Abstract
Background
Autism spectrum condition and attention-deficit/hyperactivity disorder (ADHD) are associated with a range of physical health conditions. The aim of this study was to examine the etiological components contributing to co-occurring physical health conditions in autism and ADHD.
Methods
In this nationwide Child and Adolescent Twin Study in Sweden, we analyzed data from 10,347 twin pairs aged 9 and 12. Clinical diagnoses of autism, ADHD, and physical health conditions were identified through the Swedish National Patient Register. Subclinical phenotypes of autism and ADHD were defined by symptom thresholds on a standardized parent-interview, the Autism–Tics, ADHD, and Other Comorbidities inventory. Associations between physical health conditions and autism/ADHD phenotypes were examined using generalized estimating equations. Bivariate twin models were applied to estimate the extent to which genetic and environmental risk factors accounted for physical health comorbidities.
Results
Similar patterns of association with physical health conditions were found in clinical and subclinical autism/ADHD, with odds ratios ranging from 1.31 for asthma in subclinical ADHD to 8.03 for epilepsy in clinical autism. The estimated genetic correlation (ra) with epilepsy was 0.50 for clinical autism and 0.35 for subclinical autism. In addition, a modest genetic correlation was estimated between clinical autism and constipation (ra = 0.31), functional diarrhea (ra = 0.27) as well as mixed gastrointestinal disorders (ra = 0.30). Genetic effects contributed 0.86 for mixed gastrointestinal disorders in clinical ADHD (ra = 0.21). Finally, subclinical ADHD shared genetic risk factors with epilepsy, constipation, and mixed gastrointestinal disorders (ra = 0.30, 0.17, and 0.17, respectively).
Limitations
Importantly, since medical records from primary care were not included in the registry data used, we probably identified only more severe rather than the full range of physical health conditions. Furthermore, it needs to be considered that the higher prevalence of physical health conditions among autistic children and children with ADHD could be associated with the increased number of medical visits.
Conclusions
Shared genetic effects contribute significantly to autism and ADHD phenotypes with the co-occurring physical health conditions across different organ systems, including epilepsy and gastrointestinal disorders. The shared genetic liability with co-occurring physical health conditions was present across different levels of autism and ADHD symptom severity.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13229-023-00548-3.
Keywords: Autism, ADHD, Twin study, Genetics, Comorbidity, Etiology
Background
Autism spectrum condition (ASC) and attention-deficit/hyperactivity disorder (ADHD) are conditions of altered neurodevelopment caused by an interplay of polygenic predisposition and compounding environmental factors [1]. Autistic people or individuals with ADHD are at increased risk for physical health conditions across different body systems [2–5]. Recurrently highlighted co-occurring physical health conditions include epilepsy, immune dysregulation, and gastrointestinal (GI) dysfunction. The nature of the association between autism and ADHD behavioral phenotypes and these physical health conditions, and the potential shared etiological pathways has received growing scientific interest. Pediatric epilepsy is a condition of multiple genetic, cerebral, and metabolic etiologies and associated cognitive difficulties may be somewhat similar to those found in autism and ADHD [6]. Emerging evidence also suggests adverse effects of pre- and postnatal neuroinflammation on brain development and behavioral outcomes mimicking autism and ADHD [7, 8]. Finally, altered gut-brain axis mechanisms and dietary treatments are areas of topical interest in interventions aimed at autism and ADHD [9, 10].
While shared genetic susceptibility is postulated to partly account for the overlap between physical health conditions and autism/ADHD based on the identification of somatic pleiotropic genes [11], the magnitude of genetic contributions to comorbidity remains understudied. Five previous studies had examined genetic correlations between autism or ADHD and physical health conditions or physical/physiological parameters (e.g., asthma, migraine, height, triglycerides, fasting glucose) [12–16]. Three of them focused on adult ADHD or autism [12–14], and the other two explored childhood asthma and ADHD [15, 16]. However, these studies did not cover common co-occurring physical health conditions of clinical interest, such as epilepsy and pediatric GI disorders in childhood autism and ADHD. In addition, genetic contributions to autism and ADHD are likely to be distributed continuously in the general population [17], indicating gradually convergent patterns of co-occurring physical health conditions from behavioral traits to clinical diagnosis for these conditions. Therefore, a dichotomous classification of autism/ADHD by syndromic definition in previous study designs might have been of limited sensitivity to examine shared genetic effects with physical health conditions.
To unravel the genetic and environmental contributions to phenotypic correlations between two conditions/disorders with complex etiologies (i.e., autism/ADHD and physical health conditions), twin studies are particularly valuable and have been applied on the association between neurodevelopmental and physical health conditions [16]. While genome-wide association studies (GWAS) have started to estimate genetic correlations between autism/ADHD and physical health conditions [13], they were only based on common variants and have relatively low power yielding imprecise estimates.
Therefore, the objective of this study was to provide a comprehensive picture of common co-occurring physical health conditions in autistic children and children with ADHD at clinical and subclinical levels. We used information on clinical diagnoses and symptom severity of autism/ADHD from a population-based twin cohort to estimate the association of autism/ADHD phenotypes with physical health conditions. Moreover, we applied quantitative genetic modeling to estimate the degree to which co-occurring physical health conditions in clinical and subclinical autism/ADHD are accounted for by genetic and environmental factors. In this article, we adopted “identity-first” language (i.e., autistic individuals) rather than “person-first” language (i.e., individuals with autism) to avoid ableist language which could reflect discrimination and marginalization [18].
Methods
Participants
We used data collected from participants in the population-based Child and Adolescent Twin Study in Sweden (CATSS) [19]. This ongoing longitudinal study, initiated in 2004, invites all parents of twins aged 9 in Sweden to participate. (The earlier cohorts of CATSS included also twins aged 12.) The response rate was 70%. A total of 10,347 twin pairs were included in the current study, consisting of 8,125 nine-year-old and 2222 twelve-year-old twin pairs (4767 monozygotic [MZ] and 5580 same sex dizygotic [DZ] twin pairs). Zygosity was determined by a panel of 48 single-nucleotide polymorphisms (SNPs) or a validated questionnaire composed of 5 items on twin similarity. Zygosity was only assigned using the latter method if there was at least a 95% probability of correct classification. The study was approved by the Regional Ethical Review Board in Stockholm, Sweden.
Diagnoses of autism, ADHD, and physical health conditions
Co-occurring physical health conditions [2, 3] were selected based on previous reports of associations with autism or ADHD and evidence of a strong genetic predisposition. Included physical health conditions were epilepsy, migraine, other headaches, allergic rhinitis, asthma, atopic dermatitis, allergy to specific allergen, coeliac disease, constipation, functional diarrhea, and irritable bowel syndrome [20–29]. Mixed headache (migraine and non-migraine headache) and mixed functional GI disorders (mixed FGIDs, including constipation, functional diarrhea, and irritable bowel syndrome) were also analyzed considering the ambiguous classification and idiopathic nature of these diagnoses [30, 31]. The diagnoses of autism, ADHD, and physical health conditions were identified by linking CATSS with the Swedish National Patient Register, containing medical records from in- and outpatient care. (Inpatient coverage is nationwide from 1987, and outpatient coverage is from 2001.) All diagnoses were defined based on International Classification of Diseases, Ninth Revision (ICD-9) and International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) codes. The full list of diagnostic codes is given in Additional file 1.
Subclinical phenotypes of autism and ADHD
Autism and ADHD symptoms were assessed with the autism (17 items) and ADHD modules (19 items) of the Autism–Tics, ADHD, and Other Comorbidities inventory (A-TAC), an open access instrument with full-versions in Swedish and English available on https://www.gu.se/en/gnc/gncs-resources/screening-questionnaires/a-tac-screening-questionnaire (also see Additional file 2 for the English version). The A-TAC is a standardized telephone interview with parents/caregivers as informants that has demonstrated good validity for screening the full range of neurodevelopmental disorder symptoms [32]. Each item in A-TAC is scored as 0 for “No,” 0.5 for “Yes, to some extent,” and 1 for “Yes.” The full A-TAC consists of 96 items, and the interview takes on average 32 min to administer. Subclinical phenotypes of autism were defined by scores of 4.5 or more on the A-TAC autism module (the lower cutoff value for screening autism, with sensitivity 0.85 and specificity 0.97, resulting in a prevalence of 3.6% screened positive in the general population); subclinical ADHD was defined by scores of at least 6.0 on the A-TAC ADHD module (the lower cutoff; sensitivity 0.79, specificity 0.90, resulting in a prevalence of 10.5% screened positive in the general population) [32].
Statistical analysis
MZ and same-sex DZ twins were included in the analyses for the potential differences in variance components across sex. We did not model the effects of sex in our analysis since no sex-specific genetic influences on behavioral traits of autism/ADHD have been reported in the literature [33, 34]. Conditional multivariate logistic regression analysis with twin pairs clustered was used to explore the associations between physical health conditions and autism, ADHD, as well as the overlap of autism and ADHD by calculating odds ratios, adjusting for sex and age at measurement. Physical health conditions significantly associated with autism or ADHD phenotypes entered the bivariate twin analysis. Statistical significance was set at P < 0.05. Bonferroni correction was applied for multiple comparisons.
Structural equation modeling was used to estimate the relative genetic and environmental contributions to variation in liability to the clinical (categorical) phenotypes of a disorder, with the assumption that a continuous distribution of liability underlies such disorders. Liability variance was divided into three latent components: additive genetic (A), non-additive genetic (D) or shared environments (C), and nonshared environment (E). Only C or D can be estimated in a model, as they confound one another in the classical twin design. These components are estimated based on comparing the correlations between MZ twins who share all their segregating DNA and DZ twins who share on average 50% of their segregating genes. Principles of twin design are provided in detail elsewhere [35].
Univariate analyses were used to provide variance component estimates for physical health conditions and to test assumptions of the twin design. We tested only ACE but not ADE models to increase power. Thus, the proportion of genetic effects contributing to the variance should be interpreted as broad-sense heritability. Further nested models (AE and E models) were tested and compared to ACE models using the likelihood-ratio test. The correlations of one twin’s autism or ADHD phenotypes with their co-twin’s physical health condition (cross-twin cross-trait correlations) in MZ and DZ pairs were calculated. To study whether genetic and environmental risk factors for autism and ADHD phenotypes are associated with the co-occurring physical health conditions, we fitted the bivariate Cholesky decomposition to the categorical phenotype variables, which are presented here as the mathematically equivalent correlated factors solution. The genetic correlation estimates are correlation coefficients indicating the degree to which the genetic influences on two phenotypes correlate with one another. Bivariate heritability estimates, the proportion of phenotypic correlations explained by shared genetics, were calculated. To rule out that genetic correlations between subclinical autism/ADHD and co-occurring physical health conditions are driven by clinical autism/ADHD, sensitivity analyses were conducted by excluding clinical cases from subclinical groups. All twin models were conducted in OpenMx package for R [36].
Results
The prevalence of autism, ADHD, and physical health conditions in our sample across and by sex/zygosity is presented in Table 1 (see Additional file 1: Table S1 for sex distribution and prevalence in different sex of autism/ADHD in Additional file 1). The prevalence of subclinical autism and subclinical ADHD in the sample for twin model analysis was 4.0% and 11.3%, respectively (please note that the physical health conditions in this study did not include the data from primary care health services). Table 2 summarizes the odds ratios (OR) of having physical health conditions in autism and ADHD for clinical and subclinical phenotypes. After adjusting for age and sex, both clinical and subclinical autism were associated with epilepsy, asthma, constipation, functional diarrhea, and mixed FGIDs. As for ADHD, clinical and subclinical phenotypes both displayed significant associations with epilepsy, asthma, constipation, and mixed FGIDs, while clinical ADHD was also associated with migraine and mixed headache. The ORs varied across different physical health conditions, ranging from OR = 1.31 for asthma in subclinical ADHD to OR = 8.03 for epilepsy in clinical autism. The associations between physical health conditions and the overlap of autism and ADHD as well as the mutually exclusive groups are shown in Additional file 1: Table S2.
Table 1.
Prevalence of autism/ADHD and physical health conditions in the total sample and subsamples by sex/zygosity
| Full sample (N) | Total | MZF | DZF | MZM | DZM | DZOS | Unknown | Sample for twin model analysisa | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 32,250 | 4,934 | 5,184 | 4,600 | 5,976 | 11,010 | 546 | 20,694 | |||||||||
| N | % | N | % | N | % | N | % | N | % | N | % | N | % | % | 95% CI | |
| Clinical autism | 534 | 1.7 | 33 | 0.7 | 60 | 1.2 | 85 | 1.8 | 151 | 2.5 | 189 | 1.7 | 16 | 2.9 | 1.6 | 1.4, 1.8 |
| Subclinical autism | 1,251 | 3.9 | 96 | 1.9 | 157 | 3.0 | 211 | 4.6 | 328 | 5.5 | 434 | 3.9 | 25 | 4.6 | 4.0 | 3.7, 4.3 |
| Clinical ADHD | 1,427 | 4.4 | 117 | 2.4 | 169 | 3.3 | 240 | 5.2 | 369 | 6.2 | 501 | 4.6 | 31 | 5.7 | 4.3 | 4.0, 4.7 |
| Subclinical ADHD | 3,607 | 11.2 | 340 | 6.9 | 490 | 9.5 | 602 | 13.1 | 886 | 14.8 | 1,214 | 11.0 | 75 | 13.7 | 11.3 | 10.9, 11.8 |
| Neurological disorders | ||||||||||||||||
| Epilepsy | 392 | 1.2 | 57 | 1.2 | 56 | 1.1 | 51 | 1.1 | 92 | 1.5 | 128 | 1.2 | 8 | 1.5 | 1.2 | 1.1, 1.4 |
| Migraine | 522 | 1.6 | 81 | 1.6 | 106 | 2.0 | 58 | 1.3 | 102 | 1.7 | 171 | 1.6 | 4 | 0.7 | 1.7 | 1.5, 1.9 |
| Other headache | 267 | 0.8 | 55 | 1.1 | 50 | 1.0 | 26 | 0.6 | 33 | 0.6 | 100 | 0.9 | 3 | 0.5 | 0.8 | 0.7, 0.9 |
| Mixed headache | 730 | 2.3 | 126 | 2.6 | 144 | 2.8 | 80 | 1.7 | 127 | 2.1 | 247 | 2.2 | 6 | 1.1 | 2.3 | 2.1, 2.5 |
| Immunological disorders | ||||||||||||||||
| Allergic rhinitis | 1,920 | 6.0 | 244 | 4.9 | 234 | 4.5 | 301 | 6.5 | 445 | 7.4 | 670 | 6.1 | 26 | 4.8 | 5.9 | 5.6, 6.3 |
| Asthma | 4,317 | 13.4 | 514 | 10.4 | 695 | 13.4 | 598 | 13.0 | 960 | 16.1 | 1,476 | 13.4 | 74 | 13.6 | 13.4 | 12.8, 13.9 |
| Atopic dermatitis | 1,647 | 5.1 | 242 | 4.9 | 261 | 5.0 | 205 | 4.5 | 341 | 5.7 | 565 | 5.1 | 33 | 6.0 | 5.1 | 4.7, 5.4 |
| Specific allergy | 643 | 2.0 | 90 | 1.8 | 83 | 1.6 | 107 | 2.3 | 122 | 2.0 | 234 | 2.1 | 7 | 1.3 | 1.9 | 1.7, 2.2 |
| Coeliac disease | 362 | 1.1 | 72 | 1.5 | 73 | 1.4 | 29 | 0.6 | 52 | 0.9 | 131 | 1.2 | 5 | 0.9 | 1.1 | 0.9, 1.3 |
| Gastrointestinal disorders | ||||||||||||||||
| Constipation | 2,035 | 6.3 | 271 | 5.5 | 345 | 6.7 | 258 | 5.6 | 400 | 6.7 | 728 | 6.7 | 33 | 6.0 | 6.2 | 5.8, 6.5 |
| Functional diarrhea | 187 | 0.6 | 31 | 0.6 | 35 | 0.7 | 24 | 0.5 | 39 | 0.7 | 54 | 0.5 | 4 | 0.7 | 0.6 | 0.5, 0.7 |
| Irritable bowel syndrome | 127 | 0.4 | 30 | 0.6 | 25 | 0.5 | 7 | 0.2 | 11 | 0.2 | 53 | 0.5 | 1 | 0.2 | 0.4 | 0.3, 0.4 |
| Mixed FGIDs | 2,283 | 7.1 | 320 | 6.5 | 390 | 7.5 | 283 | 6.2 | 431 | 7.2 | 821 | 7.5 | 38 | 7.0 | 6.9 | 6.5, 7.3 |
The physical health conditions in this study did not include the data from primary care health services
ADHD, attention-deficit/hyperactivity disorder; DZF, dizygotic female; DZM, dizygotic male; DZOS, dizygotic opposite sex; FGIDs, functional gastrointestinal disorders; MZF, monozygotic female; MZM, monozygotic male
aMZ and same-sex DZ twins
Table 2.
Association between autism/ADHD and physical health conditions in our sample (N = 20,964)
| Co-occurring physical health conditions | Autism | ADHD | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinical diagnosis | Subclinical phenotype | Clinical diagnosis | Subclinical phenotype | |||||||||
| Odds ratioa | 95% CI | p | Odds ratioa | 95% CI | p | Odds ratioa | 95% CI | p | Odds ratioa | 95% CI | p | |
| Neurological disorders | ||||||||||||
| Epilepsy | 8.03 | 5.32, 12.11 | < 0.001 | 6.75 | 4.82, 9.45 | < 0.001 | 3.52 | 2.46, 5.04 | < 0.001 | 3.83 | 2.91, 5.06 | < 0.001 |
| Migraine | 0.75 | 0.28, 2.03 | 0.513 | 1.00 | 0.57, 1.74 | 0.985 | 2.22 | 1.52, 3.25 | < 0.001 | 1.28 | 0.93, 1.76 | 0.126 |
| Other headache | 1.79 | 0.66, 4.87 | 0.258 | 1.06 | 0.46, 2.43 | 0.885 | 1.80 | 0.96, 3.35 | 0.066 | 1.21 | 0.73, 1.99 | 0.456 |
| Mixed headache | 1.13 | 0.56, 2.30 | 0.733 | 1.03 | 0.64, 1.65 | 0.911 | 2.08 | 1.46, 2.97 | < 0.001 | 1.22 | 0.92, 1.62 | 0.166 |
| Immunological disorders | ||||||||||||
| Allergic rhinitis | 1.10 | 0.71, 1.72 | 0.665 | 1.01 | 0.74, 1.37 | 0.959 | 1.32 | 1.02, 1.71 | 0.037 | 1.05 | 0.87, 1.27 | 0.597 |
| Asthma | 1.55 | 1.16, 2.08 | 0.003 | 1.45 | 1.23, 1.82 | < 0.001 | 1.63 | 1.36, 1.96 | < 0.001 | 1.31 | 1.16, 1.49 | < 0.001 |
| Atopic dermatitis | 1.28 | 0.78, 2.08 | 0.329 | 1.20 | 0.87, 1.65 | 0.264 | 1.52 | 1.15, 2.12 | 0.004 | 1.11 | 0.91, 1.36 | 0.303 |
| Specific allergy | 1.21 | 0.55, 2.65 | 0.644 | 0.93 | 0.54, 1.63 | 0.808 | 1.30 | 0.80, 2.12 | 0.294 | 0.87 | 0.62, 1.24 | 0.451 |
| Coeliac disease | 2.67 | 1.21, 5.85 | 0.015 | 1.45 | 0.75, 2.80 | 0.267 | 0.91 | 0.45, 1.83 | 0.784 | 1.24 | 0.81, 1.91 | 0.328 |
| Gastrointestinal disorders | ||||||||||||
| Constipation | 2.03 | 1.43, 2.88 | < 0.001 | 2.17 | 1.73, 2.72 | < 0.001 | 1.91 | 1.53, 2.39 | < 0.001 | 1.65 | 1.41, 1.93 | < 0.001 |
| Functional diarrhea | 5.51 | 2.89, 10.52 | < 0.001 | 2.67 | 1.48, 4.83 | 0.001 | 2.13 | 1.15, 3.94 | 0.017 | 1.36 | 0.83, 2.25 | 0.226 |
| Irritable bowel syndrome | 2.25 | 0.55, 9.18 | 0.257 | 1.29 | 0.41, 4.11 | 0.667 | 1.58 | 0.57, 4.38 | 0.379 | 1.86 | 1.01, 3.42 | 0.047 |
| Mixed FGIDs | 2.34 | 1.69, 3.24 | < 0.001 | 2.08 | 1.67, 2.59 | < 0.001 | 1.92 | 1.55, 2.38 | < 0.001 | 1.59 | 1.37, 1.84 | < 0.001 |
The physical health conditions in this study did not include the data from primary care health services
ADHD, attention-deficit/hyperactivity disorder; FGIDs, functional gastrointestinal disorders
Statistical significance for this table was set at p < 0.0038 (Bonferroni correction for multiple comparisons)
Bold value: statistically significant and being included in bivariate twin model analysis
aAdjusted for age and sex
Cross-twin correlations and etiological components for each physical health disorder are provided in Table 3. The MZ twin correlations exceeded the DZ correlations for all the physical health conditions, indicating that the variation in liability to each condition was associated with genetic factors. Neurological conditions displayed heritability (h2) ranging from 0.41 for migraine to 0.61 for epilepsy, with no indication of shared environmental effects (see Additional file 1: Table S3 for model fit statistics). Immunological conditions, except for coeliac disease, showed similar heritability between 0.68 and 0.73. The proportions of etiological components for coeliac disease were different from those of others, in which A and C contributed to the etiology equally (0.49 and 0.47, respectively). As for GI conditions, genetics accounted for the variation in liability predominantly in three of the four conditions (range from 0.51 to 0.77), while the heritability of irritable bowel syndrome was estimated as 0.26.
Table 3.
Univariate models for physical health conditions
| Physical health conditions | Cross-twin correlation | The proportions explained by each etiology component | Heritability reported by other studies | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Genetic effects | Shared environmental effects | Nonshared environmental effects |
||||||||
| r | 95% CI | A/V | 95% CI | C/V | 95% CI | E/V | 95% CI | h2 | ||
| Neurological disorders | ||||||||||
| Epilepsy | MZ | 0.62 | 0.47, 0.75 | 0.61 | 0.24, 0.73 | 0 | 0.39 | 0.27, 0.54 | range from 0.69 to 0.88 [20–22] | |
| DZ | 0.25 | 0.04, 0.44 | ||||||||
| Migraine | MZ | 0.45 | 0.26, 0.60 | 0.41 | 0.07, 0.56 | 0 | 0.59 | 0.44, 0.75 |
0.45 (95% CI = 0.41, 0.49) [23] (meta-analysis) |
|
| DZ | 0.13 | –0.06, 0.31 | ||||||||
| Other headache | MZ | 0.54 | 0.35, 0.70 | 0.53 | 0, 0.69 | 0 | 0.47 | 0.31, 0.66 |
range from 0.40 to 0.45 [23] (tension-type headache) |
|
| DZ | 0.18 | –0.19, 0.47 | ||||||||
| Mixed headache | MZ | 0.49 | 0.36, 0.61 | 0.45 | 0.23, 0.57 | 0 | 0.55 | 0.43, 0.67 | ||
| DZ | 0.12 | –0.05, 0.28 | ||||||||
| Immunological disorders | ||||||||||
| Allergic rhinitis | MZ | 0.76 | 0.71, 0.81 | 0.73 | 0.55, 0.81 | 0.04 | 0, 0.19 | 0.24 | 0.19, 0.29 | range from 0.33 to 0.91 [24] |
| DZ | 0.40 | 0.32, 0.47 | ||||||||
| Asthma | MZ | 0.83 | 0.80, 0.85 | 0.68 | 0.57, 0.79 | 0.15 | 0.05, 0.25 | 0.17 | 0.15, 0.20 |
0.54 (95% CI = 0.44, 0.63) [25] (meta-analysis) |
| DZ | 0.49 | 0.44, 0.54 | ||||||||
| Atopic dermatitis | MZ | 0.79 | 0.74, 0.83 | 0.73 | 0.55, 0.83 | 0.06 | 0, 0.22 | 0.21 | 0.20a, 0.26 |
0.74 (95% CI = 0.64, 0.83) [25] (meta-analysis) |
| DZ | 0.43 | 0.35, 0.50 | ||||||||
| Specific allergy | MZ | 0.90 | 0.85, 0.93 | 0.71 | 0.48, 0.93 | 0.19 | 0, 0.40 | 0.10 | 0.07, 0.14 |
0.63 (95% CI = 0.55, 0.71) [25] (meta-analysis) |
| DZ | 0.54 | 0.42, 0.65 | ||||||||
| Coeliac disease | MZ | 0.96 | 0.92, 0.98 | 0.49 | 0.30, 0.72 | 0.47 | 0.24, 0.65 | 0.04 | 0.02, 0.08 | range from 0.57 to 0.87 [27–29] |
| DZ | 0.71 | 0.60, 0.80 | ||||||||
| Gastrointestinal disorders | ||||||||||
| Constipation | MZ | 0.51 | 0.43, 0.59 | 0.51 | 0.30, 0.58 | 0 | 0.49 | 0.43, 0.49a | ||
| DZ | 0.25 | 0.17, 0.33 | ||||||||
| Functional diarrhea | MZ | 0.93 | 0.86, 0.97 | 0.77 | 0.42, 0.97 | 0.16 | 0, 0.49 | 0.07 | 0.03, 0.14 | |
| DZ | 0.54 | 0.33, 0.71 | ||||||||
| Irritable bowel syndrome | MZ | 0.69 | 0.46, 0.85 | 0.26 | 0, 0.83 | 0.43 | 0, 0.77 | 0.31 | 0.15, 0.53 |
0.22 (95% CI = 0.13, 0.30) [25] (meta-analysis) |
| DZ | 0.57 | 0.25, 0.79 | ||||||||
| Mixed FGIDs | MZ | 0.55 | 0.48, 0.62 | 0.56 | 0.39, 0.62 | 0 | 0.44 | 0.38, 0.51 | 0.57 (95% CI = 0.41, 0.76) [29] | |
| DZ | 0.27 | 0.19, 0.34 | ||||||||
The physical health conditions in this study did not include the data from primary care health services
A, additive genetic variance; C, shared environmental variance; DZ, dizygotic twins; E, nonshared environmental variance; FGIDs, functional gastrointestinal disorders; MZ, monozygotic twins; V, phenotypic variance
aalpha level not reached
The co-occurrence rates and proband-wise cross-concordances for each physical health disorder associated with autism and ADHD are given in Table 4. Proband-wise cross-concordances denote the probability of co-twins of autism or ADHD probands having the diagnosis of each physical disorder. The MZ proband-wise cross-concordances were higher than DZ estimates for all GI disorders in clinical autism and functional diarrhea in subclinical autism, as well as epilepsy in both clinical and subclinical ADHD. Additional file 1: Table S4 in Additional file 1 presents the cross-twin cross-trait correlations between the associated physical health disorder and autism/ADHD. There were higher MZ than DZ cross-disorder correlations for all the physical health conditions in clinical autism, and for epilepsy, mixed headache, constipation, and mixed FGIDs in clinical ADHD, suggesting that genetic factors influence each condition and the covariance between them. However, regarding subclinical phenotypes of autism and ADHD, the MZ cross-disorder correlations were relatively equivalent to DZ estimates for all the physical health conditions.
Table 4.
The co-occurrence rates and proband-wise cross-concordances for physical health conditions in autism and ADHD
| Physical health conditions | Affected probands who also have physical health conditions | Proportion of co-twins with physical health conditions | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinical diagnosis | Subclinical phenotype | Clinical diagnosis | Subclinical phenotype | |||||||||
| MZ | DZ | MZ | DZ | |||||||||
| % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | |
| Autism | ||||||||||||
| Neurological disorders | ||||||||||||
| Epilepsy | 8.5 | 5.5, 11.5 | 4.8 | 3.1, 7.3 | 4.2 | 0.6, 7.9 | 4.3 | 1.5, 7.0 | 1.6 | 0.2, 3.0 | 2.3 | 1.0, 3.6 |
| Immunological disorders | ||||||||||||
| Asthma | 20.4 | 15.6, 25.1 | 21.6 | 17.8, 25.6 | 19.5 | 11.1, 27.8 | 22.3 | 16.4, 28.1 | 16.6 | 11.7, 21.5 | 16.5 | 13.0, 20.0 |
| Coeliac disease | 2.4 | 0.6, 4.3 | 1.5 | 0.7, 3.2 | 0.8 | 0, 2.5 | 2.4 | 0, 4.8 | 0.7 | 0, 1.6 | 1.2 | 0.1, 2.4 |
| Gastrointestinal disorders | ||||||||||||
| Constipation | 11.6 | 8.0, 15.1 | 13.8 | 10.8, 17.5 | 11.0 | 4.8, 17.2 | 8.1 | 4.4, 11.7 | 10.1 | 6.5, 13.7 | 10.9 | 8.2, 13.7 |
| Functional diarrhea | 3.0 | 1.2, 4.9 | 1.3 | 0.5, 2.9 | 2.5 | 0, 5.4 | 1.9 | 0.1, 3.7 | 2.0 | 0.4, 3.5 | 1.0 | 0.1, 1.9 |
| Mixed FGIDs | 14.3 | 10.4, 18.2 | 14.3 | 11.2, 18.1 | 13.6 | 7.0, 21.2 | 9.5 | 5.4, 13.5 | 11.1 | 7.3, 14.8 | 11.5 | 8.7, 14.3 |
| ADHD | ||||||||||||
| Neurological disorders | ||||||||||||
| Epilepsy | 3.9 | 2.6, 5.2 | 3.1 | 2.3, 4.3 | 3.1 | 1.3, 4.9 | 2.8 | 1.4, 4.2 | 2.2 | 1.2, 3.2 | 1.9 | 1.1, 2.6 |
| Migraine | 3.4 | 2.2, 4.5 | 1.9 | 1.3, 2.9 | 2.0 | 0.5, 3.4 | 3.7 | 2.1, 5.3 | 1.6 | 0.7, 2.4 | 2.0 | 1.2, 2.7 |
| Mixed headache | 4.2 | 2.9, 5.6 | 2.5 | 1.8, 3.6 | 3.4 | 1.2, 5.5 | 4.1 | 2.4, 5.7 | 2.1 | 1.1, 3.1 | 2.7 | 1.8, 3.5 |
| Immunological disorders | ||||||||||||
| Asthma | 20.7 | 17.8, 23.6 | 19.1 | 17.0, 21.5 | 20.7 | 15.9, 25.6 | 20.6 | 17.4, 24.2 | 16.7 | 13.8, 19.5 | 16.9 | 14.9, 19.0 |
| Gastrointestinal disorders | ||||||||||||
| Constipation | 10.7 | 8.7, 12.8 | 10.2 | 8.6, 12.1 | 8.7 | 5.5, 11.9 | 10.2 | 7.7, 12.7 | 7.1 | 5.3, 8.9 | 8.7 | 7.3, 10.2 |
| Mixed FGIDs | 11.8 | 9.7, 14.0 | 10.9 | 9.2, 12.8 | 10.6 | 7.2, 14.1 | 11.2 | 8.5, 13.8 | 8.3 | 6.4, 10.2 | 9.5 | 8.0, 11.0 |
The physical health conditions in this study did not include the data from primary care health services
ADHD, attention-deficit/hyperactivity disorder; DZ, dizygotic twins; FGIDs, functional gastrointestinal disorders; MZ, monozygotic twins
Bold values: Higher MZ than DZ estimates
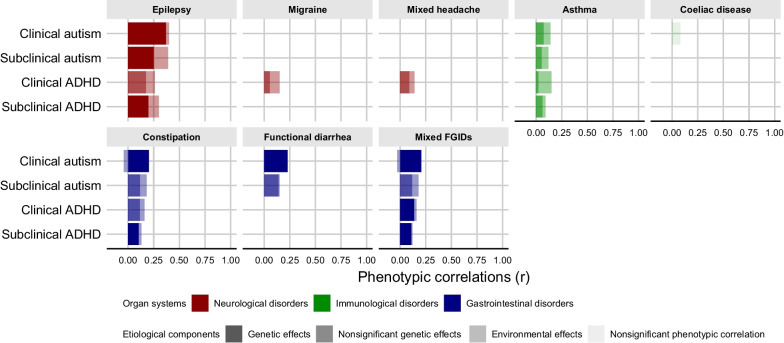
The phenotypic correlations, etiological correlations, and bivariate heritability between associated physical health conditions and autism/ADHD from each model are summarized in Fig. 1 and in Additional file 1: Table S5. Except for coeliac disease, there were significant phenotypic correlations (rPH) between all the physical health conditions and autism/ADHD for both clinical and subclinical phenotypes, ranging from 0.09 for asthma and subclinical ADHD to 0.40 for epilepsy and clinical autism. For autism, a common genetic liability was of major importance for co-occurring epilepsy in both clinical and subclinical phenotypes (proportions = 0.93 and 0.64, respectively). The estimated genetic correlation (ra) with epilepsy was 0.50 (95% CI = 0.27 − 0.76) for clinical autism and 0.35 (95% CI = 0.19 − 0.54) for subclinical autism. In addition, a modest genetic correlation was estimated between clinical autism and other physical health conditions, including constipation, functional diarrhea, and mixed FGIDs ([ra] as 0.27 [95% CI = 0.11 − 0.50], 0.31 [95% CI = 0.22 − 0.43], and 0.30 [95% CI = 0.12 − 0.63], respectively). For ADHD, subclinical phenotype displayed genetic correlations (ra) with epilepsy (0.30 [95% CI = 0.17 − 0.53]), constipation (0.17 [95% CI = 0.04 − 0.29]), and mixed FGIDs (0.17 [95% CI = 0.07 − 0.28]), while clinical ADHD showed significant genetic correlations (ra) only with mixed FGIDs (0.21 [95% CI = 0.02 − 0.44]). Model fit statistics are given in Additional file 1(Table S6-S9). Estimates derived from sensitivity analyses for subclinical autism/ADHD are presented in Additional file 1: Table S10.
Fig. 1.
Etiological components contributing to phenotypic correlations between autism/ADHD and physical health conditions. ADHD, attention-deficit/hyperactivity disorder; FGIDs, functional gastrointestinal disorders
Discussion
To our knowledge, this is the first twin study to systematically examine the shared etiologies of common physical health conditions in children with clinical and subclinical autism and ADHD phenotypes. We found that both clinical and subclinical autism and ADHD displayed phenotypic correlations with neurological, immunological, and GI conditions, which were found also moderately to highly heritable. Our findings indicate that children with higher liability for clinical autism and ADHD have an increased risk for even various co-occurring physical health conditions, and that the risk extends to subclinical variants of autism and ADHD. In addition, the majority of co-occurring physical health conditions in autism and ADHD were explained by shared genetic factors. Taken together, the results of this study support the notion that autism and ADHD are quantitative extremes of continuously altered neurodevelopment, where cumulative genetic effects also contribute to an increased liability for co-occurring physical health conditions.
In addition to clinically diagnosed autism and ADHD, our findings suggest that subclinical phenotypes are also associated with a range of physical complications, with comorbidity profiles similar to those with clinical variants. Despite the under-threshold symptom severity, the potential clinical relevance of subclinical phenotypes of autism and ADHD has been discussed in the context of risk of psychiatric disorders and negative long-term outcomes [37–39]. Presently, little is known about co-occurring physical health conditions in subclinical manifestations of autism and ADHD. Physical health conditions have been linked to ADHD symptoms [40, 41], as well as to increased functional impairment and poorer clinical trajectories in clinical autism [42–45]. In addition, it has been reported that physical health conditions might play a role as the nonshared environmental factor contributing to the emergence of autism and ADHD symptoms among individuals with genetic predisposition [46, 47]. Based on the multifactorial model of autism and ADHD, the liability for clinical diagnosis is contingent on the accumulation and interplay of genetic and environmental risk factors [48, 49]. Individuals with subclinical variants, who have high levels of risk, are likely to be susceptible to develop symptoms meeting clinical diagnosis once the accumulated effects exceed the threshold [50, 51]. Therefore, the identification of potential indicators of subsequent autism/ADHD among the at-risk population might be clinically meaningful in terms of intervention. Our results highlight the need of further investigation on the impact and clinical implication of physical health conditions in subclinical autism and ADHD.
The univariate twin modeling analysis of epilepsy, coeliac disease, and the selected GI conditions was conducted with a child twin sample for the first time. The heritability of pediatric epilepsy and irritable bowel syndrome was found similar to the results derived from other adult samples [20–22, 25], while coeliac disease in children showed relatively lower heritability but higher proportions of common environmental variance [26–28]. It has been noted that there is a true rise of coeliac disease in recent decades, independent of the genetic background of the surveyed population [52]. The increased incidence of coeliac disease was attributed to emerging environmental elements which affected the immune tolerance to gluten in genetically predisposed children [53]. Our result might reflect the stronger role of environmental triggers contributing to coeliac disease among the young generation in contrast to adult cohorts. As for other FGIDs, despite some evidence on the familial aggregation [54, 55], the heritability of specific diseases has not been explored previously. The moderate to high heritability in our study suggests that genetic effects explain at least half of the liability variance to pediatric constipation and functional diarrhea.
Our results do not support an association between coeliac disease and clinical autism and ADHD. Although the potential benefit of gluten-free diet on symptoms of autism and ADHD has recently received considerable scientific attention [56–58], the epidemiological links are unclear. Positive associations have only been reported in large population-based samples with all manifestations of traits, subclinical and clinical forms of autism/ADHD, but not in smaller samples [59–62]. These discrepancies may reflect both autism and ADHD heterogeneity, and relatively small effect sizes of the associations with physical health conditions that could only be detected by samples with higher statistical power. Compared to the high co-occurrence of neurodevelopmental conditions [63], the comorbidity rates of physical health conditions in autism and ADHD were relatively low. Also, the phenotypic correlations between physical health conditions and autism as well as ADHD were low to moderate. Thus, we were unable to observe differences in MZ and DZ cross-concordances among the associated physical health conditions despite significant genetic correlations. These results emphasize the multifaceted nature and diverse somatic phenotypes of autism and ADHD, which could also reflect the disparity in treatment responses [9, 64]. Hence, it is recommended for future research to focus on defining the potential biological subtypes, the associated biomarkers, and the specific targeted interventions [65, 66].
The present study provides evidence that the risk for co-occurring physical health conditions increased with the higher liability to autism and ADHD diagnosis. These findings are consistent with a biomedical approach to autism and ADHD [2, 3], in light of somatic pleiotropy of genes associated with autism as well as ADHD [11]. The common etiology of neurodevelopmental conditions and physical health conditions might imply a broad phenotyping strategy in future genetic studies, in order to enhance gene discovery and the understanding of genetic architectures by phenotype-to-genotype approaches [67–70]. In addition, the continuous genetic effects on liability to co-occurring physical health conditions in autism and ADHD are in line with the concept of endophenotypes [71]. Therefore, genetically informative designs such as polygenic risk scores and linkage disequilibrium score regression might be applied to refined somatic phenotypes to aid in more straightforward and powerful genetic architecture analysis. Furthermore, an insight into the associated genetic variants for somatic phenotypes in autism/ADHD may facilitate the mapping of biological mechanisms onto behaviors, which is crucial for a molecular taxonomy [72], as well as the tailored pharmacological treatments for the specific subgroups [73].
On the other hand, the co-occurring physical health conditions in autism/ADHD could also be related to other factors than exclusively genetic liability. For instance, internalizing symptoms [74, 75], medications for behavior problems, including stimulants and antipsychotics, as well as behaviors such as food selectivity and toileting problems [76], have been shown to be associated with physical health. The same is true for healthcare access/quality, socioeconomic status, and lifestyle behaviors [77]. Therefore, a comprehensive assessment and awareness of the possibly environmentally related contributors to physical health are imperative for the adequate individualized support and intervention.
Our study had several strengths. First, we included both clinical diagnosis and subclinical phenotypes of autism and ADHD. The study design allowed us to examine the continuum model of genetics influence on co-occurring physical health conditions in autism and ADHD. Second, both autism and ADHD were analyzed in one cohort, resulting in a wealth of information on clinical and etiological profiles of somatic phenotypes in neurodevelopmental disorders.
Limitations
However, our results should be interpreted taking into account some of the study’s limitations. The prevalence of headaches and GI conditions in our study could be underestimated compared to previous epidemiological studies which utilized systematic screening for data collection [31, 78]. Since the Swedish National Patient Register does not include the medical records in primary care, we probably only identified children with more severe physical diseases and thus the overall coverage of physical health conditions across the full range of severity might be incomplete. There is also a possibility that the increased number of physical health conditions detected in autistic children or children with ADHD is associated with their increased number of medical visits [79–82]. In addition, our results may also not generalize beyond children in light of the differences in nature and profiles of physical health conditions between children and adults. For example, congenital structural alterations are usually present in epilepsy which develops in childhood, while head trauma, infection, and brain tumors might cause epilepsy at any age [83]. Also, pediatric headache phenotypes are difficult to classify and could continuously evolve into adulthood [84]. Future work should seek to assess whether the etiology of co-occurring physical health conditions in autism and ADHD changes across the life span. Moreover, we did not include other demographic characteristics in the analysis of the association between physical health conditions and autism/ADHD, such as geographic distribution, socioeconomic status, and ethnicity, which might confound our results [2, 3]. The aforementioned demographic variables were not available in our dataset, and thus, we are unable to determine how representative this sample is. Furthermore, the prevalence of autism/ADHD and physical health conditions could influence the estimates of heritability [85, 86]. Therefore, the generalizability of our results to countries outside Northern Europe should be considered. Other factors which could impact the representativeness of our sample include non-response bias (response rate 70%), the sampling (to sample only twins within a population), and that twins have increased risk for low birth weight, which is associated with neurodevelopmental and physical health conditions [87–89]. Finally, children with co-occurring autism and ADHD tended to have similar risks for physical health conditions to those with autism or ADHD in our sample, for both clinical diagnosis and subclinical phenotypes. Nevertheless, we were unable to examine the complete profile of physical health conditions in this group as well as the mutually exclusive samples of autism and ADHD due to limited statistical power. In view of the shared genetic liability between autism and ADHD [63], it could be of value to explore in more detail and a larger sample the manifestation and etiology of physical health conditions in individuals with “exclusive autism,” “exclusive ADHD,” and “overlapping of autism and ADHD” in future research.
Conclusions
This study endorses the quantitative nature of autism and ADHD with genetic effects forming a continuum of behavioral traits that are associated with physical health conditions in different body systems. These associations vary in strength, and further research into the genetic links, biological pathways, and clinical implications of somatic phenotypes are required for a more complete picture of physical health in autism and ADHD.
Supplementary Information
Additional file 1. ICD code list. Table S1: Sex distribution of clinical and subclinical autism/ADHD in our sample. Table S2: Associations between physical health conditions and the overlap of autism and ADHD in our sample. Table S3: Univariate twin model fit statistics. Table S4: Bivariate models for autism/ADHD and physical health conditions. Table S5: Etiological component contributing to phenotypic correlation between autism/ADHD and physical health conditions. Table S6: Bivariate twin model of clinical autism and physical health conditions fit statistics. Table S7: Bivariate twin model of subclinical autism and physical health conditions fit statistics. Table S8: Bivariate twin model of clinical ADHD and physical health conditions fit statistics. Table S9: Bivariate twin model of subclinical ADHD and physical health conditions fit statistics. Table S10: Etiological component contributing to phenotypic correlation between subclinical autism/ADHDand physical health conditions.
Additional file 2. The English version of the Autism–Tics, ADHD, and Other Comorbidities inventory (A-TAC).
Acknowledgements
Not applicable.
Abbreviations
- ASC
Autism spectrum condition
- ADHD
Attention-deficit/hyperactivity disorder
- GI
Gastrointestinal
- GWAS
Genome-wide association studies
- CATSS
Child and Adolescent Twin Study in Sweden
- MZ
Monozygotic
- DZ
Dizygotic
- SNP
Single-nucleotide polymorphisms
- FGIDs
Functional gastrointestinal disorders
- ICD-9
International Classification of Diseases, Ninth Revision
- ICD-10
International Statistical Classification of Diseases and Related Health Problems, Tenth Revision
- A-TAC
The Autism-Tics, ADHD, and Other Comorbidities inventory
- OR
Odds ratios
Author contributions
PP, MT and SB conceived the research question and study design and acquired the data. HL, PL, and SB obtained funding. MT, HL, PL, and SL provided administrative, technical, or material support. PP and MT ran all analyses. PP wrote the first draft of the article. CA and all other co-authors contributed to data interpretation, critically reviewed drafts and edited the article for important intellectual content, and approved the final draft. MT and SB supervised this study. PP and SB had full access to all data in the study and had final responsibility for the decision to submit for publication. All authors read and approved the final manuscript.
Funding
Open access funding provided by Karolinska Institute. We acknowledge financial support from the Swedish Research Council and Swedish Queen Silvia's Anniversary Fund. PP is supported by Tri-Service General Hospital, the Ministry of National Defense, Taiwan (R.O.C), and the Swedish Research Council. We also acknowledge the Swedish Twin Registry for access to data. The Swedish Twin Registry is managed by Karolinska Institutet and receives funding through the Swedish Research Council under the Grant No. 2017-00641.
Availability of data and materials
The Public Access to Information and Secrecy Act in Sweden prohibits us from making individual-level data publicly available. Researchers who are interested in replicating our work can apply for individual-level data through The Child and Adolescent Twin Study in Sweden (CATSS) at: https://ki.se/en/meb/the-child-and-adolescent-twin-study-in-sweden-catss.
Declarations
Ethics approval and consent to participate
Informed consent was obtained from all children and their parents, and the study was approved by the Regional Ethical Review Board in Stockholm, Sweden.
Consent for publication
Not applicable.
Competing interests
HL has served as a speaker for Medice, Evolan Pharma and Shire/Takeda and has received research grants from Shire/Takeda; all outside the submitted work. SB discloses that he has in the last 5 years acted as an author, consultant or lecturer for Medice, and Roche. He receives royalties for textbooks and diagnostic tools from Hogrefe, Kohlhammer and UTB publishers. The authors declare no other potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Pei-Yin Pan, Email: pei-yin.pan@ki.se.
Mark J. Taylor, Email: mark.taylor@ki.se
Henrik Larsson, Email: henrik.larsson@ki.se.
Catarina Almqvist, Email: catarina.almqvist@ki.se.
Paul Lichtenstein, Email: paul.lichtenstein@ki.se.
Sebastian Lundström, Email: sebastian.lundstrom@gnc.gu.se.
Sven Bölte, Email: sven.bolte@ki.se.
References
- 1.Thapar A, Cooper M, Rutter M. Neurodevelopmental disorders. Lancet Psychiatry. 2017;4(4):339–346. doi: 10.1016/S2215-0366(16)30376-5. [DOI] [PubMed] [Google Scholar]
- 2.Davignon MN, Qian Y, Massolo M, Croen LA. Psychiatric and medical conditions in transition-aged individuals with ASD. Pediatrics. 2018;141(Suppl 4):S335–S345. doi: 10.1542/peds.2016-4300K. [DOI] [PubMed] [Google Scholar]
- 3.Akmatov MK, Ermakova T, Bätzing J. Psychiatric and nonpsychiatric comorbidities among children with ADHD: an exploratory analysis of nationwide claims data in Germany. J Atten Disord. 2021;25(6):874–884. doi: 10.1177/1087054719865779. [DOI] [PubMed] [Google Scholar]
- 4.Croen LA, Zerbo O, Qian Y, Massolo ML, Rich S, Sidney S, et al. The health status of adults on the autism spectrum. Autism. 2015;19(7):814–823. doi: 10.1177/1362361315577517. [DOI] [PubMed] [Google Scholar]
- 5.Instanes JT, Klungsøyr K, Halmøy A, Fasmer OB, Haavik J. Adult ADHD and comorbid somatic disease: a systematic literature review. J Atten Disord. 2018;22(3):203–228. doi: 10.1177/1087054716669589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nickels KC, Zaccariello MJ, Hamiwka LD, Wirrell EC. Cognitive and neurodevelopmental comorbidities in paediatric epilepsy. Nat Rev Neurol. 2016;12(8):465–476. doi: 10.1038/nrneurol.2016.98. [DOI] [PubMed] [Google Scholar]
- 7.Meltzer A, Van de Water J. The role of the immune system in autism spectrum disorder. Neuropsychopharmacology. 2017;42(1):284–298. doi: 10.1038/npp.2016.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunn GA, Nigg JT, Sullivan EL. Neuroinflammation as a risk factor for attention deficit hyperactivity disorder. Pharmacol Biochem Behav. 2019;182:22–34. doi: 10.1016/j.pbb.2019.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pelsser LM, Frankena K, Toorman J, Rodrigues PR. Diet and ADHD, reviewing the evidence: a systematic review of meta-analyses of double-blind placebo-controlled trials evaluating the efficacy of diet interventions on the behavior of children with ADHD. PLoS ONE. 2017;12(1):e0169277. doi: 10.1371/journal.pone.0169277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fraguas D, Díaz-Caneja CM, Pina-Camacho L, Moreno C, Durán-Cutilla M, Ayora M, et al. Dietary interventions for autism spectrum disorder: a meta-analysis. Pediatrics. 2019;144(5):e20183218. doi: 10.1542/peds.2018-3218. [DOI] [PubMed] [Google Scholar]
- 11.Vorstman JAS, Parr JR, Moreno-De-Luca D, Anney RJL, Nurnberger JI, Jr, Hallmayer JF. Autism genetics: opportunities and challenges for clinical translation. Nat Rev Genet. 2017;18(6):362–376. doi: 10.1038/nrg.2017.4. [DOI] [PubMed] [Google Scholar]
- 12.Wang K, Gaitsch H, Poon H, Cox NJ, Rzhetsky A. Classification of common human diseases derived from shared genetic and environmental determinants. Nat Genet. 2017;49(9):1319–1325. doi: 10.1038/ng.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bulik-Sullivan B, Finucane HK, Anttila V, Gusev A, Day FR, Loh PR, et al. An atlas of genetic correlations across human diseases and traits. Nat Genet. 2015;47(11):1236–1241. doi: 10.1038/ng.3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du Rietz E, Brikell I, Butwicka A, Leone M, Chang Z, Cortese S, et al. Mapping phenotypic and aetiological associations between ADHD and physical conditions in adulthood in Sweden: a genetically informed register study. Lancet Psychiatry. 2021;8(9):774–783. doi: 10.1016/S2215-0366(21)00171-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mogensen N, Larsson H, Lundholm C, Almqvist C. Association between childhood asthma and ADHD symptoms in adolescence–a prospective population-based twin study. Allergy. 2011;66(9):1224–1230. doi: 10.1111/j.1398-9995.2011.02648.x. [DOI] [PubMed] [Google Scholar]
- 16.Holmberg K, Lundholm C, Anckarsäter H, Larsson H, Almqvist C. Impact of asthma medication and familial factors on the association between childhood asthma and attention-deficit/hyperactivity disorder: a combined twin- and register-based study. Clin Exp Allergy. 2015;45(5):964–973. doi: 10.1111/cea.12529. [DOI] [PubMed] [Google Scholar]
- 17.Martin J, Taylor MJ, Lichtenstein P. Assessing the evidence for shared genetic risks across psychiatric disorders and traits. Psychol Med. 2018;48(11):1759–1774. doi: 10.1017/S0033291717003440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bottema-Beutel K, Kapp SK, Lester JN, Sasson NJ, Hand BN. Avoiding Ableist language: suggestions for autism researchers. Autism Adulthood. 2021;3(1):18–29. doi: 10.1089/aut.2020.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anckarsäter H, Lundström S, Kollberg L, Kerekes N, Palm C, Carlström E, et al. The child and adolescent twin study in Sweden (CATSS) Twin Res Hum Genet. 2011;14(6):495–508. doi: 10.1375/twin.14.6.495. [DOI] [PubMed] [Google Scholar]
- 20.Miller LL, Pellock JM, DeLorenzo RJ, Meyer JM, Corey LA. Univariate genetic analyses of epilepsy and seizures in a population-based twin study: the Virginia twin registry. Genet Epidemiol. 1998;15(1):33–49. doi: 10.1002/(SICI)1098-2272(1998)15:1<33::AID-GEPI3>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 21.Kjeldsen MJ, Kyvik KO, Christensen K, Friis ML. Genetic and environmental factors in epilepsy: a population-based study of 11900 Danish twin pairs. Epilepsy Res. 2001;44(2–3):167–178. doi: 10.1016/S0920-1211(01)00196-6. [DOI] [PubMed] [Google Scholar]
- 22.Kjeldsen MJ, Corey LA, Christensen K, Friis ML. Epileptic seizures and syndromes in twins: the importance of genetic factors. Epilepsy Res. 2003;55(1–2):137–146. doi: 10.1016/S0920-1211(03)00117-7. [DOI] [PubMed] [Google Scholar]
- 23.Nielsen CS, Knudsen GP, Steingrímsdóttir ÓA. Twin studies of pain. Clin Genet. 2012;82(4):331–340. doi: 10.1111/j.1399-0004.2012.01938.x. [DOI] [PubMed] [Google Scholar]
- 24.Ober C, Yao TC. The genetics of asthma and allergic disease: a 21st century perspective. Immunol Rev. 2011;242(1):10–30. doi: 10.1111/j.1600-065X.2011.01029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Polderman TJ, Benyamin B, de Leeuw CA, Sullivan PF, van Bochoven A, Visscher PM, et al. Meta-analysis of the heritability of human traits based on fifty years of twin studies. Nat Genet. 2015;47(7):702–709. doi: 10.1038/ng.3285. [DOI] [PubMed] [Google Scholar]
- 26.Skov J, Eriksson D, Kuja-Halkola R, Höijer J, Gudbjörnsdottir S, Svensson AM, et al. Co-aggregation and heritability of organ-specific autoimmunity: a population-based twin study. Eur J Endocrinol. 2020;182(5):473–480. doi: 10.1530/EJE-20-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuja-Halkola R, Lebwohl B, Halfvarson J, Wijmenga C, Magnusson PK, Ludvigsson JF. Heritability of non-HLA genetics in coeliac disease: a population-based study in 107 000 twins. Gut. 2016;65(11):1793–1798. doi: 10.1136/gutjnl-2016-311713. [DOI] [PubMed] [Google Scholar]
- 28.Nisticò L, Fagnani C, Coto I, Percopo S, Cotichini R, Limongelli MG, et al. Concordance, disease progression, and heritability of coeliac disease in Italian twins. Gut. 2006;55(6):803–808. doi: 10.1136/gut.2005.083964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morris-Yates A, Talley NJ, Boyce PM, Nandurkar S, Andrews G. Evidence of a genetic contribution to functional bowel disorder. Am J Gastroenterol. 1998;93(8):1311–1317. doi: 10.1111/j.1572-0241.1998.440_j.x. [DOI] [PubMed] [Google Scholar]
- 30.Dao JM, Qubty W. Headache diagnosis in children and adolescents. Curr Pain Headache Rep. 2018;22(3):17. doi: 10.1007/s11916-018-0675-7. [DOI] [PubMed] [Google Scholar]
- 31.Robin SG, Keller C, Zwiener R, Hyman PE, Nurko S, Saps M, et al. Prevalence of pediatric functional gastrointestinal disorders utilizing the Rome IV criteria. J Pediatr. 2018;195:134–139. doi: 10.1016/j.jpeds.2017.12.012. [DOI] [PubMed] [Google Scholar]
- 32.Larson T, Anckarsäter H, Gillberg C, Ståhlberg O, Carlström E, Kadesjö B, et al. The Autism–Tics, AD/HD and other Comorbidities inventory (A-TAC): further validation of a telephone interview for epidemiological research. BMC Psychiatry. 2010;10:1. doi: 10.1186/1471-244X-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Constantino JN, Todd RD. Autistic traits in the general population: a twin study. Arch Gen Psychiatry. 2003;60(5):524–530. doi: 10.1001/archpsyc.60.5.524. [DOI] [PubMed] [Google Scholar]
- 34.Larsson H, Anckarsater H, Råstam M, Chang Z, Lichtenstein P. Childhood attention-deficit hyperactivity disorder as an extreme of a continuous trait: a quantitative genetic study of 8,500 twin pairs. J Child Psychol Psychiatry. 2012;53(1):73–80. doi: 10.1111/j.1469-7610.2011.02467.x. [DOI] [PubMed] [Google Scholar]
- 35.Rijsdijk FV, Sham PC. Analytic approaches to twin data using structural equation models. Brief Bioinform. 2002;3(2):119–133. doi: 10.1093/bib/3.2.119. [DOI] [PubMed] [Google Scholar]
- 36.Neale MC, Hunter MD, Pritikin JN, Zahery M, Brick TR, Kirkpatrick RM, et al. OpenMx 2.0: extended structural equation and statistical modeling. Psychometrika. 2016;81(2):535–549. doi: 10.1007/s11336-014-9435-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kanne SM, Christ SE, Reiersen AM. Psychiatric symptoms and psychosocial difficulties in young adults with autistic traits. J Autism Dev Disord. 2009;39(6):827–833. doi: 10.1007/s10803-008-0688-x. [DOI] [PubMed] [Google Scholar]
- 38.Howlin P, Moss P, Savage S, Bolton P, Rutter M. Outcomes in adult life among siblings of individuals with autism. J Autism Dev Disord. 2015;45(3):707–718. doi: 10.1007/s10803-014-2224-5. [DOI] [PubMed] [Google Scholar]
- 39.Kirova AM, Kelberman C, Storch B, DiSalvo M, Woodworth KY, Faraone SV, et al. Are subsyndromal manifestations of attention deficit hyperactivity disorder morbid in children? A systematic qualitative review of the literature with meta-analysis. Psychiatry Res. 2019;274:75–90. doi: 10.1016/j.psychres.2019.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vega C, Vestal M, Desalvo M, Berman R, Chung M, Blumenfeld H, et al. Differentiation of attention-related problems in childhood absence epilepsy. Epilepsy Behav. 2010;19(1):82–85. doi: 10.1016/j.yebeh.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Austin JK, Harezlak J, Dunn DW, Huster GA, Rose DF, Ambrosius WT. Behavior problems in children before first recognized seizures. Pediatrics. 2001;107(1):115–122. doi: 10.1542/peds.107.1.115. [DOI] [PubMed] [Google Scholar]
- 42.Turk J, Bax M, Williams C, Amin P, Eriksson M, Gillberg C. Autism spectrum disorder in children with and without epilepsy: impact on social functioning and communication. Acta Paediatr. 2009;98(4):675–681. doi: 10.1111/j.1651-2227.2008.01184.x. [DOI] [PubMed] [Google Scholar]
- 43.Viscidi EW, Johnson AL, Spence SJ, Buka SL, Morrow EM, Triche EW. The association between epilepsy and autism symptoms and maladaptive behaviors in children with autism spectrum disorder. Autism. 2013;18(8):996–1006. doi: 10.1177/1362361313508027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Doshi-Velez F, Ge Y, Kohane I. Comorbidity clusters in autism spectrum disorders: an electronic health record time-series analysis. Pediatrics. 2014;133(1):e54–63. doi: 10.1542/peds.2013-0819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Greenlee JL, Mosley AS, Shui AM, Veenstra-VanderWeele J, Gotham KO. Medical and behavioral correlates of depression history in children and adolescents with autism spectrum disorder. Pediatrics. 2016;137:S105–S114. doi: 10.1542/peds.2015-2851I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pan PY, Tammimies K, Bölte S. The association between somatic health, autism spectrum disorder, and autistic traits. Behav Genet. 2020;50(4):233–246. doi: 10.1007/s10519-019-09986-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pan PY, Bölte S. The association between ADHD and physical health: a co-twin control study. Sci Rep. 2020;10(1):22388. doi: 10.1038/s41598-020-78627-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chang Z, Lichtenstein P, Asherson PJ, Larsson H. Developmental twin study of attention problems: high heritabilities throughout development. JAMA Psychiat. 2013;70(3):311–318. doi: 10.1001/jamapsychiatry.2013.287. [DOI] [PubMed] [Google Scholar]
- 49.Elsabbagh M. Linking risk factors and outcomes in autism spectrum disorder: Is there evidence for resilience? BMJ. 2020;368:l6880. doi: 10.1136/bmj.l6880. [DOI] [PubMed] [Google Scholar]
- 50.Lecendreux M, Konofal E, Cortese S, Faraone SV. A 4-year follow-up of attention-deficit/hyperactivity disorder in a population sample. J Clin Psychiatry. 2015;76(6):712–719. doi: 10.4088/JCP.14m09555. [DOI] [PubMed] [Google Scholar]
- 51.Georgiades S, Szatmari P, Zwaigenbaum L, Bryson S, Brian J, Roberts W, et al. A prospective study of autistic-like traits in unaffected siblings of probands with autism spectrum disorder. JAMA Psychiat. 2013;70(1):42–48. doi: 10.1001/2013.jamapsychiatry.1. [DOI] [PubMed] [Google Scholar]
- 52.Liu E, Lee HS, Aronsson CA, Hagopian WA, Koletzko S, Rewers MJ, et al. Risk of pediatric celiac disease according to HLA haplotype and country. N Engl J Med. 2014;371(1):42–49. doi: 10.1056/NEJMoa1313977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lebwohl B, Murray JA, Verdu EF, Crowe SE, Dennis M, Fasano A, et al. Gluten introduction, breastfeeding, and celiac disease: back to the drawing board. Am J Gastroenterol. 2016;111(1):12–14. doi: 10.1038/ajg.2015.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chan AO, Hui WM, Lam KF, Leung G, Yuen MF, Lam SK, et al. Familial aggregation in constipated subjects in a tertiary referral center. Am J Gastroenterol. 2007;102(1):149–152. doi: 10.1111/j.1572-0241.2006.00886.x. [DOI] [PubMed] [Google Scholar]
- 55.Ostwani W, Dolan J, Elitsur Y. Familial clustering of habitual constipation: a prospective study in children from West Virginia. J Pediatr Gastroenterol Nutr. 2010;50(3):287–289. doi: 10.1097/MPG.0b013e3181a0a595. [DOI] [PubMed] [Google Scholar]
- 56.Piwowarczyk A, Horvath A, Łukasik J, Pisula E, Szajewska H. Gluten- and casein-free diet and autism spectrum disorders in children: a systematic review. Eur J Nutr. 2018;57(2):433–440. doi: 10.1007/s00394-017-1483-2. [DOI] [PubMed] [Google Scholar]
- 57.Niederhofer H. Association of attention-deficit/hyperactivity disorder and celiac disease: a brief report. Prim Care Companion CNS Disord. 2011 doi: 10.4088/PCC.10br01104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kristensen VA, Valeur J, Brackmann S, Jahnsen J, Brunborg C, Tveito K. Attention deficit and hyperactivity disorder symptoms respond to gluten-free diet in patients with coeliac disease. Scand J Gastroenterol. 2019;54(5):571–576. doi: 10.1080/00365521.2019.1608467. [DOI] [PubMed] [Google Scholar]
- 59.Butwicka A, Lichtenstein P, Frisen L, Almqvist C, Larsson H, Ludvigsson JF. Celiac disease is associated with childhood psychiatric disorders: a population-based study. J Pediatr. 2017;184:87–93.e1. doi: 10.1016/j.jpeds.2017.01.043. [DOI] [PubMed] [Google Scholar]
- 60.Alkhayyat M, Qapaja T, Aggarwal M, Almomani A, Abureesh M, Al-Otoom O, et al. Epidemiology and risk of psychiatric disorders among patients with celiac disease: a population-based national study. J Gastroenterol Hepatol. 2021;36(8):2165–2170. doi: 10.1111/jgh.15437. [DOI] [PubMed] [Google Scholar]
- 61.Batista IC, Gandolfi L, Nobrega YK, Almeida RC, Almeida LM, Campos Junior D, et al. Autism spectrum disorder and celiac disease: no evidence for a link. Arq Neuropsiquiatr. 2012;70(1):28–33. doi: 10.1590/S0004-282X2012000100007. [DOI] [PubMed] [Google Scholar]
- 62.Ertürk E, Wouters S, Imeraj L, Lampo A. Association of ADHD and celiac disease: what is the evidence? A systematic review of the literature. J Atten Disord. 2020;24(10):1371–1376. doi: 10.1177/1087054715611493. [DOI] [PubMed] [Google Scholar]
- 63.Lichtenstein P, Carlström E, Råstam M, Gillberg C, Anckarsäter H. The genetics of autism spectrum disorders and related neuropsychiatric disorders in childhood. Am J Psychiatry. 2010;167(11):1357–1363. doi: 10.1176/appi.ajp.2010.10020223. [DOI] [PubMed] [Google Scholar]
- 64.Ousley O, Cermak T. Autism spectrum disorder: defining dimensions and subgroups. Curr Dev Disord Rep. 2014;1(1):20–28. doi: 10.1007/s40474-013-0003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tye C, Runicles AK, Whitehouse AJO, Alvares GA. Characterizing the interplay between autism spectrum disorder and comorbid medical conditions: an integrative review. Front Psychiatry. 2019;9:751. doi: 10.3389/fpsyt.2018.00751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Luo Y, Weibman D, Halperin JM, Li X. A review of heterogeneity in attention deficit/hyperactivity disorder (ADHD) Front Hum Neurosci. 2019;13:42. doi: 10.3389/fnhum.2019.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu H, Li H, Bai T, Han L, Ou J, Xun G, et al. Phenotype-to-genotype approach reveals head-circumference-associated genes in an autism spectrum disorder cohort. Clin Genet. 2020;97(2):338–346. doi: 10.1111/cge.13665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li J, Wang L, Guo H, Shi L, Zhang K, Tang M, et al. Targeted sequencing and functional analysis reveal brain-size-related genes and their networks in autism spectrum disorders. Mol Psychiatry. 2017;22(9):1282–1290. doi: 10.1038/mp.2017.140. [DOI] [PubMed] [Google Scholar]
- 69.Diaz-Beltran L, Esteban FJ, Varma M, Ortuzk A, David M, Wall DP. Cross-disorder comparative analysis of comorbid conditions reveals novel autism candidate genes. BMC Genomics. 2017;18(1):315. doi: 10.1186/s12864-017-3667-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nazeen S, Palmer NP, Berger B, Kohane IS. Integrative analysis of genetic data sets reveals a shared innate immune component in autism spectrum disorder and its co-morbidities. Genome Biol. 2016;17(1):228. doi: 10.1186/s13059-016-1084-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160(4):636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 72.Rapin I. Classification of behaviorally defined disorders: biology versus the DSM. J Autism Dev Disord. 2014;44(10):2661–2666. doi: 10.1007/s10803-014-2127-5. [DOI] [PubMed] [Google Scholar]
- 73.Mota NR, Poelmans G, Klein M, Torrico B, Fernàndez-Castillo N, Cormand B, et al. Cross-disorder genetic analyses implicate dopaminergic signaling as a biological link between attention-deficit/hyperactivity disorder and obesity measures. Neuropsychopharmacology. 2020;45(7):1188–1195. doi: 10.1038/s41386-019-0592-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jacobs H, Singhi S, Gladstein J. Medical comorbidities in pediatric headache. Semin Pediatr Neurol. 2016;23(1):60–67. doi: 10.1016/j.spen.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 75.Mazefsky CA, Schreiber DR, Olino TM, Minshew NJ. The association between emotional and behavioral problems and gastrointestinal symptoms among children with high-functioning autism. Autism. 2014;18(5):493–501. doi: 10.1177/1362361313485164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Marí-Bauset S, Zazpe I, Mari-Sanchis A, Llopis-González A, Morales-Suárez-Varela M. Food selectivity in autism spectrum disorders: a systematic review. J Child Neurol. 2014;29(11):1554–1561. doi: 10.1177/0883073813498821. [DOI] [PubMed] [Google Scholar]
- 77.Zare H, Gilmore DR, Meyerson NS, Thorpe RJ., Jr Income inequality, race/ethnicity, and obesity in U.S. men 20 years and older: 1999 to 2016. Am J Mens Health. 2022;16(5):15579883221123852. doi: 10.1177/15579883221123852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Krogh AB, Larsson B, Linde M. Prevalence and disability of headache among Norwegian adolescents: a cross-sectional school-based study. Cephalalgia. 2015;35(13):1181–1191. doi: 10.1177/0333102415573512. [DOI] [PubMed] [Google Scholar]
- 79.Croen LA, Najjar DV, Ray GT, Lotspeich L, Bernal P. A comparison of health care utilization and costs of children with and without autism spectrum disorders in a large group-model health plan. Pediatrics. 2006;118(4):e1203–e1211. doi: 10.1542/peds.2006-0127. [DOI] [PubMed] [Google Scholar]
- 80.Du Rietz E, Jangmo A, Kuja-Halkola R, Chang Z, D'Onofrio BM, Ahnemark E, et al. Trajectories of healthcare utilization and costs of psychiatric and somatic multimorbidity in adults with childhood ADHD: a prospective register-based study. J Child Psychol Psychiatry. 2020;61(9):959–968. doi: 10.1111/jcpp.13206. [DOI] [PubMed] [Google Scholar]
- 81.Mason D, Ingham B, Urbanowicz A, Michael C, Birtles H, Woodbury-Smith M, et al. A systematic review of what barriers and facilitators prevent and enable physical healthcare services access for autistic adults. J Autism Dev Disord. 2019;49(8):3387–3400. doi: 10.1007/s10803-019-04049-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Swift KD, Sayal K, Hollis C. ADHD and transitions to adult mental health services: a scoping review. Child Care Health Dev. 2014;40(6):775–786. doi: 10.1111/cch.12107. [DOI] [PubMed] [Google Scholar]
- 83.Thijs RD, Surges R, O'Brien TJ, Sander JW. Epilepsy in adults. Lancet. 2019;393(10172):689–701. doi: 10.1016/S0140-6736(18)32596-0. [DOI] [PubMed] [Google Scholar]
- 84.Guidetti V, Faedda N. From 0° to 18°: how headache changes over time. Neurol Sci. 2017;38(Suppl 1):103–106. doi: 10.1007/s10072-017-2865-1. [DOI] [PubMed] [Google Scholar]
- 85.Tick B, Bolton P, Happé F, Rutter M, Rijsdijk F. Heritability of autism spectrum disorders: a meta-analysis of twin studies. J Child Psychol Psychiatry. 2016;57(5):585–595. doi: 10.1111/jcpp.12499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Faraone SV, Larsson H. Genetics of attention deficit hyperactivity disorder. Mol Psychiatry. 2019;24(4):562–575. doi: 10.1038/s41380-018-0070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Losh M, Esserman D, Anckarsäter H, Sullivan PF, Lichtenstein P. Lower birth weight indicates higher risk of autistic traits in discordant twin pairs. Psychol Med. 2012;42(5):1091–1102. doi: 10.1017/S0033291711002339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lim KX, Liu CY, Schoeler T, Cecil CAM, Barker ED, Viding E, et al. The role of birth weight on the causal pathway to child and adolescent ADHD symptomatology: a population-based twin differences longitudinal design. J Child Psychol Psychiatry. 2018;59(10):1036–1043. doi: 10.1111/jcpp.12949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hassan S, Jahanfar S, Inungu J, Craig JM. Low birth weight as a predictor of adverse health outcomes during adulthood in twins: a systematic review and meta-analysis. Syst Rev. 2021;10(1):186. doi: 10.1186/s13643-021-01730-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. ICD code list. Table S1: Sex distribution of clinical and subclinical autism/ADHD in our sample. Table S2: Associations between physical health conditions and the overlap of autism and ADHD in our sample. Table S3: Univariate twin model fit statistics. Table S4: Bivariate models for autism/ADHD and physical health conditions. Table S5: Etiological component contributing to phenotypic correlation between autism/ADHD and physical health conditions. Table S6: Bivariate twin model of clinical autism and physical health conditions fit statistics. Table S7: Bivariate twin model of subclinical autism and physical health conditions fit statistics. Table S8: Bivariate twin model of clinical ADHD and physical health conditions fit statistics. Table S9: Bivariate twin model of subclinical ADHD and physical health conditions fit statistics. Table S10: Etiological component contributing to phenotypic correlation between subclinical autism/ADHDand physical health conditions.
Additional file 2. The English version of the Autism–Tics, ADHD, and Other Comorbidities inventory (A-TAC).
Data Availability Statement
The Public Access to Information and Secrecy Act in Sweden prohibits us from making individual-level data publicly available. Researchers who are interested in replicating our work can apply for individual-level data through The Child and Adolescent Twin Study in Sweden (CATSS) at: https://ki.se/en/meb/the-child-and-adolescent-twin-study-in-sweden-catss.