Abstract
A visible-light-induced Pd-catalyzed stereoselective synthesis of alkylated ester hydrazones has been developed. This method operates via generation of a nucleophilic carbon-centered radical from alkyl bromide, iodide, or redox-active ester, followed by its addition to hydrazone, and a subsequent desaturation by palladium. The majority of products have E configuration, which are inaccessible by conventional condensation methods. In addition, a sequential C,N-alkylation protocol has been developed: a reaction between 1,3-dihalides and glyoxylate-derived hydrazone, delivering tetrahydropyridazines.
Graphical Abstract
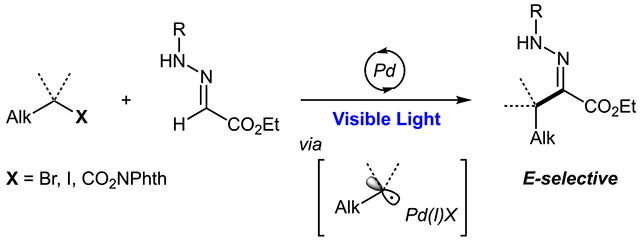
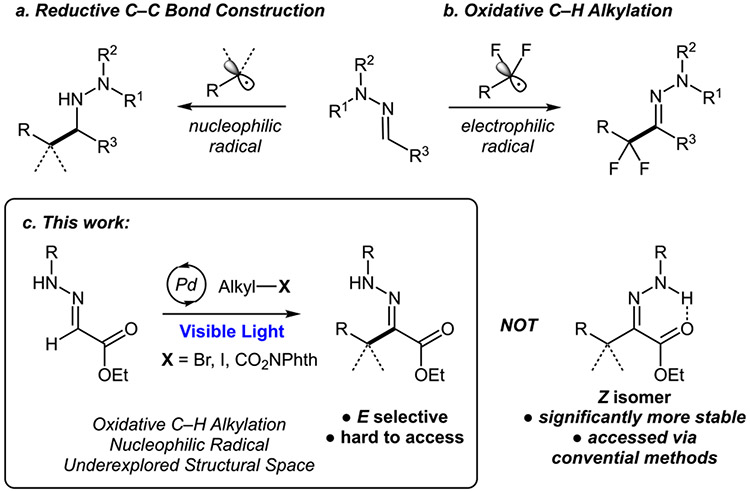
Hydrazone is a highly versatile functional group within the organic chemistry domain. It is most famous for use as a precursor/intermediate in synthesis of various electron-rich heterocycles, such as pyrazoles1 and indoles,2 as well as in azaenolate reactions3 and carbene chemistry.4 Beyond synthetic chemistry, hydrazones are used in bioconjugation,5 polymer,6 and material7 chemistry. A number of hydrazone-containing molecules exhibit biological activity, which paves the way for drug discovery.8 Classically, hydrazones are accessed via condensation reactions of carbonyls with hydrazines.9 Quite simple, this method is reliant on availability of corresponding aldehyde or ketone and may not be a facile route for diversification. Moreover, sometimes forcing conditions (heat and acid) are required to achieve the desired transformation, which may become troublesome in the presence of certain sensitive functional groups. A direct C─H functionalization of aldehyde-derived hydrazones appears as an attractive alternative for rapid assembly of fully substituted hydrazones, which could bypass these challenges. Primarily, such functionalization can be achieved via radical chemistry of hydrazones. Hydrazones have long been recognized as good nucleophilic radical acceptors.10 On the basis of this property, a number of reductive C─C bond-forming reactions have emerged (Scheme 1a),10,11 including asymmetric reactions,12 ultimately leading to chiral amines. The oxidative version of this transformation was recently realized in the form of perfluorinated radical addition (Scheme 1b).13 Use of a copper or photoredox catalyst or stoichiometric oxidant ensures regeneration of the carbon─nitrogen double bond to restore valuable hydrazone functionality. Alternatively, the C═N double bond could be preserved via the radical addition/elimination strategy used in closely related oxime-type substrates;14 however, this route has not been realized for hydrazones. As a result, at this point, there are only few non-fluorinated radical examples present in the literature,15 which means that, even though oxidative C─H alkylation is possible, it remains quite limited in the choice of radical precursors to date. In addition, these reactions most often rely on use of dialkyl-substituted hydrazones, which may prevent further functionalization, such as employment in indole synthesis.13 A non-radical C─H alkylation/arylation pathway is also possible, usually via pseudo-enolate-type reactions, but these examples are scarce and limited to very specific systems.16 On the basis of the broad application of hydrazones within synthetic chemistry1-4 and beyond,5-8 development of a more general C─H alkylation protocol is warranted. Herein, we report a mild visible-light-induced stereoselective Pd-catalyzed Heck-type C─H alkylation of glyoxylate-derived hydrazones proceeding via nucleophilic radical addition (Scheme 1c).17 The reaction exhibits a remarkable stereoselectivity, yielding a significantly less thermodynamically stable E isomer, allowing for the exploration of previously inaccessible chemical space.
Scheme 1. Radical Reactions of Hydrazones.
In last few years, the visible-light-induced photoexcited chemistry of palladium has emerged as a powerful tool for combining carbon-centered radical chemistry with innate properties of palladium complexes.18 As a part of the field, previously hard-to-realize intermolecular alkyl-Heck-type19 transformations of styrenes and other activated alkenes20 and oximes21 have been developed using an array of alkyl radical precursors. Motivated by these findings, we were eager to explore potential application of this approach to achieve a critical C─H alkylation of hydrazones.
A set of conditions was identified for coupling hydrazone 1 with cyclohexyl bromide 2 (see the Supporting Information for details). Suprisingly, the obtained product had an E configuration, despite the fact that the Z isomer is significantly more thermodynamically stable as a result of internal hydrogen bonding.22 Importantly, in a typical condensation reaction, the Z isomer is either a sole or a predominant product in most cases.23 While it is possible to synthesize the E isomer via other routes, typically, these involve reactions between organolithium reagents and diazo compounds24 or silyl-enol ethers with diazonium salts25 and, thus, have very low functional group tolerance. This means that our method not only allows for an unprecedented radical C─H alkylation of hydrazones but also allows for the synthesis of stereoisomers, which are not available otherwise.
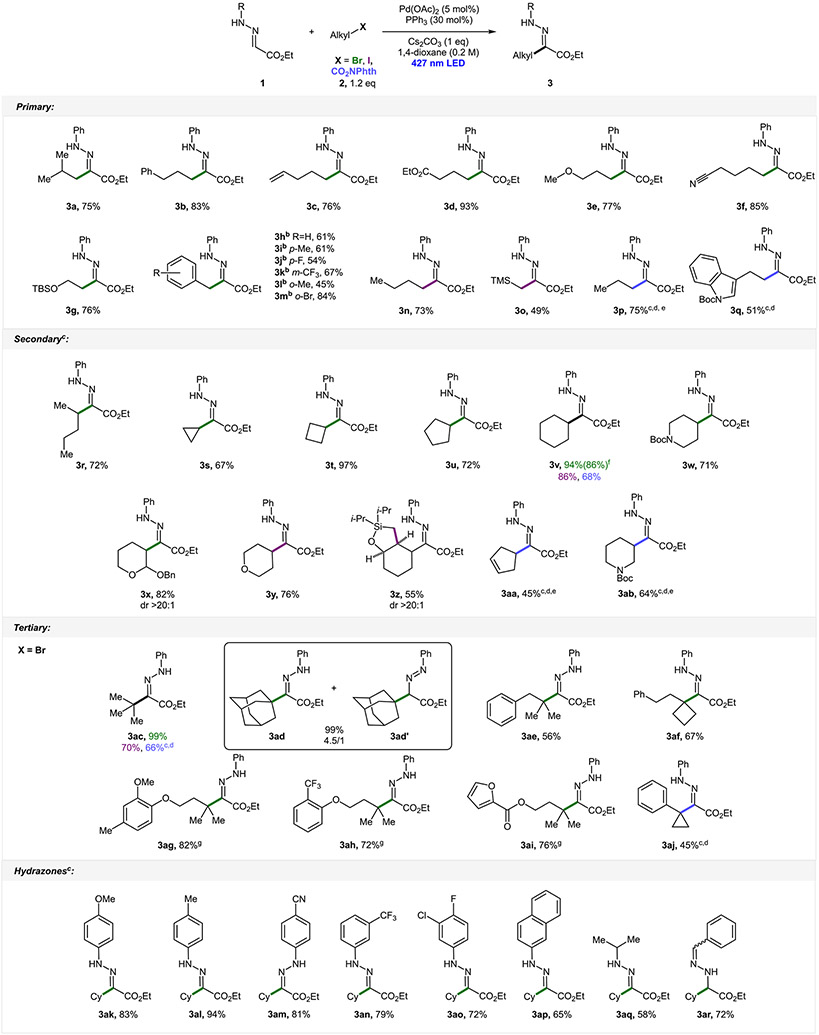
With the optimized conditions in hand, we began investigation of the reaction scope by interrogating reactions of various primary alkyl electrophiles with benchmark hydrazone 1 (Scheme 2). Alkyl bromide (3a), iodide (3n), and redox-active ester (3p) all have been found to be suitable radical precursors. Functional groups, such as phenyl (3b), terminal alkene (3c), ester (3d), ether (3e), and nitrile (3f), were all tolerated under these reaction conditions. The synthesis of bromoethanol derivative 3g, however, required a protecting group. The slightly more electrophilic trimethylsilyl-methylene radical has also engaged in the reaction with hydrazone, although delivering a product with a slightly diminished yield (3o). Perhaps the most surprising results in the investigation of primary electrophiles were reactions of benzylic bromides (3h–3m). Engagement of benzylic electrophiles in alkyl-Heck reactions via hybrid Pd radical chemistry remains a challenge. Excitingly, under a slightly modified procedure,26 we were able to achieve the desired transformation with moderate efficiency. Even more surprising was the fact that, out of all tested substrates, ortho-bromobenzyl bromide has proven to be the best reactant, with bromide functionality remaining untouched (3m). Moreover, secondary alkyl substrates, including acyclic (3r) or cyclic compounds with various ring sizes (3s–3v), could all be used via this protocol. A direct comparison between cyclohexyl bromide, iodide, and redox-active ester (3v) led to the conclusion that alkyl bromides are the most effective substrates in general. Hydrazones possessing various six-membered saturated heterocycles (3w–3y and 3ab) could also be accessed. Furthermore, this transformation can be incorporated in a cascade reaction, where the first radical 1,5-exo-trig cyclization is followed by the addition to hydrazone, resulting in a bicyclic product (3z). Excitingly, reactions leading to compounds 3x and 3z proceeded with high degrees of diastereocontrol. Tertiary substrates, as opposed to primary and secondary substrates, with few exceptions (3af and 3aj), resulted in products with a Z configuration. An increased steric repulsion as a result of a 1,3-allylic strain exhibited in a potential E isomer could be the reason for the reversed stereochemistry in these cases. Intriguingly, initial isolation of adamantyl-containing product 3ad revealed the presence of the corresponding diazene 3ad’. Diazene isomerized into the product overnight in slightly acidic non-neutralized CDCl3.26 Next, the scope of hydrazones was studied with emphasis placed on N-aryl hydrazones, en route to substituted indoles. In general, different functional groups on the aromatic ring did not influence the reaction outcome greatly, except for the para-nitrile substituent, where a Z stereoisomer 3am was obtained. It could be explained by an increased acidity of the NH proton, making this hydrazine susceptible to base-induced isomerization. Excitingly, alkyl-substituted hydrazone 3aq could also be obtained in a moderate yield via this protocol. Surprisingly, when benzyl-substituted hydrazone was employed, a regioisomeric non-ester-conjugated hydrazone product 3ar was obtained.
Scheme 2. Scope of Hydrazone in the Alkylation Reactiona.
aAt a 0.3 mmol scale. bXantphos (15 mol %) was used instead of PPh3. cHexamethylphosphoramide (HMPA, 0.2 M) was used as a solvent (if X = Br). dPd(PPh3)4 (5 mol %) was used instead of Pd(OAc)2/PPh3. eA 1.5 equiv of redox-active ester used. fA 2 mmol scale yield. gA 1 equiv of tertiary bromide and 1.2 equiv of compound 1.
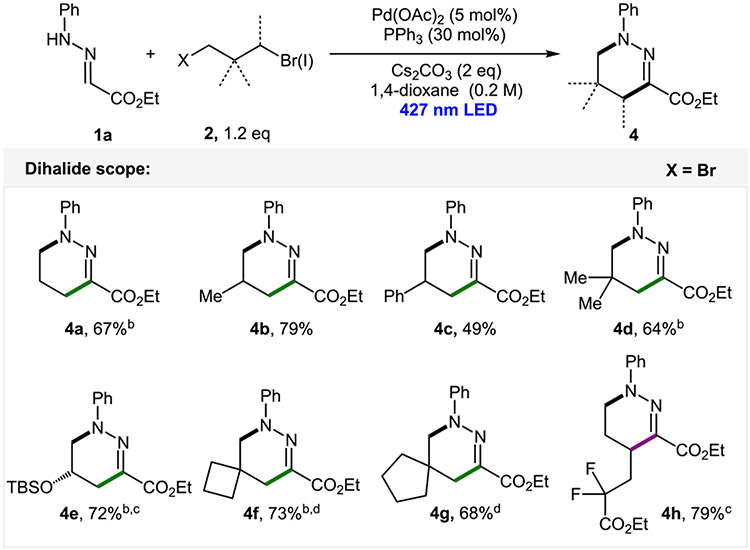
Importantly, because these reaction conditions lead to E products, the nitrogen atom is preset for a potential intramolecular SN2 reaction if an appropriate electrophile is present.27 Indeed, employment of simple 1,3-dibromopropane under standard reaction conditions with an addition of another equivalent of a base led to 1,4,5,6-tetrahydropyridazine (4a) in a good yield (Scheme 3). Via this manner, several six-membered heterocycles were synthesized, including the phenyl-substituted product (4c), (S)-epiclorohydrin derivative (4e), 4,6- and 5,6-spirocycles (4f and 4g), and difluoro-containing product (4h).
Scheme 3. Scope of the Sequential C,N-Alkylation Reactiona.
aAt a 0.3 mmol scale. b1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU) was used as a base. cX = Cl. dA 1.5 equiv of dibromide.
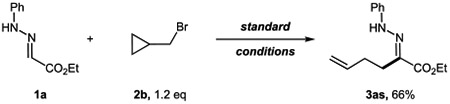
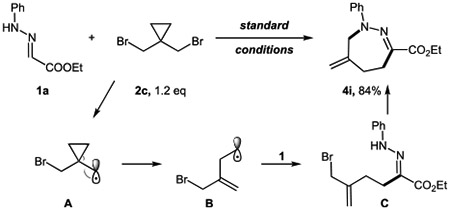
The formation of the carbon-centered radical was validated by reactions of (bromomethyl)cyclopropane under standard reaction conditions, which led to a ring-opening product 3as in a moderate yield (eq 1). Notably, when bis(bromomethyl)cyclopropane 2c was employed, a seven-membered heterocycle containing an endocyclic alkene 4i was obtained (eq 2).
 |
(1) |
 |
(2) |
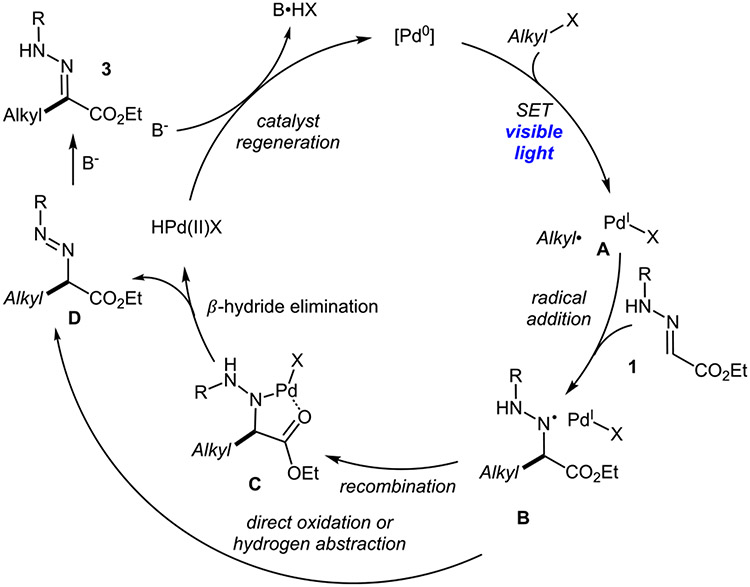
On the basis of the relevance of the reported light-induced Heck reactions of olefins18,20a and C─H alkylation of oximes,21 together with the above-mentioned observations, the following mechanism is proposed (Scheme 4). Initially, visible-light-induced alkyl Pd hybrid species A is produced, followed by the favorable10 radical addition to the C═N bond of hydrazone. The formed intermediate B could be either directly oxidized into diazene D or recombined with Pd(I) to form Pd(II) complex C, which, in turn, would undergo a β-hydride-type elimination to deliver diazene D. Diazene intermediacy is supported by its observation in the synthesis of adamantyl derivative 3ad (vide supra) as well as obtaining the non-ester conjugated regioisomer 3ar. The formation of the latter presumes intermediacy of the proposed diazene intermediate, which forms upon selective isomerization, driven by conjugation with the aromatic ring, as opposed to that with the ester group. It must be noted, however, that, in cases of primary and secondary alkyl radicals, the diazene intermediate was not detected during the reaction progress. This may imply that base-assisted isomerization of diazenes into products is very facile. E selectivity of obtained products could be explained by palladium-assisted isomerization, where Pd is chelated by oxygen and nitrogen atoms, thus pushing the other nitrogen away from the ester group. Alternatively, E hydrazone may arise from fast kinetically favorable isomerization of diazene, which appears irreversible under basic conditions. Otherwise, even in slightly acidic CDCl3, a rapid complete isomerization of E products to Z products occurs at room temperature.26
Scheme 4. Proposed Reaction Mechanism.
In conclusion, we developed a mild visible-light-induced Pd-catalyzed Heck-type alkylation of glyoxylate-derived hydrazones proceeding via a hybrid Pd radical mechanism. The major highlight of the presented work is an excellent stereoselectivity, enabling synthesis of previously hard-to-access E isomers of ester-containing hydrazones. Unique stereoselectivity of the process was also used in a sequential C,N-alkylation reaction toward one-pot construction of tetrahydropyridazines. We hope that this work will spark further interest in development of new C─H functionalization of imine-type compounds.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the National Institute of Health (GM120281), the National Science Foundation (CHE-1955663), and the Welch Foundation (Chair, AT-0041) for financial support.
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.orglett.2c01409.
Experimental procedures and analytical data for all new compounds (PDF)
The authors declare no competing financial interest.
REFERENCES
- (1).Karrouchi K; Radi S; Ramli Y; Taoufik J; Mabkhot YN; Al-aizari FA; Ansar M. h. Synthesis and Pharmacological Activities of Pyrazole Derivatives: A Review. Molecules 2018, 23, 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2) (a).Robinson B The Fischer Indole Synthesis. Chem. Rev 1963, 63, 373–401. [Google Scholar]; (b) Humphrey GR; Kuethe JT Practical Methodologies for the Synthesis of Indoles. Chem. Rev 2006, 106, 2875–2911. [DOI] [PubMed] [Google Scholar]; (c) Taber DF; Tirunahari PK Indole Synthesis: A Review and Proposed Classification. Tetrahedron 2011, 67, 7195–7210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3) (a).Lazny R; Nodzewska A N,N-Dialkylhydrazones in Organic Synthesis. From Simple N,N-Dimethylhydrazones to Supported Chiral Auxiliaries. Chem. Rev 2010, 110, 1386–1434. [DOI] [PubMed] [Google Scholar]; (b) de Gracia Retamosa M; Matador E; Monge D; Lassaletta JM; Fernández R Hydrazones as Singular Reagents in Asymmetric Organocatalysis. Chem.—Eur. J 2016, 22, 13430–13445. [DOI] [PubMed] [Google Scholar]
- (4) (a).Barluenga J; Moriel P; Valdés C; Aznar F N-Tosylhydrazones as Reagents for Cross-Coupling Reactions: A Route to Polysubstituted Olefins. Angew. Chem., Int. Ed 2007, 46, 5587–5590. [DOI] [PubMed] [Google Scholar]; (b) Radolko J; Ehlers P; Langer P Recent Advances in Transition-Metal-Catalyzed Reactions of N-Tosylhydrazones. Adv. Synth. Catal 2021, 363, 3616–3654. [Google Scholar]
- (5).Kölmel DK; Kool ET Oximes and Hydrazones in Bioconjugation: Mechanism and Catalysis. Chem. Rev 2017, 117, 10358–10376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).García F; Smulders MMJ Dynamic Covalent Polymers. J. Polym. Sci., Part A: Polym. Chem 2016, 54, 3551–3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7) (a).Shao B; Aprahamian I Hydrazones as New Molecular Tools. Chem 2020, 6, 2162–2173. [Google Scholar]; (b) Tatum LA; Su X; Aprahamian I Simple Hydrazone Building Blocks for Complicated Functional Materials. Acc. Chem. Res 2014, 47, 2141–2149. [DOI] [PubMed] [Google Scholar]
- (8) (a).de Oliveira Carneiro Brum J; França CCT; LaPlante RS; Villar DFJ Synthesis and Biological Activity of Hydrazones and Derivatives: A Review. Mini-Rev. Med. Chem 2020, 20, 342–368. [DOI] [PubMed] [Google Scholar]; (b) Rollas S; Küçükgüzel SG Biological Activities of Hydrazone Derivatives. Molecules 2007, 12, 1910–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Verma G; Marella A; Shaquiquzzaman M; Akhtar M; Ali M; Alam M A Review Exploring Biological Activities of Hydrazones. J. Pharm. Bioallied Sci 2014, 6, 69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Sayer JM; Pinsky B; Schonbrunn A; Washtien W Mechanism of Carbinolamine Formation. J. Am. Chem. Soc 1974, 96, 7998–8009. [Google Scholar]
- (10).Friestad GK Addition of Carbon-Centered Radicals to Imines and Related Compounds. Tetrahedron 2001, 57, 5461–5496. [Google Scholar]
- (11) (a).Cullen STJ; Friestad GK Alkyl Radical Addition to Aliphatic and Aromatic N-Acylhydrazones Using an Organic Photo-redox Catalyst. Org. Lett 2019, 21, 8290–8294. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Friestad GK; Ji A Mn-Mediated Coupling of Alkyl Iodides and Ketimines: A Radical Addition Route to α,α-Disubstituted α-Aminoesters. Org. Lett 2008, 10, 2311–2313. [DOI] [PubMed] [Google Scholar]
- (12) (a).Rono LJ; Yayla HG; Wang DY; Armstrong MF; Knowles RR Enantioselective Photoredox Catalysis Enabled by Proton-Coupled Electron Transfer: Development of an Asymmetric Aza-Pinacol Cyclization. J. Am. Chem. Soc 2013, 135, 17735–17738. [DOI] [PubMed] [Google Scholar]; (b) Friestad GK Radical Additions to Chiral Hydrazones: Stereoselectivity and Functional Group Compatibility. In Radicals in Synthesis III; Heinrich M, Gansäuer A, Eds.; Springer: Berlin, Germany, 2012; Topics in Current Chemistry, Vol. 320, pp 61–91, DOI: 10.1007/128_2011_163. [DOI] [PubMed] [Google Scholar]
- (13).Xu X; Zhang J; Xia H; Wu J C(sp2)─H Functionalization of Aldehyde-Derived Hydrazones Via a Radical Process. Org. Biomol Chem 2018, 16, 1227–1241. [DOI] [PubMed] [Google Scholar]
- (14) (a).Clive DLJ; Subedi R Radical Cyclization of O-Trityl Oximino Esters: A Ring Closure That Preserves the Oxime Function. Chem. Commun 2000, 237–238. [Google Scholar]; (b) Kim S; Lee IY; Yoon J-Y; Oh DH Novel Radical Reaction of Phenylsulfonyl Oxime Ethers. A Free Radical Acylation Approach. J. Am. Chem. Soc 1996, 118, 5138–5139. [Google Scholar]; (c) Kim S; Yoon J-Y Free Radical-Mediated Ketone Synthesis from Alkyl Iodides Via Sequential Radical Acylation Approach. J. Am. Chem. Soc 1997, 119, 5982–5983. [Google Scholar]
- (15) (a).Prieto A; Bouyssi D; Monteiro N Copper-Catalyzed Double C–H Alkylation of Aldehyde-Derived N,N-Dialkylhydrazones with Polyhalomethanes: Flexible Access to 4-Functionalized Pyrazoles. ACS Catal. 2016, 6, 7197–7201. [Google Scholar]; (b) Fan X-W; Lei T; Zhou C; Meng Q-Y; Chen B; Tung C-H; Wu L-Z Radical Addition of Hydrazones by α-Bromo Ketones to Prepare 1,3,5-Trisubstituted Pyrazoles Via Visible Light Catalysis. J. Org. Chem 2016, 81, 7127–7133. [DOI] [PubMed] [Google Scholar]; (c) Cheng J; Xu P; Li W; Cheng Y; Zhu C The Functionalization of a Cascade of C(sp2)-H/C(sp3)─H Bonds: Synthesis of Fused Dihydropyrazoles Via Visible-Light Photoredox Catalysis. Chem. Commun 2016, 52, 11901–11904. [DOI] [PubMed] [Google Scholar]; (d) Zhou N; Liu J; Yan Z; Wu Z; Zhang H; Li W; Zhu C Synthesis of Cyclohexylidenehydrazine-Fused Polycyclics Via a Photocatalytic Radical Cascade Reaction of 2-Ethynylaldehyde Hydrazones. Chem. Commun 2017, 53, 2036–2039. [DOI] [PubMed] [Google Scholar]; (e) Cheng J; Li W; Duan Y; Cheng Y; Yu S; Zhu C Relay Visible-Light Photoredox Catalysis: Synthesis of Pyrazole Derivatives Via Formal [4 + 1] Annulation and Aromatization. Org. Lett 2017, 19, 214–217. [DOI] [PubMed] [Google Scholar]; (f) Luescher MU; Songsichan T; Hsieh S-Y; Bode JW Copper Promoted Oxidative Coupling of Snap Hydrazines and Aldehydes to Form Chiral 1,4,5-Oxadiazepanes and 1,2,5-Triazepanes. Helv. Chim. Acta 2017, 100, e1700199. [Google Scholar]
- (16) (a).Cheng D; Shen Y; Wu Z; Xu X; Yan J Metal-Free Tandem Oxidative Cyclization for the Synthesis of 1,2-Dihydropyridazines and Pyrazoles. J. Org. Chem 2021, 86, 8563–8575. [DOI] [PubMed] [Google Scholar]; (b) Fernández M; Uria U; Vicario JL; Reyes E; Carrillo L Enantioselective Conjugate Addition of Donor–Acceptor Hydrazones to α,β-Unsaturated Aldehydes through Formal Diaza–Ene Reaction: Access to 1,4-Dicarbonyl Compounds. J. Am. Chem. Soc 2012, 134, 11872–11875. [DOI] [PubMed] [Google Scholar]; (c) Hashimoto T; Hirose M; Maruoka K Asymmetric Imino Aza-Enamine Reaction Catalyzed by Axially Chiral Dicarboxylic Acid: Use of Arylaldehyde N,N-Dialkylhydrazones as Acyl Anion Equivalent. J. Am. Chem. Soc 2008, 130, 7556–7557. [DOI] [PubMed] [Google Scholar]; (d) Kaïm LE; Gautier L; Grimaud L; Harwood LM; Michaut V The Mannich Reaction of Hydrazones: Improved Reactivity under Solvent-Free Conditions. Green Chem. 2003, 5, 477–479. [Google Scholar]; (e) Singh K; Ralhan S; Sharma PK; Dhawan SN Vilsmeier–Haack Reaction on Hydrazones: A Convenient Synthesis of 4-Formylpyrazoles. J. Chem. Res 2005, 2005, 316–318. [Google Scholar]; (f) Xu P; Wang G; Wu Z; li S; Zhu C Rh(III)-Catalyzed Double C–H Activation of Aldehyde Hydrazones: A Route for Functionalized 1H-Indazole Synthesis. Chem. Sci 2017, 8, 1303–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Xu X; Zavalij PY; Hu W; Doyle MP Vinylogous Reactivity of Enol Diazoacetates with Donor–Acceptor Substituted Hydrazones. Synthesis of Substituted Pyrazole Derivatives. J. Org. Chem 2013, 78, 1583–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Zabaleta N; Uria U; Reyes E; Carrillo L; Vicario JL Ion-Pairing Catalysis in the Enantioselective Addition of Hydrazones to N-Acyldihydropyrrole Derivatives. Chem. Commun 2018, 54, 8905–8908. [DOI] [PubMed] [Google Scholar]
- (17) (a).Blackwell JH; Kumar R; Gaunt MJ Visible-Light-Mediated Carbonyl Alkylative Amination to All-Alkyl α-Tertiary Amino Acid Derivatives. J. Am. Chem. Soc 2021, 143, 1598–1609. [DOI] [PubMed] [Google Scholar]; (b) Aguilar Troyano FJ; Merkens K; Anwar K; Gómez-Suárez A Radical-Based Synthesis and Modification of Amino Acids. Angew. Chem., Int. Ed 2021, 60, 1098–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18) (a).Chuentragool P; Kurandina D; Gevorgyan V Catalysis with Palladium Complexes Photoexcited by Visible Light. Angew. Chem., Int. Ed 2019, 58, 11586–11598. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Zhou W-J; Cao G-M; Zhang Z-P; Yu D-G Visible Light-Induced Palladium-Catalysis in Organic Synthesis. Chem. Lett 2019, 48, 181–191. [Google Scholar]
- (19) (a).McMahon CM; Alexanian EJ Palladium-Catalyzed Heck-Type Cross-Couplings of Unactivated Alkyl Iodides. Angew. Chem., Int. Ed 2014, 53, 5974–5977. [DOI] [PubMed] [Google Scholar]; (b) Zou Y; Zhou J Palladium-Catalyzed Intermolecular Heck Reaction of Alkyl Halides. Chem. Commun 2014, 50, 3725–3728. [DOI] [PubMed] [Google Scholar]; (c) McAtee JR; Yap GPA; Watson DA Rational Design of a Second Generation Catalyst for Preparation of Allylsilanes Using the Silyl-Heck Reaction. J. Am. Chem. Soc 2014, 136, 10166–10172. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Reid WB; Spillane JJ; Krause SB; Watson DA Direct Synthesis of Alkenyl Boronic Esters from Unfunctionalized Alkenes: A Boryl-Heck Reaction. J. Am. Chem. Soc 2016, 138, 5539–5542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20) (a).Kurandina D; Chuentragool P; Gevorgyan V Transition-Metal-Catalyzed Alkyl Heck-Type Reactions. Synthesis 2019, 51, 985–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Lee GS; Kim D; Hong SH Pd-Catalyzed Formal Mizoroki–Heck Coupling of Unactivated Alkyl Chlorides. Nat. Commun 2021, 12, 991. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Zhang Z; Kvasovs N; Dubrovina A; Gevorgyan V Visible Light Induced Brønsted Acid Assisted Pd-Catalyzed Alkyl Heck Reaction of Diazo Compounds and N-Tosylhydrazones. Angew. Chem., Int. Ed 2022, 61, e202110924. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Zhao B; Shang R; Wang G-Z; Wang S; Chen H; Fu Y Palladium-Catalyzed Dual Ligand-Enabled Alkylation of Silyl Enol Ether and Enamide under Irradiation: Scope, Mechanism, and Theoretical Elucidation of Hybrid Alkyl Pd(I)-Radical Species. ACS Catal. 2020, 10, 1334–1343. [Google Scholar]; (e) Kancherla R; Muralirajan K; Maity B; Zhu C; Krach PE; Cavallo L; Rueping M Oxidative Addition to Palladium(0) Made Easy through Photoexcited-State Metal Catalysis: Experiment and Computation. Angew. Chem., Int. Ed 2019, 58, 3412–3416. [DOI] [PubMed] [Google Scholar]
- (21).Kvasovs N; Iziumchenko V; Palchykov V; Gevorgyan V Visible Light-Induced Pd-Catalyzed Alkyl-Heck Reaction of Oximes. ACS Catal. 2021, 11, 3749–3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Saeed A; Ifzan Arshad M; Bolte M; Fantoni AC; Delgado Espinoza ZY; Erben MF On the Roles of Close Shell Interactions in the Structure of Acyl-Substituted Hydrazones: An Experimental and Theoretical Approach. Spectrochim. Acta A Mol. Biomol. Spectrosc 2016, 157, 138–145. [DOI] [PubMed] [Google Scholar]
- (23) (a).Isar P; Ravikanth M Facile Synthesis of Fused Oxasapphyrins. Org. Lett 2019, 21, 9502–9505. [DOI] [PubMed] [Google Scholar]; (b) Prieto A; Bouyssi D; Monteiro N Copper-Catalyzed Trifluoromethylation of Hydrazones Leading to the Formation of Quaternary α-Trifluoromethyl Diazenes. Asian J. Org. Chem 2016, 5, 742–745. [Google Scholar]
- (24) (a).Yasui E; Wada M; Takamura N Novel Approach to Arylhydrazones, the Precursor for Fischer Indole Synthesis, Via Diazo Esters Derived from α-Amino Acid Esters. Tetrahedron Lett. 2006, 47, 743–746. [Google Scholar]; (b) Yasui E; Wada M; Takamura N Novel Method for Synthesis of Aryl Hydrazones from α-Diazo Esters: Scope and Limitations of Nucleophiles. Tetrahedron 2009, 65, 461–468. [Google Scholar]
- (25).Sakakura T; Hara M; Tanaka M Reaction of Silyl Enol Ethers with Arenediazonium Salts. Part 2. α-Amination of Esters. J. Chem. Soc., Perkin Trans 1 1994, 289–293. [Google Scholar]
- (26).See the Supporting Information for details.
- (27) (a).Pantaine LRE; Milligan JA; Matsui JK; Kelly CB; Molander GA Photoredox Radical/Polar Crossover Enables Construction of Saturated Nitrogen Heterocycles. Org. Lett 2019, 21, 2317–2321. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Slater KA; Friestad GK Mn-Mediated Radical-Ionic Annulations of Chiral N-Acylhydrazones. J. Org. Chem 2015, 80, 6432–6440. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.