Abstract
Background:
Central nervous system (CNS) metastases develop in nearly half of patients and cause morbidity and mortality in RET fusion-positive non-small cell lung cancers (NSCLCs). The selective RET inhibitor selpercatinib treats existing intracranial disease, but no studies have investigated whether early initiation of selpercatinib is associated with decreased development of CNS metastases.
Patients and methods:
Sixty-one patients with RET fusion-positive advanced NSCLC with and without CNS metastases treated with selpercatinib on the LIBRETTO-001 trial (NCT03157128) or the LIBRETTO-201 expanded access program (EAP, NCT03906331) were identified. Cumulative incidence rates (CIRs) for CNS metastases were assessed as an event-of-interest; systemic progression of disease and death were considered competing risks.
Results:
The median age was 65 years, and the most common 5’ fusion partners were KIF5B (67%) and CCDC6 (18%). Twenty-four patients (39%) received prior platinum chemotherapy; twenty patients (33%) received prior multikinase inhibition. The median time on selpercatinib was 21.8 months. Thirty patients (49%) had CNS disease at baseline, and thirty-one patients (51%) had no baseline CNS disease. CIRs of CNS progression among patients with baseline CNS disease were 3% (95% CI: 0–10%), 10% (95% CI: 0–22%), 17% (3–30%), 17% (3–30%), and 20% (5–35%) at 6, 12, 18, 24, and 36 months, respectively. CIRs for CNS progression among patients without baseline CNS disease were 0% at 6, 12, 18, 24, and 36 months (95% CI: 0–0%).
Conclusions:
CNS progression was not observed with selpercatinib therapy in patients without baseline CNS disease. CNS progression on selpercatinib was rare in patients with baseline CNS disease. Early initiation of selpercatinib is associated with decreased rates of CNS metastasis formation and progression, and may play a preventive role.
Keywords: brain metastases, central nervous system, intracranial disease, non-small cell lung cancer, RET fusion-positive, selpercatinib
Introduction
Fusions involving the RET tyrosine kinase drive 1–2% of non-small cell lung cancers (NSCLCs).1–3 Breakpoints in chromosome 10, which encodes the RET gene, lead to recurrent fusions of the kinase domain with a variety of genetic partners, most commonly KIF5B.3–6 These fusions, in turn, drive downstream oncogenesis, including via constitutive activation of RET kinase.3, 4, 7 Many RET fusion-positive lung cancers occur in younger patients and non-smokers.1–3, 5
One of the most devastating complications of RET fusion-positive NSCLC is central nervous system (CNS) disease. We previously reported that nearly 46% of patients with RET fusion-positive lung cancers develop intracranial disease8, demonstrating the significant clinical need for therapeutic options that prevent and/or treat CNS metastases. Selpercatinib (LOXO-292) is an oral, selective tyrosine kinase inhibitor (TKI) directed against RET’s adenosine triphosphate binding site. Regulatory approval of selpercatinib for patients with RET fusion-positive NSCLC was based on the LIBRETTO-001 trial which reported an overall response rate (ORR) of 64% in patients with prior exposure to platinum-based chemotherapy and an ORR of 85% in those with no prior treatments.9 An intracranial response rate of 82% was reported for selpercatinib in 22 patients with measurable CNS metastases,10 and the drug is active in leptomeningeal disease.11 Although preliminary studies are promising, little is known about (1) the pattern of CNS spread in patients treated with selpercatinib who do not have CNS disease at baseline, or (2) the time to CNS progression in those with baseline CNS disease.
Determining whether selpercatinib can prevent or delay the acquisition of CNS disease constitutes a significant question for multiple reasons. First, targeted therapies for NSCLC are increasingly being studied as part of the treatment for earlier stage disease. The risk-benefit calculation for adding neoadjuvant or adjuvant targeted therapy to the treatment regimen of patients with early-stage disease differs from the calculation for patients with later stage disease. Knowing whether a drug can prevent CNS metastases, one of the most challenging complications of progressive NSCLC, is therefore critical to understanding the preventive benefits of the drug. More generally, early initiation of selpercatinib becomes increasingly important if the drug can help prevent CNS disease for patients who have not yet developed metastases to the brain. Second, many systemic treatments, such as chemotherapeutic agents, used for patients with RET fusion-positive NSCLCs that have progressed through selpercatinib, have limited CNS penetration. Whether there is a potential role for continuation of selpercatinib together with chemotherapy to optimize the prevention of CNS metastases formation or progression remains unknown.
This study is the first report of the rates of de novo CNS metastasis and progression of CNS disease in a large cohort of patients with RET fusion-positive NSCLC treated with selpercatinib. Patients were treated on either the multicenter, global LIBRETTO-001 trial, or the expanded access LIBRETTO-201 trial of the drug.
Methods
Patients.
Patients were considered eligible for inclusion in this study if they (1) had confirmed NSCLC harboring a RET fusion, (2) were enrolled on a trial of selpercatinib as part of either the LIBRETTO-001 trial (NCT03157128)9 or the multi-center expanded access program (EAP; LIBRETTO-201; NCT03906331). Given that both protocols initially did not require serial CNS imaging in patients without baseline CNS disease, the analysis solely focused on accrual at a single center (Memorial Sloan Kettering Cancer Center) at which standard of care CNS imaging complementary to protocol-prescribed imaging was performed serially as outlined in detail in the following section. Participants were enrolled between May of 2017 and March of 2020 and were followed through the data cut-off of October 13, 2022.
Because selpercatinib was approved by the US Food and Drug Administration for the treatment of RET fusion-positive NSCLC in May 2020, some patients went from being treated on either LIBRETTO-001 or the EAP to being treated with selpercatinib using standard of care commercial supply. Development of CNS or systemic progression was assessed throughout patients’ time on treatment, whether selpercatinib was received on or off trial.
Patients provided written informed consent to clinical trial participation and both trials were approved by an Institutional Review Board at MSK. The full trial protocol for LIBRETTO-001 was previously published.9 Key inclusion criteria included a RET fusion-positive cancer in patients of at least 18 years of age. Patients with symptomatic CNS disease were excluded unless they had 14 days of stable symptoms, imaging, and steroid dosage. Patients had to have completed a washout of 28 days from any CNS surgery or radiation, except for stereotactic surgery where the washout was 14 days prior to selpercatinib initiation. For LIBRETTO-201, patients were considered eligible if they had a RET fusion-positive lung cancer but were not able to go on the LIBRETTO-001 trial for either clinical reasons (such as not meeting eligibility criteria due to comorbidities or concomitant medications) or logistic reasons.
Assessments and CNS imaging.
The multicenter LIBRETTO-001 trial initially did not require baseline or serial CNS imaging in patients without known brain metastases. As such, a single center effort to secure baseline and serial CNS imaging that accompanied protocol-defined extracranial imaging was undertaken when feasible until the protocol was later amended to include serial CNS imaging. Regarding the latter, in phase 2 of the trial, all patients with RET fusion-positive NSCLC regardless of the presence of baseline CNS metastases had baseline magnetic resonance imaging (MRI) of the brain. Subsequent MRIs were performed every eight weeks during the first year of treatment and at least every 12 weeks thereafter.
For LIBRETTO-201, MRIs of the CNS were carried out at the discretion of the treating physician. Again, even in patients without known brain metastases at baseline, an effort was made to secure serial CNS imaging at the single center to accompany extracranial imaging, and was performed up to approximately every 8 weeks at the discretion of the investigator.
On LIBRETTO-001, investigator assessment of the presence of CNS metastases and response was performed by the study radiologist according to RECIST 1.1 criteria. While not required by LIBRETTO-201, a centralized investigator-assessed evaluation of response and extent of disease was performed for this analysis.
Statistical Analysis.
Categorical variables, including sex, histology, and stage at diagnosis, were analyzed using Fisher’s exact test. Cumulative incidence rates (CIRs) for CNS disease were calculated using a competing risk methodology,12 with CNS progression of disease (PD) considered the event of interest and systemic PD as well as death considered as competing risks. Where CNS progression was detected concomitantly with systemic progression, the patient was counted as having had CNS progression for purposes of analysis. Gray’s test was used to compare the CIRs for progression between patients with and without baseline brain metastases.
For sensitivity analyses, progression-free survival was assessed from the time of trial enrollment to progression using the Kaplan-Meier method, with censoring at the time of death, coming off treatment, or being lost to follow-up. Statistical analyses were performed using STATA (version 16, College Park, Texas), GraphPad Prism (version 8, San Diego, California), or R (version 4, Vienna, Austria). Results were considered statistically significant if P < 0.05.
Molecular profiling.
An exploratory descriptive analysis of molecular alterations associated with selpercatinib progression or CNS tropism was performed, with data collected on molecular alterations that have been well-characterized in these contexts including bypass mutations in the MAPK pathway (e.g. MET amplification), EGFR, TERT, TP53, and RBM10. We explored whether patients with and without baseline CNS metastases had alterations in any of these genes, as well as whether any of these developed at the time of CNS progression. Alterations classified as variants of unknown significance were excluded. Patients whose cancers underwent next generation sequencing (NGS), either of tumor tissue or liquid biopsies, were eligible for inclusion.
Results
Patients.
Sixty-one patients with RET fusion-positive lung cancers met criteria for inclusion and were treated with selpercatinib (47 on LIBRETTO-001 and 14 on LIBRETTO-201). Baseline clinicopathologic features and prior therapies are summarized in Table 1 along with a comparison of these features in patients with (n=30) and without (n=31) baseline brain metastases. The median age of the cohort was 65 years (interquartile range (IQR): 56–71 years) and thirty-two (52%) patients were female. Most patients (75%; 46/61) had stage IV disease at the time of their lung cancer diagnosis. KIF5B was the most common 5’ fusion partner, occurring in 41 patients (67%) and CCDC6 was found in 11 patients (18%) (Table S-1). The median line of treatment at which selpercatinib was given was 2 (IQR: 1–3). Twenty-four patients (39%) had received prior platinum chemotherapy, and 20 had received prior treatment with a multikinase inhibitor (MKI), with some patients having received multiple lines of MKIs. Prior MKI treatments included cabozantinib (n=13), ponatinib (n=1), vandetanib (n=1), or an experimental inhibitor (n=5).
Table 1. Baseline characteristics.
The clinicopathologic features of patients with RET fusion-positive lung cancers treated with selpercatinib are summarized according to presence or absence of central nervous system (CNS) metastases at baseline. Statistical comparisons of the difference between these features in both populations (no CNS metastases and CNS metastases at baseline) are shown. Abbreviations: IQR – interquartile range.
| Clinicopathologic Features | All Cases (n=61) | No CNS metastases (n=31) | CNS Metastases (n=30) | P value |
|---|---|---|---|---|
| Age (median, IQR) | 65 (56, 71) | 64 (57, 71) | 65 (55–70) | 0.76 |
| Sex n, % | 0.13 | |||
| Male | 29 (48%) | 18 (58%) | 11 (37%) | |
| Female | 32 (52%) | 13 (42%) | 19 (63%) | |
| Smoking n, % | 0.24 | |||
| Former | 15 (25%) | 10 (32%) | 5 (17%) | |
| Never | 46 (75%) | 21 (68%) | 25 (83%) | |
| Histology n, % | 0.61 | |||
| Adenocarcinoma | 58 (95%) | 30 (97%) | 28 (90%) | |
| Other | 3 (5%) | 1 (3%) | 2 (6%) | |
| 5’ partner* n, % | ||||
| KIF5B present | 41 (67%) | 20 (65%) | 21 (70%) | 0.79 |
| Prior RET-directed tyrosine kinase inhibitor# n, % | 20 (33%) | 8 (26%) | 12 (40%) | 0.28 |
| Selpercatinib therapy line mean, (range) | 2 (1, 3) | 2 (1, 3) | 2 (2, 3) | 0.37 |
| CNS treatment | ||||
| Prior CNS RT | 12 (20%) | 0 (0%) | 12 (40%) | <0.001 |
| Prior CNS surgery | 3 (5%) | 0 (0%) | 3 (10%) | 0.11 |
Some patients had >1 fusion.
some patients had more than one prior line of TKI therapy.
The course of each patient on selpercatinib therapy is summarized in Figure 1. Median follow-up for the 29 surviving patients was 41 months (IQR: 26–50); median follow-up was 33 months (IQR: 16–45) in the 11 surviving patients with baseline CNS disease, and 45 months (IQR 36–52) in the 18 surviving without baseline CNS disease. At the time of the data lock, 19 patients (31%) were still being treated with selpercatinib, including patients who went on commercial supply of selpercatinib following regulatory approval. One additional patient was lost to follow-up. The median time-to-treatment discontinuation was 22 months (95% CI: 14–33) for the whole cohort. The median time on treatment was 14 months (95% CI: 7–25) for patients with baseline CNS disease, and was 37 months in patients without baseline CNS disease (95% CI: 17-not reached). Median progression-free survival was 16 months for the whole cohort (11–25).
Figure 1. Selpercatinib therapy duration.
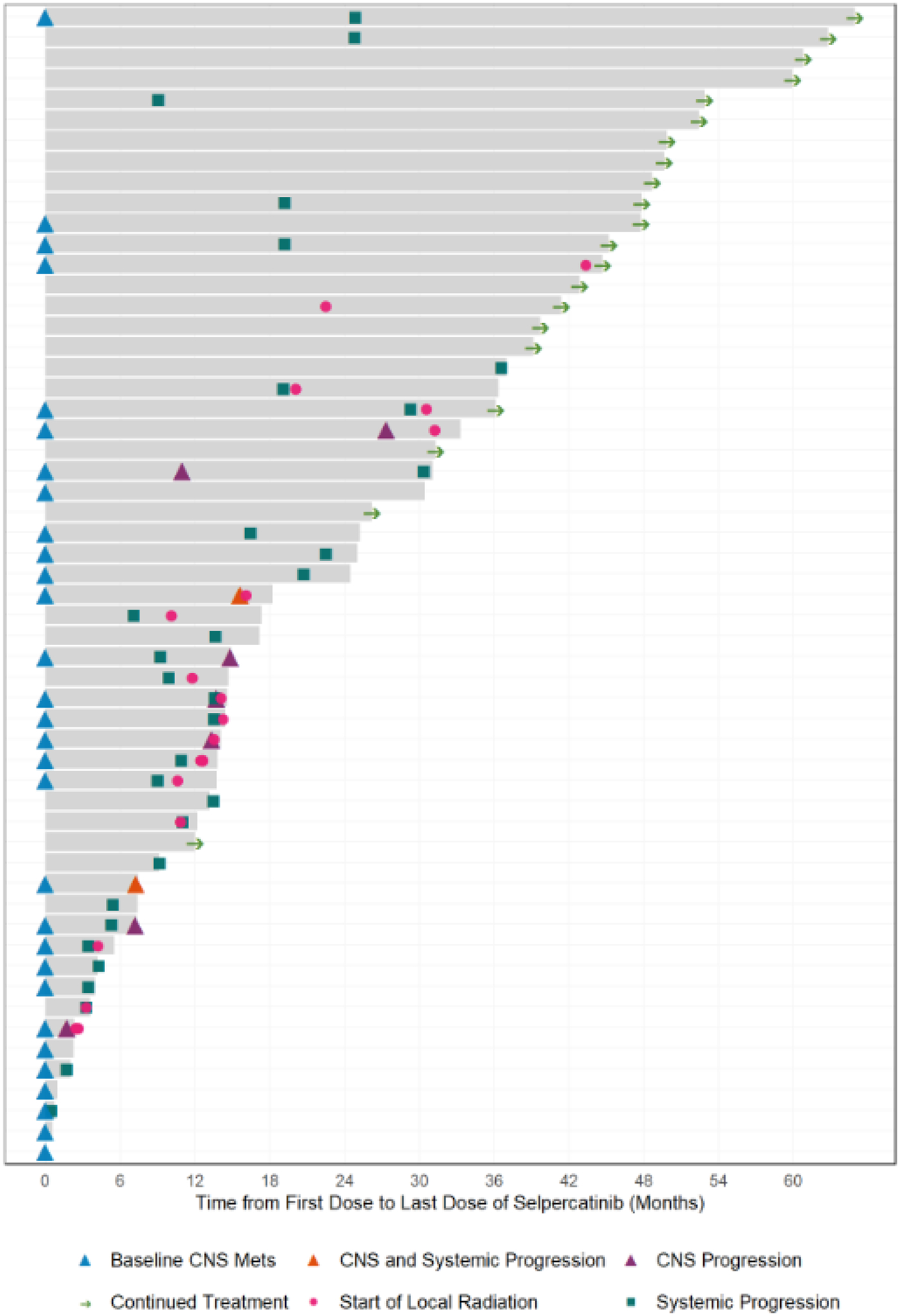
A swimmer’s plot showing time on therapy, systemic and/or CNS progression of disease, and radiation treatments administered while on selpercatinib is shown. Grey bars show time on treatment.
CNS activity.
Thirty-one patients (51%) did not have baseline CNS metastases. Interestingly, none exhibited CNS progression on selpercatinib for the duration of therapy. As of the data cut, twelve patients without baseline CNS metastases experienced progression on selpercatinib, but progression was extracranial in all cases. CIRs of CNS progression on selpercatinib are shown in Figure 2. The CIR of CNS metastases was 0% at 6, 12, 18, 24, and 36 months on selpercatinib. The median overall progression-free survival among patients without baseline CNS metastases was 25 months (95% CI: 14-not reached) as shown in Figure S-1.
Figure 2: Intracranial activity of selpercatinib.
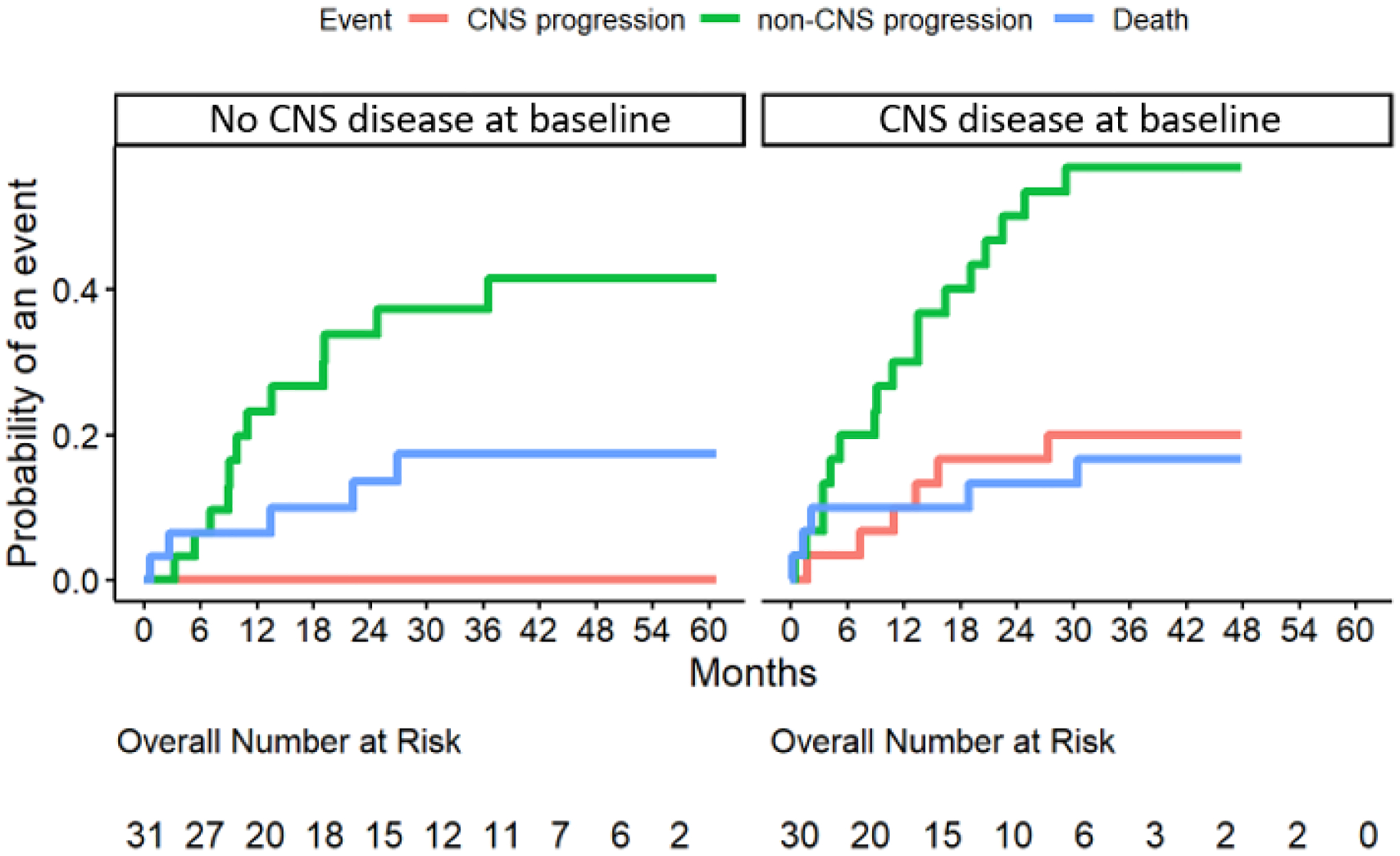
The cumulative incidence rates (CIRs) of central nervous system (CNS) progression in patients with (right) and without (left) metastases to the CNS at baseline are shown.
Of 30 patients with CNS metastases at baseline (49%), 23 experienced progression during the follow-up period, with 14 patients progressing exclusively outside the CNS, 6 progressing in both the CNS and systemically, and only 3 progressing in the CNS alone. The CIRs for CNS progression at 6, 12, 18, 24, and 36 months were 3% (95% CI: 0–10%), 10% (95% CI: 0–21%), 17% (95% CI: 3–30%), 17% (95% CI: 3–30%), and 20 (5–35%), respectively (Figure 2). The median overall progression-free survival among patients with CNS metastases at baseline was 12 months (95% CI: 7–19) as shown in Figure S-1.
Patients with CNS disease at baseline were significantly more likely to have progression of disease in the CNS than those without baseline CNS disease (P=0.01, Figure 2). There was no significant difference in the risk of progression outside of the CNS between patients with and without baseline CNS metastases (P=0.20), with a 6-month CIR for non-CNS PD of 6% (95% CI: 0–15%) and 20% (5–35%) in patients without and with CNS metastases at baseline, respectively. At 12 months, the CIRs were 23% (8–38%) and 30% (13–47%) for patients without and with baseline CNS disease, respectively. At 18 months, the CIRs were 27% (10–43%) and 40% (22–58%), respectively; at 24 months, these were 34 (16–51%) and 50 (31–69%), respectively, while at 36 months they were 37 (19–55%) and 57 (38–75%). There was also no significant difference in the CIR of death between patients with and without baseline CNS disease (P=0.99, Figure 2).
Notably, our cohort included one patient with baseline brain metastases who developed both systemic and CNS progression of disease on selpercatinib who was later treated with selpercatinib plus chemotherapy with bevacizumab, remaining on this combination for over a year. The patient did not developed clear evidence of further intracranial progression, though infarcts were observed on bevacizumab.
Molecular profiling.
Of the 9 patients in our cohort who developed CNS progression, 7 (78%) had NGS available from around the time of CNS PD (range: 175 days after CNS PD to 7 days before). Sequencing results are shown in Tables S-2 and S-3. One patient had an alteration in the MAPK pathway following CNS progression that was not detected at the time of treatment initiation, namely a KRAS G12V mutation. Two patients without pre-treatment alterations in TP53 alterations had alterations detected at the time of CNS progression. Given the known association of MET alterations with resistance,13 MET copy number gain and MET fusions were assessed and were observed pre-treatment in two patients, neither of whom developed CNS PD. No MET alterations were detected at the time of CNS progression.
Discussion
Rational drug design has increasingly maximized the likelihood of preventing CNS metastasis by optimizing the intracranial coverage of novel agents.14 CNS metastases are an especially devastating and frequent complication of RET fusion-positive cancers, with intracranial disease occurring in nearly half of patients with RET fusion-positive lung cancers in a historical series.8 Preventing the acquisition and attenuating the progression of CNS metastases remain critical goals of therapy at all stages of lung cancer.
We studied patterns of CNS disease in patients with RET fusion-positive NSCLC treated with selpercatinib on the registrational LIBRETTO-001 trial or the LIBRETTO-201 EAP trial. This study makes the seminal observation that among patients with no prior history of CNS disease, no new cases of CNS metastasis were observed with the selective RET inhibitor selpercatinib. This striking result augments existing data we previously published showing a high response rate of existing CNS lesions to selpercatinib10 and affirms the role of selective RET inhibition as a preferred first-line therapy for metastatic RET fusion-positive lung cancers. Furthermore, this activity justifies the investigation of this agent in earlier stage settings for patients who have not yet developed stage IV disease. To prospectively address the role of selpercatinib in earlier stage disease, the LIBRETTO-432 clinical trial is now underway randomizing patients to selpercatinib or placebo in the adjuvant setting (NCT04819100). An analysis of patterns of CNS metastasis acquisition on this trial will complement the results we present here.
These data also raise questions about whether selpercatinib may play a CNS-protective role as part of combination therapy. This approach could be considered in patients who experience extracranial progression on single-agent selpercatinib in the absence of intracranial progression of known CNS metastases, or in those without known CNS metastases. Such a strategy of combining a TKI with chemotherapy has gained traction in other oncogene-driven contexts such as EGFR-mutant NSCLC. Proof-of-concept studies combining chemotherapy and first-generation EGFR TKIs have been published,15–19 and osimertinib, a third-generation EGFR TKI with CNS activity, is now being investigated in combination with chemotherapy in an ongoing trial (NCT04410796).20, 21
In patients with baseline brain metastases, the low CIRs of CNS progression with selpercatinib (11% at 12 months) compare favorably to that of next-generation TKIs for other fusion-positive NSCLC (Table S-4).22 In ALK fusion-positive lung cancers with baseline CNS metastases, 12-month CIR estimates of CNS progression are ~21% with alectinib,23, 24 and 27–33% with brigatinib.25 In ALK fusion-positive lung cancers without baseline CNS metastases, historical CIRs for CNS progression are ~9–13% at 12 months,23, 25, 26 although in one study of alectinib, the 24-month CIR was 8%.24 Recent updated data on lorlatinib has shown particular success at reducing brain metastases if used earlier in a patients’ treatment course; 12-month CIRS for CNS progression on lorlatinib have been estimated as approaching 22–23% in patients with prior crizotinib and/or other TKIs, but were just 1% in treatment-naïve patients with no prior brain metastases and 7% in those who had prior brain metastases.26–28 These results in ALK fusion-positive NSCLC parallel the results of the present study in RET fusion-positive disease in suggesting that earlier initiation of brain penetrant drugs may be especially beneficial.
Our study also included exploratory analyses of mutations found on NGS of patients’ tumors and liquid biopsies. Reported selpercatinib resistance alterations include RET kinase domain mutations, as well as bypass mutations in the MAPK pathway (e.g. MET amplification).13 Acquisition of CNS metastases in patients with lung cancer have also been associated with alterations in EGFR, TERT, and TP53. By contrast, RBM10 has been correlated with lower rates of CNS metastases in this population.29 One patient developed KRAS G12V alterations at the time of CNS progression. This is in keeping with previous work we published showing that alterations in the MAPK pathway can serve as resistance mutations for selpercatinib,13 suggesting that bypass pathway resistance may unsurprisingly also play an important role in CNS progression. Direct interrogation of circulating tumor DNA in cerebrospinal fluid or sequencing of brain metastases at progression in future studies may provide further evidence in support of these mechanisms of resistance.
Our study has several limitations. While it analyzes a large dataset of patients, including data from the registrational trial that led to FDA approval of selpercatinib, it is nonetheless the experience of a single institution. Furthermore, many of the patients in our cohort had received prior lines of therapy, including approximately a third of patients who received a prior MKI. Selpercatinib is now approved for first-line therapy, and the co-mutational profile of the tumors that were treated on our study may differ somewhat from those that are treatment-naive. Despite this, the more heavily pre-treated nature of our cohort actually underscores the efficacy of selpercatinib in the CNS. Given that rates of CNS disease may increase with the prolonged selective pressure owing of multiple prior lines of therapy, we expect that our study may consequently overestimate the rate of CNS disease development with selpercatinib. In conclusion, CIRs of CNS progression on selpercatinib were low in patients with RET fusion-positive lung cancers with baseline CNS metastases and zero in patients without baseline CNS metastases. These new observations add to a growing body of literature demonstrating the substantial intracranial activity of this selective RET inhibitor that is currently approved or authorized by multiple regulatory agencies. The ongoing LIBRETTO-432 study (NCT04194944) randomizing treatment-naïve patients with RET fusion-positive lung cancers to selpercatinib or placebo in the adjuvant setting for earlier stage disease will help serve to confirm the data presented here on the association of selpercatinib with decreased occurrence of CNS disease.
Supplementary Material
ACKNOWLEDGEMENTS
Funding.
This research was supported through the National Cancer Institute at the National Institutes of Health Cancer Center Support Grant [P30 CA008748] to Memorial Sloan Kettering Cancer Center. Y.R. Murciano-Goroff acknowledges receipt of training through an institutional K30 grant from the National Institutes of Health [CTSA UL1TR00457] and has received a Kristina M. Day Young Investigator Award from Conquer Cancer, the ASCO Foundation, funded by Dr. Charles M. Baum and Carol A. Baum. She is funded by a Paul Calabresi Career Development Award for Clinical Oncology (NIH/NCI K12 CA184746) as well as by the Andrew Sabin Family Foundation, and is receiving funding from the Fiona and Stanley Druckenmiller Center for Lung Cancer Research. A. Drilon acknowledges support from the National Cancer Institute at the National Institutes of Health [R01 CA251591], LUNGevity, and the Happy Lungs Project.
Conflicts of Interest:
Y.R. Murciano-Goroff reports travel, accommodation, and expenses from AstraZeneca and honoraria from Virology Education and Projects in Knowledge (for a CME program funded by an educational grant from Amgen). She acknowledges associated research funding to the institution from Loxo Oncology at Eli Lilly, Elucida Oncology, Taiho Oncology, Hengrui USA, Ltd/ Jiangsu Hengrui Pharmaceuticals, Luzsana Biotechnology, and Endeavor Biomedicines. She acknowledges royalties from Rutgers University Press and Wolters Kluwer. D. Liu reports consulting fees from Invitae. A. Iasonos reports royalties from Chapman and Hall, and consulting fees from Mirati (data safety and monitoring board) and Intelligencia (scientific advisor). A. Drilon reports Honoraria/Advisory Boards: Ignyta, Genentech, Roche, MORE Health, AXIS, Loxo, Eli Lilly and Company, Bayer, AbbVie, EPG Health, Takeda, Ariad, Millenium, 14ner, Elevation Oncology, Harborside Nexus, TP Therapeutics, ArcherDX, Liberum, AstraZeneca, Monopteros, RV More, Pfizer, Novartis, Ology, Blueprint Medicines, EMD Serono, Amgen, Helsinn, Medendi, TouchIME, BeiGene, Repare RX, Janssen, BergenBio, Nuvalent, Entos, Hengrui Therapeutics, Merus, Treeline Bio, Exelixis, Chugai Pharmaceutical, Prelude, Tyra Biosciences, Remedica Ltd, Applied Pharmaceutical Science, Inc., Verastem, mBrace, Treeline, MonteRosa, AXIS; Associated Research Paid to Institution: Pfizer, Exelixis, GlaxoSmithKlein, Teva, Taiho, PharmaMar; Royalties: Wolters Kluwer; Other (Food/Beverage): Merck, Puma, Merus, Boehringer Ingelheim; and CME Honoraria: Medscape, OncLive, PeerVoice, Physicians Education Resources, Targeted Oncology, Research to Practice, Axis,Peerview Institute, Paradigm Medical Communications, WebMD, MJH Life Sciences, AXIS, EPG Health, JNCC/Harborside, I3 Health. The remaining authors have no disclosures to report.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Schram AM, Chang MT, Jonsson P, et al. Fusions in solid tumours: diagnostic strategies, targeted therapy, and acquired resistance. Nature reviews Clinical oncology 2017;14:735–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drilon A, Hu ZI, Lai GGY, et al. Targeting RET-driven cancers: lessons from evolving preclinical and clinical landscapes. Nature reviews Clinical oncology 2018;15:151–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Subbiah V, Yang D, Velcheti V, et al. State-of-the-Art Strategies for Targeting RET-Dependent Cancers. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2020:Jco1902551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kohno T, Ichikawa H, Totoki Y, et al. KIF5B-RET fusions in lung adenocarcinoma. Nature medicine 2012;18:375–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang R, Hu H, Pan Y, et al. RET Fusions Define a Unique Molecular and Clinicopathologic Subtype of Non–Small-Cell Lung Cancer. Journal of Clinical Oncology 2012;30:4352–4359. [DOI] [PubMed] [Google Scholar]
- 6.Takeuchi K, Soda M, Togashi Y, et al. RET, ROS1 and ALK fusions in lung cancer. Nature medicine 2012;18:378–381. [DOI] [PubMed] [Google Scholar]
- 7.Zhang X, Li Y, Liu C, et al. Identification of a novel KIF13A-RET fusion in lung adenocarcinoma by next-generation sequencing. Lung cancer (Amsterdam, Netherlands) 2018;118:27–29. [DOI] [PubMed] [Google Scholar]
- 8.Drilon A, Lin JJ, Filleron T, et al. Frequency of Brain Metastases and Multikinase Inhibitor Outcomes in Patients With RET-Rearranged Lung Cancers. J Thorac Oncol 2018;13:1595–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drilon A, Oxnard GR, Tan DSW, et al. Efficacy of Selpercatinib in RET Fusion-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2020;383:813–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Subbiah V, Gainor JF, Oxnard GR, et al. Intracranial Efficacy of Selpercatinib in RET Fusion-Positive Non-Small Cell Lung Cancers on the LIBRETTO-001 Trial. Clin Cancer Res 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo R, Schreyer M, Chang JC, et al. Response to Selective RET Inhibition With LOXO-292 in a Patient With RET Fusion-Positive Lung Cancer With Leptomeningeal Metastases. JCO Precis Oncol 2019;3: 10.1200/PO.1219.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Satagopan JM, Ben-Porat L, Berwick M, et al. A note on competing risks in survival data analysis. British journal of cancer 2004;91:1229–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosen EY, Won HH, Zheng Y, et al. The evolution of RET inhibitor resistance in RET-driven lung and thyroid cancers. Nat Commun 2022;13:1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blackburn EH. Cancer interception. Cancer Prev Res (Phila) 2011;4:787–792. [DOI] [PubMed] [Google Scholar]
- 15.Wu Q, Luo W, Li W, et al. First-Generation EGFR-TKI Plus Chemotherapy Versus EGFR-TKI Alone as First-Line Treatment in Advanced NSCLC With EGFR Activating Mutation: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Frontiers in Oncology 2021;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mok TSK, Kim S-W, Wu Y-L, et al. Gefitinib Plus Chemotherapy Versus Chemotherapy in Epidermal Growth Factor Receptor Mutation–Positive Non–Small-Cell Lung Cancer Resistant to First-Line Gefitinib (IMPRESS): Overall Survival and Biomarker Analyses. Journal of Clinical Oncology 2017;35:40274034. [DOI] [PubMed] [Google Scholar]
- 17.Soria J-C, Wu Y-L, Nakagawa K, et al. Gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR-mutation-positive non-small-cell lung cancer after progression on first-line gefitinib (IMPRESS): a phase 3 randomised trial. The Lancet Oncology 2015;16:990–998. [DOI] [PubMed] [Google Scholar]
- 18.Noronha V, Patil VM, Joshi A, et al. Gefitinib Versus Gefitinib Plus Pemetrexed and Carboplatin Chemotherapy in EGFR-Mutated Lung Cancer. Journal of Clinical Oncology 2019;38:124–136. [DOI] [PubMed] [Google Scholar]
- 19.Hosomi Y, Morita S, Sugawara S, et al. Gefitinib Alone Versus Gefitinib Plus Chemotherapy for Non–Small-Cell Lung Cancer With Mutated Epidermal Growth Factor Receptor: NEJ009 Study. Journal of Clinical Oncology 2019;38:115–123. [DOI] [PubMed] [Google Scholar]
- 20.White MN, Piotrowska Z, Stirling K, et al. Combining Osimertinib With Chemotherapy in EGFR-Mutant NSCLC at Progression. Clinical Lung Cancer 2021;22:201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng M-M, Li Y-S, Tu H-Y, et al. Subsequent treatments beyond progression on osimertinib in EGFR-mutated NSCLC and leptomeningeal metastases. BMC Med 2022;20:197–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murciano-Goroff YR, Harada G, Drilon A. An Ascendant Challenge: Central Nervous System Metastases in ALK+ Lung Cancers. Clin Cancer Res 2022;28:2477–2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin JJ, Jiang GY, Joshipura N, et al. Efficacy of Alectinib in Patients with ALK-Positive NSCLC and Symptomatic or Large CNS Metastases. J Thorac Oncol 2019;14:683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gadgeel S, Shaw AT, Barlesi F, et al. Cumulative incidence rates for CNS and non-CNS progression in two phase II studies of alectinib in ALK-positive NSCLC. British journal of cancer 2018;118:38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ou SHI, Tiseo M, Camidge R, et al. Intracranial efficacy of brigatinib (BRG) in patients (Pts) with crizotinib (CRZ)-refractory anaplastic lymphoma kinase (ALK)-positive non-small cell lung cancer (NSCLC) and baseline CNS metastasis. Annals of Oncology 2017;28:V480–V481. [Google Scholar]
- 26.Bauer TM, Shaw AT, Johnson ML, et al. Brain Penetration of Lorlatinib: Cumulative Incidences of CNS and Non-CNS Progression with Lorlatinib in Patients with Previously Treated ALK-Positive Non-Small-Cell Lung Cancer. Target Oncol 2020;15:55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Solomon BJ, Bauer TM, Ignatius Ou SH, et al. Post Hoc Analysis of Lorlatinib Intracranial Efficacy and Safety in Patients With ALK-Positive Advanced Non-Small-Cell Lung Cancer From the Phase III CROWN Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2022;40:3593–3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagasaka M, Ou SI. CROWN 2022 Second Interim Updates: When Will Be the Coronation of Lorlatinib? Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2022. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen B, Fong C, Luthra A, et al. Genomic characterization of metastatic patterns from prospective clinical sequencing of 25,000 patients. Cell 2022;185:563–575.e511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gadgeel S, Peters S, Mok T, et al. Alectinib versus crizotinib in treatment-naive anaplastic lymphoma kinase-positive (ALK+) non-small-cell lung cancer: CNS efficacy results from the ALEX study. Ann Oncol 2018;29:2214–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


