Abstract
Objectives
The objective of the study was to compare the antibiotic treatment failure and recurrence rates between antibiotic agents (amoxicillin, amoxicillin-clavulanate, cefdinir, and azithromycin) for children with uncomplicated acute otitis media (AOM).
Study design
We completed a retrospective cohort study of children 6 months-12 years of age with uncomplicated AOM identified in a nationwide claims database. The primary exposure was the antibiotic agent, and the primary outcomes were treatment failure and recurrence. Logistic regression was used to estimate ORs, and analyses were stratified by primary exposure, patient age, and antibiotic duration.
Results
Among the 1 051 007 children included in the analysis, 56.6% were prescribed amoxicillin, 13.5% were prescribed amoxicillin-clavulanate, 20.6% were prescribed cefdinir, and 9.3% were prescribed azithromycin. Most prescriptions (93%) were for 10 days, and 98% were filled within 1 day of the medical encounter. Treatment failure and recurrence occurred in 2.2% (95% CI: 2.1, 2.2) and 3.3% (3.2, 3.3) of children, respectively. Combined failure and recurrence rates were low for all agents including amoxicillin (1.7%; 1.7, 1.8), amoxicillin-clavulanate (11.3%; 11.1, 11.5), cefdinir (10.0%; 9.8, 10.1), and azithromycin (9.8%; 9.6, 10.0).
Conclusions
Despite microbiologic changes in AOM etiology, treatment failure and recurrence were uncommon for all antibiotic agents and were lower for amoxicillin than for other agents. These findings support the continued use of amoxicillin as a first-line agent for AOM when antibiotics are prescribed. (J Pediatr 2022;251:98-104).
Acute otitis media (AOM) is the most common indication for antibiotics in children and affects 60% of children by 3 years of age.1-3 The use of the pneumococcal conjugate vaccines (PCVs) 7 and 13 has resulted in an overall reduction in AOM episodes and decreased rates of Streptococcus pneumoniae nasopharyngeal colonization in children.3 This has caused a shift of the predominant otopathogen associated with AOM from S pneumoniae (~40% pre-PCV to 30% PCV) to Haemophilus influenzae (~5% pre-PCV to 40% PCV), as well as an increase in the proportion of AOM cases associated with Moraxella catarrhalis (~10% pre-PCV to 20% PCV).3,4
The change in the distribution of otopathogens has significant implications for AOM management. First, although most AOM episodes associated with S pneumoniae benefit from antibiotics, nearly one-half of infections with H influenzae and 75% of those with M catarrhalis self-resolve and do not require antibiotics.5,6 Thus, fewer children with AOM are likely to benefit from taking antibiotics. Additionally, 30%-50% of H influenzae and over 90% of M catarrhalis isolates produce beta-lactamase, rendering them nonsusceptible to amoxicillin, which is currently recommended as the first-line antibiotic treatment for AOM by the American Academy of Pediatrics (AAP).3,7 Consequently, the shift in otopathogens has caused some to question if the first-line treatment for AOM should be changed to broader-spectrum antibiotics, such as amoxicillin-clavulanate, to more effectively treat H influenzae and M catarrhalis.4 Though single-region data suggest that the clinical failure rate for AOM treated with narrow-spectrum antibiotics remains low8, no national-level studies in the PCV era have evaluated clinical failure or recurrence rates of AOM treated with amoxicillin compared with broad-spectrum antibiotics. Additionally, clinical trials evaluating treatment failure and recurrence rates for antibiotics for AOM have typically used stringent inclusion criteria, which may not be generalizable to patients diagnosed with AOM in usual clinical practice environments.9,10
Unfortunately, antibiotics are associated with numerous potential harms for children including increasing the risk for adverse drug events (ADEs),8 Clostridioides difficile infections,11 chronic diseases later in life,12-14 and future infections with antimicrobial-resistant organisms that are difficult to treat.15 Thus, the benefits of using any antibiotic, broader-spectrum antibiotics, or long durations of antibiotics must be weighed against the potential harms. Because treatment of AOM accounts for 25% of antibiotic use for children, the prescribing of unnecessary broad-spectrum antibiotics for AOM likely contributes significantly to the global burden of antibiotic-resistant organisms. Therefore, we aimed to compare antibiotic treatment failure and recurrence rates between amoxicillin and broad-spectrum antibiotic agents for children 6 months-12 years of age with uncomplicated AOM in typical clinical practice environments.
Methods
We conducted a 1-year retrospective cohort study using the 2018 IBM MarketScan Commercial Database (IBM Watson Health). The MarketScan Commercial Database is a large convenience sample of reconciled medical and prescription claims data for individuals ≤65 years of age with private, employer-sponsored health insurance from over 300 employers and 24 health plans.16 The database contains data for >40 million unique individuals, including employees and their dependents.
Study Population
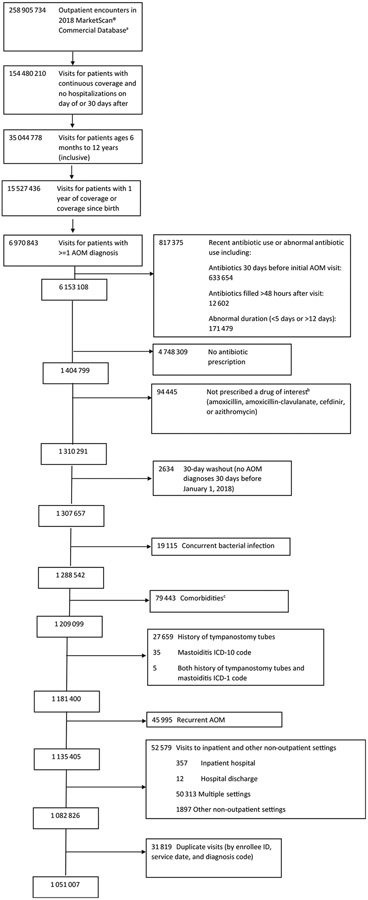
Visits from January 1, 2018 to December 31, 2018, were identified by the service date and unique enrollee identification number for children aged 6 months to 12 years at the time of the visit. To be included for analysis, children had to have at least one diagnosis of AOM using International Classification of Diseases, 10th Edition (ICD-10) codes (eText 1 in Supplementary Material) and be prescribed one of the four most commonly prescribed oral antibiotics for AOM (amoxicillin, amoxicillin-clavulanate, cefdinir, or azithromycin). Additional inclusion criteria included medical and drug insurance coverage for 30 days after the index AOM encounter date and continuous coverage since birth or 1 year prior to the index encounter. We excluded any children with an AOM diagnosis 30 days before the start of the study period on January 1, 2018, as well as any children who were prescribed an antibiotic 30 days prior to the index visit. To limit the analysis to uncomplicated AOM, we removed any visits for children with a bacterial co-diagnosis that may warrant an antibiotic,2 a history of tympanostomy tubes within 2 years prior or 30 days after the visit, an ICD-10 code for mastoiditis on the day of or 30 days prior to the visit (eText 2 in Supplementary Material), or a hospitalization on the day of or 30 days after the visit. Children with recurrent AOM, defined as ≥3 AOM visits in the preceding year, were also excluded. We used the pediatric complex chronic conditions classification system,17 to identify any comorbidities documented in either the inpatient or outpatient records from 2017 to 2018. Children with ≥1 comorbidity were excluded.
We captured all visits to outpatient care settings, including retail health clinics, urgent care centers, emergency departments, physicians’ offices, hospital-associated clinics, and ambulatory surgical centers. Visits with multiple setting types present for the same service date were excluded to ensure that only outpatient settings were included. The region (Northeast, Midwest, South, West, unknown) was defined as the US census region where the visit occurred. The provider type was categorized according to specialty (adult general practitioner, pediatrician, specialist, emergency department, urgent care, dentist, nurse practitioner, physician assistant, other, or unknown). Providers with several specialties present during the year were categorized as “multiple.” Prescription drug claims were linked to the child’s most recent visit to any setting on the same day or up to 3 days prior to the antibiotic fill date.18 Only patients with oral antibiotics and prescriptions not marked as refills at the index encounter were included. We removed visits for children who received a topical (otic) antibiotic 30 days before or after the index visit or ceftriaxone on the day of the index encounter. We also excluded visits for children who received an antibiotic prescription for <5 days or >12 days of therapy or where an antibiotic was filled >2 days after the visit.
Exposure and Outcomes
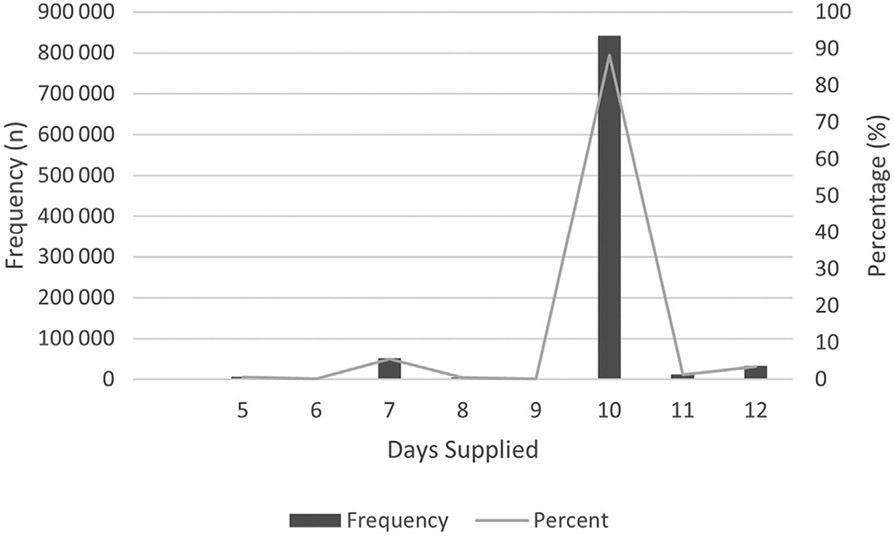
Exposure was an antibiotic prescription for amoxicillin, amoxicillin-clavulanate, cefdinir, or azithromycin filled for a visit to an urgent care, retail health, emergency department, office, or other outpatient setting in 2018. The primary outcomes were treatment failure and infection recurrence. Treatment failure was defined in accordance with other studies as another antibiotic (same or different) filled for any visit within 2-14 days after the index AOM encounter date.8,19 Infection recurrence was defined as another antibiotic (same or different) filled for any visit between 15 and 30 days after the index AOM encounter date. Secondary exposures were patient age and antibiotic duration. Antibiotic duration was divided into two groups: short course (5-9 days of therapy) and long course (10-12 days of therapy). Durations were selected to align with AAP guidelines that recommend short durations (5-7 days) for children 2 years of age and older with uncomplicated, nonsevere AOM and long durations (10 days) for children younger than 2 years of age and those with severe AOM.7 Days of therapy were determined from the total days supplied in IBM MarketScan. Duration cutoffs were then refined based on the histogram distribution of antibiotic durations along with the knowledge that suspensions may be dispensed for duration volumes slightly longer than those prescribed to account for measurement error (Table I and Figure 1; available at www.jpeds.com). Patient age was divided into three groups: 6 months-<2 years, 2-5 years, and 6-12 years.
Table I.
Histogram distribution of antibiotic durations prescribed for nonazithromycin antibiotic agents
| Maximum days supplied, oral antibiotics | ||||
|---|---|---|---|---|
| Day supply |
Frequency | Percent | Cumulative frequency |
Cumulative percent |
| 5 | 6374 | 0.67 | 6374 | 0.67 |
| 6 | 1299 | 0.14 | 7673 | 0.8 |
| 7 | 52 297 | 5.49 | 59 970 | 6.29 |
| 8 | 4465 | 0.47 | 64 435 | 6.76 |
| 9 | 1974 | 0.21 | 66 409 | 6.97 |
| 10 | 841 181 | 88.25 | 907 590 | 95.22 |
| 11 | 12 674 | 1.33 | 920 264 | 96.55 |
| 12 | 32 913 | 3.45 | 953 177 | 100 |
Figure 1.
Frequency and percentage of antibiotic durations supplied.
Statistical Analyses
We described visits by antibiotic type, patient age and sex, clinical setting, and provider type. We did not describe visits by race/ethnicity because these data are not included in the IBM MarketScan Commercial Database. We determined the percentage of children with AOM who experienced antibiotic treatment failure and infection recurrence for each of the antibiotics of interest (amoxicillin, amoxicillin-clavulanate, cefdinir, or azithromycin). We used logistic regression to estimate ORs and 95% CIs for the primary and secondary outcomes with amoxicillin as the reference. We also adjusted for patient age group, patient sex, encounter region, and clinical setting. Treatment failure and infection recurrence were stratified by the primary exposure, antibiotic type, as well as secondary exposures, antibiotic duration, and patient age group. Analyses were conducted using SAS 9.4 (SAS Institute). The study did not require institutional review board review, as the data were de-identified and deemed nonhuman subjects by the National Center for Emerging and Zoonotic Infectious Diseases’ human subjects’ advisor.
Results
A total of 6 970 843 children with an ICD-10 code for AOM were identified. After applying exclusion criteria, 1 051 007 children were included in the analysis (Figure 2; available at www.jpeds.com). Most children were diagnosed by a pediatrician (53.1%) in a clinical office setting (87.9%) and were between 6 months and 5 years of age (61.5%; Table II).
Figure 2.
Flowchart of study population based on inclusion and exclusion criteria. aHansen L. White Paper: IBM MarketScan Research Databases for life sciences researchers. IBM Watson Health. Accessed June 8, 2021. bIncludes antibiotics other than amoxicillin, cefdinir, azithromycin, and amoxicillin-clavulanate that were infrequently prescribed including but not limited to clindamycin, sulfamethoxazole-trimethoprim, cephalexin, etc. cFeudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. Aug 82 014; 14:199. https://doi.org/10.1186/1471-2431-14-199.
Table II.
Demographics and clinical features of outpatient AOM visits, MarketScan Commercial Database, 2018
| Encounter characteristics | Amoxicillin N (%) n = 594 467 (56.6) |
Amoxicillin-clavulanate N (%) n = 142 027 (13.5) |
Cefdinir N (%) n = 216 683 (20.6) |
Azithromycin N (%) n = 97 830 (9.3) |
Total n = 1 051 007 |
|---|---|---|---|---|---|
| Age | |||||
| 6 m to <2 y | 134 879 (22.7) | 40 522 (28.5) | 58 384 (26.9) | 17 957 (18.4) | 251 742 (24.0) |
| 2-5 y | 223 454 (37.6) | 52 107 (36.7) | 83 304 (38.5) | 34 685 (35.5) | 393 550 (37.5) |
| 6-12 y | 236 134 (39.7) | 49 398 (34.8) | 74 995 (34.6) | 45 188 (46.2) | 405 715 (38.6) |
| Sex | |||||
| Male | 301 741 (50.8) | 76 435 (53.8) | 113 201 (52.2) | 52 366 (53.5) | 543 743 (51.7) |
| Female | 292 726 (49.2) | 65 592 (46.2) | 103 482 (47.8) | 45 464 (46.5) | 507 264 (48.3) |
| Geographic region | |||||
| Northeast | 108 549 (18.3) | 22 300 (15.7) | 28 739 (13.3) | 14 765 (15.1) | 174 353 (16.6) |
| North central | 130 436 (21.9) | 24 431 (17.2) | 40 455 (18.7) | 20 190 (20.6) | 215 512 (20.5) |
| South | 253 761 (42.7) | 72 106 (50.8) | 117 256 (54.1) | 44 997 (46.0) | 488 120 (46.4) |
| West | 79 774 (13.4) | 16 618 (11.7) | 20 217 (9.3) | 11 807 (12.1) | 128 416 (12.2) |
| Unknown | 2947 (3.7) | 6572 (4.6) | 10 016 (4.6) | 6071 (6.2) | 44 606 (4.2) |
| Clinical setting | |||||
| Retail health clinic | 1950 (0.3) | 230 (0.2) | 434 (0.2) | 285 (0.3) | 2899 (0.3) |
| Urgent care | 63 394 (10.7) | 9391 (6.6) | 15 745 (7.3) | 9233 (9.4) | 97 763 (9.3) |
| Emergency department | 6468 (1.1) | 1313 (0.9) | 1163 (0.5) | 743 (0.8) | 9687 (0.9) |
| Office | 512 315 (86.2) | 128 996 (90.8) | 196 534 (90.7) | 86 163 (88.1) | 924 008 (87.9) |
| Other* | 10 340 (1.7) | 2097 (1.5) | 2807 (1.3) | 1406 (1.4) | 16 650 (1.6) |
| Provider type | |||||
| Adult general practitioner | 84 780 (14.3) | 16 459 (11.6) | 26 105 (12.1) | 20 622 (21.1) | 147 966 (14.1) |
| Pediatrician | 306 263 (51.5) | 85 628 (60.3) | 124 140 (57.3) | 42 451 (43.4) | 558 482 (53.1) |
| Specialist†1 | 27 503 (4.6) | 8047 (5.7) | 12 193 (5.6) | 5393 (5.5) | 53 136 (5.1) |
| Emergency department | 10 931 (1.8) | 1726 (1.2) | 2789 (1.3) | 1743 (1.8) | 17 189 (1.6) |
| Urgent care | 48 180 (8.1) | 6627 (4.7) | 11 635 (5.4) | 6777 (6.9) | 73 219 (7.0) |
| Dentist | 410 (0.1) | 128 (0.1) | 161 (0.1) | 64 (0.1) | 763 (0.1) |
| Nurse practitioner | 32 103 (5.4) | 6115 (4.3) | 11 265 (5.2) | 5585 (5.7) | 55 068 (5.2) |
| Physician assistant | 10 821 (1.8) | 1753 (1.2) | 3566 (1.7) | 1918 (2.0) | 18 058 (1.7) |
| Other‡ | 33 188 (5.6) | 5939 (4.2) | 10 650 (4.9) | 5608 (5.7) | 55 385 (5.3) |
| Multiple | 30 808 (5.2) | 7690 (5.4) | 11 433 (5.3) | 6087 (6.2) | 56 018 (5.3) |
| Unknown | 9480 (1.6) | 1915 (1.4) | 2746 (1.3) | 1582 (1.6) | 15 723 (1.5) |
Other outpatient setting—ambulatory surgical setting, outpatient hospital.
Specialist—dermatologists, surgeons, adult and pediatric specialist.
Other provider type—radiology, physical therapist, physical medicine and rehab, psychiatry, dietician, psychiatric nurse, optician, pharmacist, renal dialysis therapy, psychologist, acupuncturist, health educator/agency, home health agency, public health agency, case manager.
Antibiotic Prescribing Patterns
Amoxicillin was the most frequently prescribed antibiotic (56.6%) followed by cefdinir (20.6%), amoxicillin-clavulanate (13.5%), and azithromycin (9.3%). Children 6-12 years of age were prescribed azithromycin more often than younger children (46.2% vs 35.5% 2-5 years, 18.4% 6 months-<2 years). Long durations (10 days) of antibiotics were prescribed to 93% of children. Before application of the exclusion criteria of prescription fill within 2 days of the encounter, 98% of antibiotic prescriptions were filled within 1 day of the medical encounter.
Treatment Failure and Recurrence by Antibiotic Agent
Of children included, 5.5% had treatment failure or recurrence (Tables III and IV). Treatment failure occurred in 2.2% of children, and recurrence occurred in 3.3% of children. Treatment failure and recurrence occurred more often in children who were prescribed amoxicillin-clavulanate (11.3% [11.1, 11.5]; P < .001), cefdinir (10.0% [9.8, 10.1]; P < .001), and azithromycin (9.8% [9.6, 10.0]; P < .001) compared with those prescribed amoxicillin (1.7% [1.7, 1.8]; Tables III and V; available at www.jpeds.com). This finding was observed across all age groups. Treatment failure and recurrence also occurred more often among children 6 months-<2 years of age than in older children (Tables VI and VII; available at www.jpeds.com). In the adjusted model, the odds of treatment failure or recurrence were higher for patients prescribed broad-spectrum antibiotics than those for patients prescribed amoxicillin (aOR; amoxicillin-clavulanate: 7.0 [6.8, 7.2], cefdinir: 6.2 [6.0, 6.3], azithromycin: 6.6 [6.4, 6.8], amoxicillin-reference; P < .0001; Tables IV and V).
Table III.
Outcomes of outpatient AOM visits with antibiotic prescriptions, MarketScan Commercial Database, 2018
| Total |
Amoxicillin |
Amoxicillin-clavulanate |
Cefdinir |
Azithromycin |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n = 1 051 007 |
n = 594 467 |
n = 142 027 |
n = 216 683 |
n = 97 830 |
||||||
| Outcomes | N | % (95% CI) | N | % (95% CI) | N | % (95% CI) | N | % (95% CI) | N | % (95% CI) |
| Treatment failure | 23 029 | 2.19 (2.16, 2.22) | 2591 | 0.44 (0.42, 0.45) | 6977 | 4.91 (4.80, 5.02) | 8960 | 4.14 (4.05, 4.22) | 4501 | 4.60 (4.47, 4.73) |
| Recurrence | 34 540 | 3.29 (3.25, 3.32) | 7767 | 1.31 (1.28, 1.34) | 9059 | 6.38 (6.25, 6.51) | 12 666 | 5.85 (5.75, 5.94) | 5048 | 5.16 (5.02, 5.30) |
| Either failure or recurrence | 57 569 | 5.48 (5.43, 5.52) | 10 358 | 1.74 (1.71, 1.78) | 16 036 | 11.29 (11.13, 11.46) | 21 626 | 9.98 (9.85, 10.11) | 9549 | 9.76 (9.57, 9.95) |
Table IV.
Odds of primary and secondary outcomes for outpatient AOM visits by antibiotic type*, MarketScan Commercial Database, 2018
| Antibiotics | Treatment failure |
Recurrence |
||||
|---|---|---|---|---|---|---|
| OR (95% CI) | aOR (95% CI) | P | OR (95% CI) | aOR (95% CI) | P | |
| Amoxicillin | Ref | – | – | Ref | – | – |
| Amoxicillin-clavulanate | 11.80 (11.27, 12.35) | 11.36 (10.82, 11.93) | <.0001 | 5.15 (4.99, 5.31) | 4.96 (4.80, 5.13) | <.0001 |
| Cefdinir | 9.85 (9.43, 10.29) | 9.66 (9.22, 10.12) | <.0001 | 4.69 (4.56, 4.83) | 4.59 (4.45, 4.74) | <.0001 |
| Azithromycin | 11.01 (10.49, 11.56) | 11.79 (11.19, 12.43) | <.0001 | 4.11 (3.96, 4.26) | 4.36 (4.19, 4.54) | <.0001 |
Adjusted for age (ref—6 mo to <2 y), sex (ref—male), visit region (ref—West), setting.
Table V.
Odds of combined treatment and recurrence rates for outpatient AOM visits by antibiotic type
| Either failure or recurrence | |||
|---|---|---|---|
| Antibiotics | OR (95% CI) | aOR (95% CI) | P |
| Amoxicillin | Ref | – | – |
| Amoxicillin-clavulanate | 7.18 (7.00, 7.36) | 6.96 (6.77, 7.15) | <.0001 |
| Cefdinir | 6.25 (6.10, 6.40) | 6.16 (6.00, 6.32) | <.0001 |
| Azithromycin | 6.10 (5.93, 6.28) | 6.58 (6.38, 6.78) | <.0001 |
Table VI.
Outcomes of AOM treatment stratified by antibiotic duration, MarketScan Commercial Database, 2018
| Antibiotics | Duration | Treatment failure |
Recurrence |
||||||
|---|---|---|---|---|---|---|---|---|---|
| N (, 95 CI) | OR (95 CI) | aOR (95 CI) | P | N (, 95 CI) | OR (95 CI) | aOR (95 CI) | P | ||
| Amoxicillin | Long | 2250 (0.41, 0.39, 0.43) | Ref | – | – | 7265 (1.33, 1.30, 1.36) | Ref | – | – |
| Short | 341 (0.73, 0.66, 81) | 1.80 (1.60, 2.01) | 1.88 (1.64, 2.13) | <.0001 | 502 (1.08, 0.99, 1.18) | 0.81 (0.74, 0.89) | 0.84 (0.76, 0.93) | .001 | |
| Amoxicillin-clavulanate | Long | 6482 (4.82, 4.70, 4.93) | Ref | – | – | 8709 (6.47, 6.34, 6.61) | Ref | – | – |
| Short | 495 (6.59, 6.03, 7.16) | 1.40 (1.27, 1.53) | 1.58 (1.42, 1.75) | <.0001 | 350 (4.66, 4.19, 5.14) | 0.71 (0.63, 0.79) | 0.83 (0.74, 0.93) | .002 | |
| Cefdinir | Long | 8290 (4.06, 3.97, 4.15) | Ref | – | – | 12 118 (5.93, 5.83, 6.04) | Ref | – | – |
| Short | 670 (5.36, 4.97, 5.76) | 1.34 (1.24, 1.45) | 1.50 (1.37, 1.63) | <.0001 | 548 (4.38, 4.03, 4.74) | 0.73 (0.67, 0.79) | 0.81 (0.73, 0.89) | <.0001 | |
| Total | Long | 17 022 (1.92, 1.89, 1.95) | Ref | – | – | 28 092 (3.17, 3.13, 3.20) | Ref | – | – |
| Total (Cont.) | Short | 1506 (2.27, 2.15, 2.38) | 1.19 (1.12, 1.25) | 1.43 (1.35, 1.51) | <.0001 | 1400 (2.11, 2.00, 2.22) | 0.66 (0.62, 0.70) | 0.78 (0.73, 0.82) | <.0001 |
Table VII.
Primary outcome measures for outpatient AOM visits stratified by age
| Age | Antibiotic | Treatment failure |
Recurrence |
||||||
|---|---|---|---|---|---|---|---|---|---|
| N (, 95 CI) | OR (95 CI) | aOR (95 CI) | P | N (, 95 CI) | OR (95 CI) | aOR (95 CI) | P | ||
| 6 m to <2 y | Amoxicillin | 724 (0.54, 0.50, 0.58) | Ref | – | – | 2090 (1.55, 1.48, 1.62) | Ref | – | – |
| Amoxicillin-clavulanate | 2652 (6.54, 6.30, 6.79) | 12.98 (11.94, 14.10) | 13.06 (11.96, 14.26) | <.0001 | 3719 (9.18, 8.90, 9.46) | 6.42 (6.08, 6.78) | 6.59 (6.21, 6.99) | <.0001 | |
| Cefdinir | 3368 (5.77, 5.58, 5.96) | 11.34 (10.46, 12.30) | 11.57 (10.61, 12.61) | <.0001 | 5008 (8.58, 8.35, 8.80) | 5.96 (5.66, 6.28) | 6.12 (5.79, 6.48) | <.0001 | |
| Azithromycin | 1329 (7.40, 7.02, 7.78) | 14.81 (13.51, 16.24) | 15.36 (13.92, 16.94) | <.0001 | 1412 (7.86, 7.47, 8.26) | 5.42 (5.06, 5.81) | 5.60 (5.19, 6.04) | <.0001 | |
| Total | 8073 (3.21, 3.14, 3.28) | – | – | – | 12 229 (4.86, 4.77, 4.94) | – | – | – | |
| 2-5 y | Amoxicillin | 1005 (0.45, 0.42, 0.48) | Ref | – | – | 3019 (1.35, 1.30, 1.40) | Ref | – | – |
| Amoxicillin-clavulanate | 2466 (4.73 4.55, 4.91) | 10.99 (10.21, 11.84) | 10.81 (9.98, 11.71) | <.0001 | 1966 (3.98, 3.81, 4.15) | 5.06 (4.81, 5.32) | 4.94 (4.68, 5.22) | <.0001 | |
| Cefdinir | 3230 (3.88, 3.75, 4.01) | 8.93 (8.31, 9.59) | 8.86 (8.21, 9.57) | <.0001 | 4779 (5.74, 5.58, 5.89) | 4.44 (4.24, 4.65) | 4.42 (4.20, 4.65) | <.0001 | |
| Azithromycin | 1576 (4.54, 4.32, 4.76) | 10.53 (9.72, 11.41) | 10.82 (9.93, 11.79) | <.0001 | 1951 (5.62, 5.38, 5.87) | 4.35 (4.11, 4.61) | 4.36 (4.09, 4.65) | <.0001 | |
| Total | 8277 (2.10, 2.06, 2.15) | – | – | – | 13 123 (3.33, 3.28, 3.39) | – | – | – | |
| 6-12 y | Amoxicillin | 862 (0.37, 0.34, 0.39) | Ref | – | – | 2658 (1.13, 1.08, 1.17) | Ref | – | – |
| Amoxicillin-clavulanate | 1859 (3.76, 3.60, 3.93) | 10.67 (9.84, 11.58) | 10.64 (9.77, 11.60) | <.0001 | 1966 (3.98, 3.81, 4.15) | 3.64 (3.43, 3.86) | 3.60 (3.38, 3.84) | <.0001 | |
| Cefdinir | 2362 (3.15, 3.02, 3.27) | 8.87 (8.21, 9.60) | 8.94 (8.23, 9.72) | <.0001 | 2879 (3.84, 3.70, 3.98) | 3.51 (3.32, 3.70) | 3.53 (3.33, 3.74) | <.0001 | |
| Azithromycin | 1596 (3.53, 3.36, 3.70) | 9.99 (9.19, 10.86) | 10.32 (9.45, 11.28) | <.0001 | 1685 (3.73, 3.55, 3.90) | 3.40 (3.20, 3.62) | 3.45 (3.23, 3.69) | <.0001 | |
| Total | 6679 (1.65, 1.61, 1.69) | – | – | – | 9188 (2.26, 2.22, 2.31) | – | – | ||
Treatment Failure and Recurrence by Antibiotic Duration
In total, 5.1% of children prescribed a long duration of antibiotics and 4.4% prescribed short durations had treatment failure or recurrence. Treatment failure occurred in 1.9% (1.9, 2.0) of children who were prescribed a long duration of antibiotics and 2.3% (2.2, 2.4) of children who were prescribed a short duration of antibiotics (absolute difference: 0.4%). Infection recurrence occurred in 3.2% (3.1, 3.2) of children who were prescribed a long duration of antibiotics and 2.1% (2.0, 2.2) of children who were prescribed a short duration of antibiotics (absolute difference: 1.0%). After adjusting for confounders, odds of treatment failure and recurrence remained low for children who were prescribed short and long durations (Table VI).
Discussion
In this study of over a million children with AOM identified in a national insurance claims database, we found high prescribing rates for broad-spectrum antibiotics (44%) and long durations of antibiotics (93%). Most antibiotic prescriptions were filled immediately (within 1 day of diagnosis; 98%). For all antibiotic agents, including amoxicillin, overall treatment failure (2.2%) and recurrence rates (3.3%) were low. Additionally, children who were prescribed short durations of antibiotics had similar outcomes to those who were prescribed longer durations.
No clinical trials in the PCV era have evaluated treatment failure or recurrence rates for AOM treated with amoxicillin compared with placebo or amoxicillin compared with broader-spectrum antibiotics. Clinical trials utilizing amoxicillin-clavulanate have reported failure rates of >15%.20 We found that overall failure and recurrence rates in this cohort of children receiving care in typical clinical practice environments were low (<5%). One potential explanation is that children in usual clinical practice environments are diagnosed with AOM using less stringent criteria than those included in clinical trials. For example, clinical trials have typically required diagnosis of AOM by a trained otoscopist for inclusion.9,10 As a result, in a typical practice environment, children diagnosed with AOM may have less severe illness, may have higher probability of viral AOM, and may be more likely to be incorrectly diagnosed with AOM than in clinical trials. Additionally, the epidemiologic shift in otopathogens to those that are more likely to self-resolve probably contributes to low treatment failure and recurrence rates.3,6
Nonguideline concordant antibiotic prescribing for AOM was common, including high rates of prescribing of broad-spectrum antibiotics and prescribing of unnecessarily long durations of antibiotics for older children. The AAP recommends that most children with nonsevere AOM should be observed or prescribed a delayed antibiotic.7 Although we were unable to identify delayed antibiotic prescriptions in this data source, the fact that 98% of antibiotic prescriptions were filled within 1 day suggests the majority were either not written as delayed, most of those written as delayed were immediately filled, or only 2% of prescriptions written as delayed were filled after 24 hours using insurance. We suspect the latter to be unlikely. This complements electronic health record data from Colorado and Virginia practice cohorts that identified that 95%-98% of antibiotic prescriptions for AOM were written as immediate.21,22 In clinical trials and quasi-experimental studies, interventions for AOM to reduce prescribing of immediate,21-23 broad-spectrum,24 and longer than necessary durations of antibiotics19 have proven effective. Additionally, free, effective stewardship tools are available including the following: the Antimicrobial Change Package by AAP,25 the Core Elements of Outpatient Stewardship by the Centers for Disease Control and Prevention,26 and Dialogue Around Respiratory Tract Prescribing by the University of Washington27 and OASIS for audit and feedback by Denver Health and Hospital Authority.28
Some authors have recommended the routine use of broader-spectrum antibiotics as the first-line treatment for AOM to minimize treatment failure and recurrence.4 In contrast, we found low treatment failure and recurrence rates for all antimicrobial agents, and children who were prescribed amoxicillin did not have higher treatment failure or recurrence rates than those who were prescribed broader-spectrum antibiotics. We attempted to only include children with uncomplicated, nonrecurrent infections as defined by AAP criteria.7 Nevertheless, it is likely that differences in characteristics among children prescribed narrow-vs broad-spectrum antibiotics could not be fully captured and may be responsible for differences in outcomes. This includes potential differences in the severity of presentation, underlying medical conditions that could not be accounted for in the claims database, and/or prior history of antibiotics for AOM that caregivers paid for out of pocket rather than billing to insurance. Though not previously studied, it is possible that providers who prescribe broader-spectrum antibiotics as a first-line therapy for uncomplicated AOM may also be more likely to inappropriately prescribe additional antibiotics when they are not indicated (eg, for persistent symptoms or serous effusions). Thus, our findings of relatively higher treatment failure with broader-spectrum antibiotics may not necessarily reflect true clinical failure. Regardless, given the low treatment failure and recurrence rates for AOM treated with all antibiotic agents, the difference is unlikely to be clinically important.
Most antibiotics were prescribed for ≥10 days (93%), despite AAP recommendations that most children ≥2 years with nonsevere AOM should be prescribed shorter durations.7 Treatment failure and recurrence were uncommon (<2.5%) for those prescribed short or long durations of therapy with an absolute difference in failure or recurrence of <1%. The small difference is similar to that reported in a prior meta-analysis.29 Importantly, children prescribed longer durations of antibiotics are significantly more likely to experience ADEs than those prescribed shorter durations.29 Thus, providers should strongly consider prescribing shorter durations of antibiotics for children ≥2 years given the marginal benefit and increased risk of ADEs with longer durations. For children <2 years, randomized controlled trials have reported that shorter durations of antibiotics are inferior to longer durations in preventing treatment failure. Given the stringent inclusion criteria of these studies, it is not clear how these findings should be applied to typical clinical practice environments.20 Pragmatic trials that compare shorter vs longer durations of antibiotics would be beneficial, particularly because a large portion of AOM episodes are among children <2 years, and younger children are more likely to have long-term sequelae from antibiotic-associated microbiome changes than older children.12
The strengths of this study include a large sample size of children from geographic and demographically diverse settings in the US. This study also has important limitations. First, claims data only provide information on filled antibiotic prescriptions that were billed to insurance; thus, if caregivers paid for antibiotics out of pocket or the antibiotic prescription was not linked to an encounter diagnosis (eg, called into the pharmacy after the encounter), we may have underestimated initial prescription volumes, treatment failure, and recurrence rates. A high proportion of encounters (77%) were excluded for no associated filling of antibiotic prescription data. It is possible these children were inherently different than those included. Second, we were not able to evaluate if the antibiotic prescription was filled but not taken by the child. Third, we were also unable to capture certain clinical characteristics including vital signs, symptoms, and examination findings that could contribute to differences in prescribing. Thus, children with comorbidities that may be associated with more severe infections may have been disproportionately prescribed nonamoxicillin agents. We also could not verify the accuracy of AOM diagnoses and could not evaluate for reported penicillin allergy. Similarly, only medically attended outcomes were included, and other outcomes that are important to children and families including adverse drug events managed at home, symptom severity, etc. could not be evaluated. Fourth, because our goal was to assess failure and recurrence for children with uncomplicated infections, the results may not be generalizable to those with recurrent infections including those with tympanostomy tubes. Fifth, the database included only commercially insured children, and results may not be generalizable to those with public insurance (eg, Medicaid) or who are uninsured.
In conclusion, treatment failure and recurrence rates were low for all antibiotic agents, including amoxicillin. Our data support the AAP AOM guidelines, which recommend amoxicillin as the first-line therapy for most children who warrant an antibiotic and a short duration of antibiotics for older patients with nonsevere AOM. Future efforts should be directed toward widespread dissemination of effective stewardship tools, particularly to community-based practice settings. ■
Supplementary Material
Acknowledgments
H.F. received salary support from the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under award number K23HD099925. The other authors received no external funding. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the National Institutes of Health. The authors declare no conflicts of interest.
Glossary
- AAP
American Academy of Pediatrics
- ADE
Adverse drug event
- AOM
Acute otitis media
- ICD-10
International Classification of Diseases, 10th Edition
- PCV
Pneumococcal conjugate vaccine
References
- 1.Hersh AL, Shapiro DJ, Pavia AT, Shah SS. Antibiotic prescribing in ambulatory pediatrics in the United States. Pediatrics 2011;128:1053–61. [DOI] [PubMed] [Google Scholar]
- 2.Fleming-Dutra KE, Hersh AL, Shapiro DJ, Bartoces M, Enns EA, File TM Jr, et al. Prevalence of inappropriate antibiotic prescriptions among ITS ambulatory care visits, 2010-2011. JAMA 2016;315:1864–73. [DOI] [PubMed] [Google Scholar]
- 3.Kaur R, Morris M, Pichichero ME. Epidemiology of acute otitis media in the postpneumococcal conjugate vaccine era. Pediatrics 2017;140:e20170181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wald ER, DeMuri GP. Antibiotic recommendations for acute otitis media and acute bacterial sinusitis: conundrum no more. Pediatr Infect Dis J 2018;37:1255–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Howie VM, Ploussard JH. Efficacy of fixed combination antibiotics versus separate components in otitis media. Effectiveness of erythromycin estrolate, triple sulfonamide, ampicillin, erythromycin estolate-triple sulfonamide, and placebo in 280 patients with acute otitis media under two and one-half years of age. Clin Pediatr 1972;11:205–14. [DOI] [PubMed] [Google Scholar]
- 6.Klein JO. Otitis media. Clin Infect Dis 1994;19:823–33. [DOI] [PubMed] [Google Scholar]
- 7.Lieberthal AS, Carroll AE, Chonmaitree T, Ganiats TG, Hoberman A, Jackson MA, et al. The diagnosis and management of acute otitis media. Pediatrics 2013;13l:e964–99. [DOI] [PubMed] [Google Scholar]
- 8.Gerber JS, Ross RK, Bryan M, Localio AR, Szymczak JE, Wasserman R, et al. Association of broad- vs narrow-spectrum antibiotics with treatment failure, adverse events, and quality of life in children with acute respiratory tract infections. JAMA 2017;318:2325–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoberman A, Paradise JL, Rockette HE, Shaikh N, Wald ER, Kearney DH, et al. Treatment of acute otitis media in children under 2 years of age. N Engl J Med 2011;364:105–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tahtinen PA, Laine MK, Huovinen P, Jalava J, Ruuskanen O, Ruohola A. A placebo-controlled trial of antimicrobial treatment for acute otitis media. N Engl J Med 2011;364:116–26. [DOI] [PubMed] [Google Scholar]
- 11.Miranda-Katz M, Parmar D, Dang R, Alabaster A, Greenhow TL. Epidemiology and risk factors for community associated clostridioides difficile in children. J Pediatr 2020;221:99–106. [DOI] [PubMed] [Google Scholar]
- 12.Kronman MP, Zaoutis TE, Haynes K, Feng R, Coffin SE. Antibiotic exposure and IBD development among children: a population-based cohort study. Pediatrics 2012;130:e794–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horton DB, Scott FI, Haynes K, Putt ME, Rose CD, Lewis JD, et al. Antibiotic exposure and juvenile idiopathic arthritis: a case-control study. Pediatrics 2015;136:e333–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ni J, Friedman H, Boyd BC, McGurn A, Babinski P, Markossian T, et al. Early antibiotic exposure and development of asthma and allergic rhinitis in childhood. BMC Pediatr 2019;19:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention Antibiotic Resistance Threats in the United States, 2013. Accessed December 31, 2018. https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf
- 16.Hansen L. White Paper: IBM MarketScan Research Databases for Life Sciences Researchers; 2018. Accessed June 8, 2021. https://www.ibm.com/downloads/cas/0NKLE57Y
- 17.Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr 2014;14:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.King L, Tsay S, Hicks L, Bizune D, Hersh A, Fleming-Dutra K. Changes in outpatient antibiotic prescribing for acute respiratory illnesses, 2011 to 2018. Antimicrob Steward Healthc Epidemiol 2021;1:E66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frost HM, Lou Y, Keith A, Byars A, Jenkins TC. Increasing guideline-concordant durations of antibiotic therapy for acute otitis media. J Pediatr 2022;240:221–7.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoberman A, Paradise JL, Rockette HE, Kearney DH, Bhatnagar S, Shope TR, et al. Shortened antimicrobial treatment for acute otitis media in young children. N Engl J Med 2016;375:2446–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Norlin C, Fleming-Dutra K, Mapp J, Monti J, Shaw A, Bartoces M, et al. A learning collaborative to improve antibiotic prescribing in primary care pediatric practices. Clin Pediatr (Phila) 2021;60:230–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frost HM, Monti JD, Andersen LM, Norlin C, Bizune DJ, Fleming-Dutra KE, et al. Improving delayed antibiotic prescribing for acute otitis media. Pediatrics 2021;147:e2020026062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun D, Rivas-Lopez V, Liberman DB. A multifaceted quality improvement intervention to improve watchful waiting in acute otitis media management. Pediatr Qual Saf 2019;4:e177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gerber JS, Prasad PA, Fiks AG, Localio AR, Grundmeier RW, Bell LM, et al. Effect of an outpatient antimicrobial stewardship intervention on broad-spectrum antibiotic prescribing by primary care pediatricians: a randomized trial. JAMA 2013;309:2345–52. [DOI] [PubMed] [Google Scholar]
- 25.American Academy of Pediatrics. Improving antibiotic prescribing for children change package. 2020. Accessed April 3, 2021. https//www.aap.org/en-us/professional-resources/quality-improvement/quality-improvement-resources-and-tools/Pages/antibiotic-stewardship.aspx
- 26.Sanchez GV, Fleming-Dutra KE, Roberts RM, Hicks LA. Core elements of outpatient antibiotic stewardship. MMWR Recomm Rep 2016;65:1–12. [DOI] [PubMed] [Google Scholar]
- 27.Kronman MP, Gerber JS, Grundmeier RW, Zhou C, Robinson JD, Heritage J, et al. Reducing antibiotic prescribing in primary care for respiratory illness. Pediatrics 2020;146:e20200038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frost HM, Munsiff SS, Lou Y, Jenkins TC. Simplifying outpatient antibiotic stewardship. Infect Control Hosp Epidemiol 2022;43:260–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kozyrskyj A, Klassen TP, Moffatt M, Harvey K. Short-course antibiotics for acute otitis media. Cochrane Database Syst Rev 2010;2010:Cd001095. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.