Abstract
Background:
Lyme borreliosis (LB), a tick bite-transmitted infection caused by Borrelia burgdorferi sensu lato (Bbsl) complex spirochetes, is the most common tickborne disease in Europe. Studies in European countries have reported LB seroprevalence (prevalence of antibodies to Bbsl infection) and diagnostic strategies used for testing. We conducted a systematic literature review to summarize contemporary LB seroprevalence in Europe.
Methods:
PubMed, Embase, and CABI Direct (Global Health) databases were searched from 2005 to 2020 to identify studies reporting LB seroprevalence in European countries. Reported single-tier and two-tier test results were summarized; algorithms (standard or modified) were used to interpret final test results from studies that used two-tier testing.
Results:
The search yielded 61 articles from 22 European countries. Studies used a range of diagnostic testing methods and strategies (48% single-tier, 46% standard two-tier, and 6% modified two-tier). In 39 population-based studies, of which 14 were nationally representative, seroprevalence estimates ranged from 2.7% (Norway) to 20% (Finland). There was substantial heterogeneity among studies in terms of design, cohort types, periods sampled, sample sizes, and diagnostics, which limited cross-study comparisons. Nevertheless, among studies that reported seroprevalence in persons with greater exposure to ticks, LB seroprevalence was higher among these groups than in the general population (40.6% vs. 3.9%). Furthermore, among studies that used two-tier testing, general population LB seroprevalence was higher in Western Europe (13.6%) and Eastern Europe (11.1%) than in Northern Europe (4.2%) and Southern Europe (3.9%).
Conclusion:
Despite variations in LB seroprevalence between and within European subregions and countries, high seroprevalence was observed in certain geographic regions and particular risk groups, suggesting significant disease burden and supporting the need for improved, targeted public health interventions such as vaccination. Harmonized approaches to serologic testing and more nationally representative seroprevalence studies are needed to better understand the prevalence of Bbsl infection in Europe.
Keywords: Lyme borreliosis, seroprevalence, seropositivity, Europe, epidemiology, diagnostics
Introduction
Lyme borreliosis (LB), the most common tickborne disease in Europe, is caused by an infection with spirochetes of the Borrelia burgdorferi sensu lato (Bbsl) complex, which is transmitted to humans through the bite of a vector-competent, infected tick (Bennet et al. 2007, Bobe et al. 2021). Although a proportion of infections are reported to be asymptomatic (median proportion of asymptomatic infected persons from studies in Europe was 50%) (Stanek et al. 2011, Hofhuis et al. 2013, Markowicz et al. 2021), asymptomatic persons can still seroconvert (Huegli et al. 2011, Hofhuis et al. 2013, Wilhelmsson et al. 2016). Clinical manifestations of LB typically begin with development of erythema migrans at the site of a tick bite, with signs and symptoms such as fatigue, fever, arthralgia, and myalgia (Bobe et al. 2021). The infection may then disseminate and present with a range of clinical syndromes, including neurologic (Lyme neuroborreliosis), rheumatologic (Lyme arthritis), dermatologic (acrodermatitis chronica atrophicans), hematologic (borrelial lymphocytoma), and/or cardiac (Lyme carditis) (Steere et al. 2016).
The distribution and density of vector-competent ticks infected with clinically relevant Bbsl in Europe has been widely investigated. Vector-competent ticks mainly include Ixodes ricinus and Ixodes persulcatus (Margos et al. 2019, Perry 2021), and eight clinically relevant genospecies belonging to Bbsl complex have been reported: B. burgdorferi sensu stricto, Borrelia afzelii, Borrelia garinii, Borrelia valaisiana, Borrelia lusitaniae, Borrelia bissettii, Borrelia bavariensis, and Borrelia spielmanii (Rauter and Hartung 2005, Richter and Matuschka 2006, Margos et al. 2009). Living or working in highly endemic geographic areas and undertaking outdoor occupations or leisure activities (e.g., forestry work, farming, hunting, hiking) lead to greater risk of exposure (Piacentino and Schwartz 2002, Magnavita et al. 2022).
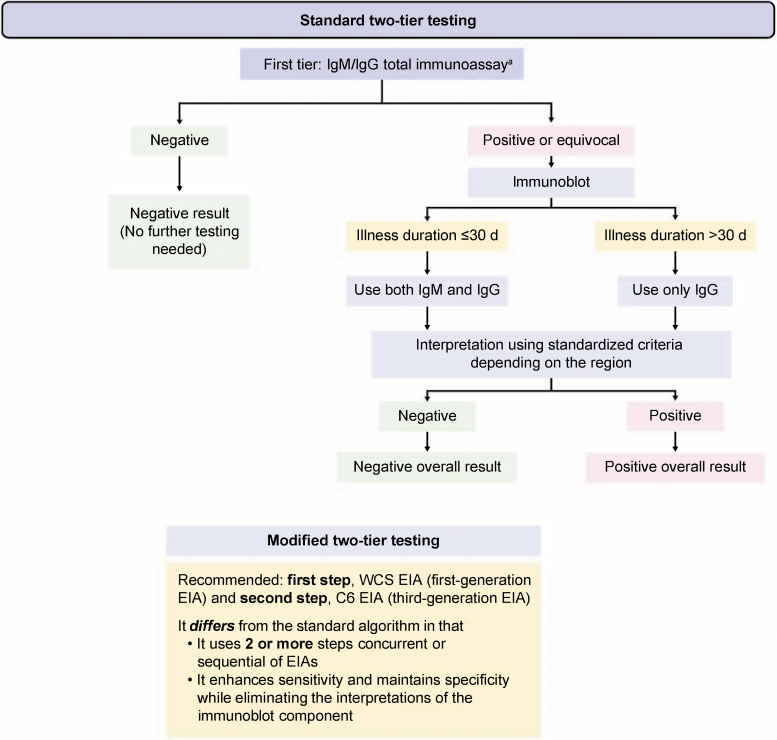
The serologic response to Bbsl infection begins with immunoglobulin M (IgM) antibodies, which initially become detectable within a few days to a few weeks after infection (Steere et al. 2016). Immunoglobulin class switching occurs, and immunoglobulin G (IgG) antibodies become detectable within 1–2 months; serum IgG and IgM antibodies can still be detected 10–20 years after infection (Kalish et al. 2001, Peltomaa et al. 2003, Glatz et al. 2006). Most individuals infected by Bbsl develop detectable antibodies; therefore, serology is the standard laboratory method for confirming an LB diagnosis (Wilske et al. 2007, Steere et al. 2016). Serologic confirmation of an LB diagnosis uses a two-tier strategy to optimize sensitivity and specificity (Fig. 1) (Branda and Steere 2021). For standard two-tier testing, the first-tier screen for IgG or IgM is an enzyme immunoassay (EIA; or enzyme-linked immunoassay [ELISA]) or an immunofluorescence assay (IFA).
FIG. 1.
Algorithm for the serologic testing of LB (Centers for Disease Control and Prevention 2011, Marques 2018, Branda and Steere 2021). Overall test results should be used from two-tier testing, with the original denominator. aEIA or IFA WCS. C6, C6 protein of the variable major protein-like sequence lipoprotein; EIA, enzyme immunoassay; IFA, immunofluorescence assay; IgG, immunoglobulin G; IgM, immunoglobulin M; LB, Lyme borreliosis; WCS, whole-cell sonicate.
To maximize specificity in the second tier, first-tier positive samples are then tested by western blot (WB) for IgM and/or IgG (Branda and Steere 2021). EIAs that detect antibodies to the highly conserved variable major protein-like sequence expressed (VlsE) peptides can improve specificity (Branda et al. 2013). Alternatively, modified two-tier testing methods that use two or more different EIA or ELISA methods as the second tier can improve diagnostic sensitivity without sacrificing specificity (Branda and Steere 2021).
Population-level seroprevalence can be used to monitor the prevalence of current or past infection in European countries without public health surveillance for LB. Furthermore, seroprevalence estimates, which indicate exposure to Bbsl at some point, can be compared with the number of surveillance-reported symptomatic LB cases to assess under-ascertainment of human contact with vector-competent, Bbsl-infected ticks. Seroprevalence data are capable of identifying geographic localities and outdoor occupations or leisure activities at higher risk of infection and monitoring population-level changes over time, such as those related to public health interventions. Consequently, we conducted a systematic literature review to obtain contemporary estimates of the seroprevalence of LB in Europe.
Methods
The methodology, search strategy, and inclusion and exclusion criteria for the systematic literature review and analysis are described in detail in a protocol developed by the Pfizer/P95 Lyme Review Group according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA, 2020) guidelines and registered in PROSPERO (CRD42021236906).
Search strategy
We conducted a multidatabase, systematic literature review across PubMed, Embase, and CABI Direct (Global Health) databases from January 1, 2005, to November 20, 2020, using the following search terms (with no language restrictions): Lyme, Borrelia, and borreliosis. After duplicates were removed, titles and abstracts were screened independently by two reviewers for their relevance to the study objectives, and the selected full-text articles were assessed based on predefined inclusion and exclusion criteria by two reviewers. Full-text articles published in other European languages were translated into English using DeepL (DeepL SE 2021).
Inclusion and exclusion criteria
Articles reporting results of serologic testing for Bbsl complex infection were included if the articles clearly reported a defined numerator (number of seropositive cases), denominator (size of the population tested), and diagnostic testing strategy based on at least one diagnostic test of either an EIA or ELISA, IFA, or western immunoblot.
Health-economic and cost studies, studies of biomedical mechanisms, mathematical models, and diagnostic guidelines, and studies without human serologic results were excluded, as were data only in abstract form from conferences, letters, perspective or opinion articles, or commentaries. Literature review articles were not included but were scanned for additional references.
Articles retrieved using the above criteria were further assessed to select articles reporting seroprevalence estimates. Studies were included if they reported estimates of seroprevalence (and not disease incidence) based on rigorous population sampling methods such as random, cross-sectional, or survey cluster sampling. If multiple seroprevalence results were obtained over time, only the first seroprevalence estimate was used to avoid mixing seroprevalence with seroconversion or incidence. Studies were excluded if they comprised participants who were selected for participation because they had suspected or confirmed LB or if they reported data from cohorts recruited based on tick bite exposure.
Data extraction
Relevant variables from selected articles were extracted into DistillerSR (Evidence Partners 2021). A reviewer independently confirmed the extraction by reviewing 20% of the articles. Predefined outcomes of interest for extraction were testing method, diagnostic strategy, results of serologic testing, population characteristics, and risk status. All discrepancies were discussed and resolved by the authors. The outcome of interest was seropositivity by at least one serologic test. Population risk level was defined by likelihood of exposure to ticks due to residence in specific regions or by occupational or leisure activities.
Data interpretation and analysis
Study results were synthesized by relevant descriptive variables and seroprevalence results. When studies used several types of single-tier tests (e.g., measurement of IgM or IgG), IgG tests results were preferred. This is because IgM-based tests, although useful for clinical diagnosis of early infection, are more likely to yield false positives (Branda and Steere 2021). In this review, therefore, IgG results were used to report seroprevalence. When studies used a two-tier testing strategy, final test results were based on calculations from standard or modified algorithms (Fig. 1). More prominence was given to test results from two-tier strategies due to the optimal sensitivity and specificity of this system (Branda and Steere 2021).
The 95% confidence intervals (CIs) (Cisak et al. 2008) for seroprevalence estimates were calculated using the binomial exact CI for a single proportion. Seroprevalence results were stratified by age, sex, and intracountry region (Supplementary Table S1).
We calculated the population-weighted mean seroprevalence for each of the European regions using data available for countries belonging to that region. If a study reported data on both the national and regional levels, we used only the data on the national level as a summary of that country. For studies that reported regional, subnational data only, seroprevalence from the largest reported region was used to calculate the weighted mean seroprevalence. We stratified weighted mean seroprevalence by diagnostic strategy and antibody testing method (Tables 5–8). Results were also stratified by exposure risk group. A population was considered a high-risk group for LB if it included a group with high exposure to ticks, such as forestry workers, farmers, or residents in high-risk regions (Tables 1–4).
Table 5.
Weighted Mean Lyme Borreliosis Seroprevalence in Eastern Europe from Published Literature, 2005–2020
| Diagnostic testing strategy | Antibody type | Risk group | Mean SP (95% CI), % |
|---|---|---|---|
| Single tier | IgG+IgM | Low risk | 34.7 (32.8–36.6) |
| IgG | 12.5 (9.4–16.7) | ||
| IgM | 10.7 (7.3–15.2) | ||
| IgG+IgM | High risk | 37.2 (35.2–39.2) | |
| IgG | 16.1 (14.3–18.1) | ||
| IgM | 12.6 (11.3–14.2) | ||
| Standard two-tier | IgG+IgM | Low risk | 11.1 (7.1–17.2) |
| IgG+IgM | High risk | 40.6 (33.6–48) | |
| IgG | 24.7 (20.6–29.2) | ||
| IgM | 7.6 (5.6–10.3) |
CI, confidence interval.
Table 6.
Weighted Mean Lyme Borreliosis Seroprevalence in Northern Europe from Published Literature, 2005–2020
| Diagnostic testing strategy | Antibody type | Exposure group | Mean SP (95% CI), % |
|---|---|---|---|
| Single tier | IgG+IgM | Low risk | 23.2 (20.3–26.3) |
| IgG | 12.0 (10.9–13.2) | ||
| IgG+IgM | High risk | 7.1 (4.2–11.0) | |
| IgG | 26.9 (21.6–2.9) | ||
| IgM | 1.1 (0.2–3.4) | ||
| Standard two-tier | IgG+IgM | Low risk | 4.2 (3.3–5.5) |
| IgG | 4.7 (3.8–5.8) | ||
| IgM | 3.8 (3.0–4.9) | ||
| Modified two-tier | IgG | Low risk | 9.5 (8.3–10.8) |
Table 7.
Weighted Mean Lyme Borreliosis Seroprevalence in Southern Europe from Published Literature, 2005–2020
| Diagnostic testing strategy | Antibody type | Exposure group | Mean SP (95% CI), % |
|---|---|---|---|
| Single tier | IgG+IgM | Low risk | 4.4 (3.5–5.7) |
| IgG | 1.3 (0.6–3.0) | ||
| IgM | 5.9 (4.1–8.0) | ||
| IgG+IgM | High risk | 8.5 (7.3–10.0) | |
| IgG | 3.2 (1.7–5.6) | ||
| Standard two-tier | IgG+IgM | Low risk | 3.9 (2.2–6.6) |
| IgG | 10.2 (8.3–12.6) | ||
| IgM | 8.2 (5.6–11.4) | ||
| IgG+IgM | High risk | 5.7 (3.8–8.3) | |
| IgG | 5.3 (4.0–7.3) | ||
| IgM | 6.5 (4.0–10.3) | ||
| Modified two-tier | IgG | Low risk | 2.5 (0.4–7.8) |
| IgG | High risk | 2.0 (0.3–6.1) |
Table 8.
Weighted Mean Lyme Borreliosis Seroprevalence in Western Europe from Published Literature, 2005–2020
| Diagnostic testing strategy | Antibody type | Exposure group | Mean SP (95% CI), % |
|---|---|---|---|
| Single tier | IgG | High risk | 16.9 (13.1–22.0) |
| Standard two-tier | IgG+IgM | Low risk | 13.6 (9.2–19.1) |
| IgG | 5.4 (5.0–5.8) | ||
| IgG+IgM | High risk | 14.1 (13.0–15.2) | |
| IgG | 37.0 (34.9–39.1) |
Table 1.
Estimates of Lyme Borreliosis Seroprevalence in Eastern Europe from Published Literature, 2005–2020
| Country | National or region within country | References | Study design (data collection period) | Sampling method | Sample size, N | Cohort description | Age group, years | Diagnostic testing strategy | Type of diagnostic test | Final SP result, %a |
|---|---|---|---|---|---|---|---|---|---|---|
| Czech Republic | Plzen, Vysocina, South Bohemia, Central Bohemia, South Moravia, Moravia-Silesia | Kříž et al. (2018) | Cross-sectional (1978–1989) Cross-sectional (2001) |
Random | 434 | Serum bank samplesb | Not described | Single tier | ELISA IgG | 25.1 |
| 270 | Serum bank samplesb | Single tier | ELISA IgG | 10.3 | ||||||
| Czech Republic | Prague | Hajek et al. (2006) | Cross-sectional (1995–1999) | Consecutive | 890 | Patients with psychiatric disordersb | Mean (SD): 33.6 (13.9) | Single tier | ELISA IgM and/or IgG | 35.7 |
| Czech Republic | Prague | Kuchynka et al. (2016) | Cross-sectional (2013–2014) | Convenience | 50 | Adults with normal left ventricular systolic function and no history suggestive of myocarditisb | Mean (SD): 67 (9) | Standard two-tier | ELISA IgG and/or IgM+WB | 14 |
| Hungary | Nationalc | Lakos et al. (2012) | Cross-sectional (1992–1995) | Convenience | 1670 | Forestry workersd | Mean (SD): 40.7 (10.2) | Single tier | ELISA IgG+IgM | 37.2 |
| Poland | Southern Podlasie Lowland, Lublin Polesie | Tokarska-Rodak et al. (2014) | Cross-sectional (2013) | Not described | 172 | Forestry workersd | Range: 25–72 | Standard two-tier | ELISA+WB IgG and/or IgM | 54.9 |
| 104 | Farmersd | Standard two-tier | ELISA+WB IgG and/or IgM | 28.0 | ||||||
| 45 | Persons not occupationally exposed to ticksb | Standard two-tier | ELISA+WB IgG and/or IgM | 5.0 | ||||||
| Poland | Warsaw | Machcińska et al. (2013) | Cross-sectional (2013) | Not described | 90 | Blood donorsb | Range: 18–70 | Single tier | ELISA IgM | 2.2 |
| ELISA IgG | 10.0 | |||||||||
| Poland | Six forest districts in Ostróda | Kocbach and Kocbach (2014) | Cross-sectional (2011–2012) | Convenience | 332 | Forestry workersd | Mean (range): 37 (25–67) | Standard two-tier | ELISA+WB IgG | 27.4 |
| St. Jablonki | 42 | Standard two-tier | ELISA+WB IgG | 11.9 | ||||||
| Kudypy | 47 | Standard two-tier | ELISA+WB IgG | 25.5 | ||||||
| Wipsowo | 79 | Standard two-tier | ELISA+WB IgG | 22.8 | ||||||
| Wichrowo | 54 | Standard two-tier | ELISA+WB IgG | 31.5 | ||||||
| Iława | 47 | Standard two-tier | ELISA+WB IgG | 21.3 | ||||||
| Miłomłyn | 63 | Standard two-tier | ELISA+WB IgG | 23.8 | ||||||
| Poland | Western Poland (Międzychód) | Bura et al. (2018) | Cross-sectional (2014) | Not described | 48 | Forest rangersd | Mean (SD): 45.0 (9.6) | Standard two-tier | ELISA+WB IgM | 8.3 |
| Standard two-tier | ELISA+WB IgG | 37.5 | ||||||||
| Poland | Nationalc | Pawelczyk et al. (2019) | Cross-sectional (2013/2016) | Not described | 227 | HIV-infected personsb | Mean (range): 33 (20–51) | Single tier | ELISA IgM | 29.1 |
| ELISA IgG | 4.8 | |||||||||
| 199 | Healthy blood donorsb | Mean (range): 36 (18–71) | Single tier | ELISA IgM | 13.1 | |||||
| ELISA IgG | 5.0 | |||||||||
| Poland | Northeastern (Białowieza Primeval forest), Southern (Radomsko forest) | Podsiadly et al. (2011) | Prospective cohort (2008–2009) | Not described | 129 | Forestry workersd | Not described | Standard two-tier | ELISA+WB IgG | 34.0 |
| Poland | Lublin | Cisak et al. (2008) | Cross-sectional (1998–2007) | Not described | 94 | Farmersd | Mean (SD): 56.3 (14.3) | Standard two-tier | ELISA+WB IgG and/or IgM | 32.9 |
| 50 | Blood donorsb | Mean (SD): 29.7 (5.0) | Standard two-tier | ELISA+WB IgG and/or IgM | 6.0 | |||||
| Poland | Nationalc | Zając et al. (2017) | Cross-sectional (2015–2016) | Convenience | 3597 | Farmersd | Mean (SD): 51.3 (11.4) | Single tier | ELISA IgM | 11.5 |
| ELISA IgG | 13.7 | |||||||||
| Gostynin | 150 | Single tier | ELISA IgM | 13.3 | ||||||
| Gostynin | 150 | Single tier | ELISA IgG | 14.7 | ||||||
| Siedlce | 329 | Single tier | ELISA IgM | 10.3 | ||||||
| Siedlce | 329 | Single tier | ELISA IgG | 16.7 | ||||||
| Kolno | 120 | Single tier | ELISA IgG | 14.2 | ||||||
| Kolno | 120 | Single tier | ELISA IgM | 6.7 | ||||||
| Siemiatycze | 106 | Single tier | ELISA IgM | 10.4 | ||||||
| Siemiatycze | 106 | Single tier | ELISA IgG | 19.8 | ||||||
| Biała Podlaska | 120 | Single tier | ELISA IgM | 14.2 | ||||||
| Biłgoraj | 59 | Single tier | ELISA IgM | 11.9 | ||||||
| Chełm | 120 | Single tier | ELISA IgM | 8.3 | ||||||
| Kraśnik | 317 | Single tier | ELISA IgM | 8.5 | ||||||
| Puławy | 103 | Single tier | ELISA IgM | 9.7 | ||||||
| Radzyń Podlaski | 114 | Single tier | ELISA IgM | 19.3 | ||||||
| Włodawa | 150 | Single tier | ELISA IgM | 25.3 | ||||||
| Zamość | 99 | Single tier | ELISA IgM | 8.1 | ||||||
| Węgrów | 182 | Single tier | ELISA IgM | 8.8 | ||||||
| Hajnówka | 103 | Single tier | ELISA IgM | 20.4 | ||||||
| Augustów | 150 | Single tier | ELISA IgM | 6.0 | ||||||
| Mońki | 99 | Single tier | ELISA IgM | 7.1 | ||||||
| Suwałki | 135 | Single tier | ELISA IgM | 12.6 | ||||||
| Biała Podlaska | 120 | Single tier | ELISA IgG | 20.0 | ||||||
| Biłgoraj | 59 | Single tier | ELISA IgG | 10.2 | ||||||
| Chełm | 120 | Single tier | ELISA IgG | 5.8 | ||||||
| Kraśnik | 317 | Single tier | ELISA IgG | 11.4 | ||||||
| Puławy | 103 | Single tier | ELISA IgG | 14.0 | ||||||
| Radzyń Podlaski | 114 | Single tier | ELISA IgG | 16.7 | ||||||
| Włodawa | 150 | Single tier | ELISA IgG | 24.0 | ||||||
| Zamość | 99 | Single tier | ELISA IgG | 16.2 | ||||||
| Węgrów | 182 | Single tier | ELISA IgG | 13.7 | ||||||
| Hajnówka | 103 | Single tier | ELISA IgG | 20.4 | ||||||
| Augustów | 150 | Single tier | ELISA IgG | 12.7 | ||||||
| Mońki | 99 | Single tier | ELISA IgG | 10.2 | ||||||
| Suwałki | 135 | Single tier | ELISA IgG | 18.5 | ||||||
| Poland | Southern Poland | Buczek et al. (2009) | Cross-sectional (2003–2006) | Convenience | 291 | Office workersb | Not described | Single tier | ELISA IgM | 10.0 |
| ELISA IgG | 13.7 | |||||||||
| 864 | Forestry workersd | ELISA IgM | 13.8 | |||||||
| ELISA IgG | 25.0 | |||||||||
| Gidle | 216 | Single tier | ELISA IgM | 6.0 | ||||||
| Herby | 203 | Single tier | ELISA IgM | 4.9 | ||||||
| Kłobuck | 160 | Single tier | ELISA IgM | 1.25 | ||||||
| Koniecpol | 211 | Single tier | ELISA IgM | 6.2 | ||||||
| Przedbórz | 141 | Single tier | ELISA IgM | 6.4 | ||||||
| Złoty Potok | 224 | Single tier | ELISA IgM | 17.4 | ||||||
| Gidle | 216 | Single tier | ELISA IgG | 21.3 | ||||||
| Herby | 203 | Single tier | ELISA IgG | 22.2 | ||||||
| Kłobuck | 160 | Single tier | ELISA IgG | 20.6 | ||||||
| Koniecpol | 211 | Single tier | ELISA IgG | 23.7 | ||||||
| Przedbórz | 141 | Single tier | ELISA IgG | 14.2 | ||||||
| Złoty Potok | 224 | Single tier | ELISA IgG | 14.3 | ||||||
| Poland | Eleven forest inspectorates | Kiewra et al. (2018) | Cross-sectional (2016) | Not described | 646 | Forestry workersd | Range: 21–67 | Standard two-tier | ELISA+WB IgM | 8.6 |
| Standard two-tier | ELISA+WB IgG | 17.8 | ||||||||
| Bardo | 49 | Standard two-tier | ELISA+WB IgM | 8.1 | ||||||
| Legnica | 51 | Standard two-tier | ELISA+WB IgM | 15.6 | ||||||
| Milicz | 89 | Standard two-tier | ELISA+WB IgM | 6.7 | ||||||
| Henryków | 34 | Standard two-tier | ELISA+WB IgM | 2.9 | ||||||
| Jugów | 59 | Standard two-tier | ELISA+WB IgM | 8.4 | ||||||
| Oleśnica | 96 | Standard two-tier | ELISA+WB IgM | 12.5 | ||||||
| Ruszów | 65 | Standard two-tier | ELISA+WB IgM | 6.1 | ||||||
| Śnieżka | 48 | Standard two-tier | ELISA+WB IgM | 8.3 | ||||||
| Świeradów | 55 | Standard two-tier | ELISA+WB IgM | 9.0 | ||||||
| Świętoszów | 37 | Standard two-tier | ELISA+WB IgM | 2.7 | ||||||
| Wołów | 63 | Standard two-tier | ELISA+WB IgM | 9.5 | ||||||
| Bardo | 49 | Standard two-tier | ELISA+WB IgG | 24.4 | ||||||
| Legnica | 51 | Standard two-tier | ELISA+WB IgG | 19.6 | ||||||
| Milicz | 89 | Standard two-tier | ELISA+WB IgG | 12.3 | ||||||
| Jugów | 59 | Standard two-tier | ELISA+WB IgG | 22.0 | ||||||
| Oleśnica | 96 | Standard two-tier | ELISA+WB IgG | 16.6 | ||||||
| Ruszów | 65 | Standard two-tier | ELISA+WB IgG | 15.3 | ||||||
| Śnieżka | 48 | Standard two-tier | ELISA+WB IgG | 27.0 | ||||||
| Świeradów | 55 | Standard two-tier | ELISA+WB IgG | 16.3 | ||||||
| Świętoszów | 37 | Standard two-tier | ELISA+WB IgG | 10.8 | ||||||
| Wołów | 63 | Standard two-tier | ELISA+WB IgG | 11.1 | ||||||
| Poland | Lublin Province | Pańczuk et al. (2019) | Cross-sectional (N/A) | Not described | 150 | Hunters and occupationally exposed persons (agriculture, collecting groundcover fruits, recreational activity in forested areas)d | Range: 17–80 | Standard two-tier | ELISA IgG VlsE+WB IgG and/or IgM | 38.0 |
| Standard two-tier | ELISA IgG VlsE+WB IgG | 36.7 | ||||||||
| Standard two-tier | ELISA IgG VlsE+WB IgM | 2.7 | ||||||||
| Russia | Northeastern Siberia (Sakha Republic) | Magnaval et al. (2016) | Cross-sectional (2012) | Random | 77 | Healthy volunteersb | ≥18 | Single tier | ELISA IgG | 10.3 |
| WB IgG | 1.6 | |||||||||
| Slovakia | Eastern Slovakia | Zákutná et al. (2015b) | Cross-sectional (2011) | Convenience | 124 | Blood donorsb | ≥30 | Single tier | ELISA IgG | 15.3 |
| WB IgG | 1.6 | |||||||||
| Slovakia | Eastern Slovakia | Zákutná et al. (2015a) | Cross-sectional (2011–2012) | Convenience | 193 | Agriculture and forestry workersd | ≥30 | Single tier | ELISA IgG | 29.2 |
| 36 | Police and border customs agentsd | Single tier | ELISA IgG | 11.1 | ||||||
| 48 | Persons frequently staying in the countrysided | Single tier | ELISA IgG | 20.8 | ||||||
| Slovakia | Senec and Senica districts | Bazovská et al. (2010) | Cross-sectional (2008–2009) | Not described | 302 | Blood donorsb | Not described | Single tier | ELISA IgG+WB IgG VlsE | 8.6 |
| Slovakia | Eastern Slovakia | Bušová et al. (2018) | Cross-sectional (2013–2016) | Not described | 135 | Gardeners and soldiers working with occupational exposure to ticksd | Mean (SD): 35.76 (11.17) | Single tier | ELISA IgG | 15.6 |
| ELISA IgM | 5.9 | |||||||||
| Slovakia | Slovakia | Bazovska et al. (2005) | Cross-sectional (1987–2004) | Not described | 250 | Blood donorsb | Not described | Standard two-tier | ELISA+WB IgG and/or IgM | 12.8 |
| Slovenia | Five establishments of the Slovenian Forest Service | Rojko et al. (2005) | Cross-sectional (March to November 2002) | Random | 112 | Forestry workersd | Median (range): 40 (22–62) | Single tier | ELISA IgG | 25.8 |
| ELISA IgM | 16.4 | |||||||||
| IFA | 9.8 | |||||||||
| 93 | Indoor workers from the same regionb | Median (range): 42 (23–65) | Single tier | ELISA IgG | 9.7 | |||||
| ELISA IgM | 16.2 | |||||||||
| IFA | 4.3 | |||||||||
| Ukraine | Nationalb | Biletska et al. (2008) | Cross-sectional (2003–2006) | Not described | 2393 | Healthy peopleb | Not described | Single tier | IFA IgG+IgM | 34.3 |
| Ukrainian Polissya | 567 | Single tier | IFA IgG+IgM | 32.6 | ||||||
| Forest steppe | 498 | Single tier | IFA IgG+IgM | 35.3 | ||||||
| Steppe | 967 | Single tier | IFA IgG+IgM | 38.2 | ||||||
| Carpathian region | 361 | Single tier | IFA IgG+IgM | 25.2 |
Includes single-tier test results and two-tier overall test results based on a standard or modified algorithm (Branda and Steere 2021, Marques et al. 2021).
General population.
Nationally representative general population.
High-risk population.
ELISA, enzyme-linked immunoassay; IgG, immunoglobulin G; IgM, immunoglobulin M; IFA, immunofluorescence assay; N/A, not available; SD, standard deviation; SP, seroprevalence; VlsE, variable major protein-like sequence, expressed; WB, Western blot.
Table 2.
Estimates of Lyme Borreliosis Seroprevalence in Northern Europe from Published Literature, 2005–2020
| Country | National or region within country | References | Study design (data collection period) | Sampling method | Sample size, N | Cohort description | Age group, years | Diagnostic testing strategy | Type of diagnostic test | Final SP result, %a |
|---|---|---|---|---|---|---|---|---|---|---|
| Baltic States | ||||||||||
| Estonia | Saaremaa | Parm et al. (2015) | Cross-sectional (2012) | Clusterb | 184 | Huntersc | Median (IQR): 41 (29–50) | Single tier | ELISA IgG | 46.7 |
| Single tier | ELISA IgM | 1.0 | ||||||||
| Single tier | ELISA IgM+IgG | 7.0 | ||||||||
| Lithuania | Nationald | Motiejunas et al. (1994) | Cross-sectional (1988) | Random | 268 | Forestersc | Not described | Single tier | IFA IgG | 14.0 |
| 115 | Field workersc | Single tier | IFA IgG | 22.0 | ||||||
| 68 | Veterinariansc | Single tier | IFA IgG | 32.0 | ||||||
| Scandinavia | ||||||||||
| Finland | Nationald | Cuellar et al. (2020) | Cross-sectional (1968–1972) | Convenience | 994 | General populatione | Median (range): 57 (15–86) | Modified two-tierf | Whole-cell sonicate IgG+C6 Lyme ELISA+RecomBead IgG | 20.0 |
| Finland | Nationald | van Beek et al. (2018) | Cross-sectional (2011) | Cluster | 2000 | General populatione | ≥29 | Modified two-tierg | Whole-cell sonicate IgG+C6 Lyme ELISA+RecomBead IgG | 4.3 |
| Norway | Sogn and Fjordane | Hjetland et al. (2014) | Cross-sectional (2010) | Convenience | 1213 | Blood donorse | Mean (range): 45.8 (19–69) | Standard two-tier | ELISA IgG VlsE+WB IgG | 6.1 |
| Standard two-tier | ELISA IgG VlsE+WB IgM | 2.8 | ||||||||
| Standard two-tier | ELISA IgM VlsE+WB IgM | 4.9 | ||||||||
| Standard two-tier | C6 ELISA+WB IgG | 5.8 | ||||||||
| Standard two-tier | C6 ELISA+WB IgM | 2.3 | ||||||||
| Norway | Nationald | Vestrheim et al. (2016) | Cross-sectional (2011–2013) | Not described | 3057 | Residual sera (pertussis study)e | ≥2 | Single tier | ELISA IgG VlsE | 2.7 |
| Single tier | EIA IgG | 3.4 | ||||||||
| Akershus | 601 | Single tier | ELISA IgG VlsE | 2.0 | ||||||
| Akershus | 601 | Single tier | EIA IgG | 1.8 | ||||||
| Oslo | 547 | Single tier | ELISA IgG VlsE | 4.0 | ||||||
| Oslo | 547 | Single tier | EIA IgG | 3.5 | ||||||
| Telemark | 178 | Single tier | ELISA IgG VlsE | 3.9 | ||||||
| Telemark | 178 | Single tier | EIA IgG | 4.5 | ||||||
| Vest-Agder | 198 | Single tier | ELISA IgG VlsE | 7.6 | ||||||
| Vest-Agder | 198 | Single tier | EIA IgG | 9.6 | ||||||
| Hordaland | 499 | Single tier | ELISA IgG VlsE | 2.4 | ||||||
| Hordaland | 499 | Single tier | EIA IgG | 3.8 | ||||||
| Sogn og Fjordane | 120 | Single tier | ELISA IgG VlsE | 2.5 | ||||||
| Sogn og Fjordane | 120 | Single tier | EIA IgG | 4.2 | ||||||
| Hedmark | 194 | Single tier | ELISA IgG VlsE | 1.5 | ||||||
| Hedmark | 194 | Single tier | EIA IgG | 1.0 | ||||||
| Nordland | 239 | Single tier | ELISA IgG VlsE | 0.8 | ||||||
| Nordland | 239 | Single tier | EIA IgG | 2.1 | ||||||
| Troms | 180 | Single tier | ELISA IgG VlsE | 0 | ||||||
| Troms | 180 | Single tier | EIA IgG | 1.1 | ||||||
| Sør-Trøndelag | 301 | Single tier | ELISA IgG VlsE | 2.7 | ||||||
| Sør-Trøndelag | 301 | Single tier | EIA IgG | 2.7 | ||||||
| Norway | Søgne | Thortveit et al. (2020) | Cross-sectional (2015–2016) | Consecutive | 2968 | General populatione | Range: 18–69 | Single tier | ELISA IgG | 22.9 |
| Sweden | Kalmar County | Carlsson et al. (2018) | Cross-sectional (2012–2013) | Consecutive | 873 | Blood donors with no LB historye | Median (range): 45 (18–72) | Single tier Single tier |
IFA IgG (≥8) IFA IgG (≥12) |
11.0 8.0 |
| 115 | Blood donors with unknown history of LBe | Median (range): 48 (18–68) | Single tier | IFA IgG (≥8) | 17.0 | |||||
| Sweden | Kalmar County | Johansson et al. (2017) | Cross-sectional (2011/2014) | Consecutive | 573 | Blood donorse | Range: 18–69 | Single tier | C6 ELISA IgG and/or IgM | 23.2 |
| Scotland | Edinburgh, Southeast Scotland, West of Scotland | Munro et al. (2015) | Cross-sectional (2010–2011) | Convenience | 1440 | Blood donors | Not described | Standard two-tier | ELISA IgG and/or IgM+WB IgG | 4.2 |
Includes single-tier test results and two-tier overall test results based on a standard or modified algorithm (Branda and Steere 2021, Marques et al. 2021).
Cluster sampling: methodology that involves (1) dividing the population into subgroups or clusters that are not necessarily (and preferably not) homogeneous, (2) drawing a random sample of the clusters, and (3) selecting all or a random sample of persons in each cluster.
High-risk population.
Nationally representative general population.
General population.
Sample taken ∼50 years ago and then tested with current diagnostic test.
Modified based on current guidelines for articles published after modified testing strategies were published (Branda and Steere 2021).
C6, C6 protein of the variable major protein-like sequence lipoprotein; EIA, enzyme immunoassay; IQR, interquartile range; LB, Lyme borreliosis.
Table 3.
Estimates of Lyme Borreliosis Seroprevalence in Southern Europe from Published Literature, 2005–2020
| Country | National or region within country | References | Study design (data collection period) | Sampling method | Sample size, N | Cohort description | Age group, years | Diagnostic testing strategy | Type of diagnostic test | Final SP result, %a |
|---|---|---|---|---|---|---|---|---|---|---|
| Italy | Arezzo, Florence, and Siena | Tomao et al. (2005) | Prospective cohort (1999–2001) | Not described | 365 | Blood donorsb | Mean (SD): 43.36 (8.16) | Standard two-tierc | ELISA+WB IgG and/or IgM | 3.5 |
| Standard two-tierd | ELISA+WB IgG and/or IgM | 1.6 | ||||||||
| 412 | Forestry workerse | Mean (SD): 43.71 (11.13) | Standard two-tierc | ELISA+WB IgG and/or IgM | 7.0 | |||||
| Standard two-tierd | ELISA+WB IgG and/or IgM | 3.8 | ||||||||
| Italy | Lazio region | Di Renzi et al. (2010) | Prospective cohort (2008) | Not described | 145 | Forestry rangerse | Mean (SD): 41.0 (7.8) | Standard two-tier | ELISA+WB IgG | 0.68 |
| Standard two-tier | ELISA+WB IgM | 13.1 | ||||||||
| 282 | Blood donorsb | Mean (SD): 40.4 (9.7) | Standard two-tier | ELISA+WB IgG | 1.1 | |||||
| Standard two-tier | ELISA+WB IgM | 8.2 | ||||||||
| Serbia | Belgrade | Jovanovic et al. (2015) | Prospective cohort (2014) | Convenience | 34 | Forestry workerse | Range: 25–45 | Standard two-tier | ELISA+WB IgM and/or IgG | 11.8 |
| 35 | Blood donorsb | Standard two-tier | ELISA+WB IgM and/or IgG | 8.6 | ||||||
| Serbia | Belgrade | Krstić and Stajković (2007) | Cross-sectional (2005) | Cluster | 34 | Occupationally exposed to ticks (public utility workers)e | Not described | Single tier | ELISA IgG and/or IgM | 23.5 |
| 35 | Not occupationally exposed to ticks (military medical cadets)b | Single tier | ELISA IgG and/or IgM | 2.9 | ||||||
| Spain | Guadalajara province | Lledo et al. (2019) | Cross-sectional (2019) | Not described | 100 | Occupationally exposed to ticks (forestry, agriculture, cattle raising)e | Median (IQR): 33 (29.5–40.25) | Single tier | IFA IgG | 7.0 |
| Spain | Asturias | Barreiro-Hurle et al. (2020) | Cross-sectional (2014) | Convenience | 316 | Blood donorsb | Mean (SD): 46 (8.5) | Standard two-tier | ELISA+WB IgG | 5.1 |
| 432 | Outpatients without infectious diseaseb | Mean (SD): 54 (14.1) | Standard two-tier | ELISA+WB IgG | 14.4 | |||||
| Spain | Navarra | Oteiza-Olaso et al. (2011) | Cross-sectional (1996) | Random | 1429 | Residents of Navarrab | ≥15 | Single tier | ELISA C6 | 4.4 |
| Stockbreeders | 13.2 | |||||||||
| Farmers | 3.5 | |||||||||
| Turkey | Erzurum Center and Pasinler district | Uyanık et al. (2009) | Prospective cohort (2007–2008) | Convenience | 101 | Residents with a high risk of tick exposure living in a high-risk area (Erzurum Province)e | Male mean: 39.5; female mean: 33.7 | Modified two-tierf | ELISA+ELFA IgG | 2.0 |
| 79 | Blood donors with low risk of exposure to ticksb | Male mean: 37.9; female mean: 35.8 | Modified two-tierf | ELISA+ELFA IgG | 2.5 | |||||
| Turkey | Düzce | Kaya et al. (2008) | Prospective cohort (2007) | Convenience | 349 | Forestry workerse | Median (range): 46.9 (14–92) | Standard two-tier | ELISA+WB IgG | 1.1 |
| 193 | Blood donorsb | ≥10 | Standard two-tier | ELISA+WB IgG | 0.0 | |||||
| Turkey | Düzce | Akar et al. (2019) | Cross-sectional (2016) | Convenience | 193 | Residents of a high-risk area (Duzce)e | Mean (SD): 47.4 (13.5) | Standard two-tier | ELISA+WB IgM | 1.5 |
| Standard two-tier | ELISA+WB IgG | 6.2 | ||||||||
| Turkey | Van region | Parlak et al. (2015) | Cross-sectional (2012) | Cluster (random sampling in clusters) | 446 | Residents of a high-risk area (Van region)e | Mean (SD): 39.6 (15.5) | Standard two-tier | ELISA+WB IgG | 0.9 |
| Turkey | Tekkekoy district | Aslan Başbulut et al. (2012) | Cross-sectional (2006) | Cluster (random sampling in clusters) | 419 | Tekkeköy (high tick population)e | Mean (SD): 33.07 (19.58) | Standard two-tier | ELISA+WB IgG | 3.3 |
| Turkey | Province of Bolu | Bucak et al. (2016) | Cross-sectional (August to October 2013) | Stratified random | 196 | Residents of Bolub | Adults and children | Standard two-tier | ELISA+WB IgG and/or IgM | 8.1 |
| Turkey | Erzincan | Cikman et al. (2019) | Cross-sectional (2014) | Cluster | 368 | Residents of Erzincan (high tick population)e | Mean (SD): 51.43 (16.91) | Standard two-tier | ELISA+WB IgG | 2.1 |
| Turkey | Sivas region | Güneş et al. (2005) | Cross-sectional (2005) | Random | 270 | Persons with contact with livestocke | Mean (range): 38.2 (13–80) | Single tier | ELISA IgG | 0.4 |
| 135 | Healthy controlsb | Single tier | ELISA IgG | 0.7 | ||||||
| Turkey | Van region | Bozkurt et al. (2008) | Prospective cohort (2008) | Random | 460 | Residents of the Van regionb | Single tier | EIA IgM | 5.8 | |
| EIA IgG | 1.5 | |||||||||
| Özalp | 31 | Single tier | EIA IgG | 6.5 | ||||||
| Gevaf | 14 | Single tier | EIA IgG | 7.1 | ||||||
| Muradiye | 29 | Single tier | EIA IgG | 3.4 | ||||||
| Van | 185 | Single tier | EIA IgG | 1.6 | ||||||
| Ozalp | 31 | Single tier | EIA IgM | 19.4 | ||||||
| Çaldran | 32 | Single tier | EIA IgM | 18.8 | ||||||
| Baflkale | 30 | Single tier | EIA IgM | 10.0 | ||||||
| Edremit | 11 | Single tier | EIA IgM | 9.1 | ||||||
| Gevaf | 14 | Single tier | EIA IgM | 7.1 | ||||||
| Muradiye | 29 | Single tier | EIA IgM | 6.9 | ||||||
| Van | 185 | Single tier | EIA IgM | 3.8 | ||||||
| Ercis | 77 | Single tier | EIA IgM | 1.3 | ||||||
| Turkey | Trabzon city and counties | Cora et al. (2017) | Retrospective, cross-sectional (2007–2008) | Convenience | 884 | Residents of Trabzon | Range: 20–79 | Standard two-tier | ELISA+WB IgG | 14.5 |
| 555 | Occupationally exposed to tickse | Standard two-tier | ELISA+WB IgG | 15.8 | ||||||
| 329 | Not occupationally exposed to ticksb | Standard two-tier | ELISA+WB IgG | 12.2 | ||||||
| Akçaabat | 95 | Standard two-tier | ELISA+WB IgG | 4.2 | ||||||
| Araklı | 49 | Standard two-tier | ELISA+WB IgG | 22.4 | ||||||
| Çaykara | 6 | Standard two-tier | ELISA+WB IgG | 50 | ||||||
| Düzköy | 12 | Standard two-tier | ELISA+WB IgG | 0 | ||||||
| Maçka | 22 | Standard two-tier | ELISA+WB IgG | 9.1 | ||||||
| Of | 48 | Standard two-tier | ELISA+WB IgG | 14.6 | ||||||
| Sürmene | 37 | Standard two-tier | ELISA+WB IgG | 13.5 | ||||||
| Vakfıkebir | 44 | Standard two-tier | ELISA+WB IgG | 22.7 | ||||||
| Yomra | 30 | Standard two-tier | ELISA+WB IgG | 26.7 | ||||||
| Turkey | Manisa | Gazi et al. (2016) | Cross-sectional (2012) | Random | 324 | Farmers | Mean (SD): 49.16 (16.78) | Single tier | IFA IgG VlsE | 4.3 |
Includes single test results and two-tier overall test results based on a standard or modified algorithm (Branda and Steere 2021, Marques et al. 2021).
General population.
According to manufacturer's instructions.
According to CDC-recommended criteria.
High-risk population.
Modified based on current guidelines for articles published after modified testing strategies were published (Branda and Steere 2021).
CDC, Centers for Disease Control and Prevention; ELFA, enzyme-linked fluorescent assay.
Table 4.
Estimates of Lyme Borreliosis Seroprevalence in Western Europe from Published Literature, 2005–2020
| Country | National or region within country | References | Study design (data collection period) | Sampling method | Sample size, N | Cohort description | Age group, years | Diagnostic testing strategy | Type of diagnostic test | Final SP result, %a |
|---|---|---|---|---|---|---|---|---|---|---|
| Austria | Districts of Burgenland | Cetin et al. (2006) | Cross-sectional (2002–2003) | Convenience | 1253 | Huntersb | Mean (SD): 51 (13) | Standard two-tier | ELISA+WB IgG | 53.7 |
| Austria | Nationalc | Sonnleitner et al. (2015) | Cross-sectional (2009) | Not described | 1607 | Blood donorsd | ≥18 | Standard two-tier | ELISA+WB IgG | 5.2 |
| Lech Valley | 58 | Standard two-tier | ELISA+WB IgG | 9.0 | ||||||
| Upper Inn Valley | 120 | Standard two-tier | ELISA+WB IgG | 3.0 | ||||||
| Central Inn Valley | 411 | Standard two-tier | ELISA+WB IgG | 6.0 | ||||||
| Lower Inn Valley | 317 | Standard two-tier | ELISA+WB IgG | 10.0 | ||||||
| East Tyrol | 104 | Standard two-tier | ELISA+WB IgG | 7.0 | ||||||
| Pustertal | 95 | Standard two-tier | ELISA+WB IgG | 1.0 | ||||||
| Eisack Valley | 79 | Standard two-tier | ELISA+WB IgG | 0 | ||||||
| Upper Eisack Valley | 277 | Standard two-tier | ELISA+WB IgG | 2.0 | ||||||
| Lower Eisack Valley | 144 | Standard two-tier | ELISA+WB IgG | 2.0 | ||||||
| Belgium | Wallonia | De Keukeleire et al. (2016) | Cross-sectional (2011) | Convenience | 31 | Farmersb Veterinariansb |
Single tier | ELISA IgG | 9.7 | |
| 96 | Single tier | ELISA IgG | 4.1 | |||||||
| Hainaut | 26 | Single tier | ELISA IgG | 11.5 | ||||||
| Leige | 37 | Single tier | ELISA IgG | 8.1 | ||||||
| Luxemburg | 26 | Single tier | ELISA IgG | 3.9 | ||||||
| Belgium | Wallonia | De Keukeleire et al. (2018) | Cross-sectional (2000–2013) | Convenience | 310 | Forestry workersb | Mean (range): 49 (24–65) | Single tier | ELISA IgG | 21.6 |
| Namur | 50 | Single tier | ELISA IgG | 34.0 | ||||||
| Arlon | 24 | Single tier | ELISA IgG | 29.0 | ||||||
| Malmedy | 42 | Single tier | ELISA IgG | 14.0 | ||||||
| Neufchâteau | 44 | Single tier | ELISA IgG | 14.0 | ||||||
| Belgium | Nationalc | Lernout et al. (2019) | Cross-sectional (2013–2015) | Convenience | 3215 | Serum bank samples | All ages | Standard two-tier | CLIA IgG+WB IgG | 1.1 |
| Region | ||||||||||
| Brussels | 378 | Standard two-tier | CLIA IgG+WB IgG | 1.0 | ||||||
| Flanders | 2052 | Standard two-tier | CLIA IgG+WB IgG | 1.3 | ||||||
| Wallonia | 785 | Standard two-tier | CLIA IgG+WB IgG | 0.7 | ||||||
| Provinces | ||||||||||
| West Flanders | 522 | Standard two-tier | CLIA IgG+WB IgG | 0.3 | ||||||
| East Flanders | 431 | Standard two-tier | CLIA IgG+WB IgG | 0.4 | ||||||
| Flemish Brabant | 532 | Standard two-tier | CLIA IgG+WB IgG | 1.5 | ||||||
| Antwerp | 551 | Standard two-tier | CLIA IgG+WB IgG | 1.2 | ||||||
| Limburg | 394 | Standard two-tier | CLIA IgG+WB IgG | 3.0 | ||||||
| Hainaut | 298 | Standard two-tier | CLIA IgG+WB IgG | 0 | ||||||
| Walloon Brabant | 69 | Standard two-tier | CLIA IgG+WB IgG | 2.8 | ||||||
| Liège | 175 | Standard two-tier | CLIA IgG+WB IgG | 1.7 | ||||||
| Namur | 62 | Standard two-tier | CLIA IgG+WB IgG | 0 | ||||||
| Luxembourg | 181 | Standard two-tier | CLIA IgG+WB IgG | 0.5 | ||||||
| France | Nationalc | Thorin et al. (2008) | Cross-sectional (2002–2003) | Convenience | 2975 | Forestry field professionalsb | Range: 17–81 | Standard two-tier | ELISA+WB IgG and/or IgM | 14.1 |
| Alsace | 636 | Standard two-tier | ELISA+WB IgG and/or IgM | 26.9 | ||||||
| Lorraine | 885 | Standard two-tier | ELISA+WB IgG and/or IgM | 16.5 | ||||||
| Champagne-Ardenne | 485 | Standard two-tier | ELISA+WB IgG and/or IgM | 8.2 | ||||||
| Burgundy | 400 | Standard two-tier | ELISA+WB IgG and/or IgM | 7.5 | ||||||
| Franche-Comté | 554 | Standard two-tier | ELISA+WB IgG and/or IgM | 5.6 | ||||||
| France | Southwestern France | Ruiz et al. (2020) | Cohort study (2007–2016) | Random | 689 | Retired farmers | ≥65 | Standard two-tier | ELISA IgG+WB IgG | 6.5 |
| Germany | Nationalc | Wilking et al. (2015) | Cross-sectional (2008–2011) | Not described | 6945 | General populationd | ≥18 | Standard two-tier | ELISA+WB IgG | 10.6 |
| Middle states | 3087 | Standard two-tier | ELISA+WB IgG | 9.8 | ||||||
| Western states | 4748 | Standard two-tier | ELISA+WB IgG | 10.1 | ||||||
| Eastern states | 2197 | Standard two-tier | ELISA+WB IgG | 11.6 | ||||||
| Northern states | 1767 | Standard two-tier | ELISA+WB IgG | 10.2 | ||||||
| Germany | Nationalc | Dehnert et al. (2012) | Cross-sectional (2003–2006) | Not described | 12,614 | Volunteersd | 1–17 | Standard two-tier | ELISA+WB IgG | 3.6 |
| West | 8248 | Standard two-tier | ELISA+WB IgG | 4.0 | ||||||
| East | 4272 | Standard two-tier | ELISA+WB IgG | 4.2 | ||||||
| North | 3294 | Standard two-tier | ELISA+WB IgG | 3.6 | ||||||
| Central | 5522 | Standard two-tier | ELISA+WB IgG | 3.7 | ||||||
| South | 3704 | Standard two-tier | ELISA+WB IgG | 5.1 | ||||||
| Rural area | 2745 | Standard two-tier | ELISA+WB IgG | 5.1 | ||||||
| Small town | 3322 | Standard two-tier | ELISA+WB IgG | 4.6 | ||||||
| Mid-size town | 3666 | Standard two-tier | ELISA+WB IgG | 3.7 | ||||||
| Metropolitan area | 2787 | Standard two-tier | ELISA+WB IgG | 3.0 | ||||||
| Netherlands | Nationalc | van Gorkom et al. (2017) | Cross-sectional (2013–2015) | Convenience | 147 | Healthy individualsd | Mean (IQR): 42.3 (28.0–53.4) | Standard two-tier | ELISA C6+WB IgG and/or IgM | 13.6 |
As a secondary analysis, to explore the impact of risk status on seroprevalence, seroprevalence odds ratios (ORs) and corresponding 95% CIs were calculated in R using the approximate Bayesian CIs (Laud 2021). The OR was calculated as seroprevalence of positive serologic test results in the risk group under study (high risk of exposure to ticks) compared with the seroprevalence in a control group (low or unknown risk of exposure to ticks) (Supplementary Table S2). Criteria used to classify study participants by their risk of exposure to ticks is defined in Supplementary Table S5. ORs >1 (with CIs also >1) indicate significantly higher seroprevalence among the high-risk group compared with the low-risk group.
Results
Search results
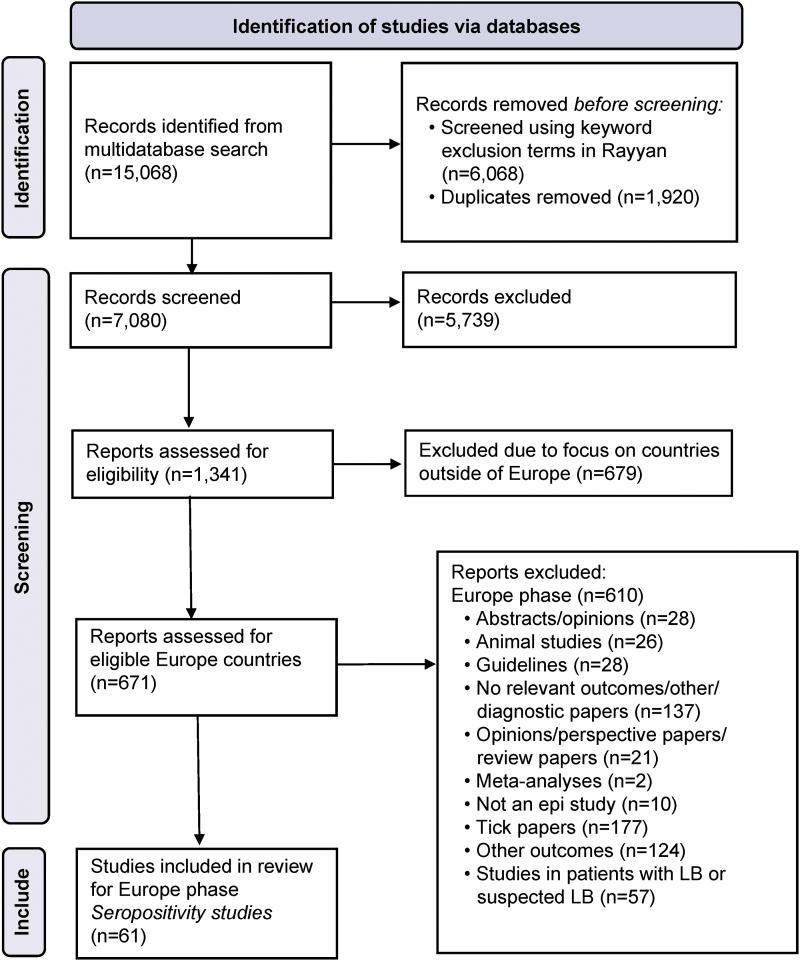
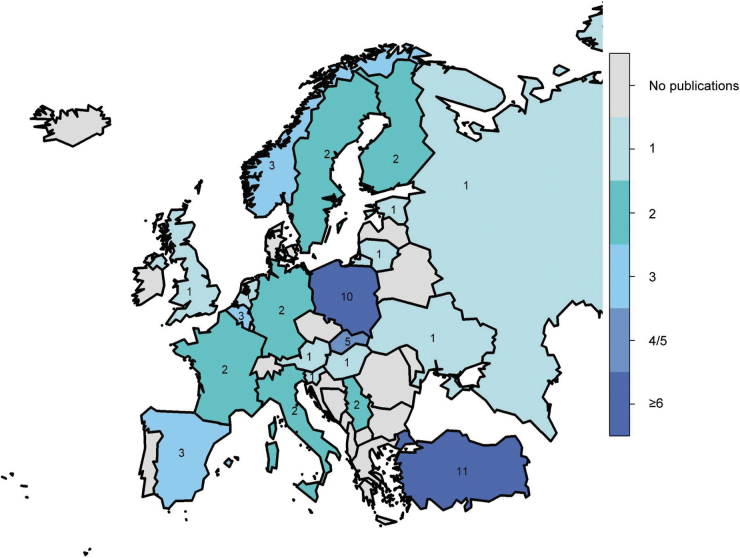
We included 61 articles from 22 European countries for analysis (Fig. 2); of these countries, seven had only 1 study, while Poland had 10 articles and Turkey had 11. The number of publications by country is shown in a heat map (Fig. 3). Fifty-two studies used a cross-sectional design, eight used a prospective cohort design, and one used a retrospective cohort design. Some studies contained both groups, general and high-risk populations (Supplementary Table S3).
FIG. 2.
PRISMA flow diagram. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
FIG. 3.
Heat map of number of publications by country from literature published between 2005 and 2020. N = 61.
A summary of the included articles with reported seroprevalence estimates and corresponding diagnostic tests and strategies is provided (Tables 1–4). The results are organized by European Region per World Health Organization (WHO) Regional Classifications (Supplementary Table S4) (World Health Organization 2022).
Study populations
Of the 61 total studies, 39 (64%) were population based, with blood samples collected from the general population (Supplementary Table S3), including office workers, healthy volunteers, and patients without clinical suspicion of LB, while 34 (56%) studies collected blood samples from populations with high risk of exposure to ticks (Supplementary Tables S3 and S5), including hunters, forestry and field workers, rangers, veterinarians, farmers and retired farmers, and soldiers. Among the 39 population-based studies, 14 collected nationally representative blood samples from the general population. Some studies were conducted among both general and high-risk groups, so there was overlap (N > 61).
Diagnostic testing strategies
Diagnostic testing methods and strategies varied among studies (Tables 1–4). Many studies used more than one test. Of the 61 studies, 28 used single-tier testing (23 used ELISA or EIA; 5 used IFA), and 30 studies utilized standard two-tier testing (ELISA with WB [IgG, IgM, or both]). Eight articles reported seroprevalence as a combined result using IgM and/or IgG detected by WB, and three studies used modified two-tier testing, including two studies from Finland (whole-cell sonicate IgG+C6 [C6 protein of the variable major protein-like sequence lipoprotein] Lyme ELISA+IFA IgG) and one study from Turkey (ELISA+enzyme-linked IFA [IgG]).
Five of the 61 studies used the VlsE-based EIA as either single-tier testing or part of a two-tier method. This included two studies in Norway (Hjetland et al. 2014, Vestrheim et al. 2016), one in Slovakia (Bazovská et al. 2010), one in Turkey (Gazi et al. 2016), and one in Poland (Pańczuk et al. 2019).
Many studies tested blood samples collected from participants in more than one period. Tests included both commercial (49 studies) and in-house (3 studies) assays; 8 studies did not give information on the source.
Seroprevalence estimates by European region
Eastern Europe (Czech Republic, Hungary, Poland, Russia, Slovakia, Slovenia, Ukraine)
Seroprevalence among general populations
Twenty-three articles reported seroprevalence of LB in seven Eastern European countries (Table 1) (Bazovska et al. 2005, Rojko et al. 2005, Hajek et al. 2006, Biletska et al. 2008, Cisak et al. 2008, Buczek et al. 2009, Bazovská et al. 2010, Podsiadly et al. 2011, Lakos et al. 2012, Machcińska et al. 2013, Kocbach and Kocbach 2014, Tokarska-Rodak et al. 2014, Zákutná et al. 2015a, 2015b, Kuchynka et al. 2016, Magnaval et al. 2016, Zając et al. 2017, Bura et al. 2018, Bušová et al. 2018, Kiewra et al. 2018, Kříž et al. 2018, Pańczuk et al. 2019, Pawelczyk et al. 2019). National seroprevalence estimates using a single-tier testing strategy on healthy persons and/or indoor workers were 5% in Poland (ELISA IgG, healthy blood donors) (Pawelczyk et al. 2019), 9.7% in Slovenia (ELISA IgG) (Rojko et al. 2005), 10.3% in Russia (ELISA IgG) (Magnaval et al. 2016), and 34.3% in Ukraine (IFA IgG+IgM) (Biletska et al. 2008).
Seroprevalence in high-risk groups
The seroprevalence of LB in populations with high-risk occupations using standard two-tier testing was 17.8% (ELISA+WB IgG) in forestry workers in Świętoszów, Poland (Kiewra et al. 2018) and 38% (ELISA IgG VlsE+WB IgG and/or IgM) in hunters and occupationally exposed persons in Lublin Province, Poland (Pańczuk et al. 2019). In studies with single-tier testing, the seroprevalence in forestry workers was 25.8% (ELISA IgG) in Slovenia (Rojko et al. 2005), 37.2% (ELISA IgG+IgM) in Hungary (Lakos et al. 2012), and 29.2% (ELISA IgG) in Slovakia.
Subnational seroprevalence
Subnational data from five regions in Ukraine were available from a study that assessed seroprevalence in healthy persons based on a single-tier testing strategy (IFA IgM+IgG) (Table 1). Seroprevalence ranged from 25.2% in the Carpathian region to 38.2% in the Steppe and up to 70% in the local administrative district of Kiverci in the region of Volyn Oblast (Biletska et al. 2008).
Northern Europe (Estonia, Finland, Lithuania, Norway, Scotland, Sweden)
Seroprevalence among general populations
Seven articles reported LB seroprevalence in Finland, Norway, and Sweden in the general population (Table 2) (Hjetland et al. 2014, Vestrheim et al. 2016, Johansson et al. 2017, Carlsson et al. 2018, van Beek et al. 2018, Cuellar et al. 2020, Thortveit et al. 2020), with testing based on samples from blood donors and residual sera from clinical trials and population-based surveys. General seroprevalence estimates were 6.1% (ELISA IgG VlsE+WB IgG) and 5.8% (C6 ELISA+WB IgG) among healthy blood donors in Norway (Hjetland et al. 2014). Among the general population in Finland, LB seroprevalence was 4.3% using modified two-tier testing (van Beek et al. 2018) versus 20% using a three-step testing method (as part of a modified two-tier testing strategy) in serum samples collected between 1968 and 1972 (Cuellar et al. 2020).
Seroprevalence in high-risk groups
Two studies of high-risk groups that used single-tier testing strategies (Motiejunas et al. 1994, Parm et al. 2015) reported seroprevalence estimates of 46.7% (ELISA IgG) in Estonian hunters and 14–32% (IFA IgG) in Lithuanian forestry workers, outdoor field workers, and veterinarians (Table 2).
Subnational seroprevalence
Five studies reported seroprevalence estimates from regions within Norway and Sweden. In Norway, seroprevalence ranged from 0% to 22.9% using single-tier testing strategies and various testing methods (Thortveit et al. 2020, Vestrheim et al. 2016) (Table 2). In Sweden, LB seroprevalence in Kalmar County in the southeast using single-tier testing (IFA IgG) was estimated to be between 8% and 17% depending on the assay cutoff employed and blood donor population studied (Carlsson et al. 2018). One study (Munro et al. 2015) reported a seroprevalence of 4.2% in blood donors by a standard two-tier testing method in the regions of West of Scotland, Edinburgh, and South East Scotland (Table 2).
Southern Europe (Italy, Serbia, Spain, Turkey)
Seroprevalence among general populations
Eighteen articles reported seroprevalence of LB in four countries in Southern Europe (Güneş et al. 2005, Tomao et al. 2005, Krstić and Stajković 2007, Bozkurt et al. 2008, Kaya et al. 2008, Uyanık et al. 2009, Di Renzi et al. 2010, Oteiza-Olaso et al. 2011, Aslan Başbulut et al. 2012, Jovanovic et al. 2015, Parlak et al. 2015, Bucak et al. 2016, Gazi et al. 2016, Cora et al. 2017, Akar et al. 2019, Cikman et al. 2019, Lledo et al. 2019, Barreiro-Hurle et al. 2020). All studies reported subnational rather than country-wide estimates (Table 3); 15 reported seroprevalence in groups at high risk of tick exposure based on occupation or residential area, and 11 reported on populations at low risk.
Estimates of LB seroprevalence were <10% for the general population in most regions of Italy, Serbia, Spain, and Turkey. An exception to this, in Turkey, determined using two-tier testing (ELISA+WB IgG), was an overall seroprevalence of 14.5% in residents of the city and environs of Trabzon, including Araklı (22.4%), Maçka (9.1%), Of (14.6%), Sürmene (13.5%), Vakfıkebir (22.7%), and Yomra (26.7%) (Cora et al. 2017). Furthermore, seroprevalence determined using a single-tier strategy was reported to be 10.0–19.4% in residents of three areas in the Van region (Ozalp, Çaldran, and Baflkaleup) (Bozkurt et al. 2008).
Seroprevalence in high-risk groups
In high-risk populations, LB seroprevalence was <1% using standard two-tier testing (ELISA+WB IgG) both in forestry rangers in Lazio, Italy (Di Renzi et al. 2010) and in residents of the Van region of Turkey (Parlak et al. 2015), but was 23.5% in public utility workers in Belgrade, Serbia (Krstić and Stajković 2007). In low-risk populations in Southern Europe, seroprevalence using standard two-tier testing (measured by different EIAs+WB IgG and/or IgM) ranged from <1% in Lazio, Italy (Di Renzi et al. 2010) and the Düzce, Van, Sivas, and Düzköy regions of Turkey (Güneş et al. 2005, Kaya et al. 2008, Parlak et al. 2015, Cora et al. 2017) to 50% in Çaykara, Turkey (Cora et al. 2017).
Western Europe (Austria, Belgium, France, Germany, the Netherlands)
Seroprevalence among general populations
Ten articles reported estimates of LB seroprevalence in countries in Western Europe, as summarized in Table 4 (Cetin et al. 2006, Thorin et al. 2008, Dehnert et al. 2012, Sonnleitner et al. 2015, Wilking et al. 2015, De Keukeleire et al. 2016, 2018, van Gorkom et al. 2017, Lernout et al. 2019, Ruiz et al. 2020). Six articles reported seroprevalence in the general population and five reported seroprevalence in groups at high risk of occupational tick exposure. The national seroprevalence of LB in volunteers or blood donors was 3.6% (ELISA+WB IgG) in Germany (Dehnert et al. 2012) and 1.1% (chemiluminescent immunoassay [CLIA] IgG+WB IgG) in Belgium (Lernout et al. 2019) based on a two-tier testing strategy.
As observed in other parts of Europe, there was substantial heterogeneity in seroprevalence of antibodies against Bbsl within individual countries according to region and occupational exposure. In Austria, seroprevalence among blood donors ranged from 0% in the Eisack Valley to 10% in the Lower Inn Valley (ELISA+WB IgG) based on two-tier testing (Sonnleitner et al. 2015).
Seroprevalence in high-risk groups
The seroprevalence of LB in populations with high-risk occupations was 14.1% among a nationally representative cohort of forestry field professionals in France, and 53.7% among hunters in Austria (ELISA+WB IgG), using two-tier testing (Cetin et al. 2006). In Wallonia, Belgium, seroprevalence estimates based on single-tier testing (ELISA IgG) among farmers and veterinarians was 11.54% in Hainaut and 9.68% in Wallonia (De Keukeleire et al. 2016), whereas in forestry workers it reached 34% in Namur and 21.6% in Wallonia (De Keukeleire et al. 2018).
Seroprevalence estimates by European region and diagnostic strategy
Eastern Europe
The weighted mean seroprevalence of LB in Eastern Europe among studies that used (standard) two-tier testing strategies in general populations was 11.1% (Table 5). Five studies (Bazovska et al. 2005, Cisak et al. 2008, Tokarska-Rodak et al. 2014, Kuchynka et al. 2016, Pańczuk et al. 2019) used the IgG and IgM standard two-tier test; the weighted mean of the high-risk group was higher (40.6%) than in the low-risk group (11.1%). Four of 23 studies (Podsiadly et al. 2011, Kocbach and Kocbach 2014, Bura et al. 2018, Kiewra et al. 2018) measured IgG in high-risk populations only, giving a weighted mean of 24.7%. When stratified by single-tier testing (IgM and IgG), seroprevalence was higher in high-risk groups (37.2%) than low-risk groups (34.7%). For IgG alone, it was 16.1% in high-risk groups versus 12.6% in low-risk groups.
Northern Europe
The weighted mean seroprevalence of LB in Northern Europe among studies that used (standard) two-tier testing strategies in general populations was 4.2% (Table 6). Additionally, two studies from Finland (van Beek et al. 2018, Cuellar et al. 2020) used a modified algorithm in low-risk groups, yielding a weighted mean seroprevalence of 9.5%. Different algorithms were evaluated in this region, including one study in Norway (Hjetland et al. 2014) using a standard two-tier test with a weighted mean of 4.7% in the low-risk group. When stratified by single-tier testing, the weighted mean seroprevalence for IgG was higher in high-risk groups (26.9%) than low-risk groups (12.0%).
Southern Europe
The weighted mean seroprevalence of LB in Southern Europe among studies that used (standard) two-tier testing strategies in general populations (Tomao et al. 2005, Kaya et al. 2008, Di Renzi et al. 2010, Aslan Başbulut et al. 2012, Jovanovic et al. 2015, Parlak et al. 2015, Bucak et al. 2016, Cora et al. 2017, Akar et al. 2019, Cikman et al. 2019, Barreiro-Hurle et al. 2020) was 3.9% (Table 7). The standard IgM and IgG weighted mean seroprevalence was 5.7% in high-risk groups versus 3.9% in low-risk groups. For single-tier testing (IgG and IgM), the mean seroprevalence was 8.9% in high-risk groups versus 4.4% in low-risk groups (Güneş et al. 2005, Krstić and Stajković 2007, Bozkurt et al. 2008, Oteiza-Olaso et al. 2011, Gazi et al. 2016, Lledo et al. 2019).
Western Europe
The weighted mean seroprevalence of LB in Western Europe among studies that used (standard) two-tier testing strategies in general populations was 13.6% (Dehnert et al. 2012, Sonnleitner et al. 2015, Wilking et al. 2015, Lernout et al. 2019) (Table 8). This was lower than the mean seroprevalence of high-risk groups (14.1%) based on one study in France (Thorin et al. 2008) and two studies that assessed seroprevalence by two-tiered IgG testing (37%) (Cetin et al. 2006, Ruiz et al. 2020). Two studies in Belgium (De Keukeleire et al. 2016, 2018) that used single-tier IgG testing in high-risk populations gave a weighted mean seroprevalence of 16.9%.
Seroprevalence estimates by age and sex
Overall Europe
Seroprevalence results stratified by age group and sex are presented in Supplementary Table S1.
Age
Thirteen studies reported age-stratified LB seroprevalence; all observed increasing seroprevalence for antibodies against Bbsl with age. In adults, a seroprevalence of at least 20% was observed in older age groups: ≥50 years of age in Finland (Cuellar et al. 2020), ≥55 years of age in France (Thorin et al. 2008), ≥60 years of age in Norway and Turkey (Hjetland et al. 2014, Cora et al. 2017), and ≥70 years of age in Germany and Poland (Wilking et al. 2015, Zając et al. 2017). The highest seroprevalence was observed in Austrian hunters, in whom 59.3% of 50- to 59-year-olds and 83.3% of >70-year-olds were seropositive (Cetin et al. 2006).
One study reported seroprevalence rates by age group in children ≥2 years of age using sera collected during a pertussis vaccine study in Norway. The seroprevalence (single tier, IgG) was 3.6% in 2- to 4-year-olds, 4.1% in 5- to 9-year-olds, and 2.1% in 10- to 19-year-olds versus 3.7% in 20- to 39-year-olds and 6.0% in >50-year-olds (Vestrheim et al. 2016).
Sex
In 17 studies that reported seroprevalence by sex, there was a trend toward higher seroprevalence in men than women (Supplementary Table S1) regardless of risk cohort.
Among high-risk groups in Estonia, seroprevalence (ELISA IgG) in male hunters was 52.6% versus 18.7% in female hunters (ELISA IgG) (Parm et al. 2015). The seroprevalence estimate was 6.4% in male Belgian farmers and veterinarians versus 0.0% in women (ELISA IgG) (De Keukeleire et al. 2018), 16.5% in male Polish farmers versus 11.7% in women (ELISA IgG) (Zając et al. 2017), and 14.3% in male forest and field professionals in France versus 3.4% in women (ELISA+WB IgG and/or IgM) (Thorin et al. 2008).
Similar trends were observed in low-risk groups in most countries. In Spain and Norway, the seroprevalence estimates were 6.5% (ELISA+WB IgG) and 13.0% (ELISA IgG VlsE) in male blood donors versus 5.5% and 3.1% in female blood donors, respectively (Hjetland et al. 2014, Barreiro-Hurle et al. 2020). The seroprevalence in the general population of Germany was 15.0% in men and 6.6% in women (ELISA+WB IgG) (Wilking et al. 2015). In contrast, the trend was reversed in Turkey and the United Kingdom, where seroprevalence was consistently higher in women than in men. While one study reported a seroprevalence of 6.1% in male residents of a high-risk area versus 2.5% in women (ELISA+ELFA [enzyme-linked fluorescent assay] IgG) (Uyanık et al. 2009), six other studies of residents in high-risk areas reported higher seroprevalence in women, with a difference between sexes of 0.2% to 6.0% (Aslan Başbulut et al. 2012, Parlak et al. 2015, Bucak et al. 2016, Cora et al. 2017, Akar et al. 2019, Cikman et al. 2019).
None of the studies showed a statistically significant difference between sex, except for one study in high-risk residents in Turkey, including ELISA and WB test results showing more statistically significant (p < 0.05) greater positive results in females (5.2%) than males (0.7%; ELISA IgG) (Parlak et al. 2015).
Seroprevalence estimates by risk groups
The high heterogeneity of the data (I2 > 90%, even after subanalyses by diagnostic methods and strategies, age, and sex) prevented a meta-analysis, but we did explore analysis by risk group. Among studies that analyzed persons with greater risk for tick exposure, LB seroprevalence (using standard two-tier for IgG+IgM) was higher among these groups than in the general population. While the difference in Eastern Europe was statistically significant (40.6% [33.6–48.0%] vs. 11.1% [7.1–17.2%] (Supplementary Table S5) where the two 95% CI did not overlap), in Southern Europe and Western Europe, we observed higher LB seroprevalence in high-risk group compared with low-risk group but this difference was not statistically significant (95% CI overlapped).
There were 12 studies that allowed calculation of ORs to evaluate whether tick exposure risk group was associated with seroprevalence (Supplementary Tables S2 and S3) by comparison with populations at low risk of exposure to ticks. Seven reported that the odds of LB seropositivity were statistically significantly increased by 2- to 21-fold in high-risk populations, suggesting that high-risk groups, including forestry workers and rural residents and workers, share a greater risk of seropositivity compared with low-risk populations. However, CIs were wide for many of the estimates.
Discussion
This review provides a comprehensive overview of the seroprevalence of LB in Europe. The main finding of our analysis is that LB is a disease of place (local setting) and activity (both occupational and leisure). National-level seroprevalence estimates may ignore potentially much higher subnational disease burdens, and conversely, subnational higher-risk areas do not necessarily indicate high national disease burden. Overall, LB seroprevalence estimates ranged from 0% to 70% (the latter in an administrative region in the Ukraine) (Biletska et al. 2008). There was a general trend toward higher seroprevalence for general population data in Western Europe and Eastern Europe, but on closer inspection there was substantial variation within the countries in Southern and Northern Europe; this may also be due to differences in Bbsl genospecies and I. ricinus abundance and distribution (Strnad et al. 2017, Van den Wijngaard et al. 2017, Estrada-Pena et al. 2018).
LB seroprevalence trended higher, although inconsistently, in persons undertaking at-risk occupational or leisure activities in some studies, with the highest estimate (83.3%) among hunters >70 years of age in Austria (Cetin et al. 2006) The seroprevalence in people with higher exposure to ticks was higher among these groups than in the general population (40.6% vs. 3.9%); the same occurs in a systematic review in human population where the high-risk population shows a seroprevalence of 18.8% (95% CI 10.1–29.4%) compared with a much lower seroprevalence in the general population of 5.7% (95% CI 4.3–7.3%) (Dong et al. 2022).
A 15-study meta-analysis reported that the OR for seroprevalence was 1.9 (95% CI: 1.2–3.2) worldwide in outdoor workers compared with controls (Magnavita et al. 2022). Interestingly, the risk changed over time and apparently decreased after 2010, possibly due to better education and/or occupational protection, but this trend was not consistently observed herein. A recent systematic review of global seroprevalence data and characteristics of B. burgdorferi in human populations (Dong et al. 2022) estimated seroprevalence to be 20.7% in Central Europe and 13.5% in Western Europe.
That review used a longer search time frame (1999–2021), whereas we captured additional studies in Europe and were ultimately more comprehensive. We also present diagnostic testing methods and strategies used, as well as our calculations of final two-tier test results, to enhance the interpretation of seroprevalence results while considering the limitations of single-tier testing (e.g., more false positives). The review by Dong and colleagues was also limited by including population cohorts suspected to have LB, which we excluded herein to reduce selection bias in seroprevalence estimates and ensure that we captured estimates in populations without existing LB.
We found that LB seroprevalence increased with age, reflecting both the cumulative risk of exposure to an infected tick over time and the persistence of antibodies to Bbsl, which show varying and unpredictable kinetics but may persist for years or even decades after acute infection (Kalish et al. 2001, Peltomaa et al. 2003, Glatz et al. 2006). Among 61 studies, only one study demonstrated statistically significant differences between sexes, with higher positive results in females in a high-risk region of Turkey (Parlak et al. 2015).
A key data gap in the epidemiology of LB is the true percentage of infections that are symptomatic versus asymptomatic. This information is needed to compare the number of symptomatic LB cases derived from seroprevalence data with the number of surveillance-reported LB cases to estimate the under-ascertainment of symptomatic LB cases by public health surveillance. However, the proportion of asymptomatic infections is difficult to estimate given the lack of prospective studies measuring the incidence of LB.
Nevertheless, three studies in Europe have estimated the proportion of asymptomatic infected persons by measuring seroconversion following a tick bite and the development of clinical signs and symptoms of LB. The median proportion of asymptomatic infected persons from these studies was 50% (Hofhuis et al. 2013, Wilhelmsson et al. 2016, Markowicz et al. 2021). A large, placebo-controlled study of a Lyme vaccine in the United States tested blood samples in intervals of months after vaccination. In this population, asymptomatic Bbsl infections were recorded in 11% of participants over the study period, which dropped to 7% when adjusted for those who met the criteria for LB (Steere et al. 2003).
However, differences between the proportions of asymptomatic infections in the United States and Europe may be related to differences in study design, duration of follow-up, diagnostic criteria, timing of postinfection treatment, antibody waning, and circulating genospecies. More data are needed to understand the proportion of asymptomatic LB infections, whether different genospecies vary in virulence, and the impact of age, sex, and other factors on susceptibility to clinical disease.
Our review has several limitations. Variations in study design, populations sampled, time frame, sample size, and diagnostic methods and strategies limit data interpretation and comparability between studies. First, the articles we reviewed encompassed countries of high and low endemicity and groups at differing risk of exposure to ticks among the general population. For example, categorization of a sample as high risk based on occupation may be inaccurate if the density of infected ticks is not substantial in the region where the study was conducted. Furthermore, seroprevalence data provide an estimate of the prevalence of infection, but these results need to be qualified based on testing accuracy and the duration of antibodies. Seropositivity for antibodies to Bbsl reflects the prevalence of prior or current Bbsl infection but is not necessarily equivalent to clinical disease because not all infections are symptomatic. Hence, seroprevalence may overestimate the proportion of true clinical burden of the population that has had symptomatic LB (Hammers-Berggren et al. 1994).
Immunoassays can yield false-positive results due to detection of crossreacting antibodies to unrelated antigens from bacterial, viral, parasitic, and some inflammatory conditions (Branda and Steere 2021). The lack of a gold standard test has significantly hampered the standardization of laboratory tools to diagnose LB. Available assays may use whole-cell or one or more recombinant antigens from a variety of Bbsl species and show marked variability in sensitivity and specificity (Kodym et al. 2018). Studies using single-tier testing are thus prone to overestimation of LB seroprevalence (Branda and Steere 2021). Additionally, heterogeneity between studies in terms of antibody testing strategies and methods (including commercial vs. in-house) limits the extent of meaningful comparisons.
Our review contributes to our understanding of the epidemiology of LB in Europe and highlights shortfalls and impediments to understanding the disease burden. Standardized LB testing strategies to monitor seroprevalence are needed to facilitate geographic and temporal data comparisons. LB is a disease of place and needs to be monitored in such a way that localized differences in tick exposure risk and burden of disease can be detected and reported.
This study complements another systematic literature review that evaluated the incidence of LB in European countries (Burn et al. 2023 in this issue) and provides information for some countries, such as Turkey, for which incidence data are not currently available. Although the ECDC started surveillance on Lyme neuroborreliosis in 2018, studies measuring the prevalence of antibodies to Bbsl infection in European populations in some countries are currently the only available source to monitor LB exposure and estimate disease burden (European Center for Disease Prevention and Control 2018). Seroprevalence, by providing a measure of the prevalence of Bbsl infection, provides data that can be used to estimate exposure to Bbsl among populations.
Our study provides an up-to-date picture of LB seroprevalence estimates in European countries. Seroprevalence is also an important component of the body of information that could guide intervention strategies, because monitoring the disease burden at the local level is a necessity, particularly in countries where LB is emerging.
Supplementary Material
Acknowledgments
The authors thank Margarita Riera-Montes (Chief Operations Officer and Medical Epidemiologist at P95) for her support in developing the study protocol and in study implementation. The authors thank Jo Wolter (Independent Medical Writer, on behalf of P95 Pharmacovigilance & Epidemiology) for her support in formatting this article.
Data Availability
All results of this systematic literature review are derived from the published literature as referenced.
Authors' Contributions
L.B.: conceptualization, methodology, data curation, software, formal analysis, investigation validation, resources, writing original draft, writing review and editing, visualization, supervision, and project administration. J.H.S.: conceptualization, methodology, writing—review and editing, project administration, supervision, and funding acquisition. A.V.G.R.: data curation, formal analysis, and writing—review and editing. T.M.P.T.: formal analysis, visualization. A.P.: conceptualization, methodology, and writing—review and editing. A.V.: conceptualization, methodology, and writing—review and editing. M.A.F.: conceptualization, methodology, and writing—review and editing. F.J.A.: methodology, and writing—review and editing. B.D.G.: writing—review and editing. J.C.M.: writing—review and editing.
Author Disclosure Statement
J.H.S., F.J.A., B.D.G., A.P., A.V., M.A.F., and J.C.M. are all employees of Pfizer and may hold stock options or stock in Pfizer. All other authors declare no conflicts of interest.
Funding Information
This study was supported and jointly funded by Valneva and Pfizer as part of their co-development of a Lyme Disease vaccine.
Supplementary Material
References
- Akar N, Çalişkan E, Öztürk CE, Ankarali H, et al. Seroprevalence of hantavirus and Borrelia burgdorferi in Düzce (Turkey) forest villages and the relationship with sociodemographic features. Turk J Med Sci 2019; 49:483–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslan Başbulut E, Gözalan A, Sönmez C, Çöplü N, et al. [Seroprevalence of Borrelia burgdorferi and tick-borne encephalitis virus in a rural area of Samsun, Turkey]. Mikrobiyol Bul 2012; 46:247–256. [PubMed] [Google Scholar]
- Barreiro-Hurle L, Melon-Garcia S, Seco-Bernal C, Munoz-Turrillas C, et al. Seroprevalence of Lyme disease in southwest Asturias. Enferm Infecc Microbiol Clin 2020; 38:155–158. [DOI] [PubMed] [Google Scholar]
- Bazovská S, Guryčová D, Výrosteková V, Jareková J, et al. [Antibodies against the causative agents of some natural focal infections in blood donor sera from western Slovakia]. Epidemiol Mikrobiol Immunol 2010; 59:168–171. [Google Scholar]
- Bazovska S, Machacova E, Spalekova M, Kontrosova S. Reported incidence of Lyme disease in Slovakia and antibodies to B. burgdorferi antigens detected in healthy population. Bratisl Lek Listy 2005; 106:270–273. [PubMed] [Google Scholar]
- Bennet L, Stjernberg L, Berglund J. Effect of gender on clinical and epidemiologic features of Lyme borreliosis. Vector Borne Zoonotic Dis 2007; 7:34–41. [DOI] [PubMed] [Google Scholar]
- Biletska H, Podavalenko L, Semenyshyn O, Lozynskyj I, et al. Study of Lyme borreliosis in Ukraine. Int J Med Microbiol 2008; 298:154–160. [Google Scholar]
- Bobe JR, Jutras BL, Horn EJ, Embers ME, et al. Recent progress in Lyme disease and remaining challenges. Front Med (Lausanne) 2021; 8:666554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozkurt H, Çiftçi HI, Güdücüělu H, Berktaş M, et al. Investigation of Borrelia burgdorferi seroprevalence in Van region of Turkey. Turk J Immunol 2008; 13:5–9. [Google Scholar]
- Branda JA, Steere AC. Laboratory diagnosis of Lyme borreliosis. Clin Microbiol Rev 2021; 34:e00018-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branda JA, Strle F, Strle K, Sikand N, et al. Performance of United States serologic assays in the diagnosis of Lyme borreliosis acquired in Europe. Clin Infect Dis 2013; 57:333–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucak Ö, Koçoğlu ME, Taș T, Mengeloğlu FZ. Evaluation of Borrelia burgdorferi sensu lato seroprevalence in the province of Bolu, Turkey. Turk J Med Sci 2016; 46:727–732. [DOI] [PubMed] [Google Scholar]
- Buczek A, Rudek A, Bartosik K, Szymańska J, et al. Seroepidemiological study of Lyme borreliosis among forestry workers in southern Poland. Ann Agric Environ Med 2009; 16:257–261. [PubMed] [Google Scholar]
- Bura M, Bukowska A, Michalak M, Bura A, et al. Exposure to hepatitis E virus, hepatitis A virus and Borrelia spp. infections in forest rangers from a single forest district in western Poland. Adv Clin Exp Med 2018; 27:351–355. [DOI] [PubMed] [Google Scholar]
- Bušová A, Dorko E, Feketeová E, Rimárová K, et al. Association of seroprevalence and risk factors in Lyme disease. Cent Eur J Public Health 2018; 26:S61–S66. [DOI] [PubMed] [Google Scholar]
- Carlsson H, Ekerfelt C, Henningsson AJ, Brudin L, et al. Subclinical Lyme borreliosis is common in south-eastern Sweden and may be distinguished from Lyme neuroborreliosis by sex, age and specific immune marker patterns. Ticks Tick Borne Dis 2018; 9:742–748. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Two-Tiered Testing Decision Tree. 2011. Available at https://www.cdc.gov/lyme/healthcare/clinician_twotier.html
- Cetin E, Sotoudeh M, Auer H, Stanek G. Paradigm Burgenland: risk of Borrelia burgdorferi sensu lato infection indicated by variable seroprevalence rates in hunters. Wien Klin Wochenschr 2006; 118:677–681. [DOI] [PubMed] [Google Scholar]
- Cikman A, Aydin M, Gulhan B, Karakecili F, et al. Geographical features and seroprevalence of Borrelia burgdorferi in Erzincan, Turkey. J Arthropod Borne Dis 2019; 12:378–386. [PMC free article] [PubMed] [Google Scholar]
- Cisak E, Chmielewska-Badora J, Zwoliñski J, Wójcik-Fatla A, et al. Study on Lyme borreliosis focus in the Lublin region (eastern Poland). Ann Agric Environ Med 2008; 15:327–332. [PubMed] [Google Scholar]
- Cora M, Kaklıkkaya N, Topbaș M, Çan G, et al. Determination of seroprevalence of Borrelia burgdorferi IgG in adult population living in Trabzon. Balkan Med J 2017; 34:47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuellar J, Dub T, Sane J, Hytonen J. Seroprevalence of Lyme borreliosis in Finland 50 years ago. Clin Microbiol Infect 2020; 26:632–636. [DOI] [PubMed] [Google Scholar]
- De Keukeleire M, Robert A, Kabamba B, Dion E, et al. Individual and environmental factors associated with the seroprevalence of Borrelia burgdorferi in Belgian farmers and veterinarians. Infect Ecol Epidemiol 2016; 6:32793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Keukeleire M, Robert A, Luyasu V, Kabamba B, et al. Seroprevalence of Borrelia burgdorferi in Belgian forestry workers and associated risk factors. Parasit Vectors 2018; 11:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeepL SE. DeepL Translator, pro version. 2021. Available at https://www.deepl.com/translator
- Dehnert M, Fingerle V, Klier C, Talaska T, et al. Seropositivity of Lyme borreliosis and associated risk factors: a population-based study in children and adolescents in Germany (KiGGS). PLoS One 2012; 7:e41321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Renzi S, Martini A, Binazzi A, Marinaccio A, et al. Risk of acquiring tick-borne infections in forestry workers from Lazio, Italy. Eur J Clin Microbiol Infect Dis 2010; 29:1579–1581. [DOI] [PubMed] [Google Scholar]
- Dong Y, Zhou G, Cao W, Xu X, et al. Global seroprevalence and sociodemographic characteristics of Borrelia burgdorferi sensu lato in human populations: a systematic review and meta-analysis. BMJ Global Health 2022; 7:e007744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada-Pena A, Cutler S, Potkonjak A, Vassier-Tussaut M, et al. An updated meta-analysis of the distribution and prevalence of Borrelia burgdorferi s.l. in ticks in Europe. Int J Health Geogr 2018; 17:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Centre for Disease Prevention and Control. ECDC comment: European Commission updates communicable disease surveillance list—Lyme neuroborreliosis now under EU/EEA surveillance. 2018. Available at https://www.ecdc.europa.eu/en/news-events/ecdc-comment-european-commission-updates-communicable-disease-surveillance-list-lyme
- Evidence Partners. DistillerSR version 2.35. 2021. Available at https://www.evidencepartners.com
- Gazi H, Özkütük N, Ecemis Ö, Atasoylu G, et al. Seroprevalence of West Nile virus, Crimean-Congo hemorrhagic fever virus, Francisella tularensis and Borrelia burgdorferi in rural population of Manisa, western Turkey. J Vector Borne Dis 2016; 53:112–117. [PubMed] [Google Scholar]
- Glatz M, Golestani M, Kerl H, Mullegger RR. Clinical relevance of different IgG and IgM serum antibody responses to Borrelia burgdorferi after antibiotic therapy for erythema migrans: long-term follow-up study of 113 patients. Arch Dermatol 2006; 142:862–868. [DOI] [PubMed] [Google Scholar]
- Güneş T, Poyraz O, Kaya S, Gençer L, et al. [Investigation of vectors for Borrelia burgdorferi and Lyme seropositivity in Sivas region]. Mikrobiyol Bul 2005; 39:503–508. [PubMed] [Google Scholar]
- Hajek T, Libiger J, Janovska D, Hajek P, et al. Clinical and demographic characteristics of psychiatric patients seropositive for Borrelia burgdorferi. Eur Psychiatry 2006; 21:118–122. [DOI] [PubMed] [Google Scholar]
- Hammers-Berggren S, Lebech AM, Karlsson M, Svenungsson B, et al. Serological follow-up after treatment of patients with erythema migrans and neuroborreliosis. J Clin Microbiol 1994; 32:1519–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjetland R, Nilsen RM, Grude N, Ulvestad E. Seroprevalence of antibodies to Borrelia burgdorferi sensu lato in healthy adults from western Norway: risk factors and methodological aspects. APMIS 2014; 122:1114–1124. [DOI] [PubMed] [Google Scholar]
- Hofhuis A, Herremans T, Notermans DW, Sprong H, et al. A prospective study among patients presenting at the general practitioner with a tick bite or erythema migrans in the Netherlands. PLoS One 2013; 8:e64361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huegli D, Moret J, Rais O, Moosmann Y, et al. Prospective study on the incidence of infection by Borrelia burgdorferi sensu lato after a tick bite in a highly endemic area of Switzerland. Ticks Tick Borne Dis 2011; 2:129–136. [DOI] [PubMed] [Google Scholar]
- Johansson M, Manfredsson L, Wistedt A, Serrander L, et al. Significant variations in the seroprevalence of C6 ELISA antibodies in a highly endemic area for Lyme borreliosis: evaluation of age, sex and seasonal differences. APMIS 2017; 125:476–481. [DOI] [PubMed] [Google Scholar]
- Jovanovic D, Atanasievska S, Protic-Djokic V, Rakic U, et al. Seroprevalence of Borrelia burgdorferi in occupationally exposed persons in the Belgrade area, Serbia. Braz J Microbiol 2015; 46:807–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalish RA, McHugh G, Granquist J, Shea B, et al. Persistence of immunoglobulin M or immunoglobulin G antibody responses to Borrelia burgdorferi 10–20 years after active Lyme disease. Clin Infect Dis 2001; 33:780–785. [DOI] [PubMed] [Google Scholar]
- Kaya AD, Parlak AH, Ozturk CE, Behcet M. Seroprevalence of Borrelia burgdorferi infection among forestry workers and farmers in Duzce, north-western Turkey. New Microbiol 2008; 31:203–209. [PubMed] [Google Scholar]
- Kiewra D, Szymanowski M, Zalewska G, Dobracka B, et al. Seroprevalence of Borrelia burgdorferi in forest workers from inspectorates with different forest types in Lower Silesia, SW Poland: preliminary study. Int J Environ Health Res 2018; 28:502–510. [DOI] [PubMed] [Google Scholar]
- Kocbach PP, Kocbach BP. [Prevalence of Lyme disease among forestry workers]. Med Pr 2014; 65:335–341. [PubMed] [Google Scholar]
- Kodym P, Kurzova Z, Berenova D, Picha D, et al. Serological diagnostics of Lyme norreliosis: comparison of universal and borrelia species-specific tests based on whole-cell and recombinant antigens. J Clin Microbiol 2018; 56:e00601-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kříž B, Malý M, Balátová P, Kodym P, et al. A serological study of antibodies to Anaplasma phagocytophilum and Borrelia burgdorferi sensu lato in the sera of healthy individuals collected two decades apart. Acta Parasitol 2018; 63:33–39. [DOI] [PubMed] [Google Scholar]
- Krstić M, Stajković N.. [Risk for infection by Lyme disease cause in green surfaces maintenance workers in Belgrade]. Vojnosanit Pregl 2007; 64:313–318. [DOI] [PubMed] [Google Scholar]
- Kuchynka P, Palecek T, Grus T, Lindner J, et al. Absence of Borrelia burgdorferi in the myocardium of subjects with normal left ventricular systolic function: a study using PCR and electron microscopy. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2016; 160:136–139. [DOI] [PubMed] [Google Scholar]
- Lakos A, Igari Z, Solymosi N. Recent lesson from a clinical and seroepidemiological survey: low positive predictive value of Borrelia burgdorferi antibody testing in a high risk population. Adv Med Sci 2012; 57:356–363. [DOI] [PubMed] [Google Scholar]
- Laud P. Confidence intervals for comparisons of binomial or Poisson rates. 2021. Available at https://cran.r-project.org/web/packages/ratesci/ratesci.pdf
- Lernout T, Kabamba-Mukadi B, Saegeman V, Tré-Hardy M, et al. The value of seroprevalence data as surveillance tool for Lyme borreliosis in the general population: the experience of Belgium. BMC Public Health 2019; 19:597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lledo L, Gimenez-Pardo C, Gegundez MI. Screening of forestry workers in Guadalajara province (Spain) for antibodies to lymphocytic choriomeningitis virus, hantavirus, Rickettsia spp. and Borrelia burgdorferi. Int J Environ Res Public Health 2019; 16:4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machcińska M, Noworyta J, Brasse-Rumin M, Gago J, et al. Prevalence of Yersinia spp., Chlamydia trachomatis, Chlamydophila pneumoniae and Borrelia burgdorferi antibodies in healthy blood donors' sera. Reumatologia 2013; 51:422–428. [Google Scholar]
- Magnaval JF, Leparc-Goffart I, Gibert M, Gurieva A, et al. A serological survey about zoonoses in the Verkhoyansk area, northeastern Siberia (Sakha Republic, Russian Federation). Vector Borne Zoonotic Dis 2016; 16:103–109. [DOI] [PubMed] [Google Scholar]
- Magnavita N, Capitanelli I, Ilesanmi O, Chirico F. Occupational Lyme disease: a systematic review and meta-analysis. Diagnostics (Basel) 2022; 12:296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margos G, Fingerle V, Reynolds S. Borrelia bavariensis: Vector switch, niche invasion, and geographical spread of a tick-borne bacterial parasite. Front Ecol Evol 2019; 7:401. [Google Scholar]
- Margos G, Vollmer SA, Cornet M, Garnier M, et al. A new Borrelia species defined by multilocus sequence analysis of housekeeping genes. Appl Environ Microbiol 2009; 75:5410–5416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowicz M, Reiter M, Gamper J, Stanek G, et al. Persistent anti-Borrelia IgM antibodies without Lyme borreliosis in the clinical and immunological context. Microbiol Spectr 2021; 9:e0102021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques AR. Revisiting the Lyme disease serodiagnostic algorithm: the momentum gathers. J Clin Microbiol 2018; 56:e00749-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques AR, Strle F, Wormser GP. Comparison of Lyme disease in the United States and Europe. Emerg Infect Dis 2021; 27:2017–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motiejunas L, Bunikis J, Barbour AG, Sadziene A. Lyme borreliosis in Lithuania. Scand J Infect Dis 1994; 26:149–155. [DOI] [PubMed] [Google Scholar]
- Munro H, Mavin S, Duffy K, Evans R, et al. Seroprevalence of Lyme borreliosis in Scottish blood donors. Transfus Med 2015; 25:284–286. [DOI] [PubMed] [Google Scholar]
- Oteiza-Olaso J, Tiberio-Lopez G, Martinez de Artola V, Belzunegui-Otano T. [Seroprevalence of Lyme disease in Navarra, Spain]. Med Clin (Barc) 2011; 136:336–339. [DOI] [PubMed] [Google Scholar]
- Pańczuk A, Tokarska-Rodak M, Plewik D, Paszkiewicz J. Tick exposure and prevalence of Borrelia burgdorferi antibodies among hunters and other individuals exposed to vector ticks in eastern Poland. Rocz Panstw Zakl Hig 2019; 70:161–168. [DOI] [PubMed] [Google Scholar]
- Parlak M, Bayram Y, Çıkman A, Ceylan N, et al. [Seropositivity of Borrelia burgdorferi in risky groups in Van region, Turkey]. Mikrobiyol Bul 2015; 49:439–445. [DOI] [PubMed] [Google Scholar]
- Parm Ü, Niitvägi E, Beljaev K, Aro T, et al. Lyme borreliosis in Saaremaa. Eesti Arst 2015; 94:203–210. [Google Scholar]
- Pawelczyk A, Bednarska M, Kowalska JD, Uszynska-Kaluza B, et al. Seroprevalence of six pathogens transmitted by the Ixodes ricinus ticks in asymptomatic individuals with HIV infection and in blood donors. Sci Rep 2019; 9:2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltomaa M, McHugh G, Steere AC. Persistence of the antibody response to the VlsE sixth invariant region (IR6) peptide of Borrelia burgdorferi after successful antibiotic treatment of Lyme disease. J Infect Dis 2003; 187:1178–1186. [DOI] [PubMed] [Google Scholar]
- Perry KC. DigitalCommons@UMaine. Acting out of Lyme: characterizing the human dimensions of Lyme disease interventions. 2021. Available at https://digitalcommons.library.umaine.edu/etd/3485
- Piacentino JD, Schwartz BS. Occupational risk of Lyme disease: an epidemiological review. Occup Environ Med 2002; 59:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podsiadly E, Chmielewski T, Karbowiak G, Kedra E, et al. The occurrence of spotted fever rickettsioses and other tick-borne infections in forest workers in Poland. Vector Borne Zoonotic Dis 2011; 11:985–989. [DOI] [PubMed] [Google Scholar]
- PRISMA. Transparent Reporting of Systematic Reviews and Meta-Analyses. 2020. Available at https://www.prisma-statement.org
- Rauter C, Hartung T. Prevalence of Borrelia burgdorferi sensu lato genospecies in Ixodes ricinus ticks in Europe: a metaanalysis. Appl Environ Microbiol 2005; 71:7203–7216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter D, Matuschka FR. Perpetuation of the Lyme disease spirochete Borrelia lusitaniae by lizards. Appl Environ Microbiol 2006; 72:4627–4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojko T, Ruzic-Sabljic E, Strle F, Lotric-Furlan S. Prevalence and incidence of Lyme borreliosis among Slovene forestry workers during the period of tick activity. Wien Klin Wochenschr 2005; 117:219–225. [DOI] [PubMed] [Google Scholar]
- Ruiz VH, Edjolo A, Roubaud-Baudron C, Jaulhac B, et al. Association of seropositivity to Borrelia burgdorferi with the risk of neuropsychiatric disorders and functional decline in older adults: the aging multidisciplinary investigation study. JAMA Neurol 2020; 77:210–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnleitner ST, Margos G, Wex F, Simeoni J, et al. Human seroprevalence against Borrelia burgdorferi sensu lato in two comparable regions of the eastern Alps is not correlated to vector infection rates. Ticks Tick Borne Dis 2015; 6:221–227. [DOI] [PubMed] [Google Scholar]
- Stanek G, Fingerle V, Hunfeld KP, Jaulhac B, et al. Lyme borreliosis: clinical case definitions for diagnosis and management in Europe. Clin Microbiol Infect 2011; 17:69–79. [DOI] [PubMed] [Google Scholar]
- Steere AC, Sikand VK, Schoen RT, Nowakowski J. Asymptomatic infection with Borrelia burgdorferi. Clin Infect Dis 2003; 37:528–532. [DOI] [PubMed] [Google Scholar]
- Steere AC, Strle F, Wormser GP, Hu LT, et al. Lyme borreliosis. Nat Rev Dis Primers 2016; 2:16090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strnad M, Honig V, Ruzek D, Grubhoffer L, et al. Europe-wide meta-analysis of Borrelia burgdorferi sensu lato prevalence in questing Ixodes ricinus ticks. Appl Environ Microbiol 2017; 83:e00609-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorin C, Rigaud E, Capek I, André-Fontaine G, et al. [Seroprevalence of Lyme borreliosis and tick-borne encephalitis in workers at risk, in eastern France]. Med Mal Infect 2008; 38:533–542. [DOI] [PubMed] [Google Scholar]
- Thortveit EA-O, Aase A, Petersen LB, Lorentzen Å RA-O, et al. Subjective health complaints and exposure to tick-borne infections in southern Norway. Acta Neurol Scand 2020; 142:260–266. [DOI] [PubMed] [Google Scholar]
- Tokarska-Rodak M, Plewik D, Kozioł-Montewka M, Szepeluk A, et al. [Risk of occupational infections caused by Borrelia burgdorferi among forestry workers and farmers]. Medycyna Pracy 2014; 65:109–118. [DOI] [PubMed] [Google Scholar]
- Tomao P, Ciceroni L, D'Ovidio MC, De Rosa M, et al. Prevalence and incidence of antibodies to Borrelia burgdorferi and to tick-borne encephalitis virus in agricultural and forestry workers from Tuscany, Italy. Eur J Clin Microbiol Infect Dis 2005; 24:457–463. [DOI] [PubMed] [Google Scholar]
- Uyanık MH, Yazgı H, Ayyıldız A. Seropositivity of Lyme disease in Erzurum province, Turkey. Turk J Infect 2009; 23:69–72. [Google Scholar]
- van Beek J, Sajanti E, Helve O, Ollgren J, et al. Population-based Borrelia burgdorferi sensu lato seroprevalence and associated risk factors in Finland. Ticks Tick Borne Dis 2018; 9:275–280. [DOI] [PubMed] [Google Scholar]
- Van den Wijngaard CC, Hofhuis A, Simões M, Rood E, et al. Surveillance perspective on Lyme borreliosis across the European Union and European economic area. Eurosurveillance 2017; 22:30569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gorkom T, Kremer K, Voet W, Notermans DW, et al. Disagreement between the results from three commercial tests for the detection of Borrelia-specific serum antibodies in the Netherlands associated with antibiotic treatment for Lyme borreliosis: a retrospective study. Eur J Clin Microbiol Infect Dis 2017; 36:2137–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestrheim DF, White RA, Aaberge IS, Aase A. Geographical differences in seroprevalence of Borrelia burgdorferi antibodies in Norway, 2011–2013. Ticks Tick Borne Dis 2016; 7:698–702. [DOI] [PubMed] [Google Scholar]
- Wilhelmsson P, Fryland L, Lindblom P, Sjowall J, et al. A prospective study on the incidence of Borrelia burgdorferi sensu lato infection after a tick bite in Sweden and on the Aland Islands, Finland (2008–2009). Ticks Tick Borne Dis 2016; 7:71–79. [DOI] [PubMed] [Google Scholar]
- Wilking H, Fingerle V, Klier C, Thamm M, et al. Antibodies against Borrelia burgdorferi sensu lato among adults, Germany, 2008–2011. Emerg Infect Dis 2015; 21:107–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilske B, Fingerle V, Schulte-Spechtel U. Microbiological and serological diagnosis of Lyme borreliosis. FEMS Immunol Med Microbiol 2007; 49:13–21. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Annex: regional classifications. 2022. Available at https://cdn.who.int/media/docs/default-source/air-pollution-documents/air-quality-and-health/country-groupings-database-2022.pdf
- Zając V, Pinkas J, Wójcik-Fatla A, Dutkiewicz J, et al. Prevalence of serological response to Borrelia burgdorferi in farmers from eastern and central Poland. Eur J Clin Microbiol Infect Dis 2017; 36:437–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zákutná L, Dorko E, Mattová E, Rimárová K. Sero-epidemiological study of Lyme disease among high-risk population groups in eastern Slovakia. Ann Agric Environ Med 2015a; 22:632–636. [DOI] [PubMed] [Google Scholar]
- Zákutná L, Dorko E, Rimárová K, Kizeková M. Pilot cross-sectional study of three zoonoses (Lyme disease, tularaemia, leptospirosis) among healthy blood donors in eastern Slovakia. Cent Eur J Public Health 2015b; 23:100–106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All results of this systematic literature review are derived from the published literature as referenced.