Abstract
Objectives
We sought to investigate how race, ethnicity, and socioeconomic status relate to tracheostomy insertion and post-tracheostomy mortality among infants with bronchopulmonary dysplasia.
Methods
The Vizient Clinical Database/Resource Manager was queried to identify infants born ≤32 weeks with bronchopulmonary dysplasia admitted to United States hospitals from January 2012-December 2020. Markers of socioeconomic status were linked to patient records from the Agency for Healthcare Research and Quality’s Social Determinants of Health Database. Regression models were used to assess trends in annual tracheostomy insertion rate and odds of tracheostomy insertion and post-tracheostomy mortality, adjusting for sociodemographic and clinical factors.
Results
There were 40,021 ex-premature infants included in the study, 1,614 (4.0%) of whom received a tracheostomy. Tracheostomy insertion increased from 2012-2017 (3.1% to 4.1%), but decreased from 2018-2020 (3.3% to 1.6%). Non-Hispanic Black infants demonstrated a 25% higher odds (aOR 1.25, 1.09-1.43) and Hispanic infants demonstrated a 20% lower odds (aOR 0.80, 0.65-0.96) of tracheostomy insertion compared to non-Hispanic White infants. Patients receiving public insurance had increased odds of tracheostomy insertion (aOR 1.15, 1.03-1.30), but there was no relation between other metrics of socioeconomic status and tracheostomy insertion within our cohort. In-hospital mortality among the tracheostomy-dependent was 14.1% and was not associated with sociodemographic factors.
Conclusions
Disparities in tracheostomy insertion are not accounted for by differences in socioeconomic status or the presence of additional neonatal morbidities. Post-tracheostomy mortality does not demonstrate the same relationships. Further investigation is needed to explore the source and potential mitigators of the identified disparities.
Keywords: Chronic lung disease, Prematurity, Healthcare inequities
Introduction
Bronchopulmonary dysplasia (BPD) is the most common complication of premature birth, affecting over 40% of infants born before 30 weeks’ gestation.1 Recent studies have demonstrated a growing population of children with BPD supported with tracheostomy insertion and chronic mechanical ventilation, with estimates peaking at 3.5 tracheostomy insertions for patients with BPD per 100,000 live births in 2017.2 Evidence-based guidelines to direct whether and when to offer tracheostomy are lacking, but typical indications in patients with BPD include failure to separate from invasive ventilation, intolerance of noninvasive interfaces, acquired structural airway abnormalities including subglottic stenosis or tracheobronchomalacia, and poor growth or compromised neurodevelopment.3 Despite the growing population of chronically ventilated patients with BPD, little is known about the factors associated with tracheostomy insertion or post-tracheostomy clinical outcomes in these children.
The effects of sociodemographic factors on prematurity-related morbidities and outcomes have been extensively studied.4,5 Regarding BPD specifically, results of studies evaluating race as a risk factor for the disease have been inconsistent. Findings have ranged from reports of a lower incidence among children of Black women6, to no association with race/ethnicity7, to a recent investigation demonstrating in a “fetuses-at-risk” analysis that Black mothers have a greater than 4 times increased risk of their infant being diagnosed with BPD.5 Studies evaluating outcomes within the BPD population are more uniform.8,9 Black infants demonstrate increased risk of hospital admission, longer lengths of hospital stay, and increased odds of mortality.8,9 Socioeconomic status as measured by neighborhood median household income10,11 and neighborhood deprivation index12 has also been associated with higher rates of readmission and mortality in patients with BPD. There has been limited assessment, however, of how sociodemographic factors relate specifically to tracheostomy insertion or how race/ethnicity and socioeconomic status affect post-tracheostomy outcomes in patients with BPD.
In a large United States-based inpatient database, we sought to identify the relationships between race/ethnicity, socioeconomic status, and tracheostomy placement in patients with BPD. Additionally, we provide a contemporary assessment of practice trends in tracheostomy insertion for patients with BPD in the US. We hypothesized that the increasing frequency of tracheostomy insertion previously described among patients with BPD has continued. Further, we hypothesized that the disparities in outcomes for patients from historically marginalized racial or ethnic backgrounds or who are socioeconomically disadvantaged would manifest as differences in tracheostomy insertion and post-tracheostomy mortality rates.
Materials and Methods
We performed a retrospective multicenter cohort analysis of inpatient data from hospitals contributing to the Vizient Clinical Database/Resource Manager (CDB/RM™). Vizient CDB/RM collects administrative, financial, and clinical outcomes data from hospital discharge records.13 Data are compiled from over 8.5 million admissions per year and from over 95% of the country’s academic medical centers and their community affiliates. Individual patient records are linked across readmissions and then deidentified prior to release to the study team. Since all data were deidentified, the study was deemed exempt from review by the local Institutional Review Board. The data that support the findings of this study are available from Vizient. Restrictions apply to the availability of these data, which were used under license for this study. Data are available from the authors with the permission of Vizient.
CDB/RM was queried for admissions from January 1, 2012 - December 31, 2020 of ex-premature children <18 years old with a diagnosis of bronchopulmonary dysplasia. We included only admissions with diagnosis codes for BPD and prematurity (≤ 32 weeks’ gestation), defined by International Classification of Disease (ICD) 9 and 10 coding (Supplemental Table 1). Vizient patient records include sociodemographic data and comorbid diagnoses. Race and ethnicity data are extracted from each institution’s electronic medical record. Details regarding how patient-level race was collected at each institution were not available to the study team. Data on social determinants of health were linked from the Agency for Healthcare Research and Quality’s (AHRQ) Social Determinants of Health Database by the household zip code listed in each patient’s index admission.14 Z-scores were calculated from the mean and standard deviation of the overall distribution for the following sociodemographic variables: patient’s neighborhood median household income, percentage living below the poverty line, and percentage over 25 years old with a bachelor’s degree.15 The Z-scores were used to cohort into 3 groups for each of the variables (Z-score less than −1.0, Z-score −1.0 to 1.0, and Z-score greater than 1.0). The primary outcome was tracheostomy insertion during any admission, as indicated by ICD procedure coding (Supplemental Table 1). These tracheostomy procedure ICD codes have been previously validated.2,16,17 Mortality, defined as an in-hospital death at one of the Vizient institutions, was additionally assessed.
Trends in the rate of tracheostomy insertion per year were assessed using logistic regression models applied to 2 time periods within the data: 2012-2017 (to replicate prior literature)2 and 2018-2020. Next, associations between sociodemographic or clinical factors and tracheostomy insertion were assessed using crude odds ratios (OR). Adjusted odds ratios (aOR) were determined from models adjusting for sex, race/ethnicity, primary insurance provider (private versus public/self-pay/other), and neighborhood sociodemographic characteristics, specifically median household income and percentage with a bachelor’s degree. Variables included in multivariable modeling were chosen a priori with the intention of focusing our primary analysis on the effects of sociodemographic factors. Neighborhood percentage with income below the poverty level was excluded from multivariable models due to its collinearity with median household income. A sensitivity analysis was performed using generalized estimating equations that incorporated hospital site as the clustering variable to assess how between-hospital variation in tracheostomy practices might affect the population-level associations between tracheostomy and the included covariates.
Clinical factors were then serially added to the multivariable model to determine how clinical differences might account for differences between sociodemographic groups. Gestational age, followed by an indicator for the presence of non-respiratory neonatal morbidities (necrotizing enterocolitis, intraventricular hemorrhage grade >2, periventricular leukomalacia, and/or retinopathy of prematurity stage >2)18, followed by additional comorbid diagnoses of pulmonary hypertension, structural airway abnormalities, and congenital heart disease were added to the model and effects on the odds of tracheostomy insertion were assessed (Supplemental Table 1). Lastly, mortality was assessed stratified by tracheostomy status. Crude ORs for mortality were determined, followed by aORs adjusting for the same sociodemographic factors as above. All analyses were performed using R statistical software.19
Results
Our data query yielded records from 75,699 admissions of 55,642 patients with a diagnosis of BPD. After including only those with an ICD code indicating gestational age ≤ 32 weeks, we were left with 40,021 patients with BPD, 1,614 (4.0%) of whom received a tracheostomy (Figure 1). Table 1 contains baseline characteristics of patients with and without tracheostomy. Overall, most patients were male, completed ≤ 26 weeks of gestation, and utilized Medicaid/self-pay/other primary insurance (versus private insurance). The race/ethnicity of the plurality of patients was reported as non-Hispanic White, followed by non-Hispanic Black. Median postnatal age at tracheostomy insertion was 4.6 months (IQR 3.6-5.9 months). Tracheostomy insertion rates per BPD patients per year ranged from a minimum of 1.6% in 2020 to a maximum of 4.1% in 2016 (Figure 2). There was a significant upward trend in the rate of tracheostomy insertion per year from 2012-2017 (p=0.021). This trend reversed, however, from 2018-2020 when there was a significant downward trend in tracheostomy (p<0.001). There were 227 (14.1%) deaths in the tracheostomy cohort and 1,857 (4.8%) deaths in the non-tracheostomy cohort (p<0.001).
Figure 1:
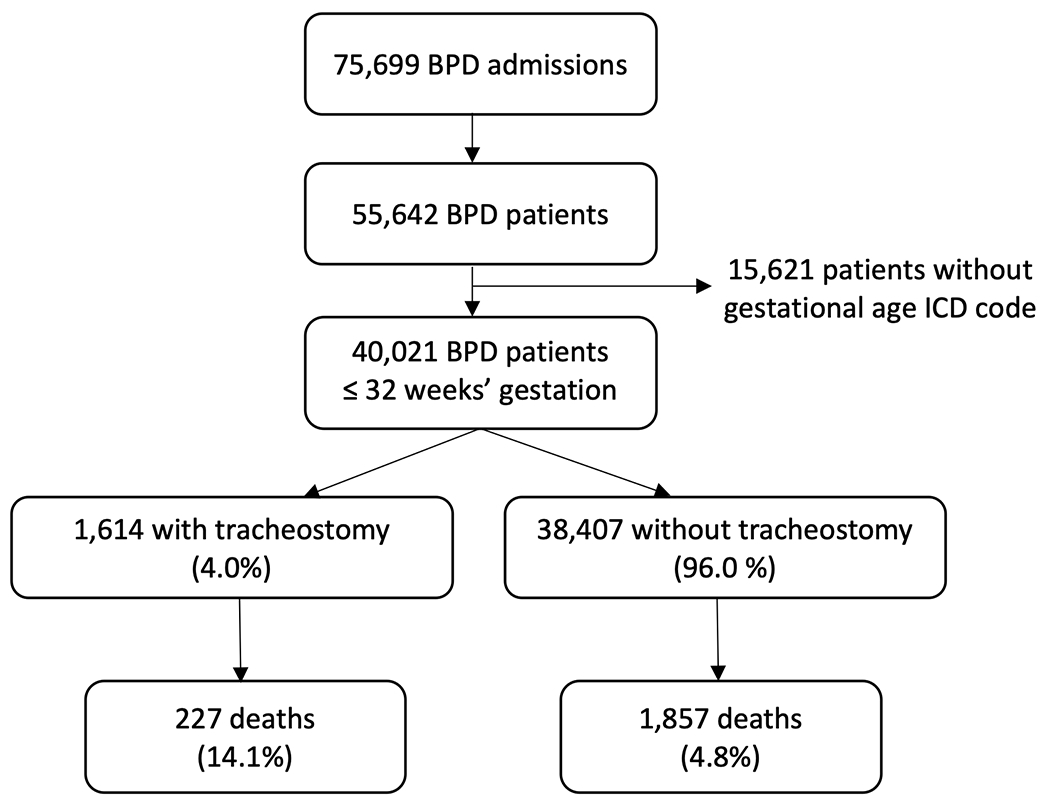
Flow chart depicting the inclusion of 40,021 ex-premature infants with bronchopulmonary dysplasia and rates of tracheostomy insertion and mortality.
Table 1:
Patient characteristics
Baseline characteristics of all BPD patients and those with and without tracheostomy.
| All BPD patients (n=40,021) |
BPD patients with tracheostomy (n=1,614) |
BPD patients without tracheostomy (n=38,407) |
|
|---|---|---|---|
| Sex, n (%) a | |||
| Male | 21981 (54.9) | 949 (58.8) | 21032 (54.8) |
| Female | 18037 (45.1) | 665 (41.2) | 17372 (45.2) |
| Race/ethnicity, n (%) | |||
| Asian | 1007 (2.5) | 40 (2.5) | 967 (2.5) |
| Non-Hispanic Black | 11467 (28.7) | 603 (37.4) | 10864 (28.3) |
| Non-Hispanic White | 16192 (40.5) | 613 (38.0) | 15579 (40.6) |
| Hispanic | 5406 (13.5) | 164 (10.2) | 5242 (13.6) |
| Other | 3713 (9.3) | 141 (8.7) | 3572 (9.3) |
| Unknown | 2236 (5.6) | 53 (3.3) | 2183 (5.7) |
| Gestational Age | |||
| Extreme prematurity, unspecified | 47 (11.7) | 5 (0.3) | 42 (0.1) |
| Less than 24 weeks | 3497 (8.7) | 234 (14.5) | 3262 (8.5) |
| 24 completed weeks | 5568 (13.9) | 323 (20.0) | 5245 (13.7) |
| 25-26 completed weeks | 12645 (31.6) | 571 (35.4) | 12074 (31.4) |
| 27-28 completed weeks | 10604 (26.5) | 285 (17.7) | 10319 (26.9) |
| 29-30 completed weeks | 5741 (14.3) | 137 (8.5) | 5604 (14.6) |
| 31-32 completed weeks | 1919 (4.8) | 59 (3.7) | 1860 (4.8) |
| Birth weight (grams; median, IQR) b | 871 (690-1125) | 710 (566-901) | 880 (700-1130) |
| Age at trach insertion (months; median, IQR) c | 4.6 (3.6-5.9) | ||
| Primary insurance, n (%) d | |||
| Medicaid/self-pay/other | 25758 (64.4) | 1106 (68.5) | 24652 (64.2) |
| Private | 14171 (35.4) | 506 (31.4) | 13665 (35.6) |
| Neighborhood median household income ($; median, IQR) e | 53,318.00 (41,675.00-70,405) | 50,826.50 (40,171.50-65,900.00) | 53,464.00 (41,741.50-70,592.50) |
| % Population with income below poverty level (median, IQR) f | 14.9 (8.9-22.7) | 16.0 (9.9-24.3) | 14.8 (8.9-22.6) |
| % Population with bachelor’s degree (median, IQR) g | 15.6 (10.7-22.7) | 14.8 (10.5-21.3) | 15.6 (10.7-22.8) |
| Comorbid diagnoses, n (%) | |||
| Other non-resp neonatal morbidityh | 11674 (29.2) | 694 (43.0) | 10980 (28.6) |
| Pulmonary hypertensioni | 1869 (4.7) | 452 (28.0) | 1417 (3.7) |
| Airway abnormalityj | 1933 (4.8) | 617 (38.2) | 1316 (3.4) |
| Congenital heart diseasek | 2742 (6.9) | 197 (12.2) | 2545 (6.6) |
| Death, n (%) | 2084 (5.2) | 227 (14.1) | 1857 (4.8) |
Missing data from 3 non-trach patients
Missing data from 510 trach patients and 5393 non-trach patients
Missing data from 36 trach patients
Missing data from 2 trach patients and 90 non-trach patients
Missing data from 14 trach patients and 651 non-trach patients
Missing data from 11 trach patients and 598 non-trach patients
Missing data from 11 trach patients and 588 non-trach patients
Necrotizing enterocolitis, intraventricular hemorrhage grade >2, periventricular leukomalacia, retinopathy of prematurity stage >2
Excluding persistent pulmonary hypertension of the newborn
Tracheo-/broncho-/laryngomalacia, subglottic stenosis, laryngeal web
Excluding isolated atrial septal defect, ventricular septal defect, patent ductus arteriosus, or unspecified congenital lesions
Figure 2:
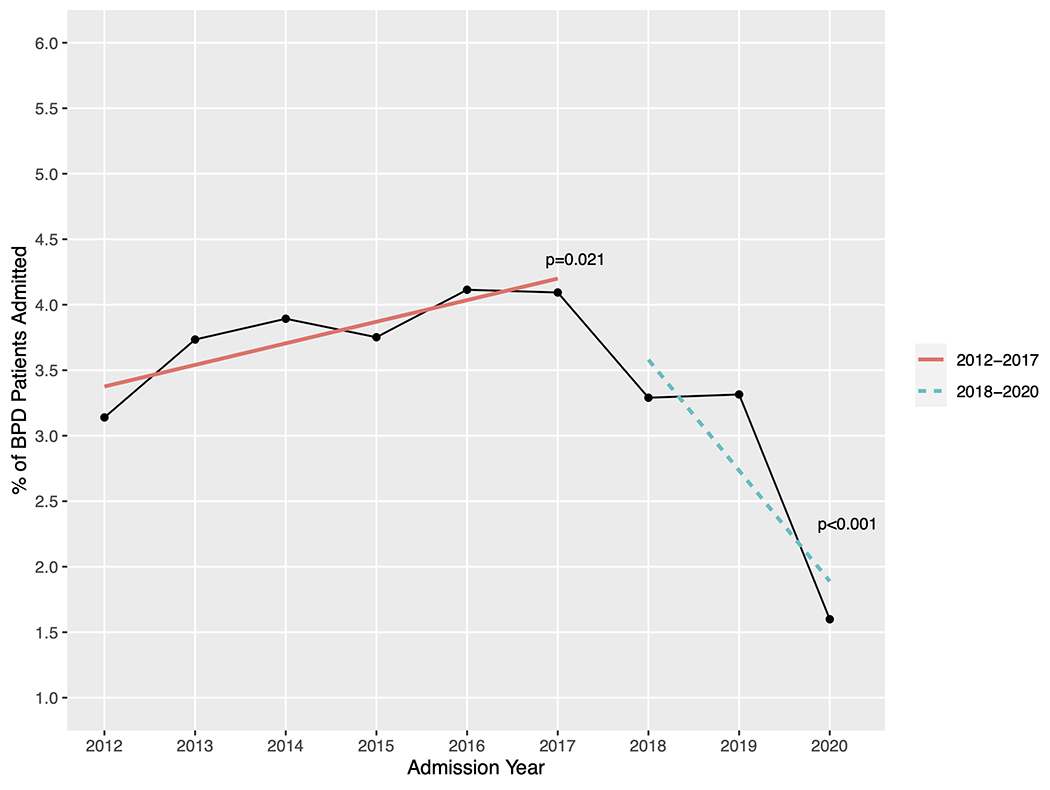
Tracheostomy insertion rates per year, represented as percentage of total BPD patient admissions. Regression lines depict an increasing rate of insertion from 2012-2017 and a decreasing rate from 2018-2020.
Associations between sociodemographic and clinical factors and tracheostomy insertion are presented in Figure 3. Crude analyses closely mirrored the results of multivariable modeling adjusting for sex, race/ethnicity, insurance provider, and relative neighborhood median household income and neighborhood percentage of population with a bachelor’s degree (Supplemental Table 2). We demonstrated greater odds of tracheostomy insertion among males (aOR 1.20, 95% CI 1.08-1.33, p<0.001), non-Hispanic Black patients (aOR 1.38, 95% CI 1.22-1.56, p<0.001; reference: non-Hispanic White patients), and patients with Medicaid/self-pay/other insurance (aOR 1.15, 95% CI 1.03-1.30, p=0.018). In crude analyses, tracheostomy insertion was additionally associated with gestational age ≤ 26 weeks and comorbid diagnoses of non-respiratory major neonatal morbidity, pulmonary hypertension, structural airway abnormalities, and congenital heart disease. Tracheostomy insertion was less likely in Hispanic patients (aOR 0.77, 95% CI 0.64-0.92, p=0.005). Within our cohort, tracheostomy insertion was not associated with relative neighborhood median household income, neighborhood percentage with income below the poverty line, or neighborhood percentage with a bachelor’s degree. In our model clustered by hospital site to account for tracheostomy practice variation between centers, point estimates did not differ from the values in the non-clustered model (Supplemental Table 3).
Figure 3:
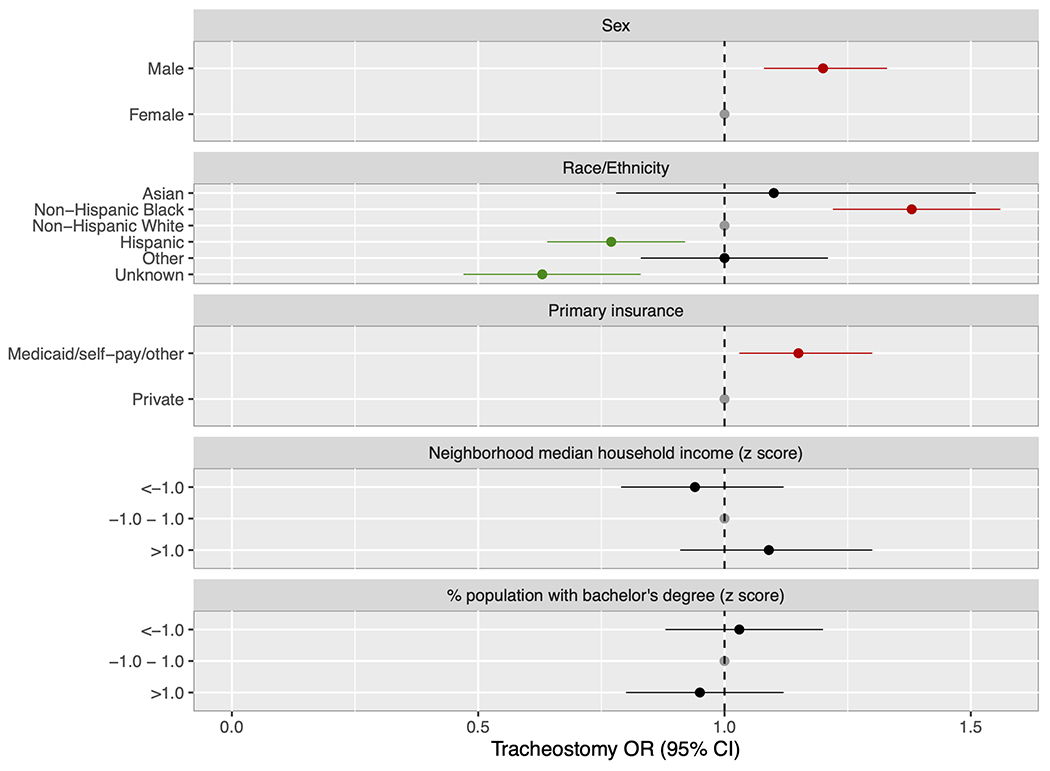
Forest plot of adjusted odds ratios for tracheostomy insertion. The model was adjusted for patient sex, race/ethnicity, primary insurance provider, relative neighborhood median household income, and relative neighborhood percentage of the population with a bachelor’s degree. Males, non-Hispanic Black, and patients with Medicaid/self-pay/other primary insurance had increased odds while Hispanic patients had decreased odds of tracheostomy insertion.
We next serially added gestational age, presence of a non-respiratory major neonatal morbidity, and then additional comorbidities to the adjusted model (Table 2). The observed associations of tracheostomy placement with race/ethnicity persisted with limited attenuation of the increased odds for non-Hispanic Black patients (aOR 1.25, 95% CI 1.09-1.43; p=0.001) and decreased odds for Hispanic patients (aOR 0.80, 95% CI 0.65-0.96; p=0.02) in the fully adjusted models (Supplemental Table 4).
Table 2:
Relationship of race/ethnicity to tracheostomy insertion, adjusting for markers of illness severity
Model A presents odds ratio of tracheostomy insertion by race/ethnicity adjusting for sex, primary insurance, neighborhood median household income, and neighborhood percent of the population with a bachelor’s degree. Model B adjusts for all covariates in Model A, plus gestational age. Model C adjusts for all covariates in Model B, plus the variable indicating non-respiratory major neonatal comorbidities. Model D adjusts for all variables in Model C, plus additional cardiorespiratory comorbidities (pulmonary hypertension, airway abnormality, and congenital heart disease.
| Model A aOR (95% CI) |
p value | Model B aOR (95% CI) |
p value | Model C aOR (95% CI) |
p value | Model D aOR (95% CI) |
p value | |
|---|---|---|---|---|---|---|---|---|
| Race/ethnicity | ||||||||
| Asian | 1.10 (0.78-1.51) | 0.56 | 1.06 (0.75-1.45) | 0.73 | 1.03 (0.73-1.42) | 0.84 | 0.87 (0.59-1.23) | 0.44 |
| Non-Hispanic Black | 1.38 (1.22-1.56) | <0.001 | 1.25 (1.10-1.42) | <0.001 | 1.26 (1.11-1.43) | <0.001 | 1.25 (1.09-1.43) | 0.001 |
| Non-Hispanic White | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| Hispanic | 0.77 (0.64-0.92) | 0.005 | 0.74 (0.62-0.88) | 0.001 | 0.74 (0.62-0.88) | 0.001 | 0.80 (0.65-0.96) | 0.020 |
| Other | 1.00 (0.83-1.21) | 0.97 | 0.97 (0.80-1.17) | 0.74 | 0.97 (0.80-1.17) | 0.75 | 1.01 (0.82-1.24) | 0.91 |
| Unknown | 0.63 (0.47-0.83) | 0.002 | 0.62 (0.46-0.82) | 0.001 | 0.62 (0.46-0.82) | 0.001 | 0.76 (0.56-1.02) | 0.08 |
Lastly, we analyzed factors associated with mortality (Supplemental Table 5 and Figure 4). In crude analyses of patients with tracheostomy, the odds of mortality were lower for patients with a comorbid diagnosis of airway abnormality and higher for patients with race unknown. In multivariable analysis, there were no significant associations between mortality and race/ethnicity, nor were there associations with primary insurance provider or relative neighborhood metrics of socioeconomic position for the tracheostomy cohort. In the non-tracheostomy group, Hispanic ethnicity was associated with lower odds of mortality, as was higher neighborhood proportion with a bachelor’s degree, while public insurance and lower neighborhood median household income were associated with higher odds of mortality.
Figure 4:
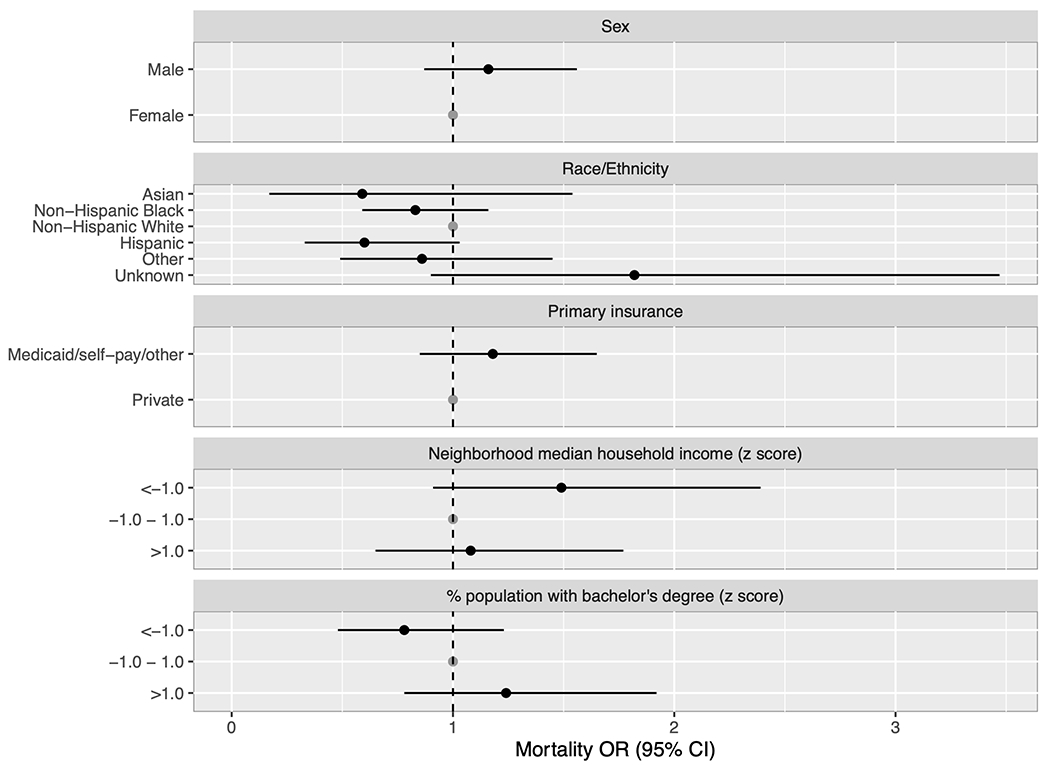
Forest plot of adjusted odds ratios for mortality among tracheostomy-dependent patients. The model was adjusted for patient sex, race/ethnicity, primary insurance provider, relative neighborhood median household income, and relative neighborhood percentage of the population with a bachelor’s degree. There were no significant differences in mortality between subgroups.
Discussion
Our findings provide updated insight into tracheostomy practice trends and the factors associated with tracheostomy insertion and post-tracheostomy mortality for infants with BPD. Surprisingly, though tracheostomy insertion rates increased from 2012-2017, there was a notable drop from 2018-2020. We found that non-Hispanic Black infants demonstrated a 25% higher odds of tracheostomy insertion that was not explained by differences in gestational age at birth or the presence of additional prematurity-related or cardiopulmonary comorbidities. Hispanic patients, on the other hand, had a 20% lower odds of tracheostomy insertion. Patients on Medicaid/self-pay/other primary insurance plans also had increased odds of tracheostomy insertion, but differences in neighborhood metrics of socioeconomic status were not associated with tracheostomy placement. The overall mortality rate post-tracheostomy was low, suggesting a growing cohort of living BPD tracheostomy-dependent patients. Sociodemographic disparities were not evident in evaluation of post-tracheostomy mortality. These findings begin to elucidate when and how inequity manifests among patients with BPD, which is an important first step in addressing the undue burden of disease that affects structurally disadvantaged communities.
We provide updated insight into nationwide tracheostomy practice trends. Our findings replicate those of Donda et al, demonstrating an increase in the rate of tracheostomy insertion per year through 2017.2 From 2018-2020, however, the rate of tracheostomy insertion per year decreased significantly to an overall low of 1.6% of BPD admissions in 2020. It is unclear what contributed to this change in the last several years and this observational study is unfortunately limited in its capacity to meaningfully explore this finding. Several prior studies have demonstrated a significant decline in preterm birth that coincided with the onset of the COVID-19 pandemic and national lockdowns.20,21 In the United States, the decrease in preterm birth was found to be related to a decline in preterm cesarean sections and induced deliveries.20 There have also been studies citing decreased rates of transmission of other respiratory viral illnesses during the COVID-19 pandemic, which may have limited the risk of viral respiratory illnesses contributing to respiratory failure in the particularly fragile BPD population.22 Our data do demonstrate a decrease in the total number of admissions of patients with BPD during 2020, but the drop in tracheostomy rate preceded this. Further understanding of why the practice of tracheostomy insertion has changed in recent years is needed, especially in light of our findings that patients with BPD fare well with low mortality rates post-tracheostomy.
A number of prior studies have demonstrated an outsized burden of respiratory disease in the Black infant population. In 2017, Keller et al reported that Black infants born < 29 weeks had a 1.6-fold increased odds of developing post-prematurity respiratory disease requiring hospitalization, medications, or home respiratory support through 1 year of life compared to White premature infants.23 Studies evaluating tracheostomy insertion rates and post-tracheostomy outcomes across indications for tracheostomy also demonstrate disparities. Black race has previously been associated with a 1.2 times increased odds of tracheostomy placement compared to children of other races,24 while Hispanic patients have been shown to receive proportionally fewer tracheostomies in a recent assessment of tracheostomy practices at a single center.25 In the neonatal tracheostomy-dependent population specifically, a multicenter study previously demonstrated a 1.3 times higher odds of tracheostomy insertion during the first hospitalization for non-Hispanic Black infants compared to non-Hispanic White infants.26 Studies evaluating how race/ethnicity is associated with post-tracheostomy outcomes show mixed findings, with some demonstrating a longer time to decannulation for Asian and Hispanic patients27,28 and others showing no differences in decannulation, neurocognitive ability, or mortality between racial/ethnic groups.25
Our study extends and refines the existing literature by examining these relationships specifically within the BPD population and across a wide breadth of hospital systems. Given the growing recognition of the benefits of tracheostomy for patients with BPD and the well-established disparities in BPD outcomes such as length of stay, readmissions, and mortality, it is important to evaluate how race/ethnicity might relate to tracheostomy practices and post-tracheostomy outcomes. We identified similar patterns of increased tracheostomy insertion in Black patients and decreased tracheostomy in Hispanic patients as has been described previously outside of BPD.24–26 These differences could not be explained by controlling for degree of prematurity or burden of prematurity-related and other comorbidities and were unchanged after accounting for possible center-specific practice variation. Post-tracheostomy in-hospital mortality rates between these groups, however, were similar. It is unclear what leads to the disparities in the use of tracheostomy in this population, but given the similar rates of post-tracheostomy mortality, one possibility may be differing clinical thresholds for the intervention between different racial/ethnic groups. There also may be differences in severity of BPD not captured by our analyses controlling for gestational age and comorbid diagnoses. A phenomenon termed the “Hispanic paradox” has previously been described based on observations that despite disparities with respect to prenatal care, education, and socioeconomic status, birth outcomes tend to be encouraging in the Hispanic population.25 Our finding of a lower tracheostomy rate among Hispanic patients fits with these observations. Various factors could contribute to this “paradox”, including self-selection of the healthiest immigrants, protective cultural factors, and strong community support systems. Related to the latter two, it may be that the support afforded or available to patients of different demographic backgrounds post-tracheostomy differs, leveling the outcomes once a tracheostomy is in place. In the non-tracheostomy population, we found no difference in mortality for non-Hispanic Black patients relative to non-Hispanic White patients, and lower mortality for Hispanic patients. Consistent with prior studies, markers of lower socioeconomic status were associated with increased mortality.10–12 The fact that these differences are absent in the tracheostomy cohort speaks towards effective efforts to provide care that levels disparities among the technology-dependent. Our data was limited in its capacity to address this area further or to evaluate alternative longitudinal outcomes, but further investigation is warranted.
Within our cohort, differences in socioeconomic status as measured by neighborhood median household income, percentage living below the poverty line, and percentage with a bachelor’s degree were not associated with tracheostomy insertion or post-tracheostomy mortality, although we did find that patients with Medicaid/self-pay/other insurance providers had increased odds of tracheostomy. Higher household income has been associated with improved health outcomes in children across a variety of other diseases, including asthma, obesity, and injury.29–31 Within the BPD population, Cristea, et al reported in 2015 concern for a serious health disparity in a cohort of 94 patients with BPD on chronic home ventilation.10 Though only 61.7% of the cohort were from families living in an area with a median annual income below the state median, 14 of the 15 (93%) patients who died were from low income areas. Our nationwide data encouragingly did not replicate such a disparity within a more recent cohort. It is possible that this is due to a learned selection bias, whereby physicians have grown more selective of the families to whom they offer tracheostomy. However, it also may reflect effective efforts to more adequately support patients from socioeconomically disadvantaged homes. Interestingly, our cohort of patients with tracheostomy demonstrated worse averages compared to national statistics in each of the socioeconomic markers studied. In 2020, the nationwide median household income, percentage living below the poverty line, and percentage over 25 years old with a bachelor’s degree in the US were $67,521, 11.4%, and 32.1%, respectively, compared to $50,827, 16.0%, and 14.8% in our tracheostomy cohort.32,33 Poverty and lower education level have been related to increased risk for premature birth itself in both the US and Canada, however the differences between our technology-dependent BPD cohort and the national averages are stark.34,35 It is encouraging that within-cohort differences in these measures did not relate to the outcomes studied, however it remains to be seen how this evident disproportion of low income, low education families may be influencing the care and outcomes of ex-premature infants.
There were a number of limitations to our study. First, our data were limited to what could be obtained from administrative records (i.e. demographics and ICD codes) and lacked important clinical details to better describe BPD severity, indication for tracheostomy, and other important risk factors such as birth weight, fetal growth restriction, maternal health, smoke exposure, and other environmental factors. We also did not capture information about out-of-hospital mortality. A recent single center report noted post-tracheostomy 5-year mortality rates of 21% for preterm and 27% for extremely preterm patients, higher rates than what we identify in this cohort.36 Race and ethnicity were not collected in a standardized manner across all institutions contributing to the Vizient CDB/RM. Misclassification of race/ethnicity in administrative data is a well-described limitation in health equity research.37 The data obtained from the AHRQ’s Social Determinants of Health Database provide only a partial, surrogate representation of socioeconomic status. Nonetheless, the income and education metrics evaluated are established factors associated with health outcomes in children.35 While we did our best to address the effects of inter-center tracheostomy practice variation in the analysis phase of our study, it is important to acknowledge that there is no absolute indication for tracheostomy and the decision is often driven by local practice.38 Lastly, though we are able to report important disparities in the BPD population, we were unable to explore the mechanisms of the inequity underlying these findings. There is insightful work being done exploring how structural and institutional racism39–46 and differences in peripartum and neonatal quality of care4,47–51 may drive these findings. We hope that our results continue to inspire clinicians and researchers to question and address the underpinnings of these health inequities.
In conclusion, our findings provide valuable insight into tracheostomy practices amongst the BPD population, highlighting important differences in insertion rates between racial and ethnic groups that are not accounted for by differences in clinical factors or socioeconomic status. The reasons for such disparities must be further investigated in order to develop targeted strategies to mitigate inequities in this vulnerable population.
Supplementary Material
Acknowledgements
Thank you to Ernie Shippey of Vizient, Inc for his assistance with data extraction.
Funding/Support:
MASmith is supported by NICHD (T32HL160508-01A1). MSZ is supported by NICHD (K12HD000850), NHLBI (K23HL146936), and the ATS Foundation Grant.
Footnotes
Conflict of Interest Disclosures: The authors have no conflicts of interest relevant to this article to disclose.
References
- 1.Jensen EA, Edwards EM, Greenberg LT, Soll RF, Ehret DEY, Horbar JD. Severity of Bronchopulmonary Dysplasia Among Very Preterm Infants in the United States. Pediatrics. 2021;148(1):e2020030007. doi: 10.1542/peds.2020-030007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donda K, Agyemang CO, Adjetey NA, et al. Tracheostomy trends in preterm infants with bronchopulmonary dysplasia in the United States: 2008–2017. Pediatric Pulmonology. 2021;56(5):1008–1017. doi: 10.1002/ppul.25273 [DOI] [PubMed] [Google Scholar]
- 3.Baker CD. Long-term ventilation for children with chronic lung disease of infancy: Current Opinion in Pediatrics. 2019;31(3):357–366. doi: 10.1097/MOP.0000000000000757 [DOI] [PubMed] [Google Scholar]
- 4.Sigurdson K, Mitchell B, Liu J, et al. Racial/Ethnic Disparities in Neonatal Intensive Care: A Systematic Review. Pediatrics. 2019;144(2):e20183114. doi: 10.1542/peds.2018-3114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Janevic T, Zeitlin J, Auger N, et al. Association of Race/Ethnicity With Very Preterm Neonatal Morbidities. JAMA Pediatr. 2018;172(11):1061–1069. doi: 10.1001/jamapediatrics.2018.2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ryan RM, Feng R, Bazacliu C, et al. Black Race Is Associated with a Lower Risk of Bronchopulmonary Dysplasia. J Pediatr. 2019;207:130–135.e2. doi: 10.1016/j.jpeds.2018.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petrova A, Mehta R, Anwar M, Hiatt M, Hegyi T. Impact of race and ethnicity on the outcome of preterm infants below 32 weeks gestation. J Perinatol. 2003;23(5):404–408. doi: 10.1038/sj.jp.7210934 [DOI] [PubMed] [Google Scholar]
- 8.Karvonen KL, Baer RJ, Rogers EE, et al. Racial and ethnic disparities in outcomes through 1 year of life in infants born prematurely: a population based study in California. J Perinatol. 2021;41(2):220–231. doi: 10.1038/s41372-021-00919-9 [DOI] [PubMed] [Google Scholar]
- 9.Lewis TR, Kielt MJ, Walker VP, et al. Association of Racial Disparities With In-Hospital Outcomes in Severe Bronchopulmonary Dysplasia. JAMA Pediatr. Published online August 1, 2022. doi: 10.1001/jamapediatrics.2022.2663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cristea AI, Ackerman VL, Davis SD, et al. Median Household Income: Association with Mortality in Children on Chronic Ventilation at Home Secondary to Bronchopulmonary Dysplasia. Pediatr Allergy Immunol Pulmonol. 2015;28(1):41–46. doi: 10.1089/ped.2014.0406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fierro J, Piccione J, Lorch S. Clinical Factors Influencing Time to Decannulation in Children with Tracheostomy and Ventilator Dependence Secondary to Bronchopulmonary Dysplasia. The Journal of Pediatrics. 2021;228:31–35. doi: 10.1016/j.jpeds.2020.08.068 [DOI] [PubMed] [Google Scholar]
- 12.Deschamps J, Boucekine M, Fayol L, et al. Neighborhood Disadvantage and Early Respiratory Outcomes in Very Preterm Infants with Bronchopulmonary Dysplasia. J Pediatr. 2021;237:177–182.e1. doi: 10.1016/j.jpeds.2021.06.061 [DOI] [PubMed] [Google Scholar]
- 13.CDB | healthcare analytics platform for clinical benchmarking. Accessed July 18, 2022. https://www.vizientinc.com/what-we-do/operations-and-quality/clinical-data-base
- 14.Social Determinants of Health Database (Beta Version). Accessed July 18, 2022. https://www.ahrq.gov/sdoh/data-analytics/sdoh-data.html
- 15.Warner RA. Chapter 2 - Using Z Scores for the Display and Analysis of Data. In: Warner RA, ed. Optimizing the Display and Interpretation of Data. Elsevier; 2016:7–51. doi: 10.1016/B978-0-12-804513-8.00002-X [DOI] [Google Scholar]
- 16.Bennett TD, DeWitt PE, Dixon RR, et al. Development and Prospective Validation of Tools to Accurately Identify Neurosurgical and Critical Care Events in Children With Traumatic Brain Injury. Pediatr Crit Care Med. 2017;18(5):442–451. doi: 10.1097/PCC.0000000000001120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lahiri S, Mayer SA, Fink ME, et al. Mechanical Ventilation for Acute Stroke: A Multi-state Population-Based Study. Neurocrit Care. 2015;23(1):28–32. doi: 10.1007/s12028-014-0082-9 [DOI] [PubMed] [Google Scholar]
- 18.Steurer MA, Baer RJ, Chambers CD, et al. Mortality and Major Neonatal Morbidity in Preterm Infants with Serious Congenital Heart Disease. J Pediatr. 2021;239:110–116.e3. doi: 10.1016/j.jpeds.2021.08.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.R Core Team. R: A language and environment for statistical computing.
- 20.Dench D, Joyce T, Minkoff H. United States Preterm Birth Rate and COVID-19. Pediatrics. 2022;149(5):e2021055495. doi: 10.1542/peds.2021-055495 [DOI] [PubMed] [Google Scholar]
- 21.Hedley PL, Hedermann G, Hagen CM, et al. Preterm birth, stillbirth and early neonatal mortality during the Danish COVID-19 lockdown. Eur J Pediatr. 2022;181(3):1175–1184. doi: 10.1007/s00431-021-04297-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haddadin Z, Schuster JE, Spieker AJ, et al. Acute Respiratory Illnesses in Children in the SARS-CoV-2 Pandemic: Prospective Multicenter Study. Pediatrics. 2021;148(2):e2021051462. doi: 10.1542/peds.2021-051462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keller RL, Feng R, DeMauro SB, et al. Bronchopulmonary Dysplasia and Perinatal Characteristics Predict 1-Year Respiratory Outcomes in Newborns Born at Extremely Low Gestational Age: A Prospective Cohort Study. J Pediatr. 2017;187:89–97.e3. doi: 10.1016/j.jpeds.2017.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown C, Shah GB, Mitchell RB, Lenes-Voit F, Johnson RF. The Incidence of Pediatric Tracheostomy and Its Association Among Black Children. Otolaryngol Head Neck Surg. 2021;164(1):206–211. doi: 10.1177/0194599820947016 [DOI] [PubMed] [Google Scholar]
- 25.Johnson RF, Brown CM, Beams DR, et al. Racial Influences on Pediatric Tracheostomy Outcomes. Laryngoscope. 2022;132(5):1118–1124. doi: 10.1002/lary.29847 [DOI] [PubMed] [Google Scholar]
- 26.Han SM, Watters KF, Hong CR, et al. Tracheostomy in Very Low Birth Weight Infants: A Prospective Multicenter Study. Pediatrics. 2020;145(3):e20192371. doi: 10.1542/peds.2019-2371 [DOI] [PubMed] [Google Scholar]
- 27.Cristea AI, Carroll AE, Davis SD, Swigonski NL, Ackerman VL. Outcomes of Children With Severe Bronchopulmonary Dysplasia Who Were Ventilator Dependent at Home. PEDIATRICS. 2013;132(3):e727–e734. doi: 10.1542/peds.2012-2990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salley J, Kou Y, Shah GB, Mitchell RB, Johnson RF. Survival analysis and decannulation outcomes of infants with tracheotomies. The Laryngoscope. 2020;130(10):2319–2324. doi: 10.1002/lary.28297 [DOI] [PubMed] [Google Scholar]
- 29.Chen E, Martin AD, Matthews KA. Understanding health disparities: the role of race and socioeconomic status in children’s health. Am J Public Health. 2006;96(4):702–708. doi: 10.2105/AJPH.2004.048124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown RL. Epidemiology of injury and the impact of health disparities. Curr Opin Pediatr. 2010;22(3):321–325. doi: 10.1097/MOP.0b013e3283395f13 [DOI] [PubMed] [Google Scholar]
- 31.Case A, Paxson C. Parental behavior and child health. Health Aff (Millwood). 2002;21(2):164–178. doi: 10.1377/hlthaff.21.2.164 [DOI] [PubMed] [Google Scholar]
- 32.Bureau UC. Income and Poverty in the United States: 2020. Census.gov. Accessed August 23, 2022. https://www.census.gov/library/publications/2021/demo/p60-273.html
- 33.Bureau UC. Bachelor’s Degree Attainment in the United States: 2005 to 2019. Census.gov. Accessed August 23, 2022. https://www.census.gov/library/publications/2021/acs/acsbr-009.html
- 34.DeFranco EA, Lian M, Muglia LA, Schootman M. Area-level poverty and preterm birth risk: A population-based multilevel analysis. BMC Public Health. 2008;8:316. doi: 10.1186/1471-2458-8-316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luo ZC, Wilkins R, Kramer MS, Fetal and Infant Health Study Group of the Canadian Perinatal Surveillance System. Effect of neighbourhood income and maternal education on birth outcomes: a population-based study. CMAJ. 2006;174(10):1415–1420. doi: 10.1503/cmaj.051096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wood W, Wang CS, Mitchell RB, Shah GB, Johnson RF. A Longitudinal Analysis of Outcomes in Tracheostomy Placement Among Preterm Infants. Laryngoscope. 2021;131(2):417–422. doi: 10.1002/lary.28864 [DOI] [PubMed] [Google Scholar]
- 37.Jarrín OF, Nyandege AN, Grafova IB, Dong X, Lin H. Validity of Race and Ethnicity Codes in Medicare Administrative Data Compared With Gold-standard Self-reported Race Collected During Routine Home Health Care Visits. Med Care. 2020;58(1):e1–e8. doi: 10.1097/MLR.0000000000001216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murthy K, Porta NFM, Lagatta JM, et al. Inter-center variation in death or tracheostomy placement in infants with severe bronchopulmonary dysplasia. J Perinatol. 2017;37(6):723–727. doi: 10.1038/jp.2016.277 [DOI] [PubMed] [Google Scholar]
- 39.Wallace ME, Mendola P, Liu D, Grantz KL. Joint Effects of Structural Racism and Income Inequality on Small-for-Gestational-Age Birth. Am J Public Health. 2015;105(8):1681–1688. doi: 10.2105/AJPH.2015.302613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wallace M, Crear-Perry J, Richardson L, Tarver M, Theall K. Separate and unequal: Structural racism and infant mortality in the US. Health Place. 2017;45:140–144. doi: 10.1016/j.healthplace.2017.03.012 [DOI] [PubMed] [Google Scholar]
- 41.Krieger N, Van Wye G, Huynh M, et al. Structural Racism, Historical Redlining, and Risk of Preterm Birth in New York City, 2013–2017. Am J Public Health. 2020;110(7):1046–1053. doi: 10.2105/AJPH.2020.305656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vilda D, Hardeman R, Dyer L, Theall KP, Wallace M. Structural racism, racial inequities and urban-rural differences in infant mortality in the US. J Epidemiol Community Health. 2021;75(8):788–793. doi: 10.1136/jech-2020-214260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mehra R, Boyd LM, Ickovics JR. Racial residential segregation and adverse birth outcomes: A systematic review and meta-analysis. Soc Sci Med. 2017;191:237–250. doi: 10.1016/j.socscimed.2017.09.018 [DOI] [PubMed] [Google Scholar]
- 44.Burris HH, Hacker MR. Birth outcome racial disparities: A result of intersecting social and environmental factors. Semin Perinatol. 2017;41(6):360–366. doi: 10.1053/j.semperi.2017.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Horbar JD, Edwards EM, Greenberg LT, et al. Racial Segregation and Inequality in the Neonatal Intensive Care Unit for Very Low-Birth-Weight and Very Preterm Infants. JAMA Pediatr. 2019;173(5):455–461. doi: 10.1001/jamapediatrics.2019.0241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burris HH, Lorch SA, Kirpalani H, Pursley DM, Elovitz MA, Clougherty JE. Racial disparities in preterm birth in USA: a biosensor of physical and social environmental exposures. Arch Dis Child. 2019;104(10):931–935. doi: 10.1136/archdischild-2018-316486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Profit J, Gould JB, Bennett M, et al. Racial/Ethnic Disparity in NICU Quality of Care Delivery. Pediatrics. 2017;140(3):e20170918. doi: 10.1542/peds.2017-0918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parker MG, Gupta M, Melvin P, et al. Racial and Ethnic Disparities in the Use of Mother’s Milk Feeding for Very Low Birth Weight Infants in Massachusetts. J Pediatr. 2019;204:134–141.e1. doi: 10.1016/j.jpeds.2018.08.036 [DOI] [PubMed] [Google Scholar]
- 49.Patel AL, Johnson TJ, Meier PP. Racial and socioeconomic disparities in breast milk feedings in US neonatal intensive care units. Pediatr Res. 2021;89(2):344–352. doi: 10.1038/s41390-020-01263-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Howell EA, Janevic T, Hebert PL, Egorova NN, Balbierz A, Zeitlin J. Differences in Morbidity and Mortality Rates in Black, White, and Hispanic Very Preterm Infants Among New York City Hospitals. JAMA Pediatr. 2018;172(3):269–277. doi: 10.1001/jamapediatrics.2017.4402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Glazer KB, Zeitlin J, Egorova NN, et al. Hospital Quality of Care and Racial and Ethnic Disparities in Unexpected Newborn Complications. Pediatrics. 2021;148(3):e2020024091. doi: 10.1542/peds.2020-024091 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


