Abstract
Objectives
Despite safe and effective multiple vaccines, the COVID-19 pandemic continued to cause morbidity, mortality, and healthcare burden. Pregnant women are among the high-risk population for COVID-19 infection and bad outcomes. Vaccination is one of the most critical public health interventions to halt the devastating impact of a pandemic. However, hesitancy, unwillingness, and refusal to take the COVID-19 vaccines are global health challenges to vaccination roll-out, especially in Africa, including Ethiopia. Country-specific evidence is essential to take appropriate context-specific actions. Some single studies with inconsistent findings are available in Ethiopia. Therefore, this meta-analysis aims to determine pooled COVID-19 vaccine acceptance among pregnant women in Ethiopia.
Study design
Systematic review and meta-analysis study design was used to synthesize evidence and overall COVID-19 vaccine acceptance and predictors among pregnant women.
Methods
A search of literature from PubMed, Scopus, Web of Science, EMBASE, Cochrane Library, and Google Scholar was conducted until January 30, 2023. All studies that met eligibility criteria were screened, and eight primary studies with 4419 total subjects were included in the meta-analysis. Two authors (DT and MK) independently extracted all the required data using a standardized form. We analyzed the data using STATA version 17 software. Heterogeneity was checked using Chocrane (Q-test) and I2 tests. Finally, the overall COVID-19 vaccine acceptance and predictors were computed using a random-effect model.
Result
The meta-analysis revealed that a pooled COVID-19 vaccine acceptance among pregnant women in Ethiopia is 42.46% (95%CI: 28.75–56.18). Further subgroup analysis stratified by region of the primary studies showed that the pooled level of COVID-19 Acceptance among pregnant women in the Amhara region is 35.16% (95% CI: 20.49–49.82), South Nation Nationality and People 50.95% (95%C:12.24–89.67) and Oromia region 62.02% (95%CI: 58.27–65.76). Predictors for COVID-19 vaccine acceptance among pregnant women in Ethiopia were awareness/knowledge of pregnant women to COVID-19 vaccine (OR 3.33, 95%CI:2.13–4.14), maternal education (OR 3.09, 95%CI: 1.67–4.51 and chronic disease (OR 2.81, 95%CI: 1.82–3.79. The lowest level of vaccine acceptance was reported in the Amhara region, while the relatively highest was observed in the Oromia region.
Conclusion
The study found a low level of COVID-19 vaccine acceptance among pregnant women in Ethiopia and emphasized the significance of improving awareness and education to increase vaccine uptake. It is crucial to provide interventions that create awareness about the COVID-19 vaccine and promote the importance of vaccination during antenatal care follow-up.
Keywords: COVID-19 vaccine acceptance, Pregnant women, Predictors, Ethiopia, Systematic review and meta-analysis
1. Background
Despite the availability of safe and effective multiple COVID-19 vaccines, the coronavirus disease 2019 (COVID-19) continued to cause morbidity, mortality, and healthcare burden [1]. Its persistence as a global health threat is due to changes over time in severe acute respiratory syndrome coronavirus 2 (SARS-CoV2), a causative agent for COVID-19(2).
Vaccination is one of the most critical public health interventions to halt morbidity and mortality and reduce the healthcare burden. However, COVID-19 vaccination progress is hampered by hesitancy, unwillingness, and refusal to take the vaccines, which leads to new concern variants [3]. COVID-19 vaccine uptake improved gradually with uneven distribution across the world. Globally 70.6% of the population had shot at least one dose of a vaccine. A survey among 23 countries showed that Acceptance for the COVID-19 vaccine increased by 5.2% from June 2021, and the overall acceptance rate worldwide is 79.1% [4].
Research findings indicated that COVID-19's susceptibility, morbidity, and mortality rate are higher among pregnant women than their nonpregnant counterparts [3]. Pregnant women are a high-risk population with a worse prognosis for viral infections than nonpregnant women. Physiological changes during pregnancy predispose pregnant women to more severe respiratory diseases, including COVID-19. The latest meta-analysis found that pregnant women with COVID-19 infection had more intensive care unit admission and poor prognosis. They also encountered a higher preterm birth rate, preeclampsia, cesarean delivery, and perinatal death [5].
Following the roll-out of the COVID-19 vaccination program, low vaccine acceptance and vaccine hesitancy have started to be a global challenge. A latest worldwide study found that vaccine hesitancy among the general population ranges from 1% in the UK to 21,1% in South Africa [4]. Lancet health commission announced a call for action against COVID-19 vaccine hesitancy in Africa. It states that low vaccine coverage in Africa with ubiquitous vaccine hesitancy across countries is a challenge. Vaccine nationalism and vaccine diplomacy are cited as a driver for inadequate coverage. In contrast, insufficient vaccine knowledge, a history of colonial medical and vaccine research abuse, and misinformation are drivers for vaccine hesitancy [6].
As of February 9, 2023, Africa CDC reported only 27.3% of the population in the African continent is fully vaccinated, even If a target is 70% to control the pandemic. While in Ethiopia, only 35.1% of the population is fully vaccinated [7]. A global study showed that COVID-19 vaccine acceptance among pregnant women is low (49%) [8] compared to the general population(79.1%) [4]. In Ethiopia, there are single studies with inconsistent findings ranging from 18.5% [7] to 70.7% [9] on COVID-19 vaccine acceptance among pregnant women. To our knowledge, no meta-analysis in Ethiopia estimates overall COVID-19 vaccine acceptance among pregnant women. Therefore determining pooled national-level COVID-19 vaccine acceptance and predictors among pregnant women is essential to raising vaccine uptake and coverage. Therefore, this meta-analysis aims to assess the pooled COVID-19 vaccine acceptance and predictors among pregnant women in Ethiopia.
2. Methods
Study design: Systematic review and meta-analysis study design was used to synthesize evidence, overall COVID-19 vaccine acceptance, and predictors among pregnant women in Ethiopia.
Study setting: This meta-analysis includes primary studies conducted in Ethiopia that assessed COVID-19 vaccine acceptance, willingness, and intention to use.
Ethiopia is an African country found within the low and middle-income economic categories. It is the second largest populous nation in the continent, with 122 inhabitants and more than 86 diverse ethnic groups.
To avoid duplication, we checked the title to determine whether systematic review and meta-analysis were already done or not using the trial registration number and Cochrane database. We followed the PRISMA (preferred reporting items for systematic review and meta-analysis) protocol to review the literature [8]. The protocol is registered on the PROSPERO (prospective international register of systematic review) database with the registration number CRD2078234564. PubMed, Scopus, Web of Science, EMBASE, Cochrane library, African Journal Online, and Google scholar are major databases that we use to identify all relevant literature. EndNote version 8 citation manager to facilitate review and citation are applied. We extended our search to retrieve additional literature using the references list of identified studies. We also searched the World Health Organization (WHO), African CDC, and Ethiopian Public Health Institute websites. Furthermore, unpublished literature from Addis Ababa Universities' online database is accessed.
We applied PICO/PECO mnemonic (population, exposure, comparison, and outcome statement) to frame and answer systematic review and meta-analysis questions. Populations: pregnant women, exposure: a determinant of vaccine acceptance, comparator: reported reference group in each included study, and outcome: level of COVID-19 vaccine acceptance.
From January 1 to 30, 2023, we searched the literature using the following terms ‘COVID-19 OR SARS CoV-2 AND vaccine AND Acceptance OR willingness OR intention AND pregnant women AND Ethiopia'.
2.1. Eligibility criteria
Inclusion criteria include all observational epidemiological studies with defined outcomes of COVID-19 vaccine acceptance, willingness, and intention to use reported using the language of English among pregnant women. The criteria also considered all published and unpublished studies in Ethiopia. At the same time, studies with methodological problems, those not fully accessed, with no defined results, letters, reviews, commentary, and studies done outside Ethiopia were excluded from a meta-analysis.
2.2. Measurement of outcome variables
Overall this meta-analysis had two outcomes. The primary outcome variable is COVID-19 vaccine acceptance, which statistically measures the number of pregnant women accepting to be vaccinated divided by the total number of pregnant women in the study multiplied by 100. The second outcome is predictors associated with vaccine acceptance, which were measured using odds ratio (OR) and calculated based on the binary result from primary studies included in the analysis.
2.3. Quality assessment and data abstraction
Two authors (DTW and MKT) screened titles and abstracts independently. Those two same authors conducted full-length article reviews for inclusion and exclusion, quality appraisal, and data collection for systematic review and meta-analysis. Then all studies identified via databases and grey literature were subject to full-text assessment. For any discrepancy raised during abstract screening, full-text review, quality appraisal, and data collection, authors met with a third researcher (BE) to discuss and resolve in consensus. The quality of each study was appraised using the Joanna Briggs Institute (JBI) quality check tool of observational studie. The tool has eight-item checklists to assess the quality of studies, including; 1. assessing inclusion and exclusion criteria, 2. description of study subject and setting, 3. measurement of outcome, 4. measurement of exposure, 5. identification of confounding factors, 6. approaches for controlling confounders, 7. appropriate statistical analysis, and 8. objective and standard criteria used. We collected study details, including the name of the first author, year of publication, study design, region (location), sample size, cases (number of pregnant women accepting to be vaccinated), and study outcome from primary studies Studies with JBI ≥7 out of 8 scales were considered high quality for this systematic review and meta-analysis. All necessary data were abstracted using a standardized data extraction Excel form (Table: 1).
2.4. Data processing and analysis
Analysis was done using STATA version 17 software after importing excel data. Cochran Q test (x2 statistic) and inverse variance (I2) test on forest plots were used to check heterogeneity. P-value ≤0.05 was considered a statistically significant heterogeneity. A heterogeneity test (I2) results for studies were considered 0%, 25%, 50%, and 75% as no, low, moderate, and high degrees of heterogeneity, respectively [10]. We used the random effect model, as the model shows better assumptions in the presence of heterogeneity, which considers both within and between study variances. Pooled effect with 95%CI was generated using the Der Simonian methods. The sub-group meta-analysis by location of the primary studies was done to minimize the random variations between the point estimates. Meta-regression was done to identify the possible source of heterogeneity. Publication bias was assessed using Egger's tests at a 5% significant level. Point estimate and 95% confidence intervals were presented in the forest plot format in this plot; the size of each box indicated the weight of the study, while each crossed line refers to 95% confidence intervals.
3. Results
3.1. Study selection
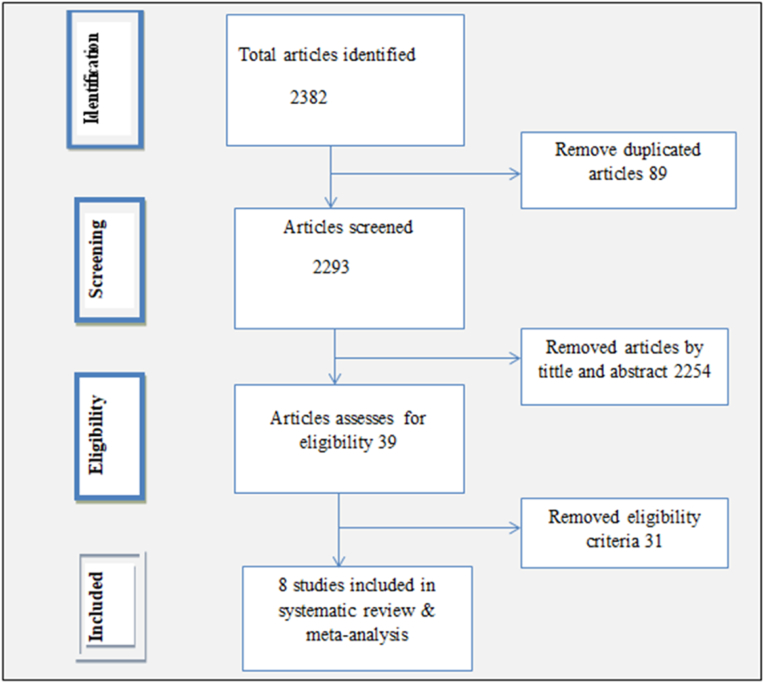
Of all 2382 published studies identified via major databases, 89 and 2254 were removed as work duplication and unrelated titles and abstracts, respectively. The remaining 39 full-text articles were assessed and screened for eligibility. Of these screened articles, 31 were excluded from the meta-analysis based on eligibility criteria: that is, if the outcome of the study were not COVID-19 vaccine acceptance, willingness and intention to use and articles with a methodological problem, those not fully accessed, without precise results, letters, reviews, commentary and studies conducted outside Ethiopia. Finally, eight studies [8,9,[13], [14], [15], [16], [17], [18]] with scores ≥7 out of 10 on the JBI quality score were included for systematic review and meta-analysis(Fig. 1).
Figure: 1.
A flow diagram depicting articles included in a meta-analysis to estimate the pooled level of COVID-19 vaccine acceptance among pregnant women in Ethiopia.
3.2. Study characteristics
[11,12] Eight primary studies in Ethiopia's three largest regions were included in a meta-analysis. Of these, five, one, and two were from Amhara [8,13,14,17,18], Oromia [15], SNNPs [9,16], respectively, with 4419 total study sizes and 350 to 702 sample range (Table: 1). All studies were cross-sectional epidemiologic studies published from 2021 to 2022. Two studies [8,18] used mixed (qualitative and quantitative) data collection techniques that generated quantitative and qualitative data. Both these studies were conducted among purposely selected pregnant women, and the sample size was determined based on desired saturation point (Table 1).
Table: 1.
Summary of articles included for systematic review and meta-analysis to estimate pooled COVID-19 vaccine acceptance among pregnant women in Ethiopia.
| ID | Author | YP | region | study design | N | n | RR | Outcome |
| 1 | Getachew, T [13] | 2022 | Oromia | cross-sectional | 645 | 400 | 100 | acceptance |
| 2 | Aynalem, Z [12] | 2022 | Amhara | cross-sectional | 350 | 55 | 100 | acceptance |
| 3 | Taye, E [14] | 2022 | Amhara | cross-sectional | 527 | 327 | 98.5 | acceptance |
| 4 | Mose, A [9] | 2021 | SNNPs | cross-sectional | 396 | 280 | 100 | acceptance |
| 5 | Tefera, Z [11] | 2022 | Amhara | cross-sectional | 702 | 159 | 100 | acceptance |
| 6 | Hailemariam,S(15) | 2021 | SNNPs | cross-sectional | 423 | 132 | 97.4 | Intention |
| 7 | Asratie, H [16] | 2022 | Amhara | cross-sectional | 851 | 291 | 100 | perception |
| 8 | Aynalem, B [17] | 2022 | Amhara | cross-sectional | 525 | 217 | 97.1 | willingness |
YP=Year of publication, N=Sample size, n = Cases, RR = Response Rate.
3.3. COVID-19 vaccine acceptance among pregnant women in Ethiopia
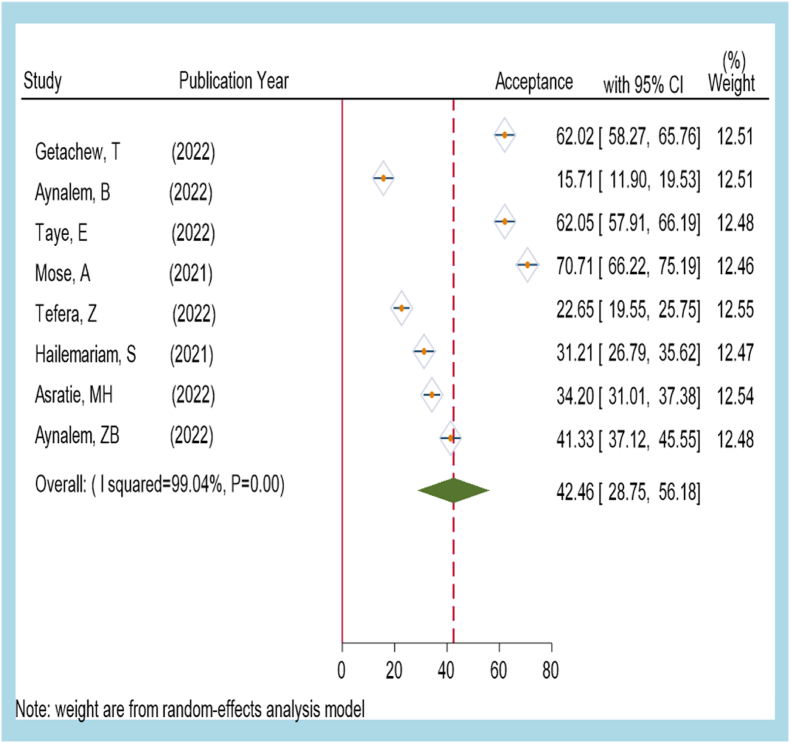
We measured COVID-19 vaccine acceptance among pregnant women as the number of pregnant women willing to receive a vaccine divided by study size multiplied by 100. We used a random effect model for meta-analysis due to significant heterogeneity among studies (I2 = 99.04%). Eight primary studies that reported the proportion of COVID-19 vaccine acceptance among pregnant women were included in the meta-analysis, with a range of 22.65%(95%CI: 19.55–25.75) to 70.71% (95%CI: 66.22–75.19). The highest weight among studies was observed in studies conducted by Zenebe T [18] Asratie, MH(13) [16]. Egger's statistical test result showed the absence of publication bias with a p-value of 0.65. The overall pooled COVID-19 vaccine acceptance among pregnant women in Ethiopia is 42.46%, with a 95% confidence interval of: 28.75–56.18. A forest plot indicated that better vaccine acceptance was reported by Mose A (70.71%) in SNNPs, followed AEden. B (62.05%) in the Amhara region and T. Getachew (62.02%) in the Oromia region [13] (Fig. 2). The result of this analysis implied that only 42 of 100 pregnant women are willing to be vaccinated [18] (Fig 2).
Figure: 2.
A forest plot indicating the pooled level of COVID-19 vaccine acceptance among pregnant women in Ethiopia(dotted red line and dark green diamond indicated overall national level COVID-19 vaccine acceptance). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
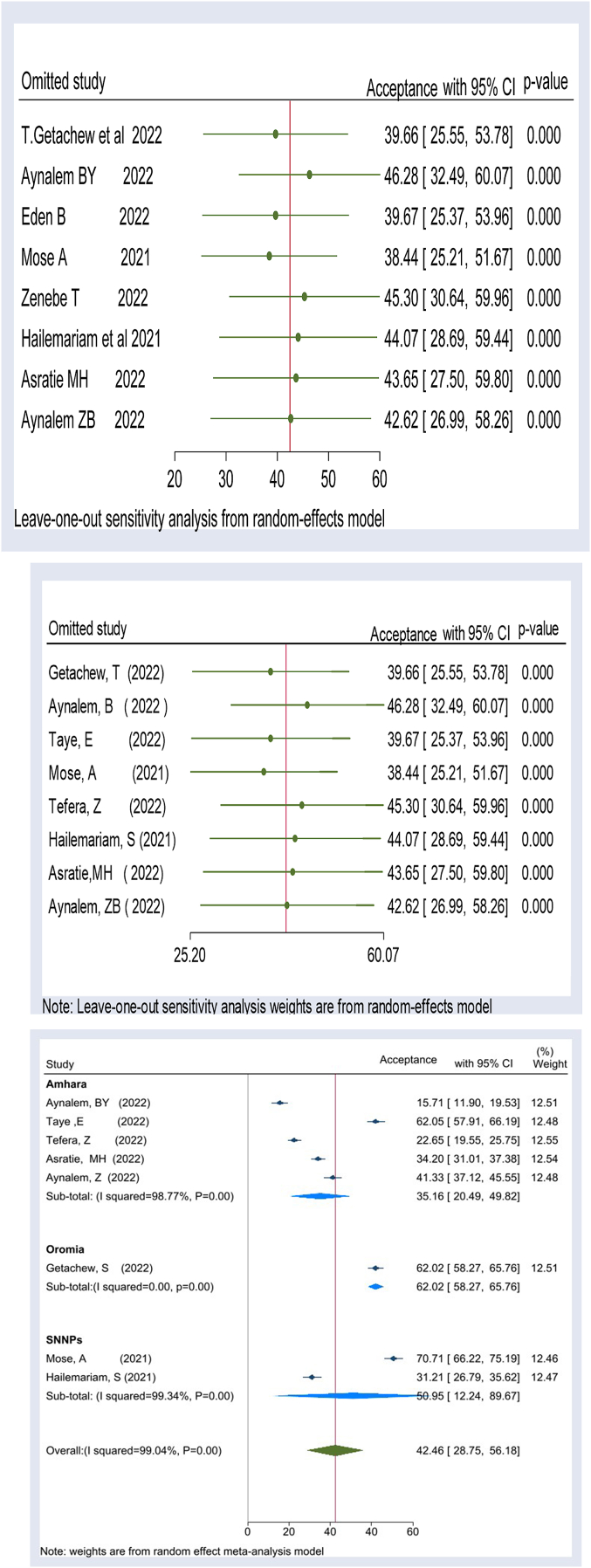
Figure: 3.
Forest plot showing the leave-one-out analysis for heterogeneity among studies included to assess COVID-19 vaccine acceptance among pregnant women in Ethiopia (red line indicated overall national COVID-19 vaccine acceptance). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Our meta-analysis on COVID-19 vaccine acceptance among pregnant women in Ethiopia exhibited a high degree of heterogeneity between primary studies, as indicated by the I2 test (I2 = 99.34, p-value <0.00). To assess the stability of our estimated effect sizes and identify any potential outlier studies, we conducted a sensitivity analysis using a “leave-one-out” approach. This iterative procedure involved omitting one study at a time and calculating the effect sizes using the remaining studies.
Our sensitivity analysis showed that the estimated overall effect size was relatively stable and not significantly impacted by any single study. For each study included in our analysis, we presented an effect size that corresponded to the overall effect size calculated from a meta-analysis that excluded that particular study. To visually display the results of the sensitivity analysis, we included a leave-one-out forest plot that featured a vertical line representing the overall effect size based on the complete set of studies (with no omission). Our findings revealed that the pooled estimate of COVID-19 vaccine acceptance among pregnant women in Ethiopia varied from 38.44 (95%CL: 25.21–51.67) to 46.28 (955CI:32.49–60.07) when each study was excluded iteratively. However, our sensitivity analysis demonstrated that the overall effect size was relatively stable, regardless of which study was omitted (Fig. 3).
3.4. Subgroup analysis by region of study for COVID-19 vaccine acceptance among pregnant women in Ethiopia
A subgroup analysis stratified by location of the primary studies showed that the level of COVID-19 Acceptance among pregnant women in Amhara is 35.16% with a 95% confidence interval of: 20.49 to 49.82 (I2 = 98.77 P = 0.000), South Nation Nationality and Peoples(SNNPs) 50.95% with 95% confidence interval of: 12.24 to 89.67 (I2 = 99.34% P = 0.00) in SNNPs, and Oromia 62.02% with 95% confidence interval of: 58.27 to 65.76 (I2 = 88.00, P = 0.00). The lowest COVID-19 vaccine acceptance among pregnant women is observed in Amhara, whereas the highest is in Oromia (Fig. 4).
Fig. 4.
Forest plot depicting subgroup analysis by region for COVID-19 vaccine acceptance among pregnant women in Ethiopia.
3.5. Predictors of COVID-19 vaccine acceptance among pregnant women
Eight primary studies were included in the meta-analysis to assess predictors for COVID-19 vaccine acceptance among pregnant women; 5 out of 8 showed that there is a significant association between COVID-19 vaccine acceptance and awareness of COVID-19 vaccine among pregnant women with a pooled odds ratio of 3.33 (95%CI:2.13–4.14) [9,[11], [12], [13],[15], [16], [17]]. This finding indicated that pregnant women who were aware of the COVID-19 vaccine were 3.33 times more likely to accept the COVID-19 vaccine. From 5 studies [9,11,13,15,16] included to asses whether education is a predictor variable, only one study indicated a significant association with COVID-19 vaccine acceptance with a pooled odds ratio of 3.09(95%CI: 1.67–4.51). Furthermore, four primary studies were included in a meta-analysis to assess the association of chronic disease with COVId-19 vaccine acceptance, 2 of 4 reported that chronic disease is a predictor for vaccine acceptance. The finding demonstrated that pregnant women with chronic disease are 2.81 fold more likely to accept the COVID-19 vaccine than their counterparts without chronic disease with a 95% confidence interval of 1.82–3.79(11, 12, 15, 17). In contrast, three primary studies were included to assess the effect of maternal age on COVID-19 vaccine acceptance, none of which reported a significant association. The pooled odds ratio from the fixed effect model also showed that there is an insignificant association with the outcome variable (OR 1.99; 95%CI: 0.27–3.70) [9,11,12,17](Table 2).
Table: 2.
A table showing Predictors of COVID-19 vaccine acceptance among pregnant women in Ethiopia.
| Variables | No. studies | Study size | OR(95%CI) | I2 (%) | P-value |
|---|---|---|---|---|---|
| Awareness about COVID vaccine | |||||
| Yes | 8 | 4419 | 3.33(2.13–4.14) | 77.39 | 0.00 |
| No | 1 | ||||
| Chronic disease | |||||
| Yes | 4 | 2222 | 2.81(1.82–3.79) | 0.00 | 0.48 |
| No | 1 | ||||
| Education | |||||
| Read and write | 5 | 2691 | 1 | ||
| 9-12+ | 3.09(1.67–4.51) | 23.8 | 0.42 | ||
| Maternal age | |||||
| 20–31 | 3 | 1271 | 1.99(0.27–3.70) | 0.00 | 0.48 |
| 31+ | 1 | ||||
| Residence | |||||
| urban | 2 | 950 | 1.10(0.3–2.67) | 0.00 | 0.11 |
| Rural | 1 | ||||
4. Discussion
Pregnant women are a particularly vulnerable subset of the population for COVID-19 morbidity and mortality, even if the availability of safe and effective multiple COVID-19 vaccines. Recent real-world meta-analysis studies on COVID-19 vaccine effectiveness and safety among pregnant women found that COVID-19 vaccination prevented women from SARS-CoV-2 infection and COVID-19-related hospitalization. It also stated that vaccination had no adverse effect on pregnant women and their fetal or neonatal outcomes [19]. A global study showed that COVId-19 vaccine acceptance among pregnant women is low (49%) [2] compared to the general population(79.1%) [4]. The reason for the lower acceptance rate might be that pregnant women fear vaccine effects on maternal-fetal complications and effects on fertility. In Ethiopia, there are single studies with inconsistent findings on vaccine acceptance among pregnant women. Therefore providing robust evidence on the national-level COVID-19 vaccine acceptance among pregnant women is paramount to raising vaccine uptake in Ethiopia, where very low vaccine coverage and ubiquitous hesitance is reported. To the best of our knowledge, this is the first meta-analysis that estimated national-level pooled COVID-19 vaccine acceptance and predictors among pregnant in the country.
Our systematic review and meta-analysis demonstrated that the national-level pooled COVID-19 vaccine acceptance among pregnant women is 42.46% with a 95% confidence interval of: 28.75 to 56.18. The finding is lower than the finding of studies conducted in Ethiopia among the general population (56.02%) [20] and (88%) [21]. The discrepancy might be because pregnant women are more concerned about unwanted vaccines' effect on maternal-fetal health and fertility [22]. This meta-analysis's finding is lower than the global survey conducted across 16 countries worldwide (52%) [23]. This finding is also lower than the finding of the meta-analysis studies conducted worldwide (49%) [1],(53.46%) [2], and (54%) [8]. This finding is also lower than the meta-analysis conducted in Latin America and the Caribbean (69.0%) [24]. Similarly, the result of this meta-analysis is lower than the study conducted in the United Kingdom (62.1%) [25], China (77.4%) [26], Czech Republic (70.2%) [27], two studies in Saudi Arabia (54.7%) [28],(68%) [29], Pennsylvania (65%) [30], Thailand (60.8%) [31], Vietnam(60.45) [32], India (78.2%) [33], and in Colombia (44.3%) [34] and California San Diego (43%) in the US [35]. In contrast, the finding of this meta-analysis is higher than the finding of studies conducted in Cameron (31%) [36], Afghanistan (8.6%) [37], Switzerland (29.7%) [38], and Turkey (37%)(39). Differences in the socio-economic status of countries, study period, awareness creation/campaign on COVID-19 vaccine, the impact of the COVID-19 pandemic on each country, and source of information might justify the discrepancies in the studies.
A subgroup meta-analysis stratified by location of the primary studies showed that the overall level of COVID-19 Acceptance among pregnant women in Amhara, SNNPs, and Oromia is 35.16%, 50.95%, and 62.02%, respectively. The lowest COVID-19 vaccine acceptance is observed in the Amhara region, whereas the highest is in the Oromia. The difference in sociocultural practices, beliefs, and attitudes of the communities towards COVID-19 vaccines could explain the different Acceptance rates among pregnant women. Since Ethiopia has more than 80 diverse ethnic groups, various cultural practices might have hampered vaccine acceptance.
In the current meta-analysis, pregnant women who were aware of the COVID-19 vaccine were more likely to accept the COVID-19 vaccination than non-aware pregnant women. This finding agreed with the studies conducted in Pennsylvania [30], China [26], and India [33]. The result might be because awareness could help pregnant women know the benefit of vaccines to them and their pregnancy, so they decided to accept the vaccination. Pregnant women comorbid with chronic disease had more likely to get the COVID-19 vaccine than pregnant women without chronic illness. This finding is in line with many studies conducted elsewhere in China [26], the UK, India [33], the US(24), Saudi Arabia [28], and Afghanistan [37]. The similarity might be due to the impact of COVID-19 being severe among people, including pregnant women who are comorbid with chronic disease, so they decide to accept to prevent hospitalization and death. And also, people with comorbidity might be more aware of the condition and the importance of vaccination, influencing their decision to get a vaccine. Pregnant women who had completed grade-9 and above education were more likely to receive COVID-19 vaccination than those who had not attended school. This finding is consistent with the result of studies conducted in Vietnam [32], India [33], Cameron [36], and Saudi Arabia [28]. The possible explanation might be that mothers who had completed primary education and above could read news and follow social media related to the COVID-19 virus's impact on the general population and its fatality. Therefore, they might use the COVID-19 vaccine compared to their counterparts.
Two studies that reported qualitative findings indicated that religious beliefs, fear of fetal side effects, and misconceptions about vaccines explained the lower level of vaccine acceptance [11]. The other study also found that misconception, fear of medical complications, lack of trust in vaccine effectiveness, and religious aspects were the factors for hesitancy/low level of vaccine acceptance [12].
4.1. Strength of the study
The strength of this study includes the use of multiple databases to search articles (both manual and electronic search), using standard critical appraisal tools, and the abstraction of information uniformly using a predetermined and pretested standard format. Finally, qualitative findings supported quantitative results.
4.2. Limitations of the study
In most of the primary studies on COVID-19, Vaccine acceptance, intention to use, and willingness to use might not be exhaustive. We only find studies from three regions of the country, even if they account for more than 80% of the population. To address the issue of potential variability across studies, we used a random effect model, which tends to give a more conservative estimate.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Data can be accessed upon reasonable request.
Competing interests
The authors declare that they have no competing interests.
Funding
The authors received no specific funding for this work.
Author contributions
All authors conceived the study, reviewed the literature, extracted the data, and did analysis, interpretation, and write-up. Finally, after preparing the manuscript, they all approved it.
Competing interests
The authors declare that we have no competing interests.
References
- 1.Bhattacharya O., Siddiquea B.N., Shetty A., Afroz A., Billah B. COVID-19 vaccine hesitancy among pregnant women: a systematic review and meta-analysis. BMJ Open. 2022;12(8) doi: 10.1136/bmjopen-2022-061477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azami M., Nasirkandy M.P., Gouvarchin Ghaleh H Esmaeili, Ranjbar R. COVID-19 vaccine acceptance among pregnant women worldwide: a systematic review and meta-analysis. PLoS One. 2022;17(9) doi: 10.1371/journal.pone.0272273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karafillakis E, Van Damme P, Hendrickx G, Larson HJ. COVID-19 in Europe: New Challenges for Addressing Vaccine Hesitancy. (1474-547X (Electronic)). [DOI] [PMC free article] [PubMed]
- 4.Lazarus JV, Wyka K, White TM, Picchio CA, Rabin K, Ratzan SC, Parsons Leigh J, Hu J, El-Mohandes A. Revisiting COVID-19 vaccine hesitancy around the world using data from 23 countries in 2021. Nat Commun. Jul 2022;13(1) doi: 10.1038/s41467-022-31441-x. 3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Samadi P, Alipour ZA-O, Ghaedrahmati M, Ahangari R. The Severity of COVID-19 Among Pregnant Women and the Risk of Adverse Maternal Outcomes. (1879-3479 (Electronic)). [DOI] [PMC free article] [PubMed]
- 6.Mutombo P.N., Fallah M.P., Munodawafa D., Kabel A., Houeto D., Goronga T., et al. COVID-19 vaccine hesitancy in Africa: a call to action. Lancet Global Health. 2022;10(3):e320–e321. doi: 10.1016/S2214-109X(21)00563-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Africa-CDC . 2023. Africa CDC COVID-19 Vaccine Dashboard. [Google Scholar]
- 8.Nikpour M., Sepidarkish M., Omidvar S., Firouzbakht M. Global prevalence of acceptance of COVID-19 vaccines and associated factors in pregnant women: a systematic review and meta-analysis. Expet Rev. Vaccine. 2022;21(6):843–851. doi: 10.1080/14760584.2022.2053677. [DOI] [PubMed] [Google Scholar]
- 9.Mose AA-O, Yeshaneh AA-O. COVID-19 Vaccine Acceptance and its Associated Factors Among Pregnant Women Attending Antenatal Care Clinic in Southwest Ethiopia: Institutional-Based Cross-Sectional Study. (1178-7074 (Print)). [DOI] [PMC free article] [PubMed]
- 10.Higgins JP, Thompson SG. Quantifying Heterogeneity in a Meta-Analysis. (0277-6715 (Print)). [DOI] [PubMed]
- 11.Tefera ZA-O, Assefaw MA-O. A Mixed-Methods Study of COVID-19 Vaccine Acceptance and its Determinants Among Pregnant Women in Northeast Ethiopia. (1177-889X (Print)). [DOI] [PMC free article] [PubMed]
- 12.Aynalem BY, Melesse MF, Zeleke LB. COVID-19 Vaccine Acceptability and Determinants Among Pregnant Mothers Attending Antenatal Care Services at Debre Markos Town Public Health Institutions, Debre Markos Northwest Ethiopia: Mixed Study. (1937-8688 (Electronic)). [DOI] [PMC free article] [PubMed]
- 13.Getachew T, Balis B, Eyeberu A, Debella A, Nigussie S, Habte S, et al. COVID-19 Vaccine Acceptance Among Pregnant Women Attending Antenatal Care in Public Hospitals in Eastern Ethiopia: A Multi-Center Facility-Based Cross-Sectional Study. (2666-5352 (Electronic)). [DOI] [PMC free article] [PubMed]
- 14.Taye EB, Taye ZW, Muche HA, Tsega NT, Haile TT, Tiguh AE. COVID-19 Vaccine Acceptance and Associated Factors Among Women Attending Antenatal and Postnatal Cares in Central Gondar Zone Public Hospitals, Northwest Ethiopia. (2452-0918 (Print)). [DOI] [PMC free article] [PubMed]
- 15.Hailemariam SA-O, Mekonnen B, Shifera N, Endalkachew B, Asnake MA-O, Assefa A, et al. Predictors of Pregnant Women's Intention to Vaccinate against Coronavirus Disease 2019: A Facility-Based Cross-Sectional Study in Southwest Ethiopia. (2050-3121 (Print)). [DOI] [PMC free article] [PubMed]
- 16.Asratie MA-O, Kassie BA, Belay DG, Endalew M, Gashaw MA-O, Assegie GM. Perception of Risk Regarding the Use of COVID-19 Vaccine Among Pregnant Women in Motta Town and Hulet Eji Enese District, Northwest Ethiopia. (1932-6203 (Electronic)). [DOI] [PMC free article] [PubMed]
- 17.Aynalem ZA-O, Bogale TW, Bantie GM, Ayalew AF, Tamir W, Feleke DG, et al. Factors Associated with Willingness to Take COVID-19 Vaccine Among Pregnant Women at Gondar Town, Northwest Ethiopia: A Multicenter Institution-Based Cross-Sectional Study. (1932-6203 (Electronic)). [DOI] [PMC free article] [PubMed]
- 18.Ma Y., Deng J., Liu Q., Du M., Liu M., Liu J. Effectiveness and safety of COVID-19 vaccine among pregnant women in real-world studies: a systematic review and meta-analysis. Vaccines. 2022;10(2) doi: 10.3390/vaccines10020246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma Y., Deng J., Liu Q., Du M., Liu M., Liu J. Effectiveness and safety of COVID-19 vaccine among pregnant women in real-world studies: a systematic review and meta-analysis. Vaccines. 2022;10(2) doi: 10.3390/vaccines10020246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mekonnen B.D., Mengistu B.A. COVID-19 vaccine acceptance and its associated factors in Ethiopia: a systematic review and meta-analysis. Clinical Epidemiology and Global Health. 2022;14 doi: 10.1016/j.cegh.2022.101001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strupat CA-O, Shigute Z, Bedi AS, Rieger M. Willingness to Take COVID-19 Vaccination in Low-Income Countries: Evidence from Ethiopia. (1932-6203 (Electronic)). [DOI] [PMC free article] [PubMed]
- 22.Odedokun T., Marquez R., Thakkar M., Dinglas C., Kady D.E. COVID-19 vaccine acceptance in pregnancy. Am. J. Perinatol. 2022 doi: 10.1055/s-0042-1757275. [DOI] [PubMed] [Google Scholar]
- 23.Skjefte M., Ngirbabul M., Akeju O., Escudero D., Hernandez-Diaz S., Wyszynski D.F., et al. COVID-19 vaccine acceptance among pregnant women and mothers of young children: results of a survey in 16 countries. Eur. J. Epidemiol. 2021;36(2):197–211. doi: 10.1007/s10654-021-00728-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alarcón-Braga E.A., Hernandez-Bustamante E.A., Salazar-Valdivia F.E., Valdez-Cornejo V.A., Mosquera-Rojas M.D., Ulloque-Badaracco J.R., et al. Acceptance towards COVID-19 vaccination in Latin America and the Caribbean: a systematic review and meta-analysis. Trav. Med. Infect. Dis. 2022;49 doi: 10.1016/j.tmaid.2022.102369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Skirrow H., Barnett S., Bell S., Riaposova L., Mounier-Jack S., Kampmann B., et al. Women's views on accepting COVID-19 vaccination during and after pregnancy, and for their babies: a multi-methods study in the UK. BMC Pregnancy Childbirth. 2022;22(1):33. doi: 10.1186/s12884-021-04321-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tao LA-O, Wang R, Han N, Liu J, Yuan C, Deng L, et al. Acceptance of a COVID-19 Vaccine and Associated Factors Among Pregnant Women in China: a Multi-Center Cross-Sectional Study Based on Health Belief Model. (2164-554X (Electronic)). [DOI] [PMC free article] [PubMed]
- 27.Riad AA-O, Jouzová A, Üstün BA-O, Lagová E, Hruban L, Janků P, et al. COVID-19 vaccine acceptance of pregnant and lactating women (PLW) in Czechia: an analytical cross-sectional study. LID - 10.3390/ijerph182413373 [doi] LID - 13373. (1660-4601 (Electronic)). [DOI] [PMC free article] [PubMed]
- 28.Bagalb A.S., Almazrou D., Albraiki A.A., Alflaih L.I., Bamunif L.O. COVID-19 vaccine acceptance among pregnant and lactating women in Saudi Arabia. Cureus. 2022;14(12) doi: 10.7759/cureus.32133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghamri R.A., Othman S.S., Alhiniah M.H., Alelyani R.H., Badawi A.M., Alshahrani A.A. Acceptance of COVID-19 vaccine and associated factors among pregnant women in Saudi Arabia. Patient Prefer. Adherence. 2022;16:861–873. doi: 10.2147/PPA.S357653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sznajder K.K., Kjerulff K.H., Wang M., Hwang W., Ramirez S.I., Gandhi C.K. Covid-19 vaccine acceptance and associated factors among pregnant women in Pennsylvania 2020. Preventive medicine reports. 2022;26 doi: 10.1016/j.pmedr.2022.101713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pairat K., Phaloprakarn C. Acceptance of COVID-19 vaccination during pregnancy among Thai pregnant women and their spouses: a prospective survey. Reprod. Health. 2022;19(1):74. doi: 10.1186/s12978-022-01383-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nguyen L.H., Hoang M.T., Nguyen L.D., Ninh L.T., Nguyen H.T.T., Nguyen A.D., et al. Acceptance and willingness to pay for COVID-19 vaccines among pregnant women in Vietnam. Tropical medicine & international health. TM & IH. 2021;26(10):1303–1313. doi: 10.1111/tmi.13666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumari A., Kumari S., Kujur M., Tirkey S., Singh S.B. Acceptance rate of COVID-19 vaccine and its determinants among Indian pregnant women: a hospital-based cross-sectional analysis. Cureus. 2022;14(10) doi: 10.7759/cureus.30682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sutton D., D'Alton M., Zhang Y., Kahe K., Cepin A., Goffman D., et al. COVID-19 vaccine acceptance among pregnant, breastfeeding, and nonpregnant reproductive-aged women. American journal of obstetrics & gynecology MFM. 2021;3(5) doi: 10.1016/j.ajogmf.2021.100403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perrotta K., Messer A., Alvarado S., Gaudette M., Tran C., Bandoli G. COVID-19 vaccine hesitancy and acceptance among pregnant people contacting a teratogen information service. J. Genet. Counsel. 2022;31(6):1341–1348. doi: 10.1002/jgc4.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gunawardhana N., Baecher K., Boutwell A., Pekwarake S., Kifem M., Ngong M.G., et al. COVID-19 vaccine acceptance and perceived risk among pregnant and non-pregnant adults in Cameroon, Africa. PLoS One. 2022;17(9) doi: 10.1371/journal.pone.0274541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nemat A., Yaftali S., Danishmand T.J., Nemat H., Raufi N., Asady A. High rates of COVID-19 vaccine refusal among Afghan pregnant women: a cross sectional study. Sci. Rep. 2022;12(1) doi: 10.1038/s41598-022-18497-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stuckelberger S., Favre G., Ceulemans M., Nordeng H., Gerbier E., Lambelet V., et al. SARS-CoV-2 vaccine willingness among pregnant and breastfeeding women during the first pandemic wave: a cross-sectional study in Switzerland. Viruses. 2021;13(7) doi: 10.3390/v13071199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data can be accessed upon reasonable request.