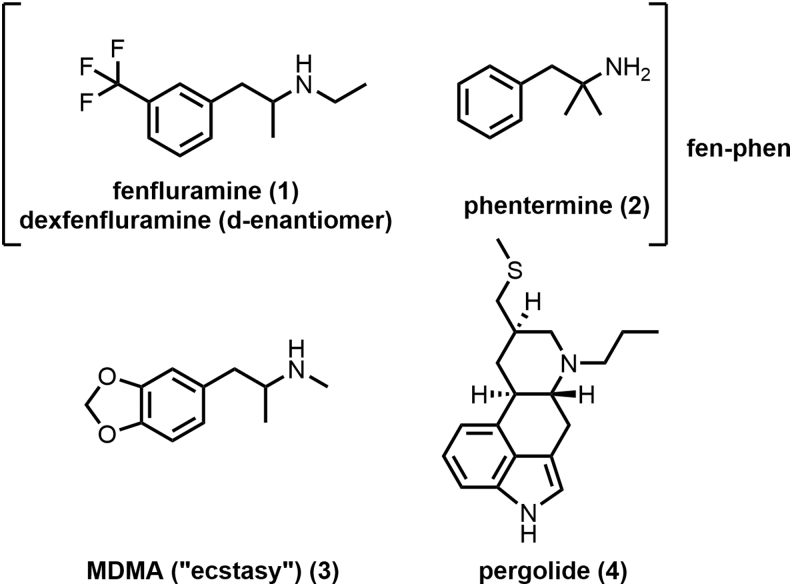
That this type of screening is now commonplace in drug discovery is due in no small part to the postclinical failure of fen-phen (fenfluramine/phentermine; Fig. 1), a previously approved anti-obesity regimen that was withdrawn from the market due to the observed development of fatal pulmonary arterial hypertension (PAH) and heart valve abnormalities in patients. The resulting legal fallout from the drug's withdrawal (with awarded damages exceeding $20 billion) led to widespread interest regarding the mechanism of action involved in the observed side effects.7
Fig. 1.
Fen-phen and additional 5-HT2B agonists. 5-HT2B, serotonin receptor 2B; fen-phen, fenfluramine/phentermine.
Multiple studies have since confirmed the excessive activation of 5-HT2B to be a likely culprit; specifically the N-des-ethyl metabolite of fenfluramine (1) and dexfenfluramine has been shown to be a potent 5-HT2B agonist.3,4 Numerous follow-up studies8–10 have since confirmed the link between 5-HT2B activation and PAH (and related disorders), and many such agonists, including 3,4-methylenedioxymethamphetamine (3)11 and pergolide (4),12 have recapitulated the deleterious effects of fen-phen in animal models and human subjects (Fig. 1).
Conversely, the development of 5-HT2B antagonists has shown great promise for the treatment of PAH and related disorders, including vessel wall stiffness and pulmonary microvasculature cell contractility.8,9,13 Although there are currently several FDA-approved drugs for the treatment of PAH, all of these treatments are characterized by high cost, low tolerability, and mechanisms of action that are not disease-modifying.
Because PAH is both progressive and often lethal (characterized by pulmonary vascular remodeling and right heart failure), there still exists a great unmet need to develop novel and disease-modifying treatment strategies.14–17 To this end, deficiencies in both 5-HT2B and the serotonin transporter (5-HTT) have been demonstrated to be protective and therapeutic in the context of hypoxic PAH in mice,4,18 and the 5-HT2B antagonist SB204741 prevents elevated right ventricular systolic pressure in mouse models of both hypoxia-induced PAH and Bmpr2 (heritable) PAH.8,9 In addition, 5-HT2B antagonism can reduce both hypoxia-induced and heritable PAH to normal levels, with treated mice ultimately failing to develop PAH.8,9
An important caveat in the development of 5-HT2B antagonists for the treatment of such disorders is that these compounds must be peripherally restricted (limited potential for permeation through the blood–brain barrier [BBB]). Centrally-mediated 5-HT2B antagonism is associated with a number of consequences in animal models, including impulsivity19 and impaired sleep.20 In addition, the presence of a 5-HT2B stop codon in specific human populations has been linked to severe impulsivity, including suicidal ideation.21 Toward the goal of developing peripherally restricted 5-HT2B antagonists, we undertook a high-throughput screen (HTS) utilizing compounds from the Vanderbilt Institute of Chemical Biology (VICB) Discovery Collection.
MATERIALS AND METHODS
Calcium Mobilization Assays
Calcium mobilization assays were conducted as previously described.22 The stable HEK293T cell line with TET-inducible 5-HT2B receptor expression was generously provided by Dr. Bryan Roth (University of North Carolina). Cells were dispensed as follows: 20,000 cells in 20 μL per well in black 384-well amine coated clear bottom plates (Corning) in Dulbecco's Modified Eagle's Medium (Life Technologies) with 1% fetal bovine serum (Dialyzed), 20 mM N-2-hydroxyethylpiperazine-N-2-ethane sulfonic acid (HEPES), 1% penicillin/streptomycin and 2 μg/mL tetracycline using a Multidrop Combi dispenser (Thermo). Cells were incubated at 37°C under 5% CO2 and used for screening between 18 and 22 h (not exceeding 24 h).
For screening, medium was removed and replaced with 1 μM Calbryte520 AM (AAT Bioquest®, Sunnyvale, CA, USA) in assay buffer (Hank's Balanced Salt Solution supplemented with 20 mM HEPES) mixed with 10% pluronic acid (Life Technologies) using a BioTek ELx405 plate washer, and plates were incubated 60 min at 37°C under 5% CO2. Dye was then removed and replaced with 20 μL of fresh assay buffer and equilibrated to ambient temperature for 10 min before loading into the Panoptic (Wavefront Biosciences, Franklin TN, USA) kinetic imaging plate reader.
Compound libraries at a stock of 10 mM in dimethyl sulfoxide (DMSO) were transferred to compound plates using the ECHO OMICS (Labcyte), and diluted to 2 × of the final assay concentration. The compound plate, the dye-loaded cell plate, and plate containing EC80 serotonin (5-HT) concentrations were placed in the Panoptic kinetic imaging plate reader equipped to measure Ex482/35 Em536/40 fluorescence. Data were collected at 1 frame/s. After 14 s of collecting baseline fluorescence, 20 μL of the compound solutions were added to the cell plate for 133 s.
This was followed by the addition of an EC80 concentration of 5-HT along with a maximally effective 5-HT concentration in wells not containing a compound to allow data normalization. The fluorescence signal was collected for a total of 290 s. Each screening set started with an initial plate with a full dose response of 5-HT ranging from 5 μM to 80 pM to measure the suitable 5-HT EC80 for the given screen day and to monitor the consistency of expression and activity of 5-HT2B. Compound concentration response curves (CRCs) were collected in triplicate within each plate.
Data were imported and analyzed using custom-built Vanderbilt HTS software (Waveguide) and exported for plotting data in GraphPad Prism. First, each compound and control well's data were normalized to the initial baseline fluorescence. Then, each well value was calculated as maximal values within the 5-HT stimulation time window (145–290 s) subtracting the prestimulation timeframe minimum (135–140 s). Normalized values were then calculated as a percentage of maximal 5-HT response for each screen plate.
Likewise, the activity for compound stimulation alone was calculated as maximal values within the compound stimulation time window (10–125 s) subtracting the prestimulation timeframe minimum (1–5 s) with a subset for putative agonist peak (15–60 s). For CRCs, this percentage of maximum response was plotted against log[compound] and fit to a four parameter logistical equation to determine log(IC50) (see Table 1 for further details).
Table 1.
Protocols for High-Throughput Screen Calcium Mobilization Assay
| Step | Parameter | Value | Description |
|---|---|---|---|
| 1 | Plate cells | 20 mL | Stable HEK293T cell line with TET-inducible 5-HT2B receptor |
| 2 | Calcium indicator dye | 20 mL | Exchange media for 1 μM Calbryte520 AM in assay buffer |
| 3 | Incubation | 60 m | 37°C |
| 4 | Assay buffer | 20 mL | Remove dye and replace with HBSS +20 mM HEPES |
| 5 | Library compounds | 20 mL | 10 mM in DMSO stocks diluted to 2 × (20 mM) for live imaging dispense |
| 6 | Control | 20 mL | 10 mM in DMSO stock SB204741 (VU0254140) diluted fresh to 2 × (20 mM) |
| 7 | 5-HT | 10 mL | EC80 5-HT or Emax 5-HT |
| 8 | Incubation | 10 m | ambient equilibration period |
| 9 | Panoptic reader | 482ex/536em | Live kinetic imaging |
| Step | Notes | ||
|---|---|---|---|
| 1 |
|
|
Corning 384 well amine coated plates, plated with Multidrop Combi |
| 2 |
|
|
Exchange from media to assay buffer using ELX405 plate washer |
| 3 |
|
|
Lidded plates in incubator at 37°C and 5%CO2 |
| 4 |
|
|
Exchange from dye to assay buffer using ELX405 plate washer |
| 5 |
|
|
Create aliquot from DMSO stock library into aqueous 2 × working solution using ECHO OMICS |
| 6 |
|
|
Create aliquot from DMSO stock into aqueous 2 × working solution using ECHO OMICS |
| 7 |
|
|
Create aliquot from DMSO stock into aqueous 5 × working solution using ECHO OMICS |
| 8 |
|
|
Unlidded plate |
| 9 | 2 × plate with compounds, controls; 5 × plate with 5-HT; and cell plate load into Panoptic |
5-HT2B, serotonin receptor 2B; DMSO, dimethyl sulfoxide; HBSS, Hanks' Balanced Salt Solution; HEPES, N-2-hydroxyethylpiperazine-N-2-ethane sulfonic acid; TET, tetracycline.
Radioligand Binding Assays
Radioligand binding assays for 5-HT2A, 5-HT2B, and 5-HT2C were performed through contract by Eurofins Discovery (Taiwan) in human recombinant CHO-K1 cells with 0.5 nM [3H]ketanserin, 1.2 nM [3H]lysergic acid diethylamide (LSD), and 1.0 nM [3H]mesulergine as the respective specific ligands in accordance with their established standard assay methodology. 5-HT2B IC50s were generated across five concentration points (0.1 nM, 1 nM, 10 nM, 0.1 μM, and 10 μM), and 5-HT2A/2C counterscreening was assessed as % inhibition at a single-point concentration of 10 μM.
P-Glycoprotein Efflux
Initial determination of test compounds' potential for efflux by human P-glycoprotein (P-gp) at a single-point concentration (5 μM; in duplicate) was performed through contract by Absorption Systems (Exton, PA, USA) using a bidirectional permeability assay with MDCK-MDR1 cells in accordance with their established standard assay methodology.
Compounds
VU0530244, VU0544894, and VU0631019 were purchased from Life Chemicals, Inc. (Ontario, Canada) and assigned Vanderbilt-specific ID numbers for purposes of chemical inventory (see Supplementary Data ). Compounds were determined to have >95% purity by liquid chromatography-mass-spectrometry analysis at 215 nm, and structures were confirmed by proton nuclear magnetic resonance analysis. Low-resolution mass spectra were obtained on a Waters QDa (Performance) SQ mass spectrometer (MS) with ESI source. MS parameters were as follows: cone voltage: 15 V, capillary voltage: 0.8 kV, probe temperature: 600°C. Samples were introduced through an Acquity I-Class PLUS UPLC comprising a binary solvent manager, fixed loop sample manager, column compartment, and photodiode array detector. UV absorption was generally observed at 215 and 254 nm; 4 nm bandwidth.
Column: Phenomenex EVO C18, 1.0 × 50 mm, 1.7 μm. Column temperature: 55°C. Flow rate: 0.4 mL/min. Default gradient: 5%–95% CH3CN (0.05% trifluoroacetic acid [TFA]) in H2O (0.05% TFA) over 1.4 min, hold at 95% CH3CN for 0.1 min. Nuclear magnetic resonance spectra were recorded on a 400 MHz Brüker AV-400 instrument. 1H chemical shifts are reported as δ values in ppm relative to the residual solvent peak (CDCl3 = 7.26, DMSO = 2.50). Data are reported as follows: chemical shift, multiplicity (br = broad, s = singlet, d = doublet, t = triplet, q = quartet, p = pentet, dd = doublet of doublets, ddd = doublet of doublet of doublets, td = triplet of doublets, dt = doublet of triplets, m = multiplet), coupling constant, and integration.
RESULTS AND DISCUSSION
Preparation for screening was first conducted using a series of validation experiments to demonstrate feasibility for HTS. Tet-induced 5-HT2B HEK293T cells were tested at multiple tetracycline concentrations and incubation times for optimal 5-HT2B expression and response in the calcium mobilization assay, finding 2 μg/mL and 18–24 h to be optimal. Cells were then stimulated with a dose response of 5-HT with or without tetracycline preincubation, which indicated that increasing 5-HT concentration stimulated calcium response only with induced expression of 5-HT2B (see Supplementary Fig. S1A).
Cells were tested in optimal conditions for evaluating HTS standard metrics of 5-HT response with DMSO alone (neutral control) versus antagonist SB204741 (VU0254140, positive control) in a checkerboard pattern, for demonstrating HTS readiness (Z′ > 0.5, coefficients of variations <10%), in single-well formatted 384-well plates (Supplementary Fig. S1C). DMSO tolerance was tested and evaluated to be suitable for testing the small molecule library up to 1% v/v. The Selleck Chemical's FDA drug set library was used for a pilot screen to evaluate the ability to identify hits selected from single-point 10 μM screening and retest in our calcium mobilization assay.
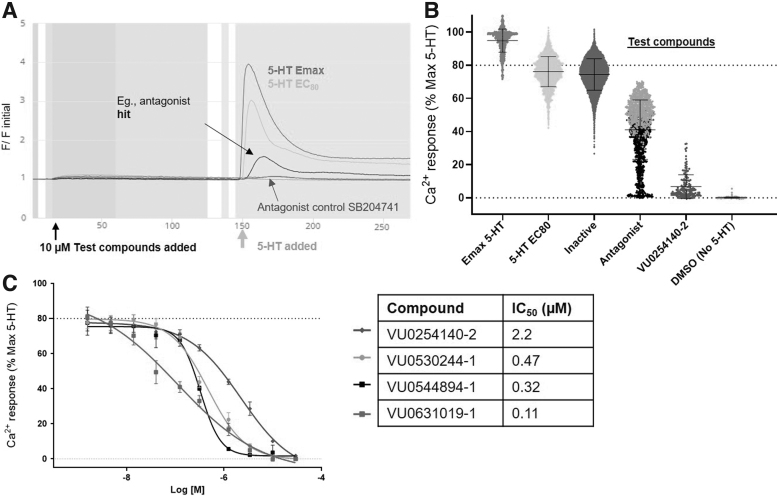
Identified hits were revealed as known 5-HT receptor antagonists, such as pizotifen malate. Overall, the pilot screen included 1,181 drugs, from which was found 14 putative agonists, 59 antagonists, and 6 undesignated hits (see Supplementary Fig. S1). After these validation experiments, the primary screen included 24,592 compounds from the VICB Discovery Collection at 10 μM single-point concentration. Compounds that significantly decreased the 5-HT EC80 responses were selected as hits when the measured response was 3 standard deviations or 3 median absolute deviations from the plate compound population mean or median response, respectively (Fig. 2).
Fig. 2.
Results of screening HEK-5-HT2B cells.. (A) Example traces from the calcium assay showing representative wells of a 384-well screen plate over time (s); test compound addition (14 s), 5-HT stimulation (147 s). Fluorescence values are normalized as a ratio of initial value at time 0 s (F/F initial). Control maximal response of serotonin (“5-HT Emax”), EC80 stimulation with 5-HT (“5-HT EC80”), example hit compound, antagonist control, SB204741 (VU0254140-2). (B) Screening results of 24,592 small molecules shown as individual well values in presence of EC80 5-HT, unless otherwise indicated. Values normalized to percentage of maximal 5-HT response. Putative antagonist hit candidates identified as values decreasing 3SD or 3MAD below the mean or median response per 384-well plate tested. Five hundred seventy-six hits have completed testing in duplicate and confirmed hits have undergone CRCs. Additional 631 antagonist hits have not been further tested. Additional hits not shown: putative agonists (206) identified as increasing calcium response 3SD or 3MAD above mean or median before stimulation with 5-HT as well as other undesignated hits or flagged as fluorescent (78) were identified. (C) Selected antagonist candidates measured in triplicate compared with control antagonist VU0254140-2; all activity shown in presence of EC80 5-HT and values normalized to maximal 5-HT response. Values are mean and SD (n = 3) modeled with four parameter logistic curve-fitting, least squares regression (GraphPad Prism v.9.2). CRCs, concentration response curves; MAD, median absolute deviation; SD, standard deviation.
Discovery of 1,207 putative antagonists and 206 agonist candidates were identified. In addition, 78 undesignated hits were found to respond independent of 5-HT, had increased 5-HT response, or were flagged as an active fluorescent compound. All screening plates were required to have an acceptable Z′, with overall mean Z′ 0.74 ± 0.04. Hit compounds were replated from a source tube, rather than cherry-picked from the library plate and retested in duplicate at 10 μM with 478 of 576 (83%) compounds retested positive for antagonist activity.
Compounds that reduced the 5-HT EC80 response but did not modify calcium response alone were triaged for further studies (n = 411). Candidate compounds were then profiled in 10-point CRCs in the calcium mobilization assay to calculate potencies (IC50 values calculated from the change in 5-HT EC80 responses). Thirty-six compounds had IC50 values <1 μM. Of these, 15 were reordered as dry powders from Life Chemicals (Ontario, Canada) and retested in counterscreens using 5-HT2A or 5-HT2C receptor expressed cell lines (Eurofins Discovery; see Materials and Methods section).
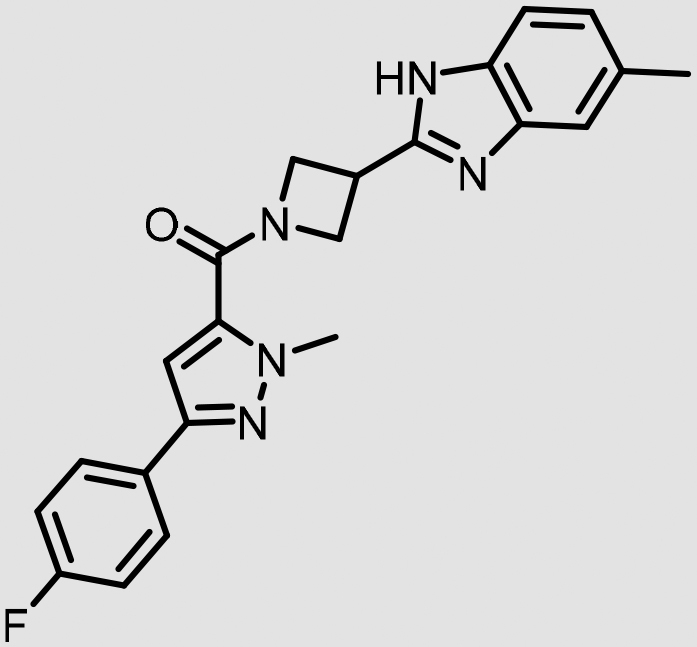
After the generation of CRCs for 315 of these antagonist hits, compounds with potencies ranging from 9.4 nM to >10 μM were ultimately identified, with many hits displaying 5-HT2B potencies in the low nanomolar range (Fig. 2C). From this set, VU0530244 (5), VU0544894 (6), and VU0631019 (7) (Table 2) were among the top potency compounds chosen for selectivity profiling relative to the serotonin receptor 2A and 2C subtypes (5-HT2A and 5-HT2C), as well as a preliminary assessment for predicted ability to cross the BBB and induce centrally-mediated antagonism (Table 2). Encouragingly, all three hits were found to be robustly selective for 5-HT2B relative to 5-HT2A and 5-HT2C in radioligand binding assays, and are among the most potent and selective 5-HT2B antagonists yet detailed in the drug discovery literature.
Table 2.
Structure, Serotonin Subtype Potency, and Predicted P-Glycoprotein Efflux for Hit Compounds VU0530244, VU0544894, and VU0631019
| Compound | Structure | 5-HT2B IC50 (nM)a | 5-HT2A % inh. (10 μM)b | 5-HT2C % inh. (10 μM)c | P-gp efflux ratio (PappA-B, 10–6 cm/s)d |
|---|---|---|---|---|---|
| VU0530244 (5) |
|
17.3 | <50% | <50% | 12.3 (5.4) |
| VU0544894 (6) |
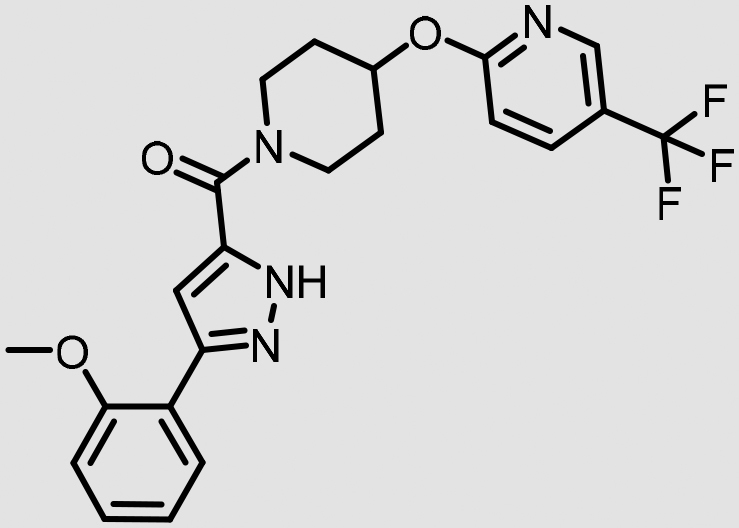
|
5.3 | <50% | <50% | 0.59 (6.7) |
| VU0631019 (7) |
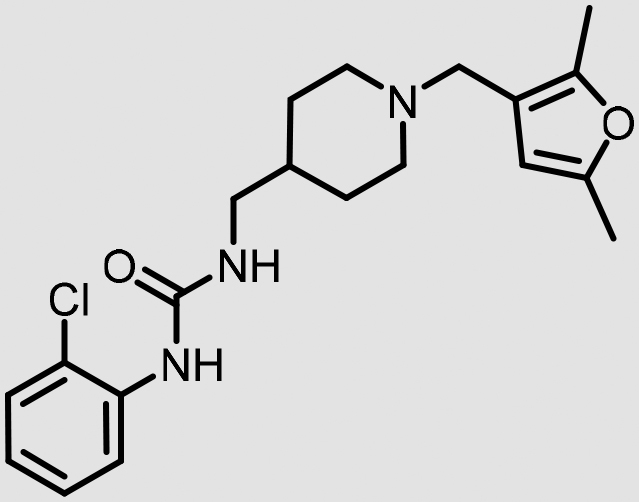
|
29 | <50% | <50% | 25 (2.6) |
Radioligand binding assay with 1.20 nM [3H]lysergic acid diethylamide (LSD) and 10 μM serotonin (5-HT) as the nonspecific ligand across five concentration points (0.1 nM, 1 nM, 10 nM, 0.1 μM, 10 μM).
Radioligand binding assay with 0.5 nM [3H]ketanserin and 1.0 μM mianserin as the nonspecific ligand at single-point 10 μM concentration.
Radioligand binding assay with 1.0 nM [3H]mesulergine and 1.0 μM mianserin as the nonspecific ligand at single-point 10 μM concentration (Eurofins Discovery).
BBB penetration potential using Madin Darby canine kidney cells with overexpression of MDR1 gene (encoding for P-gp) in cell monolayers; ER is defined as Papp(B-to-A)/Papp(A-to-B); see Materials and Methods section for further details.
BBB, blood–brain barrier; ER, efflux ratio.
In addition, VU0530244 and VU0631019 are predicted to have very limited potential for brain penetration in human subjects, as both are predicted to be substrates for P-gp-mediated efflux in humans (Table 2). A strong correlation exists between the measured efflux ratios and membrane permeability in such assays with the observed in vivo brain to plasma exposure.23 Although some structural similarity is noted between VU0530244 and VU0544894, the latter is predicted to be highly brain-penetrant; consequently, VU0544894 may find use as a tool to study the effects of centrally mediated 5-HT2B antagonism. Follow-up studies to rigorously characterize the potential for central penetration will be necessary to further de-risk these and related analogs for clinical development.
CONCLUSIONS
In summary, we have conducted a successful HTS campaign for the purpose of detailing new chemical matter for the development of potent, selective, and peripherally restricted 5-HT2B antagonists. From the list of most potent antagonists after full CRC profiling, three compounds are detailed in this article. It is our hope that the hit compounds described herein will prove useful as starting scaffolds for medicinal chemists interested not only in the development of selective 5-HT2B tool compounds, but also for the development of next-generation and disease-modifying agents for PAH, valvular heart disease, and related disorders.
Supplementary Material
ACKNOWLEDGMENTS
We thank the Vanderbilt HTS Core (VHTSC) staff members Liangping Li, Dehui Mi, and Corbin Whitwell for their remarkable assistance.
Abbreviations Used
- 5-HT2B
serotonin receptor 2B
- BBB
blood–brain barrier
- DMSO
dimethyl sulfoxide
- ER
efflux ratio
- fen-phen
fenfluramine/phentermine
- HBSS
Hanks' Balanced Salt Solution
- HEPES
N-2-hydroxyethylpiperazine-N-2-ethane sulfonic acid
- HTS
high-throughput screen
- MAD
median absolute deviation
- MS
mass spectrometry
- PAH
pulmonary arterial hypertension
- P-gp
P-glycoprotein
- SD
standard deviation
- TFA
trifluoroacetic acid
- VICB
Vanderbilt Institute of Chemical Biology
AUTHORs' CONTRIBUTIONS
Data curation, formal analysis, investigation, project supervision, validation visualization, and writing—review and editing (medicinal chemistry) by A.M.B. Data curation, formal analysis, investigation, methodology, validation, and visualization (molecular pharmacology) by M.S.V. Data curation, formal analysis, investigation, methodology, project administration, supervision, validation, visualization, and writing—review and editing (HTS lead) by J.A.B. Data curation, formal analysis, methodology, validation, visualization, and writing—review and editing (HTS supporting) by E.D. Project administration and supervision by C.W.L and W.D.M.
DISCLOSURE STATEMENT
No competing financial interests exist.
FUNDING INFORMATION
This study was funded by a Discovery Grant from Vanderbilt University to W.D.M. and C.W.L. A.M.B. and C.W.L. thank the William K. Warren Family and Foundation for funding the William K. Warren, Jr. Chair in Medicine and support of our programs. The Vanderbilt HTS Core (VHTSC) receives support from the Vanderbilt Institute of Chemical Biology and the Vanderbilt Ingram Cancer Center (P30CA68485). The WaveFront Biosciences Panoptic kinetic imaging plate reader within the VHTSC was funded by NIH Shared Instrumentation Grant (S10OD021734). The FDA-approved library was provided by the Vanderbilt CTSA (UL1TR00044) and distributed by the VHTSC. J.A.B. is supported by an NCI award (R50CA211206). W.D.M. is supported by HL135790 from the NIH.
SUPPLEMENTARY MATERIAL
REFERENCES
- 1. Setola V, Roth BL. The Emergence of Serotonin 5-HT2B Receptors as DRUG Antitargets. In: Antitargets: Prediction and Prevention of Drug Side Effects. (Vaz RJ, Klabunde T. eds.) Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany; 2008. [Google Scholar]
- 2. Huang XP, Setola V, Yadav PM, et al. Parallel functional activity profiling reveals valvulopathogens are potent 5-hydroxytryptamine2b receptor agonists: Implications for Drug Safety Assessment. Mol Pharmacol 2009;76(4):710–722; doi: 10.1124/mol.109.058057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rothman RB, Baumann MH, Savage JE, et al. Evidence for possible involvement of 5-HT2B receptors in the cardiac valvulopathy associated with fenfluramine and other serotonergic medications. Circulation 2000;102:2836–2841; doi: 10.1161/01.CIR.102.23.2836 [DOI] [PubMed] [Google Scholar]
- 4. Launay JM, Hervé P, Peoc'h K, et al. Function of the serotonin 5-hydroxytryptamine 2B receptor in pulmonary hypertension. Nat Med 2002;8:1129–1135; doi: 10.1038/nm764 [DOI] [PubMed] [Google Scholar]
- 5. Porter MR, Xiao H, Wang J, et al. 3-Amino-chromanes and tetrahydroquinolines as selective 5-HT2B, 5-HT7, or σ1 receptor ligands. ACS Med Chem Lett 2019;10(10):1436–1442; doi: 10.1021/acsmedchemlett.9b00225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dosa PI, Ward T, Walters MA, et al. Synthesis of novel analogs of cabergoline: Improving cardiovascular safety by removing 5-HT2B receptor agonism. ACS Med Chem Lett 2013;4(2):254–258; doi: 10.1021/ml3003814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stola V, Roth BL. Screening the receptorome reveals molecular targets responsible for drug-induced side effects: Focus on ‘fen–phen’. Expert Opin Drug Metab Toxicol 2005;1(3);377–387; doi: DOI: 10.1517/17425255.1.3.377 [DOI] [PubMed] [Google Scholar]
- 8. West JD, Carrier EJ, Bloodworth NC, et al. Serotonin 2B receptor antagonism prevents heritable pulmonary arterial hypertension. PLoS One 2016;11(2):e0148657; doi: 10.1371/journal.pone.0148657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bloodworth NC, Clark CR, West JD, et al. Bone marrow–derived proangiogenic cells mediate pulmonary arteriole stiffening via serotonin 2B receptor dependent mechanism. Circ Res 2018;123:e51–e64; doi: 10.1161/CIRCRESAHA.118.313397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Launay JM, Hervé P, Callebert J, et al. Serotonin 5-HT2B receptors are required for bone-marrow contribution to pulmonary arterial hypertension. Blood 2012;119(7):1772–1780; doi: 10.1182/blood-2011-06-358374 [DOI] [PubMed] [Google Scholar]
- 11. Setola V, Hufeisen SJ, Grande-Allen J, et al. 3,4-Methylenedioxymethamphetamine (MDMA, “Ecstasy”) induces fenfluramine-like proliferative actions on human cardiac valvular interstitial cells in vitro. Mol Pharmacol 2003;63(6):1223–1229; doi: 10.1124/mol.63.6.1223 [DOI] [PubMed] [Google Scholar]
- 12. Schade R, Andersohn F, Suissa S, et al. Dopamine agonists and the risk of cardiac-valve regurgitation. N Eng J Med 2007;356;29–38; doi: 10.1056/NEJMoa062222 [DOI] [PubMed] [Google Scholar]
- 13. Bloodworth NC, West JD, Merryman WD. Microvessel mechanobiology in pulmonary arterial hypertension: Cause and effect. Hypertension 2015;65:483–489; doi: 10.1161/HYPERTENSIONAHA.114.04652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rabinovitch M. Molecular pathogenesis of pulmonary arterial hypertension. J Clin Invest 2008;118:2372–2379; doi: 10.1172/JCI60658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thenappan T, Ormiston ML, Ryan JJ, et al. Pulmonary arterial hypertension: pathogenesis and clinical management. BMJ 2018;360:j5492; doi: 10.1136/bmj.j5492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sutendra G, Michelakis ED. Pulmonary arterial hypertension: Challenges in translational research and a vision for change. Sci Transl Med 2013;5:208sr5–208sr5. [DOI] [PubMed] [Google Scholar]
- 17. Marc H, Olivier S, Gérald S. Treatment of pulmonary arterial hypertension. N Engl J Med 2004;12; doi: 10.1056/NEJMra040291 [DOI] [Google Scholar]
- 18. Eddahibi S, Hanoun N, Lanfumey L, et al. Attenuated hypoxic pulmonary hypertension in mice lacking the 5-hydroxytryptamine transporter gene. J Clin Invest 2000;105:1555–1562; doi: 10.1172/JCI8678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Popova NK, Tsybko AS, Naumenko VS. The implication of 5-HT receptor family members in aggression, depression and suicide: Similarity and difference. Int J Mol Sci 2022;23;8814; doi: 10.3390/ijms23158814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Popa D, Léna C, Fabre V, et al. Contribution of 5-HT2 receptor subtypes to sleep-wakefulness and respiratory control, and functional adaptations in knock-out mice lacking 5-HT2A receptors. J Neurosci 2005;25(49);11231–11238; doi: 10.1523/JNEUROSCI.1724-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bevilacqua L, Doly S, Kaprio J, et al. A population-specific HTR2B stop codon predisposes to severe impulsivity. Nature 2010;468;1061–1066; doi: 10.1038/nature09629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rodriguez AL, Grier MD, Jones CK, et al. Discovery of novel allosteric modulators of metabotropic glutamate receptor subtype 5 reveals chemical and functional diversity and in vivo activity in rat behavioral models of anxiolytic and antipsychotic activity. Mol Pharmacol 2010;78(6);1105–1123. doi: 10.1124/mol.110.067207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Feng B, Mills JB, Davidson RE, et al. In vitro P-glycoprotein assays to predict the in vivo interactions of P-glycoprotein with drugs in the central nervous system. Drug Metab Dispos 2008;36(2):268–275; doi: 10.1124/dmd.107.017434 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.