Abstract
Background/Objectives
We examined the prevalence of anaemia, iron deficiency, and inflammation during pregnancy and their associations with adverse pregnancy and infant outcomes in India.
Subjects/Methods
Three hundred and sixty-six women participating in a randomised trial of vitamin B12 supplementation were monitored to assess haemoglobin (Hb), serum ferritin (SF), hepcidin, C-reactive protein (CRP), and alpha-1-acid glycoprotein (AGP) during pregnancy. Women received vitamin B12 supplementation (50 μg per day) or placebo daily; all women received daily prenatal iron–folic acid supplementation. Binomial and linear regression models were used to examine the associations of maternal iron biomarkers with pregnancy and infant outcomes.
Results
Thirty percent of women were anaemic (Hb < 11.0 g/dl), 48% were iron deficient (SF < 15.0 μg/l), and 23% had iron deficiency anaemia at their first prenatal visit. The prevalence of inflammation (CRP > 5.0 mg/l: 17%; AGP > 1.0 g/l: 11%) and anaemia of inflammation (Hb < 11.0 g/dl, SF > 15.0 μg/l, plus CRP > 5.0 mg/l or AGP > 1.0 g/l: 2%) were low. Infants born to anaemic women had a twofold higher risk of low birth weight (<2500 g; risk ratio [RR]: 2.15, 95%CI: 1.20–3.84, p = 0.01), preterm delivery (RR: 2.67 (1.43–5.00); p = 0.002), underweight (WAZ < −2; RR: 2.20, 95%CI: 1.16–4.15, p = 0.02), and lower MUAC (β(SE): −0.94 (0.45)cm, p = 0.03). Similarly, maternal Hb concentrations predicted higher infant birth weight (p = 0.02) and greater gestational age at delivery (β(SE): 0.28 (0.08) weeks, p = 0.001), lower risk of preterm delivery (<37 weeks; RR: 0.76, 95%CI: 0.66–86, p < 0.0001); and higher infant MUAC (β(SE): 0.36 (0.13) cm, p = 0.006). Maternal SF concentrations were associated with greater birth length (β(SE): 0.44 (0.20) cm, p < 0.03). Findings were similar after adjusting SF concentrations for inflammation. IDA was associated with higher risk of low birth weight (RR: 1.99 (1.08–3.68); p = 0.03) and preterm birth (RR: 3.46 (1.81–6.61); p = 0.0002); and lower birth weight (p = 0.02), gestational age at birth (p = 0.0002), and infant WAZ scores (p = 0.02).
Conclusions
The prevalence of anaemia and iron deficiency was high early in pregnancy and associated with increased risk of adverse pregnancy and infant outcomes. A comprehensive approach to prevent anaemia is needed in women of reproductive age, to enhance haematological status and improve maternal and child health outcomes.
Introduction
Anaemia is a major public health problem worldwide, affecting over 1.6 billion people [1]. Anaemia has been associated with increased risk of maternal and infant mortality [1], impaired neurodevelopment in offspring [2] and reduced cognitive function [3, 4] and physical work capacity [3, 5] later in life. The burden of anaemia in India is among the highest in the world, where it exacts a heavy toll in terms of mortality, disability, and lost productivity [1, 6]. It is estimated that anaemia affects 56% of women of reproductive age, 59% of pregnant women, 63% of lactating women, and 70% of young children in India [1].
Iron deficiency is the leading cause of anaemia world-wide, and iron supplementation is the standard of care for prevention and treatment of anaemia [1]. It has been estimated that ~50% of anaemia is due to iron deficiency (50%, 95%CI: 47–53%) [1]. However, recent findings indicate that the proportion of anaemia due to iron deficiency may be considerably lower [7], particularly in settings where the anaemia prevalence is high, and in settings with very high inflammation exposure [7]. Other nutritional factors, such as vitamin B12 and folate, and non-nutritional factors such as inflammation also contribute to the aetiology of anaemia and impact human health [8, 9]. Elucidation of the aetiology of anaemia early in pregnancy and its impact on perinatal outcomes could help to inform screening and targeted interventions for anaemia prevention.
We, therefore, conducted a prospective observational analysis among pregnant women and their infants participating in a randomised trial of vitamin B12 supplementation in Bangalore, India. The objectives of this study were to: (1) determine the prevalence of anaemia, iron deficiency, iron deficiency anaemia (IDA), and inflammation in pregnant women; and (2) examine the associations between maternal biomarkers of iron status with the risks of adverse pregnancy and infant outcomes.
Subjects and methods
Study population
Participants were pregnant women, who were enrolled in a randomised, double-blind, placebo-controlled trial of vitamin B12 supplementation in Bangalore, India. This trial was conducted to examine the effects of daily vitamin B12 supplementation on biomarkers of maternal vitamin B12 status during pregnancy. The design and main results of the trial have been previously described (#NCT00641862) [10]. Briefly, pregnant women (n = 366) were recruited from Hosahalli Referral Hospital in Bangalore, India, and randomised to receive vitamin B12 supplementation (50 μg per day) or placebo, daily during pregnancy through 6 weeks postpartum. Findings demonstrated that maternal prenatal vitamin B12 supplementation significantly increased maternal, breast milk, and infant vitamin B12 concentrations [10]. All women also received daily iron (60 mg) and folic acid (500 μg) supplementation, beginning at their first prenatal visit, as per standard of care.
Pregnant women were eligible to participate in this study if they were at least 18 years of age, ≤14 weeks of gestation at enrolment, healthy, and carrying a single fetus. Women were excluded if they had any known medical complications, including HIV infection, hepatitis B, or syphilis. Women with a serious preexisting medical condition (defined by the need for regular medication use), or who had a previous caesarean delivery, or who were taking daily vitamin supplements in addition to iron and folic acid were also excluded.
Follow-up procedures
Structured interviews were conducted at the baseline clinic visit to collect information on sociodemographic characteristics, including maternal age, educational level, socioeconomic status, and obstetric history. A clinical examination was performed, including measurement of vital signs and blood pressure. Obstetric, reproductive, and neurological examinations were conducted. Detailed clinical, sociodemographic, and anthropometric data were collected prospectively. Weights of all the mothers were recorded using a digital balance to the nearest 0.1 kg (Salter’s 9016; Tonbridge), and maternal height was measured using a stadiometer to the nearest 0.1 cm. Maternal mid-upper arm circumference and triceps, biceps, and subscapular skinfold thickness measurements were recorded by trained research assistants.
Infant weight was recorded on an electronic weighing scale to the nearest 0.01 kg; measurements were recorded by trained study staff. Infant supine length, mid-upper arm circumference, head and chest circumferences, and triceps and biceps skinfolds were recorded by trained research assistants, using standardised procedures and calibrated instruments [10–12].
Laboratory investigations. Blood sample collection
Maternal blood samples were collected at study visits at enrolment (≤14 weeks), and during the second and third trimesters, timed to coincide with early (median [IQR]: 10.6 (9.1, 12.6) weeks), mid- (24.1 (23.7, 25.0) weeks), and late- (33.1 (32.7, 33.6) weeks) gestation. Infant blood samples were collected at 6 weeks of age by venipuncture. The laboratory procedures and biochemical analyses in this trial have previously been described [10]. Briefly, ~10 ml of blood was drawn from mothers during pregnancy and their infants at 6 weeks of age by venipuncture and collected in both EDTA and plain vacutainers (BD Biosciences), which were stored on ice until separated in a refrigerated centrifuge, within 4 h of being drawn. Haemoglobin and complete blood count were analysed on whole-blood samples in an automated Coulter counter (ABX Pentra C+; Horiba Medicals). Plasma and red blood cells (RBCs) were separated and stored at or below −80 °C until analysis.
Biomarkers
Serum ferritin was measured by electrochemiluminescence (Elecsys 2010, Roche Diagnostics Mannheim, USA). Serum C-reactive protein and alpha-1-acid glycoprotein (AGP) were analysed via the particle-enhanced immune-turbidometric assay in a Roche Hitachi 902 analyzer (Roche Diagnostics, Germany). Hepcidin was measured by the competitive enzyme immunoassay method (Peninsula Laboratories, LLC, Bachem Group).
Anaemia was defined as low haemoglobin concentrations based on trimester-specific cutoffs (i.e., Hb < 11.0 g/dl during the first trimester, Hb < 10.5 g/dl during the second trimester, Hb < 11.0 g/dl during the third trimester) [13, 14]. Iron deficiency was defined as SF < 15.0 μg/l, and iron insufficiency was defined as SF < 30.0 μg/l. Inflammation was defined as elevated CRP or AGP using established cutoffs (CRP > 5.0 mg/l, AGP > 1.0 g/l). Anaemia of inflammation was defined as anaemia in the presence of normal serum ferritin concentrations and elevated inflammatory biomarkers (i.e., Hb < 11.0 g/dl, SF > 15.0 μg/l, plus CRP > 5.0 mg/l, or AGP > 1.0 g/l) [15].
Maternal body mass index (BMI) was defined as the ratio of weight in kilograms to height in meters squared (m2), and categorised using World Health Organization classifications [16]. Preterm delivery was defined as less than 37 completed weeks gestation. Infant low birth weight was defined as <2.5 kg. Small for gestational age (SGA) was defined as birth weight <10th percentile of the gestational age, using the INTERGROWTH reference [17]. Infant ponderal index was defined as weight in grams divided by length in centimeters cubed (g/cm3). Length-for-age (LAZ), weight-for-age (WAZ), and weight-for-length (WLZ) z-scores were calculated using World Health Organization (WHO) standards [18–20]. Stunting, wasting, and underweight were defined as standardised, sex-specific z-scores < −2, using LAZ, WLZ, and WAZ, respectively.
Statistical analyses
Variables were defined using conventional cutoffs where available; otherwise, medians of variables were defined based on their distributions in the population. Serum ferritin concentrations were adjusted for inflammation, considering Thurnham [21, 22] and Biomarkers Reflecting Inflammation and Nutritional Determinants of Anaemia (BRINDA) [23, 24] methods. Non-normally distributed variables were natural logarithmically transformed to ensure normality before further analysis; non-transformed values are presented in Table 1 for interpretation purposes.
Table 1.
Characteristics of the study populationa
| Variables | Median (IQR) or n (%) |
|---|---|
| Maternal characteristics | |
| Sociodemographic | |
| Age, y | 22 (20, 24) |
| Monthly household income, INRb | 6000 (4500, 9000) |
| <6000 INR, n (%) | 161 (44) |
| Standard of living index | |
| 0–22, n (%) | 127 (35) |
| 23–28, n (%) | 124 (34) |
| 29–64, n (%) | 115 (31) |
| Gestational age at randomisation, weeks | 10.6 (9.1, 12.6) |
| Parity | |
| Nulliparous, n (%) | 236 (64) |
| Primiparous or multiparous, n (%) | 130 (36) |
| Anthropometric | |
| Weight, kg | 46.7 (41.6, 53.0) |
| Height, cm | 153 (149, 157) |
| <150 cm, n (%) | 102 (28) |
| Body mass index, kg/m2 | 19.6 (18.1, 22.5) |
| <18.5 kg/m2, n (%) | 114 (31) |
| Mid-upper arm circumference, cm | 23.0 (21.5, 25.5) |
| Biochemical, at enrolmentc | |
| Haemoglobin, g/dl | 11.7 (10.8, 12.6) |
| Hb <11.0 g/dl | 109 (30) |
| Hb <8.5 g/dl | 19 (5) |
| Hb <7.0 g/dl | 4 (1) |
| Serum ferritin (SF), μg/l | 16.2 (7.4, 36.4) |
| <30.0 μg/l | 234 (68) |
| <15.0 μg/l | 166 (48) |
| <12.0 μg/l | 139 (40) |
| Serum ferritin (BRINDA adjusted), μg/l | 12.4 (5.7, 28.3) |
| <30.0 μg/l | 268 (78) |
| <15.0 μg/l | 202 (59) |
| <12.0 μg/l | 166 (48) |
| Serum ferritin (Thurnham adjusted), μg/l | 14.9 (6.7, 33.5) |
| <30.0 μg/l | 241 (70) |
| <15.0 μg/l | 174 (51) |
| <12.0 μg/l | 147 (43) |
| Iron deficiency anaemia | |
| Anaemia and SF <15.0 μg/l | 81 (23) |
| Anaemia and SF <12.0 μg/l | 75 (22) |
| C-reactive protein (CRP), mg/l | 1.7 (0.9, 3.7) |
| CRP >1.0 mg/l | 238 (69) |
| CRP >5.0 mg/l | 59 (17) |
| Alpha-1-acid glycoprotein (AGP), g/l | 0.77 (0.66, 0.90) |
| AGP >1.0 g/l | 39 (11) |
| CRP >5.0mg/L and AGP >1.0 g/l | 21 (6) |
| Hepcidin, ng/ml | 4.6 (1.9, 13.2) |
| Anaemia of inflammationd | 6 (2) |
| Hematocrit, % | 35.0 (32.4, 37.4) |
| Mean corpuscular volume, fl | 83 (78, 87) |
| Plasma vitamin B12, pmol/l | 149 (110, 204) |
| <150.0 pmol/l, n (%) | 180 (51) |
| Plasma MMA, μmol/l | 0.47 (0.28, 0.67) |
| >0.26 μmol/l, n (%) | 273 (76) |
| Plasma tHcy, μmol/l | 9.23 (5.75, 15.08) |
| >15.0 μmol/l, n (%) | 91 (25) |
| Erythrocyte folate, nmol/l | 387 (291, 496) |
| <340.0 nmol/l, n (%) | 136 (38) |
| Pregnancy outcomes | |
| Males, n (%) | 122 (47.5) |
| Birth weight, kg | 2.8 (2.5, 3.0) |
| Low birth weight (<2.5 kg), n (%) | 38 (15.1) |
| Gestational age at birth, weeks | 39.4 (38.3, 40.1) |
| Preterm (<37 weeks), n (%) | 33 (12.8) |
| Preterm and low birth weight, n (%) | 15 (6.0) |
| Small for gestational agee, n (%) | 85 (33.9) |
| Infant outcomes | |
| Haemoglobin, g/dl | 10.9 (9.9, 11.7) |
| Anaemia (<11.0 g/dl), n (%) | 37 (50.7) |
| Length, cm | 47.0 (46.0, 49.0) |
| Length-for-age z-score (LAZ) | −1.3 (−2.2, −0.3) |
| LAZ < −2 | 65 (28.9) |
| Weight-for-age z-score (WAZ) | −1.0 (−1.6, −0.5) |
| WAZ < −2 | 30 (12.0) |
| Weight-for-length z-score (WLZ) | −0.3 (−1.5, 0.8) |
| WLZ < −2 | 28 (15.1) |
| BMI z-score (BMIZ) | −0.4 (−1.6, 0.6) |
| BMIZ < −2 | 35 (15.6) |
| Ponderal Index, g/cm3 | 0.027 (0.024, 0.030) |
| Head circumference, cm | 33.5 (32.5, 34.5) |
| Chest circumference, cm | 31.5 (30.0, 32.5) |
| Waist circumference, cm | 28.0 (26.0, 30.0) |
| Hip circumference, cm | 28.0 (26.5, 29.7) |
| Mid-upper arm circumference, cm | 9.5 (9.0, 10.5) |
| Biceps skinfold, mm | 4.0 (3.4, 4.8) |
| Triceps skinfold, mm | 5.3 (4.6, 6.2) |
| Subscapular skinfold, mm | 5.2 (4.4, 6.2) |
Values are median (IQR) and n (%); n = 366 mothers, n = 258 infants
100 INR was equivalent to approximately US$2 at the time the study was conducted
Enrolment: ≤14 weeks gestation
Anaemia of inflammation was defined as anaemia (Hb < 11.0 g/dl) + serum ferritin ≥ 15.0 μg/l + (either CRP > 5.0 mg/l or AGP > 1.0 g/l)
Small for gestational age (SGA) was defined as birth weight < 10th percentile of the gestational age, using INTERGROWTH reference [17]
Linear and binomial regression models were used to examine the associations of maternal biomarkers of iron status at enrolment (i.e., ≤14 weeks) with pregnancy and infant outcomes, for continuous and categorical outcomes, respectively, including birth weight (continuous), low birth weight (categorical), gestational age at birth (continuous), preterm delivery (categorical), SGA (categorical), and infant WHO z-scores (continuous). Associations between maternal iron status biomarkers and perinatal outcomes were examined independently in separate models. All models included an adjustment for the intervention regimen and gestational age at sample collection, to account for the study design and timing of sample collection. The approach proposed by Rothman and Greenland was used to adjust for confounding, in which all known or suspected risk factors for the outcome that lead to a greater than 10% change-in-estimate were included in the models [25]. The missing indicator method was used for covariates, to retain observations with missing covariate data in multivariate analyses [26]. Statistical analyses were performed using SAS software, version 9.4 (SAS Institute, Inc., Cary, NC, USA).
Results
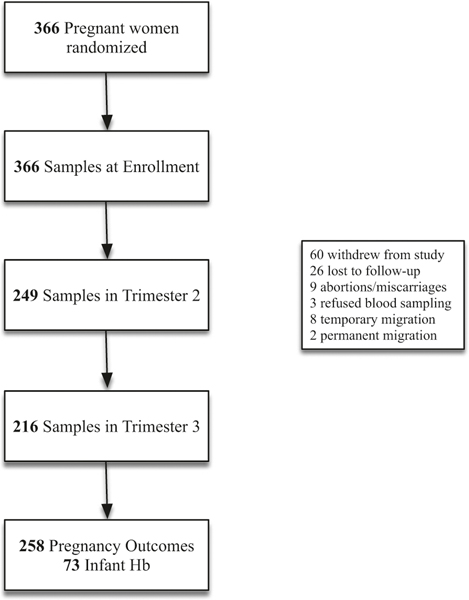
Characteristics of participants included in this study are presented in Table 1, and a flow chart of participants is presented in Fig. 1. At enrolment (≤14 weeks gestation), 30% of mothers were anaemic (Hb < 11.0 g/dl), 48% were iron deficient (SF < 15.0 μg/l), 68% had insufficient iron status (SF < 30.0 μg/l), and 23% had IDA. The prevalence of inflammation (CRP > 5.0 mg/l: 17%; AGP > 1.0 g/l: 11%) and AI (Hb < 11.0 g/dl, SF > 15.0 μg/l, plus CRP > 5.0 mg/l or AGP > 1.0 g/l: 2%) was relatively low. Findings were similar after adjusting serum ferritin for inflammation (Supplementary Table I, e.g., BRINDA, Thurnham); the prevalence of iron deficiency was 59% (SF < 15.0 μg/l), and 78% of pregnant women had insufficient iron status (SF < 30.0 μg/l), after adjusting for inflammation.
Fig. 1.
Participant flow chart
Maternal serum ferritin concentrations (β(SE): 0.78 (0.07) μg/l; p < 0.0001), hepcidin concentrations (β(SE): 0.37 (0.05) ng/ml; p < 0.0001), and iron deficiency (β(SE): −1.29 (0.15); p < 0.0001) at baseline were significantly associated with higher maternal haemoglobin concentrations in linear regression analyses. Findings were similar after adjusting serum ferritin concentrations for inflammation (SF: (β(SE): 0.79 (0.08) μg/L, p < 0.0001; ID: β(SE): −1.19 (0.16), p < 0.0001). Similarly, higher maternal serum ferritin concentrations (Risk Ratio [RR]: 0.48, 95%CI: 0.40–0.57, p < 0.0001) and hepcidin concentrations (RR: 0.73, 95%CI: 0.67–0.79, p < 0.0001) at baseline were associated with lower risk of maternal anaemia; whereas maternal iron deficiency was associated with a 3.6 times higher risk of anaemia (RR: 3.62, 95%CI: 2.09–6.36, p < 0.0001). Findings were similar after adjusting serum ferritin concentrations for inflammation (SF: RR: 0.49, 95%CI: 0.41–0.58, p < 0.0001; ID: RR: 3.41, 2.16–5.41, p < 0.0001).
The associations between maternal anaemia and haemoglobin concentrations and risk of adverse pregnancy and infant outcomes are presented in Tables 2 and 3, respectively. Women who were anaemic at enrolment had infants with lower birth weight (β(SE): −166.8 (61.1) g; p = 0.006) and 2.15 times higher risk of low birth weight (<2500 g; RR: 2.15, 95%CI: 1.20–3.84, p = 0.01), compared with the women who were not anaemic, in multivariate analyses adjusting for gestational age at sample collection, vitamin B12 intervention, maternal BMI, socioeconomic status, and inflammation. Infants born to women who were anaemic had significantly lower gestational age at birth (β(SE): −0.95 (0.28); p = 0.0007), and 2.67 times greater risk of being born prematurely (<37 weeks; RR: 2.67, 95%CI: 1.43–5.00, p = 0.002). Infants born to women who were anaemic had significantly greater risk of being born underweight (WAZ < −2; RR: 2.20, 95%CI: 1.16–4.15, p = 0.02), lower WAZ scores at birth (β(SE): −0.41 (0.14), p = 0.004), and lower mid-upper arm circumference (β(SE): −0.94 (0.45), p = 0.03), compared with the mothers who were not anaemic. Similarly, maternal haemoglobin concentrations (Table 3) at the first prenatal visit were associated with significantly greater gestational age at delivery (β(SE): 0.28 (0.08) weeks; p = 0.001), and lower risk of preterm delivery (<37 weeks; RR: 0.76, 95%CI: 0.66–86, p < 0.0001). Higher maternal haemoglobin concentrations were also associated with higher infant mid-upper arm circumference (β(SE): 0.36 (0.13); p = 0.006) in multivariate analyses.
Table 2.
Associations between maternal anaemia at enrolment and pregnancy and infant outcomes
| Model 1b | Model 2c | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Variablesa | n | β (SE) or RR (95%CI) | P-value | n | β (SE) or RR (95%CI) | P-value |
| Pregnancy outcomes | ||||||
| Birth weight, g | 252 | −174.7 (61.2) | 0.004 | 243 | −166.8 (61.1) | 0.006 |
| Low birth weight (<2500 g), n (%) | 252 | 1.84 (1.03, 3.29) | 0.04 | 243 | 2.15 (1.20, 3.84) | 0.01 |
| Gestational age at birth, weeks | 258 | −0.91 (0.28) | 0.001 | 249 | −0.95 (0.28) | 0.0007 |
| Preterm (<37 weeks), n (%) | 258 | 2.53 (1.35, 4.74) | 0.004 | 249 | 2.67 (1.43, 5.00) | 0.002 |
| Preterm and low birth weight, n (%) | 252 | 2.01 (0.75, 5.39) | 0.17 | 243 | 2.15 (0.80, 5.78) | 0.13 |
| Small for gestational aged, n (%) | 251 | 1.24 (0.87, 1.76) | 0.23 | 242 | 1.25 (0.87, 1.81) | 0.23 |
| Infant outcomes | ||||||
| Haemoglobin, g/dl | 73 | 0.14 (0.44) | 0.76 | 69 | −0.11 (0.44) | 0.80 |
| Anaemia (<11.0 g/dl), n (%) | 73 | 0.83 (0.49, 1.40) | 0.49 | 69 | 1.02 (0.57, 1.83) | 0.95 |
| Length, cm | 227 | −0.41 (0.41) | 0.31 | 218 | −0.35 (0.41) | 0.39 |
| Length-for-age z-score (LAZ) | 225 | −0.08 (0.20) | 0.67 | 216 | −0.03 (0.20) | 0.89 |
| LAZ < −2 | 225 | 0.96 (0.62, 1.50) | 0.87 | 216 | 0.85 (0.54, 1.33) | 0.47 |
| Weight-for-age z-score (WAZ) | 251 | −0.41 (0.14) | 0.004 | 242 | −0.41 (0.14) | 0.004 |
| WAZ < −2 | 251 | 1.99 (1.03, 3.87) | 0.04 | 242 | 2.20 (1.16, 4.15) | 0.02 |
| Weight-for-length z-score (WLZ) | 185 | −0.45 (0.27) | 0.09 | 178 | −0.40 (0.27) | 0.15 |
| WLZ < −2 | 185 | 1.52 (0.78, 2.97) | 0.22 | 178 | 1.50 (0.76, 2.95) | 0.25 |
| BMI z-score (BMIZ) | 224 | −0.48 (0.22) | 0.03 | 215 | −0.45 (0.22) | 0.04 |
| BMIZ < −2 | 224 | 2.42 (1.34, 4.39) | 0.004 | 215 | 2.49 (1.37, 4.56) | 0.003 |
| Ponderal index, g/cm3 | 226 | −0.001 (0.0007) | 0.17 | 217 | −0.001 (0.0007) | 0.18 |
| Head circumference, cm | 227 | −0.27 (0.24) | 0.24 | 218 | −0.021 (0.24) | 0.38 |
| Chest circumference, cm | 227 | −0.56 (0.29) | 0.05 | 218 | −0.61 (0.30) | 0.04 |
| Waist circumference, cm | 227 | −0.61 (0.40) | 0.13 | 218 | −0.61 (0.41) | 0.13 |
| Hip circumference, cm | 227 | 0.03 (0.58) | 0.96 | 218 | −0.06 (0.57) | 0.91 |
| Mid-upper arm circumference, cm | 225 | −0.94 (0.46) | 0.04 | 216 | −0.94 (0.45) | 0.03 |
| Biceps skinfold, mm | 226 | −0.07 (0.14) | 0.65 | 217 | −0.09 (0.15) | 0.54 |
| Triceps skinfold, mm | 226 | 0.06 (0.22) | 0.80 | 217 | 0.03 (0.22) | 0.90 |
Hb < 11.0 g/dl at enrolment (≤14 weeks gestation); beta (SE) is reported for continuous pregnancy outcomes; RR (95%CI) is reported for binary pregnancy outcomes
Models adjusted for gestational age of sample and vitamin B12 intervention
Models adjusted for gestational age of sample, vitamin B12 intervention, maternal BMI, standard of living index (SLI) >28, educational level, and ln(CRP)
Small for gestational age (SGA) was defined as birth weight <10th percentile of the gestational age, using INTERGROWTH reference [17]
Table 3.
Associations between maternal haemoglobin concentrations at enrolment and pregnancy and infant outcomes
| Model 1b | Model 2c | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Variablesa | n | β (SE) or RR (95% CI) | P-value | n | β (SE) or RR (95%CI) | P-value |
| Pregnancy outcomes | ||||||
| Birth weight, g | 252 | 37.4 (18.2) | 0.04 | 243 | 35.0 (18.2) | 0.05 |
| Low birth weight (<2500 g), n (%) | 252 | 0.91 (0.77, 1.07) | 0.27 | 243 | 0.87 (0.73, 1.04) | 0.13 |
| Gestational age at birth, weeks | 258 | 0.27 (0.08) | 0.001 | 249 | 0.28 (0.08) | 0.001 |
| Preterm (<37 weeks), n (%) | 258 | 0.76 (0.67, 0.87) | <0.0001 | 249 | 0.76 (0.66, 0.86) | <0.0001 |
| Preterm and low birth weight, n (%) | 252 | 0.90 (0.68, 1.20) | 0.48 | 243 | 0.86 (0.64, 1.15) | 0.30 |
| Small for gestational aged, n (%) | 251 | 0.98 (0.88, 1.09) | 0.72 | 242 | 0.98 (0.88, 1.09) | 0.67 |
| Infant outcomes | ||||||
| Haemoglobin, g/dl | 73 | 0.14 (0.14) | 0.33 | 69 | 0.22 (0.14) | 0.13 |
| Anaemia (<11.0 g/dl), n (%) | 73 | 1.02 (0.86, 1.22) | 0.81 | 69 | 0.94 (0.78, 1.13) | 0.52 |
| Length, cm | 227 | 0.14 (0.12) | 0.27 | 218 | 0.10 (0.12) | 0.42 |
| Length-for-age z-score (LAZ) | 225 | 0.04 (0.06) | 0.47 | 216 | 0.01 (0.06) | 0.81 |
| LAZ < −2 | 225 | 0.98 (0.87, 1.11) | 0.77 | 216 | 1.01 (0.90, 1.14) | 0.84 |
| Weight-for-age z-score (WAZ) | 251 | 0.08 (0.04) | 0.05 | 242 | 0.08 (0.04) | 0.06 |
| WAZ < −2 | 251 | 0.89 (0.74, 1.07) | 0.22 | 242 | 0.82 (0.67, 1.01) | 0.06 |
| Weight-for-length z-score (WLZ) | 185 | 0.07 (0.08) | 0.38 | 178 | 0.07 (0.08) | 0.40 |
| WLZ < −2 | 185 | 1.02 (0.83, 1.25) | 0.87 | 178 | 1.03 (0.85, 1.25) | 0.78 |
| BMI z-score (BMIZ) | 224 | 0.09 (0.07) | 0.16 | 215 | 0.09 (0.07) | 0.19 |
| BMIZ < −2 | 224 | 0.90 (0.76, 1.05) | 0.18 | 215 | 0.88 (0.74, 1.05) | 0.16 |
| Ponderal Index, g/cm3 | 226 | 0.0002 (0.0002) | 0.46 | 217 | 0.0002 (0.0002) | 0.44 |
| Head circumference, cm | 227 | 0.08 (0.07) | 0.28 | 218 | 0.06 (0.07) | 0.38 |
| Chest circumference, cm | 227 | 0.14 (0.09) | 0.11 | 218 | 0.14 (0.09) | 0.11 |
| Waist circumference, cm | 227 | 0.18 (0.12) | 0.13 | 218 | 0.19 (0.12) | 0.11 |
| Hip circumference, cm | 227 | −0.06 (0.17) | 0.72 | 218 | −0.04 (0.17) | 0.82 |
| Mid-upper arm circumference, cm | 225 | 0.36 (0.14) | 0.008 | 216 | 0.36 (0.13) | 0.006 |
| Biceps skinfold, mm | 226 | 0.04 (0.04) | 0.32 | 217 | 0.05 (0.04) | 0.26 |
| Triceps skinfold, mm | 226 | 0.05 (0.06) | 0.45 | 217 | 0.06 (0.07) | 0.35 |
Hb, g/dl enrolment (≤14 weeks gestation); beta (SE) is reported for continuous pregnancy outcomes; RR (95%CI) is reported for binary pregnancy outcomes
Models adjusted for gestational age of sample and vitamin B12 intervention
Models adjusted for gestational age of sample, vitamin B12 intervention, maternal BMI, standard of living index (SLI) >28, educational level, and ln(CRP)
Small for gestational age (SGA) was defined as birth weight < 10th percentile of the gestational age, using INTERGROWTH reference [17]
The associations between maternal serum ferritin concentrations (Table 4A–C) and iron deficiency (Table 5) at enrolment and adverse pregnancy and infant outcomes are presented in subsequent tables. Maternal serum ferritin concentrations were associated with increased infant length at birth (β(SE): 0.44 (0.20) cm, p = 0.03) in multivariate analyses, including adjusting for gestational age at sample collection, vitamin B12 intervention, maternal BMI, socioeconomic status, and inflammation. Findings were similar after adjusting maternal serum ferritin concentrations for inflammation, considering BRINDA (β(SE): 0.47 (0.20) cm, p = 0.02), and Thurnham (β(SE): 0.45 (0.20) cm, p = 0.02) methods (Tables 4B, C). Maternal iron deficiency was not significantly associated with risk of adverse pregnancy or infant outcomes, before or after adjusting for inflammation.
Table 4A.
Associations between maternal serum ferritin concentrations at enrolment and pregnancy and infant outcomes
| Model 1b | Model 2c | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Variablesa | n | β (SE) or RR (95%CI) | P-value | n | β (SE) or RR (95%CI) | P-value |
| Pregnancy outcomes | ||||||
| Birth weight, g | 244 | 34.0 (30.5) | 0.26 | 242 | 31.6 (30.1) | 0.29 |
| Low birth weight (<2500 g), n (%) | 244 | 0.97 (0.70, 1.33) | 0.83 | 242 | 0.96 (0.70, 1.32) | 0.80 |
| Gestational age at birth, weeks | 250 | 0.14 (0.14) | 0.32 | 248 | 0.17 (0.14) | 0.22 |
| Preterm (<37 weeks), n (%) | 250 | 0.80 (0.57, 1.13) | 0.21 | 248 | 0.77 (0.55, 1.09) | 0.14 |
| Preterm and low birth weight, n (%) | 244 | 1.06 (0.63, 1.77) | 0.83 | 242 | 1.02 (0.61, 1.71) | 0.93 |
| Small for gestational aged, n (%) | 243 | 0.94 (0.77, 1.14) | 0.54 | 241 | 0.93 (0.76, 1.14) | 0.49 |
| Infant outcomes | ||||||
| Haemoglobin, g/dl | 68 | 0.18 (0.23) | 0.42 | 68 | 0.25 (0.23) | 0.28 |
| Anaemia (<11.0 g/dl), n (%) | 68 | 0.81 (0.61, 1.07) | 0.13 | 68 | 0.82 (0.63, 1.08) | 0.17 |
| Length, cm | 219 | 0.46 (0.20) | 0.02 | 217 | 0.44 (0.20) | 0.03 |
| Length-for-age z-score (LAZ) | 217 | 0.19 (0.10) | 0.048 | 215 | 0.16 (0.10) | 0.09 |
| LAZ < −2 | 217 | 0.83 (0.67, 1.03) | 0.09 | 215 | 0.86 (0.69, 1.08) | 0.20 |
| Weight-for-age z-score (WAZ) | 243 | 0.07 (0.07) | 0.35 | 241 | 0.06 (0.07) | 0.36 |
| WAZ < −2 | 243 | 0.92 (0.64, 1.33) | 0.66 | 241 | 0.90 (0.63, 1.30) | 0.58 |
| Weight-for-length z-score (WLZ) | 179 | −0.10 (0.14) | 0.46 | 177 | −0.11 (0.14) | 0.44 |
| WLZ < −2 | 179 | 1.00 (0.69, 1.43) | 0.98 | 177 | 1.03 (0.70, 1.50) | 0.90 |
| BMI z-score (BMIZ) | 216 | −0.13 (0.11) | 0.25 | 214 | −0.13 (0.11) | 0.23 |
| BMIZ < −2 | 216 | 1.05 (0.76, 1.44) | 0.77 | 214 | 1.07 (0.78, 1.47) | 0.68 |
| Ponderal Index, g/cm3 | 218 | −0.0006 (0.0004) | 0.10 | 216 | −0.0006 (0.0004) | 0.09 |
| Head circumference, cm | 219 | −0.03 (0.12) | 0.78 | 217 | −0.05 (0.12) | 0.69 |
| Chest circumference, cm | 219 | −0.03 (0.14) | 0.85 | 217 | −0.06 (0.15) | 0.69 |
| Waist circumference, cm | 219 | −0.06 (0.20) | 0.75 | 217 | −0.08 (0.20) | 0.68 |
| Hip circumference, cm | 219 | −0.16 (0.27) | 0.55 | 217 | −0.20 (0.28) | 0.46 |
| Mid-upper arm circumference, cm | 217 | 0.37 (0.21) | 0.08 | 215 | 0.38 (0.22) | 0.08 |
| Biceps skinfold, mm | 218 | 0.05 (0.07) | 0.45 | 216 | 0.05 (0.07) | 0.48 |
| Triceps skinfold, mm | 218 | −0.14 (0.10) | 0.16 | 216 | −0.15 (0.11) | 0.15 |
Serum ferritin, natural log-transformed at enrolment (≤14 weeks gestation); beta (SE) is reported for continuous pregnancy outcomes; RR (95% CI) is reported for binary pregnancy outcomes
Models adjusted for gestational age of sample and vitamin B12 intervention
Models adjusted for gestational age of sample, vitamin B12 intervention, maternal BMI, standard of living index (SLI) >28, educational level, and ln(CRP)
Small for gestational age (SGA) was defined as birth weight < 10th percentile of the gestational age, using INTERGROWTH reference [17]
Table 4C.
Associations between maternal serum ferritin concentrations at enrolment (Thurnham adjusted) and pregnancy and infant outcomes
| Model 1b | Model 2c | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Variablesa | n | β (SE) or RR (95%CI) | P-value | n | β (SE) or RR (95%CI) | P-value |
| Pregnancy outcomes | ||||||
| Birth weight, g | 242 | 32.1 (30.5) | 0.29 | 242 | 32.3 (29.9) | 0.28 |
| Low birth weight (<2500 g), n (%) | 242 | 0.95 (0.69, 1.31) | 0.74 | 242 | 0.95 (0.69, 1.32) | 0.77 |
| Gestational age at birth, weeks | 248 | 0.14 (0.14) | 0.30 | 248 | 0.15 (0.14) | 0.28 |
| Preterm (<37 weeks), n (%) | 248 | 0.83 (0.59, 1.16) | 0.27 | 248 | 0.81 (0.57, 1.14) | 0.22 |
| Preterm and low birth weight, n (%) | 242 | 1.04 (0.63, 1.74) | 0.87 | 242 | 1.03 (0.61, 1.71) | 0.92 |
| Small for gestational aged, n (%) | 241 | 0.93 (0.76, 1.12) | 0.43 | 241 | 0.91 (0.74, 1.11) | 0.35 |
| Infant outcomes | ||||||
| Haemoglobin, g/dl | 68 | 0.28 (0.23) | 0.23 | 68 | 0.33 (0.23) | 0.16 |
| Anaemia (<11.0 g/dl), n (%) | 68 | 0.77 (0.57, 1.03) | 0.07 | 68 | 0.79 (0.60, 1.04) | 0.09 |
| Length, cm | 217 | 0.47 (0.20) | 0.02 | 217 | 0.45 (0.20) | 0.02 |
| Length-for-age z-score (LAZ) | 215 | 0.17 (0.10) | 0.07 | 215 | 0.16 (0.10) | 0.10 |
| LAZ < −2 | 215 | 0.85 (0.69, 1.06) | 0.14 | 215 | 0.87 (0.69, 1.09) | 0.22 |
| Weight-for-age z-score (WAZ) | 241 | 0.07 (0.07) | 0.34 | 241 | 0.07 (0.07) | 0.33 |
| WAZ < −2 | 241 | 0.89 (0.61, 1.28) | 0.52 | 241 | 0.89 (0.62, 1.29) | 0.55 |
| Weight-for-length z-score (WLZ) | 177 | −0.13 (0.14) | 0.35 | 177 | −0.12 (0.14) | 0.37 |
| WLZ < −2 | 177 | 1.02 (0.71, 1.48) | 0.90 | 177 | 1.06 (0.74, 1.53) | 0.74 |
| BMI z-score (BMIZ) | 214 | −0.15 (0.11) | 0.18 | 214 | −0.14 (0.11) | 0.18 |
| BMIZ < −2 | 214 | 1.07 (0.78, 1.47) | 0.67 | 214 | 1.09 (0.80, 1.50) | 0.58 |
| Ponderal Index, g/cm3 | 216 | −0.0007 (0.0004) | 0.06 | 216 | −0.0007 (0.0004) | 0.06 |
| Head circumference, cm | 217 | −0.05 (0.12) | 0.70 | 217 | −0.05 (0.12) | 0.68 |
| Chest circumference, cm | 217 | −0.05 (0.14) | 0.73 | 217 | −0.06 (0.14) | 0.70 |
| Waist circumference, cm | 217 | −0.07 (0.20) | 0.72 | 217 | −0.05 (0.20) | 0.78 |
| Hip circumference, cm | 217 | −0.18 (0.27) | 0.52 | 217 | −0.20 (0.27) | 0.46 |
| Mid-upper arm circumference, cm | 215 | 0.33 (0.22) | 0.13 | 215 | 0.36 (0.22) | 0.09 |
| Biceps skinfold, mm | 216 | 0.04 (0.07) | 0.57 | 216 | 0.04 (0.07) | 0.60 |
| Triceps skinfold, mm | 216 | −0.15 (0.11) | 0.16 | 216 | −0.15 (0.11) | 0.15 |
Baseline serum ferritin concentrations were adjusted for inflammation using Thurnham [21, 22] methods; natural log-transformed; beta (SE) is reported for continuous pregnancy outcomes; RR (95%CI) is reported for binary pregnancy outcomes
Models adjusted for gestational age of sample and vitamin B12 intervention
Models adjusted for gestational age of sample, vitamin B12 intervention, maternal BMI, standard of living index (SLI) >28, educational level, and ln(CRP)
Small for gestational age (SGA) was defined by sex and gestational age <10th percentile using INTERGROWTH methods [17]
Table 5.
Associations between maternal iron deficiency at enrolment and pregnancy and infant outcomes
| Model 1b | Model 2c | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Variablesa | n | β (SE) or RR (95%CI) | P-value | n | β (SE) or RR (95%CI) | P-value |
| Pregnancy outcomes | ||||||
| Birth weight, g | 244 | −54.3 (58.6) | 0.35 | 242 | −45.4 (57.9) | 0.43 |
| Low birth weight (<2500 g), n (%) | 244 | 1.38 (0.76, 2.51) | 0.30 | 242 | 1.36 (0.75, 2.46) | 0.31 |
| Gestational age at birth, weeks | 250 | −0.29 (0.26) | 0.28 | 248 | −0.35 (0.26) | 0.18 |
| Preterm (<37 weeks), n (%) | 250 | 1.61 (0.84, 3.09) | 0.15 | 248 | 1.75 (0.91, 3.37) | 0.09 |
| Preterm and low birth weight, n (%) | 244 | 1.19 (0.44, 3.16) | 0.73 | 242 | 1.26 (0.47, 3.35) | 0.64 |
| Small for gestational aged, n (%) | 243 | 0.96 (0.67, 1.38) | 0.84 | 241 | 0.97 (0.68, 1.38) | 0.85 |
| Infant outcomes | ||||||
| Haemoglobin, g/dl | 68 | −0.41 (0.44) | 0.36 | 68 | −0.64 (0.44) | 0.15 |
| Anaemia (<11.0 g/dl), n (%) | 68 | 1.17 (0.70, 1.95) | 0.54 | 68 | 1.20 (0.72, 1.98) | 0.48 |
| Length, cm | 219 | −0.77 (0.39) | 0.048 | 217 | −0.71 (0.39) | 0.07 |
| Length-for-age z-score (LAZ) | 217 | −0.29 (0.19) | 0.12 | 215 | −0.25 (0.19) | 0.18 |
| LAZ < −2 | 217 | 1.45 (0.94, 2.23) | 0.09 | 215 | 1.41 (0.92, 2.18) | 0.11 |
| Weight-for-age z-score (WAZ) | 243 | −0.12 (0.14) | 0.38 | 241 | −0.10 (0.13) | 0.46 |
| WAZ < −2 | 243 | 1.49 (0.74, 2.98) | 0.26 | 241 | 1.46 (0.74, 2.88) | 0.27 |
| Weight-for-length z-score (WLZ) | 179 | 0.07 (0.26) | 0.79 | 177 | 0.10 (0.26) | 0.69 |
| WLZ < −2 | 179 | 1.30 (0.66, 2.53) | 0.45 | 177 | 1.19 (0.62, 2.31) | 0.60 |
| BMI z-score (BMIZ) | 216 | 0.21 (0.21) | 0.33 | 214 | 0.25 (0.21) | 0.24 |
| BMIZ < −2 | 216 | 0.99 (0.54, 1.83) | 0.98 | 214 | 0.90 (0.50, 1.65) | 0.74 |
| Ponderal Index, g/cm3 | 218 | 0.001 (0.0007) | 0.11 | 216 | 0.001 (0.0007) | 0.09 |
| Head circumference, cm | 219 | −0.03 (0.23) | 0.89 | 217 | 0.01 (0.23) | 0.97 |
| Chest circumference, cm | 219 | 0.18 (0.28) | 0.52 | 217 | 0.23 (0.28) | 0.41 |
| Waist circumference, cm | 219 | 0.13 (0.39) | 0.73 | 217 | 0.10 (0.39) | 0.79 |
| Hip circumference, cm | 219 | 0.33 (0.53) | 0.54 | 217 | 0.41 (0.54) | 0.45 |
| Mid-upper arm circumference, cm | 217 | −0.29 (0.42) | 0.50 | 215 | −0.32 (0.42) | 0.45 |
| Biceps skinfold, mm | 218 | 0.12 (0.14) | 0.39 | 216 | 0.13 (0.14) | 0.34 |
| Triceps skinfold, mm | 218 | 0.56 (0.20) | 0.01 | 216 | 0.59 (0.20) | 0.004 |
Serum ferritin < 15.0 μg/l at enrolment (≤14 weeks gestation); beta (SE) is reported for continuous pregnancy outcomes; RR (95%CI) is reported for binary pregnancy outcomes
Models adjusted for gestational age of sample and vitamin B12 intervention
Models adjusted for gestational age of sample, vitamin B12 intervention, maternal BMI, standard of living index (SLI) >28, educational level, and ln(CRP)
Small for gestational age (SGA) was defined as birth weight <10th percentile of the gestational age, using INTERGROWTH reference [17]
Table 4B.
Associations between maternal serum ferritin concentrations at enrolment (BRINDA adjusted)a and pregnancy and infant outcomes
| Model 1b | Model 2c | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Variablesa | n | β (SE) or RR (95%CI) | P-value | n | β (SE) or RR (95%CI) | P-value |
| Pregnancy outcomes | ||||||
| Birth weight, g | 244 | 35.0 (30.9) | 0.26 | 242 | 35.0 (30.4) | 0.25 |
| Low birth weight (<2500 g), n (%) | 244 | 0.96 (0.69, 1.33) | 0.80 | 242 | 0.96 (0.69, 1.33) | 0.81 |
| Gestational age at birth, weeks | 250 | 0.11 (0.14) | 0.44 | 248 | 0.12 (0.14) | 0.38 |
| Preterm (<37 weeks), n (%) | 250 | 0.84 (0.60, 1.18) | 0.32 | 248 | 0.82 (0.58, 1.15) | 0.25 |
| Preterm and low birth weight, n (%) | 244 | 1.06 (0.63, 1.78) | 0.84 | 242 | 1.03 (0.61, 1.74) | 0.92 |
| Small for gestational aged, n (%) | 243 | 0.92 (0.76, 1.12) | 0.42 | 241 | 0.90 (0.74, 1.10) | 0.32 |
| Infant outcomes | ||||||
| Haemoglobin, g/dl | 68 | 0.29 (0.23) | 0.22 | 68 | 0.35 (0.23) | 0.13 |
| Anaemia (<11.0 g/dl), n (%) | 68 | 0.78 (0.59, 1.04) | 0.10 | 68 | 0.78 (0.59, 1.03) | 0.08 |
| Length, cm | 219 | 0.49 (0.20) | 0.01 | 217 | 0.47 (0.20) | 0.02 |
| Length-for-age z-score (LAZ) | 217 | 0.19 (0.10) | 0.05 | 215 | 0.17 (0.10) | 0.08 |
| LAZ < −2 | 217 | 0.84 (0.68, 1.05) | 0.12 | 215 | 0.86 (0.69, 1.08) | 0.20 |
| Weight-for-age z-score (WAZ) | 243 | 0.07 (0.07) | 0.31 | 241 | 0.07 (0.07) | 0.30 |
| WAZ < −2 | 243 | 0.89 (0.61, 1.29) | 0.54 | 241 | 0.89 (0.61, 1.29) | 0.55 |
| Weight-for-length z-score (WLZ) | 179 | −0.12 (0.14) | 0.40 | 177 | −0.11 (0.14) | 0.44 |
| WLZ < −2 | 179 | 1.00 (0.69, 1.45) | 1.00 | 177 | 1.04 (0.71, 1.52) | 0.84 |
| BMI z-score (BMIZ) | 216 | −0.15 (0.11) | 0.19 | 214 | −0.14 (0.11) | 0.20 |
| BMIZ < −2 | 216 | 1.04 (0.75, 1.44) | 0.81 | 214 | 1.05 (0.76, 1.45) | 0.76 |
| Ponderal Index, g/cm3 | 218 | −0.0007 (0.0004) | 0.07 | 216 | −0.0006 (0.0004) | 0.07 |
| Head circumference, cm | 219 | −0.04 (0.12) | 0.71 | 217 | −0.05 (0.12) | 0.66 |
| Chest circumference, cm | 219 | −0.06 (0.15) | 0.69 | 217 | −0.07 (0.15) | 0.63 |
| Waist circumference, cm | 219 | −0.10 (0.20) | 0.62 | 217 | −0.08 (0.20) | 0.67 |
| Hip circumference, cm | 219 | −0.22 (0.28) | 0.42 | 217 | −0.26 (0.28) | 0.36 |
| Mid-upper arm circumference, cm | 217 | 0.38 (0.22) | 0.08 | 215 | 0.42 (0.22) | 0.05 |
| Biceps skinfold, mm | 218 | 0.06 (0.07) | 0.38 | 216 | 0.06 (0.07) | 0.40 |
| Triceps skinfold, mm | 218 | −0.14 (0.11) | 0.20 | 216 | −0.14 (0.11) | 0.18 |
Baseline serum ferritin concentrations were adjusted for inflammation using BRINDA [23, 24] methods; natural log-transformed; beta (SE) is reported for continuous pregnancy outcomes; RR (95%CI) is reported for binary pregnancy outcomes
Models adjusted for gestational age of sample and vitamin B12 intervention
Models adjusted for gestational age of sample, vitamin B12 intervention, maternal BMI, standard of living index (SLI) >28, educational level, and ln(CRP)
Small for gestational age (SGA) was defined by sex and gestational age < 10th percentile using INTERGROWTH methods [17]
The associations between maternal IDA and pregnancy and infant outcomes are presented in Table 6. Women who had IDA at enrolment had infants with significantly lower birth weight (β(SE): −159.7 (67.7) g, p = 0.02) and increased risk of low birth weight (RR: 1.99 (1.08–3.68); p = 0.03) in multivariate analyses. They also had significantly lower gestational age at delivery (β(SE): −1.16 (0.31) weeks, p = 0.0002) and 3.46 times higher risk of preterm delivery (RR: 3.46 (1.81–6.61); p = 0.0002). Infants born to women who were iron-deficient anaemic also had significantly lower WAZ (β(SE): −0.39 (0.16), p = 0.01), compared with infants born to women without IDA.
Table 6.
Associations between maternal iron deficiency anaemia at enrolment and pregnancy and infant outcomes
| Model 1b | Model 2c | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Variablesa | n | β (SE) or RR (95%CI) | P-value | n | β (SE) or RR (95%CI) | P-value |
| Pregnancy outcoems | ||||||
| Birth weight, g | 244 | −169.7 (67.9) | 0.01 | 243 | −159.7 (67.7) | 0.02 |
| Low birth weight (<2500 g), n (%) | 244 | 1.77 (0.96, 3.27) | 0.07 | 243 | 1.99 (1.08, 3.68) | 0.03 |
| Gestational age at birth, weeks | 250 | −1.02 (0.31) | 0.001 | 249 | −1.16 (0.31) | 0.0002 |
| Preterm (<37 weeks), n (%) | 250 | 2.90 (1.56, 5.39) | 0.001 | 249 | 3.46 (1.81, 6.61) | 0.0002 |
| Preterm and low birth weight, n (%) | 244 | 2.20 (0.79, 6.10) | 0.13 | 243 | 2.43 (0.88, 6.71) | 0.09 |
| Small for gestational aged, n (%) | 243 | 1.16 (0.79, 1.73) | 0.45 | 242 | 1.18 (0.80, 1.76) | 0.40 |
| Infant outcomes | ||||||
| Haemoglobin, g/dl | 68 | −0.35 (0.48) | 0.47 | 69 | −0.49 (0.47) | 0.30 |
| Anaemia (<11.0 g/dl), n (%) | 68 | 1.05 (0.61, 1.81) | 0.87 | 69 | 1.25 (0.79, 2.00) | 0.34 |
| Length, cm | 219 | −0.87 (0.44) | 0.05 | 218 | −0.86 (0.45) | 0.06 |
| Length-for-age z-score (LAZ) | 217 | −0.29 (0.22) | 0.18 | 216 | −0.25 (0.22) | 0.25 |
| LAZ < −2 | 217 | 1.28 (0.82, 2.00) | 0.28 | 216 | 1.17 (0.74, 1.83) | 0.51 |
| Weight-for-age z-score (WAZ) | 243 | −0.40 (0.16) | 0.01 | 242 | −0.39 (0.16) | 0.01 |
| WAZ < −2 | 243 | 1.73 (0.85, 3.53) | 0.13 | 242 | 1.88 (0.95, 3.70) | 0.07 |
| Weight-for-length z-score (WLZ) | 179 | −0.16 (0.31) | 0.61 | 178 | −0.08 (0.31) | 0.80 |
| WLZ < −2 | 179 | 1.15 (0.53, 2.49) | 0.72 | 178 | 1.10 (0.50, 2.44) | 0.81 |
| BMI z-score (BMIZ) | 216 | −0.27 (0.25) | 0.27 | 215 | −0.21 (0.25) | 0.40 |
| BMIZ < −2 | 216 | 1.23 (0.63, 2.39) | 0.54 | 215 | 1.18 (0.61, 2.30) | 0.62 |
| Ponderal Index, g/cm3 | 218 | −0.0002 (0.0008) | 0.78 | 217 | −0.0001 (0.0008) | 0.93 |
| Head circumference, cm | 219 | −0.31 (0.26) | 0.24 | 218 | −0.26 (0.27) | 0.33 |
| Chest circumference, cm | 219 | −0.39 (0.32) | 0.23 | 218 | −0.32 (0.33) | 0.33 |
| Waist circumference, cm | 219 | −0.16 (0.44) | 0.73 | 218 | −0.11 (0.45) | 0.80 |
| Hip circumference, cm | 219 | 0.02 (0.61) | 0.97 | 218 | 0.12 (0.62) | 0.84 |
| Mid-upper arm circumference, cm | 217 | −0.82 (0.48) | 0.09 | 216 | −0.88 (0.49) | 0.07 |
| Biceps skinfold, mm | 218 | 0.01 (0.16) | 0.94 | 217 | 0.01 (0.16) | 0.97 |
| Triceps skinfold, mm | 218 | 0.32 (0.23) | 0.17 | 217 | 0.34 (0.24) | 0.15 |
Hb < 11.0 g/dl and serum ferritin < 15.0 μg/l at enrolment (≤14 weeks gestation); beta (SE) is reported for continuous pregnancy outcomes; RR (95%CI) is reported for binary pregnancy outcomes
Models adjusted for gestational age of sample and vitamin B12 intervention
Models adjusted for gestational age of sample, vitamin B12 intervention, maternal BMI, standard of living index (SLI) >28, educational level, and ln(CRP)
Small for gestational age (SGA) was defined as birth weight <10th percentile of the gestational age, using INTERGROWTH reference [17]
The prevalence of elevated inflammatory biomarkers (CRP > 5.0 mg/l: 17%; AGP > 1 g/l: 11%) and anaemia of inflammation (2%) were low in pregnancy (Supplemental tables I–IV). Maternal inflammatory biomarkers during pregnancy, CRP and AGP, were not associated with maternal haematological status or risk of adverse pregnancy or infant outcomes. Maternal hepcidin concentrations during pregnancy were associated with increased infant length (β(SE): 0.31 (0.13), p = 0.02), LAZ (β(SE): 0.13 (0.06), p = 0.04), WAZ (β(SE): 0.09 (0.05), p = 0.04), and mid-upper arm circumference (β(SE): 0.40 (0.15), p = 0.007).
Discussion
In this prospective analysis among pregnant women and their infants, anaemia and iron deficiency were common early in gestation (≤14 weeks) and were associated with increased risk of adverse pregnancy and infant outcomes. Maternal anaemia at enrolment predicted a twofold higher risk of infant low birth weight, preterm delivery, and underweight; similarly, maternal haemoglobin concentrations were associated with higher birth weight and gestational age at delivery, lower risk of preterm birth, and higher infant weight-for-age z-scores. Infants born to women with IDA had a significantly higher risk of preterm birth and low birth weight and had lower WAZ scores.
The prevalence of anaemia and iron deficiency at the first prenatal visit in this study was high: 30% of pregnant women were anaemic (Hb < 11.0 g/dl), 48% were iron deficient (SF < 15.0 μg/l), and 23% of women had IDA (Hb < 11.0 g/dl and SF < 15.0 μg/l); and 51% of infants were anaemic at 6 weeks of age. Findings were similar after adjusting for inflammation, considering Thurnham [21, 22] and BRINDA [23, 24] methods. These findings are consistent with previous studies that reported a high prevalence of anaemia and iron deficiency in pregnant women and young children in India. The National Family Health Survey in India reported 56% of women of reproductive age, 59% of pregnant women, and 70% of young children were anaemic [27]. The burden of anaemia in rural areas is estimated to be higher than in urban settings, ranging from 77% in Maharashtra state to over 90% in Northeast India [28–30]. Previous studies have also noted a high prevalence of iron deficiency and IDA in pregnancy. For example, a multicenter study among pregnant women in India reported 27% of women presented with IDA [31]. The high burden of anaemia and iron deficiency early in pregnancy, and its association with risk of adverse pregnancy and infant outcomes—despite daily iron and folic acid (and vitamin B12) supplementation during pregnancy—is cause for concern. Findings are consistent with the recent Women First Pre-conception Maternal Nutrition Trial [32], and suggest that screening and interventions are urgently needed in women of reproductive age (pre-conceptionally) and early in pregnancy to prevent maternal anaemia and associated adverse pregnancy and infant outcomes.
The prevalence of elevated inflammatory biomarkers (CRP > 5 mg/l: 17%; AGP > 1 g/l: 11%) and anaemia of inflammation (2%) were low in pregnancy and were not significantly associated with risk of adverse pregnancy or infant outcomes. However, pregnant women were excluded from participating in this study if they presented with any known medical complications, including severe anaemia and acute infections which might impair vitamin B12 absorption. Few studies to date have conducted comprehensive investigations of anaemia of inflammation in pregnant women early in gestation, with measurements of nutritional biomarkers for differential diagnosis, particularly in South Asia. In contrast, other studies in sub-Saharan Africa have noted a high prevalence of inflammation and anaemia of inflammation in pregnancy. For example, a cross-sectional study in pregnant women in Uganda found a high prevalence of anaemia of inflammation: over two-thirds of women who were not iron deficient had anaemia of inflammation [33]. Similarly, another study in Malawi found that 73.5% of pregnant women who were not iron deficient had anaemia of inflammation [34]. Inflammation may play an important role in the aetiology of anaemia, particularly in the context of infection or chronic diseases [35–37]. Recent studies suggest that the proportion of anaemia associated with iron deficiency may be lower in settings where the anaemia prevalence is high, and in settings with very high inflammation exposure [7].
In this current study, maternal anaemia and IDA were associated with increased risk of adverse perinatal outcomes. Maternal anaemia at enrolment predicted a twofold higher risk of preterm delivery, infant low birth weight, and underweight. Infants born to women with IDA also had significantly higher risk of low birth weight and preterm birth and lower WAZ scores. These findings are consistent with previous studies in India and in other settings in low- and middle-income countries. For example, in a prospective cohort study in Assam, India, maternal IDA was associated with significantly increased risk of infant low birth weight and small for gestational age [38]. Similarly, a recent meta-analysis found that pregnant women with anaemia had a significantly higher risk of preterm birth and low birth weight, compared with non-anaemic women [39]. In this review, in low- and middle-income countries, 12% of low birth weight, 19% of preterm births, and 18% of perinatal mortality were attributable to maternal anaemia, highlighting the importance of targeting maternal anaemia to reduce the risk of adverse perinatal outcomes. Lower maternal haemoglobin in the first and third trimesters has also been associated with increased risk of preterm birth and low birth weight [40].
In this study, maternal serum ferritin concentrations were associated with increased infant length at birth, and findings were similar before and after adjusting for inflammation. The observed association of maternal haemoglobin and serum ferritin concentrations early in pregnancy and improved pregnancy and neonatal outcomes are consistent with findings from several research studies [32, 41, 42], including the recent Women First Preconception Maternal Nutrition Trial [32]. In the Women’s First trial, a multi-country (India, DRC, Guatemala, Pakistan) randomized clinical trial, lipid-based maternal nutritional supplements administered pre-conception or early in pregnancy (first trimester) improved fetal growth-related birth outcomes (e.g., birth length, LAZ), compared with no supplementation [32]. In the current study, maternal iron deficiency (SF < 15.0 μg/l) was not significantly associated with increased risk of adverse pregnancy or infant outcomes, before or after adjusting for inflammation.
The aetiology of anaemia is multifactorial and complex, and includes nutritional and non-nutritional factors [43]. Findings from this study are consistent with previous evidence of the importance of iron deficiency as the leading cause of anaemia [1], as maternal serum ferritin concentrations and iron deficiency were significantly associated with maternal haemoglobin levels (p < 0.0001). The risk of iron deficiency is increased in pregnancy due to greater physiological demand for iron to support development of the placenta and fetus, increased maternal RBC mass volume, and requirements for maternal and infant metabolism [42, 44]. The low prevalence of inflammation and anaemia of inflammation in this study, and lack of association with risk of adverse pregnancy or infant outcomes are in contrast to previous studies among pregnant women in sub-Saharan Africa. However, the exclusion of pregnant women with active infections (and chronic conditions which might impair vitamin B12 absorption) in the parent vitamin B12 randomised trial constrained our ability to examine the role of infection and inflammation in the context of anaemia in this setting. This is an important study limitation which limits the generalisability of findings to healthy pregnant women. Findings suggest that a comprehensive assessment of nutritional biomarkers early in pregnancy may help to inform screening, differential diagnosis, and interventions for prevention and treatment of anaemia in pregnant women and their children [43].
There were several other limitations to this study. Although the sample was the same cohort as the parent randomised trial, these observational analyses do not inform causal interpretation of findings. Although maternal biomarkers were available for all women early in pregnancy (n = 366), data for pregnancy outcomes (n = 258), and infant blood measurements (n = 73) were available on a smaller subset of the total sample (n = 366). Although this population did not differ in terms of measured sociodemographic and nutritional variables, it is possible that they differ on other unmeasured variables. This analysis included a comprehensive assessment of iron status, including haemoglobin, serum ferritin, C-reactive protein, and AGP. However, the lack of assessment of soluble transferrin receptor and total body iron remains a study limitation.
In summary, in this large cohort of pregnant women participating in a randomised trial of vitamin B12 supplementation in Southern India, maternal anaemia and iron deficiency were common in pregnancy and predicted increased risk of adverse pregnancy and neonatal outcomes. Maternal anaemia at enrolment predicted a two to four times greater risk of low birth weight and preterm delivery. Findings suggest that, although prenatal iron–folic acid supplementation improves haematological status during pregnancy, maternal anaemia and IDA early in pregnancy have an important role in determining the risk of adverse outcomes early in life. Further research is needed to understand the complex aetiology of anaemia in women of reproductive age, to inform screening and interventions to prevent anaemia and improve the health of mothers and their children.
Supplementary Material
Acknowledgements
We thank the mothers and children, and field teams, including physicians, nurses, midwives, research and laboratory, and administrative staff, who made this study possible; Tinu Samuel, Ramya Rajendran, Sumithra Muthayya, Ammu Lukose, Wafaie Fawzi, and Ron Bosch for their contributions to the parent randomised trial of vitamin B12 supplementation; and St. John’s Medical College, Bangalore, India, for its institutional support.
Funding
National Institutes of Health (NICHD R03HD054123 and K24DK104676); Indian Council of Medical Research (ICMR: 5/7/192/06-RHN); Rose Fellowship in Chronic Disease Epidemiology and Biostatistics; Harvard Institute for Global Health; Uwe Brinkmann Memorial Fellowship; Michael von Clemm Fellowship; South Asia Initiative Graduate Grant; Division of Nutritional Sciences, Cornell University
Footnotes
Compliance with ethical standards
Conflict of interest The authors declare that they have no conflict of interest.
Ethics The research protocols and study procedures were approved by the Institutional Ethical Board of St. John’s Medical College and the Harvard T.H. Chan School of Public Health Human Subjects Committee, and written informed consent was obtained from all participants. A Data Safety and Monitoring Board met twice annually during the course of the trial.
Supplementary information The online version of this article (https://doi.org/10.1038/s41430-019-0464-3) contains supplementary material, which is available to authorised users.
References
- 1.WHO The global prevalence of anaemia in 2011. Geneva: WHO; 2015. [Google Scholar]
- 2.Janbek J, Sarki M, Specht IO, Heitmann BL A systematic literature review of the relation between iron status/anemia in pregnancy and offspring neurodevelopment. Eur J Clin Nutr. 2019. [DOI] [PubMed] [Google Scholar]
- 3.McClung JP, Murray-Kolb LE. Iron nutrition and premenopausal women: effects of poor iron status on physical and neuropsychological performance. Annu Rev Nutr. 2013;33:271–88. [DOI] [PubMed] [Google Scholar]
- 4.Murray-Kolb LE, Beard JL. Iron treatment normalizes cognitive functioning in young women. Am J Clin Nutr. 2007;85:778–87. [DOI] [PubMed] [Google Scholar]
- 5.Haas JD, Brownlie Tt. Iron deficiency and reduced work capacity: a critical review of the research to determine a causal relationship. J Nutr. 2001;131:676S–88S. [DOI] [PubMed] [Google Scholar]
- 6.Kalaivani K. & Prevalence consequences of anaemia in pregnancy. Indian J Med Res. 2009;130:627–33. [PubMed] [Google Scholar]
- 7.Petry N, Olofin I, Hurrell RF, Boy E, Wirth JP, Moursi M, et al. The proportion of anemia associated with iron deficiency in low, medium, and high human development index countries: a systematic analysis of national surveys. Nutrients 2016;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finkelstein JL, Layden AJ, Stover PJ. Vitamin B-12 and perinatal health. Adv Nutr. 2015;6:552–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finkelstein JL, Kurpad AV, Thomas T, Srinivasan K, Duggan C. Vitamin B12 status in pregnant women and their infants in South India. Eur J Clin Nutr. 2017;71:1046–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duggan C, Srinivasan K, Thomas T, Samuel T, Rajendran R, Muthayya S, et al. Vitamin B-12 supplementation during pregnancy and early lactation increases maternal, breast milk, and infant measures of vitamin B-12 status. J Nutr. 2014;144:758–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization Training course on child growth assessment. Geneva: World Health Organization; 2008. [Google Scholar]
- 12.Gibson RS. Principles of nutritional assessment. 2nd ed. New York, NY: Oxford University Press, Inc; 2005. [Google Scholar]
- 13.Assessing the iron status of populations: report of a joint World Health Organization/Centers for Disease Control and Prevention technical consultation on the assessment of iron status at the population level, 2nd ed. Geneva: World Health Organization; 2007. [Google Scholar]
- 14.Technical meeting: use and interpretation of haemoglobin concentrations for assessing anaemia status in individuals and populations. Geneva: World Health Organization; 2017. [Google Scholar]
- 15.Nemeth E, Ganz T. Anemia of inflammation. Hematol/Oncol Clin North Am. 2014;28:671–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.WHO. Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. Geneva: World Health Organization; 1995. [PubMed] [Google Scholar]
- 17.Villar J, Cheikh Ismail L, Victora CG, Ohuma EO, Bertino E, Altman DG, et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the newborn cross-sectional study of the INTERGROWTH-21st project. Lancet 2014;384:857–68. [DOI] [PubMed] [Google Scholar]
- 18.de Onis M, Blossner M. The World Health Organization Global Database on Child Growth and Malnutrition: methodology and applications. Int J Epidemiol. 2003;32:518–26. [DOI] [PubMed] [Google Scholar]
- 19.WHO Multicentre Growth Reference Study Group WHO Child Growth Standards: growth velocity based on weight, length and head circumference: methods and development. Geneva: World Health Organization; 2009. [Google Scholar]
- 20.WHO Multicentre Growth Reference Study Group WHO Child Growth Standards: methods and development: length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age. Geneva: World Health Organization; 2006 [Google Scholar]
- 21.Thurnham DI, McCabe LD, Haldar S, Wieringa FT, Northrop-Clewes CA, McCabe GP. Adjusting plasma ferritin concentrations to remove the effects of subclinical inflammation in the assessment of iron deficiency: a meta-analysis. Am J Clin Nutr. 2010;92:546–55. [DOI] [PubMed] [Google Scholar]
- 22.Thurnham DI, Northrop-Clewes CA, Knowles J. The use of adjustment factors to address the impact of inflammation on vitamin A and iron status in humans. J Nutr. 2015;145:1137S–43S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Namaste SM, Rohner F, Huang J, Bhushan NL, Flores-Ayala R, Kupka R, et al. Adjusting ferritin concentrations for inflammation: biomarkers reflecting inflammation and nutritional determinants of anemia (BRINDA) project. Am J Clin Nutr. 2017;106:359S–71S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suchdev PS, Williams AM, Mei Z, Flores-Ayala R, Pasricha SR, Rogers LM, et al. Assessment of iron status in settings of inflammation: challenges and potential approaches. Am J Clin Nutr. 2017;106:1626S–33S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Modeling Greenland S. and variable selection in epidemiologic analysis. Am J public health. 1989;79:340–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miettinen O. Theoretical Epidemiology. New York: John Wiley & Sons; 1985. 107 [Google Scholar]
- 27.NFHS. National Family Health Survey-4: 2015–16. Government of India, 2016. [Google Scholar]
- 28.Ahankari AS, Myles PR, Dixit JV, Tata LJ, Fogarty AW. Risk factors for maternal anaemia and low birth weight in pregnant women living in rural India: a prospective cohort study. Public health. 2017;151:63–73. [DOI] [PubMed] [Google Scholar]
- 29.Toteja GS, Singh P, Dhillon BS, Saxena BN, Ahmed FU, Singh RP, et al. Prevalence of anemia among pregnant women and adolescent girls in 16 districts of India. Food Nutr Bull. 2006;27:311–5. [DOI] [PubMed] [Google Scholar]
- 30.Bora R, Sable C, Wolfson J, Boro K, Rao R. Prevalence of anemia in pregnant women and its effect on neonatal outcomes in Northeast India. J Matern-fetal Neonatal Med. 2014;27:887–91. [DOI] [PubMed] [Google Scholar]
- 31.Mohanty D, Gorakshakar AC, Colah RB, Patel RZ, Master DC, Mahanta J, et al. Interaction of iron deficiency anemia and hemoglobinopathies among college students and pregnant women: a multi center evaluation in India. Hemoglobin. 2014;38:252–7. [DOI] [PubMed] [Google Scholar]
- 32.Hambidge KM, Westcott JE, Garces A, Figueroa L, Goudar SS, Dhaded SM, et al. A multicountry randomized controlled trial of comprehensive maternal nutrition supplementation initiated before conception: the women first trial. Am J Clin Nutr. 2019;109:457–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baingana RK, Enyaru JK, Tjalsma H, Swinkels DW, Davidsson L. The aetiology of anaemia during pregnancy: a study to evaluate the contribution of iron deficiency and common infections in pregnant Ugandan women. Public health Nutr. 2015;18:1423–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van den Broek NR, Letsky EA. Etiology of anemia in pregnancy in south Malawi. Am J Clin Nutr. 2000;72:247S–56S. [DOI] [PubMed] [Google Scholar]
- 35.Suchdev PS, Namaste SM, Aaron GJ, Raiten DJ, Brown KH, Flores-Ayala R. Overview of the biomarkers reflecting inflammation and nutritional determinants of anemia (BRINDA) project. Adv Nutr. 2016;7:349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bah A, Pasricha SR, Jallow MW, Sise EA, Wegmuller R, Armitage AE, et al. Serum hepcidin concentrations decline during pregnancy and may identify iron deficiency: analysis of a longitudinal pregnancy cohort in the Gambia. J Nutr. 2017;147:1131–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shaw JG, Friedman JF. Iron deficiency anemia: focus on infectious diseases in lesser developed countries. Anemia. 2011;2011:260380:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nair M, Choudhury MK, Choudhury SS, Kakoty SD, Sarma UC, Webster P, et al. Association between maternal anaemia and pregnancy outcomes: a cohort study in Assam, India. BMJ Glob health. 2016;1:e000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rahman MM, Abe SK, Rahman MS, Kanda M, Narita S, Bilano V, et al. Maternal anemia and risk of adverse birth and health outcomes in low- and middle-income countries: systematic review and meta-analysis. Am J Clin Nutr. 2016;103:495–504. [DOI] [PubMed] [Google Scholar]
- 40.Sukrat B, Wilasrusmee C, Siribumrungwong B, McEvoy M, Okascharoen C, Attia J, et al. Hemoglobin concentration and pregnancy outcomes: a systematic review and meta-analysis. Biomed Res int. 2013;2013:769057:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alwan NA, Cade JE, McArdle HJ, Greenwood DC, Hayes HE, Simpson NA. Maternal iron status in early pregnancy and birth outcomes: insights from the baby’s vascular health and iron in pregnancy study. Br J Nutr. 2015;113:1985–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Breymann C. Iron deficiency anemia in pregnancy. Semin Hematol. 2015;52:339–47. [DOI] [PubMed] [Google Scholar]
- 43.Camaschella C. New insights into iron deficiency and iron deficiency anemia. Blood Rev. 2017;31:225–33. [DOI] [PubMed] [Google Scholar]
- 44.Fisher AL, Nemeth E. Iron homeostasis during pregnancy. Am J Clin Nutr. 2017;106:1567S–74S. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.