Key Clinical Message
Oral mucosal leishmaniasis is a rare finding posing challenges in the diagnosis and treatment in a nonendemic setting. This disease is present in dental clinics as nonhealing chronic growth. Timely diagnosis and appropriate treatment are crucial to prevent further complications and death.
Keywords: chronic growth, leishmaniasis, Oral cavity
Leishmaniasis is one of the most neglected tropical diseases. The disease affects the rural community and people living in poverty. Mucosal leishmaniasis (ML) is a very rare disease in the world, even in endemic areas. It has a progressive course and can cause deformity and even mutilation in the affected areas. Mucosal leishmaniasis usually becomes clinically evident within several years or even takes decades after cutaneous lesions. The disease can occur in different clinical forms such as cutaneous, mucosal, and visceral. In a few instances, mucocutaneous forms are found to be more severe than others and are usually chronic and often occur years after the presentation of skin lesions. It can also cause severe respiratory complications including the the destruction of lung tissue. Malnutrition is common in leishmaniasis, thus good nutrition is important to prevent remission of the disease. The progression of the disease leads to immune deficiency thereby weakening the patient further. Oral mucosal leishmaniasis being a very rare finding poses diagnostic challenges and treatment in a nonendemic setting. Oral mucosal disease presents in dental clinics as nonhealing chronic growth. Clinicians need to be very vigilant about such presentations. Timely diagnosis and appropriate treatment are crucial to prevent further complications and death.
1. INTRODUCTION
Leishmaniasis is a neglected tropical disease with an annual estimated 700,000 to 1 million new cases worldwide. 1 The disease affects the rural community and people living in poverty. 1 Mucosal leishmaniasis (ML) is a very rare disease in the world, even in endemic areas such as Iran and the American continent. 2 , 3 It has a progressive course and can cause deformity and even mutilation in the affected areas. 3 Leishmaniasis is a parasitic disease transmitted by phlebotamine sand flies or Lutzomyia species and caused by a protozoan Leishmania and its 22 different species. 4 , 5 , 6 Of these, Leishmania braziliensis and Leishmania guyanensis are the species that causes mucocutaneous leishmaniasis. 7 , 8 Rarely, Leishmania donovani causes mucosal leishmaniasis especially in patients with immune suppression. 2 , 9 Mucosal leishmaniasis usually becomes clinically evident within several years or even takes decades after cutaneous lesions which were not treated at all or were incompletely treated. 10 , 11 Sporadic cases of visceral leishmaniasis have been reported in Bhutan and it has been demonstrated to be endemic in certain areas. 12
The disease can occur in different clinical forms such as cutaneous, mucosal, and visceral. 6 In a few instances, mucocutaneous forms are found to be more severe than others and are usually chronic and often occur years after the presentation of skin lesions. 7 , 13 It can also cause severe respiratory complications including the destruction of lung tissue. Malnutrition is common in leishmaniasis, thus good nutrition is important to prevent remission of the disease. 14 Advent of the disease leads to immune deficiency thereby weakening the patient further. 6 , 14 Studies have documented depletion of T lymphocytes that plays an important role in curing and progression of the disease and is more severe in malnutrition. 6 Various laboratory methods can be used to diagnose leishmaniasis—to detect the parasite and to identify the Leishmania species. 10 , 11
2. CASE PRESENTATION
A 76‐year‐old man was referred to us from a district hospital. He presented with an oral lesion growth and swelling of the lower lip since 2018. He complained of abnormal taste in the mouth, drooling, and difficulty in swallowing. He had visited hospitals several times, the last biopsy was performed in 2019 suspecting malignancy but it was reported as nonspecific growth. He was treated several times for oral candidiasis without a definitive diagnosis. He also complained of chronic productive cough with occasional shortness of breath. There was no history of fever, night sweats, or loss of weight. He did not smoke but he used to drink in moderation until a few years ago. He is in hypertensive treatment and undergoing treatment for age‐related macular degeneration.
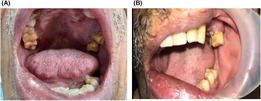
On extra‐oral examinations, there was facial asymmetry with edema on the left side of the face and the lips. Cutaneous findings were normal and there was no regional lymphadenopathy. On intraoral examination, there was a large ill‐defined vegetative growth on the anterior palate and the left buccal mucosa with maxillary gingivial hyperplasia (Figure 1). There were features of xerostomia but there was no infection or suppuration.
FIGURE 1.

(A) Large ill‐defined vegetative growth on the anterior palate (B) Large ill‐defined vegetative growth on the left buccal mucosa with maxillary gingivial hyperplasia.
His blood workup was normal except for mild neutrophilia and lymphopenia and raised erythrocyte sedimentation rate. Ultrasound abdomen was normal. However, chest X‐ray and CT chest showed some consolidation on the left lower lobe. A bone marrow biopsy was not done. Incisional biopsies were performed from the palate and the buccal mucosa and specimens were sent for histopathological examination (HPE). Histopathological examination showed focal mucosal ulceration lined by granulation tissue densely infiltrated with acute and chronic inflammatory cells (Figure 2). The intact squamous epithelium showed keratinization and was mildly hyperplastic. Subepithelial regions showed many round‐ to oval‐shaped organisms within the cytoplasm of histiocytes and some within the stroma, histologically consistent with LD bodies as seen with H & E and Giemsa stain (Figure 3). There was no microscopic evidence of fungal infection. Kala‐azar serology using rk‐39 was done which turned out to be positive for Leishmaniasis.
FIGURE 2.

H & E section showing focal mucosal ulceration lined by granulation tissue densely infiltrated with acute and chronic inflammatory cells (×40).
FIGURE 3.

Subepithelial region with numerous round‐ to oval‐shaped organisms within the cytoplasm of histiocytes and some within the stroma, histologically consistent with LD bodies as seen with H & E (A) and Giemsa stain (B).
He was admitted to the ward and liposomal Amphotericin B was started at 2 mg/kg body weight (150 mg) but his creatinine level started rising after the third dose. Infusion frequency was paced according to his renal function which stabilized once adequate hydration was maintained after the infusion. His compliance with the injection was poor with the main complaint of pain at the injection site. A total of 1350 mg of Amphotericin B was given. His lungs condition were evaluated. Sputum culture grew Pseudomonas. His cough and shortness of breath improved after a course of antibiotics. After the 9th infusion of amphotericin B, the patient refused any further injections. At this point, there was a 50% posttreatment resolution of the lesion on the palate and left buccal mucosa (Figure 4). There was no drooling, lip swelling had reduced, and he was able to eat without difficulty. He was discharged with the plan to start on oral miltefosine as other alternative treatment is not available in our country.
FIGURE 4.

(A) Posttreatment resolution of lesion on the palate (B) posttreatment resolution of lesion on the left buccal mucosa.
3. DISCUSSION
This case is a rare clinical presentation of the disease in an immune‐competent person representing a clinical and laboratory diagnostic challenge. The case was initially evaluated as a malignant growth due to its clinical presentation and intractable course with several incisional biopsies. The biopsies that we performed from the palate and buccal mucosa showed no evidence of malignancy. Special stains for acid fast bacilli and fungal organisms were done which were negative. Kala‐azar serology was positive. Dental full mouth X‐ray/Orthopentomogram showed some degree of bone loss on the palate and 2 loose teeth (grade I mobility) on the left side.
Mucosal leishmaniasis is a serious integumentary disease considering its diagnostic challenges, treatment, and coinfections. 14 Diagnosis of oral leishmaniasis is a challenge mainly because it is rarely encountered, and in fact, this is the first case at our facility in 2022. Secondly, we the clinicians never think of mucosal leishmaniasis since we do not fall in the endemic area. Studies have shown that Leishmania parasites can remain dormant for a long period of time even though the antimonials have been used and thereby recurrences can occur. 15 , 16 The clinical form of the disease depends on the type of infecting species of Leishmania and the patient's immune status. Leishmaniasis in humans is classified into three major clinical types: visceral, cutaneous, and mucocutaneous. The visceral form of leishmaniasis is caused mainly by L. infantum and L. donovani, which is endemic in the western Mediterranean. Cutaneous leishmaniasis is caused by L. tropica or L. major and the mucocutaneous variant is mainly due to L. braziliensis, but also L. panamensis, L. guyanensis, and L. amazonensis, which is endemic in South America. 11 , 17 , 18 L. braziliensis is the species that causes serious disease and often leads to a chronic state. 17 , 19 Cutaneous leishmaniasis is the most common form of New World Leishmaniasis; mucosal legions may occur simultaneously or after years of the disease. 10 Organism for mucosal leishmaniasis in Asia is donovani species. Mucosal forms are destructive and can affect the oral and nasal cavity. 2 Oral lesions usually appear as ulceration in the hard or soft palate. However, they can affect any site and also may present as exophytic or nodular growths as in our current case. 11 , 17
In our case, a potent inflammatory response was seen with neutrophilia that contributed to tissue damage besides lymphocytopenia. The uncontrolled and nonspecific inflammatory process is characteristic of mucosal leishmaniasis. 20 , 21 Anemia and malnutrition are a result of progression of mucosal leishmaniasis. A 3%–5% of cutaneous leishmaniasis can develop into mucosal leishmaniasis as per literature. 17 , 22 , 23
4. CONCLUSION
This case is a classic example of diagnostic challenge, in settings without adequate laboratory backup and appropriate tests. The patient underwent several biopsies over long period of time before a definitive diagnosis was made. A high degree of clinical suspicion is required as the disease is a public health problem. Clinicians at the periphery and dentists should be aware of rare presentations and have a robust system of cross‐consultation with a specialist. An early diagnosis and treatment can prevent complications and reduce patient morbidity and mortality.
AUTHOR CONTRIBUTIONS
Gyan Prasad Bajgai: Conceptualization; investigation; methodology; resources; supervision; validation; visualization; writing – original draft; writing – review and editing. Sangay Tshering: Conceptualization; methodology; resources; software; validation; visualization; writing – original draft; writing – review and editing. Birendra Pradhan: Conceptualization; investigation; methodology; validation; visualization; writing – review and editing. Ambika Rani Pradhan: Conceptualization; investigation; methodology; supervision; validation; visualization; writing – review and editing. Pema Yangzom: Conceptualization; investigation; methodology; supervision; validation; visualization; writing – review and editing.
CONFLICT OF INTEREST STATEMENT
The authors have no conflict of interest to declare.
ETHICS STATEMENT
Ethical approval is not needed for case reports in de‐identified patients.
CONSENT STATEMENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
ACKNOWLEDGMENTS
We would like to acknowledge the staff and doctors and specialists of the dental outpatient department (OPD), Dermatology OPD, and inpatient department (IPD) and thank them for their immense support during the patient's treatment and follow‐up in the hospital.
Bajgai GP, Tshering S, Pradhan B, Pradhan AR, Yangzom P. Oral mucosal leishmaniasis presenting as a nonhealing chronic oral growth: A case report. Clin Case Rep. 2023;11:e7234. doi: 10.1002/ccr3.7234
DATA AVAILABILITY STATEMENT
Data sharing is not applicable as this article did not generate or analyzed the data set.
REFERENCES
- 1. Tobgay T, Dorjee S, Pradhan A, et al. Is leishmaniasis donovani elimination feasible in Bhutan? A review of current prevention and control mechanisms in Bhutan. Bhutan Heal J. 2020;6(2):27‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Daneshbod Y, Oryan A, Davarmanesh M, Shirian S. Mucosal leishmaniasis and literature review. Arch Pathol Lab Med. 2011;135:478‐482. [DOI] [PubMed] [Google Scholar]
- 3. Muvdi‐Arenas S, Ovalle‐Bracho C. Mucosal leishmaniasis: a forgotten disease. Description and identification of species in 50 Colombian cases. Biomedica. 2019;39:58‐65. [DOI] [PubMed] [Google Scholar]
- 4. Varghese L, Laxmanan S, Varghese GM. Mucosal leishmaniasis due to leishmania donovani—a rare presentation. Ear Nose Throat J. 2020;101(4):226‐227. doi: 10.1177/0145561320952186 [DOI] [PubMed] [Google Scholar]
- 5. Strazzulla A, Cocuzza S, Pinzone MR, et al. Mucosal leishmaniasis: an underestimated presentation of a neglected disease. Biomed Res Int. 2013;2013:805108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jaimes JR. Severe Mucosal Leishmaniasis with Torpid and Fatal Evolution. Clin Case Rep. 2022;10:e6220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Oliveira‐Neto MP, Schubach A, Mattos M, da Costa SC, Pirmez C. Intralesional therapy of American cutaneous leishmaniasis with pentavalent antimony in Rio de Janeiro, Brazil‐‐an area of leishmania (V.) braziliensis transmission. Int J Dermatol. 1997;36(6):463‐468. [DOI] [PubMed] [Google Scholar]
- 8. Cardoso DT, de Souza DC, de Castro VN, Geiger SM, Barbosa DS. Identification of priority areas for surveillance of cutaneous leishmaniasis using spatial analysis approaches in southeastern Brazil. BMC Infect Dis. 2019;19:318. doi: 10.1186/s12879-019-3940-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mahdi M, Elamin EM, Melville SE, et al. Sudanese mucosal leishmaniasis: isolation of a parasite within the leishmania donovani complex that differs genotypically from L. donovani causing classical visceral leishmaniasis. Infect Genet Evol. 2005;5(1):29‐33. [DOI] [PubMed] [Google Scholar]
- 10. Cincurá C, De Lima CMF, Machado PRL, et al. Mucosal leishmaniasis: a retrospective study of 327 cases from an endemic area of leishmania (Viannia) braziliensis. Am J Trop Med Hyg. 2017;97(3):761‐766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Motta ACF, Lopes MA, Ito FA, Carlos‐Bregni R, De Almeida OP, Roselino AM. Oral leishmaniasis: a clinicopathological study of 11 cases. Oral Dis. 2007;13(3):335‐340. [DOI] [PubMed] [Google Scholar]
- 12. Yangzom T, Cruz I, Bern C, et al. Endemic transmission of visceral leishmaniasis in Bhutan. Am J Trop Med Hyg. 2012;87(6):1028‐1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fleissig Y, Dan‐Gur M, Michael‐Gayego A, et al. A trespasser from a foreign land? A case report of primary mucosal leishmaniasis. BMC Infect Dis. 2022;22:212. doi: 10.1186/s12879-022-07169-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Snapper I. American mucocutaneous leishmaniasis successfully treated with 2‐hydroxystilbamidine. Am J Med. 1952;13(5):655‐664. Available from: https://www.sciencedirect.com/science/article/pii/0002934352900727 [DOI] [PubMed] [Google Scholar]
- 15. Conceição‐Silva F, Leite‐Silva J, Morgado FN. The binomial parasite‐host immunity in the healing process and in reactivation of human Tegumentary leishmaniasis. Front Microbiol. 2018;9:1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Morgado FN, Schubach A, Vasconcellos E, et al. Signs of an in situ inflammatory reaction in scars of human American tegumentary leishmaniasis. Parasite Immunol. 2010;32(4):285‐295. [DOI] [PubMed] [Google Scholar]
- 17. Lucas CM, Franke ED, Cachay MI, et al. Geographic distribution and clinical description of leishmaniasis cases in Peru. Am J Trop Med Hyg. 1998;59(2):312‐317. [DOI] [PubMed] [Google Scholar]
- 18. Gupta N, Gupta R, Kumar Acharya A, et al. Nepal journal of epidemiology changing trends in oral cancer – a global scenario citation. Nepal J Epidemiol. 2016;6(4):613‐619. Available from: https://www.nepjol.info/index.php/NJE/article/viewFile/17255/14065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Llanos‐Cuentas A, Tulliano G, Araujo‐Castillo R, et al. Clinical and parasite species risk factors for pentavalent antimonial treatment failure in cutaneous leishmaniasis in Peru. Clin Infect Dis an off Publ Infect Dis Soc Am. 2008;46(2):223‐231. [DOI] [PubMed] [Google Scholar]
- 20. Kumar R, Chauhan SB, Ng SS, Sundar S, Engwerda CR. Immune checkpoint targets for host‐directed therapy to prevent and treat leishmaniasis. Front Immunol. 2017;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Quintella LP, Passos SRL, de Miranda LHM, Cuzzi T, et al. Proposal of a histopathological predictive rule for the differential diagnosis between American tegumentary leishmaniasis and sporotrichosis skin lesions. Br J Dermatol. 2012;167(4):837‐846. [DOI] [PubMed] [Google Scholar]
- 22. Zea DF, Prager M, Figueroa RA, Miranda MC. Complicación mucosa de la leishmaniasis cutánea. Biomedica. 2009;29:9‐10. [PubMed] [Google Scholar]
- 23. Azeredo‐Coutinho RBG, Pimentel MI, Zanini GM, et al. Intestinal helminth coinfection is associated with mucosal lesions and poor response to therapy in American tegumentary leishmaniasis. Acta Trop. 2016;154:42‐49. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable as this article did not generate or analyzed the data set.


