Abstract
Introduction:
Individuals with major depressive disorder (MDD) exhibit high rates of tobacco use and lower responsiveness to tobacco cessation treatments. Treatment adherence is a strong predictor of treatment outcomes in the general population but has not been evaluated in this under-served community of smokers with MDD.
Methods:
We used data from a randomized clinical trial on smoking cessation treatment among 300 smokers with MDD to examine the rate of adherence (medication and counseling), the association of adherence with cessation outcomes, and factors associated with adherence, including demographic and smoking characteristics, psychiatric characteristics, smoking cessation processes (e.g., withdrawal, reinforcers), and treatment-related side effects (e.g., nausea).
Results:
Overall, 43.7% of participants were adherent with medication and 63.0% were adherent with counseling. Medication adherence was significantly associated with cessation, with 32.1% of adherent vs. 13.0% of non-adherent participants quitting smoking at EOT. Counseling adherence was also significantly associated with cessation, with 32.3% of adherent vs. 2.7% of non-adherent participants quitting smoking. Multivariate regression models showed that medication adherence was associated with higher engagement in complementary reinforcers and higher baseline smoking reward, while counseling adherence was associated with identifying as female, lower alcohol use and nicotine dependence, higher baseline smoking reward, and higher engagement in substitute and complementary reinforcers within the first weeks of medication use.
Conclusions:
As with the general population of smokers, non-adherence to treatment in smokers experiencing depression is widespread and a significant barrier to cessation. Interventions that target reinforcers may improve rates of treatment adherence.
Keywords: smoking, major depressive disorder, adherence, reinforcers
1. Introduction
The rate of smoking is 2–3 times higher among adults experiencing past year mental health disorders than among the general population in the United States (40–60% vs. 19%; Weinberger et al., 2020). Major depressive disorder (MDD) is one of the most common mental health disorders, with a point prevalence among adults aged >20 years old of 8.1% (Brody et al., 2018). The higher rate of smoking in this population translates into higher rates of cancer and cardiovascular disease and lower overall life expectancy, vs. the general population (Druss et al., 2011).
Studies conducted over the last 10 years have found that FDA-approved medications for smoking and guideline-based behavioral interventions are safe and effective for smokers with MDD (Anthenelli et al., 2016; Hawes et al., 2021). However, compared to no treatment or placebo, smokers with MDD still show lower quit rates than the general population, even when receiving the same treatments (Anthenelli et al., 2016).
In the general population, one of the strongest predictors of response to smoking cessation treatments is treatment adherence (Fiore et al., 2008; Pacek et al., 2018). Upwards of one-quarter to one-half of smokers enrolled in clinical trials involving FDA-approved medications or behavioral interventions are non-adherent, and quit rates are nearly two times lower for these smokers vs. those who are adherent (Okuyemi et al., 2010; Grenard et al., 2011; Peng et al., 2018; Crawford et al., 2019). Regarding predictors of adherence, one review indicated that increased medication adherence is associated with demographic characteristics (e.g., male, non-Hispanic Whites, greater education), less psychiatric comorbidity, lower degree of nicotine dependence, and a lack of adverse treatment-related side effects (Pacek et al., 2018). Other studies have found medication adherence to be associated with nicotine withdrawal symptoms (Catz et al., 2011), engagement in complementary (e.g., socializing with friends) or substitute (e.g., exercise) reinforcers (Handschin et al., 2018), and reductions in the rewarding experience from smoking (Crawford et al., 2019). To date, there is a paucity of data on the rates of adherence to tobacco treatments, how variability in adherence is associated with cessation outcomes, and what factors are associated with adherence among smokers with MDD.
Given these notable gaps in the current literature, we used data from a double-blind placebo-controlled clinical trial evaluating varenicline and behavioral activation counseling for tobacco use among smokers with MDD to examine the rate of medication and counseling adherence in this population, the association between medication and counseling adherence and end-of-treatment smoking cessation (12 weeks), and baseline and early phase (i.e., across the first two weeks of treatment) changes in variables potentially associated with medication and counseling adherence. The results of this study may contribute to our understanding of why smokers with MDD show low rates of cessation following treatment and identify potential targets for intervening to promote greater adherence in this under-served community of smokers.
2. Methods
2.1. Design
We used data from a 2×2 factorial randomized clinical trial that tested behavioral activation (BA) vs. standard counseling plus varenicline (or placebo) to promote cessation among smokers with current or past MDD (ClinicalTrials.gov ID: NCT02378714). Randomization by computer was stratified by clinic site, sex, and depression level (minimal-mild vs. moderate-severe) using the Beck Depression Inventory-II (BDI-II; Beck et al., 1996) and permuted blocks of fixed size (18 participants/block). We selected BA for this trial since our meta-analysis revealed that, while varenicline had the strongest association with long-term abstinence, standard behavioral counseling (cognitive behavioral therapy; CBT) was only effective for short-term abstinence (Hitsman et al., 2013). Further, compared with CBT, BA is simpler to administer, time efficient, and less complicated for patients (Dimidjian et al., 2011; Jacobson et al., 2001; Lejuez et al., 2001). The study included a placebo control to evaluate adverse events in smokers with MDD for whom varenicline has been discouraged (Williams, 2012). We used data from the intent-to-treat (ITT) sample (N=300) and up to the end of treatment (EOT; week 14), consisting of: N=68 for BA + placebo; N=83 for BA + varenicline; N=68 for standard counseling + placebo; and N=81 for standard counseling and varenicline.
The Institutional Review Boards at Northwestern University and the University of Pennsylvania provided approval for the trial.
2.2. Interventions and Assessments
The intensive behavioral interventions were implemented using structured manuals and consisted of eight 45-minute sessions from weeks 1–12. Except for sessions 1 and 3, treatment was delivered by telephone. Standard counseling was based on established guidelines for tobacco intervention (Fiore et al., 2008) and included behavioral strategies such as identifying smoking triggers, eliciting social support, and relapse prevention. The BA intervention addressed smoking as a behavior that prevents and restricts opportunities for healthy rewarding behaviors and emphasized reducing environmental and perceived stress and lost reward due to smoking cessation and on identifying and establishing alternative reinforcers to promote abstinence. Medication was administered for 12 weeks (week 2 and 14) according to US Food and Drug Administration-approved labeling.
Assessments were conducted at weeks 0, 1, 3, 4, 6, 7, 8, 10, 12, 14, and 27. Final eligibility screening, informed consent, treatment randomization, and the baseline assessment was completed at week 0. Week 14 represented the EOT.
2.3. Participants
To be eligible for the trial, participants had to endorse an interest in quitting smoking, be ≥18 years of age, reside in the geographic area for >8 months, have access to a phone, smoke ≥1 cigarette/day, have a lifetime Diagnostic and Statistical Manual of Mental Disorders (DSM-5; APA, 2013) diagnosis of MDD without psychotic features, be able to communicate in English, and be able to provide informed consent. Individuals were excluded from the trial if they self-reported a suicide attempt in the last 12 months or active suicidal ideation with intent to act in the past 30 days, self-reported current or planned pregnancy, were women of childbearing potential who refused to use a medically acceptable method of birth control, were currently using a smoking cessation medication, or reported consuming >28 alcoholic drinks/week or a lifetime DSM-5 bipolar or psychotic disorder by self-report or the Mini International Neuropsychiatric Interview (MINI; Sheehan et al., 1998), and uncontrolled hypertension (systolic blood pressure> 185 mm Hg or diastolic blood pressure > 110 mm Hg).
2.4. Measures
2.4.1. Adherence
We assessed counseling and medication adherence using self-report, including timeline follow-back procedures and collection of used blister packs at assessments between baseline and EOT. Reports were summed to provide a total number of sessions completed (out of a possible 8) and the total number of days that assigned medication (one dose or two doses depending on the day) was taken (out of 84). As with previous studies, adherence was defined as taking the prescribed medication on ≥80% (67/84) of the total number of days prescribed (Crawford et al., 2019) and completing ≥75% (6/8) of counseling sessions (Okuyemi et al., 2010; Crawford et al., 2019).
2.4.2. Smoking Cessation
We assessed 7-day point prevalence abstinence, confirmed with a carbon monoxide (CO) sample of ≤ 6ppm at EOT.
2.4.3. Demographic and Smoking History
Demographic information was collected from all participants (e.g., sex, sexual orientation, age, race, education, employment, income, BMI). We also collected smoking history data (i.e., baseline number of years smoked, CO, average cigarettes per day, and the nicotine metabolite ratio, a biomarker measure of the rate of nicotine metabolism; Siegel et al., 2020), including the Fagerström Test for Cigarette Dependence (FTCD), a 6-item measure validated in smokers with mental health disorders (Buckley et al., 2005). The Readiness Ladder was used as a continuous measure of level of quit motivation; it asked participants to indicate what they thought about quitting on a scale from 1 (I have no interest in quitting) to 10 (I have quit and will never smoke again) (Biener et al., 1991; Hitsman et al., 2002).
2.4.4. Psychiatric Characteristics
Psychiatric status was measured with the MINI, including, among others, bipolar disorder, post-traumatic stress disorder, generalized anxiety disorder, alcohol/substance abuse, and MDD (current and past, past only, current only) and suicidality risk as measured by the Columbia Suicidality Severity Rating Scale (C-SSRS; Posner et al., 2011). Current use of antidepressants was assessed.
2.3.5. Affective and Behavioral Variables
Baseline levels and changes from pre-quit to week 3 (target quit date; over 2 weeks of treatment) in the following variables were assessed: self-reported level of depression symptoms, using the Beck Depression Inventory II (BDI-II; Beck et al., 1996); hedonic capacity, measured by the 14-item Snaith-Hamilton Pleasure Scale (SHAPS; Franken et al., 2007); the rewarding value of smoking, assessed by the cigarette reward value scale (Spring et al., 2003), craving and withdrawal, assessed using the 10-item Questionnaire of Smoking Urges-Brief (Cox et al., 2001) and the 9-item Minnesota Nicotine Withdrawal Scale (MNWS; Hughes & Hatsukami, 1986), respectively, and alternative reinforcers (complementary and substitute reinforcers), using the 45-item Pleasant Events Schedule (PES; Schnoll et al., 2016), which assesses the frequency and enjoyability of rewarding activities and events an individual has engaged in over the last 30 days. We summed the cross-products of enjoyability and frequency for each activity and then calculated the average by dividing by the total number of reinforcers to calculate a total score for complementary and substitute reinforcers that were used by participants at the assessment time-point. Values, therefore, represent the change over time in the use of complimentary (associated with smoking) and substitute (not associated with smoking) reinforcing activities. For smokers with MDD, smoking acquires a high reward value through positive reinforcement effects that support cognitive enhancement and reward functioning in the context of a limited range of positive reinforcers due to depressive symptoms. BA therapy supports the use of substitute reinforcers like exercise or increased social engagement and works to reduce engagement in complementary reinforcers such as consuming alcohol, so the PES was a key measure in this trial.
2.4.6. Side Effects
A checklist was administered at each assessment time-point to determine the presence and severity (0=none to 3=severe) of side effects. For the purpose of this study, we considered changes in side effects from pre-quit to week 3, which represented changes over 2 weeks of treatment (Peng et al., 2017). We focused on the total number of side effects reported and the nausea item alone since it is the most common side effect associated with varenicline.
2.5. Statistical Analyses
Consistent with our main trial (Hitsman et al., under review), there were no significant between arm differences in counseling adherence (BA + placebo=47.1%; BA + varenicline=57.8%; standard counseling + placebo=58.8%; and standard counseling + varenicline=64.2%; χ2[3]=4.55, p=0.21) or medication adherence (BA + placebo=35.3%; BA + varenicline=39.8%; standard counseling + placebo=51.5%; and standard counseling + varenicline=48.1%; χ2[3]=4.82, p=0.19). Thus, here, we collapsed across the counseling arms and the medication arms to assess medication and counseling adherence without consideration of treatment arm.
Descriptive statistics characterized rate of medication and counseling adherence. We used chi-square to examine the relationships between medication and counseling adherence and EOT smoking cessation. Analysis of variance (ANOVA) was used for continuous measures and chi-square was used for categorical measures to evaluate factors associated with counseling and medication adherence (e.g., demographics, side effects). Variables associated with adherence (p ≤ .10) were included in separate multivariate logistic regression models predicting counseling or medication adherence. The significance of measures was assessed using odds ratios and 95% confidence intervals with alphas set at 0.05. To facilitate interpretation, we standardized scores in the regressions with the baseline standard deviation values of the respective variables.
3. Results
3.1. Sample Characteristics
Table 1 shows the characteristics of the participants, overall and stratified by adherence to medication and counseling. Overall, 45% of the sample identified as male, 42.6% as white, 31% as ≤high school graduate, and 49% reported a current MDD diagnosis. Participants were, on average, 50 years old (SD=12.6), had been smoking for 31.2 years (SD=14.0), were highly nicotine dependent (FTCD=5.2; SD=2.1), and smoked 15.2 cigarettes/day (SD=7.9).
Table 1.
Characteristics of the Sample, Overall and by Medication and Counseling Adherence
| Variable | Varenicline Adherent (N=131) N(%) or Mean (SD) | Varenicline Non-adherent (N=169) N(%) or Mean (SD) | Counseling Adherent (N=172) N(%) or Mean (SD) | Counseling Non-adherent (N=128) N(%) or Mean (SD) | Overall (N=300) N(%) or Mean (SD) |
|---|---|---|---|---|---|
| Demographics | |||||
| Other^ | 3 (2.3)A | 24 (14.2)B | 15 (8.7) | 12 (9..4) | 27 (9.0) |
| % Male | 63 (48.1) | 72 (42.6) | 64 (37.2)A | 71 (55.5)B | 135 (45.0) |
| % Heterosexual | 109 (83.8) | 138 (82.1) | 140 (81.4) | 109 (85.1) | 247 (82.3) |
| Age (M, SD) | 52.0 (11.3)A | 48.4 (13.3)B | 50.7 (12.5) | 49.0 (12.7) | 50.0 (12.6) |
| % Married/Living as Married | 34 (26.1) | 36 (21.4%) | 44 (25.7) | 26 (20.5) | 70 (23.3) |
| % ≤High School | 41 (31.8) | 52 (30.8) | 49 (28.5) | 44 (34.4) | 93 (31.0) |
| % <20k income | 48 (36.9) | 62 (37.3) | 60 (35.3) | 50 (39.7) | 110 (36.7) |
| BMI (M, SD) | 30.3 (7.9)A | 28.8 (6.2)B | 30.1 (7.0)A | 28.6 (7.0)B | 29.4 (7.0) |
| Smoking/Psychiatric History | |||||
| Readiness to Quit (M, SD) | 6.7 (1.2) | 6.8 (1.3) | 6.8 (1.2) | 6.8 (1.2) | 6.8 (1.2) |
| Years Smoked (M, SD) | 32.9 (13.5)A | 30.0 (14.3)B | 31.4 (14.3) | 31.1 (13.5) | 31.2 (14.0) |
| % Antidepressant Use | 33 (25.2) | 49 (29.0) | 42 (24.4) | 40 (31.3) | 82 (27.3) |
| % other Psych Dx | 25 (19.2) | 24 (14.2) | 33 (19.2) | 16 (12.5) | 49 (16.3) |
| % Regular Drinker | 68 (19.1) | 92 (54.4) | 83 (48.5)A | 77 (60.1)B | 160 (53.7) |
| % Current MDD | 62 (47.3) | 85 (50.3) | 81 (47.1) | 66 (51.6) | 147 (49.0) |
| C-SSRS (M, SD) | .21 (.48) | .21 (.63) | .26 (.65)A | .15 (.42)B | .21 (.57) |
| NMR (M, SD) | .36 (.25) | .37 (.21) | .37 (.24) | .36 (.22) | .36 (.23) |
| FTCD (M, SD) | 5.1 (2.2) | 5.3 (2.1) | 5.0 (2.2)A | 5.5 (2.1)B | 5.2 (2.1) |
| CO (M, SD) | 13.0 (8.0) | 12.5 (7.2) | 13.1 (7.9) | 12.2 (7.0) | 12.7 (7.5) |
| Smoking Rate (M, SD) | 15.4 (8.1) | 15.0 (7.7) | 14.9 (7.9) | 15.6 (7.8) | 15.2 (7.9) |
| Affective, Behavioral, and Side Effects (M, SD) * | |||||
| Baseline BDI | 17.5 (10.4) | 19.6 (12.2) | 18.0 (11.3) | 19.7 (11.7) | 18.7 (11.5) |
| Baseline Comp. Reinf. | 24.4 (19) | 26.3 (19.8) | 25.8 (21.2) | 24.9 (16.8) | 25.4 (19.4) |
| Baseline Sub. Reinf. | 21.5 (19.4) | 23.0 (18.8) | 23.9 (20.9) | 20.3 (16.1) | 22.4 (19.0) |
| Baseline Craving | 37.4 (16.4) | 37.7 (17.2) | 37.3 (17.0) | 37.9 (16.6) | 37.6 (16.8) |
| Baseline Smoking Reward | 7.7 (3.6)A | 6.8 (3.8)B | 7.8 (3.6)A | 6.4 (3.7)B | 7.2 (3.7) |
| Baseline Nausea | 0.2 (0.4) | 0.2 (0.5) | 0.2 (0.5) | 0.2 (0.5) | 0.2 (0.5) |
| Baseline Side Effects | 9.0 (7.6) | 9.8 (8.1) | 9.5 (7.6) | 9.3 (8.2) | 9.4 (7.9) |
| Baseline Hedonic Capacity | 2.5 (3.0) | 2.1 (3.3) | 2.2 (3.1) | 2.3 (3.3) | 2.3 (3.2) |
| Baseline Withdrawal | 13.9 (7.5) | 13.5 (7.1) | 14.0 (7.3) | 13.2 (7.3) | 13.7 (7.3) |
| Change in BDI | −3.4 (5.8) | −2.8 (7.3) | −2.9 (6.1) | −3.5 (7.5) | −3.1 (6.6) |
| Change in Comp. Reinf. | −6.3 (16.3)A | −12.2 (17.3)B | −7.0 (16.7)A | −13.2 (17.1)B | −9.7 (17.1) |
| Change in Sub. Reinf. | 2.6 (16.3)A | −1.7 (21.6)B | 3.6 (16.2)A | −4.4 (22.5)B | .21 (19.5) |
| Change in Craving | −9.9 (14.9) | −10.2 (17.0) | −9.9 (16.6) | −10.4 (14.7) | −10.1 (16.0) |
| Change in Smoking Reward | −2.0 (3.4) | −1.8 (3.4) | −2.1 (3.3) | −1.5 (3.5) | −1.9 (3.3) |
| Change in Nausea | .11 (.74) | .15 (.66) | .10 (.71) | .22 (.71) | .13 (.71) |
| Change in Side Effects | −1.1 (6.9) | −.83 (7.8) | −1.1 (6.8) | −.46 (8.7) | −.97 (7.3) |
| Change in Hedonic Cap. | −.85 (2.7) | −.56 (3.2) | −.71 (2.8) | −.69 (3.3) | −.70 (2.9) |
| Change in Withdrawal | −2.1 (6.0) | −1.9 (5.2) | −2.0 (5.0) | −2.0 (5.6) | −2.0 (5.6) |
Note.
Other race includes participants who were either Asian (n=2), Native American (n=4), More than One Race (n=16), Unknown (n=3), or Refused (n=2);
reflects change in variables from baseline to week 3 (e.g., BDI scores decreased from baseline to week 3 across groups); variables with different subscripts are different from each other (< 0.10).
3.2. Rate of Adherence and Association with Cessation
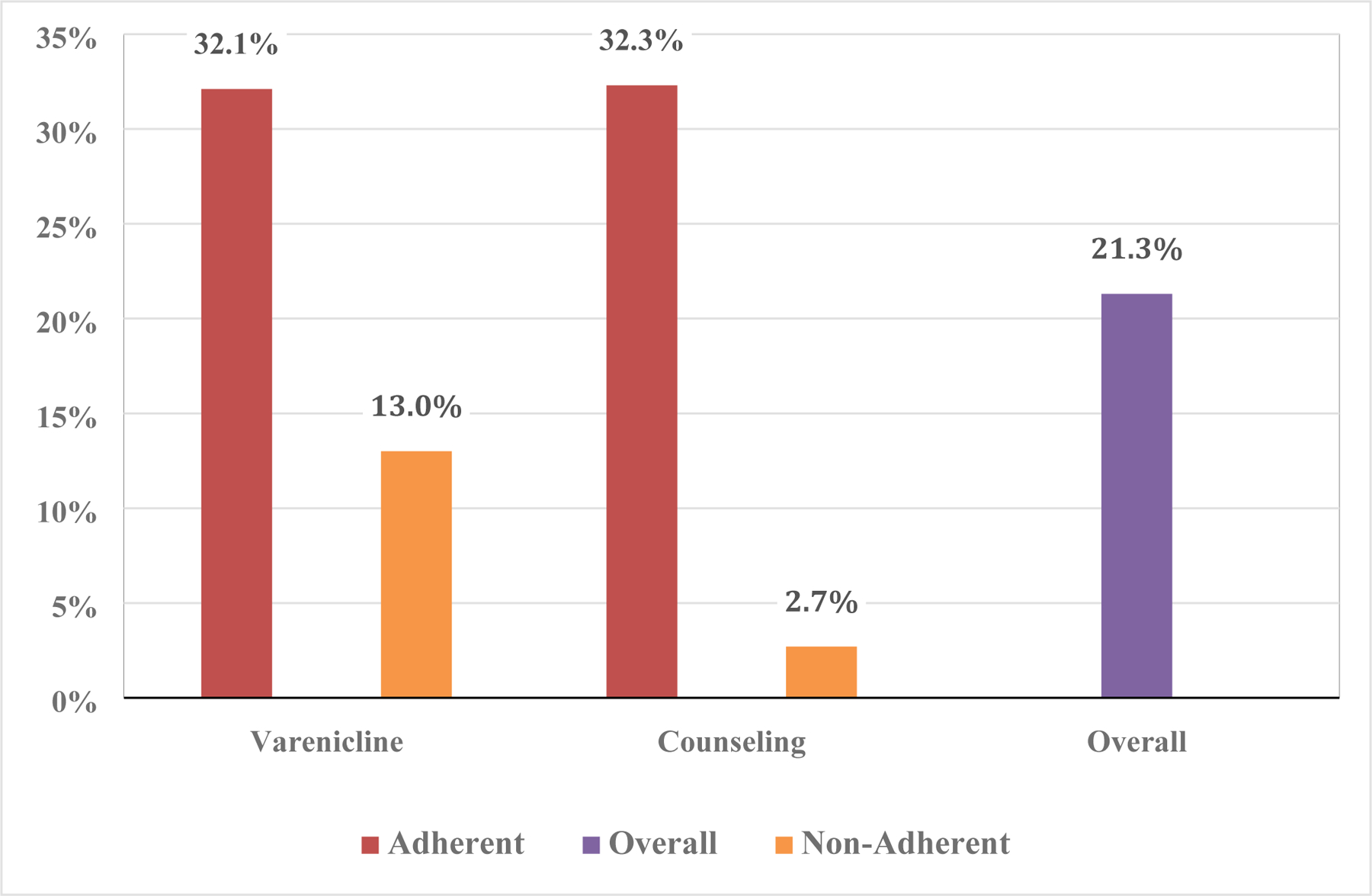
Overall, 43.7% of participants were adherent with study medication and 63% were adherent with counseling; 41% were adherent to counseling and medication and 34.3% were non-adherent to both. Adherence to counseling and medication were correlated (χ2[1]=101.51, p<.001). Figure 1 shows the rates of cessation at EOT, overall and stratified by counseling and medication adherence. Medication adherence was significantly related to EOT cessation rates (χ2[1]=15.93, p<.001); among participants adherent with medication, the EOT quit rate was 32.1%, vs. 13.0% for those who were not adherent. Counseling adherence was significantly associated with EOT cessation rates (χ2[1]=45.69, p<.001); among participants who were adherent with counseling, the EOT quit rate was 32.3%, vs. 2.7% among participants who were not adherent.
Figure 1.
Abstinence Rates, Overall and Stratified by Adherence Groups
3.3. Correlates of Medication and Counseling Adherence
Table 1 shows the univariate associations between medication and counseling adherence and demographics and clinical characteristics. Participants who were adherent with medication were more likely to identify as other race, older, had higher BMI, reported a longer history of smoking, and showed a higher baseline level of smoking reward. Adherence was also associated with a significant increase in the use of substitute reinforcers (M=2.6), vs. a decrease (M=−1.7) for participants who were non-adherent with medication. Both groups showed a decrease in the use of complementary reinforcers over time, but non-adherent participants showed a significantly greater decrease (M=−12.2) versus adherent participants (M=−6.3).
Participants who were adherent with counseling were more likely to be female, show higher BMI, report lower alcohol use, nicotine dependence, and average suicide risk, and higher baseline smoking reward, vs. participants who were non-adherent with counseling. Participants who were adherent with counseling also showed a significant increase in the use of substitute reinforcers (M=3.6), whereas participants who were non-adherent with counseling showed a significant decrease in the use of substitute reinforcers (M=−4.4). Lastly, while both participants who were adherent with counseling and those who were not adherent showed decreased engagement in complementary reinforcers, non-adherent participants exhibited a significantly greater decrease (M=−13.2) versus adherent participants (M=−7.0).
3.4. Multivariate Models of Adherence
The regression models are shown in Table 2. Participants who were classified as other race were more likely to be non-adherent with medication; higher baseline reward from smoking and higher engagement in complementary reinforcers was associated with increased medication adherence. Counseling adherence was associated with lower alcohol use, being female, higher baseline reward from smoking, and nicotine dependence, and greater engagement and higher engagement in substitute and complementary reinforcers over time (controlling for baseline).
Table 2.
Logistic Regression Predicting Medication and Counseling Adherence
| Odds Ratio | 95% CI | P | |
|---|---|---|---|
| Varenicline Adherence | |||
| Race (Reference = Black) | |||
| White | 0.94 | .52, 1.70 | .84 |
| Other | 0.16 | .04, .59 | .006 |
| Age | 1.53 | .92, 2.55 | .10 |
| BMI | 1.24 | .95, 1.61 | .11 |
| Years Smoked | 0.81 | .50, 1.32 | .40 |
| Change in Complementary Reinforcers | 1.60 | 1.11, 2.32 | .012 |
| Change in Substitute Reinforcers | 1.15 | .86, 1.54 | .33 |
| Baseline Smoking Reward | 1.42 | 1.08, 1.88 | .013 |
| Baseline Complementary Reinforcers | 1.1 | .80, 1.5 | .57 |
| Baseline Substitute Reinforcers | 1.07 | .79, 1.44 | .66 |
| Counseling Adherence | |||
| Alcohol Use (Reference = Drinker) | 2.42 | 1.32, 4.43 | .004 |
| Sex (Reference = Male) | 2.34 | 1.31, 4.18 | .004 |
| BMI | 1.12 | .84, 1.51 | .43 |
| Change in Complementary Reinforcers | 2.27 | 1.46, 3.54 | <.001 |
| Change in Substitute Reinforcers | 2.14 | 1.51, 3.02 | <.001 |
| FTCD | .62 | .45, .86 | .005 |
| C-SSRS | 1.22 | .86, 1.73 | .26 |
| Baseline Smoking Reward | 1.98 | 1.43, 2.74 | <.001 |
| Baseline Complementary Reinforcers | 1.71 | 1.15, 2.53 | .007 |
| Baseline Substitute Reinforcers | 1.99 | 1.36, 2.93 | <.001 |
Note. N = 296 for model of varenicline adherence from missing data; n = 300 for counseling adherence. Baseline measures of reinforcers included as control for change score.
4. Discussion
This study assessed level and correlates of medication and counseling adherence among smokers with current or a history of depression being treated with intensive counseling and varenicline and described the association between medication and counseling adherence and cessation rates. Overall, we found low rates of counseling and medication adherence, both of which were strongly associated with a significantly lower likelihood of cessation. Further, the findings show that engagement with reinforcers during the initial weeks of treatment and sex, nicotine dependence, alcohol use, and smoking reward are associated with adherence. These findings and their clinical and research implications are discussed below.
First, this study shows that non-adherence to medication and intensive counseling is a substantial clinical problem for smokers with MDD. The rate of varenicline adherence in the present study converges with rates seen in the general population and with clinical populations such as smokers with cancer and HIV (i.e., 52–67%; Liberman et al., 2013; Bauer et al., 2021; Crawford et al., 2019) although the rates were lower than reported in a large trial testing brief behavioral treatment plus either varenicline, bupropion or nicotine patch among smokers with clinically stable serious mental illness and no active substance use disorder (i.e., >75%; Correa et al., 2021). The rate of counseling adherence in the present study was lower than seen in the general population (i.e., 72%; Okuyemi et al., 2010), which could indicate that smokers with MDD experience more challenges complying with intensive behavioral smoking cessation interventions such as low positive affect and higher negative affect or cognitive impairment (Mathew et al., 2017). Thus, as seen in the general population of smokers and among smokers with medical comorbidities, adherence to evidence-based tobacco treatments remains a serious challenge for adults with past or present MDD.
Second, the present findings show that non-adherence to behavioral counseling and medication substantially reduces an individual’s likelihood of cessation. With regard to varenicline, participants in the present trial who were adherent had a greater than 2-fold rate of EOT cessation vs. those who were non-adherent. This result aligns with data from the general population (Catz et al., 2011; Peng et al., 2018) and from a trial involving smokers with medical (Bauer et al., 2021; Crawford et al., 2018) and substance abuse (Rohsenow et al., 2017) comorbidities. The effect of adherence on cessation was largest for behavioral counseling. Indeed the lowest quit rate was among those who were non-adherent with behavioral counseling, which is similar to a previous study that assessed the association between counseling adherence and smoking cessation (Hood et al., 2013).
The present results also indicate that certain subgroups of smokers – men, those who regularly use alcohol, those with high levels of nicotine dependence, and those who report lower reward from smoking – are at high risk of showing non-adherence to tobacco cessation treatment, particularly behavioral counseling1. Men have been found to show greater adherence to medication in past studies (e.g., Catz et al., 2011; Pacek et al., 2018) but in this study they exhibited lower adherence to counseling. This is consistent with smoking cessation counseling programs for young adults with mental health issues (Prochaska et al., 2015) and with the broader literature showing that men are more responsive to smoking cessation mediations while women are more responsive to behavioral interventions (Perkins, 2001). Likewise, our findings concerning nicotine dependence and alcohol use and counseling adherence extend the literature that shows that higher levels of nicotine dependence and alcohol use are related to lower adherence to tobacco use medications (Pacek et al., 2018). The findings concerning smoking reward may indicate that those who smoke for reasons aside from perceived rewards may be at greater risk for treatment non-adherence and may need support to engage in tobacco interventions.
Lastly, as the field considers the development of interventions to specifically address treatment compliance, the present data suggest that such interventions may consider targeting complementary and substitute reinforcers. In the present study, participants who were adherent to counseling showed a significant increase in the use of substitute reinforcers over the early weeks of the intervention, whereas non-adherent participants showed a decrease in substitute reinforcers.
Similar to a previous study that showed that higher nicotine patch adherence was associated with increased engagement with substitute reinforcers (Handschin et al., 2018), our findings further suggest that supporting smokers to engage in healthy alternative sources of reinforcement (e.g., going for walks, snacking, engaging in social support) during a quit attempt can support successful cessation potentially through treatment adherence. However, the findings concerning complementary reinforcers and adherence to both counseling and medication were not completely aligned with other studies. While both adherent and non-adherent participants have been shown to exhibit a decrease in engagement with complementary reinforcers (Handschin et al., 2018), our findings indicate that participants not adherent to counseling or medication show a significantly greater reduction in engagement with complementary reinforcers vs. adherent participants, suggesting that non-adherent participants may potentially be reducing their engagement in complementary reinforcers instead of engaging in counseling and medication to support their cessation attempt. Notably, differences across studies could be driven by the differences in the study samples since the present sample included those with psychiatric comorbidities versus the general population of smokers.
4.1. Limitations
These findings should be considered in the context of limitations. First, the sample for this study was from a clinical trial that used specific inclusion and exclusion criteria and, as such, the results may not generalize to the broader population of smokers with current or past MDD. Yet, recruitment efforts were successful in enrolling individuals with diverse comorbid psychiatric and medical conditions, were drawn from the community in two different cities, and involved individuals with limited physical and social resources. Second, medication adherence was assessed using self-report, which may be less reliable than biological measures (Peng et al., 2018).
However, we collected weekly measures of medication adherence to minimize recall bias. Lastly, although we included prospective measures for many of our predictors of adherence (e.g., change in reinforcers during the first two weeks of treatment), our study design does not permit causal inferences between predictors and adherence.
4.2. Conclusions
Nevertheless, this study fills a gap in our understanding of the impact of treatment adherence on cessation outcomes among smokers with current and past MDD. The results show that medication and counseling non-adherence in this population of smokers is highly prevalent and significantly associated with cessation outcomes and provides information about how to design and target interventions designed to increase treatment adherence. Future studies are needed to evaluate interventions developed to address treatment non-adherence in this under-served community of smokers.
Non-adherence to tobacco use medication and counseling among smokers with MDD is common
Smokers with MDD who are adherent to medication and counseling are more likely to quit
Interventions that increase substitute reinforcers to tobacco use may increase adherence
Reducing complementary reinforcers rather than adhering to treatment may undermine cessation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interests
Dr. Schnoll has received varenicline and placebo free from Pfizer and has provided consultation to Pfizer. Dr. Schnoll has provided consultation to GlaxoSmithKline and Palliatech. Dr. Hitsman has received varenicline and placebo free from Pfizer.
Author CRediT Statement
ShelDan Dalsimer and Robert Schnoll: conceptualization, formal analysis, writing – original and editing; Gabrielle Barrila: writing – original and editing; Mackenzie Hosie Quinn: writing – editing; Matthew Olonoff: investigation, writing – editing; Anna-Marika Bauer: investigation, writing – editing, project administration; Erica Fox: investigation, writing – editing, project administration; Nancy Jao: investigation, writing – editing; Mark Huffman: investigation, writing – editing; Sadiya Khan: investigation, writing – editing; Frank Leone: investigation, writing – editing; Jacqueline K Gollan: investigation, writing – editing; George D. Papandonatos: methodology, data curation, writing – editing; Brian Hitsman: conceptualization, investigation, writing – original and editing, supervision, project administration, and funding; Robert Schnoll: conceptualization, investigation, writing – original and editing, supervision, and project administration.
That “Other” race was significantly associated with lower medication adherence is challenging to interpret given the small sample and heterogeneous nature of the group but potentially important at a population level.
REFERENCES
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). 10.1176/appi.books.9780890425596 [DOI] [Google Scholar]
- Anthenelli RM, Benowitz NL, West R, St Aubin L, McRae T, Lawrence D, Ascher J, Russ C, Krishen A, & Evins AE (2016). Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet (London,England), 387(10037),2507–2520. 10.1016/S01406736(16)30272-0 [DOI] [PubMed] [Google Scholar]
- Audrain-McGovern J, Rodriguez D, Rodgers K, & Cuevas J (2011). Declining alternative reinforcers link depression to young adult smoking. Addiction (Abingdon, England), 106(1), 178–187. 10.1111/j.1360-0443.2010.03113.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, & Brown GK (1996). Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Biener L, & Abrams DB (1991). The Contemplation Ladder: validation of a measure of readiness to consider smoking cessation. Health psychology : official journal of the Division of Health Psychology, American Psychological Association, 10(5), 360–365. 10.1037//0278-6133.10.5.360 [DOI] [PubMed] [Google Scholar]
- Brody DJ, Pratt LA, & Hughes JP (2018). Prevalence of Depression Among Adults Aged 20 and Over: United States, 2013–2016. NCHS data brief, (303), 1–8. [PubMed] [Google Scholar]
- Buckley TC, Mozley SL, Holohan DR, Walsh K, Beckham JC, & Kassel JD (2005). A psychometric evaluation of the Fagerström Test for Nicotine Dependence in PTSD smokers. Addictive behaviors, 30(5),1029–1033. 10.1016/j.addbeh.2004.09.005 [DOI] [PubMed] [Google Scholar]
- Catz SL, Jack LM, McClure JB, Javitz HS, Deprey M, Zbikowski SM, McAfee T, Richards J, & Swan GE (2011). Adherence to varenicline in the COMPASS smoking cessation intervention trial. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco, 13(5), 361–368. 10.1093/ntr/ntr003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa JB, Lawrence D, McKenna BS, Gaznick N, Saccone PA, Dubrava S, Doran N, & Anthenelli RM (2021). Psychiatric Comorbidity and Multimorbidity in the EAGLES Trial: Descriptive Correlates and Associations With Neuropsychiatric Adverse Events, Treatment Adherence, and Smoking Cessation. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco, 23(10), 1646–1655. 10.1093/ntr/ntab056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, & Christen AG (2001). Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco, 3(1), 7–16. 10.1080/14622200020032051 [DOI] [PubMed] [Google Scholar]
- Crawford G, Weisbrot J, Bastian J, Flitter A, Jao NC, Carroll A, Kalhan R, Leone F, Hitsman B, & Schnoll R (2019). Predictors of Varenicline Adherence Among Cancer Patients Treated for Tobacco Dependence and its Association With Smoking Cessation. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco, 21(8), 1135–1139. 10.1093/ntr/nty133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druss BG, & Walker ER (2011). Mental disorders and medical comorbidity. The Synthesis project. Research synthesis report, (21), 1–26. [PubMed] [Google Scholar]
- Fiore MC; Jaen CR; Baker TB; Bailey WC; Benowitz NL; Curry SJ; Dorfman SF; Froelicher ES; Goldstein MG; Healton CG; Henderson PN; Heyman RB; Koh HK; Kottke TE; Lando HA; Mecklenburg RE; Mermelstein RJ; Mullen PD; Orleans CT; Robinson L Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline. www.ahrq.gov/path/tobacco.htm#clinic. Accessed November 15, 2018. [Google Scholar]
- Franken IH, Rassin E, & Muris P (2007). The assessment of anhedonia in clinical and non-clinical populations: further validation of the Snaith-Hamilton Pleasure Scale (SHAPS). Journal of affective disorders, 99(1–3), 83–89. 10.1016/j.jad.2006.08.020 [DOI] [PubMed] [Google Scholar]
- Grenard JL, Munjas BA, Adams JL, Suttorp M, Maglione M, McGlynn EA, & Gellad WF (2011). Depression and medication adherence in the treatment of chronic diseases in the United States: a meta-analysis. Journal of general internal medicine, 26(10), 1175–1182. 10.1007/s11606-011-1704-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood NE, Ferketich AK, Paskett ED, & Wewers ME (2013). Treatment adherence in a lay health adviser intervention to treat tobacco dependence. Health education research, 28(1), 72–82. 10.1093/her/cys081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handschin J, Hitsman B, Blazekovic S, Veluz-Wilkins A, Wileyto EP, Leone FT, & Schnoll RA (2018). Factors Associated with Adherence to Transdermal Nicotine Patches within a Smoking Cessation Effectiveness Trial. Journal of smoking cessation, 13(1), 33–43. 10.1017/jsc.2017.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawes MR, Roth KB, & Cabassa LJ (2021). Systematic Review of Psychosocial Smoking Cessation Interventions for People with Serious Mental Illness. Journal of dual diagnosis, 17(3), 216–235. 10.1080/15504263.2021.1944712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitsman B, Abrams DB, Shadel WG, Niaura R, Borrelli B, Emmons KM, Brown RA, Swift RM, Monti PM, Rohsenow DJ, & Colby SM (2002). Depressive symptoms and readiness to quit smoking among cigarette smokers in outpatient alcohol treatment. Psychology of addictive behaviors : journal of the Society of Psychologists in Addictive Behaviors, 16(3), 264–268. [PubMed] [Google Scholar]
- Hitsman B, Papandonatos GD, McChargue DE, DeMott A, Herrera MJ, Spring B, Borrelli B, Niaura R. Past major depression and smoking cessation outcome: a systematic review and meta-analysis update. Addiction. 2013. Feb;108(2):294–306. doi: 10.1111/add.12009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitsman B, Papandonatos G, Gollam J, Huffman M, Niaura R, Mohr D, Veluz-Wilkins A, Hole A, Lubitz SF, Leone F, Khan S, Fox E, Bauer A-M, Wileyto EP, & Schnoll R (2022). Efficacy and safety of combination behavioral activation therapy and varenicline for smoking cessation among smokers with major depression: A randomized double-blind placebo-controlled trial. Addiction. [DOI] [PubMed] [Google Scholar]
- Hughes JR, & Hatsukami D (1986). Signs and symptoms of tobacco withdrawal. Archives of general psychiatry, 43(3), 289–294. 10.1001/archpsyc.1986.01800030107013 [DOI] [PubMed] [Google Scholar]
- Liberman JN, Lichtenfeld MJ, Galaznik A, Mastey V, Harnett J, Zou KH, Leader JB, & Kirchner HL (2013). Adherence to varenicline and associated smoking cessation in a community-based patient setting. Journal of managed care pharmacy : JMCP, 19(2), 125–131. 10.18553/jmcp.2013.19.2.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew AR, Hogarth L, Leventhal AM, Cook JW, & Hitsman B (2017). Cigarette smoking and depression comorbidity: systematic review and proposed theoretical model. Addiction (Abingdon, England), 112(3), 401–412. 10.1111/add.13604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuyemi KS, Zheng H, Guo H, & Ahluwalia JS (2010). Predictors of adherence to nicotine gum and counseling among African-American light smokers. Journal of general internal medicine, 25(9), 969–976. 10.1007/s11606-010-1386-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacek LR, McClernon FJ, & Bosworth HB (2018). Adherence to Pharmacological Smoking Cessation Interventions: A Literature Review and Synthesis of Correlates and Barriers. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco, 20(10), 1163–1172. 10.1093/ntr/ntx210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng AR, Morales M, Wileyto EP, Hawk LW Jr, Cinciripini P, George TP, Benowitz NL, Nollen NL, Lerman C, Tyndale RF, & Schnoll R (2017). Measures and predictors of varenicline adherence in the treatment of nicotine dependence. Addictive behaviors, 75, 122–129. 10.1016/j.addbeh.2017.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng AR, Schnoll R, Hawk LW Jr, Cinciripini P, George TP, Lerman C, & Tyndale RF (2018). Predicting smoking abstinence with biological and self-report measures of adherence to varenicline: Impact on pharmacogenetic trial outcomes. Drug and alcohol dependence, 190, 72–81. 10.1016/j.drugalcdep.2018.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA (2001). Smoking cessation in women. Special considerations. CNS drugs, 15(5), 391–411. 10.2165/00023210-200115050-00005 [DOI] [PubMed] [Google Scholar]
- Posner K, Brown GK, Stanley B, Brent DA, Yershova KV, Oquendo MA, Currier GW, Melvin GA, Greenhill L, Shen S, & Mann JJ (2011). The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. The American journal of psychiatry, 168(12), 1266–1277. 10.1176/appi.ajp.2011.10111704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochaska JJ, Fromont SC, Ramo DE, Young-Wolff KC, Delucchi K, Brown RA, & Hall SM (2015). Gender differences in a randomized controlled trial treating tobacco use among adolescents and young adults with mental health concerns. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco, 17(4), 479–485. 10.1093/ntr/ntu205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohsenow DJ, Tidey JW, Martin RA, Colby SM, Swift RM, Leggio L, & Monti PM (2017). Varenicline versus nicotine patch with brief advice for smokers with substance use disorders with or without depression: effects on smoking, substance use and depressive symptoms. Addiction (Abingdon, England), 112(10), 1808–1820. 10.1111/add.13861 [DOI] [PubMed] [Google Scholar]
- Schnoll RA, Hitsman B, Blazekovic S, Veluz-Wilkins A, Wileyto EP, Leone FT, Audrain-McGovern JE. Longitudinal changes in smoking abstinence symptoms and alternative reinforcers predict long-term smoking cessation outcomes. Drug Alcohol Depend. 2016. Aug 1;165:245–52. doi: 10.1016/j.drugalcdep.2016.06.017. Epub 2016 Jun 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, & Dunbar GC (1998). The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. The Journal of clinical psychiatry, 59 Suppl 20, 22–57. [PubMed] [Google Scholar]
- Siegel SD, Lerman C, Flitter A, & Schnoll RA (2020). The Use of the Nicotine Metabolite Ratio as a Biomarker to Personalize Smoking Cessation Treatment: Current Evidence and Future Directions. Cancer prevention research (Philadelphia, Pa.), 13(3), 261–272. 10.1158/1940-6207.CAPR-19-0259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spring B, Pingitore R, & McChargue DE (2003). Reward value of cigarette smoking for comparably heavy smoking schizophrenic, depressed, and nonpatient smokers. The American journal of psychiatry, 160(2), 316–322. 10.1176/appi.ajp.160.2.316 [DOI] [PubMed] [Google Scholar]
- Weinberger AH, Chaiton MO, Zhu J, Wall MM, Hasin DS, & Goodwin RD (2020). Trends in the Prevalence of Current, Daily, and Nondaily Cigarette Smoking and Quit Ratios by Depression Status in the U.S.: 2005–2017. American journal of preventive medicine, 58(5), 691–698. 10.1016/j.amepre.2019.12.023 [DOI] [PubMed] [Google Scholar]


