Abstract
We present the first examples of tethered olefin functionalization reactions using a silanol auxiliary. A range of allylic alcohols are readily condensed with di-tert-butylsilyl bis(trifluoromethanesulfonate) to form allylic silanols. When treated with Hg(OTf)2 and NaHCO3, these silanols facilely transform into cyclic silanediol organomercurial compounds. In most cases, the reactions are exquisitely diastereoselective. The scale can be increased more than ten-fold without loss of yield and selectivity. We demonstrate that the product silanediols are versatile synthons for a variety of further reactions.
Graphical Abstract
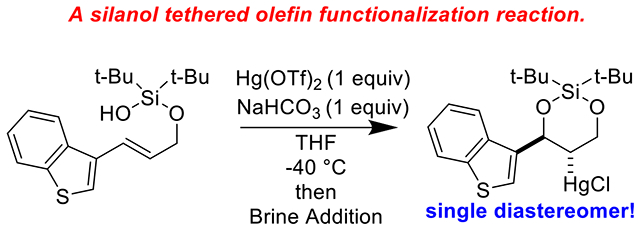
The family of organomercurials has earned an unfortunate reputation, largely due to the actions of a minority of its members.1, 2 Nevertheless, the majority of higher order organomercurial compounds are crystalline, air-stable, and, when treated with appropriate respect, no more dangerous than most chemicals encountered in the organic laboratory.3 The C–Hg linkage is essentially covalent, making it a most unique and versatile organometallic bond. 4–6 Our laboratory is deeply invested in the field of tethered olefin functionalization reactions.7–9 In such reactions, a nucleophilic auxiliary (the “tether”) is appended to an alcohol or amine and then cyclized onto a pendant alkene. Here, we disclose our most recent contribution to this area, a cyclization reaction of allylic silanols into cyclic silanediol mercury chloride compounds. Allylic alcohols can be readily transformed into allylic silanols using a combination of di-tert-butylsilyl bis(trifluoromethanesulfonate)10 and imidazole; the free OH of the silanol tether serves as a convenient nucleophile for olefin attack.
The resulting cyclic silanediol compounds, reminiscent of intermediates en route to polyol natural products (Figure 1),11–17 are synthons for a variety of further transformations.
Figure 1.
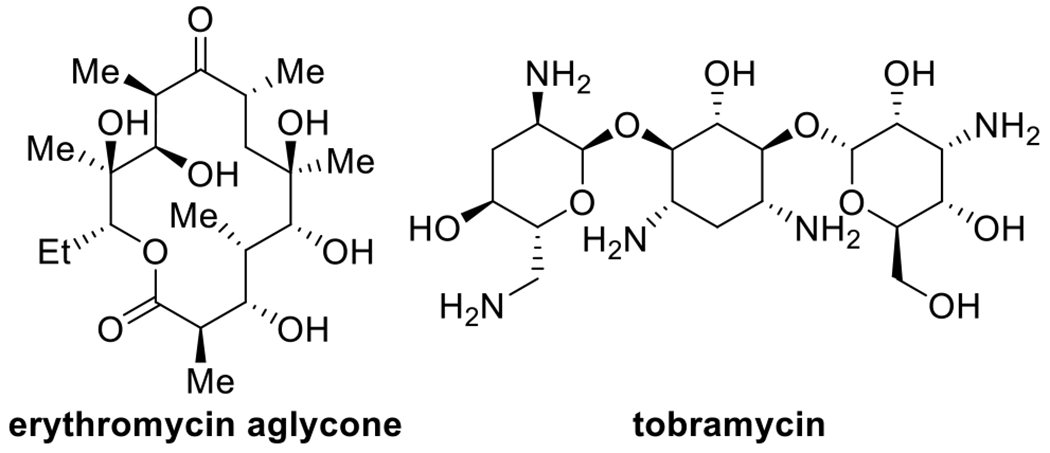
Many antibiotics are polyhydroxylated compounds.
Silanol auxiliaries have been popularized by Gevorgyan and co-workers as directing groups in C–H functionalization reactions.18, 19 There is one example from the Lee laboratory of the use of silanols for gold catalyzed alkyne functionalization20, and an example of a silylperoxidation reaction of homoallylic alcohols from the Woerpel laboratory.21 Nevertheless, our survey of the literature revealed no examples of the silanol tether employed in alkene functionalization. Our work has been directly inspired by the pioneering contributions of Overman22, 23 and Leighton24–28, who demonstrated that acetal tethers could be used for the mercuric-salt mediated functionalization of olefins (Scheme 1).29, 30
Scheme 1.
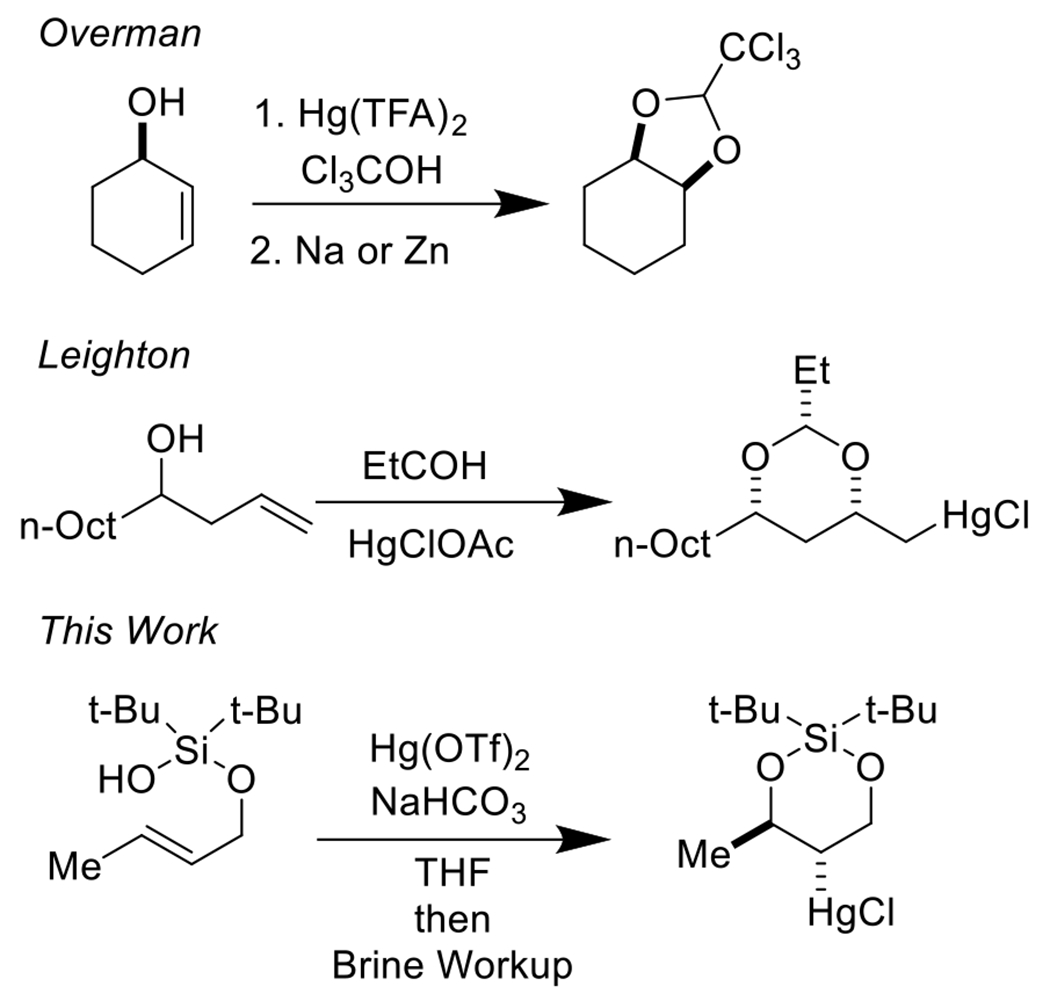
A strategy for the conversion of alkenyl alcohols into diol synthons.
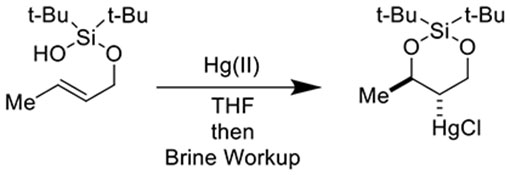
Optimization of our silanol-tethered alkene functionalization reaction (Table 1) was performed with (E)-(but-2-en-1-yloxy)di-tert-butylsilanol which was synthesized in one step from crotyl alcohol and di-tert-butylsilyl bis(trifluoromethanesulfonate) (See Supporting Information for full experimental procedures). With 1 equivalent of Hg(OCOCF3)2, we were pleased to observe formation of 22% of desired cyclic silanediol (Table 1, Entry 1), giving us hope that our conceived reaction was viable. There was little improvement with increase of temperature (Table 1, Entry 2), equivalents of Hg(OCOCF3)2 (Table 1, Entry 3), or time (Table 1, Entry 4). We hypothesized that adventitious trifluoroacetic acid, formed as a byproduct of cyclization, was deleterious to reaction progress. Accordingly, we tested K2CO3 and NaHCO3 in varying equivalents (Table 1, Entries 5-7) as base additives. Nevertheless, we observed little change in reaction performance. Reducing the temperature to −40 °C was markedly deleterious (Table 1, Entry 8). To our amazement and excitement, at this temperature, simply switching to Nishizawa’s salt (Hg(OTf)2),31 in combination with NaHCO3 (1 equivalent) afforded clean and high-yielding conversion to the desired product (Table 1, Entry 9). It should be noted that the use of 1 equivalent of NaHCO3 was critical for reaction performance! In its absence, a complex mixture of decomposition products was observed (Table 1, Entry 10).
Table 1.
Reaction Optimization
| ||||
|---|---|---|---|---|
| Entrya | Hg (II)(equiv.) | Base | Temp/Time | P/RSM |
| 1 | Hg(OCOCF3)2(1) | None | 23 °C, 1h | 22/45 |
| 2 | Hg(OCOCF3)2(1) | None | 40 °C, 1h | 25/28 |
| 3 | Hg(OCOCF3)2 (2) | None | 23 °C, 1h | 29/30 |
| 4 | Hg(OCOCF3)2 (1) | None | 23 °C, 16h | 31/13 |
| 5 | Hg(OCOCF3)2 (1) | K2CO3 (1) | 23 °C, 16h | 34/18 |
| 6 | Hg(OCOCF3)2 (1) | NaHCO3 (1) | 23 °C, 16h | 34/18 |
| 7 | Hg(OCOCF3)2 (1) | NaHCO3 (2) | 23 °C, 16h | 38/8 |
| 8 | Hg(OCOCF3)2 (1) | NaHCO3 (1) | −40 °C, 16h | 6/63 |
| 9 | Hg(OTf)2 (1) | NaHCO3 (1) | −40 °C, 16h | 67/<5 |
| 10 | Hg(OTf)2 (1) | None | −40 °C, 16h | —b |
Yield estimated with methyl phenyl sulfone as a 1H NMR internal standard.
Complex mixture of products.
Our optimized protocol utilizing Hg(OTf)2 (1 equivalent) and NaHCO3 (1 equivalent) at −40 °C in THF was compatible with a wide array of alkenyl silanol substrates (Scheme 2). The silanol auxiliary could be appended to both primary allylic alcohols as well as secondary ones (Scheme 2, Entry 9). In all but two instances (Scheme 2, Entry 10), the cyclization reaction proceeded with excellent diastereoselectivity (>20:1), with the pendant alkyl or aryl group and mercury chloride substituent in a trans relationship. A crystal structure of 31 (Scheme 2, Entry 6) unambiguously established this stereochemistry. It is remarkable to note that even with products containing 3 stereocenters (Scheme 2, Entry 9), only a single diastereomer was observed. We hypothesize that a chair-like transition state during silanoxy-mercuration is responsible for this excellent diastereoselectivity (Scheme 3). The reaction was tolerant of a wide variety of alkyl and aryl substituents attached to the olefin. Ethers (Scheme 2, Entries 2, 5, 6, 7), halogens (Scheme 2, Entry 6), and several heterocycles (Scheme 2, Entries 7 and 8) were all compatible. Di-substituted trans-olefins were the most competent substrates in this cyclization. While di-substituted cis-olefins also reacted (Scheme 2, Entry 11), the yield of cyclized product dropped. Furthermore, an endo mode of cyclization was strongly preferred; exo cyclization (Scheme 2, Entry 12) proceeded in only low yield.
Scheme 2.
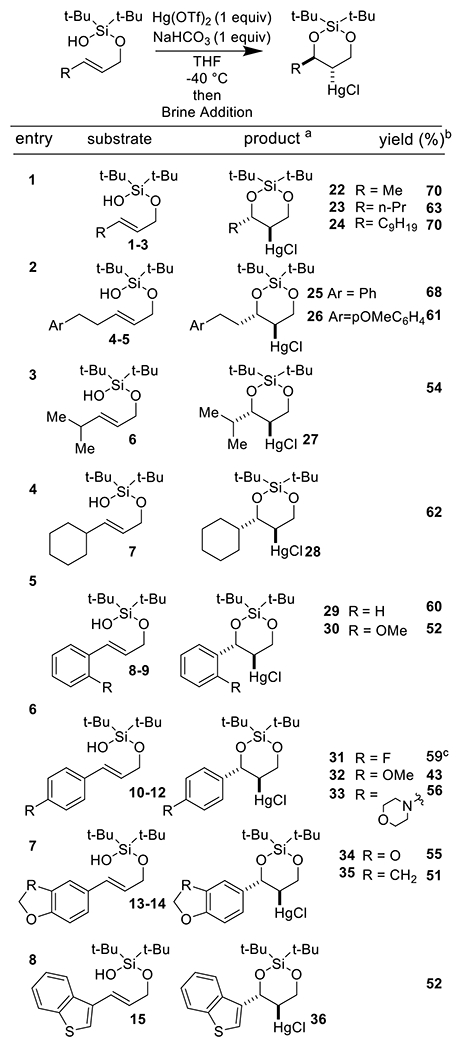
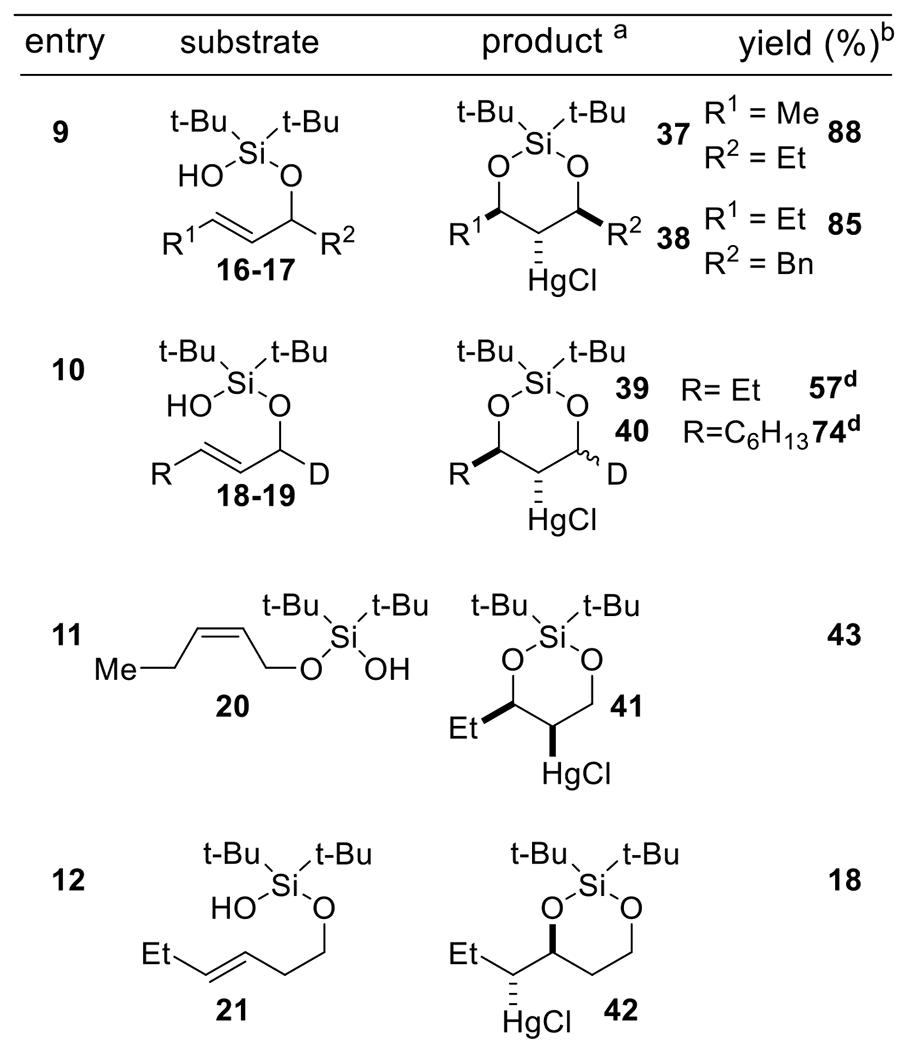
Substrate Scope
areactions conducted on a 0.2 mmol scale and relative stereochemistry is shown in all cases. bIsolated yields. cCrystallographic information deposited in the Cambridge Database (CCDC), Number 2032765 ddr = 1:1.
Scheme 3.
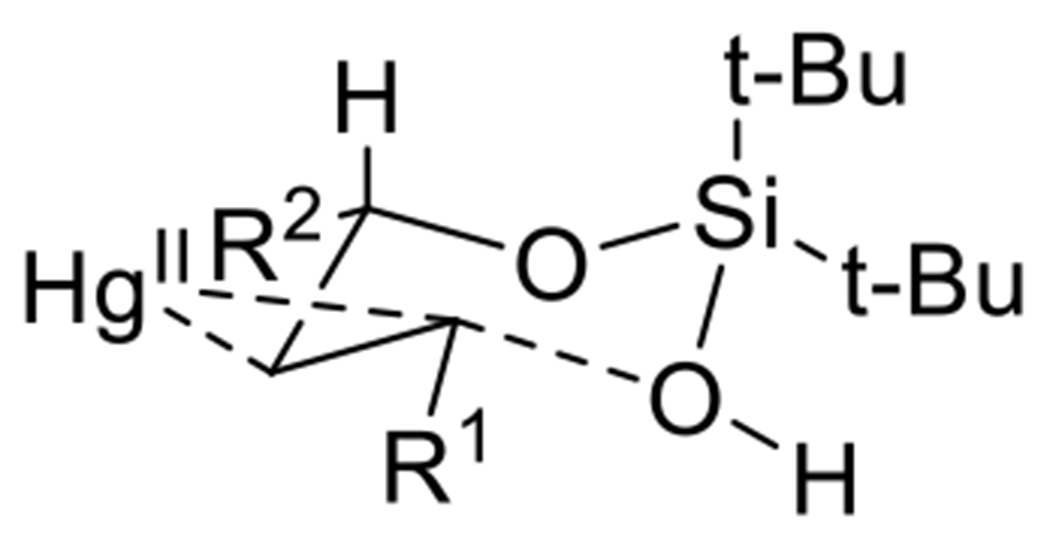
A chair-like transition state likely underlies the high diastereoselectivity of cyclization.
With tri-substituted alkenes, the cyclization reaction proceeded only partially, if at all (Scheme 4). Nevertheless, in these reactions, alcohol products were isolated, which themselves may be valuable intermediates for further derivatization.3
Scheme 4.
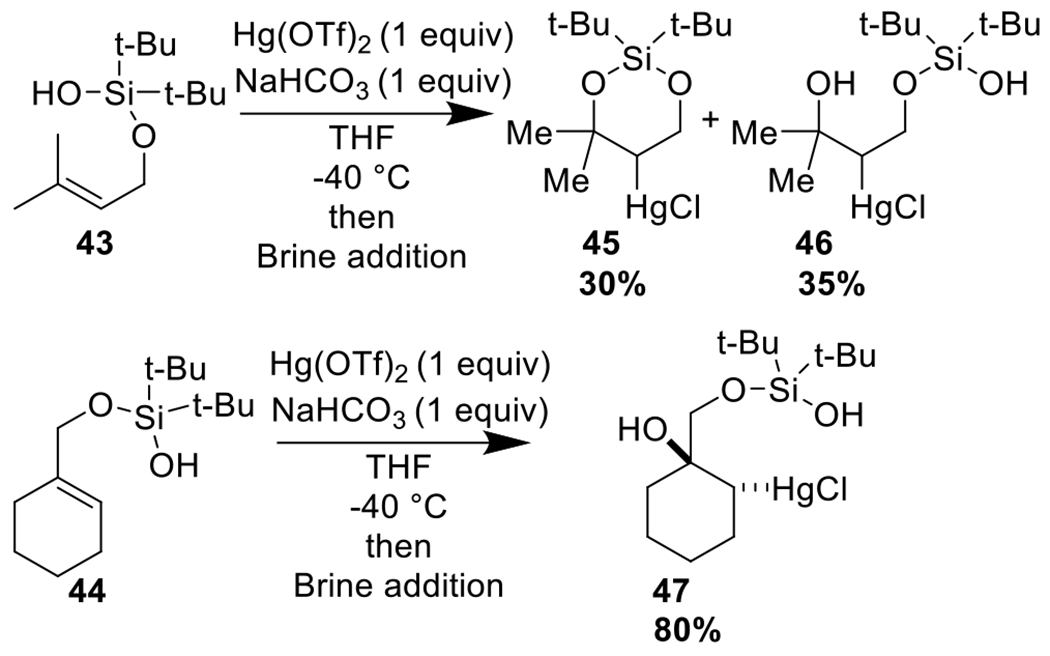
Some substrates do not fully cyclize but still form valuable diol products.
We were pleased to find that our cyclization reaction scaled greater than 10-fold with no loss in yield or selectivity (Scheme 5).
Scheme 5.
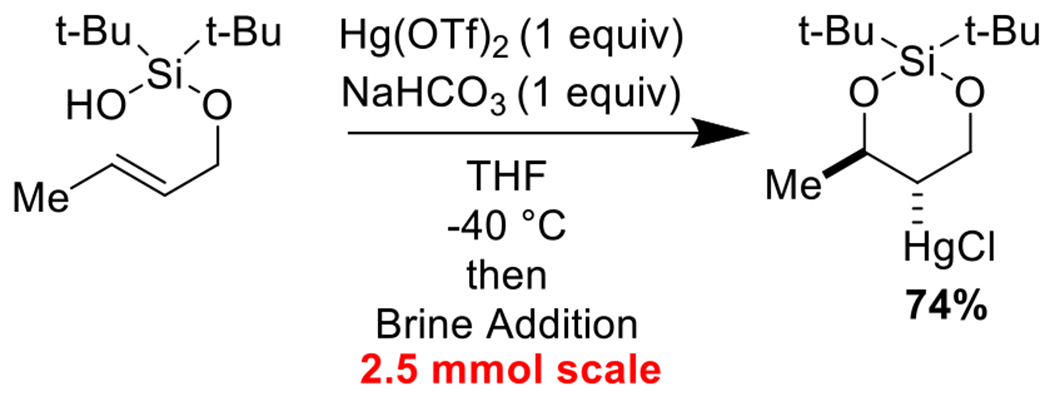
Cyclization scales greater than 10-fold with no loss of yield and selectivity.
Furthermore, as we had envisioned at the conception of this project, the cyclic silanediol organo-mercury products could be used as starting materials for a variety of further transformations, including hydroxylation,32–34 demercuration, 35–38 and iodination39–41 (Scheme 6A–C).
Scheme 6.
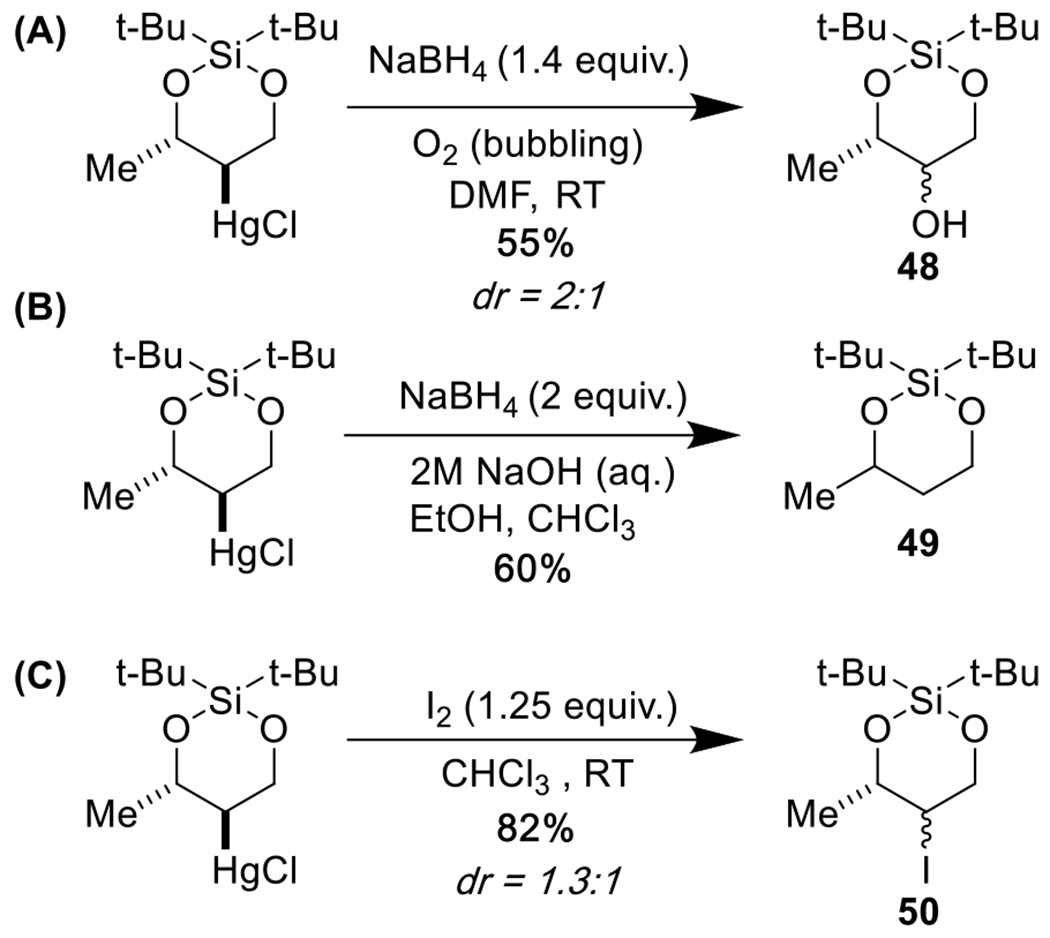
The C–Hg organometallic bond is highly versatile.
In summary, we present the first tethered olefin functionalization using a silanol auxiliary. The silanol tether could be conveniently appended to a variety of primary and secondary alcohols via condensation with di-tert-butylsilyl bis(trifluoromethanesulfonate). Hg(OTf)2 mediated cyclization proceeded with high diastereoselectivity and afforded cyclic silanediol mercury chloride products. The reaction was scalable greater than ten-fold and the products were readily amenable for further demercurative transformations. We expect this reaction to find much application in the pursuit of important polyhydroxylated compounds.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by start-up funding provided jointly by the University of Kansas Office of the Provost and the Department of Medicinal Chemistry as well as an NIH COBRE Chemical Biology of Infectious Diseases Pilot Project Grant to Shyam Sathyamoorthi (P20GM113117). We thank Dr. Victor Day (University of Kansas) for X-ray crystallography analysis. Support for the NMR instrumentation was provided by the NIH Shared Instrumentation Grant No. S10RR014767. We thank Professor Frank Schoenen, Professor Apurba Dutta, Professor Ryan Altman, and Mr. Cornelius Ndi for timely donation of a Lauda Brinkmann IC-6 immersion cooler chiller.
Footnotes
Supporting Information. Experimental and characterization data as well crystallographic data for Compound 31 is included in the supplementary information. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
REFERENCES
- 1.Yokoyama H, Lecture on Methylmercury Poisoning in Minamata (MPM). In Mercury Pollution in Minamata, Yokoyama H, Ed. Springer Singapore: Singapore, 2018; pp 5–51. [Google Scholar]
- 2.Hong Y-S; Kim Y-M; Lee K-E, Methylmercury Exposure and Health Effects. J Prev Med Public Health 2012, 45, 353–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Larock RC, Organomercury Compounds in Organic Synthesis. Angew. Chem. Int. Ed 1978, 17, 27–37. [Google Scholar]
- 4.Larock RC, Hydrogen and Halogen Substitution. In Organomercury Compounds in Organic Synthesis, Larock RC, Ed. Springer Berlin Heidelberg: Berlin, Heidelberg, 1985; pp 155–186. [Google Scholar]
- 5.Larock RC, Synthesis of Heteroatom-Containing Compounds. In Organomercury Compounds in Organic Synthesis, Larock RC, Ed. Springer Berlin Heidelberg: Berlin, Heidelberg, 1985; pp 187–239. [Google Scholar]
- 6.Larock RC, Alkene and Alkyne Addition and Substitution Reactions. In Organomercury Compounds in Organic Synthesis, Larock RC, Ed. Springer Berlin Heidelberg: Berlin, Heidelberg, 1985; pp 263–308. [Google Scholar]
- 7.Shinde AH; Sathyamoorthi S, Oxidative Cyclization of Sulfamates onto Pendant Alkenes. Org. Lett 2020, 22, 896–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shinde AH; Nagamalla S; Sathyamoorthi S, N-arylated oxathiazinane heterocycles are convenient synthons for 1,3-amino ethers and 1,3-amino thioethers. Med. Chem. Res 2020, 29, 1223–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas AA; Nagamalla S; Sathyamoorthi S, Salient features of the aza-Wacker cyclization reaction. Chem. Sci 2020, 11, 8073–8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corey EJ; Hopkins PB, Diisopropylsilyl ditriflate and di-tert-butysilyl ditriflate: new reagents for the protection of diols. Tetrahedron Lett. 1982, 23, 4871–4874. [Google Scholar]
- 11.Trost BM; Caldwell CG, The di-t-butylsilylene protecting group for diols. Tetrahedron Lett. 1981, 22, 4999–5002. [Google Scholar]
- 12.Arefolov A; Panek JS, Crotylsilane Reagents in the Synthesis of Complex Polyketide Natural Products: Total Synthesis of (+)-Discodermolide. J. Am. Chem. Soc 2005, 127, 5596–5603. [DOI] [PubMed] [Google Scholar]
- 13.Obringer M; Barbarotto M; Choppin S; Colobert F, Efficient and Stereoselective Access to the Polyol Fragment C9–C16 of Ansamycin Antibiotics. Org. Lett 2009, 11, 3542–3545. [DOI] [PubMed] [Google Scholar]
- 14.Chan C; Zheng S; Zhou B; Guo J; Heid RM; Wright BJD; Danishefsky SJ, The Solution to a Deep Stereochemical Conundrum: Studies toward the Tetrahydroisoquinoline Alkaloids. Angew. Chem. Int. Ed 2006, 45, 1749–1754. [DOI] [PubMed] [Google Scholar]
- 15.Paterson I; Mühlthau FA; Cordier CJ; Housden MP; Burton PM; Loiseleur O, Toward the Total Synthesis of the Brasilinolides: Stereocontrolled Assembly of a C1–C19 Polyol Segment. Org. Lett 2009, 11, 353–356. [DOI] [PubMed] [Google Scholar]
- 16.Chen C-L; Namba K; Kishi Y, Attempts To Improve the Overall Stereoselectivity of the Ireland–Claisen Rearrangement. Org. Lett 2009, 11, 409–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caspi DD; Garg NK; Stoltz BM, Heterogeneous Reductive Isomerization Reaction Using Catalytic Pd/C and H2. Org. Lett 2005, 7, 2513–2516. [DOI] [PubMed] [Google Scholar]
- 18.Parasram M; Gevorgyan V, Silicon-Tethered Strategies for C–H Functionalization Reactions. Acc. Chem. Res 2017, 50, 2038–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y; Gevorgyan V, General Method for the Synthesis of Salicylic Acids from Phenols through Palladium-Catalyzed Silanol-Directed C-H Carboxylation. Angew. Chem. Int. Ed 2015, 54, 2255–2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee E; Ryu T; Park Y; Park S; Lee PH, Tandem Gold-Catalyzed Hydrosilyloxylation–Aldol and –Mannich Reaction with Alkynylaryloxysilanols in 6-exo Mode. Adv. Synth. Catal 2013, 355, 1585–1596. [Google Scholar]
- 21.Oswald JP; Woerpel KA, Cobalt-Catalyzed Intramolecular Silylperoxidation of Unsaturated Diisopropylsilyl Ethers. J. Org. Chem 2019, 84, 7564–7574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Overman LE; Campbell CB, Hemiacetal mediated reactions. Directed synthesis of diols and acetals. J. Org. Chem 1974, 39, 1474–1481. [Google Scholar]
- 23.Overman LE, Intramolecular delivery of a water equivalent in the oxymercuration reaction. Conversion of an allylic alcohol into a cis- vicinal diol. J. Chem. Soc., Chem. Commun 1972, 1196–1198. [Google Scholar]
- 24.Dreher SD; Leighton JL, Formal Total Synthesis of Mycoticin A. J. Am. Chem. Soc 2001, 123, 341–342. [DOI] [PubMed] [Google Scholar]
- 25.Sarraf ST; Leighton JL, Oxymercuration of Homoallylic Alcohol Derived Hemiacetals: Diastereoselective Synthesis of Protected 1,3-Diols. Org. Lett 2000, 2, 403–405. [DOI] [PubMed] [Google Scholar]
- 26.Hornberger KR; Hamblett CL; Leighton JL, Total Synthesis of Leucascandrolide A. J. Am. Chem. Soc 2000, 122, 12894–12895. [Google Scholar]
- 27.Sarraf ST; Leighton JL, Rhodium-Catalyzed Formylation of Organomercurials: Application to Efficient Polyol Synthesis. Org. Lett 2000, 2, 3205–3208. [DOI] [PubMed] [Google Scholar]
- 28.Dreher SD; Hornberger KR; Sarraf ST; Leighton JL, Yb(OTf)3-Catalyzed Oxymercuration of Homoallylic Alcohol-Derived Hemiacetals and Hemiketals. Org. Lett 2000, 2, 3197–3199. [DOI] [PubMed] [Google Scholar]
- 29.Evans PA; Grisin A; Lawler MJ, Diastereoselective Construction of syn-1,3-Dioxanes via a Bismuth-Mediated Two-Component Hemiacetal/Oxa-Conjugate Addition Reaction. J. Am. Chem. Soc 2012, 134, 2856–2859. [DOI] [PubMed] [Google Scholar]
- 30.Bonini C; Campaniello M; Chiummiento L; Videtta V, Stereoselective synthesis of versatile 2-chloromercurium-3,5-syn-dihydroxy esters via intramolecular oxymercuration. Tetrahedron 2008, 64, 8766–8772. [Google Scholar]
- 31.Nishizawa M; Imagawa H; Yamamoto H, A new catalyst for organic synthesis: mercuric triflate. Org. Biomol. Chem 2010, 8, 511–521. [DOI] [PubMed] [Google Scholar]
- 32.Raeppel F; Weibel J-M; Heissler D, Synthesis of the trans-syn-trans perhydrobenz[e]indene moiety of the stellettins and of the stelliferins. Tetrahedron Lett. 1999, 40, 6377–6381. [Google Scholar]
- 33.Khalaf JK; Datta A, An Efficient and Highly Stereocontrolled Route to Bulgecinine Hydrochloride. J. Org. Chem 2004, 69, 387–390. [DOI] [PubMed] [Google Scholar]
- 34.Crich D; Natarajan S; Crich JZ, Synthesis of the taxol AB-system by olefination of an A-ring C1 ketone and direct B-ring closure. Tetrahedron 1997, 53, 7139–7158. [Google Scholar]
- 35.Andrey O; Glanzmann C; Landais Y; Parra-Rapado L, 1,3-Asymmetric induction in electrophilic addition onto homoallylsilanes. An approach towards the total synthesis of (+/−)-kumausyne. Tetrahedron 1997, 53, 2835–2854. [Google Scholar]
- 36.Liu T-Z; Li J-M; Isobe M, Synthetic Studies on Ciguatoxin—Synthesis of H–I–J Ring System. Tetrahedron 2000, 56, 10209–10219. [Google Scholar]
- 37.Oikawa H; Toyomasu T; Toshima H; Ohashi S; Kawaide H; Kamiya Y; Ohtsuka M; Shinoda S; Mitsuhashi W; Sassa T, Cloning and Functional Expression of cDNA Encoding Aphidicolan-16β-ol Synthase: A Key Enzyme Responsible for Formation of an Unusual Diterpene Skeleton in Biosynthesis of Aphidicolin. J. Am. Chem. Soc 2001, 123, 5154–5155. [DOI] [PubMed] [Google Scholar]
- 38.McDonald FE; Ishida K; Hurtak JA, Stereoselectivity of electrophile-promoted oxacyclizations of 1,4-dihydroxy-5-alkenes to 3-hydroxytetrahydropyrans. Tetrahedron 2013, 69, 7746–7758. [Google Scholar]
- 39.Stevens RV; Albizati KF, Synthetic approach to the amphilectane diterpenes: the use of nitriles as terminators of carbocation-olefin cyclizations. J. Org. Chem 1985, 50, 632–640. [Google Scholar]
- 40.Bloodworth AJ; Bowyer KJ; Mitchell JC, Stereochemical evidence for an alkylated perepoxide intermediate. J. Org. Chem 1987, 52, 1124–1128. [Google Scholar]
- 41.Eaton PE; Daniels RG; Casucci D; Cunkle GT; Engel P, Amide activation for cyclopropane ortho-lithiation. J. Org. Chem 1987, 52, 2100–2102. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


