Abstract
Purpose
Acupuncture can improve symptoms of autism spectrum disorder (ASD), but the potential mechanisms remain undefined. So, we aimed to explore the behavioral improvement of autism rat model after acupuncture and to describe the potential molecular mechanism underlying these changes.
Patients and Methods
Wistar rats were intraperitoneally injected with VPA 12.5 days after conception, and their offspring were considered as good models of autism. Experimental rats were divided into three groups (wild-type (WT), n = 10; VPA, n = 10; and VPA_acupuncture, n = 10). VPA_acupuncture group rat received 4 weeks of acupuncture treatment (Shéntíng (GV24), and Bilateral Běnshén (GB13)) on the 23rd day after birth. All rats were subjected to behavioral tests, including social interaction, open field, and Morris water maze tests. Afterwards, hippocampal tissues (left side) were removed and subjected to RNA sequencing (RNA-seq) analysis; ELISA was also used to detect the associated serotonin levels in the hippocampus.
Results
Behavioral tests showed that acupuncture treatment improved spontaneous activity, aberrant social interaction, and alleviated impaired learning and memory in the VPA-induced rat model. Differentially expressed genes (DGEs) analysis showed 142 significantly differentially expressed genes between WT and VPA groups, and 282 between VPA and VPA_acupuncture rats. Htr2c and Htr1a, 5-HT receptor genes, were up-regulated in the VPA group compared with WT group. Additionally, Tph1, a rate-limiting enzyme gene of 5-HT synthesis, was up-regulated after acupuncture. These genes were confirmed to have the same trend of expression obtained by RT-qPCR and RNA seq. Furthermore, the concentration of serotonin in the hippocampus in the VPA group was significantly lower than the WT and VPA_acupuncture groups.
Conclusion
Acupuncture improved abnormal behavioral symptoms in the VPA-induced rat model. Further experiments showed that the improvement of the serotonin system may be one of the main regulatory mechanisms of acupuncture for treating ASD.
Keywords: acupuncture, autism, behavioral abnormalities, RNA-sequencing
Introduction
Autism spectrum disorder (ASD) is a neurodevelopmental disorder, which is characterized by the presence of social impairment and repetitive behaviors. Its prevalence has been rising in the past 50 years.1 At present, ASD cannot be cured. Behavioral intervention, drugs and complementary and alternative medicine treatment (CAM) go some way to alleviating symptoms such as social impairment and repetitive stereotyped behavior in children with autism.2,3 Behavioral interventions are costly and time-consuming, and drugs have inevitable side effects, so they are limited in the clinical treatment of autism. Under the medical environment of the author’s country, it can be used for CAM treatment of children with autism, mainly including acupuncture, massage, Chinese herbal medicine, etc. Among them, acupuncture is a unique method in the field of traditional Chinese medicine, which can be used to treat various types of diseases and has been developed for thousands of years.4,5 Many studies have shown that acupuncture treatment can reduce brain dysfunction and improve the symptoms of various neurological disorders, including early Alzheimer’s disease (AD), ASD, Parkinson’s disease (PD) and stroke.6–8 Acupuncture has also been integrated into the treatment of pediatric diseases, such as cerebral palsy, enuresis, tic disorder, hypoxic-ischemic encephalopathy, attention deficit hyperactivity disorder and ASD. Several clinical studies have confirmed the clinical efficacy of acupuncture for children in treating the diseases mentioned above, especially in the areas of verbal communication, social function and social problems, and no fatal side effects have been reported.9–11
Although acupuncture is increasingly being used in the clinical treatment of autism, its improvement mechanism remains unclear.
Hippocampus plays an important role in the limbic system, which is involved in physiological processes such as the formation and regulation of emotions and the formation of learning and memory.12 Hippocampal dysfunction is closely related to the pathophysiology of nervous system diseases such as ASD.13,14 Evidence shows that the improvement of hippocampal function may play a key role in the treatment of the above diseases by acupuncture. For example, acupuncture may improve cognitive impairment by promoting the release of dopamine and its main metabolites in the hippocampus of AD rats, and may improve learning and memory ability by changing the levels of NAA and Cho in the hippocampus of ischemia reperfusion rats, or improve the learning and memory ability of autistic rats by regulating the expression of PSD-95 protein in the hippocampus.15–17 There are many acupuncture points, and Traditional Chinese medicine (TCM) believes that GV24 and bilateral GB13 points in scalp acupuncture to be important, as they represent “wisdom”.18 GV24 belongs to the governor vessel, which is the home of human spirit, consciousness, intelligence and cognition. GB13 belongs to the gallbladder meridian of foot Shaoyang, which is the source and foundation of human spirit, consciousness, intelligence and cognition.6 Some studies found that acupuncture at these three points can improve the learning and memory ability of rats with Alzheimer’s disease by changing the brain glucose metabolism of the hypothalamus, thalamus and brain stem, and other studies have shown that acupuncture at these points can regulate cerebral blood flow and enhance the connectivity of the hippocampus, thereby improving brain function.19,20 Serotonin, an important neurotransmitter and endogenous active substance, is critically involved in brain development and innervates the entire central nervous system, which allows serotonin to affect diverse physiological processes, including the sleep-wake cycle, learning and memory, mood, and aggression.21 It has been reported that abnormalities in the serotonin system may lead to abnormal brain development in patients.22 In addition, serotonin abnormalities in the brain have been observed in ASD patients; these include abnormalities in the serotonin transporter, alterations of serotonin levels, reduction in synthesis of serotonin, and changes in serotonin receptor expression in the brain.23,24 Acupuncture can affect the level of serotonin, which may be a crucial factor for the improvement of autism symptoms after acupuncture.
RNA-seq can effectively identify genes regulated by particular interventions, which can be used to explore ASD pathogenesis and the molecular mechanisms underlying the effectiveness of acupuncture in treating ASD.25
In the current study, we constructed classical VPA models to induce autistic-like features in a rodent model. After treating the VPA-induced rats with acupuncture, we conducted a series of behavioral tests to compare the performance among wildtype, VPA, and VPA_acupuncture rat groups. The behavioral changes, as well as the differences of the data from the RNA sequencing analysis of hippocampal tissues between the groups were compared.
Materials and Methods
Animals and Grouping
Adult male and female Wistar rats were sourced from the Slake Laboratory Animal Co., Ltd. (Shanghai, China). Male and female rats were mated in a 1:1 ratio, and their male offspring were used as the experimental models. Animals were bred and raised under 12:12 light cycle and constant temperature (22 ± 2°C) and humidity (55 ± 5%).
Eight-week-old SPF (specific pathogen free) Wistar pregnant rats (n = 10), weighing 250–260 g, were randomly selected. Five of them were injected intraperitoneally with 600 mg/kg VPA on day 12.5 post-conception and the other rats were injected intraperitoneally with saline.26–29 A total of 20 pups from pregnant rats injected with VPA were randomly assigned: VPA group (n = 10) and VPA_acupuncture group (n = 10). A total of 10 pups from pregnant rats injected with saline were assigned to the WT group (n = 10).
All experiments have been conducted in accordance with the procedures developed by the Administrative Panel on Laboratory Animal Care of Fujian Medical University, Fuzhou, China. The experimental steps were carried out following the Institutional Animal Ethics Review Board of Fujian Medical University (FJMU IACUC permit number: 2018–088), and in strict compliance with International Ethics Guideline.
Acupuncture Treatment
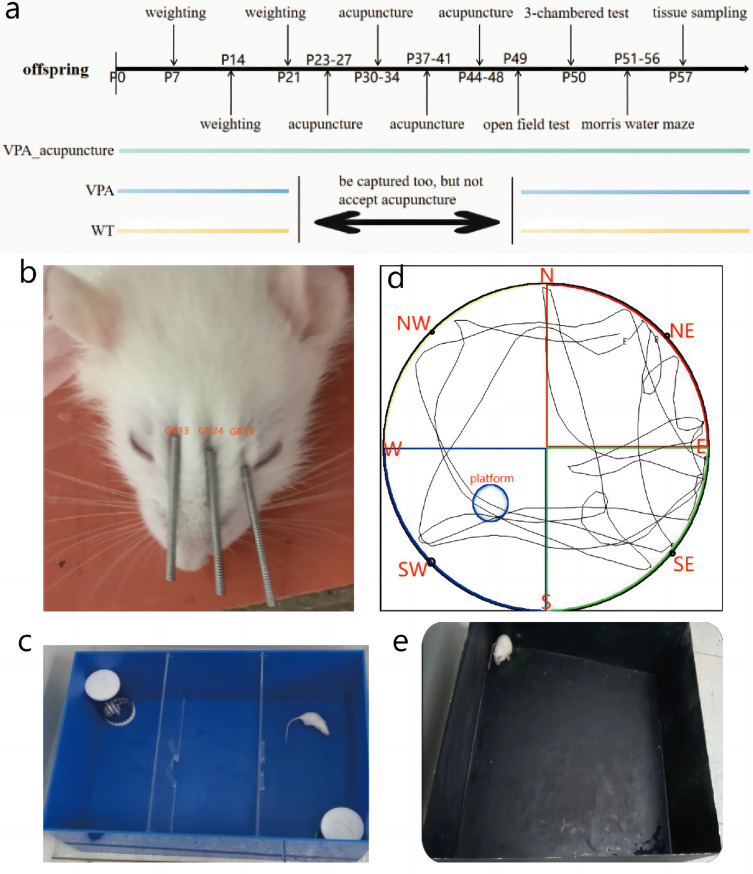
On postnatal day 23 (P23), rats from the VPA_acupuncture group received acupuncture treatment (Figure 1a), which included three acupoints: Shéntíng (GV24) and bilateral Běnshén (GB13) (Figure 1b). Rats were placed in a fixator, and the acupuncture needles were inserted with a depth of 2–3 mm into the acupoints. Acupuncture stimulation lasted for 40 minutes with manual twisting of the needles every 10 minutes. We observed the rats to ensure that the needles did not drop during acupuncture, and all rats were returned to their own cages after treatment. Acupuncture was performed 5 days per week, with 2 days off for 4 weeks. The WT and VPA groups were captured as well to reduce the error of the physiological reaction caused by the capture of rats on the experimental results and increase the reliability of the results.
Figure 1.
Experimental diagram of acupuncture and behavior tests.
Notes: (a) Time axis; (b) Acupoint localization; (c) Social interaction test; (d) Morris water maze test (the position of each direction and platform was shown); (e) open field test.
Abbreviations: E, East; W, West; N, North; S, South; NE, Northeast; NW, Northwest; SE, Southeast; SW, Southwest.
Behavioral Experiments
Open Field Test
At P49, rats were placed in an open arena (100×100×100 cm) and allowed to explore freely, and were under continuous surveillance by the AnyMaze tracking software (Stoeltingco, America) for 5 minutes after 2 minutes of adaptation (Figure 1e). The entire area was divided into the surrounding and central areas. The ratio between the two areas was 1:1. Time spent in the surrounding area and central area, and bouts in the central area were automatically collected using the analyzer software system. Experimenter use 70% ethanol to clean the arena between tests. The interval between the two tests was more than 5 minutes to allow ethanol to evaporate and prevent the rats from smelling the foul odor.
Social Interaction Test
At P50, test rats were placed in the middle chamber and permitted to explore freely in the 3-chambers through 2 gates after 5 minutes of adaptation (Figure 1c). In session I, a stranger rat (stranger 1) was placed in an enclosure and an object in the other, with 3–5 cm around the enclosure being defined as the contact area. The test rat was allowed to sniff stranger 1 or an object on the other side for 10 minutes, and the time of direct contact between the test rat and stranger 1 or the object was recorded (sociability). In session II, another stranger rat (stranger 2) replaced forementioned object, with 3–5 cm around the enclosure being defined as the contact range. The test rat was allowed to sniff stranger 2 or stranger 1 for 10 minutes, and the time of direct contact between the test rat and the stranger 1 or stranger 2 was recorded (preference for social novelty).
Morris Water Maze
At P551-56, the Morris water maze (MWM) test was performed. The diameter of the MWM was 150 cm and its inner surface was painted black (Figure 1d). The circular platform had a diameter of 10 cm and was submerged 1–2 cm below the water-surface. One day before testing, each rat was placed into MWM for adaptation time of 1 minute. They were tested 4 times a day for 5 days continuously. In each experiment, each rat was placed into the maze in the following this order: Day 1: N-E-SE-NW; day 2: SE-N-NW-E; day 3: NW-SE-E-N; day 4: E-NW-N-SE; day 5: N-SE-E-NW. The rats faced the wall of pool at 1 minute intervals. Each experiment ended exactly when the rat successfully escaped the maze and landed on the platform for 3 seconds. If the rat failed to find the platform within 1 minute, it would be guided to the target and stayed there for 15 seconds. The average latency for locating the platform, the time staying on the platform, the total swimming distance and the average speed of each rat were calculated and recorded. After 5 days of learning, the platform was removed, and each rat was introduced into the maze for 1 minute. The mean time spent in target quadrant, the mean number of bouts the rats crossed the platform, and their swimming paths were recorded.
All experimental rats enrolled, a total of 30 rats, were tested for the above-mentioned behavioral experiments. All behavioral experiments were conducted under normal laboratory lighting.
Tissue Sampling
At P57, all rats were sacrificed using cervical dislocation. The hippocampal tissues (left side) were washed with cold saline and immediately frozen in liquid nitrogen. In each group, 5 hippocampal tissues were randomly selected for RNA sequencing, 3 samples were subjected to RT-qPCR, and the remaining 2 samples were subjected to ELISA.
RNA-Seq
RNA-Sequencing Analysis and Quality Control
The RNA-seq transcriptome library was prepared by the TruSeq TM RNA sample preparation kit (Illumina, San Diego, CA) using one μ g of total RNA. Firstly, mRNA was separated by oligo (dT) beads and cleaved with fragment buffers. Secondly, cDNA was synthesized (cDNA Synthesis kit, Invitrogen, CA; random primer, Illumina). Thirdly, end-repair, phosphorylation and “A” base addition of cDNA were performed following Illumina’s library construction protocol. Fourthly, libraries were selected for cDNA target fragments of 300 bp and Phusion DNA polymerase was used for 15 PCR cycles. The paired-end RNA-seq sequencing library was sequenced after quantification.
Sequencing platform: https://www.majorbio.com/. The transcriptome analysis of 15 samples was completed, and a total of 226.21 Gb of Clean Data was obtained. The Clean Data of all samples reached above 6.82 Gb, and the base percentage of Q30 was above 93.5%. All analysis software information is listed as Table 1.
Table 1.
Analysis Software Information of RNA-Sequencing.xls
| Soft/Database | Fastx_Toolkit | Sickle | Stringtie | BLAST+ | Fastp |
| Version | Version 0.0.14 | -- | Version 1.3.3b | Version 2.9.0 | 0.19.5 |
| Soft/Database | Cufflinks | Kallisto | Samtools | SeqPrep | MSigDB data base |
| Version | Version 2.2.1 | Version 0.46.0 | Version 1.9 | -- | Version 6.2 |
| Soft/Database | Bedtools | GSEA | Star_fusion | NCBI species mclassification database | TopHat |
| Version | Version 2.27.1 | Version 3.0 | Version 1.8.1 | Version 2019. | Version v2.1.1 |
| Soft/Database | TransDecoder | maSigPro | PlantTFDB | Diamond | Rfam data base |
| Version | Version 5.5.0 | Version 1.56.0 | Version 4.0 | Version 0.9.24 | Version Rfam v14.1 |
| Soft/Database | KOBAS | GATK | RSEM | STAR | Bowtie2 |
| Version | Version 2.1.1 | Version 3.8 | Version 1.3.1 | Version 2.7.1a | Version 2.3.5 |
| Soft/Database | EdgeR | Blast2go | DESeq2 | STEM | Limma |
| Version | Version 3.24.3 | Version2.5 | Version 1.24.0 | Version 1.3.11 | Version 3.38.3 |
| Soft/Database | KEGG data base | Salmon | WGCNA | AnimalTFDB | PIR idmapping data base |
| Version | Version 2018. | Version 0.14.1 | Version 1.63 | Version 3.0 | Version 2019. |
| Soft/Database | Ipath data base | JASPAR | NOISeq | Hisat2 | DEGSeq |
| Version | Version 3 | Version 2020 | Version 2.18.0 | Version 2.1.0 | Version 1.38.0 |
| Soft/Database | STRING data base | Pfam data base | |||
| Version | Version 11.5 | Version 32 |
Differentially Expressed Genes
Based on the quantitative results of expression quantity, the inter-group differential gene analysis was performed to obtain the differentially expressed genes between the two groups. RSEM30 was used to quantify the abundance of each gene. Differential expression analysis was conducted by the DESeq231 /DEGseq32 /EdgeR33 with a Q value ≤0.05, DEGs with |log2FC|>1 and Q value ≤0.05 were called significantly differentially expressed genes.
GO and KEGG Enrichment Analyses
Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) can identify the genes that are significantly enriched in a specific pathway or GO term. The following two methods were, respectively, used for analysis: Goatools:https://github.com/tanghaibao/Goatools and KOBAS:http://kobas.cbi.pku.edu.cn/home.do.34
Cluster Analysis
Clustering was performed using fastcluster, which was based on the relative expression level of the gene log2 (ratios). Corresponding distance algorithm was used to calculate the distance between each gene. And then calculates the relative distance between the genes through repeated iterations. Finally, it divides the genes into different sub-clusters according to the relative distance of the genes.
Confirmatory Gene Expression Study Using RT-qPCR
Total RNA was isolated by Tri-Reagent in accordance with manufacturer’s instructions. Then, experimenter determined the purity and concentration of total RNA. The single strand cDNA was prepared from 1μg total RNA, and it was diluted 10 folds. β-actin was selected as reference genes. RT-qPCR was performed as follows: 50°C for 2 minutes, 95°C for 10 minutes, 95°C for 15 seconds, 60°C for 1 minute, 40 Cycles. The melting curve at 95°C for 15 minutes, 60°C for 30 seconds, and 95°C for 15 minutes. Finally, experimenter detected fluorescence signal, recorded cycle threshold value (CT), and calculated gene expression level (2−ΔΔCt). Each group had 3 biological samples (n = 3) and each sample was run with 3 technical replicates during the procedure.
Enzyme Linked Immunosorbent Assay
Finally, experimenter tested the remaining 2 samples in each group as follows (n = 2). Serotonin (5-HT) levels of hippocampus were measured by ELISA kit (ADI-900-175, USA) following manufacturer’s instructions. Specimens, standard products, and HRP labeled detection antibodies were successively added to the coated micropores pre-coated with 5-HT antibodies, which were incubated and thoroughly washed. The substrate TMB was used for color rendering. The shade of the color was positively associated with the serotonin in the sample. Finally, absorbance (OD value) was measured at 450 nm by using a microplate reader for calculating the serotonin concentration.
Statistical Analysis
Statistical analysis was performed using SPSS 25.0 (Chicago, Illinois, USA) and graphical depiction was performed using GraphPad Prism 9.0 (San Diego, California, USA). If the data was accorded with normality and homogeneity of variance, one-way ANOVA was applied to multiple group comparisons and LSD tests can be used for comparison of multiple groups. If not, a non-parametric test was adopted. All results were shown as the mean ± standard deviation, with two-tailed p < 0.05 being considered significant.
Results
Behavioral Results Analysis
Acupuncture Improved Spontaneous Activity in VPA Rat Model
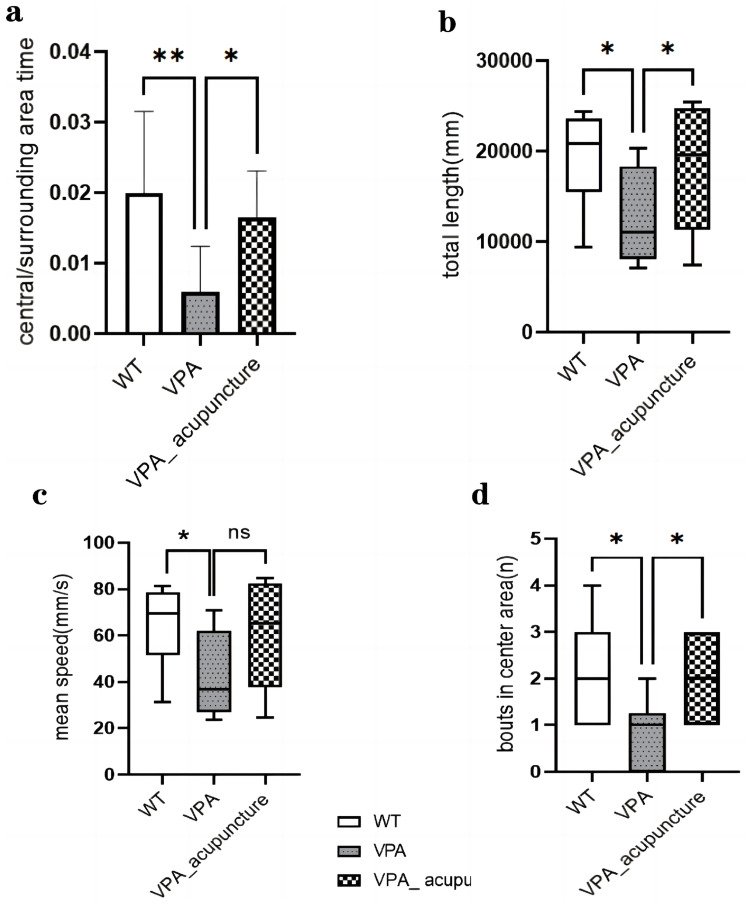
To observe the exploratory behavior and locomotor activity of rats, we assessed the activity of the rats’ activities among the WT, the VPA and the VPA_acupuncture groups in the open field. The ratio of the time spent in central area and surrounding area differed significantly across all experimental group rats (F2.27=7.390, P=0.003). The VPA group rats showed significantly less exploration of central area than the WT and VPA_acupuncture groups (VPA vs WT, P = 0.001; VAP vs VPA_acupuncture, P=0.01; Figure 2a). We observed the total length of VPA group rats was significantly shorter than that of rats in the WT and VPA_acupuncture groups (p = 0.031; WT vs VPA, P = 0.013; VPA_acupuncture vs VPA, P=0.042; Figure 2b) and the frequency of VPA group rats entering the central area less than the WT and VPA_acupuncture group rats (p = 0.01; VPA vs WT, P = 0.008; VAP vs VPA_acupuncture, P = 0.01; Figure 2d). The mean speed of VPA group rats was less than the WT group rats, and it was not significantly improved after acupuncture (p = 0.043; VPA vs WT, P=0.016; VPA vs VPA_acupuncture, P=0.054; Figure 2c).
Figure 2.
Results of open field test.
Notes: (a) Central/surrounding area time; (b) Total length; (c) Mean speed; (d) Bouts in center area (* p < 0.05; ** p < 0.01; ns p > 0.05).
Acupuncture Improved Aberrant Social Interactions in VPA Rat Model
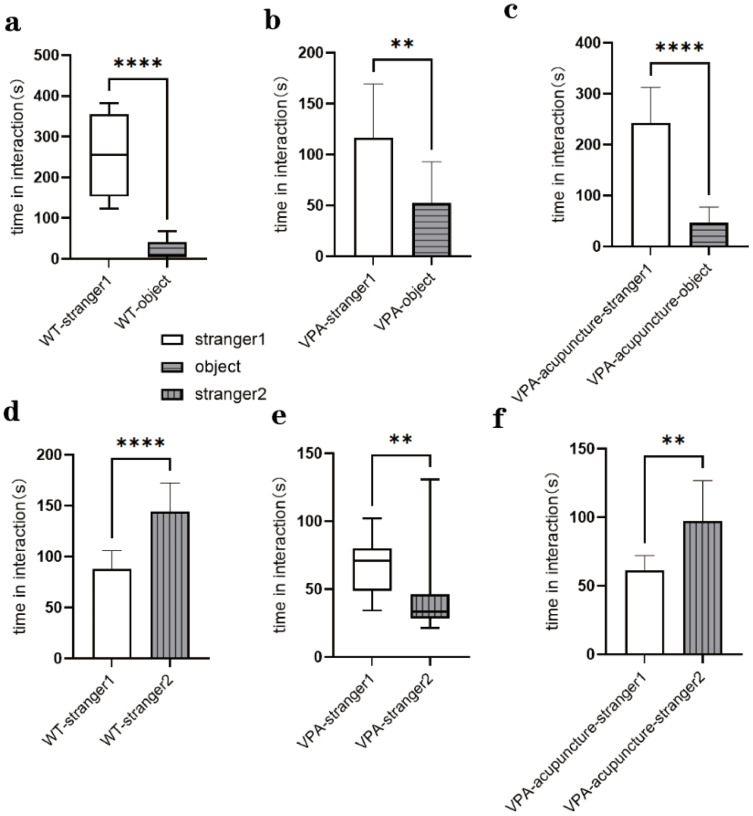
We used a three-chamber social arena to probe animals for their ability to discriminate social novelty and their voluntary initiation of social interaction. In session I, all rats spent more time interacting with stranger 1 than the object (VPA: stranger 1 vs object, P = 0.007, Figure 3b; WT: stranger 1 vs object, P<0.0001, Figure 3a; VPA_acupuncture: stranger 1 vs object, P<0.0001, Figure 3c). In session II, rats in the WT and VPA_acupuncture groups showed more interest in searching new strangers than stranger 1. However, the VPA group rats showed more interest in stranger 1 (VPA: stranger 1 vs stranger 2, P=0.008, Figure 3e; WT: stranger 1 vs stranger 2, P<0.0001, Figure 3d; VPA_acupuncture: stranger 1 vs stranger 2, P=0.004; Figure 3f).
Figure 3.
Results of social interactions test.
Notes: (a) The contact time between WT group rats and object/stranger rat 1; (b) The contact time between VPA group rats and object/stranger rat 1; (c) The contact time between VPA_acupuncture group rats and object/stranger rat 1; (d) The contact time between WT group rats and stranger rat 2/stranger rat 1; (e) The contact time between VPA group rats and stranger rat 2/stranger rat 1; (f) The contact time between VPA_acupuncture group rats and stranger rat 2/stranger rat 1 (** p < 0.01; **** p < 0.0001).
Acupuncture Alleviated Impaired Learning and Memory in VPA Rat Model
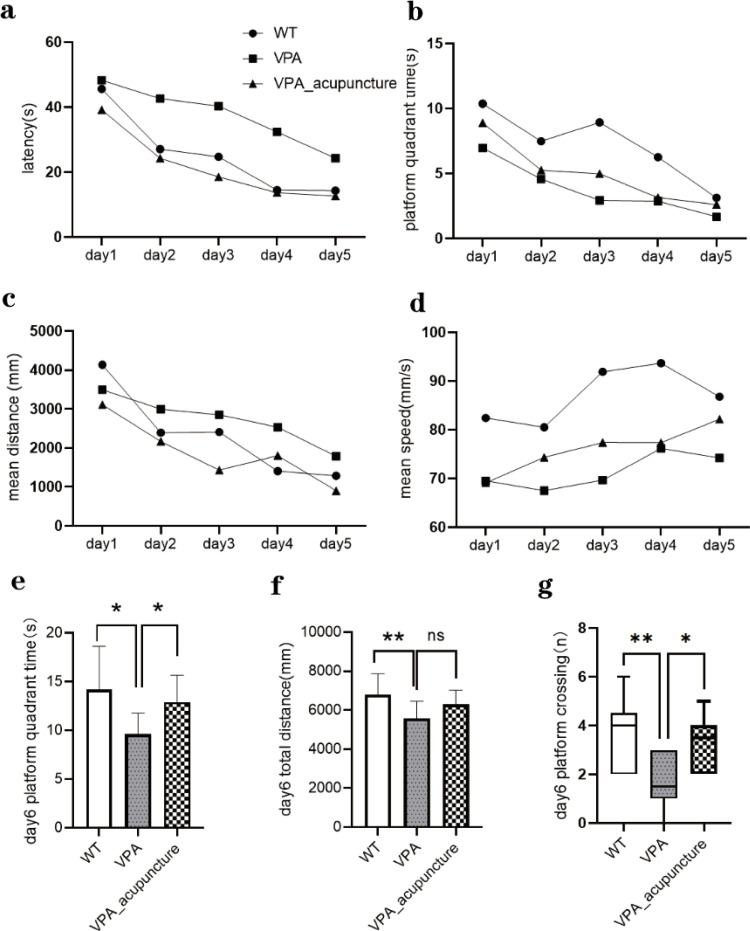
Rats were trained and tested in the MWM to assess differences in learning and memory among different groups of rats (Table 2). In the place navigation trial, three groups of rats all showed a downward trend in latency, platform quadrant time and mean distance over the five days of training. The latency, platform quadrant time and mean distance of the VPA group rats were greater than those of the WT and VPA_acupuncture group rats (Latency: time: F3.726, 435.9=43.49, P<0.0001; groups: F2117=42.26, P<0.0001, Figure 4a. Platform quadrant time: time: F3.395, 397.2=27.26, P<0.0001; groups: F2117=31.66, P<0.0001, Figure 4b. Mean distance: time: F3.747, 438.4=30.65, P<0.0001; groups: F2117=10.98, P<0.0001, Figure 4c). The mean speed of rats in all groups showed upward trend over training days, and the differences among groups were significant (Time: F3.898, 456.1=5.146, P=0.0005; Groups: F2117=29.78, P<0.0001, Figure 4d).
Table 2.
Statistical Results of MWM Test of All Rats from day1 to day5.xls
| VPA vs WT | VPA vs VPA_Acupuncture | WT | VPA | VPA_Acupuncture | |||
|---|---|---|---|---|---|---|---|
| Latency | Day1 | p=0.7673 | p=0.0849 | Day1 vs day2 | p<0.0001 | p=0.200 | p<0.0001 |
| Day2 | p=0.0009 | p=0.0001 | Day2 vs day3 | p=0.535 | p=0.606 | p=0.129 | |
| Day3 | p=0.0012 | p<0.0001 | Day3 vs day4 | p=0.007 | p=0.073 | p=0.200 | |
| Day4 | p<0.0001 | p<0.0001 | Day4 vs day5 | p=0.968 | p=0.066 | p=0.786 | |
| Day5 | p=0.0103 | p=0.0103 | |||||
| Platform quadrant time | Day1 | p=0.0560 | p=0.1289 | Day1 vs day2 | p=0.043 | p=0.003 | p<0.0001 |
| Day2 | p=0.0255 | p=0.7278 | Day2 vs day3 | p=0.307 | p=0.043 | p=0.758 | |
| Day3 | p<0.0001 | p=0.0315 | Day3 vs day4 | p=0.060 | p=0.940 | p=0.039 | |
| Day4 | p=0.0032 | p=0.8931 | Day4 vs day5 | p=0.029 | p=0.132 | p=0.547 | |
| Day5 | p=0.0178 | p=0.0858 | |||||
| Mean distance | Day1 | p=0.1700 | p=0.5325 | Day1 vs day2 | p<0.0001 | p=0.185 | p=0.01 |
| Day2 | p=0.2759 | p=0.0698 | Day2 vs day3 | p=0.967 | p=0.704 | p=0.045 | |
| Day3 | p=0.4699 | p=0.0002 | Day3 vs day4 | p=0.011 | p=0.398 | p=0.306 | |
| Day4 | p=0.0067 | p=0.1674 | Day4 vs day5 | p=0.754 | p=0.049 | p=0.014 | |
| Day5 | p=0.1586 | p=0.0033 | |||||
| Mean speed | Day1 | p=0.0074 | p=0.9914 | Day1 vs day2 | p=0.667 | p=0.615 | p=0.145 |
| Day2 | p=0.0250 | p=0.1970 | Day2 vs day3 | p=0.038 | p=0.597 | p=0.392 | |
| Day3 | p<0.0001 | p=0.0834 | Day3 vs day4 | p=0.744 | p=0.103 | p=0.994 | |
| Day4 | p=0.0011 | p=0.9304 | Day4 vs day5 | p=0.207 | p=0.623 | p=0.177 | |
| Day5 | p=0.0153 | p=0.0362 |
Figure 4.
Results of MWM test.
Notes: (a) The results of latency of MWM test of all rats from day1 to day5; (b) The results of platform quadrant of MWM test of all rats from day1 to day5; (c) The results of mean distance of MWM test of all rats from day1 to day5; (d) The results of mean speed of MWM test of all rats from day1 to day5. Test results on the sixth day: (e) platform quadrant; (f) total distance; (g) platform crossing (* p < 0.05; ** p < 0.01; ns p > 0.05).
During the probe trial, the VPA group rats spent less time in the platform quadrant, with the shorter total distance and fewer number of times crossing the platform than the WT group rats. Platform quadrant time and the number of times crossing the platform increased significantly after acupuncture. However, the total distance did not increase significantly after acupuncture (Platform quadrant time: p = 0.013, VPA vs WT, P = 0.034; VPA vs VPA_acupuncture, p = 0.025, Figure 4e; Total distance: p = 0.019, VPA vs WT, p = 0.005; VPA vs VPA_acupuncture, p = 0.083 Figure 4f; Platform crossing: p = 0.007, VPA vs WT, p = 0.003; VPA vs VPA_acupuncture, p = 0.012; Figure 4g).
RNA-Seq
DEGs
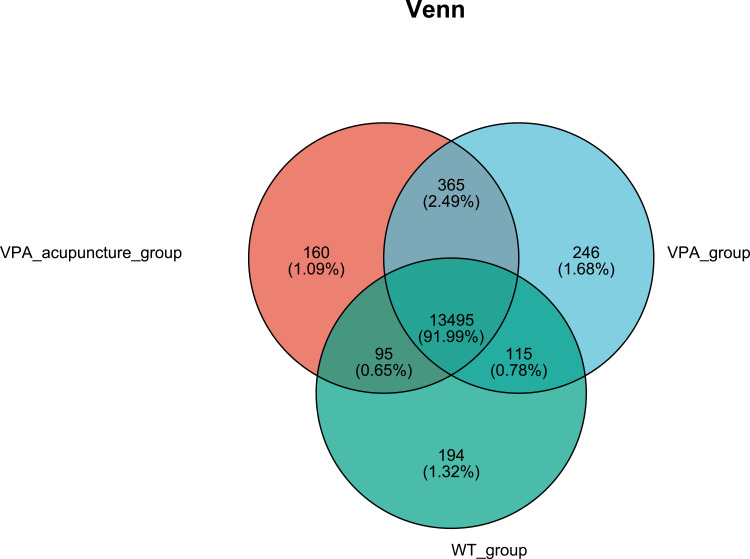
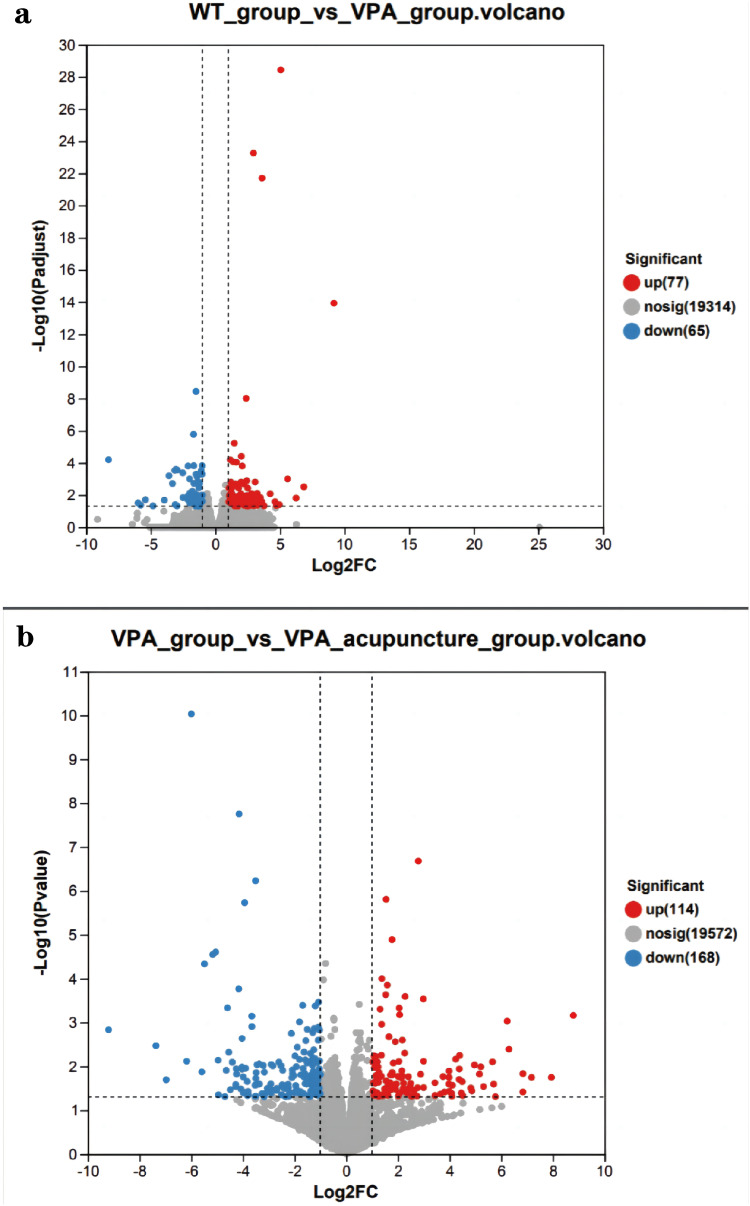
Genes that are differentially expressed between groups are represented by Venn diagrams (Figure 5). A total of 142 genes were significantly differentially expressed between the WT and VPA groups, and a total of 282 genes were significantly differentially between the VPA and VPA_acupuncture groups (Figure 6). Partial of these genes were up-regulated, while others were down-regulated (adjusted p < 0.05; |log2FC| ≥ 1).
Figure 5.
Genes differentially expressed among 3 groups of experimental rats.
Figure 6.
Genes differentially expressed between the two groups.
Notes: (a) Genes differentially expressed between WT and VPA groups; (b) Genes differentially expressed between VPA group and VPA_acupuncture group (red indicates up-regulated genes, blue indicates down-regulated genes).
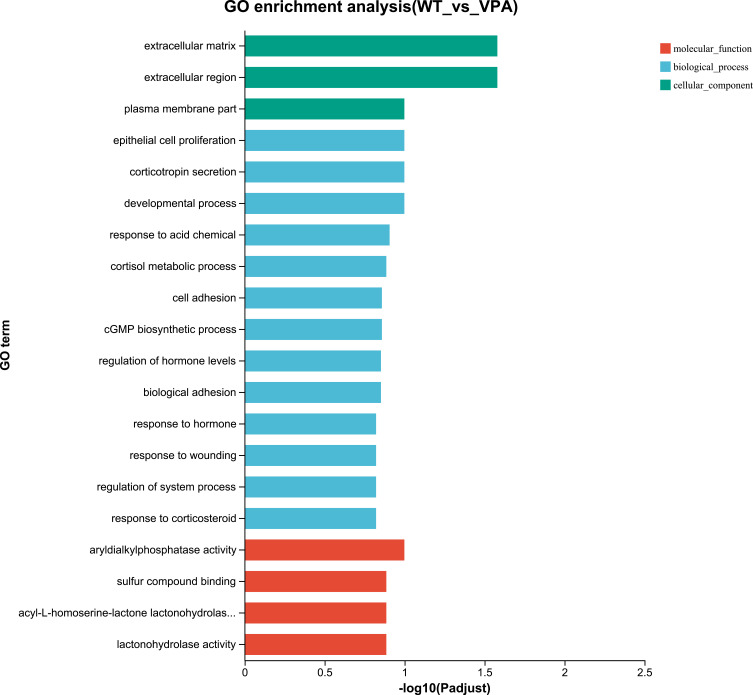
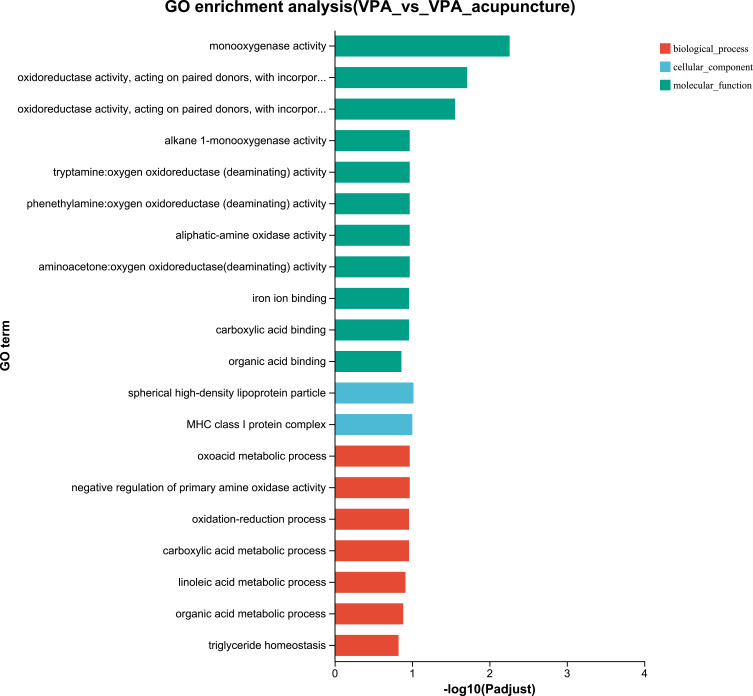
GO and KEGG Analysis
GO covers three sub-ontologies: biological processes, cellular components and molecular functions. GO functions were enriched in certain cell components between the WT group and VPA group rats, such as the extracellular matrix, extracellular regions, and the plasma membrane (Figure 7). Between the VPA and VPA_acupuncture group rats, GO functions were enriched in certain molecular functions, such as monooxygenase activity and oxidoreductase activity (Figure 8).
Figure 7.
GO enrichment analysis of differentially expressed genes between WT and VPA group.
Figure 8.
GO enrichment analysis of differentially expressed genes between VPA and VPA_acupuncture group.
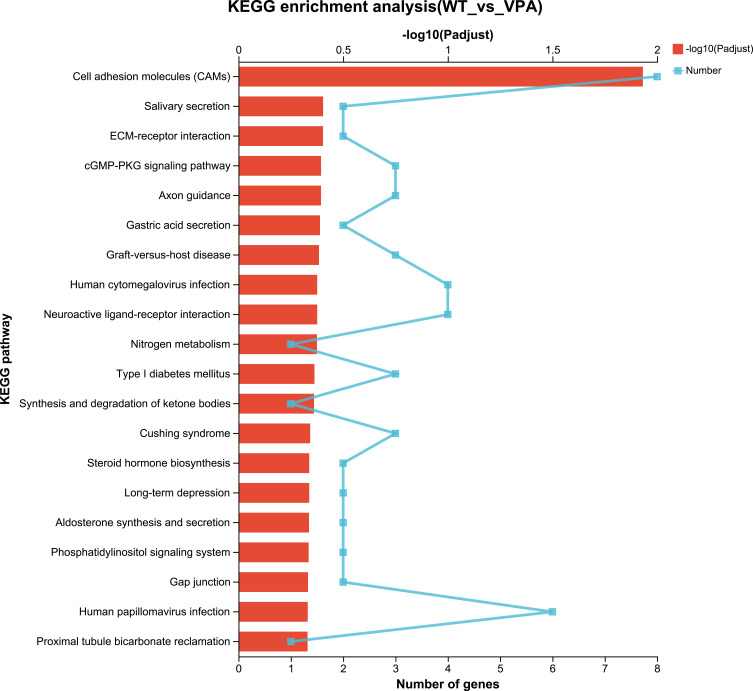
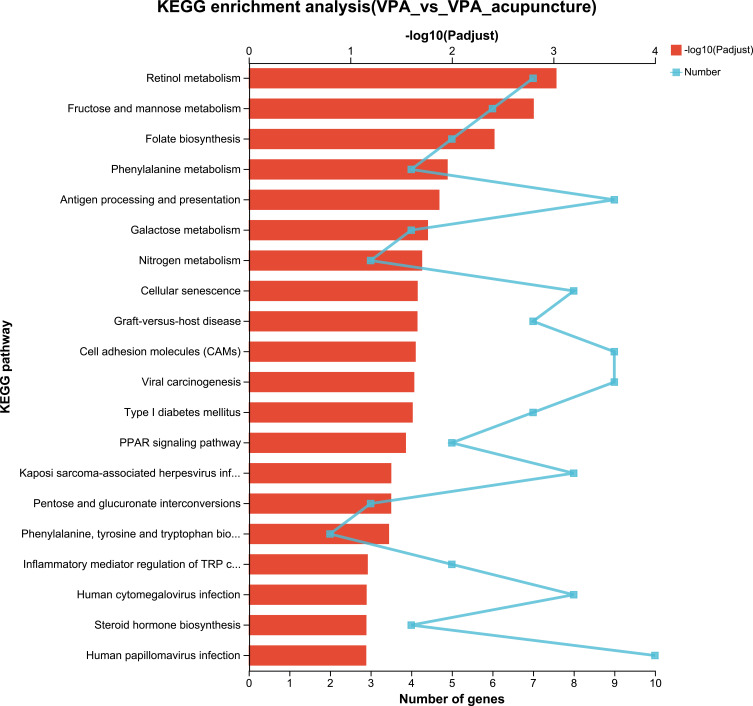
KEGG can be classified as 7 categories: metabolism, cellular processes, genetic information processing, organizational systems, human diseases, environmental information processing and drug development. KEGG analysis ranked the top 20 most important DEG pathways between the WT and VPA groups (Figure 9). To state some examples, these include cell adhesion molecules, neuroactive ligand–receptor interaction, the cGMP-PKG signaling pathway, phosphatidylinositol signaling system, long-term depression, steroid hormone biosynthesis, Cushing syndrome, and aldosterone synthesis and secretion. Analogously, the results of KEGG analysis between the VPA and VPA_acupuncture group rats were related to endogenous synthetic and metabolic pathways (Figure 10), including folate biosynthesis, retinol, fructose and mannose, phenylalanine, and galactose metabolism, antigen processing and presentation, phenylalanine and steroid hormone biosynthesis, the inflammatory mediator regulation of TRP channels, and PPAR signaling pathways.
Figure 9.
KEGG enrichment analysis of differentially expressed genes between WT and VPA group.
Figure 10.
KEGG enrichment analysis of differentially expressed genes between VPA and VPA_acupuncture group.
Cluster Analysis
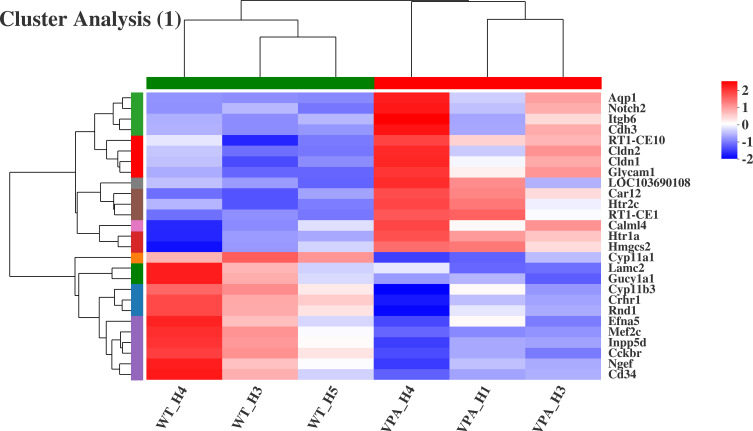
Cluster analysis was performed after GO and KEGG enrichment analysis. Twenty-seven genes in the VPA rat model were significantly different from WT rats (Figure 11). Among them, Cldn1, Cldn2, Notch2, and 5-HT receptor genes (Htr2c and Htr1a) were upregulated in the VPA group. Of these, Cldn1 mediates inflammation and tumorigenesis, while Cldn2 mediates infection. Notch2, an inhibitory signal gene, mediates cell differentiation. In contrast, the Lamc2, Mef2c, Efna5, Rnd1, and Crhr1 genes were downregulated in the VPA group. Most of these genes are involved in signal transduction, transcriptional activation, and the development and differentiation of central nervous system.
Figure 11.
Results of cluster analysis (WT group vs VPA group).
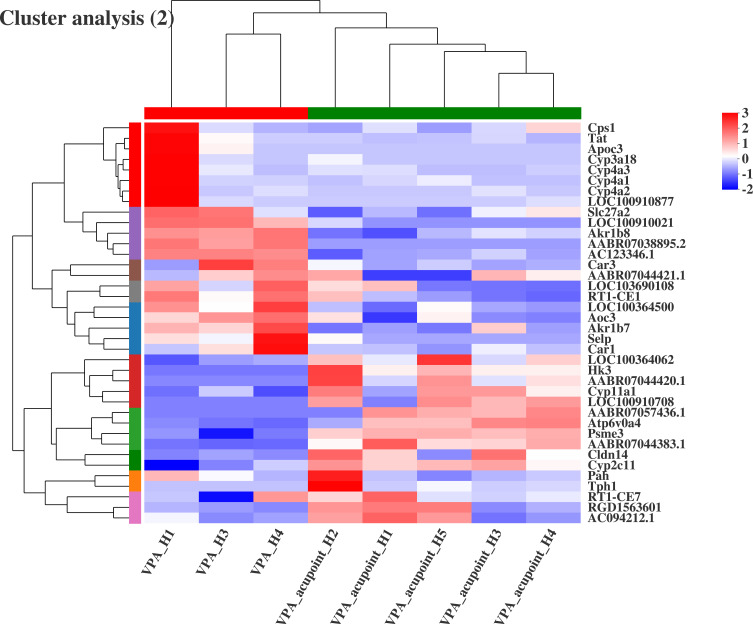
Thirty-eight genes in the VPA_acupuncture group differed significantly from the VPA rat model (Figure 12). The Hk3, Cyp2c11, Pah, and Tph1 genes, which are all involved in the endogenous metabolism, were up-regulated in the VPA_acupuncture group. In contrast, the Tat, Cyp3a18, Cyp4a3, Cyp4a1, Cyp4a2, Aoc3, Akr1b7, and Akr1b8 genes were down-regulated. Tat played a role in enhancing viral replication; Cyp3a18, Cyp4a3, Cyp4a1, and Cyp4a2 were involved in endogenous metabolism. Akr1b7 and Akr1b8 mediated tumorigenesis and Aoc3 mediated inflammation.
Figure 12.
Results of cluster analysis (VPA group vs VPA_acupuncture group).
RT-qPCR
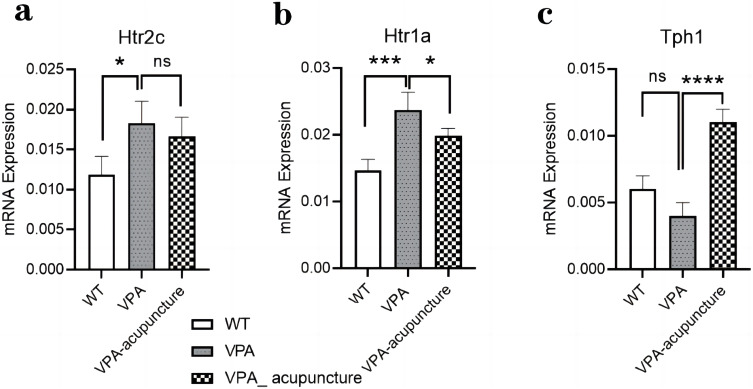
By comparing the results of RT-qPCR and RNA-seq, we confirmed that Htr2c, Htr1a and Tph1 genes had the same expression trend in the two experiments. The expression of Htr2c and Htr1a in VPA group rats were drastically up-regulated than the WT group rats (Htr2c: P<0.05, Figure 13a; Htr1a: P<0.001, Figure 13b). Most notably, the Htr1a expression was significantly down-regulated after acupuncture (P< 0.05, Figure 13b), while Htr2c expression did not significantly change after acupuncture. There was no statistical difference in the expression of Tph1 gene between the WT and VPA group, but its expression increased significantly after acupuncture (P<0.0001, Figure 13c).
Figure 13.
The results of RT-qPCR.
Notes: (a) The mRNA expression of Htr2c; (b) The mRNA expression of Htr1a; (c) The mRNA expression of Tph1 (* p < 0.05; *** p < 0.001; **** p < 0.0001; ns p > 0.05).
Acupuncture Increased Serotonin Levels of Hippocampus in VPA-Induced Rat Model
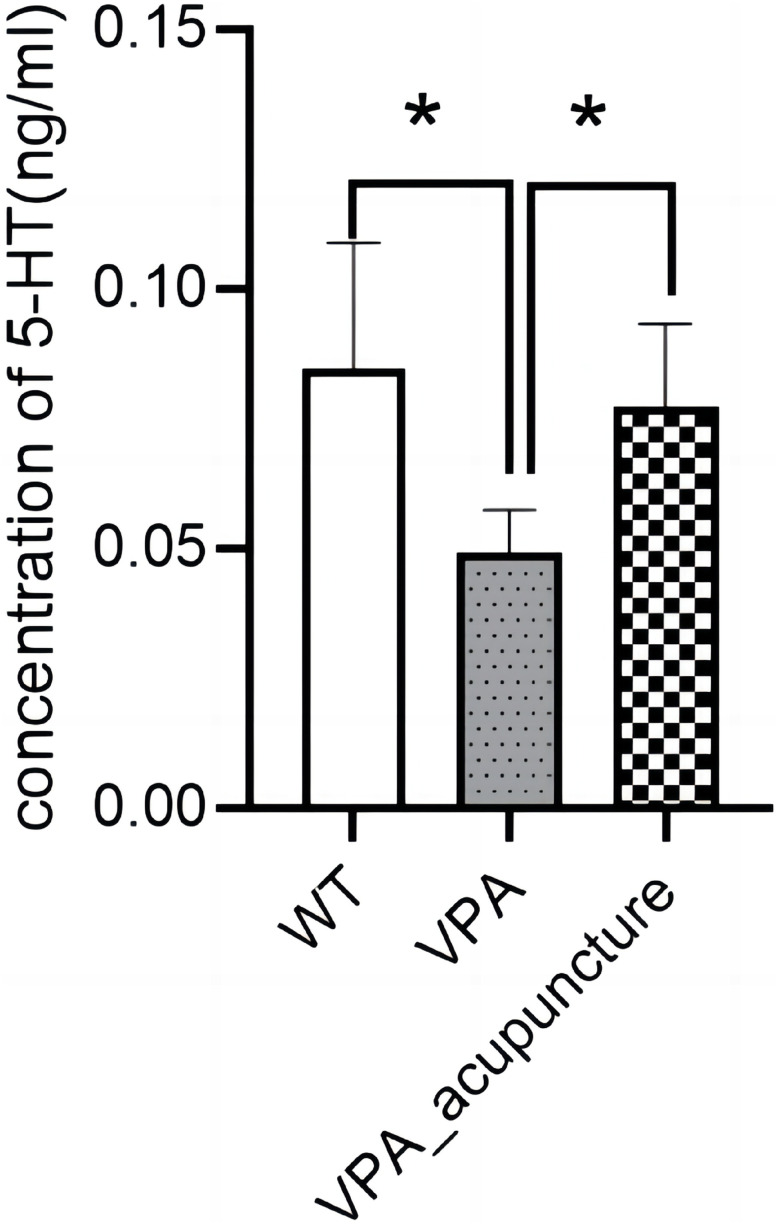
We performed an ELISA in order to ascertain whether acupuncture changed the serotonin levels of hippocampus across the different groups. The concentration of serotonin in VPA-induced rat model was significantly lower than WT and VPA_acupuncture groups (VPA vs WT, P = 0.013; VPA vs VPA_acupuncture, P = 0.047; Figure 14).
Figure 14.
Determination of serotonin level in hippocampus of three group rats.
Note: * p < 0.05.
Discussion
Childhood autism is a neurodevelopmental disorder that occurs in early childhood. It frequently affects children’s social interaction, language communication, emotional cognition, intellectual development, etc.35,36 The etiology of autism is still unclear. The combined effects of genetic factors, immune disorders, environmental factors and adverse factors during pregnancy, such as exposure to second-hand smoke, certain drugs and adverse emotions are considered to be closely related to the onset of autism.37 Although behavioral intervention, education and medication can alleviate the clinical symptoms of children with autism to a certain extent, it is regrettable that autism cannot be cured at present. As a result, many children with the condition are unable to integrate into normal life and learning, which has a serious impact on the children’s families and society.38 What we emphasize is that the timing of intervention is very important, and early intervention is considered to be of great significance to the long-term outcome of autism.39
In the situation that the diagnostic rate of childhood autism to rise, it is particularly important to find other effective intervention methods. Originating from traditional Chinese medicine, acupuncture is one of the treasures of Chinese culture that have been inherited and developed for thousands of years. Acupuncture is as widely used in children as it is in adults. There are many acupoints throughout the body, which are in charge of various symptoms, basically covering all systems of the body. For example, acupuncture at Zusanli, Hegu, and Chize on both sides and Quchi, Kongzui, Lieque can significantly improve children’s pneumonia;40 acupuncture at Ququan, Quchi, Zhongwan, Qihai, Zusanli and Sanyinjiao can improve the gastrointestinal symptoms of patients with Crohn’s disease, such as chronic dyspepsia, reflux, abdominal pain and excessive diarrhea;41 acupuncture of Fengchi, Zusanli and other acupoints can significantly improve children’s enuresis,42 and acupuncture at four Shengong and Baihui can significantly improve central nervous system diseases, such as cerebral palsy in children.43 For childhood autism, common acupuncture methods include brain tri-points, four-spirit points, spirit setting points, mental tri-points, spirit waiting-up points and foot mental points, etc.44 Acupuncture can also significantly improve the symptoms of various animal models, such as animal model of COPD,45 animal model of cerebra hemorrhage,46 animal model of PCOS47 and animal model of ASD.48 Hence, acupuncture has aroused the interest of researchers from various disciplines in its potential mechanisms of action. This study mainly selected Shéntíng (GV24) and bilateral BěNshén (GB13), mental tri-points, to treat autism model rats.
We found that in the open field experiment, the VPA-induced rat model was more willing to stay in the surrounding area compared with the WT and acupuncture group, which suggested that the basic anxiety level of the model rats was increased and acupuncture could significantly improve their anxiety level, which was consistent with the research results of Zhang Xuejie et al17 The total length movement and average speed in VPA group were smaller than those in WT group, suggesting that the VPA group had reduced locomotor capacity, which was improved by acupuncture to some extent. In the three-chamber test, all the experimental rats spent significantly more time in contact with stranger 1 than with the object, which showed certain social activities. The contact time between the test rat with stranger 2 was significantly longer than that of stranger 1 in the WT and VPA acupuncture groups, while the VPA-induced rat model apparently preferred to communicate with stranger 1, indicating that the social novelty preference of VPA-induced rat model was weakened. This may be a result of autism-induced neurological developmental impairment that reduced the desire to explore new things, resulting in the loss of social novelty preference. In contrast, acupuncture can significantly improve this social disorder, which was consistent with previous results.49,50 In Morris water maze experiment, with the increase in the number of training days, all of the rats could find the target quadrant faster than before, and the degree of improvement of the WT and VPA_acupuncture groups were significantly better than the VPA group, which suggested that the learning and memory of VPA group rats were damaged, and acupuncture treatment could significantly improve this problem. In the test on the sixth day, the WT group rats were more active in the target quadrant, such as the target quadrant time was longer than VPA group rats, the number of times of crossing the platform was more than VPA group rats, and the movement distance was also more than VPA group rats. After acupuncture, the rats in the target quadrant were more active, also indicating that their learning and memory were improved.
RNA-sequence analysis of the hippocampus was conducted after the behavioral tests. The KEGG analysis between WT and VPA groups was primarily enriched in signal transduction pathways, endogenous synthesis and metabolism pathways. Between VPA and VPA_acupuncture groups, DEGs were also mainly enriched in endogenous synthesis, metabolism and signal transduction pathways. For example, in our study, DEGs were enriched in folate biosynthesis between the VPA and VPA acupuncture groups. During neurodevelopmental periods, folate deficiency was related to increased risk of ASD, and increased folic acid synthesis has been reported to improve ASD symptoms.51,52 In conclusion, abnormal endogenous synthesis, metabolism and signal pathways may cause ASD, and acupuncture may improve the clinical manifestations and symptoms of ASD by reducing the number of abnormal pathways stated above. After cluster analysis, the author screened out the significantly different expression genes related to the pathogenesis of autism and the mechanism of acupuncture treatment by consulting a large number of documents. Notably, the 5-HT receptor genes (Htr2c and Htr1a) were up-regulated in VPA-induced rat model compared with the WT rats. The Tph1 gene was up-regulated in the VPA_acupuncture group compared with the VPA-induced rat model. Although Tph1 gene was not responsible for most of serotonin synthesis in the central nervous system of mature animals, it is thought to be associated with developmental disorders because of the effect of Tph1 on serotonin synthesis during late brain development.53,54 We inferred that serotonin may play a certain role therein. Lee EJ, Warden S et al used serotonin or serotonin antagonists/agonists to investigate the mechanisms by which acupuncture improved multiple symptoms (eg, pain, obesity, and depression), which were confirmed to be relevant for alterations of serotonin levels.55 Thus, the abnormal serotonin system may be an underlying cause of autism. Since acupuncture treatment may regulate the expression of Tph1 and thus the serotonin system. This may be the mechanism by which acupuncture improves the behavioural performance of the VPA-induced rat model. In addition, we further verified by ELISA that acupuncture can increase the level of serotonin in the hippocampus of VPA-induced rat model.
In conclusion, this study provides molecular evidence for acupuncture in treating ASD. It reveals that the pathogenesis of autism model and the rat mechanism of acupuncture to improve ASD symptoms are related to the changes in the hippocampal serotonin system. However, we have only demonstrated from a single perspective that the mechanism by which acupuncture improves autism is associated with VPA exposure during pregnancy, and more models of autism are needed to further validate our findings. The two processes are regulated by different genes (Htr2c, Htr1a, and TPH1), which were shown to share the same RT-qPCR expression trends. Therefore, further experiments are required to validate the specific role of these genes and molecular pathways during the processes.
Conclusion
Acupuncture improved abnormal behavioral symptoms in the VPA-induced rat model. Further experiments showed that the improvement of the serotonin system may be one of the main regulatory mechanisms of acupuncture for treating ASD, which provided theoretical basis for the clinical application of acupuncture of children with autism.
Acknowledgments
Thanks for the support of the authors of the present paper and the Experimental Animal Center of Fujian Medical University.
Funding Statement
The National Natural Science Foundation of China (No. 81804173), Fujian Provincial Medical Innovation Project (No. 2020CXA016), and Fujian Provincial Department of Science and Technology (No. 2020J01335) supported this study.
Ethics Approval and Consent to Participate
All animal experiments were conducted in accordance with the protocol approved by the Animal Research Committee of the Fujian Medical University (FJMU IACUC permit number: 2018-088).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Kalantarian H, Jedoui K, Dunlap K, et al. The performance of emotion classifiers for children with parent-reported autism: quantitative feasibility study. JMIR Ment Health. 2020;7(4):e13174. doi: 10.2196/13174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong VC. Use of Complementary and Alternative Medicine (CAM) in Autism Spectrum Disorder (ASD): comparison of Chinese and Western culture (part A). J Autism Dev Disord. 2009;39(3):454–463. doi: 10.1007/s10803-008-0644-9 [DOI] [PubMed] [Google Scholar]
- 3.Tayanloo-Beik A, Hamidpour SK, Abedi M, et al. Zebrafish modeling of autism spectrum disorders, current status and future prospective. Front Psychiatry. 2022;13:911770. doi: 10.3389/fpsyt.2022.911770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vanderploeg K, Yi X. Acupuncture in modern society. J Acupunct Meridian Stud. 2009;2(1):26–33. doi: 10.1016/S2005-2901(09)60012-1 [DOI] [PubMed] [Google Scholar]
- 5.Rong PJ, Wang Y, Xu NG. Brain science promotes the development of acupuncture in treating brain diseases. Zhen Ci Yan Jiu. 2019;44(12):859–862. doi: 10.13702/j.1000-0607.190691 [DOI] [PubMed] [Google Scholar]
- 6.Yang Y, Hu S, Lin H, He J, Tang C. Electroacupuncture at GV24 and bilateral GB13 improves cognitive ability via influences the levels of Aβ, p-tau (s396) and p-tau (s404) in the hippocampus of Alzheimer’s disease model rats. Neuroreport. 2020;31(15):1072–1083. doi: 10.1097/WNR.0000000000001518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wattanathorn J, Sutalangka C. Laser acupuncture at HT7 acupoint improves cognitive deficit, neuronal loss, oxidative stress, and functions of cholinergic and dopaminergic systems in animal model of Parkinson’s disease. Evid Based Complement Alternat Med. 2014;2014(22):937601. doi: 10.1155/2014/937601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sutalangka C, Wattanathorn J, Muchimapura S, et al. Laser acupuncture improves memory impairment in an animal model of Alzheimer’s disease. J Acupunct Meridian Stud. 2013;6(5):247–251. doi: 10.1016/j.jams.2013.07.001 [DOI] [PubMed] [Google Scholar]
- 9.Yau CH, Ip CL, Chau YY. The therapeutic effect of scalp acupuncture on natal autism and regressive autism. Chin Med. 2018;13:30. doi: 10.1186/s13020-018-0189-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan AS, Cheung MC, Sze SL, et al. Seven-star needle stimulation improves language and social interaction of children with autistic spectrum disorders. Am J Chin Med. 2012;37(3):495–504. doi: 10.1142/S0192415X09007004 [DOI] [PubMed] [Google Scholar]
- 11.Yang C, Hao Z, Zhang LL, Guo Q. Efficacy and safety of acupuncture in children: an overview of systematic reviews. Pediatr Res. 2015;78(2):112–119. doi: 10.1038/pr.2015.91 [DOI] [PubMed] [Google Scholar]
- 12.Thongkorn S, Kanlayaprasit S, Panjabud P, et al. Sex differences in the effects of prenatal bisphenol A exposure on autism-related genes and their relationships with the hippocampus functions. Sci Rep. 2021;11(1):1241. doi: 10.1038/s41598-020-80390-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Codagnone MG, Podest MF, Uccelli NA, et al. Differential local connectivity and neuroinflammation profiles in the medial prefrontal cortex and hippocampus in the valproic acid rat model of autism. Dev Neurosci. 2015;37(3):215–231. doi: 10.1159/000375489 [DOI] [PubMed] [Google Scholar]
- 14.Isabel BM, Octavio G, Erika CO, et al. Alterations in neuronal cytoskeletal and astrocytic proteins content in the brain of the autistic-like mouse strain C58/J. Neurosci Lett. 2018;682:32–38. doi: 10.1016/j.neulet.2018.06.004 [DOI] [PubMed] [Google Scholar]
- 15.Ye Y, Li H, Yang JW, et al. Acupuncture attenuated vascular dementia-induced hippocampal long-term potentiation impairments via activation of D1/D5 receptors. Stroke. 2017;48(4):1044–1051. doi: 10.1161/STROKEAHA.116.014696 [DOI] [PubMed] [Google Scholar]
- 16.He J, Zhao C, Liu W, et al. Neurochemical changes in the hippocampus and prefrontal cortex associated with electroacupuncture for learning and memory impairment. Int J Mol Med. 2018;41(2):709–716. doi: 10.3892/ijmm.2017.3287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang XJ, Wu Q. Effects of electroacupuncture at different acupoints on learning and memory ability and PSD-95 protein expression on hippocampus CA1 in rats with autism. Zhongguo Zhen Jiu. 2013;33(7):627–631. [PubMed] [Google Scholar]
- 18.Huang X, Yuan Q, Luo Q, et al. Clinical efficacy on mental retardation in the children treated with JIN’s three scalp needling therapy and the training for cognitive and perceptual disturbance. Chin Acupunct Moxibustion. 2015;35(7):651. [PubMed] [Google Scholar]
- 19.Cui S, Xu M, Huang J, et al. Cerebral responses to acupuncture at GV24 and bilateral GB13 in rat models of Alzheimer’s disease. Behav Neurol. 2018;2018:8740284. doi: 10.1155/2018/8740284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Z, Liang P, Zhao Z, et al. Acupuncture modulates resting state hippocampal functional connectivity in Alzheimer disease. PLoS One. 2014;9(3):e91160. doi: 10.1371/journal.pone.0091160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Connors SL, Matteson KJ, Sega GA, Lozzio CB, Carroll RC, Zimmerman AW. Plasma serotonin in autism. Pediatr Neurol. 2006;35(3):182–186. doi: 10.1016/j.pediatrneurol.2006.02.010 [DOI] [PubMed] [Google Scholar]
- 22.Lambe EK, Fillman SG, Webster MJ, et al. Serotonin receptor expression in human prefrontal cortex: balancing excitation and inhibition across postnatal development. PLoS One. 2011;6(7):e22799. doi: 10.1371/journal.pone.0022799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jaiswal P, Mohanakumar KP, Rajamma U. Serotonin mediated immunoregulation and neural functions: complicity in the aetiology of autism spectrum disorders. Neurosci Biobehav Rev. 2015;55:413–431. doi: 10.1016/j.neubiorev.2015.05.013 [DOI] [PubMed] [Google Scholar]
- 24.Israelyan N, Margolis KG. Reprint of: serotonin as a link between the gut-brain-microbiome axis in autism spectrum disorders. Pharm Res. 2019;140:115–120. doi: 10.1016/j.phrs.2018.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rahman MR, Petralia MC, Ciurleo R, et al. Comprehensive analysis of RNA-seq gene expression profiling of brain transcriptomes reveals novel genes, regulators, and pathways in autism spectrum disorder. Brain Sci. 2020;10(10):747. doi: 10.3390/brainsci10100747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nicolini C, Fahnestock M. The valproic acid-induced rodent model of autism. Exp Neurol. 2018;299(Pt A):217–227. doi: 10.1016/j.expneurol.2017.04.017 [DOI] [PubMed] [Google Scholar]
- 27.Choi CS, Gonzales EL, Kim KC, et al. The transgenerational inheritance of autism-like phenotypes in mice exposed to valproic acid during pregnancy. Sci Rep. 2016;6(12):36250–36267. doi: 10.1038/srep36250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hou QL, Yan W, Li YB, et al. A developmental study of abnormal behaviors and altered GABAergic signaling in the VPA-treated rat model of Autism. Front Behav Neurosci. 2018;12(12):182–191. doi: 10.3389/fnbeh.2018.00182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang EJ, Ahn S, Lee K, et al. Early behavioral abnormalities and perinatal alterations of PTEN/AKT pathway in valproic acid autism model mice. PLoS One. 2016;11(4):298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011;12:323. doi: 10.1186/1471-2105-12-323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. doi: 10.1186/s13059-014-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang L, Feng Z, Wang X, et al. DEGseq: an R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics. 2009;26(1):136–138. doi: 10.1093/bioinformatics/btp612 [DOI] [PubMed] [Google Scholar]
- 33.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26(1):139–140. doi: 10.1093/bioinformatics/btp616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xie C, Mao X, Huang J, et al. KOBAS 2.0: a web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011;39:W316–W322. doi: 10.1093/nar/gkr483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Konicar L, Radev S, Prillinger K, et al. Volitional modification of brain activity in adolescents with autism spectrum disorder: a bayesian analysis of slow cortical potential neurofeedback. Neuroimage Clin. 2021;29:102557. doi: 10.1016/j.nicl.2021.102557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bernard Paulais MA, Mazetto C, Thiébaut E, et al. Heterogeneities in cognitive and socio-emotional development in children with autism spectrum disorder and severe intellectual disability as a comorbidity. Front Psychiatry. 2019;10:508. doi: 10.3389/fpsyt.2019.00508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bölte S, Girdler S, Marschik PB. The contribution of environmental exposure to the etiology of autism spectrum disorder. Cell Mol Life Sci. 2019;76(7):1275–1297. doi: 10.1007/s00018-018-2988-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Correale C, Borgi M, Cirulli F, et al. The impact of health and social services on the quality of life in families of adults with Autism Spectrum Disorder (ASD): a focus group study. Brain Sci. 2022;12(2):177. doi: 10.3390/brainsci12020177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peacock G, Lin SC. Enhancing early identification and coordination of intervention services for young children with autism spectrum disorders: report from the act early regional summit project. Disabil Health J. 2012;5(1):55–59. doi: 10.1016/j.dhjo.2011.10.001 [DOI] [PubMed] [Google Scholar]
- 40.Sun Q, Yu H, Shang Y, et al. Correlation analysis of chaige qinlian decoction and acupuncture combined intervention on prognosis of children with pneumonia. J Healthc Eng. 2021;2021(Pt.15):2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lai H, Wang K, Dong Q, Zhu X, Li X, Qi S. Traditional Chinese medicine for management of recurrent and refractory Crohn disease: a case report. Medicine. 2019;98(15):e15148. doi: 10.1097/MD.0000000000015148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Radmayr C, Schlager A, Studen M, Bartsch G. Prospective randomized trial using laser acupuncture versus desmopressin in the treatment of nocturnal enuresis. Eur Urol. 2001;40(2):201–205. doi: 10.1159/000049773 [DOI] [PubMed] [Google Scholar]
- 43.Wang J, Shi W, Khiati D, et al. Acupuncture treatment on the motor area of the scalp for motor dysfunction in children with cerebral palsy: study protocol for a multicenter randomized controlled trial. Trials. 2020;21(1):29. doi: 10.1186/s13063-019-3986-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yuan C, Xiao J, Zhong Y, Qin WX, Yuan Q. YUAN Qing’s experience of Tiaoshen acupuncture in treating autism spectrum disorder. Zhongguo Zhen Jiu. 2021;41(12):1383–1386. doi: 10.13703/j.0255-2930.20201115-k0002 [DOI] [PubMed] [Google Scholar]
- 45.Li J, Wu S, Tang H, et al. Long-term effects of acupuncture treatment on airway smooth muscle in a rat model of smoke-induced chronic obstructive pulmonary disease. Acupunct Med. 2016;34(2):107–113. doi: 10.1136/acupmed-2014-010674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu H, Sun X, Zou W, et al. Scalp acupuncture attenuates neurological deficits in a rat model of hemorrhagic stroke. Complement Ther Med. 2017;32:85–90. doi: 10.1016/j.ctim.2017.03.014 [DOI] [PubMed] [Google Scholar]
- 47.Xing L, Chen Y, He Z, et al. Acupuncture improves endometrial angiogenesis by activating PI3K/AKT pathway in a rat model with PCOS. Evid Based Complement Alternat Med. 2022;2022:1790041. doi: 10.1155/2022/1790041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang X, Ding R, Song Y, et al. Transcutaneous electrical acupoint stimulation in early life changes synaptic plasticity and improves symptoms in a valproic acid-induced rat model of autism. Neural Plast. 2020;2020:8832694. doi: 10.1155/2020/8832694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cheng KJ. Neurobiological mechanisms of acupuncture for some common illnesses: a clinician’s perspective. J Acupunct Meridian Stud. 2014;7(3):105–114. doi: 10.1016/j.jams.2013.07.008 [DOI] [PubMed] [Google Scholar]
- 50.Wang L, Peng JL, Qiao FQ, et al. Clinical randomized controlled study of acupuncture treatment on children with Autism Spectrum Disorder (ASD): a systematic review and meta-analysis. Evid Based Complement Altern Med. 2021;2021:5549849. doi: 10.1155/2021/5549849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Geryk J, Macek M, Vlčková M, Havlovicová M, Macek M, Kremlíková Pourová R. The key role of purine metabolism in the folate-dependent phenotype of autism spectrum disorders: an in silico analysis. Metabolites. 2020;10(5):184. doi: 10.3390/metabo10050184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ramaekers VT, Sequeira JM, Thöny B, Quadros EV. Oxidative stress, folate receptor autoimmunity, and CSF findings in severe infantile autism. Autism Res Treat. 2020;2020:9095284. doi: 10.1155/2020/9095284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Betari N, Sahlholm K, Morató X, et al. Inhibition of tryptophan hydroxylases and monoamine oxidase-A by the proton pump inhibitor, omeprazole-in vitro and in vivo investigations. Front Pharmacol. 2020;11:593416. doi: 10.3389/fphar.2020.593416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sabir MS, Haussler MR, Sanchita M, et al. Optimal vitamin D spurs serotonin: 1,25-dihydroxyvitamin D represses serotonin reuptake transport (SERT) and degradation (MAO-A) gene expression in cultured rat serotonergic neuronal cell lines. Genes Nutr. 2018;13(1):19. doi: 10.1186/s12263-018-0605-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee EJ, Warden S. The effects of acupuncture on serotonin metabolism. Eur J Integr Med. 2016;2016:S187638201630124X. [Google Scholar]