Abstract
Background:
Thyroid hormone (triiodothyronine [T3]) is essential for development and organ metabolism in all vertebrates. T3 has both genomic and nongenomic effects on target cells. While much has been learnt on its genomic effects via T3 receptors (TRs) in vertebrate development, mostly through TR-knockout and TR-knockin studies, little is known about the effects of T3 on gene expression in animals in the absence of TR. We have been studying Xenopus metamorphosis as a model for mammalian postembryonic development, a period around birth when plasma T3 level peaks and many organs/tissues mature into their adult forms. We have recently generated TR double knockout (TRDKO) Xenopus tropicalis animals. This offers an opportunity to compare the effects of T3 on global gene expression in tadpole tissues in the presence or absence of TR.
Methods:
We analyzed the effects of T3 on gene expression in tadpole tail and intestine by using RNA-seq analysis on wild-type and TRDKO tadpoles with or without T3 treatment.
Results:
We observed that removing TRs reduced the number of genes regulated by T3 in both organs. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses revealed that T3 affected distinct biological processes and pathways in wild-type and TRDKO tadpoles. Many GO terms and KEGG pathways that were enriched among genes regulated in wild-type tissues are likely involved in mediating the effects of T3 on metamorphosis, for example, those related to development, stem cells, apoptosis, and cell cycle/cell proliferation. However, such GO terms and pathways were not enriched among T3-regulated genes in TRDKO tadpoles. Instead, in TRDKO tadpoles, GO terms and pathways related to “metabolism” and “immune response” were highly enriched among T3-regulated genes. We further observed strong divergence in the TR-independent nongenomic effects of T3 in the intestine and tail.
Conclusions:
Our data suggest that T3 has distinct and organ-dependent effects on gene expression in developing tadpoles. The TR-mediated effects are consistent with the metamorphic changes, in agreement with the fact that TR is necessary and sufficient to mediate the effects of T3 on metamorphosis. T3 appears to have a major effect on metabolism and immune response via TR-independent nongenomic processes.
Keywords: genomic, intestine, metabolism, metamorphosis, nongenomic, RNA-seq, tail, thyroid hormone receptors, Xenopus tropicalis
Introduction
Thyroid hormone (triiodothyronine [T3]) is essential for normal development as well as adult organ metabolism in vertebrates.1,2 In human, plasma T3 level peaks in the several months around birth when many organs/tissues mature into their adult forms, a period referred to as postembryonic development.3 Abnormal T3 level during postembryonic development causes serious health problems, including intellectual deficits and abnormal metabolic rate.4,5 Anuran metamorphosis is the most dramatic process regulated by T3 and mimics mammalian postembryonic development.6–8
T3 regulates transcription via T3 receptors (TRs).2,9 Studies on Xenopus laevis have suggested a dual function model for TR during anuran development. That is, unliganded TR recruits corepressors to T3-inducible genes in premetamorphic tadpoles to repress their expression and prevent premature metamorphosis, while liganded TR recruits coactivators to activate these genes to induce metamorphosis.10 There are two TR genes, TRα and TRβ, in vertebrates.9,11 Knocking out TRα or TRβ has distinct effects on different organs during metamorphosis in diploid Xenopus tropicalis.12–19
Somewhat surprisingly, tadpoles lacking both TRα and TRβ can develop to metamorphic climax with many adult organs formed, although they are then stalled at stage 61 for about 2 weeks before death.13,20 This contrasts with tadpoles that cannot synthesize T3, which are stalled before the onset of metamorphosis (stage 54).21,22 While it is possible that the depression of target genes upon TR knockout allows adult organ development, the finding may also raise the question whether T3 can regulate organ development through TR-independent nongenomic actions.
T3 has various nongenomic effects that involve binding to plasma membrane.2,23–26 For example, thyroxine (T4), the precursor for T3, can bind to integrin αvβ3 to affect angiogenesis and cell proliferation.26 T3 can modulate transport systems such as ATP-binding cassette transporters and affect the immune system and the phosphorylation in PI3K/Akt/mTOR pathway.27–30 However, little is known about the global gene expression changes induced by nongenomic action of T3 in the absence of TR, especially in developing animals.
In this study, we have carried out RNA-seq analyses on the intestine and tail of wild-type and TR double knockout (TRDKO, i.e., lacking both TRα and TRβ) X. tropicalis tadpoles treated with T3. (Note that without TR, the immediate action of T3 is to affect cellular events via nongenomic pathways. Such effects in turn affect downstream changes in mRNA levels by altering mRNA stability and/or transcription. For simplicity, we consider such changes in the absence of TR to be nongenomic effects of T3.) We discovered that TRDKO reduced T3-regulated genes in both organs with few commonly regulated genes between wild-type and TRDKO organs. We showed that many Gene Ontology (GO) terms and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways likely involved in metamorphosis were enriched among T3-regulated genes in wild-type but not TRDKO organs, while many GO terms and KEGG pathways related to metabolism and immune response were enriched in TRDKO but not wild-type organs.
Materials and Methods
All experiments involving X. tropicalis animals were carried out as approved by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Animal Use and Care Committee. The details are presented in the Supplementary Data.
Results
TRDKO reduces the number of genes regulated by T3 in both the intestine and tail
To reveal the role of TR in gene expression by T3 during Xenopus development, we carried out RNA-seq analysis on the intestine and tail from wild-type and TRDKO tadpoles at stage 54 with or without 18-hour T3 treatment to identify differentially expressed genes (DEGs), that is, T3-regulated genes (Supplementary Table S1). To validate the data, we selected 13 DEGs with T3 regulation of fivefold or more and analyzed their expression independently by reverse transcription polymerase chain reaction (RT-qPCR) and found that all were consistent with RNA-seq data (Supplementary Fig. S1). Additionally, the regulation of three well-known direct T3 target genes, hal2,31 mmp11, and thrb,20 was confirmed by both RNA-seq and RT-qPCR analyses (Supplementary Fig. S2).
There were 2060 DEGs with T3 regulation of twofold or more in wild-type intestine but only 869 DEGs in TRDKO intestine, with 356 DEGs in common (Fig. 1A and Supplementary Table S3). In wild-type intestine, there were 1002 upregulated and 1058 downregulated DEGs, compared with only 338 upregulated and 531 downregulated DEGs in TRDKO intestine (Fig. 1B). Interestingly, T3 regulated distinct genes in wild-type and TRDKO intestine (Fig. 1C). Even among 356 DEGs genes common between wild-type and TRDKO intestine, more than half were regulated by T3 in opposite manner in wild-type and TRDKO intestine (Fig. 1C).
FIG. 1.
There are more DEGs in the intestine of premetamorphic wild-type tadpoles than TRDKO tadpoles after T3 treatment. (A) Venn diagram of DEGs identified in the wild-type and TRDKO intestine. Of the 2060 DEGs in wild-type intestine and 869 in TRDKO intestine, only a small fraction of the DEGs, that is, 356 DEGs, were common in both groups. (B) Volcano plot showing the upregulated and downregulated DEGs in the wild-type and TRDKO intestine. There were 1058 downregulated (blue) and 1002 upregulated (red) DEGs in wild-type intestine, while only 531 downregulated (blue) and 338 upregulated (red) DEGs in TRDKO intestine. (C) Venn diagram analysis reveals that T3 regulates distinct genes in the intestine of premetamorphic wild-type and TRDKO tadpoles. Note that only a small fraction of the upregulated or downregulated genes were regulated similarly in the wild-type and TRDKO tadpoles. In fact, of the 356 DEGs common between wild-type and TRDKO tadpoles, about half were regulated in an opposite manner in wild-type and TRDKO tadpoles (40 DEGs were upregulated in TRDKO tadpoles but downregulated in wild-type tadpoles, while 144 were upregulated in wild-type tadpoles but downregulated in TRDKO tadpoles). DEGs, differentially expressed genes; T3, triiodothyronine; TRDKO, T3 receptor double knockout.
The reason for this is unclear. It is possible that T3 causes opposite effects on their expression via TR-dependent pathway and TR-independent pathway, with the TR-dependent pathway having a bigger effect. Thus, in the wild-type tadpoles, the TR-dependent effect dominates, while in the TRDKO tadpoles, the T3 effect from TR-independent pathway becomes detectable. In any case, our findings indicate that T3 regulates mostly different genes in the presence and absence of TR in premetamorphic tadpole intestine.
Similar analysis identified 1848 DEGs in wild-type tail while only 774 DEGs in TRDKO tail, with only 177 common DEGs (Fig. 2A and Supplementary Table S3). There were 840 upregulated and 1008 downregulated DEGs in wild-type tail compared with only 256 upregulated and 518 downregulated DEGs in TRDKO tail (Fig. 2B). Like in the intestine, T3 regulated distinct genes in wild-type and TRDKO tail (Fig. 2C). Even among the 177 common DEGs, more than half were regulated by T3 in opposite manner in wild-type and TRDKO tail (Fig. 2C).
FIG. 2.
There are more DEGs in the tail of premetamorphic wild-type tadpoles than TRDKO tadpoles after T3 treatment. (A) Venn diagram of DEGs identified in the wild-type and TRDKO tail. Of the 1848 DEGs in wild-type tail and 774 in TRDKO tail, only a small fraction of the DEGs, that is, 177 DEGs, were common in both groups. (B) Volcano plot showing the upregulated and downregulated DEGs in the wild-type and TRDKO tail. There were 1008 downregulated (blue) and 840 upregulated (red) DEGs in wild-type tail, while only 518 downregulated (blue) and 256 upregulated (red) DEGs in TRDKO tail. (C) Venn diagram analysis reveals that T3 regulates distinct genes in the tail of premetamorphic wild-type and TRDKO tadpoles. Note that only a small fraction of the upregulated or downregulated genes were regulated similarly in the wild-type and TRDKO tadpoles. In fact, of the 177 DEGs common between wild-type and TRDKO tadpoles, most were regulated in an opposite manner in wild-type and TRDKO tadpoles (50 DEGs were upregulated in TRDKO tadpoles but downregulated in wild-type tadpoles, while 55 were upregulated in wild-type tadpoles but downregulated in TRDKO tadpoles).
TRDKO affects GO terms and KEGG pathways regulated by T3 in the intestine
Earlier studies have shown that TRDKO delays/inhibits intestinal remodeling and TRDKO animals die at stage 61, before any significant tail length reduction.9,20 Thus, TRDKO likely affects T3 regulation of processes involved in metamorphosis. To investigate this, we carried out GO and KEGG pathway analyses on the 2060 and 869 DEGs in wild-type and TRDKO intestine, respectively (Supplementary Tables S4 and S5). The most significantly enriched GO term was “development process” in wild-type intestine (Fig. 3A and Supplementary Table S4) and “response to organic substance” in TRDKO intestine (Fig. 3C and Supplementary Table S5).
FIG. 3.
GO and KEGG analyses reveal that distinct GO terms/pathways are regulated by T3 in the intestine of wild-type (A, B) and knockout (C, D) tadpoles. (A, B) Top 10 GO terms/KEGG pathways significantly enriched among the 2060 T3-regulated genes in wild-type intestine. (C, D) Top 10 GO terms/KEGG pathways significantly enriched among the 869 T3-regulated genes in TRDKO intestine. GO, Gene Ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes.
In addition, there were many more development-related GO terms among the top 10 most significantly enriched GO terms in wild-type intestine than in TRDKO intestine (Fig. 3A, C). The top 10 most significantly enriched KEGG pathways were also different between wild-type and TRDKO intestine. Of note, glutathione metabolism pathways, involved in cell cycle and apoptosis, and pyrimidine metabolism pathways, important in stem cell function and DNA replication, were enriched in wild-type intestine (Fig. 3B), while the pathway for extracellular matrix (ECM)-receptor interaction, likely involved in epithelial folding, and PPAR pathway, important for lipid metabolism, were enriched in TRDKO intestine (Fig. 3D).
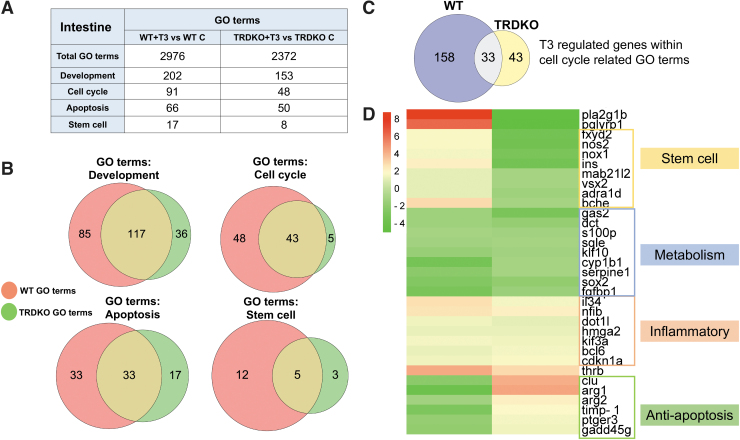
GO terms related to development, cell cycle, apoptosis, and stem cell are expected to be critical for intestinal remodeling during metamorphosis. Consistently, we found many of them enriched among DEGs in wild-type intestine, but fewer in TRDKO intestine (Fig. 4A and Supplementary Tables S4 and S5). Interestingly, most of the GO terms enriched in TRDKO intestine were also enriched in wild-type intestine. Taking cell cycle-related GO terms as an example, 43 of the 48 GO terms enriched in TRDKO intestine were also enriched in wild-type intestine (Fig. 4B). We identified T3-regulated genes in these 43 GO terms and found 191 and 76 DEGs in wild-type and TRDKO intestine, respectively.
FIG. 4.
TRDKO reduced the number of enriched GO terms related to major intestinal remodeling processes: apoptosis, cell proliferation/cell cycle, development, and stem cell. (A) GO terms (FDR <0.05) enriched among T3-regulated genes in the wild-type and TRDKO intestine: Total or those related to the major processes involved in intestinal remodeling. (B) Venn diagram comparisons of enriched GO terms between wild-type and TRDKO intestine show that TRDKO reduces the number enriched GO terms related to major intestinal remodeling processes. (C) Venn diagram comparisons of T3-regulated genes in the GO terms related to cell cycle between wild-type and TRDKO intestine. Note that there are 191 DEGs involved in wild-type intestine, while 76 DEGs involved in TRDKO intestine, with only 33 common between the 2 groups. (D) Heat map of the 33 DEGs regulated by T3 in both wild-type and TRDKO intestine in the GO terms related to cell cycle. Note that even within this group of commonly regulated genes, the regulation of many genes by T3 was opposite in TRDKO intestine compared with wild-type intestine (e.g., upregulation in one but downregulation in the other genotype). The remaining 33 DEGs were regulated by T3 similarly in wild-type and TRDKO intestine; all but one are involved in either metabolism or inflammatory response (Supplementary Fig. S3).
Among them, only 33 DEGs were common between wild-type and TRDKO intestine (Fig. 4C). Furthermore, clustered heat map comparison of gene regulation patterns showed that most of these 33 genes were regulated in opposite manner by T3 in wild-type and TRDKO tadpoles, and they could be grouped into two functional groups: stem cells and anti-apoptosis (Fig. 4D). Their opposite regulation patterns are consistent with T3-induced stem cell formation and larval epithelial cell death during intestine remodeling in wild-type but not TRDKO tadpoles. These findings suggest that T3 affects mostly distinct biological processes in the presence and absence of TRs, but there are common effects on metabolism and inflammatory response commonly in wild-type and TRDKO intestine.
Effects of TRDKO on GO terms and KEGG pathways regulated by T3 in the tail
Similar analyses on the 1848 and 774 T3-regulated DEGs in wild-type and TRDKO tail showed that many development-related GO terms were enriched in wild-type tail (Fig. 5A and Supplementary Table S6), while lipid transport and metabolism GO terms were highly enriched in TRDKO tail (Fig. 5C and Supplementary Table S7). Among KEGG pathways, cell cycle, cellular senescence, and p53 signaling pathways were enriched in wild-type tail, while lipid metabolism pathways were enriched in TRDKO tail (Fig. 5B, D and Supplementary Tables S6 and S7). Notably, a higher fraction of metabolism-related pathways (17/27) were enriched in TRDKO tail than in wild-type tail (13/40), suggesting that many more of DEGs were involved in metabolism in TRDKO tail compared with wild-type tail.
FIG. 5.
GO and KEGG analyses reveal that distinct GO terms/KEGG pathways are regulated by T3 in the tail of wild-type (A, B) and knockout (C, D) tadpoles. (A, B) Top 10 GO terms/KEGG pathways significantly enriched among the 1848 T3-regulated genes in wild-type tail. (C, D) Top 10 GO terms/KEGG pathways significantly enriched among the 774 T3-regulated genes in TRDKO tail.
Organ-specific nongenomic actions of T3
To investigate if the nongenomic effects were organ-dependent, we compared the T3-regulated DEGs in TRDKO tail and intestine. We found that 598 DEGs were unique to the tail and 693 DEGs were unique to the intestine, with only 176 common DEGs, much less than the common DEGs between the wild-type intestine and tail (Fig. 6A). Heat map of the 176 common DEGs showed that many were regulated by T3 in opposite manner in the 2 organs (i.e., upregulated [red] by T3 in one organ but downregulated [green] in the other) (Fig. 6B). In addition, of these 176 DEGs, 55 DEGs were upregulated and 97 DEGs downregulated in both organs, while 24 genes had opposite regulation patterns (Fig. 6C–F, C′–F′). Thus, TR-independent nongenomic effects of T3 on gene expression are mostly organ-specific.
FIG. 6.
The nongenomic effects of T3 affect distinct genes in the tail and intestine. (A) Venn diagram analyses reveal that vast majority of the genes regulated by T3 treatment of premetamorphic TRDKO tadpoles are different between the tail and intestine. Note that only 176 T3-regulated genes were common between the tail and intestine. This represented only 20.3% and 22.7% of the regulated genes in the TRDKO intestine and tail, respectively. In comparison, in the wild-type tadpoles, there were 584 commonly regulated genes in the tail and intestine (not shown), representing 28.3% and 31.6% of the regulated genes in wild-type intestine and tail, respectively. (B) Heat maps reveal that among the 176 genes regulated by T3 in both the tail and intestine of TRDKO tadpoles, many were regulated in an opposite manner in the intestine and tail (e.g., downregulated by T3 or green in one organ but upregulated or red in the other). The intensity of color indicates relative fold of regulation by T3 (indicated by log2-fold change). Red and green correspond to upregulation and downregulation by T3, respectively. (C–F) Venn diagram comparisons of regulation in the tail and intestine for the 176 genes regulated by T3 in both organs of TRDKO tadpoles. There are four subgroups: upregulated (C) or downregulated (F) by T3 in both organs, regulated by T3 in opposite manner in the two organs (D, E). (C′–F′) Heat maps showing subgroups within the 176 genes that were upregulated or downregulated in both (C′ or F′) or regulated by T3 in opposite manner in the two organs (D′, E′). The intensity of color indicates relative fold of regulation by T3 (indicated by log2-fold change). Red and green correspond to upregulation and downregulation by T3, respectively.
To identify common nongenomic effects of T3, we performed GO/KEGG analysis on the 152 DEGs similarly regulated by T3 in TRDKO intestine and tail and found that the enriched GO terms and pathways were mostly related to metabolism and cytokine–cytokine receptor interaction (Supplementary Fig. S4).
We next analyzed KEGG pathways enriched among 256 upregulated and 518 downregulated DEGs in TRDKO tail or 338 upregulated and 531 downregulated DEGs in TRDKO intestine (Supplementary Fig. S5). We combined the pathways enriched among the upregulated and downregulated DEGs for each organ and compared them. We found that 13 pathways were enriched in both organs, including “PPAR signaling pathway” and “Cytokine–cytokine receptor interaction” (Supplementary Fig. S5). Intriguingly, more than half of pathways were enriched from DEGs regulated by T3 in opposite patterns in the tail and intestine, such as “PPAR signaling pathway” (Supplementary Fig. S5).
Further analysis showed that the pathways enriched among DEGs regulated by T3 in either the same or opposite manner in the two organs were mainly related to different metabolic processes with exception of the pathway for “Cytokine–cytokine receptor interaction” and “FoxO signaling pathway,” both of which were enriched among DEGs upregulated in both organs. The regulation of genes such as il34/il1r2 in “Cytokine–cytokine receptor interaction pathway” and cdkn2b and cdkn2d/cdkn1a in “FoxO signaling pathway” suggests that both pathways are likely involved immune regulation in both organs (Supplementary Fig. S6).32–35
Given that integrin αvβ3 is one of the key targets of T4 for its nongenomic actions and “ECM-receptor interaction pathway” was enriched in TRDKO intestine (Supplementary Table S5), we compared the DEGs in this pathway between TRDKO tail and intestine. The results showed that all DEGs in the pathway were downregulated, such as laminin, collagen, and α8, in TRDKO intestine (Fig. 7A). However, in the tail, laminin was upregulated, while other DEGs were distinct from the DEGs in the intestine (Fig. 7B). Additionally, majority of the DEGs in “PPAR pathway,” enriched in both TRDKO intestine and tail (Figs. 3D and 5D), were distinct in the two organs (Supplementary Fig. S7A, B). Thus, nongenomic effects of T3 affect different genes in the intestine and tail.
FIG. 7.
KEGG pathway for ECM-receptor interaction is enriched among T3-regulated genes in both the tail (A) and intestine (B) of TRDKO tadpoles but involves different genes. Note that only downregulated genes were found in the intestine, and among them, laminin was actually upregulated in the tail by T3. In addition, vitronectin and cd36 were downregulated in the tail but not in the intestine. ECM, extracellular matrix.
TRDKO derepresses T3-regulated programs
Earlier studies showed that TRDKO led to precocious premetamorphic development, enabling tadpoles to enter metamorphosis prematurely, likely due to derepression of target genes.20,36 To investigate if TRDKO derepresses developmental programs in the tail and intestine, we compared gene expression between wild-type and TRDKO tail and intestine at stage 54 without T3 treatment. We obtained that 2682 (1646 upregulated, 1036 downregulated) DEGs in the tail (Supplementary Table S8A) and 2405 (1295 upregulated, 1110 downregulated) DEGs in the intestine (Supplementary Table S8D).
GO/KEGG analyses revealed that “cell cycle” and “p53 signaling pathway” were enriched among 2682 tail DEGs (Supplementary Table S8B, C and Supplementary Fig. S8A, B), while “development process” and “ECM-receptor interaction” were enriched among intestinal 2405 DEGs (Supplementary Table S8E, F and Supplementary Fig. S8C, D). These results suggest that TRDKO also causes derepression of T3-inducible genes and T3-regulated programs that are likely involved in the metamorphosis of the tail and intestine, although to an extent not sufficient to cause detectable precocious metamorphic changes in these organs.
Discussion
Thyroid hormone signals predominantly via TR to affect biological processes, such as metabolism, cell proliferation and death, and organ development.1,3,37 Extensive studies in mice have shown that TRα knockout, TRβ knockout, and TRDKO are associated with different phenotypes, suggesting that different TR subtypes may have distant roles for development and organ physiology.38,39 Similarly, studies in frog have shown that TRα and TRβ knockout have different phenotypes.3 Interestingly, TRs appear to be not required for the development of many, if not all, adult organs in both frogs and mammals, although tadpoles lacking TRs die at the climax of metamorphosis.3,9,20,38,40
This offers an interesting and important opportunity to study potential roles of T3 nongenomic actions during development. Our findings here indicate that TR-mediated genomic and TR-independent nongenomic effects of T3 affect the expression of mostly distinct genes in premetamorphic tadpoles. Furthermore, the nongenomic effects of T3 are largely organ-specific with metabolism as a major common target in both the intestine and tail.
Unliganded TRs are critical to maintain normal development and prevent premature metamorphosis.3,9,12 Consistently, our RNA-seq analysis identified many more T3-regulated gene in wild-type tadpoles than those in TRDKO tadpoles. Furthermore, majorities of the genes regulated by T3 in both wild-type and TRDKO organs exhibited opposite T3 regulation patterns. For example, dio3 (deiodinase type 3), responsible for T3 degradation and required for proper development41 and upregulated by T3 treatment in mouse cerebrocortical cells,42 is similarly regulated in both wild-type intestine and tail after T3 treatment of premetamorphic tadpoles. However, the expression was repressed in the intestine by T3 treatment of TRDKO (Supplementary Table S3). Thus, TRs are critical to mediate T3 regulation of genes important for metamorphosis, consistent with early transgenic expression of dominant-negative TRs that can block metamorphosis.43–45
TR is necessary for intestinal larval epithelial cell death and de novo formation of adult stem cells.9,12,13,15 Consistently, GO terms and KEGG pathways related to development, apoptosis, and stem cell were highly enriched among DEGs in wild-type intestine while TRDKO dramatically reduced their enrichment. Notable examples include signaling pathways such as Hedgehog, Notch, Wnt, and TGF-beta, which are important for regulation of stem cell and organ development and include DEGs such as bmp4 and wnt5a, important for cell proliferation and differentiation during intestinal remodeling.46–52
In this regard, it is worth noting that such pathways are also regulated by T3 to affect stem cells, cell proliferation, and differentiation in the mouse intestine,53–56 highlighting a conservation of T3 function in vertebrate intestine. In addition, KEGG pathways “glutathione metabolism” and “pyrimidine metabolism,” reported to be important for cell cycle and apoptosis, stem cell and DNA replication,57–59 were enriched in wild-type intestine, again consistent with their likely involvement in intestinal metamorphosis. However, “ECM-receptor interaction” and “lipid metabolism” pathways were enriched in TRDKO intestine, which agrees with our previous findings that TRDKO intestine has premature intestinal epithelial folding and muscle development but lacks larval epithelial cell apoptosis and stem cell development/proliferation.9
T3 and T4 can induce rapid nongenomic actions through plasma membrane and mitochondrial binding sites.26,60,61 For instance, T4 (and to a lesser extend T3) binds to integrin αvβ3 to activate MAPK signaling cascade, while T3 is the principal activator of the PI3K/Akt pathway, and such effects in turn affect cell proliferation, angiogenesis, and cell survival.61–64 Our analysis for the first time revealed nongenomic effects of T3 on global gene expression in developing animals. We found that GO terms and pathways related to metabolism were highly enriched among DEGs in the absence of TR in both the intestine and tail. T3 is a well-known regulator of metabolism in mammals, including amino acid catabolism and lipid metabolism.5,26,61,65–67 While it is generally believed that these effects are mediated by TR-dependent gene regulation, our findings here suggest that TR-independent nongenomic effects of T3 may also play a, if not dominant, role in regulating tissue metabolism.
For example, GO terms and pathways related to metabolism (e.g., α-linolenic acid [18:3n-3]) and immune response (e.g., Cytokine–cytokine receptor interaction) were highly enriched in both TRDKO tail and intestine, suggesting that nongenomic actions of T3 may affect immune response and metabolism of longer chain polyunsaturates, which are required for normal physiology such as brain function.68,69 Similarly, melanogenesis pathway was enriched among downregulated DEGs in TRDKO intestine and tail, consistent with reports that T3 may inhibit the production of melanin.70,71 However, despite such common effects in the intestine and tail, most of genes regulated by T3 in the absence of TRs and the corresponding enriched GO terms/pathways are different between the tail and intestine, suggesting that nongenomic actions of T3 are largely dependent on tissues/organs and/or cellular microenvironment.2,63,67
Conclusions
In conclusion, we have discovered that mostly distinct genes are regulated by T3 via TR-independent nongenomic and TR-dependent processes. While it is possible that RNA-seq of wild-type tissues may miss some genes regulated by T3 via TRs if the genes are regulated by T3 in opposite manner though TR-dependent and TR-independent processes, the enrichment of GO terms and pathways among the DEGs in wild-type tadpoles is consistent with T3-dependent metamorphic changes. However, the most noticeable nongenomic effects of T3 in the absence of TR in both organs are the regulation of GO terms and pathways involved in metabolism. Given the well-known effects of T3 on tissue metabolisms in mammals, it is templating to suggest that perhaps some of the metabolic effects of T3 in mammals are mediated by TR-independent nongenomic processes.
Supplementary Material
Acknowledgments
We thank Nga Luu for laboratory management and animal care staff for frog husbandry.
Authors' Contributions
S.W., Y.S., Y.T., and Y.-B.S. designed the research plan. S.W., Y.S., and Y.T. carried out the research and data analyses. All authors participated in the article preparation and approved the final version of the article.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This work was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), National Institutes of Health (NIH). Yuta Tanizaki and Yuki Shibata were supported in part by the Japan Society for the Promotion of Science Research Fellowship for Japanese Biomedical and Behavioral Researchers at the NIH. Yuta Tanizaki was also supported in part by an FY22 NICHD Early Career Award.
Supplementary Material
References
- 1. Cheng SY, Leonard JL, Davis PJ. Molecular aspects of thyroid hormone actions. Endocr Rev 2010;31(2):139–170; doi: 10.1210/er.2009-0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mullur R, Liu YY, Brent GA. Thyroid hormone regulation of metabolism. Physiol Rev 2014;94(2):355–382; doi: 10.1152/physrev.00030.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shi YB. Life without thyroid hormone receptor. Endocrinology 2021;162(4):bqab028; doi: 10.1210/endocr/bqab028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bernal J. Thyroid Hormones in Brain Development and Function. In: Endotext. (Feingold KR, Anawalt B, Boyce A, et al. eds.) MDText.com, Inc.: South Dartmouth, MA; 2000; pp. 1–33. [PubMed] [Google Scholar]
- 5. Ross I, Omengan DB, Huang GN, et al. Thyroid hormone-dependent regulation of metabolism and heart regeneration. J Endocrinol 2022;252(3):R71–R82; doi: 10.1530/JOE-21-0335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brown DD, Cai L, Das B, et al. Thyroid hormone controls multiple independent programs required for limb development in Xenopus laevis metamorphosis. Proc Natl Acad Sci U S A 2005;102(35):12455–12458; doi: 10.1073/pnas.0505989102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang S, Liu L, Liu J, et al. Gene expression program underlying tail resorption during thyroid hormone-dependent metamorphosis of the ornamented pygmy frog Microhyla fissipes. Front Endocrinol (Lausanne) 2019;10:11; doi: 10.3389/fendo.2019.00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shi YB, Fu L, Hsia SC, et al. Thyroid hormone regulation of apoptotic tissue remodeling during anuran metamorphosis. Cell Res 2001;11(4):245–252; doi: 10.1038/sj.cr.7290093 [DOI] [PubMed] [Google Scholar]
- 9. Shibata Y, Tanizaki Y, Zhang HE, et al. Thyroid hormone receptor is essential for larval epithelial apoptosis and adult epithelial stem cell development but not adult intestinal morphogenesis during Xenopus tropicalis metamorphosis. Cells 2021;10(3):536; doi: 10.3390/cells10030536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shi YB. Dual functions of thyroid hormone receptors in vertebrate development: The roles of histone-modifying cofactor complexes. Thyroid 2009;19(9):987–999; doi: 10.1089/thy.2009.0041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang X, Matsuda H, Shi YB. Developmental regulation and function of thyroid hormone receptors and 9-cis retinoic acid receptors during Xenopus tropicalis metamorphosis. Endocrinology 2008;149(11):5610–5618; doi: 10.1210/en.2008-0751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tanizaki Y, Shibata Y, Zhang H, et al. Analysis of thyroid hormone receptor alpha-knockout tadpoles reveals that the activation of cell cycle program is involved in thyroid hormone-induced larval epithelial cell death and adult intestinal stem cell development during Xenopus tropicalis metamorphosis. Thyroid 2021;31(1):128–142; doi: 10.1089/thy.2020.0022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shibata Y, Tanizaki Y, Shi YB. Thyroid hormone receptor beta is critical for intestinal remodeling during Xenopus tropicalis metamorphosis. Cell Biosci 2020;10:46; doi: 10.1186/s13578-020-00411-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wen L, Shibata Y, Su D, et al. Thyroid hormone receptor alpha controls developmental timing and regulates the rate and coordination of tissue-specific metamorphosis in Xenopus tropicalis. Endocrinology 2017;158(6):1985–1998; doi: 10.1210/en.2016-1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nakajima K, Tazawa I, Yaoita Y. Thyroid hormone receptor alpha- and beta-knockout Xenopus tropicalis tadpoles reveal subtype-specific roles during development. Endocrinology 2018;159(2):733–743; doi: 10.1210/en.2017-00601 [DOI] [PubMed] [Google Scholar]
- 16. Choi J, Ishizuya-Oka A, Buchholz DR. Growth, development, and intestinal remodeling occurs in the absence of thyroid hormone receptor alpha in tadpoles of Xenopus tropicalis. Endocrinology 2017;158(6):1623–1633; doi: 10.1210/en.2016-1955 [DOI] [PubMed] [Google Scholar]
- 17. Choi J, Suzuki KT, Sakuma T, et al. Unliganded thyroid hormone receptor alpha regulates developmental timing via gene repression in Xenopus tropicalis. Endocrinology 2015;156(2):735–744; doi: 10.1210/en.2014-1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sakane Y, Iida M, Hasebe T, et al. Functional analysis of thyroid hormone receptor beta in Xenopus tropicalis founders using CRISPR-Cas. Biol Open 2018;7(1):bio030338; doi: 10.1242/bio.030338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wen L, Shi YB. Unliganded thyroid hormone receptor alpha controls developmental timing in Xenopus tropicalis. Endocrinology 2015;156(2):721–734; doi: 10.1210/en.2014-1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shibata Y, Wen L, Okada M, et al. Organ-specific requirements for thyroid hormone receptor ensure temporal coordination of tissue-specific transformations and completion of Xenopus metamorphosis. Thyroid 2020;30(2):300–313; doi: 10.1089/thy.2019.0366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dodd MHI, Dodd JM.. The Biology of Metamorphosis. Academic Press: New York; 1976. [Google Scholar]
- 22. Shi Y-B. Amphibian Metamorphosis. Wiley-Liss: New York; 2000. [Google Scholar]
- 23. Damiano F, Rochira A, Gnoni A, et al. Action of thyroid hormones, T3 and T2, on hepatic fatty acids: Differences in metabolic effects and molecular mechanisms. Int J Mol Sci 2017;18(4):744; doi: 10.3390/ijms18040744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Davis PJ, Davis FB.. Nongenomic actions of thyroid hormone on the heart. Thyroid 2002;12(6):459–466; doi: 10.1089/105072502760143827 [DOI] [PubMed] [Google Scholar]
- 25. Davis PJ, Davis FB.. Nongenomic actions of thyroid hormone. Thyroid 1996;6(5):497–504; doi: 10.1089/thy.1996.6.497 [DOI] [PubMed] [Google Scholar]
- 26. Davis PJ, Goglia F, Leonard JL.. Nongenomic actions of thyroid hormone. Nat Rev Endocrinol 2016;12(2):111–121; doi: 10.1038/nrendo.2015.205 [DOI] [PubMed] [Google Scholar]
- 27. Davis PJ, Leonard JL, Lin HY, et al. Molecular basis of nongenomic actions of thyroid hormone. Vitam Horm 2018;106:67–96; doi: 10.1016/bs.vh.2017.06.001 [DOI] [PubMed] [Google Scholar]
- 28. Hammes SR, Davis PJ.. Overlapping nongenomic and genomic actions of thyroid hormone and steroids. Best Pract Res Clin Endocrinol Metab 2015;29(4):581–593; doi: 10.1016/j.beem.2015.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. De Vito P, Balducci V, Leone S, et al. Nongenomic effects of thyroid hormones on the immune system cells: New targets, old players. Steroids 2012;77(10):988–995; doi: 10.1016/j.steroids.2012.02.018 [DOI] [PubMed] [Google Scholar]
- 30. Pedrelli M, Pramfalk C, Parini P.. Thyroid hormones and thyroid hormone receptors: Effects of thyromimetics on reverse cholesterol transport. World J Gastroenterol 2010;16(47):5958–5964; doi: 10.3748/wjg.v16.i47.5958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Luu N, Fu L, Fujimoto K, et al. Direct regulation of histidine ammonia-lyase 2 gene by thyroid hormone in the developing adult intestinal stem cells. Endocrinology 2017;158(4):1022–1033; doi: 10.1210/en.2016-1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lin W, Xu D, Austin CD, et al. Function of CSF1 and IL34 in macrophage homeostasis, inflammation, and cancer. Front Immunol 2019;10:2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lacy P, Stow JL. Cytokine release from innate immune cells: Association with diverse membrane trafficking pathways. Blood 2011;118(1):9–18. [DOI] [PubMed] [Google Scholar]
- 34. Hedrick SM. The cunning little vixen: Foxo and the cycle of life and death. Nat Immunol 2009;10(10):1057–1063; doi: 10.1038/ni.1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Peng SL. Foxo in the immune system. Oncogene 2008;27(16):2337–2344; doi: 10.1038/onc.2008.26 [DOI] [PubMed] [Google Scholar]
- 36. Nieuwkoop PD, Faber J.. Normal Table of Xenopus laevis (Daudin). North-Holland publishing company: Amsterdam; 1965. [Google Scholar]
- 37. Krieger TG, Moran CM, Frangini A, et al. Mutations in thyroid hormone receptor alpha1 cause premature neurogenesis and progenitor cell depletion in human cortical development. Proc Natl Acad Sci U S A 2019;116(45):22754–22763; doi: 10.1073/pnas.1908762116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gothe S, Wang Z, Ng L, et al. Mice devoid of all known thyroid hormone receptors are viable but exhibit disorders of the pituitary-thyroid axis, growth, and bone maturation. Genes Dev 1999;13(10):1329–1341; doi: 10.1101/gad.13.10.1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yen PM, Feng X, Flamant F, et al. Effects of ligand and thyroid hormone receptor isoforms on hepatic gene expression profiles of thyroid hormone receptor knockout mice. EMBO Rep 2003;4(6):581–587; doi: 10.1038/sj.embor.embor862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Forrest D, Vennstrom B.. Functions of thyroid hormone receptors in mice. Thyroid 2000;10(1):41–52; doi: 10.1089/thy.2000.10.41 [DOI] [PubMed] [Google Scholar]
- 41. Singh SP, Dhakshinamoorthy R, Jaiswal P, et al. The thyroxine inactivating gene, type III deiodinase, suppresses multiple signaling centers in Dictyostelium discoideum. Dev Biol 2014;396(2):256–268; doi: 10.1016/j.ydbio.2014.10.012 [DOI] [PubMed] [Google Scholar]
- 42. Gil-Ibanez P, Garcia-Garcia F, Dopazo J, et al. Global transcriptome analysis of primary cerebrocortical cells: Identification of genes regulated by triiodothyronine in specific cell types. Cereb Cortex 2017;27(1):706–717; doi: 10.1093/cercor/bhv273 [DOI] [PubMed] [Google Scholar]
- 43. Buchholz DR, Hsia SC, Fu L, et al. A dominant-negative thyroid hormone receptor blocks amphibian metamorphosis by retaining corepressors at target genes. Mol Cell Biol 2003;23(19):6750–6758; doi: 10.1128/MCB.23.19.6750-6758.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Buchholz DR, Paul BD, Fu L, et al. Molecular and developmental analyses of thyroid hormone receptor function in Xenopus laevis, the African clawed frog. Gen Comp Endocrinol 2006;145(1):1–19; doi: 10.1016/j.ygcen.2005.07.009 [DOI] [PubMed] [Google Scholar]
- 45. Schreiber AM, Das B, Huang H, et al. Diverse developmental programs of Xenopus laevis metamorphosis are inhibited by a dominant negative thyroid hormone receptor. Proc Natl Acad Sci U S A 2001;98(19):10739–10744; doi: 10.1073/pnas.191361698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wen L, Hasebe T, Miller TC, et al. A requirement for hedgehog signaling in thyroid hormone-induced postembryonic intestinal remodeling. Cell Biosci 2015;5:13; doi: 10.1186/s13578-015-0004-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hasebe T, Kajita M, Shi YB, et al. Thyroid hormone-up-regulated hedgehog interacting protein is involved in larval-to-adult intestinal remodeling by regulating sonic hedgehog signaling pathway in Xenopus laevis. Dev Dyn 2008;237(10):3006–3015; doi: 10.1002/dvdy.21698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Massagué J, Xi Q. TGF-β control of stem cell differentiation genes. FEBS Lett 2012;586(14):1953–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Medici D, Hay ED, Olsen BR.. Snail and Slug promote epithelial-mesenchymal transition through beta-catenin-T-cell factor-4-dependent expression of transforming growth factor-beta3. Mol Biol Cell 2008;19(11):4875–4887; doi: 10.1091/mbc.E08-05-0506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Borretzen A, Gravdal K, Haukaas SA, et al. The epithelial-mesenchymal transition regulators Twist, Slug, and Snail are associated with aggressive tumour features and poor outcome in prostate cancer patients. J Pathol Clin Res 2021;7(3):253–270; doi: 10.1002/cjp2.202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Takiguchi G, Nishita M, Kurita K, et al. Wnt5a-Ror2 signaling in mesenchymal stem cells promotes proliferation of gastric cancer cells by activating CXCL16-CXCR6 axis. Cancer Sci 2016;107(3):290–297; doi: 10.1111/cas.12871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wang S, Liu L, Shi YB, et al. Transcriptome profiling reveals gene regulation programs underlying tail development in the Ornamented Pygmy frog Microhyla fissipes. Front Biosci (Landmark Ed) 2021;26(11):1001–1012; doi: 10.52586/5004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sirakov M, Kress E, Nadjar J, et al. Thyroid hormones and their nuclear receptors: New players in intestinal epithelium stem cell biology? Cell Mol Life Sci 2014;71(15):2897–2907; doi: 10.1007/s00018-014-1586-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Skah S, Nadjar J, Sirakov M, et al. The secreted Frizzled-Related Protein 2 modulates cell fate and the Wnt pathway in the murine intestinal epithelium. Exp Cell Res 2015;330(1):56–65; doi: 10.1016/j.yexcr.2014.10.014 [DOI] [PubMed] [Google Scholar]
- 55. Sirakov M, Boussouar A, Kress E, et al. The thyroid hormone nuclear receptor TRalpha1 controls the Notch signaling pathway and cell fate in murine intestine. Development 2015;142(16):2764–2774; doi: 10.1242/dev.121962 [DOI] [PubMed] [Google Scholar]
- 56. Kress E, Rezza A, Nadjar J, et al. The frizzled-related sFRP2 gene is a target of thyroid hormone receptor alpha1 and activates beta-catenin signaling in mouse intestine. J Biol Chem 2009;284(2):1234–1241; doi: 10.1074/jbc.M806548200 [DOI] [PubMed] [Google Scholar]
- 57. Diaz Vivancos P, Wolff T, Markovic J, et al. A nuclear glutathione cycle within the cell cycle. Biochem J 2010;431(2):169–178; doi: 10.1042/BJ20100409 [DOI] [PubMed] [Google Scholar]
- 58. Franco R, Cidlowski JA.. Apoptosis and glutathione: Beyond an antioxidant. Cell Death Differ 2009;16(10):1303–1314; doi: 10.1038/cdd.2009.107 [DOI] [PubMed] [Google Scholar]
- 59. Liu Z, Li W, Geng L, et al. Cross-species metabolomic analysis identifies uridine as a potent regeneration promoting factor. Cell Discov 2022;8(1):6; doi: 10.1038/s41421-021-00361-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Arcos MLB, Sterle HA, Paulazo MA, et al. Cooperative nongenomic and genomic actions on thyroid hormone mediated-modulation of T cell proliferation involve up-regulation of thyroid hormone receptor and inducible nitric oxide synthase expression. J Cell Physiol 2011;226(12):3208–3218; doi: 10.1002/jcp.22681 [DOI] [PubMed] [Google Scholar]
- 61. Giammanco M, Di Liegro CM, Schiera G, et al. Genomic and non-genomic mechanisms of action of thyroid hormones and their catabolite 3,5-diiodo-l-thyronine in mammals. Int J Mol Sci 2020;21(11):4140; doi: 10.3390/ijms21114140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Nappi A, Murolo M, Sagliocchi S, et al. Selective inhibition of genomic and non-genomic effects of thyroid hormone regulates muscle cell differentiation and metabolic behavior. Int J Mol Sci 2021;22(13):7175; doi: 10.3390/ijms22137175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Cayrol F, Sterle HA, Diaz Flaque MC, et al. Non-genomic actions of thyroid hormones regulate the growth and angiogenesis of T cell lymphomas. Front Endocrinol (Lausanne) 2019;10:63; doi: 10.3389/fendo.2019.00063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hiroi Y, Kim HH, Ying H, et al. Rapid nongenomic actions of thyroid hormone. Proc Natl Acad Sci U S A 2006;103(38):14104–14109; doi: 10.1073/pnas.0601600103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ferdous A, Wang ZV, Luo Y, et al. FoxO1-Dio2 signaling axis governs cardiomyocyte thyroid hormone metabolism and hypertrophic growth. Nat Commun 2020;11(1):2551; doi: 10.1038/s41467-020-16345-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zhu W, Chang L, Zhao T, et al. Remarkable metabolic reorganization and altered metabolic requirements in frog metamorphic climax. Front Zool 2020;17:30; doi: 10.1186/s12983-020-00378-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Davis PJ, Tillmann HC, Davis FB, et al. Comparison of the mechanisms of nongenomic actions of thyroid hormone and steroid hormones. J Endocrinol Invest 2002;25(4):377–388; doi: 10.1007/Bf03344022 [DOI] [PubMed] [Google Scholar]
- 68. Domenichiello AF, Kitson AP, Bazinet RP. Is docosahexaenoic acid synthesis from alpha-linolenic acid sufficient to supply the adult brain? Prog Lipid Res 2015;59:54–66; doi: 10.1016/j.plipres.2015.04.002 [DOI] [PubMed] [Google Scholar]
- 69. Sinclair AJ, Attar-Bashi NM, Li D.. What is the role of alpha-linolenic acid for mammals? Lipids 2002;37(12):1113–1123; doi: 10.1007/s11745-002-1008-x [DOI] [PubMed] [Google Scholar]
- 70. Yoo JH, Takeuchi T, Tagawa M, et al. Effect of thyroid hormones on the stage-specific pigmentation of the Japanese Flounder Paralichthys olivaceus. Zoolog Sci 2000;17(8):1101–1106; doi: 10.2108/zsj.17.1101 [DOI] [PubMed] [Google Scholar]
- 71. Saunders LM, Mishra AK, Aman AJ, et al. Thyroid hormone regulates distinct paths to maturation in pigment cell lineages. Elife 2019;8:e45181; doi: 10.7554/eLife.45181 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.