Abstract
Background
Professional community health workers (CHWs) can help achieve universal health coverage, although evidence gaps remain on how to optimise CHW service delivery. We conducted an unblinded, parallel, cluster randomised trial in rural Mali to determine whether proactive CHW delivery reduced mortality and improved access to health care among children under five years, compared to passive delivery. Here we report the secondary access endpoints.
Methods
Beginning from 26-28 February 2017, 137 village-clusters were offered care by CHWs embedded in communities who were trained, paid, supervised, and integrated into a reinforced public-sector health system that did not charge user fees. Clusters were randomised (stratified on primary health centre catchment and distance) to care during CHWs during door-to-door home visits (intervention) or based at a fixed village site (control). We measured outcomes at baseline, 12-, 24-, and 36-month time points with surveys administered to all resident women aged 15-49 years. We used logistic regression with cluster-level random effects to estimate intention-to-treat and per-protocol effects over time on prompt (24-hour) treatment within the health sector.
Results
Follow-up surveys between February 2018 and April 2020 generated 20 105 child-year observations. Across arms, prompt health sector treatment more than doubled compared to baseline. At 12 months, children in intervention clusters had 22% higher odds of receiving prompt health sector treatment than those in control (cluster-specific adjusted odds ratio (aOR) = 1.22; 95% confidence interval (CI) = 1.06, 1.41, P = 0.005), or 4.7 percentage points higher (adjusted risk difference (aRD) = 0.047; 95% CI = 0.014, 0.080). We found no evidence of an effect at 24 or 36 months.
Conclusions
CHW-led health system redesign likely drove the 2-fold increase in rapid child access to care. In this context, proactive home visits further improved early access during the first year but waned afterwards.
Registration
ClinicalTrials.gov NCT02694055.
Ensuring that all people have access to quality health services without financial hardship is central to achieving universal health coverage (UHC) and other health-related targets of the Sustainable Development Goal (SDGs). Despite progress to date, up to one-third of the world’s population may not benefit from UHC by 2030 [1]. Achieving these goals requires a fundamental shift in how primary care is organised, managed, and delivered.
Community health workers (CHWs) have the potential to contribute to the diverse, sustainable health workforce required to deliver integrated, people-centred primary care [1]. Low and middle-income countries (LMICs) are increasingly adopting integrated community case management (iCCM) (comprising the diagnosis, treatment, and referral in the community for childhood malaria, diarrhoea, pneumonia, acute malnutrition, and/or newborn illnesses [2]) as a CHW-led strategy to improve service coverage and health outcomes among children under five years of age [3,4]. This scale-up is motivated by substantial evidence that CHWs can deliver a range of preventive and curative primary care services [5-7], including community case management for malaria [8,9], diarrhoea [10], and pneumonia [10-12] to increase utilisation, improve health, and reduce mortality among under-five children in many settings.
However, iCCM programme design and implementation vary greatly between settings, to variable effects [13,14]. Evaluations of scaled iCCM in Burkina Faso, Ethiopia, and Malawi found implementation shortcomings related to CHW training and deployment, health systems, and community mobilisation, and no effects on care-seeking, treatment coverage, or child mortality [15-17]. A systematic review of iCCM found moderate quality evidence that care-seeking from an appropriate provider increased by 68%, compared to facility-based care, yet inconsistent effects on the receipt of adequate treatment from an appropriate provider and under-five mortality among included studies, few of which included payment, supervision, or information systems to support CHWs [18].
Optimising iCCM means moving beyond training and deploying CHWs to ensure that these frontline health workers are integrated into and adequately supported by the health system [18]. The World Health Organization (WHO) guidelines released in 2018 recommend CHW remuneration, functioning referral systems, supply chain management, and supportive supervision, among other health system enablers [19]. However, existing gaps in the evidence do not allow for the recommendation of specific programme design features such as CHW workflow or approaches by which community-based services like iCCM are delivered [18,19].
Across sub-Saharan Africa, including in Mali, CHWs are stationed in community health sites to provide iCCM and other community-based services to patients who seek care. An alternative to this conventional, passive approach to service delivery is a proactive workflow in which CHWs conduct routine door-to-door home visits, searching for and identifying prospective patients. Proactively offering promotive, preventive, and curative services at patients’ doorsteps may improve community engagement, service coverage, and treatment outcomes, and especially the speed with which evaluation and treatment are received.
Ensuring prompt treatment, particularly within the crucial 24-hour window after symptom onset in children under five, is a cornerstone of global iCCM and malaria control programmes. A meta-analysis estimated that almost half of severe childhood malarial anaemia cases in the included studies could have been averted if children had accessed facility-based treatment within the first day of symptom onset [20]. From Brazil to Uganda, studies using verbal and social autopsy data have uncovered how delays at various points along the trajectory to care contribute to child death due to diarrhoea, acute respiratory infection, and newborn illnesses [21-23].
Based on existing evidence, it is uncertain whether proactive case-finding home visits by CHWs can improve prompt treatment and reduce the prevalence of infectious diseases or under-five mortality [24]. We implemented a cluster randomised trial to evaluate the effects of proactive CHW home visits on child mortality (primary trial endpoint) and access to care in rural, central Mali [25]. The primary trial endpoint results will be reported separately (unpublished data). Here we report the secondary trial endpoint analysis on child health and service utilisation over the three-year trial period, including the receipt of prompt treatment within the health sector, receipt of recommended case management according to iCCM protocols, and the prevalence of common childhood illnesses in this context. We assessed whether effects differed according to population size, distance to primary health centre (PHC), or household wealth, to determine the equity of this approach.
METHODS
Study design and participants
We conducted a pragmatic, cluster randomised controlled trial, with a stratified, two-arm, parallel group design in a rural setting in the Bankass health district of central Mali’s Mopti region. The district, served by one public secondary referral hospital and 22 PHCs was chosen in partnership with the Malian Ministry of Health and Social Development based on its high under-five mortality and low health care utilisation [26,27], with few concurrent health interventions and a high interest from local authorities in collaborating. From initial geo-mapping across seven contiguous PHC catchment areas, villages and hamlets one kilometre or less apart were grouped into clusters. We randomised clusters in a 1:1 allocation to intervention and control arms to receive CHW services delivered via proactive home visits (n = 69 clusters) or only at a fixed community health site (n = 68 clusters), respectively.
To assess outcomes, we censused all permanent residents and surveyed all resident women aged 15 to 49 years at baseline and annually at 12, 24, and 36 months. Respondents provided written, informed consent (or assent, if aged 15 to 17 years and unmarried) at their first enrolment and were included in follow-up surveys if present (including those who were aged above 49 years). Any individual who sought care from study providers was eligible to receive health care throughout the trial, regardless of residency, survey enrolment, or arm assignment.
Randomisation and masking
We used the timeline cluster graphical tool to describe the sequencing and blinding of the different recruitment, randomisation, and assessment procedures implemented during the trial, and whether they were conducted at the cluster or participant level, or both (Figure S1 in the Online Supplementary Document) [28]. We stratified the randomization by health catchment area and distance to the nearest PHC. In total, we had 21 strata. Each of the seven catchment areas had three strata: one for the cluster where the PHC was located, one for clusters within five kilometres from the PHC, and one for clusters beyond this distance. Given the nature of the intervention, we could not blind the participants, providers, or outcome assessors. Statisticians were blinded throughout the trial, until the data were fully cleaned and locked by the Data Safety & Monitoring Board (DSMB).
Procedures
In each cluster, community leaders nominated individuals aged 18 to 45 years who could read and write in French to be trained, selected, and deployed as CHWs. Nominees were divided by study arm and trained separately over six weeks, with annual one-week refresher training, based on the same clinical protocols (that covered preventive and curative primary care for reproductive, maternal, newborn, and child health, including iCCM for diarrhoea, pneumonia, malaria, acute malnutrition, and newborn illnesses) and the delivery approach to which their clusters were allocated. CHWs were ultimately selected based on a post-training evaluation and deployed to serve approximately 700 people, in line with Mali’s 2016-2020 national community health strategy [29].
CHWs in the intervention arm were instructed to conduct door-to-door proactive case-finding home visits for at least two hours per day, six days per week, with the goal of visiting every household at least twice per month. In the control arm, CHWs were instructed to station themselves at community health sites for four hours per day, six days per week, to provide the same package of services to care-seeking patients. CHWs in both arms were expected to be available on-call to provide care as needed, at all times.
CHWs in both arms received the same systems support, in accordance with WHO guidelines [19]. All CHWs signed contracts with the Community Health Associations (ASACO) that manage public-sector PHCs, received part-time salaries and benefits that met local minimum wage requirements, and had performance-based opportunities to advance into the cadre of dedicated CHW supervisors. All CHWs received individual, monthly supervision that included house calls without the CHW to solicit patients’ perspectives, direct observation while conducting home visits or stationed at their site (depending on which arm they were allocated to), and one-on-one feedback aided by a personalised performance dashboard [30]. Dedicated supervisors also held group supervision meetings twice per month, separately by arm. Supervisors monitored CHWs’ supplies and equipment, including the CHW smartphone-based mobile application for recording patient encounters. All CHWs were supported by a functioning referral system, as all study PHCs received reinforcements in infrastructure (e.g. waiting area, separate general and maternity wards), equipment, supplies, and human resources (e.g. recruitments and training). Finally, user fees were removed at all points of care, from CHW to tertiary hospital, for patients in both arms. The redesigned CHW-led health system in both arms was launched February 26-28, 2017.
We assessed the outcomes at baseline (December 2016 to January 2017) and approximately 12 (February to March 2018), 24 (March to May 2019), and 36 months (January to April 2020) via surveys administered at respondents’ homes by female surveyors who were neither community residents nor involved in health care delivery. We adapted the household and women’s surveys from Mali’s Demographic and Health Survey (DHS) and programmed in Open Data Kit. They included a household roster (census) and modules on migration, mortality, and socio-economic characteristics. The women’s survey included socio-demographic characteristics, current contraceptive use, most recent pregnancy and childbirth, lifetime birth history, and symptoms and service utilisation in the two weeks preceding the survey for all the woman’s co-residing children under five years of age.
Outcomes
We assessed all outcomes using the women’s survey, measured at the child level and analysed at the child-year level. The primary outcome was prompt treatment within the health sector, defined as a child aged 0-59 months with any symptom at any time in the two weeks preceding the survey who had received CHW or public or private health centre evaluation and any treatment, including traditional or home remedies, the same or next day after symptom onset. Secondary outcomes included any prompt treatment (from any source), health sector evaluation (CHW or public or private health centre consultation, with or without prompt treatment), and any care (inside or outside the home). As the intervention was designed to improve UHC, we defined (in an appendix to the trial statistical analysis plan that was approved by the DSMB prior to unblinding) composite utilisation outcomes that assessed access to care for all sick children, regardless of illness. Consistent with endpoints defined in the trial protocol [25], we included as secondary outcomes recommended case management and prompt. According to iCCM clinical protocols [2], we defined recommended case management as a child aged 3-59 months with fever, and/or diarrhoea without blood, and/or cough with fast breathing (i.e., suspected pneumonia) who had received a rapid diagnostic test for malaria, and/or oral rehydration solution (ORS) and zinc, and/or antibiotics, respectively; newborns were excluded as their clinical protocol was different. We were unable, however, to conduct stratified analyses by illness due to fewer clusters with cases and events per illness. To contextualise the access to care results and assess intervention effects on child morbidity, we also included the prevalence of fever, diarrhoea, cough, and suspected pneumonia in the two weeks preceding the survey among all children under five years.
Statistical analysis
We based the sample size calculation, planned interim analyses, and stopping guidelines on the trial’s primary endpoint (deaths among children under five years of age per 1000 person-years at risk of mortality), as reported in the protocol [25].
For all ten outcomes, we first generated cluster-specific summaries (means) by calculating the proportion in each cluster at each time point and plotting the median per arm and cluster-level variability over time. We then estimated the intervention effects using the following mixed effects logistic regression model on the intention-to-treat (ITT) population:
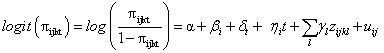 |
Here, πijkt is the probability for the kth individual in the jth cluster in the ith treatment arm, at the tth time point. α is the constant, representing the mean outcome among individuals in the control arm. (βi) is the cluster-specific odds ratio (ORCS) representing the outcome in the intervention arm (i = 1) compared to the control arm (i = 0). δt represents the time effect, with t = 1, 2, 3 corresponding to three consecutive follow-up surveys. ηit is the interaction term that estimates the differential effect of the intervention arm relative to the control arm across the three time points. For each outcome, we fit an additional model without the interaction term that estimated an overall cluster-specific effect throughout the three-year trial, controlling for the linear effect of time. γl is a vector of the estimated coefficients for the following set of covariates, represented by zijkl (l = 1,2,…,L): a cluster-level summary of the baseline value of the outcome, baseline cluster-level summaries of sample characteristics that were deemed imbalanced at baseline and likely to influence the outcome, individual’s age and sex, and variables on which randomisation was stratified. Cluster-level random effects, , accounted for within-cluster correlation. For prevalence outcomes, we included an additional random intercept, νijk, to account for repeated measure and within-individual correlation over time. We conducted all statistical analyses using Stata version 15 (StataCorp, College Station TX, USA). We reported the results following the CONSORT guidelines [31], including the presentation of both relative and absolute effect sizes (using the margins post-estimation command) and the intracluster correlation coefficient (ICC) per arm (taking the rho coefficient of models run separately by arm, or using the estat post-estimation command with multilevel models).
We assessed heterogeneous treatment effects by fitting models that included an interaction term between an arm and prespecified effect modifiers at each time point separately (to facilitate the interpretation of interaction effects; prespecified analysis) and during the three-year period overall (controlling for the linear effect of time; post-hoc analysis). We used likelihood ratio tests to determine if there was evidence to reject the assumption of no interaction/effect modification. As potential modifiers, baseline cluster population size and distance to PHC were chosen to critically examine design features of Mali’s community health strategy [29], which recommends one CHW per 700 people only in villages more than five kilometres away from a PHC. Household wealth was chosen to permit an equity sub-analysis, examining differential effects for children living in households in the poorest wealth quintile.
We conducted a prespecified per-protocol subgroup analysis by excluding (from the main model/equation above) child-year observations in the intervention arm if no female respondent in the household reported receiving at least two CHW home visits in the month preceding the survey, and then by additionally excluding child-year observations in the control arm if any female respondent in the household reported a home visit in the last month.
The main intervention effect models used complete-case analysis. However, due to missing treatment data at the 24-month time point caused by a data capture coding error, we performed multiple imputation by chained equations (MICE) in sensitivity analyses on related outcomes: primary outcome, any prompt treatment, recommended case management, and prompt, recommended case management. Furthermore, because missing outcome data exceeded the predefined 10% threshold for the 24-month subset, we performed MICE prior to assessing heterogeneous treatment effects at 24 months. Due to correlation between outcomes, we ran separate MICE models, each generating 20 imputed data sets. We included all variables and interaction terms that appeared in one or more subsequent regression analyses and two auxiliary variables (any treatment received and CHW care received) associated with missing data. Due to strong clustering for outcomes and missing data, we were unable to impute data separately by cluster or include indicator variables for clusters. Instead, we captured between-cluster variability by including all baseline cluster-level covariates and outcome summaries when creating imputations.
Patient and public involvement
We involved national and district level authorities from the Malian Ministry of Health and Social Development in the study design, implementation, and dissemination. We chose research questions (including an embedded costing analysis) and outcomes for the trial (including the primary outcome on under-five mortality) that were of key interest to our government partners. We also involved national and district health authorities in study site selection, including both the rural district within the country and the seven PHC catchment areas within the district. Within each catchment area, we held public consultation meetings with community representatives, including village chiefs and their advisors, women’s and youth association leaders, religious leaders, and politico-administrative authorities (such as mayors, PHC directors, and ASACOs), where we discussed and obtained verbal permission to conduct the trial. Communities nominated CHW candidates who participated in the training and provided a fixed health site for control arm CHWs, as well as a house if the CHW was not a resident of the village-cluster.
Once we conducted the analysis on the trial’s primary and secondary endpoints, including child health and access to care, we held results dissemination workshops with local, district, regional, and national level stakeholders, starting at the district and local level with community representatives (as listed above), including study CHWs and their dedicated supervisors.
Role of the funding source
The funders had no role in study design, data collection, analysis, interpretation, or writing of this paper. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Ethics
The trial received ethical approval from the Faculty of Medicine, Pharmacy and Odonto-Stomatology Ethics Committee at the Université des Sciences, des Techniques et des Technologies of Bamako (Ref: 2016/03/CE/FMPOS). Secondary analysis of trial data was approved by the Observational/Interventions Research Ethics Committee at the London School of Hygiene & Tropical Medicine (Ref: 13832) and exempted by the University of California, San Francisco (Ref: 154824)
RESULTS
Baseline data collection covered 137 clusters, censused 99 576 people, and surveyed 15 884 women of reproductive age who provided outcome data on 15 855 children under five years (Figure S2 in the Online Supplementary Document). Clusters , children under five years of age, sick children under five years, and children aged 3-59 months with iCCM illnesses had similar characteristics between arms at baseline (Table 1, Table S1-S3 in the Online Supplementary Document). All clusters contributed observations to the analysis. However, between the 12- and 24-month surveys, due to escalating violent conflict in the study area, three intervention and three control clusters, all relatively small and remote , were lost to follow-up (Table S4 and Figure S2 in the Online Supplementary Document). Sample characteristics were similar between observations with complete vs missing outcome data (Tables S5-S7 in the Online Supplementary Document). Analyses included 46 789 child-year observations, 20 105 sick child-year observations, and 15 278 child-year observations with iCCM illnesses during the three-year trial period. Among all child-year observations, 57% were repeated measures on the same child; 28% of sick child-year and 22% of child-year observations with iCCM illnesses were repeated measures.
Table 1.
Baseline cluster-level characteristics and summaries of the outcomes of interest*
| Intervention | Control | |
|---|---|---|
|
Characteristics, n (%)
|
n = 69 clusters |
n = 68 clusters |
| Population size, median (IQR) |
532 (305.0-1087.0) |
564 (243.5-984.0) |
| <700 |
38 (55.1) |
40 (58.8) |
| ≥700 |
31 (44.9) |
28 (41.2) |
| Distance from PHC in kilometres, median (IQR) |
6.3 (4.2-8.6) |
5.8 (3.5-8.6) |
| ≤5.0 |
28 (40.6) |
29 (42.7) |
| >5.0 |
41 (59.4) |
39 (57.4) |
| Topography |
|
|
| None |
63 (91.3) |
64 (94.1) |
| On clifftop |
1 (1.5) |
2 (2.9) |
| PHC inaccessible during rainy season (June, July, August) |
5 (7.3) |
2 (2.9) |
| CHW services available† |
|
|
| None |
51 (73.9) |
51 (75.) |
| Satellite village |
14 (20.3) |
14 (20.6) |
| Posted village |
4 (5.8) |
3 (4.4) |
| PHC catchment area |
|
|
| Dimbal |
15 (21.7) |
15 (22.1) |
| Lessagou |
14 (20.3) |
12 (17.7) |
| Doundé |
8 (11.6) |
7 (10.3) |
| Ende |
2 (2.9) |
3 (4.4) |
| Soubala |
11 (15.9) |
13 (19.1) |
| Kanibozon |
9 (13.0) |
8 (11.8) |
| Koulongon |
10 (14.5) |
10 (14.7) |
|
Outcomes, median (IQR)
|
|
|
| Prevalence |
n = 69 clusters |
n = 68 clusters |
| Fever |
0.12 (0.05-0.24) |
0.12 (0.06-0.22) |
| Diarrhoea |
0.14 (0.08-0.28) |
0.16 (0.08-0.26) |
| Cough |
0.10 (0.06-0.15) |
0.11 (0.04-0.18) |
| Suspected pneumonia |
0.03 (0-0.05) |
0.03 (0.00-0.05) |
| Health care utilisation, median (IQR) |
n = 67 clusters‡ |
n = 68 clusters |
| Prompt treatment within health sector |
0.19 (0.09-0.31) |
0.19 (0.06-0.27) |
| Any prompt treatment |
0.48 (0.29-0.60) |
0.45 (0.33-0.55) |
| Health sector evaluation |
0.21 (0.14-0.37) |
0.20 (0.08-0.33) |
| Any care |
0.55 (0.38-0.68) |
0.50 (0.42-0.66) |
| Recommended case management, median (IQR) |
n = 67 clusters |
n = 68 clusters |
| Recommended case management |
0.21 (0.93-0.30) |
0.16 (0.08-0.27) |
| Prompt, recommended case management | 0.16 (0.08-0.25) | 0.12 (0.05-0.21) |
CHW – community health care workers, IQR – interquartile range, PHC – primary health centre
*Data are presented as n (%) unless otherwise specified.
†CHWs are stationed/posted in some communities at baseline and may also serve members from neighbouring/satellite communities.
‡There are two intervention clusters with no sick children at baseline.
Prompt treatment within the health sector increased from a median of 19% across all clusters at baseline to 61% at 12 months, 44% at 24 months, and 52% at 36 months, with similar trends in both arms (Figure 1). Similarly, one in five children at baseline received health sector evaluation, which increased to two-thirds at 12 and 24 months and over one-half at 36 months across arms. Recommended case management also increased two-fold compared to baseline, and similarly between arms (Figure 1), but did not reach half of the children with iCCM illnesses during the trial. Whether sick children received any prompt treatment (from any source) or any care varied considerably between clusters at baseline, but less so during the trial, reaching as many as 66% or 72%, respectively, at 12 months across arms.
Figure 1.
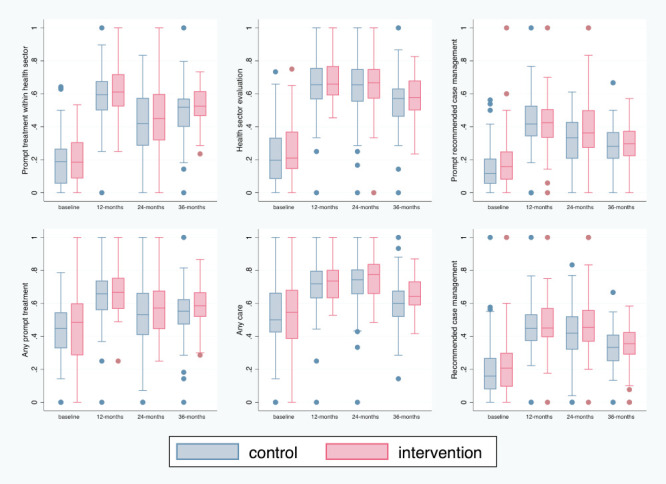
Box plots representing the variability in cluster summaries of the primary and secondary health care utilisation outcomes in intervention and control arms at each time point.
At the 12-month follow-up, the odds of receiving prompt treatment within the health sector were 22% higher in intervention compared to control clusters (AORCS = 1.22; 95% confidence interval (CI) = 1.06, 1.41, P = 0.005) (Table 2). At 12 months, children in intervention clusters were 4.7 percentage points more likely to receive prompt health sector treatment than those in control clusters (adjusted risk difference (ARD) = 0.047; 95% CI = 0.014, 0.080). However, there was no evidence of an intervention effect at 24 or 36 months. Findings were similar for any prompt treatment. Furthermore, the results were consistent in sensitivity analyses dealing with missing data, including multiple imputation (Table S8 in the Online Supplementary Document). The ICC for the primary outcome was 0.017 (95% CIs = 0.010, 0.029) in the intervention arm and 0.019 (95% CI = 0.010, 0.035) in the control arm.
Table 2.
Cluster-specific intervention effects on primary and secondary health care utilisation outcomes, including absolute risks in each arm, during the three-year trial period overall and at each follow-up time point*
| ARC | ARI | C vs I, AORCS (95% CI) | P-value | |
|---|---|---|---|---|
|
Prompt treatment within the health sector (n = 18 765)
|
|
|||
| Overall† |
0.52 |
0.55 |
1.10 (0.98-1.24) |
0.103 |
| Time point‡ |
|
|||
| 12 mo |
0.58 |
0.62 |
1.22 (1.06-1.41) |
0.005 |
| 24 mo |
0.46 |
0.45 |
0.99 (0.85-1.15) |
0.887 |
| 36 mo |
0.52 |
0.54 |
1.08 (0.94-1.25) |
0.290 |
| ICC |
|
|||
| Control |
|
|
0.019 (0.010-0.035) |
|
| Intervention |
|
|
0.017 (0.010-0.029) |
|
| LR test |
|
|
|
0.016 |
|
Any prompt treatment (n = 18 753)
|
|
|||
| Overall† |
0.59 |
0.61 |
1.12 (1.00-1.25) |
0.054 |
| Time point‡ |
|
|||
| 12 mo |
0.64 |
0.69 |
1.24 (1.08-1.42) |
0.003 |
| 24 mo |
0.55 |
0.55 |
0.98 (0.85-1.13) |
0.792 |
| 36 mo |
0.56 |
0.59 |
1.13 (0.98-1.30) |
0.100 |
| ICC |
|
|||
| Control |
|
|
0.016 (0.008-0.032) |
|
| Intervention |
|
|
0.013 (0.007-0.025) |
|
| LR test |
|
|
|
0.009 |
|
Health sector evaluation (n = 20 088)
|
|
|||
| Overall† |
0.62 |
0.64 |
1.12 (0.99-1.26) |
0.072 |
| Time point‡ |
|
|||
| 12 mo |
0.63 |
0.67 |
1.19 (1.03-1.38) |
0.016 |
| 24 mo |
0.65 |
0.66 |
1.06 (0.91-1.22) |
0.463 |
| 36 mo |
0.57 |
0.60 |
1.10 (0.95-1.27) |
0.216 |
| ICC |
|
|||
| Control |
|
|
0.020 (0.011-0.036) |
|
| Intervention |
|
|
0.019 (0.011-0.033) |
|
| LR test |
|
|
|
0.232 |
|
Any care (N = 20 104)
|
|
|||
| Overall† |
0.69 |
0.72 |
1.15 (1.03-1.28) |
0.017 |
| Time point‡ |
|
|||
| 12 mo |
0.70 |
0.74 |
1.20 (1.04-1.38) |
0.010 |
| 24 mo |
0.73 |
0.75 |
1.07 (0.92-1.23) |
0.386 |
| 36 mo |
0.63 |
0.66 |
1.17 (1.01-1.34) |
0.032 |
| ICC |
|
|||
| Control |
|
|
0.017 (0.009-0.032) |
|
| Intervention |
|
|
0.014 (0.007-0.027) |
|
| LR test |
|
|
|
0.282 |
|
Recommended case management (n = 14 613)
|
|
|||
| Overall† |
0.41 |
0.42 |
1.08 (0.96-1.21) |
0.208 |
| Time point‡ |
|
|||
| 12 mo |
0.46 |
0.47 |
1.07 (0.92-1.25) |
0.399 |
| 24 mo |
0.39 |
0.41 |
1.14 (0.97-1.34) |
0.109 |
| 36 mo |
0.38 |
0.38 |
1.02 (0.87-1.20) |
0.812 |
| ICC |
|
|||
| Control |
|
|
0.010 (0.003-0.028) |
|
| Intervention |
|
|
0.008 (0.003-0.022) |
|
| LR test |
|
|
|
0.5361 |
|
Prompt, recommended case management (n = 14 612)
|
|
|||
| Overall† |
0.36 |
0.37 |
1.09 (0.97-1.22) |
0.164 |
| Time point‡ |
|
|||
| 12 mo |
0.42 |
0.44 |
1.08 (0.93-1.26) |
0.332 |
| 24 mo |
0.31 |
0.34 |
1.21 (1.03-1.42) |
0.024 |
| 36 mo |
0.33 |
0.33 |
0.98 (0.83-1.16) |
0.787 |
| ICC |
|
|||
| Control |
|
|
0.008 (0.023- 0.025) |
|
| Intervention |
|
|
0.011 (0.005- 0.026) |
|
| LR test | 0.1106 | |||
AORCS – cluster-specific adjusted odds ratio, LR – likelihood ratio, ARC – absolute risk of events in the control arm, ARI – absolute risk of events in the intervention arm, C – control clusters, CI – confidence interval, I – intervention clusters, ICC – intracluster correlation coefficient, mo – months
*Two regression models are presented here: regression model 1 controlled for the time effect t = 1, 2, 3, to estimate the intervention effect during the three-year follow-up period overall. Regression model 2 included the interaction term ηit that estimated the intervention effect at each time point. The likelihood ratio test corresponds to the interaction term in model 2. Adjusted models controlled for child’s age (0-11, 12-23, 24-35, 36-59 mo) and sex; baseline cluster-level summary of the outcome; baseline cluster-level summary of household wealth (quintiles), mother’s decision-making power (any, none), and mother’s mobility (none, dependent mobility, independent mobility), which were deemed imbalanced at baseline and likely risk factors; PHC catchment area and cluster distance to PHC (coded as a continuous variable in the models for prompt treatment within the health sector, any prompt treatment, prompt, recommended case management, and pneumonia where the relationship with distance was linear, and otherwise coded as a dichotomous variable using a five-kilometre cut-off), which were the variables on which randomisation was stratified; and symptom (fever, diarrhoea with no blood, cough with fast breathing, combination), only for recommended case management outcomes.
†Regression model 1.
‡Regression model 2.
The results suggested no differential effect by time point for health sector evaluation and any care, although the largest effects were seen at 12 months (Table 2). During the three-year period overall, the odds of receiving any health sector evaluation was 12% higher in intervention compared to control clusters (AORCS = 1.12; 95% CI = 0.99, 1.26, P = 0.072), corresponding to an absolute difference of 2.5 percentage points (ARD = 0.025; 95% CI = -0.002, 0.052). Results were similar for any care. There was no evidence of an effect on recommended case management or prompt, recommended case management. We did not find statistical evidence for effect modification by cluster size, distance to PHC, or household wealth. However, estimated magnitudes suggest that the intervention may have been more effective in improving prompt treatment within the health sector (Table 3) and access to care across outcomes and time points (Tables S9-S10 in the Online Supplementary Document) in smaller, more remote clusters, and in the poorest households.
Table 3.
Heterogeneous treatment effects by cluster population size, cluster distance to nearest PHC, and household wealth on the primary outcome, prompt treatment within the health sector, during the three-year trial period overall*
| ARC | ARI | C vs I, AORCS (95% CI) | P-value | |
|---|---|---|---|---|
|
Cluster distance to PHC
|
|
|
|
|
| ≤5.0 km |
0.54 |
0.55 |
1.01 (0.84-1.22) |
0.918 |
| >5.0 km |
0.50 |
0.54 |
1.18 (1.01-1.38) |
0.039 |
| LR test |
|
|
|
0.2193 |
|
Cluster population size
|
|
|
|
|
| <700 people |
0.53 |
0.57 |
1.18 (0.99-1.41) |
0.072 |
| ≥700 |
0.51 |
0.53 |
1.07 (0.91-1.24) |
0.419 |
| LR test |
|
|
|
0.4132 |
|
Household wealth†
|
|
|
|
|
| Less poor |
0.53 |
0.55 |
1.08 (0.95-1.22) |
0.243 |
| Poorest |
0.49 |
0.54 |
1.23 (1.03-1.46) |
0.022 |
| LR test | 0.1000 |
PHC – public health centre, LR – likelihood ratio, AORCS – cluster-specific adjusted odds ratio, ARC – absolute risk of events in the control arm, ARI – absolute risk of events in the intervention arm, C – control clusters, CI – confidence interval, I – intervention clusters
*We ran three separate models, one for each of the predefined effect modifiers that included an interaction term between treatment arm and the modifier. We report the results of the LR tests for interaction between arm and modifier in each model. All models controlled for the same covariates as the main model for overall effects during the three-year trial period; we removed the baseline cluster-level summary of wealth in the models that assessed heterogeneous effects by this variable at the household level.
†For 20% of sick child-year observations included in the analysis, their household wealth was measured during the follow-up period rather than at baseline. The co-intervention in both arms to remove user fees could have influenced household wealth in the follow-up period.
During the trial, 47% of sick child-year observations met the per-protocol definition (at least two CHW home visits in the preceding month) in the intervention arm, while 78% met the definition (no CHW home visits in the preceding month) in the control arm (Table S11 in the Online Supplementary Document). The proportion that met the per-protocol definition waned over time in the intervention arm (53% at 12, 49% at 24, 39% at 36 months) and increased in the control arm (72% at 12, 81% at 24, 83% at 36 months). Restricted to the per-protocol subgroup, the intervention effect on prompt treatment within the health sector increased to 45% higher odds at 12 months (AORCS = 1.43; 95% CI = -1.21, 1.69, P < 0.001) and 22% over the three years (AORCS = 1.22; 95% CI = -1.06, 1.40, P = 0.005), compared to control clusters (Table 4). Health sector evaluation odds were 29% higher in the intervention compared to control clusters over the three years (AORCS = 1.29; 95% CI = -1.12, 1.48, P < 0.001). Per-protocol analyses also yielded significant effects over the three years on recommended case management (AORCS = 1.20; 95% CI = -1.06, 1.37, P = 0.005). Results were consistent whether or not we excluded control arm observations that did not meet per-protocol (Table S12 in the Online Supplementary Document).
Table 4.
Per-protocol subgroup estimates for the primary and secondary health care utilisation outcomes, excluding observations in the intervention arm that did not receive at least two CHW home visits in the month preceding the survey, during the three-year trial period overall and at each follow-up time point*
| Outcome | ARC | ARI | C vs I, AORCS (95% CI) | P-value |
|---|---|---|---|---|
|
Prompt treatment within the health sector (n = 13 500)
|
|
|||
| Overall† |
0.52 |
0.57 |
1.22 (1.06-1.40) |
0.005 |
| Time point‡ |
|
|
|
|
| 12 mo |
0.58 |
0.66 |
1.43 (1.21-1.69) |
<0.001 |
| 24 mo |
0.46 |
0.48 |
1.07 (0.89-1.28) |
0.453 |
| 36 mo |
0.52 |
0.55 |
1.13 (0.94-1.35) |
0.184 |
| LR test |
|
|
|
0.0036 |
|
Any prompt treatment (n = 13 493)
|
|
|||
| Overall† |
0.59 |
0.64 |
1.26 (1.09-1.44) |
0.001 |
| Time point‡ |
|
|
|
|
| 12 mo |
0.64 |
0.72 |
1.43 (1.21-1.69) |
<0.001 |
| 24 mo |
0.55 |
0.58 |
1.10 (0.92-1.32) |
0.315 |
| 36 mo |
0.56 |
0.61 |
1.23 (1.03-1.47) |
0.023 |
| LR test |
|
|
|
0.0205 |
|
Health sector evaluation (n = 14 518)
|
|
|||
| Overall† |
0.62 |
0.68 |
1.29 (1.12-1.48) |
<0.001 |
| Time point‡ |
|
|
|
|
| 12 mo |
0.63 |
0.70 |
1.38 (1.17-1.65) |
<0.001 |
| 24 mo |
0.65 |
0.71 |
1.30 (1.09-1.55) |
0.003 |
| 36 mo |
0.58 |
0.61 |
1.17 (0.97-1.39) |
0.094 |
| LR test |
|
|
|
0.1736 |
|
Any care (n = 14 527)
|
|
|||
| Overall† |
0.69 |
0.75 |
1.35 (1.17-1.55) |
<0.001 |
| Time point‡ |
|
|
|
|
| 12 mo |
0.70 |
0.76 |
1.37 (1.16-1.63) |
<0.001 |
| 24 mo |
0.73 |
0.79 |
1.35 (1.13-1.62) |
0.001 |
| 36 mo |
0.63 |
0.69 |
1.31 (1.10-1.57) |
0.003 |
| LR test |
|
|
|
0.8945 |
|
Recommended case management (n = 10 569)
|
|
|||
| Overall† |
0.42 |
0.45 |
1.20 (1.06-1.37) |
0.005 |
| Time point‡ |
|
|
|
|
| 12 mo |
0.46 |
0.49 |
1.19 (0.99-1.42) |
0.061 |
| 24 mo |
0.39 |
0.45 |
1.35 (1.12-1.63) |
0.001 |
| 36 mo |
0.38 |
0.39 |
1.06 (0.86-1.30) |
0.592 |
| LR test |
|
|
|
0.1435 |
|
Prompt, recommended case management (n = 10 569)
|
|
|||
| Overall† |
0.36 |
0.39 |
1.15 (1.01-1.32) |
0.040 |
| Time point‡ |
|
|
|
|
| 12 mo |
0.43 |
0.46 |
1.19 (0.99-1.43) |
0.062 |
| 24 mo |
0.31 |
0.36 |
1.26 (1.04-1.53) |
0.017 |
| 36 mo |
0.33 |
0.33 |
0.99 (0.80-1.22) |
0.890 |
| LR test | 0.1280 | |||
AORCS – cluster-specific adjusted odds ratio, ARC – absolute risk of events in the control arm, ARI – absolute risk of events in the intervention arm, C – control clusters, CI – confidence interval, I – intervention clusters, ICC – intracluster correlation coefficient, LR – likelihood ratio, mo – months
*Two regression models are presented here: regression model 1 controlled for the time effect t = 1, 2, 3, to estimate the intervention effect during the three-year follow-up period overall. Regression model 2 included the interaction term ηit that estimated the intervention effect at each time point. The likelihood ratio test corresponds to the interaction term in model 2. Adjusted models controlled for child’s age (0-11, 12-23, 24-35, 36-59 mo) and sex; baseline cluster-level summary of the outcome; baseline cluster-level summary of household wealth (quintiles), mother’s decision-making power (any, none), and mother’s mobility (none, dependent mobility, independent mobility), which were deemed imbalanced at baseline and likely risk factors; PHC catchment area and cluster distance to PHC (coded as a continuous variable in the models for prompt treatment within the health sector, any prompt treatment, prompt, recommended case management, and pneumonia where the relationship with distance was linear, and otherwise coded as a dichotomous variable using a five-kilometre cut-off), which were the variables on which randomisation was stratified; and symptom (fever, diarrhoea with no blood, cough with fast breathing, combination), only for recommended case management outcomes.
†Regression model 1.
‡Regression model 2.
Finally, infectious disease prevalence increased in both arms compared to baseline, two-fold for cough and suspected pneumonia (Figure S3 in the Online Supplementary Document). There was no intervention effect on any disease prevalence during the three years overall, although the odds of cough and suspected pneumonia were 1.16 times (95% CI = 1.04, 1.30) or 2.2 percentage points and 1.22 times (95% CI = 1.07, 1.40) or 1.6 percentage points higher, respectively, at 12 months in the intervention compared to control clusters, with consistent results in the per-protocol analyses (Table S13, S16, and S17 in the Online Supplementary Document).
DISCUSSION
Early access to health sector treatment more than doubled for sick children when study communities received care from professional CHWs and upgraded primary care clinics without user fees. In 2018, the Mali DHS found that only 21% and 55% of children under five with fever in the Mopti region received any prompt treatment and any care, respectively [32]. In that same year, our 12-month survey found that any prompt treatment and any care reached two-thirds or more of all sick children under five in the trial area of Mopti. Health care utilisation peaked at 12 months and waned over time, and many sick children still did not receive prompt, health sector, or recommended care. Nevertheless, this overall improvement in child access to care is remarkable in the context of the performance of large-scale iCCM programme [15-17] and the armed conflict that emerged after 12 months in the trial area, imposing challenges to delivering and receiving services. It is in this redesigned health system context that the results between arms on the effects of proactive CHW home visits should be interpreted.
Proactive CHW service delivery improved early health sector treatment further, compared to the fixed approach, after 12 months, but not after 24 or 36 months of implementation. These findings suggest that home visits were most important during the first year after launching the redesigned CHW-led health system, possibly by mobilising care-seeking, reinforcing the importance of prompt treatment, or building trust in the health system. After more than a year of experiencing accessible, high-quality care without fees, control communities with fixed CHWs may have themselves mobilised, adopted rapid care-seeking, and gained trust in the system, though not as quickly. There was some evidence that, over all three years, proactive CHW service delivery improved access to health sector evaluation and any care, suggesting that home visits may have helped to overcome persistent indirect cost, distance, or social barriers to care, even where fixed CHW services were available without fees. Subgroup estimates suggested that proactive home visits may improve child access to care best in smaller communities, where a CHW can achieve greater home visit coverage, in those farther from a PHC, where utilisation was lowest at baseline [27], and in the poorest households, by overcoming indirect costs to even frontline services or women’s limited resources to make health care decisions. Although these subgroup results should be interpreted with caution, they may contribute to the evidence that home visits enhance equity benefits of CHW programmes, along with the important equity impacts of free, proximal, quality service provision [33,34].
For maternal health care, our analysis of other secondary trial endpoints (reported elsewhere) [35] found that proactive CHW home visits increased the likelihood of first trimester antenatal care (ANC) by 11% (risk ratio (RR) = 1.11; 95% CI = 1.02, 1.19) and of four or more ANC visits by 25% (RR = 1.25; 95% CI = 1.08, 1.43), but had no effect on institutional delivery (RR=1.06; 95% CI = 0.91, 1.20). Across trial arms relative to baseline, any ANC attendance increased by 83% (RR = 1.83; 95% CI = 1.78, 1.86), first trimester ANC by 15% (RR = 1.15; 95% CI = 1.06, 1.25), four or more ANC visits by 2.6 times (RR = 2.59; 95% CI = 2.28, 2.91), and institutional delivery by 54% (RR = 1.54; 95% CI = 1.41, 1.66) [35]. These maternal care results are consistent with the child health care utilisation results insomuch that the bulk of the improvements occurred across both arms, with the proactive service delivery intervention yielding modest incremental benefits, which are nonetheless important for achieving timely, universal health coverage.
CHW adherence to the proactive workflow protocol, as reported at survey time points by respondents, reached only half of sick children in the intervention arm and waned over time. This could be intervention fatigue or the conflict making the proactive workflow difficult to deliver. This likely biased ITT intervention effect estimates towards the null, as per-protocol subgroup analyses showed stronger magnitudes and significance of effects across children’s utilisation outcomes at 12 months and during the trial overall. These findings suggest that had households in the intervention arm received a proactive CHW home visit at least once every two weeks throughout the trial period, home visits may have had more effect on children’s health care utilisation.
The proactive service delivery intervention effects found in this trial should be understood within the context of the co-interventions in both trial arms, including user fee removal, professional CHWs, and upgraded primary care clinics. Proactive CHW home visits’ effects may be different in other health system or social contexts. Our forthcoming process evaluation paper used mixed methods to elucidate the implementation, mechanisms, and context of the proactive home visits and co-interventions in both arms and to help to explain these trial outcome results (unpublished data).
Child morbidity, measured as disease prevalence, did not decrease over time or more so in the intervention arm as we expected it to. Rather, reported prevalence of all four illnesses increased during the trial period compared to baseline (descriptive), and cough and suspected pneumonia increased statistically at 12 months in intervention compared to control clusters. These increases could reflect mothers’ improved illness recognition given CHW care and, additionally, home visits. Mothers who received routine counselling during home visits on disease prevention, illness recognition, and rapid care-seeking may have been more likely during the first year than their control arm counterparts to recognise cough as an illness and fast breathing as an alert, and thus report it during a survey. Our study did not measure progression or severity of disease, which may link health care utilisation to survival in the pathway of change, and this is a limitation. In Ghana, home visits by volunteer CHWs focusing on health education, but who also tested febrile children for malaria and treated childhood diarrhoea with ORS, had no effect on the prevalence of these illnesses (primary outcomes) or case detection/management, compared to no volunteer CHWs [36]. Although our trial also did not find expected reductions in the prevalence of these illnesses, we did find that recommended case management of iCCM illnesses doubled during our intervention of paid, professional CHWs, compared to baseline.
With its randomised design, large number of clusters, and rigorous, baseline, and repeated outcome measurement, this trial addressed common risks of bias found in studies in this domain [24]. Contamination between arms is an important concern and could have occurred because CHWs did not always adhere to their workflow protocol; co-interventions may have triggered mechanistic pathways of proactive home visits, such as supervisor house calls without the CHW or community mobilisation by village chiefs; or study participants could have migrated between clusters. The armed conflict that emerged led to devastating death and displacement, contributing to our loss to follow-up, but all clusters and participants contributed data to the analysis. We also had missing treatment data for some sick children at 24 months, which is an important limitation, but our complete-case analysis results were robust to multiple imputation.
CONCLUSIONS
This analysis showed that proactive CHW service delivery can improve the timeliness of children’s curative treatment within the first year of implementing a redesigned CHW-led health system, and may increase sick children’s health care utilisation relative to a fixed CHW approach. In the context of user fee removal, professional CHWs, and upgraded primary care clinics, proactive CHW home visits yielded modest improvements in access to child and maternal health care. While policy-makers, public health practitioners, and clinicians may consider proactive home visits to be a low-cost intervention for optimising CHW programmes, the UHC and equity impact they seek will be primarily driven by health system enablers, such as user fee removal, professional CHWs, and reinforced primary care clinics.
Additional material
Acknowledgements
We would like to acknowledge all trial community members for their involvement in this research. We are grateful to all the health-care delivery staff, including CHWs, dedicated CHW supervisors, and PHC personnel, without whom the trial would not have been possible. We thank our partners at the Malian Ministry of Health and Social Development at district, regional, and national levels. We are grateful to Belco Poudiougou, Saibou Doumbia, Mahamadou Sogoba, Ousmane Koné, Yacouba Samaké, and Boni M Ale of Muso who coordinated study activities at different points during the trial, as well as Amadou Beydi Cissé, Youssouf Keita, Aminata dite Nene Konipo, and Seydou Sidibé who were part of the Muso team managing programme implementation. Kaledou Doumbia and Caleb Dembélé contributed to the study site selection process. We thank Idrissa Kamara, Lamine Guindo, Mahamadou Sylla, Matt Britton, Jane Yang, Faith Cole, Hailey Zuverink, Sasha Rozenshteyn, David Boettiger, Rakesh Ghosh, and Calvin Chiu for their invaluable contributions to cleaning and processing survey panel data. We are grateful to our partners at: IC4D (Issa Diarra, Boubacar Diaroumba, Alhousseyni Toure, Alhassane Toure, Hugor De Sthaël Mombo) who programmed the survey in Open Data Kit; Mass Design Group who designed PHC rehabilitation; Medic Mobile who developed the CHW mobile application; and the national, regional, and district health authorities of the government of Mali. We appreciate the attention our DSMB (Nick Jewell, Sandra McCoy, Grant Dorsey, Issaka Sagara, and their statistician Tom Hoffman) and Clinical Research Associate, Pharmalys (Victorine Mensah, Peya Gaye) accorded our trial.
Data availability: Household survey panel data that is de-identified can be made available to external researchers upon reasonable request to Muso, by contacting Ari Johnson ajohnson@musohealth.org; Ari.Johnson@ucsf.edu.
Footnotes
Funding: Funding for the trial was provided to Muso by USAID Development Innovation Ventures, CRI Foundation, Grand Challenges Canada, and Johnson & Johnson Foundation.
Authorship contributions: KK, AJ, CW, JL, ET, DD, and MG designed the trial. AJ acquired funding for the trial, with grant writing and reporting help from JL, ET, CW, DD, MBT, and KK. NK, MBT, KK, and CW trained and supervised data collectors. KK, CW, and AJ provided trial supervision and DD, MG, MB, and MC provided intervention oversight. ET and NK managed trial data, with input from MBT, CW, KK, and JL. CW developed the statistical methods for the analysis, with input from CL, ET, DC, BG, AJ, KK, and JL. CW analysed the data, with validation by CL and ET. CW generated data visualisations and wrote the manuscript. All authors helped interpret results or edited the manuscript directly. All authors reviewed and approved the final version for publication.
Disclosure of interest: The authors completed the ICMJE Disclosure of Interest Form (available upon request from the corresponding authors) and declare the following activities and relationships: AJ, CW, DD, KK, MBT, NK were employed by Muso at the time of the trial. JL and ET received grants from Muso to contribute to the trial. Muso designed and implemented the trial and the intervention evaluated. Muso received funding to support the trial from USAID Development Innovation Ventures, CRI Foundation, Grand Challenges Canada, and Johnson & Johnson Foundation. MC, MB, and MG were employed by the government of Mali during the trial period. CL is supported by the United Kingdom Medical Research Council (Skills Development Fellowship MR/T032448/1).
REFERENCES
- 1.Perry HB, Hodgins S.Health for the People: Past, Current, and Future Contributions of National Community Health Worker Programs to Achieving Global Health Goals. Glob Health Sci Pract. 2021;9:1-9. 10.9745/GHSP-D-20-00459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Young M, Wolfheim C, Marsh DR, Hammamy D.World Health Organization/United Nations Children’s Fund joint statement on integrated community case management: an equity-focused strategy to improve access to essential treatment services for children. Am J Trop Med Hyg. 2012;87:6-10. 10.4269/ajtmh.2012.12-0221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rasanathan K, Muñiz M, Bakshi S, Kumar M, Solano A, Kariuki W, et al. Community case management of childhood illness in sub-Saharan Africa - findings from a cross-sectional survey on policy and implementation. J Glob Health. 2014;4:020401-020401. 10.7189/jogh.04.020401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennett S, George A, Rodriguez D, Shearer J, Diallo B, Konate M, et al. Policy challenges facing integrated community case management in Sub-Saharan Africa. Trop Med Int Health. 2014;19:872-82. 10.1111/tmi.12319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lewin S, Munabi-Babigumira S, Glenton C, Daniels K, Bosch-Capblanch X, Van Wyk B, et al. Lay health workers in primary and community health care for maternal and child health and the management of infectious diseases. Cochrane Database Syst Rev. 2010;2010:CD004015-004015. 10.1002/14651858.CD004015.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christopher JB, Le May A, Lewin S.Ross D a. Thirty years after Alma-Ata: a systematic review of the impact of community health workers delivering curative interventions against malaria, pneumonia and diarrhoea on child mortality and morbidity in sub-Saharan Africa. Hum Resour Health. 2011;9:27. 10.1186/1478-4491-9-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilmore B, Mcauliffe E.Effectiveness of community health workers delivering preventive interventions for maternal and child health in low- and middle-income countries: a systematic review. BMC Public Health. 2013;13:847. 10.1186/1471-2458-13-847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okwundu CI, Nagpal S, Musekiwa A, Sinclair D.Home- or community-based programmes for treating malaria. Cochrane Database Syst Rev. 2013. 10.1002/14651858.CD009527.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruizendaal E, Dierickx S, Peeters Grietens K, Schallig HD, Pagnoni F, Mens PF.Success or failure of critical steps in community case management of malaria with rapid diagnostic tests: a systematic review. Malar J. 2014;13:229. 10.1186/1475-2875-13-229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Das JK, Lassi ZS, Salam RA, Bhutta ZA.Effect of community based interventions on childhood diarrhea and pneumonia: uptake of treatment modalities and impact on mortality. BMC Public Health. 2013;13:S29. 10.1186/1471-2458-13-S3-S29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sazawal S, Black RE.Effect of pneumonia case management on mortality in neonates, infants, and preschool children: a meta-analysis of community-based trials. Lancet Infect Dis. 2003;3:547-56. 10.1016/S1473-3099(03)00737-0 [DOI] [PubMed] [Google Scholar]
- 12.Theodoratou E, Al-Jilaihawi S, Woodward F, Ferguson J, Jhass A, Balliet M, et al. The effect of case management on childhood pneumonia mortality in developing countries. Int J Epidemiol. 2010;39:i155-71. 10.1093/ije/dyq032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amouzou A, Morris S, Moulton LH, Mukanga D.Assessing the impact of integrated community case management (iCCM) programs on child mortality: Review of early results and lessons learned in sub–Saharan Africa. J Glob Health. 2014;4. 10.7189/jogh.04.020411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bosch–Capblanch X, Marceau C.Training, supervision and quality of care in selected integrated community case management (iCCM) programmes: A scoping review of programmatic evidence. J Glob Health. 2014;4. 10.7189/jogh.04.020403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Munos M, Guiella G, Roberton T, Maïga A, Tiendrebeogo A, Tam Y, et al. Independent Evaluation of the Rapid Scale-Up Program to Reduce Under-Five Mortality in Burkina Faso. Am J Trop Med Hyg. 2016;94:584-95. 10.4269/ajtmh.15-0585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amouzou A, Hazel E, Shaw B, Miller NP, Tafesse M, Mekonnen Y, et al. Effects of the integrated Community Case Management of Childhood Illness Strategy on Child Mortality in Ethiopia: A Cluster Randomized Trial. Am J Trop Med Hyg. 2016;94:596-604. 10.4269/ajtmh.15-0586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amouzou A, Kanyuka M, Hazel E, Heidkamp R, Marsh A, Mleme T, et al. Independent Evaluation of the integrated Community Case Management of Childhood Illness Strategy in Malawi Using a National Evaluation Platform Design. Am J Trop Med Hyg. 2016;94:574-83. 10.4269/ajtmh.15-0584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oliphant NP, Manda S, Daniels K, Odendaal WA, Besada D, Kinney M, et al. Integrated community case management of childhood illness in low- and middle-income countries. Cochrane Database Syst Rev. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization. WHO guideline on health policy and system support to optimize community health worker programmes. Geneva: World Health Organization; 2018. [PubMed] [Google Scholar]
- 20.Mousa A, Al-Taiar A, Anstey NM, Badaut C, Barber BE, Bassat Q, et al. The impact of delayed treatment of uncomplicated P. falciparum malaria on progression to severe malaria: A systematic review and a pooled multicentre individual-patient meta-analysis. PLOS Medicine. 2020;17:e1003359. 10.1371/journal.pmed.1003359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Terra de Souza AC, Peterson KE, Andrade FM, Gardner J, Ascherio A.Circumstances of post-neonatal deaths in Ceara, Northeast Brazil: mothers’ health care-seeking behaviors during their infants’ fatal illness. Soc Sci Med. 2000;51:1675-93. 10.1016/S0277-9536(00)00100-3 [DOI] [PubMed] [Google Scholar]
- 22.Källander K, Hildenwall H, Waiswa P, Galiwango E, Peterson S, Pariyo G.Delayed care seeking for fatal pneumonia in children aged under five years in Uganda: a case-series study. Bull World Health Organ. 2008;86:332-8. 10.2471/BLT.07.049353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Waiswa P, Peterson S, Tomson G, Pariyo GW.Using the three delays model to understand why newborn babies die in eastern Uganda. Trop Med Int Health. 2010;15:964-72. 10.1111/j.1365-3156.2010.02557.x [DOI] [PubMed] [Google Scholar]
- 24.Whidden C, Thwing J, Gutman J, Wohl E, Leyrat C, Kayentao K, et al. Proactive case detection of common childhood illnesses by community health workers: a systematic review. BMJ Glob Health. 2019;4:e001799. 10.1136/bmjgh-2019-001799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whidden C, Treleaven E, Liu J, Padian N, Poudiougou B, Bautista-Arredondo S, et al. Proactive community case management and child survival: protocol for a cluster randomised controlled trial. BMJ Open. 2019;9. 10.1136/bmjopen-2018-027487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boettiger DC, Treleaven E, Kayentao K, Guindo M, Coumaré M, Johnson AD, et al. Household factors and under-five mortality in Bankass, Mali: results from a cross-sectional survey. BMC Public Health. 2021;21:244. 10.1186/s12889-021-10242-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Treleaven E, Whidden C, Cole F, Kayentao K, Traoré MB, Diakité D, et al. Relationship between symptoms, barriers to care and healthcare utilisation among children under five in rural Mali. Trop Med Int Health. 2021;26:943-52. 10.1111/tmi.13592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caille A, Kerry S, Tavernier E, Leyrat C, Eldridge S, Giraudeau B.Timeline cluster: a graphical tool to identify risk of bias in cluster randomised trials. BMJ. 2016;354:i4291. 10.1136/bmj.i4291 [DOI] [PubMed] [Google Scholar]
- 29.Ministère de la Santé et de l’Hygiène Publique. Plan Stratégique National des Soins Essentiels dans la Communauté 2016-2020. 2015. Available: https://www.unicef.org/mali/rapports/plan-strategicque-national-des-soins-essentiels-dans-la-communaute. Accessed: 14 April 2023.
- 30.Whidden C, Kayentao K, Liu JX, Lee S, Keita Y, Diakité D, et al. Improving Community Health Worker performance by using a personalised feedback dashboard for supervision: a randomised controlled trial. J Glob Health. 2018;8:020418. 10.7189/jogh.08.020418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Campbell MK, Piaggio G, Elbourne DR, Altman DG, CONSORT Group Consort 2010 statement: extension to cluster randomised trials. BMJ. 2012;345:e5661. 10.1136/bmj.e5661 [DOI] [PubMed] [Google Scholar]
- 32.Institut National de la Statistique (INSTAT). [Demographic and Health Survey in Mali 2018]. 2019. Available: https://dhsprogram.com/pubs/pdf/FR358/FR358.pdf. Accessed: 14 April 2023. [Italian].
- 33.McCollum R, Gomez W, Theobald S, Taegtmeyer M.How equitable are community health worker programmes and which programme features influence equity of community health worker services? A systematic review. BMC Public Health. 2016;16:419–419. 10.1186/s12889-016-3043-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blanchard AK, Prost A, Houweling TAJ.Effects of community health worker interventions on socioeconomic inequities in maternal and newborn health in low-income and middle-income countries: a mixed-methods systematic review. BMJ Global Health. 2019;4:e001308. 10.1136/bmjgh-2018-001308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kayentao K, Ghosh R, Guindo L, Whidden C, Treleaven E, Chiu C, et al. Effect of community health worker home visits on antenatal care and institutional delivery: an analysis of secondary outcomes from a cluster randomised trial in Mali. BMJ Glob Health. 2023;8:e011071. 10.1136/bmjgh-2022-011071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma Y, Sudfeld CR, Kim H, Lee J, Cho Y, Awppnor-Williams JK, et al. Evaluating the impact of community health volunteer home visits on child diarrhea and fever in the Volta Region, Ghana: A cluster-randomized controlled trial. PLoS Med. 2019;16:e1002830. 10.1371/journal.pmed.1002830 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


