Abstract
Introduction:
High-grade histologic patterns are associated with poor prognosis in patients with primary nonmucinous lung adenocarcinoma (ADC). We investigated whether the presence of micropapillary and/or solid patterns (MIP/SOL) in lymph node (LN) metastases has prognostic value.
Methods:
Patients who underwent lobectomy for pathologic stage II-III lung ADC with N1 or N2 LN metastases (n=360; 2000–2012) were analyzed. We assessed overall survival (OS), lung cancer–specific cumulative incidence of death (LC-CID), and cumulative incidence of recurrence (CIR) between patients with and without MIP/SOL patterns in LN metastases. Multivariable Cox regression analysis was used to quantify the association between MIP/SOL patterns and outcomes.
Results:
Micropapillary and solid patterns in LN metastases were associated with a higher incidence of smoking history (p=0.004), tumor necrosis (p=0.013), and spread of tumor through air spaces (p<0.0001), a higher prevalence of micropapillary or solid pattern in the primary tumor (p<0.0001), shorter OS (5-year OS, 40% [95% CI, 29%–56%] vs. 63% [48–83%) for no MIP/SOL in LNs; p=0.03), higher LC-CID (5-year, 43% [29%–56%] vs. 14% (4–29%); p=0.013), and higher CIR (5-year, 65% [50%–77%] vs. 43% (25–60%); p=0.057). Micropapillary and solid patterns in LN metastases were independently associated with poor outcomes: OS (hazard ratio [HR], 1.81 [95% CI, 1.00–3.29]; p=0.05), LC-CID (HR, 3.10 [1.30–7.37]; p=0.01), and CIR (HR, 2.06 [1.09–3.90]; p=0.026).
Conclusion:
MIP/SOL histological patterns in N1 or N2 LN metastases are associated with worse outcomes in patients with stage II-III lung ADC. MIP/SOL histological patterns in LN metastases can stratify patients with high-risk stage II-III lung ADC.
Keywords: Nodal metastasis, Non-small cell lung cancer, N classification, Micropapillary adenocarcinoma, Solid adenocarcinoma
INTRODUCTION
In the International Association for the Study of Lung Cancer 8th edition of the staging system, metastatic lymph nodal (N) descriptors are recommended to indicate the anatomical location of the metastasis (N1, N2, and N3), the number of lymph node (LN) stations involved (single vs. multiple), and the absence or presence of skip metastasis (N2 with or without N1 involvement), resulting in the stratification of the N category into N1a, N1b, N2a1, N2a2, N2b, and N3.1 The overlapping survival curves for N1b (multiple N1 stations), N2a1 (single N2 station with skip N1 metastasis), and N2a2 (single N2 station with N1 involvement) provide a rationale to investigate prognostic factors beyond the location and the number of LN metastases.2–5
In addition to primary tumor (T) size, predominant histologic patterns of nonmucinous lung adenocarcinoma (ADC)6, 7 are associated with prognosis and can be used to stratify lung ADC into three prognostic groups: low grade (lepidic [LEP] predominant), intermediate grade (acinar [ACI] or papillary [PAP] predominant), and high grade (micropapillary [MIP] or solid [SOL] predominant)—the latter of which is associated with the worst prognosis.7–9 We have shown that the SOL-predominant histologic subtype in patients with resected stage I lung ADC is an independent predictor of early, extrathoracic, multisite recurrence and of poor postrecurrence survival.10 We and others have shown that the presence of “nonpredominant” MIP pattern is associated with high rates of locoregional recurrence after limited resection, a high rate of occult LN metastases, and poor prognosis.11–14 Both MIP and SOL histologic patterns are associated with spread of tumor through air spaces (STAS), which is a form of invasion that has been shown to be correlated with poor prognosis.15–17 MIP/SOL tumors had higher tumor mutational burden, fraction of genome altered, copy number amplifications, rate of whole-genome doubling, and number of oncogenic pathways altered as compared with LEP and ACI/PAP tumors.18 Although the observations mentioned above lend further evidence that MIP and/or SOL histologic patterns in the primary tumor are associated with a poor prognosis, the prognostic value of histologic patterns in LN metastases remains unexplored.
Herein, we investigate the prognostic value of histologic patterns in LN metastases by use of a consecutive cohort of patients with primary nonmucinous lung ADC with LN metastases who underwent anatomic resection for pathologic stage II-III lung ADC.
MATERIALS AND METHODS
Study Population
In this retrospective study, we identified patients with pathologic stage II-III lung ADC who underwent curative-intent resection at Memorial Sloan Kettering Cancer Center (MSK) from 2000 to 2012 (n=1169). Pathologic stage was based on the eighth edition of the American Joint Committee on Cancer Staging Manual.19 Patients were excluded if they had received any induction therapy, had multiple nodules, underwent treatment for another type of lung cancer within the previous 2 years, had positive margins (R1 or R2 resection), had concurrent progressive disease other than lung cancer, or had undergone segmentectomy or wedge resection. After histologic evaluation, patients were excluded if they were diagnosed with invasive mucinous ADC or colloid-predominant ADC, had no LN metastasis, or had inadequate LN tissue for analysis (Figure 1). Of the 1169 patients identified, 360 were analyzed in this study. Clinical data were collected from MSK’s prospectively maintained Thoracic Surgery Lung Cancer database. The institutional review board at MSK approved this study.
Figure 1.
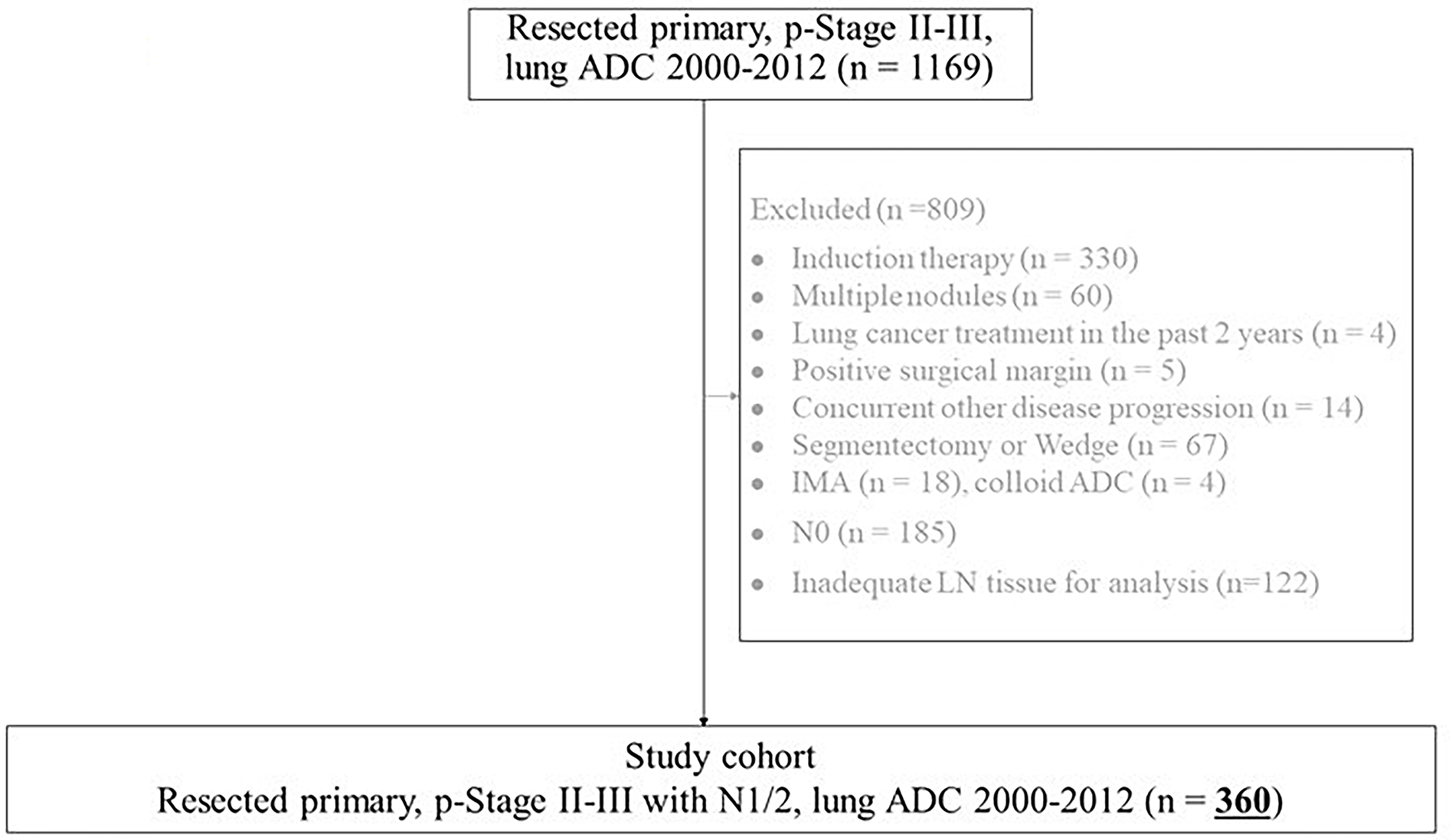
Patient flowchart. ADC, adenocarcinoma; IMA, invasive mucinous adenocarcinoma; LN, lymph node.
Histologic Evaluation
By the use of a multi-head Olympus BX51 microscope (Olympus, Tokyo, Japan) with a standard 22-mm diameter eyepiece, all available hematoxylin and eosin–stained tumor and LN slides were reviewed by three pathologists (Y.L., S.H.L., and W.D.T.), who were blinded to patient clinical outcomes. Any discrepancies in histologic evaluation among the pathologists were later resolved by consensus meeting. The percentage of each histologic pattern in primary tumors and LN metastases was recorded in 5% increments, and tumors were classified by the predominant pattern in accordance with the International Association for the Study of Lung Cancer, American Thoracic Society, and European Respiratory Society and WHO (2021) classifications: ADC in situ; minimally invasive ADC; LEP, ACI, PAP, MIP, and SOL invasive ADC; invasive mucinous ADC; and colloid ADC.6, 7, 20 We also investigated lymphatic, vascular, and pleural invasions, tumor necrosis, and the presence of STAS. STAS was defined as tumor cells within air spaces in the lung parenchyma beyond the edge of the main tumor.15, 21 Pathologic invasive tumor size was determined as the size of the invasive components, excluding LEP component, on microscopic examination.22 The location, number,1 and histologic patterns (only ACI, PAP, MIP, and SOL) of LN metastases were recorded.
Statistical Analysis
Patient demographic and clinical characteristics were summarized using frequency (percentage) or median (interquartile range, IQR) and compared between groups using chi-squared test for categorical variables and Kruskal-Wallis test for continuous variables. The primary endpoint was overall survival (OS; duration from surgery to death). The secondary endpoints were lung cancer–specific cumulative incidence of death (LC-CID), and cumulative incidence of recurrence (CIR). OS was estimated using the Kaplan-Meier approach from the time of surgery to death of any cause and compared between groups using log-rank tests. Patients were otherwise censored on the date of last follow-up. Relationships between groups and OS were quantified using Cox proportional hazards analyses.23 As a secondary endpoint, lung cancer–specific survival was evaluated using a competing-risk approach from the time of surgery to the time of death from lung cancer.24 Non–lung cancer death was considered a competing-risk event. LC-CID was compared between groups using Gray’s test, and association between variables and lung cancer-specific deaths was quantified using Fine and Gray competing-risk regression. As in the analysis for LC-CID, CIR was analyzed in the competing-risk analysis framework. CIR was used to estimate the probability of recurrence after surgical resection with curative intent.24 Death without recurrence was treated as a competing event. Patients were otherwise censored at the time of the last available follow-up (assessed in September 2016). Multivariable models for each outcome included a set of clinically important factors define a priori: all models included STAS, invasive tumor size, location of involved LN (N1 or N2), number of involved stations (single or multiple); the models for OS and LC-CID included age at surgery.
All statistical tests were two-sided, and statistical significance was defined as p<0.05. Statistical analyses were performed using R 3.1.1 (R Foundation for Statistical Computing, Vienna, Austria); the “survival” and “cmprsk” software packages were used for the analyses.
RESULTS
Patient Clinicopathologic Characteristics
Patient clinicopathologic characteristics are presented on Table 1. The median age of all patients was 68.3 years (interquartile range, 61.0–74.8 years), 68% of patients (n=244) were women, and 82% of patients (n=294) had a history of smoking. Involved LNs were p-N2 in 51% of patients (n=182); 34% of patients (n=122) had a single involved LN station, and 66% (n=238) had multiple involved LN stations. We stratified all patients by the presence or absence of a high-grade pattern (MIP and/or SOL) in LN metastases as follows: no MIP or SOL (neither), MIP but not SOL (MIP [no SOL] in LN), SOL but not MIP (SOL [no MIP] in LN), and both MIP and SOL (both in LN) in LN metastases. Patients with MIP and/or SOL patterns in LN metastases were more likely to have a history of smoking (p=0.004) and have necrosis (p=0.013) and had a higher incidence of STAS in primary tumors (p<0.0001). Patients with MIP only or SOL only in LN metastases had a higher incidence of MIP-predominant (21/75 [28%]) or SOL-predominant (91/197 [46%]) primary tumors, respectively (p<0.0001). Representative images of each histologic pattern observed in LN metastases are shown in Figure, Supplemental Data 1.
Table 1.
Clinicopathologic characteristics of patients with stage II-III lung adenocarcinoma
| Characteristic | All patients (N=360) |
Neither MIP nor SOL in LN (n=31; 8.6%) |
MIP (no SOL) in LN (n=75; 21%) |
SOL (no MIP) in LN (n=197; 55%) |
Both MIP and SOL in LN (n=57; 16%) |
p |
|---|---|---|---|---|---|---|
|
| ||||||
| Age, years | 68.3 (61.0–74.8) | 72.2 (63.4–78.7) | 68.2 (60.4–74.0) | 67.7 (59.8–74.3) | 70.0 (64.2–75.1) | 0.2 |
| Sex | 0.7 | |||||
| Female | 244 (68) | 21 (68) | 47 (63) | 135 (69) | 41 (72) | |
| Male | 116 (32) | 10 (32) | 28 (37) | 62 (31) | 16 (28) | |
| Smoking ever | 0.004 | |||||
| Absence | 66 (18) | 12 (39) | 18 (24) | 27 (14) | 9 (16) | |
| Presence | 294 (82) | 19 (61) | 57 (76) | 170 (86) | 48 (84) | |
| Primary tumor | ||||||
| LVI | ||||||
| Absence | 66 (18) | 8 (26) | 11 (15) | 37 (19) | 10 (18) | 0.6 |
| Presence | 294 (82) | 23 (74) | 64 (85) | 160 (81) | 47 (82) | |
| Necrosis (N=329) | 0.013 | |||||
| Absence | 263 (71) | 26 (87) | 53 (79) | 113 (63) | 40 (74) | |
| Presence | 97 (29) | 4 (13) | 14 (21) | 65 (37) | 14 (26) | |
| STAS (N=352) | ||||||
| Absence | 123 (33) | 17 (55) | 12 (17) | 79 (41) | 7 (12) | <0.0001 |
| Presence | 237 (67) | 14 (45) | 60 (83) | 113 (59) | 50 (88) | |
| Invasive tumor size, cm | 2.7 (1.9–3.8) | 3.1 (2.3–4.0) | 2.3 (1.8–3.2) | 2.7 (2.0–3.9) | 3.1 (2.0–3.8) | 0.088 |
| IASLC8 | <0.0001 | |||||
| Lepidic | 7 (1.9) | 0 (0) | 0 (0) | 5 (2.5) | 2 (3.5) | |
| Acinar | 155 (43) | 23 (74) | 30 (40) | 80 (41) | 22 (39) | |
| Papillary | 39 (11) | 7 (23) | 21 (28) | 7 (3.6) | 4 (7.0) | |
| MIP | 49 (14) | 0 (0) | 21 (28) | 14 (7.1) | 14 (25) | |
| SOL | 110 (31) | 1 (3.2) | 3 (4.0) | 91 (46) | 15 (26) | |
| MIP or SOL status | <0.0001 | |||||
| No MIP or SOL | 15 (4.2) | 8 (26) | 0 (0) | 7 (3.6) | 0 (0) | |
| Either MIP or SOL presence | 71 (20) | 9 (29) | 27 (36) | 24 (12) | 11 (19) | |
| Either MIP or SOL secondary predominant | 115 (32) | 13 (42) | 24 (32) | 61 (31) | 17 (30) | |
| Either MIP or SOL predominant LN | 159 (44) | 1 (3.2) | 24 (32) | 105 (53) | 29 (51) | |
| Location of involved LN | 0.13 | |||||
| N1 | 178 (49) | 18 (58) | 35 (47) | 104 (53) | 21 (37) | |
| N2 | 182 (51) | 13 (42) | 40 (53) | 93 (47) | 36 (63) | |
| No. of involved LN stations | 0.5 | |||||
| Single | 122 (34) | 13 (42) | 25 (33) | 69 (35) | 15 (26) | |
| Multiple | 238 (66) | 18 (58) | 50 (67) | 128 (65) | 42 (74) | |
| Predominant histologic pattern in LN | <0.0001 | |||||
| Acinar | 77 (21) | 24 (77) | 20 (27) | 28 (14) | 5 (8.8) | |
| Papillary | 17 (4.7) | 7 (23) | 5 (6.7) | 3 (1.5) | 2 (3.5) | |
| MIP | 70 (19) | 0 (0) | 50 (67) | 0 (0) | 20 (35) | |
| SOL | 196 (54) | 0 (0) | 0 (0) | 166 (84) | 30 (53) | |
| Adjuvant chemotherapy status (N=323) | ||||||
| No adjuvant chemotherapy | 135 (42) | 17 (57) | 25 (38) | 73 (42) | 20 (38) | 0.3 |
| Adjuvant chemotherapy | 188 (58) | 13 (43) | 41 (62) | 102 (58) | 32 (62) | |
| Outcomes, % | ||||||
| 5-year OS (95% CI) | 49 (44–55) | 63 (48–83) | 49 (38–63) | 49 (42–57) | 40 (29–56) | 0.03 |
| 5-year LC-CID (95% CI) | 32 (27–37) | 14 (4–29) | 34 (23–46) | 31 (24–38) | 43 (29–56) | 0.013 |
| 5-year CIR (95% CI) | 53 (47–58) | 43 (25–60) | 53 (40–64) | 51 (43–58) | 65 (50–77) | 0.057 |
Data are presented as no. (%) or median (interquartile range), unless otherwise noted. P values were calculated using the chi-squared test for categorical variables and the Kruskal-Wallis test for continuous variables. ACI, acinar; CI, confidence interval; CIR, cumulative incidence of recurrence; IASLC9, International Association for the Study of Lung Cancer classification, 9th edition; LC-CID, lung cancer–specific cumulative incidence of death; LN, lymph node; LVI, lymphovascular invasion; MIP, micropapillary; OS, overall survival; STAS, spread of tumor through air spaces; SOL, solid. Bold type indicates statistical significance.
Prevalence of MIP and SOL Patterns in LN Metastases and Primary Tumors
The distributions of MIP and SOL patterns in LN metastases, stratified by the location of the involved LN (N1 vs. N2) or the number of involved LN stations (single vs. multiple), are shown in Figures 2A and 2B, respectively. The percentage of MIP pattern in LN metastases increased as N stage increased (p=0.02); this relationship was not observed for SOL (p=0.40; Figure 2A). There were no significant differences in the percentages of MIP or SOL pattern between single and multiple involved LN stations (Figure 2B).
Figure 2.
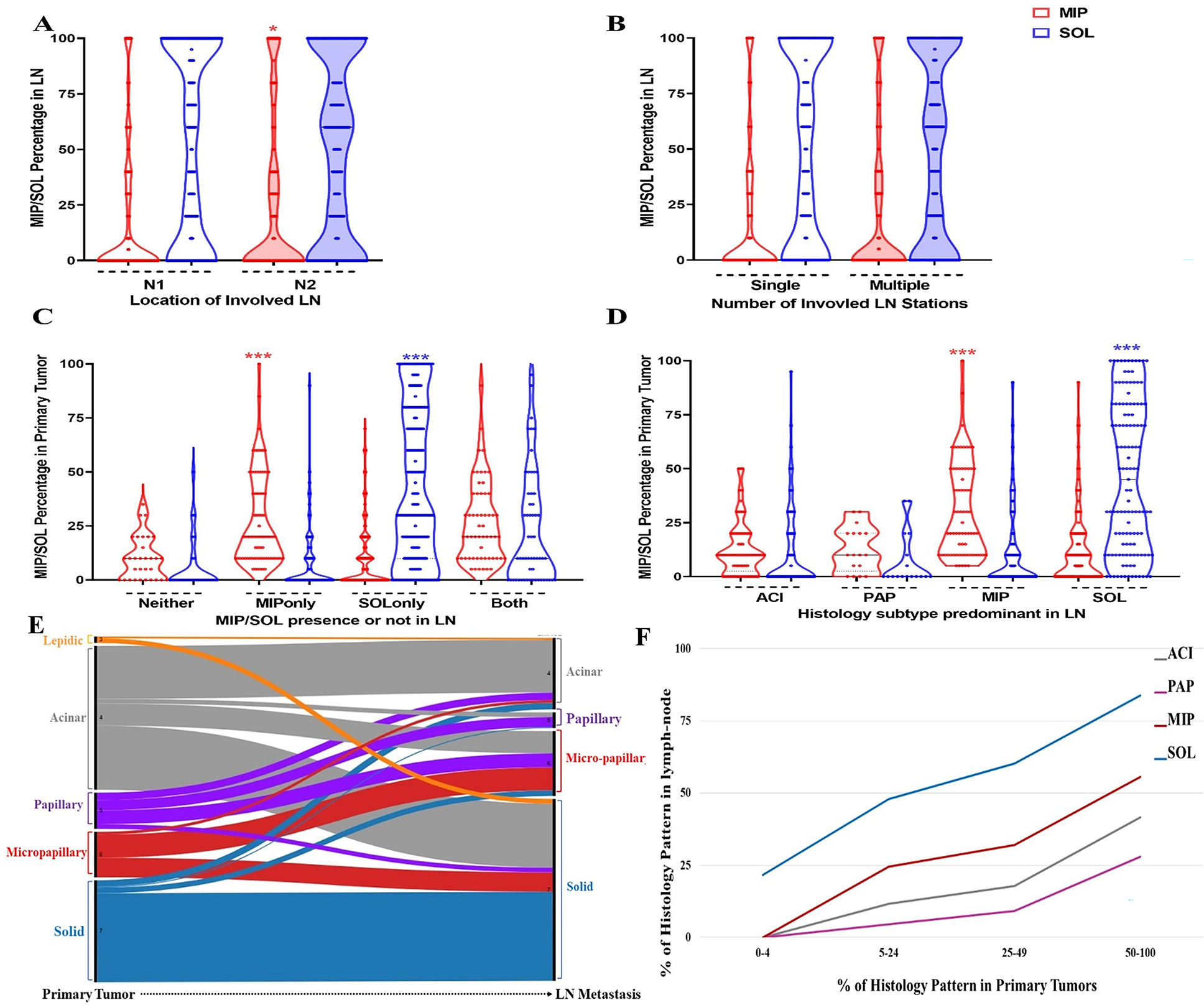
Prevalence of micropapillary (MIP) and solid (SOL) pattern in lymph nodes (LNs) and primary tumors. (A) Violin plot describing the distribution of MIP and SOL pattern in LN from patients with N1 versus N2 staging. (B) Violin plot describing the distribution of MIP and SOL pattern in LN from patients with single versus multiple involved LNs. (C) Violin plot describing the distribution of MIP and SOL pattern in primary tumors of which corresponding LNs had MIP and/or SOL pattern present. (D) Violin plot describing the distribution of MIP and SOL pattern in primary tumors of which corresponding LNs were either acinar (ACI), papillary (PAP), MIP, or SOL predominant. (E) Alluvial plot describing the proportion of patients with each predominant histologic subtype in the primary tumor and in the corresponding LNs. (F) Correlation of the percentage of histologic patterns between primary tumors and LNs. For each histological subtype (ACI, PAP, MIP and SOL), the mean percentage of the pattern in the LN across patients is calculated within four groups of increasing percentages (0–4%, 5–24%, 25–49%, 50–100%) of the pattern in the primary tumor. #, p>0.05; *p<0.05; ***p<0.0001.
The distribution of MIP and SOL pattern in primary tumors, stratified by the presence of MIP and/or SOL - in LN metastases, is shown in Figures 2C. The percentage of MIP pattern in primary tumors was highest in patients with only MIP in LN metastases (median [25th-75th percentile]: 20% [10%–40%]; p<0.0001; Figure 2C). As with MIP, the percentage of SOL pattern in primary tumors was highest in patients with only SOL in LN metastases (30% [10%–80%]; p<0.0001; Figure 2C).
Similar observations were made in patients with MIP-predominant and SOL-predominant LN metastases (Figure 2D). The percentage of MIP or SOL pattern in primary tumors was highest in patients with MIP-predominant or SOL-predominant LN metastases, respectively (p<0.0001; Figure 2D). Of note, 51% of patients with MIP-predominant LN metastases (36/70) and 36% of patients with SOL-predominant LN metastases (71/196) had <25% MIP or SOL pattern in their primary tumor, respectively.
Comparison of Histologic Patterns Between Primary Tumors and LN Metastases
The distribution of predominant patterns between primary tumors and LN metastases is shown in Figure 2E. The most common predominant pattern in LN metastases was SOL (n=196 [54%]), followed by ACI (n=77 [21%]), MIP (n=70 [19%]), and PAP (n=17 [4.7%]). The most common predominant pattern in primary tumors was ACI (n=155 [43%]), followed by SOL (n=110 [30.6%]), MIP (n=49 [13.7%]), PAP (n=39 [10.8%]), and LEP (n=7 [1.9%]).
The percentage of each histologic pattern in LN metastases in relation to the percentage of that pattern in the primary tumor is shown in Figure 2F. Across each histologic pattern, there is a positive correlation between the percent of histological pattern in LN metastases and the percent of the same pattern in the primary tumor. The average percentage of SOL pattern in LN metastases was high even when the percentage of SOL pattern in the primary tumor was low: the average percentage of SOL pattern in LN metastases was 24% when the percentage of SOL pattern in the primary tumor was 0% to 4% and 50% when the percentage of SOL pattern in the primary tumor was 5% to 24%. A similar relationship was observed for MIP pattern.
The Prognostic Value of Histologic Pattern in LN Metastases
Of the 360 patients analyzed, 186 had a recurrence, 222 died during follow-up, and 136 died from lung cancer. The median follow-up was 6.8 years (interquartile range, 4.6 to 9.7 years). When patients were stratified by N1 versus N2 status, patients with N1 status (compared with N2) had better OS (p=0.021; Figure 3D) but not LC-CID (p=0.513; Figure 3E) or CIR (p=0.335; Figure 3F).
Figure 3.
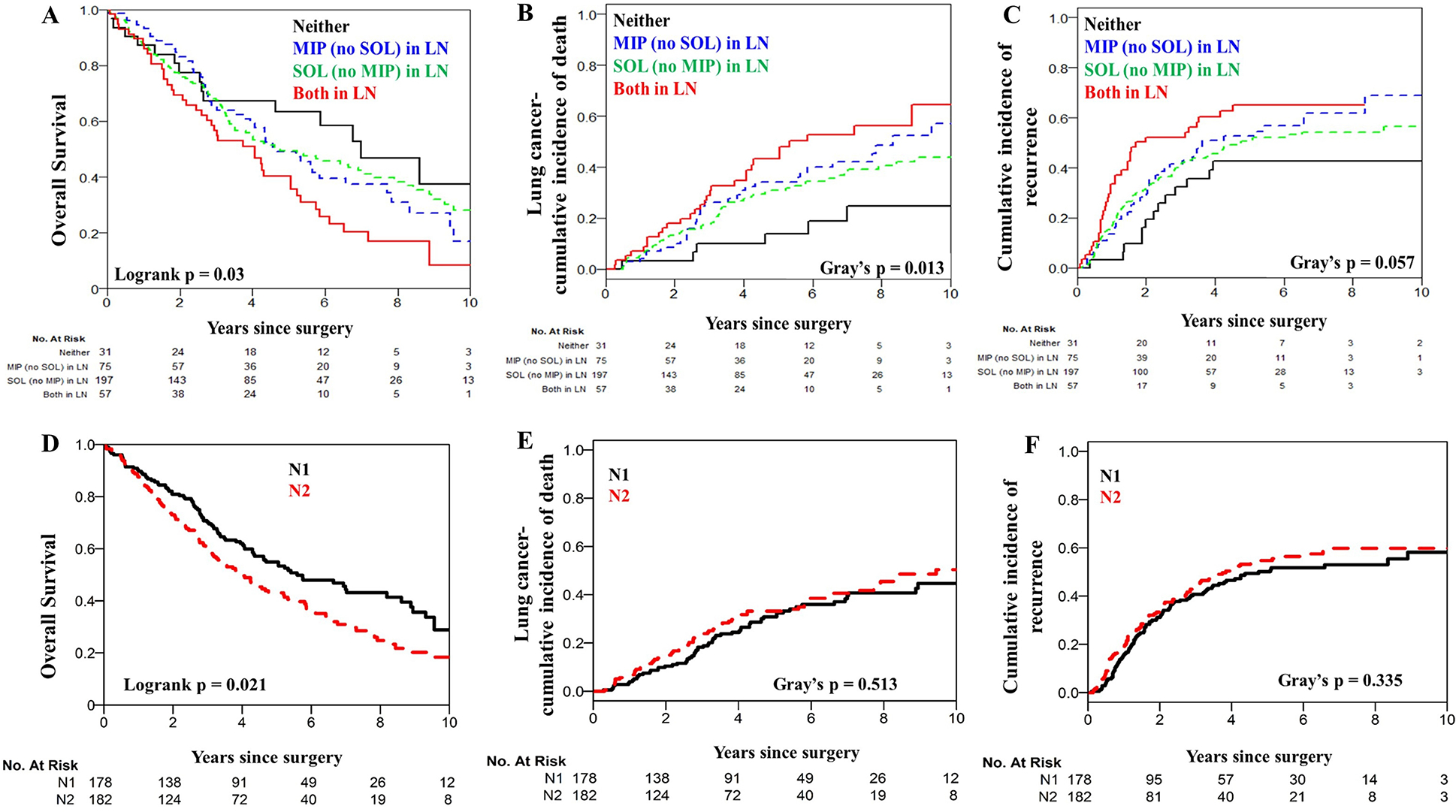
Overall survival, lung cancer–specific cumulative incidence of death, and cumulative incidence of recurrence for patients stratified by N1 versus N2 staging (A-C) or micropapillary (MIP) and/or solid (SOL) patterns in lymph nodes (LNs) (D-F).
When patients were stratified by the presence or absence of each histologic pattern in LN metastases (Figure, Supplemental Data 2), only absence of MIP (compared with presence of MIP) was associated with significantly better OS, LC-CID, and CIR. The presence of ACI or PAP pattern in LN metastases (compared with absence of each) was not associated with significant differences in OS. Although absence of SOL (compared with presence of SOL) was associated with slightly better OS, LC-CID, and CIR, the difference was not statistically significant.
When patients were stratified by predominance or lack of predominance of MIP (Figure, Supplemental Data 3A–C) or SOL (Figure, Supplemental Data 3D–F) in LN metastases, lack of MIP predominance (compared with MIP predominance) was associated with significantly better OS, LC-CID, and CIR; whereas no differences were observed with lack of SOL predominance (compared with SOL predominance).
Since both MIP and SOL are known high-grade patterns, we next aimed to determine whether the absence of the combination of MIP and SOL in LN metastases was associated with a better prognosis. Absence of both MIP and SOL in LN metastases (compared with presence of either or both) was associated with significantly better OS, LC-CID, and CIR (5-year OS, 40% [95% CI, 29%–56%]; p=0.03; LC-CID, 43% [95% CI, 29%–56%]; p=0.013; and CIR, 65% [95% CI, 50%–77%]; p=0.057) (Figure 3A–C). Subset analysis by pN1 only and pN2 only confirmed our observations in the pN1 cohort. Subset analysis in pN2 only did not reproduce the overall observations, likely due to limited sample size (Figure, Supplemental Data 4A–B).
Multivariable Competing-Risks Regression Analysis for OS, LC-CID, and CIR
After adjusting for clinical factors such as age, STAS, invasive tumor size, location of the LNs (N1 or N2), number of involved LN stations (single or multiple), the presence of both MIP and SOL in LN metastases was statistically significantly associated with worse OS (vs neither: hazard ratio [HR], 1.81 [95% CI, 1.00–3.29]; p=0.05) (Table 2). Similar relationships were observed for lung cancer specific death (adjusted HR, 3.10 [95% CI, 1.30–7.37]; p=0.01) and recurrence (adjusted HR 2.06 [95% CI, 1.09–3.90]; p=0.026). Subset analysis of patients stratified by treatment with adjuvant chemotherapy shows that patients with high-grade histological component in the LNs demonstrate improved outcomes with adjuvant chemotherapy compared to patients with high-grade histological component that did not receive adjuvant chemotherapy, particularly those with both SOL and MIP in the LNs (Figure, Supplemental Data 5A–B; Table, Supplemental Data 1).
Table 2.
Multivariable analysis of Cox proportional hazards regression model for overall survival, lung cancer–specific cumulative incidence of death, and cumulative incidence of recurrence
| OS | LC-CID | CIR | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| Variable | HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p |
|
| |||||||||
| Age, years | 1.02 | 1.00–1.03 | 0.023 | 1.00 | 0.98–1.02 | 0.9 | |||
| STAS | 1.24 | 0.90–1.71 | 0.2 | 1.26 | 0.84–1.91 | 0.3 | 1.06 | 0.75–1.50 | 0.7 |
| Invasive tumor size, cm | 1.06 | 0.98–1.14 | 0.2 | 1.09 | 0.99–1.21 | 0.093 | 1.08 | 0.98–1.18 | 0.11 |
| Location of involved LN, N2 vs. N1 | 1.22 | 0.91–1.62 | 0.2 | 0.91 | 0.63–1.33 | 0.6 | 0.93 | 0.67–1.29 | 0.7 |
| Number of involved stations, multiple vs. single | 1.22 | 0.89–1.67 | 0.2 | 1.35 | 0.89–2.03 | 0.2 | 1.50 | 1.05–2.14 | 0.025 |
| MIP or SOL status in LN | |||||||||
| MIP only vs. neither | 1.37 | 0.76–2.48 | 0.3 | 2.51 | 1.05–6.00 | 0.039 | 1.53 | 0.84–2.78 | 0.2 |
| SOL only vs. neither | 1.46 | 0.86–2.48 | 0.2 | 2.12 | 0.92–4.88 | 0.078 | 1.41 | 0.81–2.45 | 0.2 |
| MIP and SOL, both vs. neither | 1.81 | 1.00–3.29 | 0.05 | 3.10 | 1.30–7.37 | 0.010 | 2.06 | 1.09–3.90 | 0.026 |
CI, confidence interval; CIR, cumulative incidence of recurrence; HR, hazard ratio; LN, lymph node; LC-CID, lung cancer–specific cumulative incidence of death; MIP, micropapillary; OS, overall survival; STAS, spread of tumor through air spaces; SOL, solid. Bold type indicates statistical significance.
DISCUSSION
This study demonstrates the prognostic significance of high-grade histologic patterns in LN metastases. The presence of both MIP and SOL patterns in LN metastases is an independent, poor prognostic factor of OS, LC-CID, and CIR in patients with stage II-III lung ADC.
The primary strength of our study is its comprehensive assessment of histologic patterns in both primary tumors and LN metastases in a cohort of 360 patients with a median follow-up of 6.8 years. Most importantly, although the location, size, and number of LN metastases are presently considered in the nodal staging of solid tumors (head and neck, breast, esophagus, gastric, pancreas, colorectal, kidney, ovarian, uterine, or cervical, and prostate), we have demonstrated herein, for the first time in solid tumors, the significance of evaluating clinically important histologic patterns in LN metastases.
Whereas the prognostic significance of N1 and N2 LN metastases was established by the International Association for the Study of Lung Cancer,1 the results of the multivariable analysis in the present study showed that beyond N1 and N2 status, the presence of both MIP and SOL is a significant factor providing insight into high-risk factors in patients with stage II-III lung ADC. Patients who had no MIP pattern in LN metastases or whose LN metastases were not MIP predominant had significantly better OS, LC-CID, and CIR, compared with those who did. In addition, patients with neither MIP nor SOL pattern in LN metastases had better survival than patients with both patterns in LN metastases.
The inclusion of nodal metastases in the staging of lung ADC is not only prognostic, but it can also serve as an indicator of occult metastases prompting consideration of adjuvant therapy. Adjuvant chemotherapy, administered as the standard of care for patients with resected stage II-III lung ADC to target the premetastatic niche, is associated with a benefit in 5-year OS of only 4% to 15%.25–28 Biomarkers are desperately needed to identify patients with stage II-III lung ADC who may benefit from adjuvant chemotherapy—thereby avoiding the side effects of treatment in patients who are unlikely to benefit from it—N1 and N2 status alone cannot differentiate patients in this manner. Our comprehensive analysis demonstrated that the absence of any high-grade component (i.e., absence of both MIP and SOL) in LN metastases offers more prognostic information than the anatomic location (N1 vs. N2) or the number (single vs. multiple stations) of LN metastases. This observation provides a strong rationale to conduct a prospective investigation to determine the prognostic and predictive value of histologic patterns in LN metastases. The necessity of such a study is even more apparent when considered in the context of the current era of induction immunotherapy and the fact that the immune microenvironment can differ by histologic pattern.29, 30 In addition, our subset analysis by adjuvant chemotherapy shows that patients with high-grade histological component in the LNs demonstrate improved outcomes with adjuvant chemotherapy compared to patients with high-grade histological component that did not receive adjuvant chemotherapy, particularly those with both SOL and MIP in the LNs (Table, Supplemental Data 1). Our study raises awareness for the consideration of the histological component in the regional LNs while evaluating the efficacy in future clinical trials of adjuvant chemotherapy, immunotherapy, or combination therapies.
Several studies have investigated histologic patterns in primary lung ADC tumors.7, 31, 32 The few studies that investigated histologic pattern in LN metastases included relatively small cohorts (<100 patients).33, 34 For instance, Russell et al. compared predominant histologic patterns in primary tumors and corresponding N2 metastases in 69 patients with stage III lung ADC.33 In that study, the predominant pattern in MIP- and SOL-predominant primary tumors was most often observed in the N2 metastases. Survival was not observed to be significantly different by the predominant histologic pattern in N2 metastases. Suda et al. analyzed 24 patients with surgically treated lung ADC with LN metastases and found similar results.34 Given their findings, both groups concluded that the predominant pattern in the primary tumor was the main determinant of outcomes in patients with lung ADC with LN metastasis; however, the small number of patients in each study limits the solidity of these observations. In our study, we showed that predominant histological subtypes in primary tumor were not statistically significant in any of the outcomes; hence, they were not included in the multivariable analysis. Sensitivity analyses, with the inclusion of the primary tumor histological patterns as a variable in all three multivariable models, did not change our observation that high-grade histological components in LNs are independently prognostic (Table 3).
Table 3.
Multivariable analysis of Cox proportional hazards regression model for overall survival, lung cancer–specific cumulative incidence of death, and cumulative incidence of recurrence with primary tumor patterns
| Outcome 1. Overall survival | ||||||
|
| ||||||
| No IASLC9 primary tumor | IASLC9 primary tumor | |||||
|
|
||||||
| Variable | HR | 95% CI | p | HR | 95% CI | p |
|
| ||||||
| Age, years | 1.02 | 1.00, 1.03 | 0.023 | 1.02 | 1.00, 1.03 | 0.019 |
| STAS | 1.24 | 0.90, 1.71 | 0.2 | 1.26 | 0.91, 1.74 | 0.2 |
| Invasive tumor size, cm | 1.06 | 0.98, 1.14 | 0.2 | 1.06 | 0.98, 1.15 | 0.2 |
| Location of involved LN, N2 vs. N1 | 1.22 | 0.91, 1.62 | 0.2 | 1.24 | 0.93, 1.66 | 0.14 |
| Number of involved stations, multiple vs. single | 1.22 | 0.89, 1.67 | 0.2 | 1.20 | 0.88, 1.64 | 0.3 |
| MIP only vs. neither | 1.37 | 0.76, 2.48 | 0.3 | 1.46 | 0.80, 2.68 | 0.2 |
| SOL only vs. neither | 1.46 | 0.86, 2.48 | 0.2 | 1.36 | 0.78, 2.37 | 0.3 |
| MIP and SOL, both vs. neither | 1.81 | 1.00, 3.29 | 0.050 | 1.83 | 1.00, 3.37 | 0.051 |
| IASLC9 primary MIP vs. low/int | 0.83 | 0.54, 1.25 | 0.4 | |||
| IASLC9 primary SOL vs. low/int | 1.22 | 0.87, 1.70 | 0.2 | |||
|
| ||||||
| Outcome 2. Cumulative incidence of lung cancer–specific death | ||||||
|
| ||||||
| No IASLC9 primary tumor | IASLC9 primary tumor | |||||
|
|
||||||
| Variable | HR | 95% CI | p | HR | 95% CI | p |
|
| ||||||
| Age, years | 1.01 | 0.99, 1.03 | 0.4 | 1.01 | 0.99, 1.03 | 0.3 |
| STAS | 1.31 | 0.86, 1.99 | 0.2 | 1.34 | 0.88, 2.05 | 0.2 |
| Invasive tumor size, cm | 1.09 | 0.99, 1.20 | 0.067 | 1.09 | 1.00, 1.20 | 0.060 |
| Location of involved LN, N2 vs. N1 | 1.03 | 0.72, 1.49 | 0.9 | 1.06 | 0.73, 1.53 | 0.8 |
| Number of involved stations, multiple vs. single | 1.37 | 0.91, 2.07 | 0.13 | 1.35 | 0.90, 2.03 | 0.2 |
| MIP only vs. neither | 2.36 | 0.97, 5.77 | 0.059 | 2.55 | 1.03, 6.30 | 0.043 |
| SOL only vs. neither | 2.13 | 0.92, 4.94 | 0.077 | 1.94 | 0.82, 4.62 | 0.13 |
| MIP and SOL, both vs. neither | 3.08 | 1.25, 7.57 | 0.014 | 3.11 | 1.25, 7.73 | 0.015 |
| IASLC9 primary MIP vs. low/int | 0.78 | 0.47, 1.32 | 0.4 | |||
| IASLC9 primary SOL vs. low/int | 1.28 | 0.84, 1.96 | 0.3 | |||
|
| ||||||
| Outcome 3. Cumulative incidence of recurrence | ||||||
|
| ||||||
| No IASLC9 primary tumor | IASLC9 primary tumor | |||||
|
|
||||||
| Variable | HR | 95% CI | p | HR | 95% CI | p |
|
| ||||||
| Age, years | 1.01 | 0.99, 1.03 | 0.2 | 1.01 | 0.99, 1.03 | 0.2 |
| STAS | 1.10 | 0.78, 1.55 | 0.6 | 1.11 | 0.78, 1.57 | 0.6 |
| Invasive tumor size, cm | 1.07 | 0.98, 1.15 | 0.11 | 1.07 | 0.98, 1.16 | 0.12 |
| Location of involved LN, N2 vs. N1 | 0.99 | 0.72, 1.36 | 0.9 | 0.99 | 0.72, 1.37 | 1 |
| Number of involved stations, multiple vs. single | 1.63 | 1.15, 2.32 | 0.006 | 1.62 | 1.14, 2.31 | 0.007 |
| MIP only vs. neither | 1.51 | 0.79, 2.89 | 0.2 | 1.54 | 0.79, 2.98 | 0.2 |
| SOL only vs. neither | 1.50 | 0.83, 2.69 | 0.2 | 1.44 | 0.78, 2.66 | 0.2 |
| MIP and SOL, both vs. neither | 2.28 | 1.18, 4.41 | 0.015 | 2.27 | 1.16, 4.47 | 0.017 |
| IASLC9 primary MIP vs. low/int | 0.94 | 0.59, 1.48 | 0.8 | |||
| IASLC9 primary SOL vs. low/int | 1.09 | 0.76, 1.57 | 0.6 | |||
CI, confidence interval; HR, hazard ratio; IASLC9, International Association for the Study of Lung Cancer classification, 9th edition; LN, lymph node; MIP, micropapillary; SOL, solid; STAS, spread of tumor through air spaces.
Even when present in only small percentages in the primary tumor, both MIP and SOL were more likely to be present in LN metastases: 51% of patients with MIP-predominant LN metastases (36/70) and 36% of patients with SOL-predominant LN metastases (71/196) had only a small percentage (25%) of MIP or SOL, respectively, in the primary tumor. A higher percentage of either pattern in LN metastases was associated with a higher percentage of that pattern in the primary tumor. Of note, the average percentage of SOL in LN metastases was high even when the percentage of SOL in the primary tumor was low, which echoes previous findings that solid and micro-papillary patterns are tightly linked to the risk of developing nodal metastases.35–37 Yamada et al. also found that SOL was often present in LN metastases ≤2 mm, even if it was not predominant in the synchronous primary tumor.38 Yu et al. observed that, in lung tumors ≤3 cm, the presence of MIP or SOL was associated with a higher percentage of LN metastases.36 Dai et al. also reported that LN micrometastasis was more frequently observed in patients with lung ADC with MIP pattern present.39 These observations are highly clinically significant—in particular, they underline the importance of LN staging (even in the presence, and not necessarily the predominance, of MIP or SOL pattern in the primary tumor) before treatment by resection or stereotactic body radiation therapy. Furthermore, it could be speculated that MIP and SOL patterns can reflect the presence of metastasis-initiating cancer cells.40
Our data suggest there may be clinical value if pathologists can record the histologic patterns of nonmucinous lung adenocarcinoma in LN metastases in their routine clinical work. This approach is currently not standard practice and has not been recommended by the IASLC or WHO. However, this may be considered in the future, given the accumulating data, including our own, demonstrating prognostic significance to even small amounts of the micropapillary and solid patterns in LN metastases. The feasibility of including histological subtypes of each LN in resection samples may not be practical and can only be considered with validation of our data in other cohorts as well as the significance confirmed in prospective studies. However, it is our pathologist’s experience that identification of high-grade histological components in LNs is relatively easier in the background of LN architecture than in the primary tumor.
The observations from our study should be interpreted with acknowledgment of the inherent limitations of a retrospective study from a single-institution database with N1 and N2 status combined for analysis. In the current study, our observations were limited to patients with nonmucinous lung ADC; this therefore limits the application of our findings to the tumor-node-metastasis classification for nonmucinous lung ADC.
In conclusion, the co-presence of high-grade histologic patterns in LN metastases has a strong influence on patient survival, and the use of information on histologic patterns in LN metastases can achieve a better prognosis than the current pN classification in this study cohort. This observation raises the question whether recording of histologic patterns of nonmucinous lung adenocarcinoma in LN metastases should be performed in prospective clinical trials and/or in routine clinical practice.
Supplementary Material
Figure, Supplemental Data 1. Representative pictures of each histologic subtype—acinar (ACI) (A), papillary (PAP) (B), micropapillary (MIP) (C), and solid (SOL) (D)—in lymph nodes.
Figure, Supplemental Data 2. Overall survival, lung cancer–specific cumulative incidence of death (LC-CID), and cumulative incidence of recurrence (CIR) for patients stratified by presence versus absence of a single histologic pattern—acinar (ACI) (A-C), papillary (PAP) (D-F), micropapillary (MIP) (G-I), and solid (SOL) (J-L)—in lymph-nodes.
Figure, Supplemental Data 3. Overall survival, lung cancer–specific cumulative incidence of death (LC-CID), and cumulative incidence of recurrence (CIR) for patients stratified by micropapillary (MIP) (A-C) predominance or solid (D-F) predominance, versus other histologic pattern predominance, in lymph node metastases. Pre, predominance.
Figure, Supplemental Data 4. Overall survival, lung cancer–specific cumulative incidence of death (LC-CID), and cumulative incidence of recurrence (CIR) for patients stratified by pN1 (A) versus pN2 (B) status.
Figure, Supplemental Data 5. Overall survival, lung cancer–specific cumulative incidence of death (LC-CID), and cumulative incidence of recurrence (CIR) for patients stratified by the treatment with adjuvant chemotherapy (A) versus no adjuvant chemotherapy (B) in lymph nodes.
Acknowledgments
We acknowledge excellent editorial assistance from Summer Koop and David B. Sewell of the Memorial Sloan Kettering Cancer Center Thoracic Surgery Service.
Research Support:
P.S.A.’s laboratory work is supported by grants from the National Institutes of Health (P30 CA008748, R01 CA236615-01, and R01 CA235667), the U.S. Department of Defense (BC132124, LC160212, CA170630, CA180889, and CA200437), the Batishwa Fellowship, the Comedy vs. Cancer Award, the Dalle Pezze Foundation, the Derfner Foundation, the Esophageal Cancer Education Fund, the Geoffrey Beene Foundation, the Memorial Sloan Kettering Technology Development Fund, the Miner Fund for Mesothelioma Research, the Mr. William H. Goodwin and Alice Goodwin, the Commonwealth Foundation for Cancer Research, and the Experimental Therapeutics Center of Memorial Sloan Kettering Cancer Center. P.S.A.’s laboratory receives research support from ATARA Biotherapeutics. All sponsors played no role in any aspect of this work. D.R. J’s laboratory work is supported by National Institutes of Health grants R01CA217169 and R01CA240472.
Abbreviations:
- ACI
acinar
- ADC
adenocarcinoma
- CI
confidence interval
- CIR
cumulative incidence of recurrence
- HR
hazard ratio
- IQR
Interquartile range
- LC-CID
lung cancer–specific cumulative incidence of death
- LEP
lepidic
- LN
lymph node
- MIP
micropapillary
- N
nodal
- OS
overall survival
- PAP
papillary
- SOL
solid
- STAS
spread of tumor through air spaces
- T
tumor
Footnotes
Conflict of Interest Statement: D.R.J. is a member of the Advisory Council for Astra Zeneca and a member of a Clinical Trial Steering Committee for Merck. P.S.A. declares research funding from ATARA Biotherapeutics; Scientific Advisory Board Member and Consultant for ATARA Biotherapeutics, Bayer, Carisma Therapeutics, Imugene, ImmPactBio, Johnson & Johnson, Orion Pharma, Outpace Bio; Patents, royalties, and intellectual property on mesothelin-targeted CAR and other T-cell therapies, which have been licensed to ATARA Biotherapeutics, issued patent method for detection of cancer cells using virus, and pending patent applications on PD-1 dominant negative receptor, on a wireless pulse-oximetry device, and on an ex vivo malignant pleural effusion culture system.
Memorial Sloan Kettering Cancer Center has licensed intellectual property related to mesothelin-targeted CARs and T-cell therapies to ATARA Biotherapeutics and has associated financial interests.
CRediT roles:
Yan Li: Investigation, data curation, roles/writing – original draft
Alexander J. Byun: Data curation & validation
Jennie K. Choe: Data curation, investigation
Shaohua Lu: Investigation, methodology
David Restle: Validation, writing - review & editing
Takashi Eguchi: Data curation, methodology, investigation, writing - review & editing
Kay See Tan: Formal analysis, visualization
Jasmeen Saini: Investigation, writing - review & editing
James Huang: Writing - review & editing
Gaetano Rocco: Writing - review & editing
David R. Jones: Writing - review & editing
William D. Travis: Data curation, investigation & writing - review & editing
Prasad S. Adusumilli: Conceptualization, Funding acquisition, Project administration, Supervision, writing - review & editing.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Asamura H, Chansky K, Crowley J, et al. The International Association for the Study of Lung Cancer Lung Cancer Staging Project: Proposals for the Revision of the N Descriptors in the Forthcoming 8th Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2015;10:1675–1684. 10.1097/jto.0000000000000678. [DOI] [PubMed] [Google Scholar]
- 2.Li S, Yan S, Lu F, et al. Validation of the 8th Edition Nodal Staging and Proposal of New Nodal Categories for Future Editions of the TNM Classification of Non-Small Cell Lung Cancer. Ann Surg Oncol 2021;28:4510–4516. 10.1245/s10434-020-09461-y. [DOI] [PubMed] [Google Scholar]
- 3.Yun JK, Lee GD, Choi S, et al. Comparison between lymph node station- and zone-based classification for the future revision of node descriptors proposed by the International Association for the Study of Lung Cancer in surgically resected patients with non-small-cell lung cancer. Eur J Cardiothorac Surg 2019;56:849–857. 10.1093/ejcts/ezz147. [DOI] [PubMed] [Google Scholar]
- 4.Park BJ, Kim TH, Shin S, et al. Recommended Change in the N Descriptor Proposed by the International Association for the Study of Lung Cancer: A Validation Study. J Thorac Oncol 2019;14:1962–1969. 10.1016/j.jtho.2019.07.034. [DOI] [PubMed] [Google Scholar]
- 5.Chiappetta M, Lococo F, Leuzzi G, et al. External validation of the N descriptor in the proposed tumour-node-metastasis subclassification for lung cancer: the crucial role of histological type, number of resected nodes and adjuvant therapy. Eur J Cardiothorac Surg 2020;58:1236–1244. 10.1093/ejcts/ezaa215. [DOI] [PubMed] [Google Scholar]
- 6.WHO Classification of Tumours Editorial Board. WHO Classification of Tumours: Thoracic Tumours. Lyon, France: International Agency for Research on Cancer; 2021. [Google Scholar]
- 7.Nicholson AG, Tsao MS, Beasley MB, et al. The 2021 WHO Classification of Lung Tumors: Impact of Advances Since 2015. J Thorac Oncol 2022;17:362–387. 10.1016/j.jtho.2021.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Cha MJ, Lee HY, Lee KS, et al. Micropapillary and solid subtypes of invasive lung adenocarcinoma: clinical predictors of histopathology and outcome. J Thorac Cardiovasc Surg 2014;147:921–928.e2. 10.1016/j.jtcvs.2013.09.045. [DOI] [PubMed] [Google Scholar]
- 9.Yanagawa N, Shiono S, Abiko M, et al. The Clinical Impact of Solid and Micropapillary Patterns in Resected Lung Adenocarcinoma. J Thorac Oncol 2016;11:1976–1983. 10.1016/j.jtho.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 10.Ujiie H, Kadota K, Chaft JE, et al. Solid Predominant Histologic Subtype in Resected Stage I Lung Adenocarcinoma Is an Independent Predictor of Early, Extrathoracic, Multisite Recurrence and of Poor Postrecurrence Survival. J Clin Oncol 2015;33:2877–2884. 10.1200/jco.2015.60.9818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nitadori J, Bograd AJ, Kadota K, et al. Impact of micropapillary histologic subtype in selecting limited resection vs lobectomy for lung adenocarcinoma of 2cm or smaller. J Natl Cancer Inst 2013;105:1212–1220. 10.1093/jnci/djt166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang W, Hu Z, Zhao J, et al. Both the presence of a micropapillary component and the micropapillary predominant subtype predict poor prognosis after lung adenocarcinoma resection: a meta-analysis. J Cardiothorac Surg 2020;15:154. 10.1186/s13019-020-01199-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuan Y, Ma G, Zhang Y, et al. Presence of micropapillary and solid patterns are associated with nodal upstaging and unfavorable prognosis among patient with cT1N0M0 lung adenocarcinoma: a large-scale analysis. J Cancer Res Clin Oncol 2018;144:743–749. 10.1007/s00432-017-2571-7. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi Y, Eguchi T, Kameda K, et al. Histologic subtyping in pathologic stage I-IIA lung adenocarcinoma provides risk-based stratification for surveillance. Oncotarget 2018;9:35742–35751. 10.18632/oncotarget.26285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kadota K, Nitadori J, Sima CS, et al. Tumor Spread through Air Spaces is an Important Pattern of Invasion and Impacts the Frequency and Location of Recurrences after Limited Resection for Small Stage I Lung Adenocarcinomas. J Thorac Oncol 2015;10:806–814. 10.1097/jto.0000000000000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yagi Y, Aly RG, Tabata K, et al. Three-Dimensional Histologic, Immunohistochemical, and Multiplex Immunofluorescence Analyses of Dynamic Vessel Co-Option of Spread Through Air Spaces in Lung Adenocarcinoma. J Thorac Oncol 2020;15:589–600. 10.1016/j.jtho.2019.12.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vaghjiani RG, Takahashi Y, Eguchi T, et al. Tumor Spread Through Air Spaces Is a Predictor of Occult Lymph Node Metastasis in Clinical Stage IA Lung Adenocarcinoma. J Thorac Oncol 2020;15:792–802. 10.1016/j.jtho.2020.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caso R, Sanchez-Vega F, Tan KS, et al. The Underlying Tumor Genomics of Predominant Histologic Subtypes in Lung Adenocarcinoma. J Thorac Oncol 2020;15:1844–1856. 10.1016/j.jtho.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American College of Surgeons. AJCC Cancer Staging Manual. Springer International Publishing; 2017. [Google Scholar]
- 20.Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244–285. 10.1097/jto.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu S, Tan KS, Kadota K, et al. Spread through Air Spaces (STAS) Is an Independent Predictor of Recurrence and Lung Cancer-Specific Death in Squamous Cell Carcinoma. J Thorac Oncol 2017;12:223–234. 10.1016/j.jtho.2016.09.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kameda K, Eguchi T, Lu S, et al. Implications of the Eighth Edition of the TNM Proposal: Invasive Versus Total Tumor Size for the T Descriptor in Pathologic Stage I-IIA Lung Adenocarcinoma. J Thorac Oncol 2018;13:1919–1929. 10.1016/j.jtho.2018.08.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cox DR. Regression models and life tables. J R Statist Soc B 1972;34:187–220. [Google Scholar]
- 24.Dignam JJ, Zhang Q, Kocherginsky M. The use and interpretation of competing risks regression models. Clin Cancer Res 2012;18:2301–2308. 10.1158/1078-0432.ccr-11-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. Non-small Cell Lung Cancer Collaborative Group. BMJ 1995;311:899–909. [PMC free article] [PubMed] [Google Scholar]
- 26.Arriagada R, Bergman B, Dunant A, et al. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med 2004;350:351–360. 10.1056/nejmoa031644. [DOI] [PubMed] [Google Scholar]
- 27.Douillard JY, Rosell R, De Lena M, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol 2006;7:719–727. 10.1016/s1470-2045(06)70804-x. [DOI] [PubMed] [Google Scholar]
- 28.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN Guidelines): Non-small cell lung cancer v3.2020. 2020;March.2020 Available at https://www2.tri-kobe.org/nccn/guideline/lung/english/non_small.pdf. Accessed May 19, 2021.
- 29.Akhave N, Zhang J, Bayley E, et al. Immunogenomic profiling of lung adenocarcinoma reveals poorly differentiated tumors are associated with an immunogenic tumor microenvironment. Lung Cancer 2022;172:19–28. 10.1016/j.lungcan.2022.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muller S, Mayer S, Moller P, et al. Spatial distribution of immune checkpoint proteins in histological subtypes of lung adenocarcinoma. Neoplasia 2021;23:584–593. 10.1016/j.neo.2021.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsuta K, Kawago M, Inoue E, et al. The utility of the proposed IASLC/ATS/ERS lung adenocarcinoma subtypes for disease prognosis and correlation of driver gene alterations. Lung Cancer 2013;81:371–376. 10.1016/j.lungcan.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 32.Moreira AL, Ocampo PSS, Xia Y, et al. A Grading System for Invasive Pulmonary Adenocarcinoma: A Proposal From the International Association for the Study of Lung Cancer Pathology Committee. J Thorac Oncol 2020;15:1599–1610. 10.1016/j.jtho.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Russell PA, Barnett SA, Walkiewicz M, et al. Correlation of mutation status and survival with predominant histologic subtype according to the new IASLC/ATS/ERS lung adenocarcinoma classification in stage III (N2) patients. J Thorac Oncol 2013;8:461–468. 10.1097/jto.0b013e3182828fb8. [DOI] [PubMed] [Google Scholar]
- 34.Suda K, Sato K, Shimizu S, et al. Prognostic implication of predominant histologic subtypes of lymph node metastases in surgically resected lung adenocarcinoma. Biomed Res Int 2014;2014:645681. 10.1155/2014/645681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Warth A, Muley T, Meister M, et al. The novel histologic International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification system of lung adenocarcinoma is a stage-independent predictor of survival. J Clin Oncol 2012;30:1438–1446. 10.1200/jco.2011.37.2185. [DOI] [PubMed] [Google Scholar]
- 36.Yu Y, Jian H, Shen L, et al. Lymph node involvement influenced by lung adenocarcinoma subtypes in tumor size </=3 cm disease: A study of 2268 cases. Eur J Surg Oncol 2016;42:1714–1719. 10.1016/j.ejso.2016.02.247. [DOI] [PubMed] [Google Scholar]
- 37.Zhao Y, Wang R, Shen X, et al. Minor Components of Micropapillary and Solid Subtypes in Lung Adenocarcinoma are Predictors of Lymph Node Metastasis and Poor Prognosis. Ann Surg Oncol 2016;23:2099–2105. 10.1245/s10434-015-5043-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamada E, Ishii G, Aramaki N, et al. Tumor-size-based morphological features of metastatic lymph node tumors from primary lung adenocarcinoma. Pathol Int 2014;64:591–600. 10.1111/pin.12213. [DOI] [PubMed] [Google Scholar]
- 39.Dai C, Xie H, Kadeer X, et al. Relationship of Lymph Node Micrometastasis and Micropapillary Component and Their Joint Influence on Prognosis of Patients With Stage I Lung Adenocarcinoma. Am J Surg Pathol 2017;41:1212–1220. 10.1097/pas.0000000000000901. [DOI] [PubMed] [Google Scholar]
- 40.Tavernari D, Battistello E, Dheilly E, et al. Nongenetic Evolution Drives Lung Adenocarcinoma Spatial Heterogeneity and Progression. Cancer Discov 2021;11:1490–1507. 10.1158/2159-8290.cd-20-1274. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure, Supplemental Data 1. Representative pictures of each histologic subtype—acinar (ACI) (A), papillary (PAP) (B), micropapillary (MIP) (C), and solid (SOL) (D)—in lymph nodes.
Figure, Supplemental Data 2. Overall survival, lung cancer–specific cumulative incidence of death (LC-CID), and cumulative incidence of recurrence (CIR) for patients stratified by presence versus absence of a single histologic pattern—acinar (ACI) (A-C), papillary (PAP) (D-F), micropapillary (MIP) (G-I), and solid (SOL) (J-L)—in lymph-nodes.
Figure, Supplemental Data 3. Overall survival, lung cancer–specific cumulative incidence of death (LC-CID), and cumulative incidence of recurrence (CIR) for patients stratified by micropapillary (MIP) (A-C) predominance or solid (D-F) predominance, versus other histologic pattern predominance, in lymph node metastases. Pre, predominance.
Figure, Supplemental Data 4. Overall survival, lung cancer–specific cumulative incidence of death (LC-CID), and cumulative incidence of recurrence (CIR) for patients stratified by pN1 (A) versus pN2 (B) status.
Figure, Supplemental Data 5. Overall survival, lung cancer–specific cumulative incidence of death (LC-CID), and cumulative incidence of recurrence (CIR) for patients stratified by the treatment with adjuvant chemotherapy (A) versus no adjuvant chemotherapy (B) in lymph nodes.


