Abstract
Background
Several risk factors of immune checkpoint inhibitors (ICIs)-associated acute kidney injury (AKI) have been reported sporadically. To identify the risk factors of ICIs-associated AKI in a large-scale population, therefore we conducted a systematic review and a real-world retrospective study.
Methods
We search literature concerning risk factors of ICIs-associated AKI in ClinicalTrials.gov and electronic databases (PubMed, Cochrane Library, Embase) up to January 2022. Meta-analysis was performed by using odds ratios (ORs) with 95%CIs. In a separate retrospective pharmacovigilance study by extracting data from US FDA Adverse Event Reporting System (FAERS) database, disproportionality was analyzed using the reporting odds ratio (ROR).
Results
A total of 9 studies (5927 patients) were included in the meta-analysis. The following factors were associated with increased risk of ICIs-associated AKI, including proton pump inhibitors(PPIs) (OR = 2.07, 95%CI 1.78–2.42), angiotensin-converting enzyme inhibitors (ACEIs)/ angiotensin receptor blockers (ARBs) (OR = 1.56, 95%CI 1.24–1.95), nonsteroidal anti-inflammatory drugs (NSAIDs) (OR = 1.29, 95%CI 1.01–1.65), diuretics (OR = 2.00, 95%CI 1.38–2.89), diabetes mellitus (OR = 1.28, 95%CI 1.04–1.57), genitourinary cancer (OR = 1.46, 95%CI 1.15–1.85), combination therapy of ICIs (OR = 1.93, 95%CI 1.25–2.97) and extrarenal immune-related adverse events(irAEs) (OR = 2.51, 95%CI 1.96–3.20). Furthermore, analysis from FAERS database verified that concurrent exposures of PPIs (ROR = 2.10, 95%CI 1.91–2.31), ACEIs/ARBs (ROR = 3.25, 95%CI 2.95–3.57), NSAIDs (ROR = 3.06, 95%CI 2.81–3.32) or diuretics (ROR = 2.82, 95%CI 2.50–3.19) were observed significant signals associated with AKI in ICIs-treated patients.
Conclusions
Concurrent exposures of PPIs, ACEIs/ARBs, NSAIDs or diuretics, diabetes mellitus, genitourinary cancer, combination therapy, and extrarenal irAEs seem to increase the risk of AKI in ICIs-treated patients.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12882-023-03171-9.
Keywords: Immune checkpoint inhibitor, Acute kidney injury, Systematic review, FDA Adverse Event Reporting System, Real-world pharmacovigilance
Introduction
Strategies with immune checkpoint inhibitors (ICIs) to treat malignancy have been adopted with a significant improvement in patients' prognosis in recent years. However, ICIs might induce a series of immune-related adverse events(irAEs) due to the unrestricted activation of the immune system and the off-target mode of these drugs [1]. Among the irAEs, an increased risk of acute kidney injury(AKI) was reported with ICIs [2].The incidence of ICIs-associated AKI was 0.8%-4.7% obtained from randomized controlled trials(RCTs) and observational studies [2–6]. Though the incidence of ICIs-associated AKI is not high, it usually leads to discontinuation of the suspicious drugs and add-on immunosuppressive therapy [7, 8], which may add complexity to the anti-tumor therapeutic course.
Several risk factors of ICIs-associated AKI have been reported recently, including the concurrent drugs of proton pump inhibitors (PPIs), angiotensin-converting enzyme inhibitors (ACEIs)/ angiotensin receptor blockers (ARBs) or diuretics, combination therapy with different ICIs, use of ipilimumab or pembrolizumab, coexisting chronic kidney disease (CKD) or low estimated glomerular filtration rate(eGFR), hypertension, the combination of other irAEs and so on [3–5, 9–14]. Early recognition of risk factors may help to reduce the incidence of AKI in ICIs-treated patients. But the above results were only based on every single research.
To identify the risk factors of ICIs-associated AKI in a large-scale population, therefore we conducted a systematic review of observational studies and a real-world study by extracting data on concurrent drugs from the US Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS) database.
Patients and methods
Study design and data sources
Firstly, a meta-analysis of observational studies was conducted to investigate the risk factors of AKI in patients treated with ICIs. Secondly, a real-world analysis was performed by extracting data from the FAERS database to further verified the risk of concurrent drugs of ICI-associated AKI in a large-scale population.
Systematic review and meta-analysis
This study was registered prospectively in PROSPERO (CRD42021293326). The meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statement [15].
Search strategy
The search for literature was conducted in the following databases: PubMed, Embase, the Cochrane Library, and ClinicalTrials.gov. We performed a systematic literature review of observational studies about ICIs-associated AKI (up to 10th January 2022). The terms searched for literature were that combined “immune checkpoint inhibitor” or “ipilimumab” or “tremelimumab” or “pembrolizumab” or “nivolumab” or “cemiplimab” or “atezolizumab” or “durvalumab” or “avelumab” with “acute kidney injury” or “AKI” or “acute renal failure” or “renal failure acute”.
Inclusion and exclusion criteria
The inclusion criteria were as follows: 1) observational studies that compared clinical data of patients with versus without AKI after ICIs administration. The definitions and severity of AKI in each included study were demonstrated in supplementary table 1; 2) literature published in English language. Articles that did not meet our requirements (systematic reviews, case reports, case series, comments, protocols, animal studies, and in vitro studies) or lack of full text were excluded, as well as those that did not report the baseline characteristics. We also removed duplicate publications and articles comparing ICIs-associated AKI with AKI caused by other reasons.
Data extraction and qualitative assessment
After duplicate records were removed, all abstracts were screened by two independent reviewers (P.C and J.Z), and potential eligible articles were searched for full texts. These two review authors also independently extracted data from each study that fulfilled all the criteria. Discrepancies were resolved by a third investigator (M.F). The following information was collected: 1) Sample size; 2) Baseline characteristics: median age with interquartile range (IQR) or mean age with standard deviation (SD), gender, race, baseline eGFR; 3) ICI regimens; 4) Cancer types (melanoma, genitourinary cancer, lung cancer, head and neck cancer); 5) Comorbidities such as diabetes, hypertension, congestive heart failure, chronic obstructive pulmonary disease(COPD), liver disease; 6) Concurrent medications of PPIs, ACEIs/ARBs, nonsteroidal anti-inflammatory drugs(NSAIDs) and diuretics. Quality assessment of each included study was simultaneously performed by two authors(P.C and J.Z) with the Newcastle–Ottawa Scale (NOS) [16]. Discrepancies were resolved by a third investigator (M.F).
Statistical analysis
Risk factors associated with ICIs-associated AKI were assessed with odds ratio (OR) with 95% confidence interval (CI). The I2 statistic was used to present between-study heterogeneity, which of > 50% was considered substantial heterogeneity. Random-effect model was utilized when significant heterogeneity existed. Statistical analyses were performed with STATA software (version 16.0).
Pharmacovigilance study
The real-world pharmacovigilance study was based on the US FAERS database, which defines adverse drug reactions using Preferred Terms from the Medical Dictionary for Regulatory Activities (MedDRA, version 23.1). We only used the MedDRA Preferred Term “Acute kidney injury” to identify AKI cases as previous studies [17–19]. Moreover, brand names or generic names of ICIs, along with names of targeted concurrent drugs (PPIs, ACEIs/ARBs, NSAIDs, and diuretics) were exploited to identify interested records. Brand names and generic names of all searched drugs were shown in supplementary table 2. Data included in our study is recorded from January 2011 to September 2021. Information was extracted including reporter, age, gender, reported year, reported country, ICI drug name, concurrent medication, the indication of ICIs, and AKI event.
Disproportionality analysis was applied to assess whether AKI was differentially reported in ICIs-treated patients with or without target concurrent drugs. Reporting odds ratio (ROR) was utilized as reported previously [18, 20]. ROR was marked as a significant signal when the lower limit of the 95% CI (ROR025) exceeded 1 and with at least 3 reports of AKI simultaneously. Statistical analyses were performed with Microsoft Excel (2021, Microsoft).
Results
Systematic review and meta-analysis
Study selection
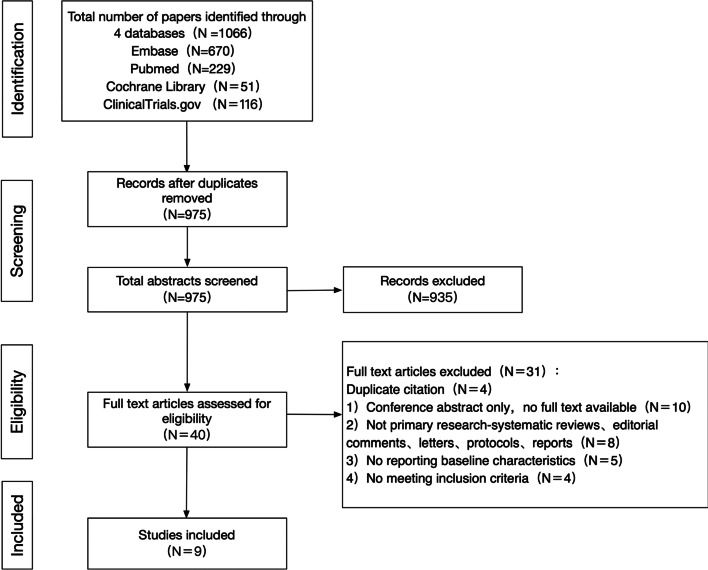
As demonstrated in Fig. 1, the initial search yielded 1066 articles. After duplicate records were removed, the number of studies was reduced to 975, then 935 studies were excluded based on titles and abstracts. Full text of 40 articles was reviewed for further investigation. Finally, 9 studies reporting a total of 5927 ICIs-treated cancer patients were included in our systematic review and meta-analysis.
Fig. 1.
Flow chart of study selection for systemic review
Study characteristics
Detailed characteristics of the included studies were summarized in Table 1. Among the 9 eligible studies, 3 studies [3, 5, 12] were reported from the United States, 1 from Canada [10], 1 from France [11], 1 from Japan [13], 1 from Netherlands[4], 1 from North America (including the United States and Canada) [9], and the other from multiregional centers (including North America, Europe, and Asia) [14].
Table 1.
Characteristics of included studies
| Study | Year | Author's country | Sample Size, N | Male, N(%) | Age, Years, Mean ± SD or Median(IQR) | Baseline eGFR, mL/min/1.73 m2, Mean ± SD or Median(IQR) |
ICIs-related AKI, N (%) | Cases underwent renal biopsy, N (%) |
|---|---|---|---|---|---|---|---|---|
| Meraz-Muñoz [10] | 2020 | Canada | 309 | 186(60.2) | 61(51–69) | 88(75–99) | 51(16.5) | 6(11.8) |
| Abdelrahim [3] | 2021 | USA | 1664 | 1098(66.0) | NA | NA | 72(4.3) | 25(34.7) |
| Seethapathy [5] | 2019 | USA | 1016 | 616(60.6) | 63 ± 13 | 82 ± 22 | 169(16.6) | 1(0.6) |
| Cortazar [9] | 2020 | USA | 414 | 254(61.4) | NA | NA | 138(33.3) | 60(43.5) |
| Koks [4] | 2021 | Netherlands | 676 | 420(62.1) | 64(53–71) | 90(75–101) | 96(14.2) | 1 (1.0) |
| Shimamura [13] | 2021 | Japan | 152 | 114(75.0) | 67 ± 10 | 72(55–87) | 27(17.8) | 1 (3.7) |
| Stein [11] | 2020 | France | 239 | 132(55.2) | 68(58.5–77) | 84(70–94) | 41(17.1) | 3(7.3) |
| Seethapathy [12] | 2020 | USA | 599 | 298(49.7) | 65 ± 13 | 88 ± 26 | 36(6.0) | 1(2.8) |
| Gupta [14] | 2021 | USA | 858 | 517(60.3) | NA | NA | 429(50.0) | 151(35.2) |
ICI Immune checkpoint inhibitor, AKI Acute kidney injury, SD Standard deviation, IQR Interquartile range, NA Not available
Risk of bias assessment
Of these 9 included studies, 5 studies attained a score of 8 with NOS method, 2 studies of 7, 1 study of 6, and 1 study of 5 (Table 2).
Table 2.
Quality assessment of eligible studies with the Newcastle–Ottawa Scale
| Study | Selection | Comparability | Outcome | Scores | |||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||
| Meraz-Muñoz A 2020 [10] | ✔ | ✔ | ✔ | ✔✔ | ✔ | ✔ | ✔ | 8 | |
| Abdelrahim 2021 [3] | ✔ | ✔ | ✔✔ | ✔ | ✔ | ✔ | 7 | ||
| Seethapathy 2019 [5] | ✔ | ✔ | ✔ | ✔✔ | ✔ | ✔ | ✔ | 8 | |
| Cortazar 2020 [9] | ✔ | ✔ | ✔✔ | ✔ | ✔ | ✔ | 7 | ||
| Koks 2021 [4] | ✔ | ✔ | ✔ | ✔✔ | ✔ | ✔ | ✔ | 8 | |
| Shimamura 2021[13] | ✔ | ✔ | ✔ | ✔✔ | ✔ | ✔ | ✔ | 8 | |
| Stein 2020 [11] | ✔ | ✔✔ | ✔ | ✔ | ✔ | 6 | |||
| Seethapathy 2020 [12] | ✔ | ✔ | ✔ | ✔ | ✔ | 5 | |||
| Gupta 2021 [14] | ✔ | ✔ | ✔ | ✔✔ | ✔ | ✔ | ✔ | 8 | |
1 Representativeness of the exposed cohort
2 Selection of the non-exposed cohort
3 Ascertainment of exposure
4 Demonstration that outcome of interest was not present at start of study
5 Comparability of cohorts on the basis of the design or analysis
6 Assessment of outcome
7 Was follow-up long enough for outcomes to occur
8 Adequacy of follow up of cohorts
Risk factors of ICI-associated AKI
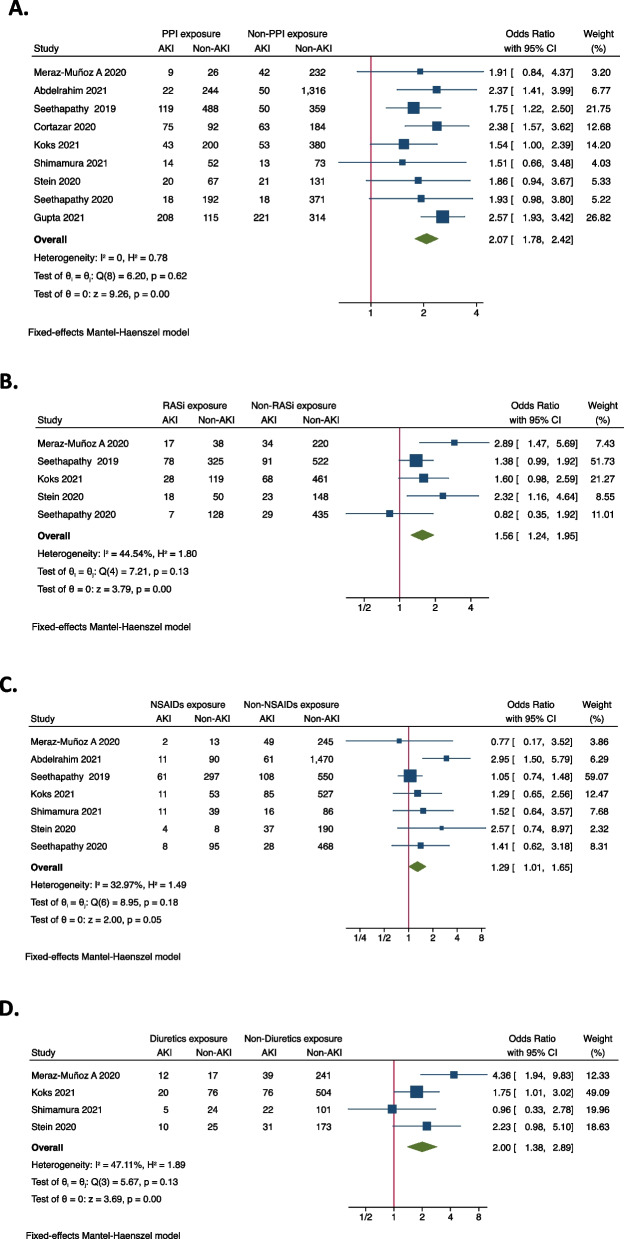
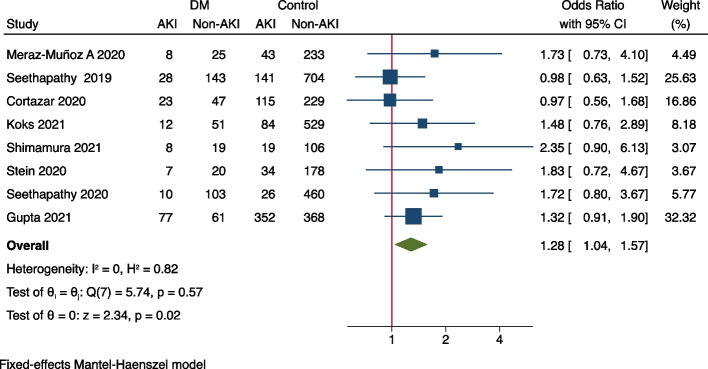
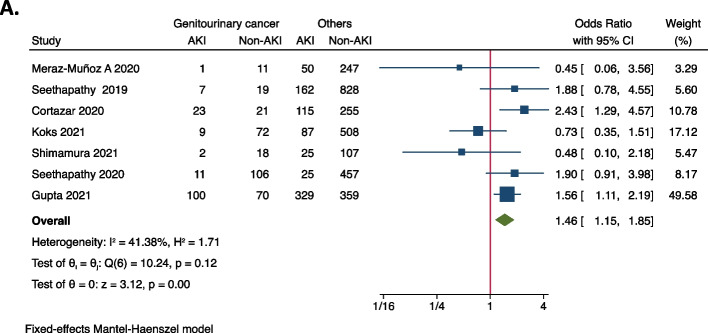
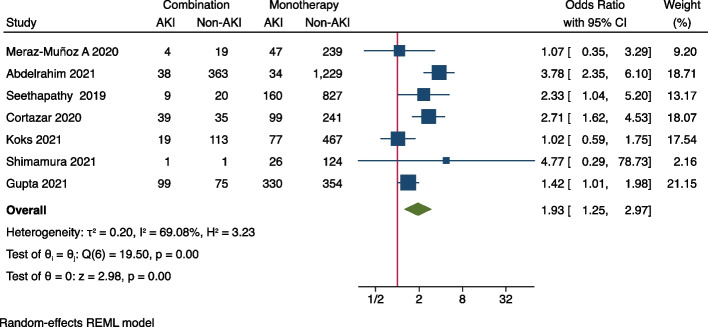
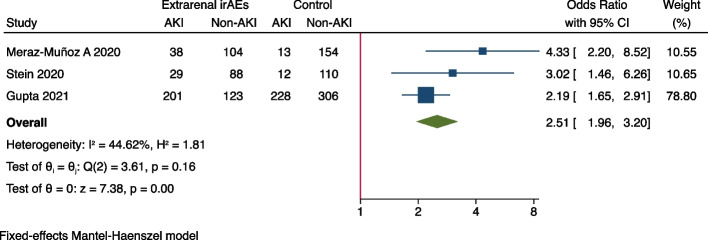
Meta-analysis was performed to identify risk factors of ICI-associated AKI. As demonstrated in Fig. 2, concurrent exposures of PPIs (OR = 2.07, 95%CI 1.78–2.42), ACEIs/ARBs (OR = 1.56, 95%CI 1.24–1.95), NSAIDs (OR = 1.29, 95%CI 1.01–1.65), and diuretics (OR = 2.00, 95%CI 1.38–2.89) were all associated with the increased occurrence of AKI in ICIs-treated patients. As for comorbidities, it was shown that coexisting diabetes mellitus (OR = 1.28, 95%CI 1.04–1.57) seems to increase the risk of ICIs-associated AKI (Fig. 3), while hypertension (OR = 1.30, 95%CI 0.91–1.85), congestive heart failure (OR = 1.26, 95%CI 0.69–2.29), liver disease (OR = 1.13, 95%CI 0.67–1.90) and COPD (OR = 0.63, 95%CI 0.26–1.51) were not significantly associated with AKI (Supplementary Fig. 1). In terms of original cancer, it was found that genitourinary cancer (OR = 1.46, 95%CI 1.15–1.85) was associated with an increased risk of AKI in ICI-treated patients (Fig. 4), whilst lung cancer (OR = 0.80, 95%CI 0.66–0.96) and melanoma didn’t increase the risk (Supplementary Fig. 2). None of any ICI category (anti-PD-1, anti-PD-L1, or anti-CTLA-4) was more likely to cause AKI than the others (Supplementary Fig. 3), but the combination of anti-PD-1/ PD-L1 with anti-CTLA-4 (OR = 1.93, 95%CI 1.25–2.97) was found to be significantly correlated with AKI (Fig. 5). It was also worth noting that extrarenal irAEs (OR = 2.51, 95%CI 1.96–3.20) were correlated with an elevated risk of AKI (Fig. 6). Otherwise, the male sex (OR = 1.00, 95%CI 0.86–1.16) and white race (OR = 0.90, 95%CI 0.70–1.16) showed no correlation with AKI in ICIs-treated patients (Supplementary Fig. 4).
Fig. 2.
Forest plot of associations between concurrent drugs exposures with risk AKI in patients with ICIs. PPI Proton pump inhibitors, ACEI Angiotensin-converting enzyme inhibitor, ARB Angiotensin receptor blocker, NSAID Nonsteroidal anti-inflammatory drug, AKI Acute kidney injury, ICIs Immune checkpoint inhibitors
Fig. 3.
Forest plot of association between DM and AKI in ICIs-treated patients. DM Diabetes mellitus, AKI Acute kidney injury, ICIs Immune checkpoint inhibitors
Fig. 4.
Forest plot of associations between genitourinary cancer and AKI in ICIs-treated patients. AKI Acute kidney injury, ICIs Immune checkpoint inhibitors
Fig. 5.
Forest plot of associations between combination therapy and AKI in ICIs-treated patients. AKI Acute kidney injury, ICIs Immune checkpoint inhibitors
Fig. 6.
Forest plot of associations between extra-renal irAEs and AKI in ICIs-treated patients. irAEs Immune checkpoint inhibitor related adverse events, AKI Acute kidney injury, ICIs Immune checkpoint inhibitors
Pharmacovigilance study of FAERS
Descriptive analysis
A total of 135,531 adverse events related to ICIs were extracted from FAERS database up to September 2021, with AKI of 2948. The baseline characteristics of these reports were summarized in Table 3. Cases were majorly reported by healthcare professionals, accounting for 83.6%. Most cases were reported from the United States (41.7%) and Japan (15.5%). Male cases account for 62.1% while old patients (≥ 65 years old) for 53.6%. The most common indication for ICIs was lung cancer (28.6%), followed by melanoma (22.1%) and renal carcinoma (8.0%). With the increasing application of ICIs in clinical practice, the number of reported cases also grows.
Table 3.
Baseline characteristics of ICIs-treated patients with or without AKI events reported from FAERS database
| Characteristics | All cases (N = 135,531) | AKI (N = 2948) | Non-AKI (N = 132,583) |
|---|---|---|---|
| Reporter | |||
| Total data | 135,186 | 2945 | 132,241 |
| Healthcare professional | 112,981(83.6%) | 2841(96.5%) | 110,140(83.3%) |
| Non-healthcare professional | 22,205(16.4%) | 104(3.5%) | 22,101(16.7%) |
| Sex | |||
| Total data | 119,777 | 2810 | 116,967 |
| Male | 74,398(62.1%) | 1882(67.0%) | 72,516(62.0%) |
| Female | 45,379(37.9%) | 928(33.0%) | 44,451(38.0%) |
| Age | |||
| Total data | 95,464 | 2679 | 92,785 |
| < 65YR | 44,321(46.4%) | 1074(40.1%) | 43,247(46.6%) |
| ≥ 65YR | 51,143(53.6%) | 1605(59.9%) | 49,538(53.4%) |
| Reported countries | |||
| Total data | 135,118 | 2938 | 132,180 |
| United States, US | 56,363(41.7%) | 1112(37.8%) | 55,251(41.8%) |
| Japan, JP | 20,905(15.5%) | 269(9.2%) | 20,636(15.6%) |
| France, FR | 11,520(8.5%) | 468(15.9%) | 11,052(8.4%) |
| Germany, DE | 7422(5.5%) | 278(9.5%) | 7144(5.4%) |
| Canada, CA | 4755(3.5%) | 58(2.0%) | 4697(3.6%) |
| United Kingdom, GB | 3600(2.7%) | 157(5.3%) | 3443(2.6%) |
| Australia, AU | 3122(2.3%) | 55(1.9%) | 3067(2.3%) |
| Italy, IT | 3012(2.2%) | 63(2.1%) | 2949(2.2%) |
| China, CN | 2613(1.9%) | 11(0.4%) | 2602(2.0%) |
| Spain, ES | 2504(1.9%) | 76(2.6%) | 2428(1.8%) |
| Other countries | 19,302(14.3%) | 391(13.3%) | 18,911(14.3%) |
| Indications | |||
| Total data | 135,531 | 2948 | 132,583 |
| Lung cancer | 38,720(28.6%) | 755(25.6%) | 37,965(28.6%) |
| Melanoma | 29,989(22.1%) | 787(26.7%) | 29,202(22.0%) |
| Renal cancer | 10,888(8.0%) | 330(11.2%) | 10,558(8.0%) |
| Hepatocellular carcinoma | 2768(2.0%) | 69(2.3%) | 2699(2.0%) |
| Head and neck cancer | 2651(2.0%) | 44(1.5%) | 2607(2.0%) |
| Gastric cancer | 2436(1.8%) | 41(1.4%) | 2395(1.8%) |
| Bladder cancer | 2065(1.5%) | 97(3.3%) | 1968(1.5%) |
| Colorectal cancer | 2034(1.5%) | 54(1.8%) | 1980(1.5%) |
| Other cancers | 43,980(32.5%) | 771(26.2%) | 43,209(32.6%) |
ICI Immune checkpoint inhibitor, AKI Acute kidney injury
Disproportionality analysis
ICIs therapy with concurrent exposures of PPIs (ROR = 2.10, 95%CI 1.91–2.31), ACEIs/ARBs (ROR = 3.25, 95%CI 2.95–3.57), NSAIDs (ROR = 3.06, 95%CI 2.81–3.32) and diuretics (ROR = 2.82, 95%CI 2.50–3.19) were observed significant signals associated with AKI (Table 4).
Table 4.
Disproportional analysis of concurrent medications with AKI
| Category | Total (N) | a (N) | b (N) | c (N) | d (N) | ROR | ROR025 | ROR975 |
|---|---|---|---|---|---|---|---|---|
| PPIs exposure | ||||||||
| ICIs as a class | 12,676 | 516 | 12,160 | 2432 | 120,423 | 2.10 | 1.91 | 2.31 |
| Anti-PD-1 | 6016 | 323 | 5693 | 1492 | 79,096 | 3.01 | 2.66 | 3.40 |
| Nivolumab | 3327 | 209 | 3118 | 976 | 51,415 | 3.53 | 3.03 | 4.12 |
| Pembrolizumab | 2605 | 113 | 2492 | 504 | 27,364 | 2.46 | 2.00 | 3.03 |
| Cemiplimab | 84 | 1 | 83 | 12 | 317 | |||
| Anti-PD-L1 | 3599 | 101 | 3498 | 362 | 17,493 | 1.40 | 1.12 | 1.74 |
| Atezolizumab | 2400 | 75 | 2325 | 273 | 10,721 | 1.27 | 0.98 | 1.64 |
| Avelumab | 223 | 4 | 219 | 43 | 1579 | 0.67 | 0.24 | 1.89 |
| Durvalumab | 976 | 22 | 954 | 46 | 5193 | 2.60 | 1.56 | 4.35 |
| Anti-CTLA-4 | 3061 | 92 | 2969 | 578 | 23,834 | 1.28 | 1.02 | 1.60 |
| Ipilimumab | 2572 | 80 | 2492 | 564 | 22,914 | 1.30 | 1.03 | 1.65 |
| Tremelimumab | 489 | 12 | 477 | 14 | 920 | 1.65 | 0.76 | 3.60 |
| ACEI/ARBs exposure | ||||||||
| ICIs as a class | 9261 | 548 | 8713 | 2400 | 123,870 | 3.25 | 2.95 | 3.57 |
| Anti-PD-1 | 4690 | 332 | 4358 | 1483 | 80,431 | 4.13 | 3.65 | 4.67 |
| Nivolumab | 2555 | 229 | 2326 | 956 | 52,207 | 5.38 | 4.63 | 6.25 |
| Pembrolizumab | 2063 | 100 | 1963 | 517 | 27,893 | 2.75 | 2.21 | 3.42 |
| Cemiplimab | 72 | 3 | 69 | 10 | 331 | 1.44 | 0.39 | 5.37 |
| Anti-PD-L1 | 2298 | 85 | 2213 | 378 | 18,778 | 1.91 | 1.50 | 2.42 |
| Atezolizumab | 1478 | 66 | 1412 | 282 | 11,634 | 1.93 | 1.47 | 2.54 |
| Avelumab | 209 | 8 | 201 | 39 | 1597 | 1.63 | 0.75 | 3.54 |
| Durvalumab | 611 | 11 | 600 | 57 | 5547 | 1.78 | 0.93 | 3.42 |
| Anti-CTLA-4 | 2273 | 131 | 2142 | 539 | 24,661 | 2.80 | 2.30 | 3.40 |
| Ipilimumab | 1967 | 122 | 1845 | 522 | 23,561 | 2.98 | 2.44 | 3.66 |
| Tremelimumab | 306 | 9 | 297 | 17 | 1100 | 1.96 | 0.87 | 4.44 |
| NSAIDs exposure | ||||||||
| ICIs as a class | 14,407 | 765 | 13,642 | 2183 | 118,941 | 3.06 | 2.81 | 3.32 |
| Anti-PD-1 | 6539 | 487 | 6052 | 1328 | 78,737 | 4.77 | 4.29 | 5.31 |
| Nivolumab | 3668 | 377 | 3291 | 808 | 51,242 | 7.26 | 6.40 | 8.25 |
| Pembrolizumab | 2721 | 104 | 2617 | 513 | 27,239 | 2.11 | 1.70 | 2.62 |
| Cemiplimab | 150 | 6 | 144 | 7 | 256 | 1.52 | 0.50 | 4.62 |
| Anti-PD-L1 | 4120 | 122 | 3998 | 341 | 16,993 | 1.52 | 1.23 | 1.88 |
| Atezolizumab | 2614 | 94 | 2520 | 254 | 10,526 | 1.55 | 1.22 | 1.97 |
| Avelumab | 396 | 9 | 387 | 38 | 1411 | 0.86 | 0.41 | 1.80 |
| Durvalumab | 1110 | 19 | 1091 | 49 | 5056 | 1.80 | 1.05 | 3.06 |
| Anti-CTLA-4 | 3748 | 156 | 3592 | 514 | 23,211 | 1.96 | 1.63 | 2.35 |
| Ipilimumab | 3150 | 146 | 3004 | 498 | 22,402 | 2.19 | 1.81 | 2.64 |
| Tremelimumab | 598 | 10 | 588 | 16 | 809 | 0.86 | 0.39 | 1.91 |
| Diuretics Exposure | ||||||||
| ICIs as a class | 5513 | 305 | 5208 | 2643 | 127,375 | 2.82 | 2.50 | 3.19 |
| Anti-PD-1 | 2452 | 163 | 2289 | 1652 | 82,500 | 3.56 | 3.01 | 4.20 |
| Nivolumab | 1237 | 112 | 1125 | 1073 | 53,408 | 4.96 | 4.04 | 6.07 |
| Pembrolizumab | 1154 | 49 | 1105 | 568 | 28,751 | 2.24 | 1.67 | 3.02 |
| Cemiplimab | 61 | 2 | 59 | 11 | 341 | |||
| Anti-PD-L1 | 1702 | 64 | 1638 | 399 | 19,353 | 1.90 | 1.45 | 2.48 |
| Atezolizumab | 1208 | 44 | 1164 | 304 | 11,882 | 1.48 | 1.07 | 2.04 |
| Avelumab | 120 | 6 | 114 | 41 | 1684 | 2.16 | 0.90 | 5.20 |
| Durvalumab | 374 | 14 | 360 | 54 | 5787 | 4.17 | 2.29 | 7.57 |
| Anti-CTLA-4 | 1359 | 78 | 1281 | 592 | 25,522 | 2.63 | 2.06 | 3.35 |
| Ipilimumab | 1173 | 72 | 1101 | 572 | 24,305 | 2.78 | 2.16 | 3.58 |
| Tremelimumab | 186 | 6 | 180 | 20 | 1217 | 2.03 | 0.80 | 5.12 |
a cases reported of AKI with target concurrent drugs;
b cases reported of non-AKI with target concurrent drugs;
c cases reported of AKI with any other concurrent drugs;
d cases reported of non-AKI with any other concurrent drugs
PPI Proton pump inhibitor, ACEI Angiotensin-converting enzyme inhibitor, ARB Angiotensin receptor blocker, NSAIDs Non-steroid anti-inflammatory drug; AKI: acute kidney injury
ROR Reporting odds ratio
ROR025 The lower limit of the 95% confidence interval (CI) of the ROR
ROR975 The upper limit of the 95% confidence interval (CI) of the ROR
Discussion
Until now, the present study seems to be the first study to identify risk factors of ICIs-associated AKI by a systematic review of clinical studies and analysis of data from a worldwide pharmacovigilance database. Our study suggested that concurrent drugs exposure (PPIs, ACEIs/ARBs, NSAIDs, and diuretics), coexisting diabetes mellitus, genitourinary cancers, combination therapy of ICIs, and extrarenal irAEs were associated with increased risk of AKI events in ICIs-treated cancer patients.
PPIs have been reported to increase the risk of AKI in the general population with an abundance of evidence [21]. Deposition of PPI metabolite in the renal tubular area and interstitium may cause acute interstitial nephritis and acute kidney injury [22]. In cancer patients with ICIs, the concomitant medication of PPIs was revealed to be a risk factor of ICIs-associated AKI in several publications [3, 5, 9, 14], but negative results were reported as well [4, 10–13]. In our study, both results of the systematic review (including 9 studies) and analysis data from FARES suggested that concomitant medication of PPIs increased the risk of ICIs-associated AKI, indicating the caution of PPI use in cancer patients treated with ICIs. Analyzed from FAERS database, PPIs exposure in drugs of anti-PD1 as a class, anti-PDL1, and anti-CTLA4 had positive signals with AKI. Regarding of individual drugs, PPIs exposure in only nivolumab, pembrolizumab, durvalumab and ipilimumab had significant positive signals with AKI, whilst negative in other ICIs. Owing to certain relatively new drugs (cemiplimab, avelumab, tremelimumab) being approved not for a long time, the clinical results of these drugs were limited, so further investigation is still needed.
ACEIs and ARBs both have effects on vasodilation of the renal efferent arterioles thus causing a reduction of glomerular filtration pressure. During the hypovolemia state, the reduced efferent vascular tone as described above may induce AKI [23]. Nevertheless, the evidence of ACEIs/ARBs directly leading to AKI is lacking [24]. Several guidelines still recommend to withhold ACEIs/ARBs during certain acute states, such as sepsis, hypovolemia, or hypotension [24]. Whether the concomitant use of ACEIs/ARBs is a risk factor of ICIs-associated AKI is still controversial based on the published studies [4, 5, 10–12]. In our study, both of the results suggested that exposure to ACEIs/ARBs is related to the increased risk of ICIs-associated AKI. From the FAERS database, regardless of the ICIs types (anti-PD1, anti-PDL1 or anti-CTLA4), ACEIs/ARBs exposure had significant positive signals with AKI. According to individual drugs, results were nearly similar with PPIs, while nivolumab, pembrolizumab, atezolizumab, and ipilimumab show positive results. These results indicate that ACEIs/ARBs would better be replaced by other anti-hypertensive agents to reduce the risk of AKI when accompanied by ICIs.
NSAIDs were reported to be correlated with an increased risk of AKI in the general population in both children and adults. The possible reasons were as follows: NSAIDs can reduce renal blood flow; and may cause tubular obstruction owing to crystal deposition then induce direct cytotoxicity and cell-mediated immune attack [25, 26]. Among the published studies in which NSAIDs were mentioned, only one study found that patients with NSAIDs exposure had a higher incidence of ICIs-associated AKI [3]. In our study we pooled data from 7 studies and found that NSAIDs exposure was associated with an increased risk of ICIs-associated AKI. Positive signals of AKI were also found in ICIs-treated patients with NSAIDs exposure, based on the analysis from FAERS. Our results indicated that, in order to minimize the risk of AKI, avoidance of NSAIDs and ICIs concurrent use is recommended.
Diuretics are reported to be a risk factor of AKI after liver transplantation and related with a higher risk of perioperative AKI [27, 28]. Diuretics use hasn’t been found to correlate with ICIs-AKI depending on existing publications [4, 10, 11, 13]. Here in our present study, we pooled data from 4 observational studies and found that diuretics use increased the risk of ICIs-AKI, though the number of eligible studies was limited. Moreover, results from FARES also supported our results of the meta-analysis mentioned above. It is implicated when diuretics and ICIs are used together, the occurrence of AKI should be vigilant.
Coexisting diabetes mellitus DM is a risk factor of AKI in a certain status, such as infection with coronavirus disease-19, liver transplantation, percutaneous coronary intervention, and so on [29–31]. However, diabetes hasn’t been verified to be an independent risk factor of AKI in patients who received ICIs treatment from individual observational study until now. Through pooling data from 8 studies, we found that coexisting diabetes increased the risk of AKI in patients who received ICIs treatment. It is indicated that monitoring urinalysis and renal function is necessary for diabetic patients treated with ICIs and with significant importance.
AKI was reported to occur in genitourinary cancers with a relatively high incidence [32–34], partially owing to the obstruction of urinary tract and destruction of the kidney. Genitourinary cancer was regarded as a potential risk factor of AKI in patients treated with ICIs in 7 studies, but no positive results were found. By pooling data from these 7 studies, AKI was prone to occur in ICIs-treated patients with genitourinary cancer. It is implied that special awareness should be paid to this subgroup of patients when treated with ICIs.
A combination of anti-CTLA-4 and anti-PD1/PDL1 was found to be an independent risk factor of ICIs-associated AKI in several studies [3, 9], whereas negative results were found in other researches [4, 5, 10, 13, 14]. By pooling data from 7 studies in our meta-analysis, the combination of ICIs was revealed to correlate with increased risk for AKI. The explanation of this phenomenon may be due to the dual immune checkpoint blockage resulting in enhanced stimulation of autoreactive T cells.
Multisystem irAEs could be induced during the treatment with ICIs, mainly attributed to the unrestricted activation of the immune system and the off-target mode of ICIs [1]. The irAEs could occur in the central nervous system, skin, liver, heart, lungs, musculoskeletal system, gastrointestinal tract, and kidneys [8]. Similar to the published observational studies [10], our research also suggested that extrarenal irAEs were correlated with the increased incidence of AKI. Extrarenal irAEs may reflect the degree of the immune system activated by ICIs, hence increasing the possibility of off-target immune reactions in the kidney.
There still be some limitations in our study. Firstly, all of the eligible studies were retrospective; thus, other possible confounding factors may influence the results. Secondly, a specific risk factor only reported in only one study was excluded in the present study due to it is impossible to pool the results in a meta-analysis. Thirdly, the relation between the factors with positive results and ICIs-related AKI could only be association. Further studies still need to be introduced to clarify the causal relationship. What’s more, reporting to FAERS was voluntary, hence the relationship between concurrent drugs (PPIs, ACEI/ARBs, NSAIDs, and diuretics) exposure in patients with ICIs and suspected adverse event (AKI event) was not clear and definite. Also, differences in the definition of AKI were unclear. Besides, detailed clinical information such as PD-1 status, patient’s baseline renal function, or other risk factors related to AKI were missing. Finally, comparisons of incidence for adverse events through a disproportionality analysis might be influenced by many confounding factors, such as reporting bias and lack of denominator data [35].
Following the increasing use of ICIs, AKI related to these new anti-tumor agents have been reported and prompted more investigations. Based on meta-analysis of observational studies and real-world pharmacovigilance study of FAERS, our results suggested that drugs exposure (PPIs, ACEIs/ARBs, NSAIDs, and diuretics), coexisting diabetes mellitus, genitourinary cancers, combination therapy of ICIs and extrarenal irAEs may increase the risk of AKI events in ICI-treated patients. Future studies are needed to investigate the mechanism and optimal management of ICIs-associated AKI.
Supplementary Information
Additional file 1. Supplementary figure.
Additional file 2. Supplementary tables.
Acknowledgements
Not applicable.
Authors’ contributions
Min Feng had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Min Feng. Acquisition of data: Pengwei Chen, Yanchun Xu, Qiuyan Huang, Jianan Su, Ziqing Gao. Analysis and interpretation of data: Pengwei Chen, Jianhong Zhu,Yanchun Xu, Qiuyan Huang, Jianan Su, Ziqing Gao and Min Feng. Drafting of the manuscript: Pengwei Chen, Jianhong Zhu and Min Feng. Statistical analysis: Pengwei Chen and Jianhong Zhu.Revision of the manuscript: Pengwei Chen, Jianhong Zhu and Min Feng. The author(s) read and approved the final manuscript.
Funding
Research grants from National Natural Science Foundation of China (81800595) and Science and Technology Project of Guangdong Province, China (2016A020215068).
Availability of data and materials
Data from the meta-analysis are freely and publicly available on published literature. Data of real-world pharmacovigilance study are collected from the FDA Adverse Event Reporting System, and can be obtained through the FAERS Public Dashboard website.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Pengwei Chen and Jianhong Zhu contributed equally to this work.
References
- 1.Martins F, Sofiya L, Sykiotis GP, et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. 2019;16(9):563–580. doi: 10.1038/s41571-019-0218-0. [DOI] [PubMed] [Google Scholar]
- 2.Manohar S, Kompotiatis P, Thongprayoon C, Cheungpasitporn W, Herrmann J, Herrmann SM. Programmed cell death protein 1 inhibitor treatment is associated with acute kidney injury and hypocalcemia: meta-analysis. Nephrol Dial Transplant. 2019;34(1):108–117. doi: 10.1093/ndt/gfy105. [DOI] [PubMed] [Google Scholar]
- 3.Abdelrahim M, Mamlouk O, Lin H, et al. Incidence, predictors, and survival impact of acute kidney injury in patients with melanoma treated with immune checkpoint inhibitors: a 10-year single-institution analysis. Oncoimmunology. 2021;10(1):1927313. doi: 10.1080/2162402X.2021.1927313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koks MS, Ocak G, Suelmann BBM, et al. Immune checkpoint inhibitor-associated acute kidney injury and mortality: An observational study. PLoS ONE. 2021;16(6):e0252978. doi: 10.1371/journal.pone.0252978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seethapathy H, Zhao S, Chute DF, et al. The Incidence, Causes, and Risk Factors of Acute Kidney Injury in Patients Receiving Immune Checkpoint Inhibitors. Clin J Am Soc Nephrol. 2019;14(12):1692–1700. doi: 10.2215/CJN.00990119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sorah JD, Rose TL, Radhakrishna R, Derebail VK, Milowsky MI. Incidence and Prediction of Immune Checkpoint Inhibitor-related Nephrotoxicity. J Immunother. 2021;44(3):127–131. doi: 10.1097/CJI.0000000000000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brahmer JR, Abu-Sbeih H, Ascierto PA, et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J Immunother Cancer. 2021;9(6):e002435. doi: 10.1136/jitc-2021-002435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Esfahani K, Elkrief A, Calabrese C, et al. Moving towards personalized treatments of immune-related adverse events. Nat Rev Clin Oncol. 2020;17(8):504–515. doi: 10.1038/s41571-020-0352-8. [DOI] [PubMed] [Google Scholar]
- 9.Cortazar FB, Kibbelaar ZA, Glezerman IG, et al. Clinical Features and Outcomes of Immune Checkpoint Inhibitor-Associated AKI: A Multicenter Study. J Am Soc Nephrol. 2020;31(2):435–446. doi: 10.1681/ASN.2019070676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meraz-Munoz A, Amir E, Ng P, et al. Acute kidney injury associated with immune checkpoint inhibitor therapy: incidence, risk factors and outcomes. J Immunother Cancer. 2020;8(1):e000467. doi: 10.1136/jitc-2019-000467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stein C, Burtey S, Mancini J, et al. Acute kidney injury in patients treated with anti-programmed death receptor-1 for advanced melanoma: a real-life study in a single-centre cohort. Nephrol Dial Transplant. 2021;36(9):1664–1674. doi: 10.1093/ndt/gfaa137. [DOI] [PubMed] [Google Scholar]
- 12.Seethapathy H, Zhao S, Strohbehn IA, et al. Incidence and Clinical Features of Immune-Related Acute Kidney Injury in Patients Receiving Programmed Cell Death Ligand-1 Inhibitors. Kidney Int Rep. 2020;5(10):1700–1705. doi: 10.1016/j.ekir.2020.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shimamura Y, Watanabe S, Maeda T, Abe K, Ogawa Y, Takizawa H. Incidence and risk factors of acute kidney injury, and its effect on mortality among Japanese patients receiving immune check point inhibitors: a single-center observational study. Clin Exp Nephrol. 2021;25(5):479–487. doi: 10.1007/s10157-020-02008-1. [DOI] [PubMed] [Google Scholar]
- 14.Gupta S, Short SAP, Sise ME, et al. Acute kidney injury in patients treated with immune checkpoint inhibitors. J Immunother Cancer. 2021;9(10):e003467. doi: 10.1136/jitc-2021-003467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wells G OCD, Peterson J, Welch V, Lossos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp2022).
- 17.Patek TM, Teng C, Kennedy KE, Alvarez CA, Frei CR. Comparing Acute Kidney Injury Reports Among Antibiotics: A Pharmacovigilance Study of the FDA Adverse Event Reporting System (FAERS) Drug Saf. 2020;43(1):17–22. doi: 10.1007/s40264-019-00873-8. [DOI] [PubMed] [Google Scholar]
- 18.Zhu J, He Z, Liang D, Yu X, Qiu K, Wu J. Pulmonary tuberculosis associated with immune checkpoint inhibitors: a pharmacovigilance study. Thorax. 2022;77(7):721–723. doi: 10.1136/thoraxjnl-2021-217575. [DOI] [PubMed] [Google Scholar]
- 19.Zhu J, Wu J, Chen P, et al. Acute kidney injury associated with immune checkpoint inhibitors: A pharmacovigilance study. Int Immunopharmacol. 2022;113(Pt A):109350. doi: 10.1016/j.intimp.2022.109350. [DOI] [PubMed] [Google Scholar]
- 20.Zhu J, Chen G, He Z, et al. Stevens-Johnson syndrome/toxic epidermal necrolysis in patients treated with immune checkpoint inhibitors: A safety analysis of clinical trials and FDA pharmacovigilance database. EClinicalMedicine. 2021;37:100951. doi: 10.1016/j.eclinm.2021.100951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salvo EM, Ferko NC, Cash SB, Gonzalez A, Kahrilas PJ. Umbrella review of 42 systematic reviews with meta-analyses: the safety of proton pump inhibitors. Aliment Pharmacol Ther. 2021;54(2):129–143. doi: 10.1111/apt.16407. [DOI] [PubMed] [Google Scholar]
- 22.Nochaiwong S, Ruengorn C, Awiphan R, et al. The association between proton pump inhibitor use and the risk of adverse kidney outcomes: a systematic review and meta-analysis. Nephrol Dial Transplant. 2018;33(2):331–342. doi: 10.1093/ndt/gfw470. [DOI] [PubMed] [Google Scholar]
- 23.Schoolwerth AC, Sica DA, Ballermann BJ, Wilcox CS, Council on the Kidney in Cardiovascular D, the Council for High Blood Pressure Research of the American Heart A Renal considerations in angiotensin converting enzyme inhibitor therapy: a statement for healthcare professionals from the Council on the Kidney in Cardiovascular Disease and the Council for High Blood Pressure Research of the American Heart Association. Circulation. 2001;104(16):1985–91. doi: 10.1161/hc4101.096153. [DOI] [PubMed] [Google Scholar]
- 24.Sharma N, Anders HJ, Gaikwad AB. Fiend and friend in the renin angiotensin system: An insight on acute kidney injury. Biomed Pharmacother. 2019;110:764–774. doi: 10.1016/j.biopha.2018.12.018. [DOI] [PubMed] [Google Scholar]
- 25.Gong J, Ma L, Li M, et al. Nonsteroidal anti-inflammatory drugs associated acute kidney injury in hospitalized children: A systematic review and meta-analysis. Pharmacoepidemiol Drug Saf. 2022;31(2):117–127. doi: 10.1002/pds.5385. [DOI] [PubMed] [Google Scholar]
- 26.Zhang X, Donnan PT, Bell S, Guthrie B. Non-steroidal anti-inflammatory drug induced acute kidney injury in the community dwelling general population and people with chronic kidney disease: systematic review and meta-analysis. BMC Nephrol. 2017;18(1):256. doi: 10.1186/s12882-017-0673-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gomelsky A, Abreo K, Khater N, et al. Perioperative acute kidney injury: Stratification and risk reduction strategies. Best Pract Res Clin Anaesthesiol. 2020;34(2):167–182. doi: 10.1016/j.bpa.2020.04.003. [DOI] [PubMed] [Google Scholar]
- 28.Zhou J, Zhang X, Lyu L, Ma X, Miao G, Chu H. Modifiable risk factors of acute kidney injury after liver transplantation: a systematic review and meta-analysis. BMC Nephrol. 2021;22(1):149. doi: 10.1186/s12882-021-02360-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang YJ, Li JH, Guan Y, Xie QH, Hao CM, Wang ZX. Diabetes mellitus is a risk factor of acute kidney injury in liver transplantation patients. Hepatobiliary Pancreat Dis Int. 2021;20(3):215–221. doi: 10.1016/j.hbpd.2021.02.006. [DOI] [PubMed] [Google Scholar]
- 30.Cai X, Wu G, Zhang J, Yang L. Risk Factors for Acute Kidney Injury in Adult Patients With COVID-19: A Systematic Review and Meta-Analysis. Front Med (Lausanne) 2021;8:719472. doi: 10.3389/fmed.2021.719472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carande EJ, Brown K, Jackson D, et al. Acute Kidney Injury Following Percutaneous Coronary Intervention for Acute Coronary Syndrome: Incidence, Aetiology. Risk Factors and Outcomes Angiology. 2022;73(2):139–145. doi: 10.1177/00033197211040375. [DOI] [PubMed] [Google Scholar]
- 32.Cardwell CR, O'Sullivan JM, Jain S, Hicks BM, Devine PA, McMenamin UC. Hormone therapy use and the risk of acute kidney injury in patients with prostate cancer: a population-based cohort study. Prostate Cancer Prostatic Dis. 2021;24(4):1055–1062. doi: 10.1038/s41391-021-00348-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zabell J, Isharwal S, Dong W, et al. Acute Kidney Injury after Partial Nephrectomy of Solitary Kidneys: Impact on Long-Term Stability of Renal Function. J Urol. 2018;200(6):1295–1301. doi: 10.1016/j.juro.2018.07.042. [DOI] [PubMed] [Google Scholar]
- 34.Sin EI, Chia CS, Tan GHC, Soo KC, Teo MC. Acute kidney injury in ovarian cancer patients undergoing cytoreductive surgery and hyperthermic intra-peritoneal chemotherapy. Int J Hyperthermia. 2017;33(6):690–695. doi: 10.1080/02656736.2017.1293304. [DOI] [PubMed] [Google Scholar]
- 35.Morice PM, Leary A, Dolladille C, et al. Myelodysplastic syndrome and acute myeloid leukaemia in patients treated with PARP inhibitors: a safety meta-analysis of randomised controlled trials and a retrospective study of the WHO pharmacovigilance database. Lancet Haematol. 2021;8(2):e122–e134. doi: 10.1016/S2352-3026(20)30360-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Supplementary figure.
Additional file 2. Supplementary tables.
Data Availability Statement
Data from the meta-analysis are freely and publicly available on published literature. Data of real-world pharmacovigilance study are collected from the FDA Adverse Event Reporting System, and can be obtained through the FAERS Public Dashboard website.