Abstract
Background
Childhood exposure to dyslipidemia is associated with adult atherosclerosis, but it is unclear whether the long‐term risk associated with dyslipidemia is attenuated on its resolution by adulthood. We aimed to address this question by examining the links between childhood and adult dyslipidemia on carotid atherosclerotic plaques in adulthood.
Methods and Results
The Cardiovascular Risk in Young Finns Study is a prospective follow‐up of children that began in 1980. Since then, follow‐up studies have been conducted regularly. In 2001 and 2007, carotid ultrasounds were performed on 2643 participants at the mean age of 36 years to identify carotid plaques and plaque areas. For childhood lipids, we exploited several risk factor measurements to determine the individual cumulative burden for each lipid during childhood. Participants were categorized into the following 4 groups based on their childhood and adult dyslipidemia status: no dyslipidemia (reference), incident, resolved, and persistent. Among individuals with carotid plaque, linear regression models were used to study the association of serum lipids with plaque area. The prevalence of plaque was 3.3% (N=88). In models adjusted for age, sex, and nonlipid cardiovascular risk factors, the relative risk for carotid plaque was 2.34 (95% CI, 0.91–6.00) for incident adult dyslipidemia, 3.00 (95% CI, 1.42–6.34) for dyslipidemia resolved by adulthood, and 5.23 (95% CI, 2.57–10.66) for persistent dyslipidemia. Carotid plaque area correlated with childhood total, low‐density lipoprotein, and non–high‐density lipoprotein cholesterol levels.
Conclusions
Childhood dyslipidemia, even if resolved by adulthood, is a risk factor for adult carotid plaque. Furthermore, among individuals with carotid plaque, childhood lipids associate with plaque size. These findings highlight the importance of primordial prevention of dyslipidemia in childhood to reduce atherosclerosis development.
Keywords: area under the curve, atherosclerosis, carotid artery plaque, child cardiovascular risk factors, dyslipidemia
Subject Categories: Risk Factors, Epidemiology, Atherosclerosis
Nonstandard Abbreviation and Acronym
- IMT
intima‐media thickness
Clinical Perspective.
What Is New?
Childhood dyslipidemia, even if resolved by adulthood, is a risk factor for adult carotid atherosclerotic plaque.
Childhood cholesterol levels have a stronger association on carotid plaque area than contemporary cholesterol levels.
What Are the Clinical Implications?
The results emphasize the role of childhood exposures for adult cardiovascular health and suggest the importance of primordial prevention targeted to lipids from an early age as subsequent prevention left to adulthood is likely to be too late to limit the risk that has accumulated earlier in life.
Atherosclerosis develops slowly during the lifespan, and it takes decades for the clinical outcomes of atherosclerotic cardiovascular disease (ASCVD), such as myocardial, cerebral, or peripheral ischemic syndrome, to emerge. 1 However, despite the progress made in identifying the causes and effective treatment of ASCVD, it remains a major cause of morbidity and mortality worldwide. 2
Exposure to cardiovascular risk factors in childhood is associated with preclinical markers of ASCVD and cardiovascular events observed in adulthood. 3 , 4 , 5 By using 1 such preclinical marker, common carotid artery intima‐media thickness (IMT) measured in adulthood, it has been shown that the association of childhood cardiovascular risk factors can be largely reversed by resolving the status of these risk factors by adulthood. 6 , 7 , 8 The data from these studies suggest that the risk of later life ASCVD associated with exposure to risk factors in childhood (such as being overweight and obesity, elevated blood pressure, and dyslipidemia) are not sustained provided the risk factors are resolved by adulthood. However, thickening of the common carotid IMT might not be entirely specific for the development of atherosclerosis as it might additionally reflect arterial medial hypertrophy. 9 , 10
Instead, carotid artery plaque and plaque area are considered highly specific phenotypes of active atherosclerosis that are strongly associated with clinical ASCVD outcomes. 10 , 11 , 12 , 13 , 14 , 15 We previously demonstrated in the Cardiovascular Risk in Young Finns (Young Finns) Study cohort that long‐term cumulative exposure to cardiovascular risk factors in childhood, including dyslipidemia, blood pressure, and smoking, are associated with carotid artery plaque in mid‐adulthood. 4 In this study, we aimed to extend these findings by examining if the resolution of dyslipidemia in the time between childhood and adulthood was associated with lower or attenuated risk of carotid plaque in adulthood. We also examined the association between childhood and adulthood serum lipid levels and carotid plaque area.
METHODS
Data access may be permitted on a case‐by‐case basis on request only. Data sharing outside the group is done in collaboration with the Young Finns Study group and requires a data‐sharing agreement. Investigators can submit an expression of interest to the chairman of the Young Finns Study steering group (Professor Olli T. Raitakari, University of Turku, Turku, Finland).
Study Population
The Young Finns Study is an ongoing follow‐up that initially aimed to study the association of childhood ASCVD risk factors and their determinants. The ongoing follow‐up of the cohort into adulthood has allowed an extension of the original aim to examine childhood factor associations with adult ASCVD risk and other outcomes. The Young Finns Study was conducted in 5 Finnish cities with medical schools (Helsinki, Kuopio, Oulu, Tampere, Turku) and their surrounding rural areas. In 1980, 4320 children aged 3, 6, 9, 12, 15, and 18 years were randomly chosen from the national population register of these areas and were invited to participate in the study. Of those invited, 3596 children participated in the first cross‐sectional field study. 16 Since then, follow‐up studies have been conducted in 1983, 1986, 1989, 1992, 2001, 2007, and 2011. 17 A flowchart for the Young Finns Study is provided in Table S1. Adulthood cardiovascular risk factors and carotid ultrasound data were collected in the 2001 and 2007 follow‐ups when 2620 and 2243 individuals participated, respectively. During these 2 follow‐ups, participants were aged 24 to 45 years. Childhood and adulthood dyslipidemia and carotid ultrasound data were available for 2643 participants, constituting the sample size for this study (complete case analysis). The study protocol was approved by locally appointed ethics committee, and the study was performed according to the Declaration of Helsinki. Written informed consent was obtained from all participants or their parents.
Cardiovascular Risk Factors
Standard methods were used to define total cholesterol and triglyceride concentrations from fasting blood samples. 18 The Friedewald formula was used to indirectly calculate low‐density lipoprotein (LDL) cholesterol. 19 Because of high triglyceride levels, the Friedewald formula could not be applied to 57 adults. However, based on elevated triglyceride levels, these participants were classified as having dyslipidemia in adulthood. All childhood triglyceride levels were within acceptable limits to be included in the Friedewald formula for the calculation of childhood LDL cholesterol. High‐density lipoprotein (HDL) cholesterol was analyzed after precipitation of very‐low‐density lipoprotein and LDL with dextran sulphate 500 000. Non‐HDL cholesterol was calculated as total cholesterol minus HDL cholesterol. Systolic blood pressure was measured in 1980 with an ultrasound scanning device (Arteriosonde 1020, Roche) in participants aged 3 years. For all other participants, blood pressure was measured with a standard mercury sphygmomanometer in 1980 and 1983 and from 1986 onward with a random‐zero sphygmomanometer. At each visit, blood pressure was measured 3 times. The average of these 3 measurements was used in the analyses. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters. Information on smoking habits and family history of coronary heart disease (CHD) were collected using self‐report questionnaires. Information on childhood smoking habits was collected from participants aged 12 to 18 years. If the participant was aged <12 years, information on smoking habits was collected in the subsequent follow‐ups. Childhood smoking status was defined as positive if the participant indicated that he or she had ever smoked daily between the ages of 12 and 18 years. Adulthood smoking status was defined as positive if the participant indicated that he or she was currently smoking daily. Information on family history of CHD was collected during the 2001 and 2007 follow‐ups. Family history was classified as positive if the participant indicated that their father or mother had been diagnosed with CHD, experienced myocardial infarction, or had percutaneous coronary intervention or coronary bypass surgery performed at age <55 years. In the 2007 questionnaire, the mother's age was raised to <65 years.
Area Under the Curve—Childhood Risk Factor Cumulative Burden
At the time of the first field study, participants were aged 3 to 18 years. During puberty, there is a significant decrease in serum lipid levels, 18 , 20 thus 1980 lipid baseline values measured at different age points were not comparable with each other. To obtain comparable childhood lipid levels for participants, we used the area under the curve (AUC) method, which allowed us to determine the individual cumulative burden for each serum lipid during childhood. The method used to create AUC variables is explained in detail in Data S1. In brief, we exploited several risk factor measurements from 1980 to 2011 to calculate the AUC separately for each serum lipid as well as blood pressure and BMI. First, participant‐specific curves for the cardiovascular risk factors were estimated by mixed‐model regression splines. 21 Then, similar to the approach of Lai et al, 22 AUCs were evaluated for each cardiovascular risk factor as a measure of risk factor cumulative burden. AUC variables were defined for the age period of 6 to 18 years, indicating the individual cumulative burden for the risk factor in childhood (Figure S1).
For adulthood, risk factor measurements and carotid ultrasound data from a single time point, either 2001 or 2007, was used; if carotid artery plaque was present in the 2001 ultrasound or if the individual did not participate in the 2007 field study, then risk factor and carotid ultrasound data from 2001 were used. Otherwise, risk factors and carotid ultrasound data from 2007 were used. This approach enabled the comparison of childhood risk factors with those collected at the time of the carotid imaging.
Dyslipidemia Classification
To study the associations of change in dyslipidemia status from childhood to adulthood with the risk of having carotid plaque, both childhood and adulthood lipids were classified according to clinical practice guidelines. Cutoff values comparable with childhood AUC variables were calculated by using guideline‐based cutoffs of total cholesterol ≥200 mg/dL (5.17 mmol/L), LDL cholesterol ≥130 mg/dL (3.36 mmol/L), non‐HDL cholesterol ≥145 mg/dL (3.75 mmol/L), and triglycerides ≥100 mg/dL (1.13 mmol/L) for ages ≤9 years and ≥130 mg/dL (1.47 mmol/L) for ages 10 to 18 years, and HDL cholesterol <40 mg/dL (1.03 mmol/L). 20 Childhood lipids were classified as dyslipidemia if the cumulative burden of any lipid exceeded or in the case of HDL cholesterol was below the comparable cutoff value. Adulthood dyslipidemia was defined using guideline‐based cutoffs of total cholesterol ≥240 mg/dL (6.21 mmol/L), LDL cholesterol ≥160 mg/dL (4.14 mmol/L), non‐HDL cholesterol ≥190 mg/dL (4.91 mmol/L), triglycerides ≥200 mg/dL (2.26 mmol/L), and HDL cholesterol <40 mg/dL (1.03 mmol/L). 23 Adulthood lipids were classified as dyslipidemia if any lipid exceeded or in case of HDL cholesterol was below the comparable cutoff value. Participants self‐reporting the current use of lipid‐lowering medication were considered as having dyslipidemia. Altogether, there were 43 participants who were taking lipid‐lowering medication in adulthood.
For the comparison of risk for having carotid plaque, participants were categorized into 4 groups based on their dyslipidemia status in childhood and in adulthood. Group I included participants who did not have dyslipidemia in childhood and in adulthood, group II included those who did not have dyslipidemia in childhood but had dyslipidemia in adulthood, group III included participants who had dyslipidemia in childhood but did not have dyslipidemia in adulthood, and group IV consisted of those who had dyslipidemia both in childhood and in adulthood.
Carotid Plaque and Carotid Plaque Area
Carotid ultrasound studies were performed in the 2001 and 2007 follow‐ups on the left common carotid artery and carotid bifurcation using B‐mode ultrasound (Sequoia 512; Acuson) with a 13.0 MHz linear‐array transducer according to a standardized protocol. 24 Digitally stored images were scanned for the existence of carotid plaques, defined as focal thickenings of the arterial wall protruding into the lumen >50% compared with the adjacent IMT. 25 Carotid plaque area was measured from the longitudinal view at the end‐diastolic (incident with the R wave on a continuously recorded ECG) still frame. Plaque lumen–intima and media–adventitia boundaries were manually traced around the perimeter, and the area inside the boundaries was automatically measured. If >1 plaque was detected, then the plaque areas were summed. 10 For assessing reproducibility of plaque area measurements, 27 plaque areas were remeasured by a second observer. The between‐observer coefficient of variation was 6.8%.
Statistical Analysis
To evaluate the association of the dyslipidemia group with carotid plaque, an overall test (Wald type III) was first performed. Then, modified Poisson regression models with robust SEs, adjusted for sex and adult age, were used to calculate the relative risks and 95% CIs for having carotid plaque according to dyslipidemia group. 26 Group I (no dyslipidemia in childhood and in adulthood) was used as the reference category. Finally, to evaluate the independent association of dyslipidemia group with carotid plaque, the model was additionally adjusted for childhood and adulthood BMI and systolic blood pressure z scores, smoking status, and family history of CHD.
The association between childhood and adulthood lipids and carotid plaque area was studied among individuals with carotid plaque. Because of skewness, adult triglycerides and carotid plaque area were log‐transformed. Carotid plaque area, childhood and adulthood lipids, systolic blood pressure, and BMI z scores were created, resulting in variables with a mean of 0 and SD of 1. Linear regression models were used to study the association of z scores of childhood and adulthood lipids with carotid plaque area z scores. Individuals with a missing value for the variable were excluded from analyses where that variable was used. Models were adjusted for potential confounders (those variables likely associated with both lipids and atherosclerosis) of age, sex, and BMI. First, linear regression models, adjusted for sex and adult age, for each childhood and adulthood lipid were examined. Second, to evaluate the independent association of childhood total, LDL, and non‐HDL cholesterol with carotid plaque area, multivariable models including the corresponding adulthood cholesterol, childhood and adulthood BMI and systolic blood pressure, smoking status, and family history of CHD were examined.
In sensitivity analyses, we refitted the models replacing family history of premature CHD with family history of CHD at any age; after dividing participants into 4 groups based on their childhood and adulthood total, LDL, and non‐HDL cholesterol status; after using alternate adult dyslipidemia cutoffs from the guidelines of the European Society of Cardiology/European Atherosclerosis Society for the management of dyslipidemias 27 ; and after excluding participants with lipid‐lowering medication. Carotid plaque size was defined as small or large by using the median plaque area as a cutoff. Large plaque was defined as greater than, and small plaque as smaller than, the median area. Modified Poisson regression models with robust SEs were used to calculate the relative risks and their 95% CIs for large plaque in adulthood for child and adult total, LDL, and non‐HDL cholesterol z scores. Participants with a small plaque were used as the reference category. To evaluate the independent association of childhood cholesterol level with the development of large plaque in adulthood, the corresponding child and adult lipid was included in the same model. To test if sex modified the association between lipids and carotid plaque area, sex by lipid interaction terms were examined separately for each lipid. No significant interactions were observed (P always >0.1). To evaluate collinearity between childhood and adulthood lipids, variance inflation factor values were examined. 28 All variance inflation factor values were <1.5, indicating no apparent collinearity. Statistical significance was inferred at a 2‐sided P value <0.05. All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
The study cohort consisted of 2643 participants of whom 1209 (45.7%) were male and 1434 (54.3%) were female participants. The mean (SD) length of follow‐up from the first risk factor measurement to outcome measurement was 25.9 (2.3) years. With respect to the dyslipidemia groups defined previously, there were 813 participants in group I (no dyslipidemia in childhood and in adulthood), 311 in group II (no dyslipidemia in childhood and dyslipidemia in adulthood), 910 in group III (dyslipidemia in childhood and no dyslipidemia in adulthood), and 609 in group IV (dyslipidemia both in childhood and in adulthood). Table 1 shows the characteristics of the participants according to dyslipidemia group.
Table 1.
Characteristics of Participants Across Dyslipidemia Groups
| Group I | Group II | Group III | Group IV | |
|---|---|---|---|---|
| No. (%) | 813 (31) | 311 (12) | 910 (34) | 609 (23) |
| Carotid plaque, n (%) | 9 (1.1) | 8 (2.6) | 31 (3.4) | 40 (6.6) |
| Age, y, mean (SD) | 34.6 (5.2) | 34.4 (5.1) | 38.0 (5.5) | 37.6 (5.5) |
| Sex, n (%) | ||||
| Female | 451 (55) | 85 (27) | 662 (73) | 236 (39) |
| Male | 362 (45) | 226 (73) | 248 (27) | 373 (61) |
| Serum lipids and lipoproteins, mg/dL, mean (SD) | ||||
| Total cholesterol | ||||
| Childhood | 179.3 (14.4) | 177.0 (15.1) | 216.0 (15.8) | 221.2 (22.9) |
| Adulthood | 173.3 (24.0) | 186.9 (34.6) | 197.5 (21.6) | 230.5 (40.5) |
| LDL cholesterol | ||||
| Childhood | 106.8 (14.5) | 108.2 (14.2) | 144.6 (15.5) | 151.3 (21.7) |
| Adulthood | 101.1 (21.4) | 113.0 (27.8)* | 122.8 (19.6) | 150.8 (35.2)† |
| HDL cholesterol | ||||
| Childhood | 58.5 (7.0) | 52.8 (6.2) | 59.1 (8.4) | 54.6 (9.0) |
| Adulthood | 54.5 (10.0) | 39.4 (9.5)‡ | 56.0 (10.3) | 46.5 (14.9)§ |
| Non‐HDL cholesterol | ||||
| Childhood | 119.1 (14.6) | 122.5 (14.3) | 156.6 (15.9) | 166.1 (21.8) |
| Adulthood | 118.8 (23.5) | 147.4 (31.8)‡ | 141.5 (21.4) | 184.1 (37.1)§ |
| Triglycerides | ||||
| Childhood | 62.1 (15.6) | 68.5 (16.7) | 61.8 (15.9) | 70.3 (21.5) |
| Adulthood | 89.7 (32.9) | 178.6 (90.7) | 95.4 (34.8) | 185.9 (122.8) |
| Systolic blood pressure, mm Hg, mean (SD) | ||||
| Childhood | 110 (5.9) | 111 (6.0) | 111 (5.3) | 113 (5.9) |
| Adulthood | 118 (13.4)∥ | 124 (13.4) | 119 (13.3)¶ | 125 (15.1)# |
| Diastolic blood pressure, mm Hg, mean (SD) | ||||
| Childhood | 64 (5.2)** | 65 (5.1) | 66 (4.5) | 66 (4.6) |
| Adulthood | 73 (10.5)†† | 78 (12.0)‡‡ | 74 (10.7)¶ | 79 (12.3)# |
| Body mass index, kg/m2, mean (SD) | ||||
| Childhood | 18.2 (2.0) | 18.8 (2.2) | 18.2 (1.8) | 18.7 (2.3) |
| Adulthood | 24.6 (4.0)§§ | 28.6 (5.0)∥∥ | 24.9 (4.3)¶¶ | 27.9 (5.0)## |
| Smoking, n (%) | ||||
| Childhood | 168 (21) | 73 (23) | 186 (20) | 128 (21) |
| Adulthood | 169 (21) | 79 (25) | 152 (17) | 147 (24) |
| Family history of CHD, n (%) | 113 (14) | 50 (16) | 185 (20) | 135 (22) |
Values of childhood lipids and lipoproteins, blood pressure, and body mass index represent the estimate of the cumulative exposure in childhood between ages 6 and 18 years. These have been calculated for each individual on the basis of the modeling of serial measurements. Adulthood values represent single time point measured at the time that the carotid ultrasound study (outcome) was performed. For all variables, n=2643 unless stated otherwise. To convert cholesterol and triglycerides units from mg/dL to mmol/L, multiply by 0.02586 and 0.01129, respectively. Group I=no dyslipidemia in childhood and in adulthood, group II=no dyslipidemia in childhood and dyslipidemia in adulthood, group III=dyslipidemia in childhood and no dyslipidemia in adulthood, and group IV=dyslipidemia in childhood and in adulthood. CHD indicates coronary heart disease; LDL, low‐density lipoprotein; and HDL, high‐density lipoprotein.
n=297.
n=566.
n=310.
n=600.
n=811.
n=905.
n=608.
n=812.
n=810.
n=310.
n=801.
n=306.
n=898.
n=601.
Association Between Dyslipidemia Group and Carotid Plaque
Carotid plaque was present in 88 (3.3%) individuals. All plaques were detected in the carotid bifurcation. The prevalence of carotid plaque was higher among male participants than female participants (4.7% versus 2.2%; P<0.001), and those with carotid plaque were, on average, older (38.2 [4.6] versus 36.4 [5.6] years; P<0.001). Across dyslipidemia groups I, II, III, and IV, the carotid plaque prevalence increased from 1.1%, 2.6%, 3.4%, to 6.6%, respectively (FigureFigure). An overall test (Wald type III) for the effect of the dyslipidemia group on carotid plaque was highly significant (P<0.001). When using participants in group I as the reference category, the relative risk for carotid plaque increased across dyslipidemia groups II, III, and IV (Table 2). In comparison to the reference group I, the relative risk of carotid plaque was significantly higher in groups III and IV. The model was repeated after adjusting for childhood and adulthood systolic blood pressure, BMI, smoking status, and family history of CHD with essentially similar results (Table 3).
Figure . Carotid plaque prevalence across dyslipidemia groups.
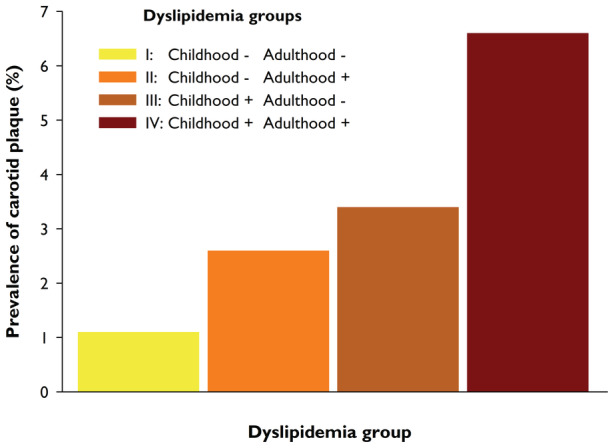
In groups I, II, III, and IV, the carotid plaque prevalence increased from 1.1%, 2.6%, 3.4%, to 6.6%, respectively.
Table 2.
RRs of Carotid Plaque in Adulthood According to Dyslipidemia Group, Adjusted for Sex and Adult Age
| RR | 95% CI | P value | |
|---|---|---|---|
| Group I | 1.00 | Reference | … |
| Group II | 1.93 | 0.75–4.96 | 0.17 |
| Group III | 3.11 | 1.46–6.62 | 0.003 |
| Group IV | 4.73 | 2.30–9.72 | <0.001 |
Group I=no dyslipidemia in childhood and in adulthood, group II=no dyslipidemia in childhood and dyslipidemia in adulthood, group III=dyslipidemia in childhood and no dyslipidemia in adulthood, and group IV=dyslipidemia in childhood and in adulthood. RR indicates relative risk.
Table 3.
RRs of Carotid Plaque in Adulthood According to Dyslipidemia Group, Adjusted for Sex, Adult Age, Childhood and Adulthood Body Mass Index and Systolic Blood Pressure z Scores, Smoking Status, and Family History of Coronary Heart Disease
| RR | 95% CI | P value | |
|---|---|---|---|
| Group I | 1.00 | Reference | … |
| Group II | 2.34 | 0.91–6.00 | 0.08 |
| Group III | 3.00 | 1.42–6.34 | 0.004 |
| Group IV | 5.23 | 2.57–10.66 | <0.001 |
Group I=no dyslipidemia in childhood and in adulthood, group II=no dyslipidemia in childhood and dyslipidemia in adulthood, group III=dyslipidemia in childhood and no dyslipidemia in adulthood, and group IV=dyslipidemia in childhood and in adulthood. RR indicates relative risk.
In sensitivity analyses, participants were divided into 4 groups as described previously for dyslipidemia based solely on their childhood and adulthood total, LDL, and non‐HDL cholesterol status. Overall tests for the effects of total, LDL, and non‐HDL cholesterol groups on carotid plaque were highly significant (P always <0.001). Again, those without elevated cholesterol levels in childhood and in adulthood served as the reference category. When adjusted for sex, adult age, childhood and adulthood systolic blood pressure, BMI, smoking status, and family history of CHD, the relative risks for carotid plaque for those with resolving cholesterol levels were 1.89 (95% CI, 1.12–3.17) for total cholesterol, 3.23 (95% CI, 1.80–5.79) for LDL cholesterol, and 1.75 (95% CI, 1.03–2.96) for non‐HDL cholesterol dyslipidemia.
In addition, in sensitivity analyses, adult dyslipidemia was classified according to the European Society of Cardiology/European Atherosclerosis Society guidelines: total cholesterol >190 mg/dL (5.0 mmol/L), LDL cholesterol >115 mg/dL (3.0 mmol/L), non‐HDL cholesterol >145 mg/dL (3.8 mmol/L), triglycerides >150 mg/dL (1.7 mmol/L), and HDL cholesterol <46 mg/dL (1.2 mmol/L) in women and <39 mg/dL (1.0 mmol/L) in men. 27 Participants self‐reporting current use of lipid‐lowering medication were considered as having dyslipidemia. Again, the Wald type III test was first performed yielding a P value of <0.001 for the effect of the dyslipidemia group on carotid plaque. When the modified Poisson regression model was adjusted for sex and adult age, the relative risks of carotid plaque in groups II, III, and IV were 2.53 (95% CI, 0.83–7.72), 3.43 (95% CI, 0.94–12.49), and 5.92 (95% CI, 2.12–16.53) compared with group I participants, respectively.
Association Between Serum Lipids and Carotid Plaque Area
Data of 88 participants with carotid plaque were used in these analyses. The median plaque area was 9.97 (5.42–14.62) mm2. Carotid plaque area was significantly associated with childhood and adulthood total, LDL, and non‐HDL cholesterol, but not with triglycerides or HDL cholesterol (Table 4). In multivariable linear regression models, the association of childhood total, LDL, and non‐HDL cholesterol remained statistically significant, whereas the association for the equivalent adulthood cholesterol level was attenuated and no longer statistically significant (Table 4). We additionally estimated the relative risks for large plaque (defined as greater than the median area) development for total, LDL, and non‐HDL cholesterol using participants with a small plaque (defined as smaller than the median area) as the reference category. First, modified Poisson regression models were examined for each childhood cholesterol. For 1‐SD unit, change in childhood cholesterol level yielded relative risks for large plaque of 1.42 (95% CI, 1.19–1.69; P<0.001) for total cholesterol, 1.39 (95% CI, 1.16–1.67; P<0.001) for LDL cholesterol, and 1.37 (95% CI, 1.15–1.63; P<0.001) for non‐HDL cholesterol. Second, models were repeated using adulthood cholesterol, yielding relative risks of 1.32 (95% CI, 1.12–1.56; P=0.001), 1.32 (95% CI, 1.14–1.53; P<0.001), and 1.26 (95% CI, 1.07–1.49; P=0.005), respectively. Finally, models were repeated after including the equivalent childhood and adulthood cholesterol in the same model. Relative risks for childhood total, LDL, and non‐HDL cholesterol for large plaque decreased only slightly and remained significant: 1.32 (95% CI, 1.08–1.62; P=0.007), 1.30 (95% CI, 1.03–1.64; P=0.03), and 1.30 (95% CI, 1.07–1.58; P=0.008), respectively. However, the association between adulthood cholesterol and large plaque decreased markedly and was no longer statistically significant: 1.14 (95% CI, 0.94–1.38; P=0.19), 1.14 (95% CI, 0.95–1.37; P=0.16), and 1.10 (95% CI, 0.92–1.32; P=0.31), respectively.
Table 4.
Associations of Carotid Plaque Area With Childhood and Adulthood Serum Lipids Among 88 Individuals With Carotid Plaque
| Childhood | Adulthood | |||
|---|---|---|---|---|
| β (SE) | P value | β (SE) | P value | |
| Total cholesterol | 0.41 (0.10) | <0.001 | 0.34 (0.10) | 0.001 |
| LDL cholesterol* | 0.38 (0.10) | <0.001 | 0.36 (0.10) | <0.001 |
| Non‐HDL cholesterol† | 0.37 (0.10) | <0.001 | 0.31 (0.10) | 0.004 |
| HDL cholesterol† | 0.18 (0.10) | 0.09 | 0.18 (0.11) | 0.10 |
| Triglycerides | 0.02 (0.11) | 0.82 | −0.06 (0.11) | 0.57 |
| Multivariable models‡ | ||||
| Total cholesterol | 0.31 (0.13) | 0.02 | 0.18 (0.13) | 0.19 |
| LDL cholesterol | 0.29 (0.14) | 0.04 | 0.21 (0.14) | 0.13 |
| Non‐HDL cholesterol | 0.31 (0.13) | 0.02 | 0.14 (0.14) | 0.32 |
Childhood lipid values represent the estimate of the cumulative exposure in childhood between ages 6 and 18 years. This is calculated for each individual on the basis of the modeling of serial lipid measurements. Adulthood lipid values represent a single time point measured at the time that the carotid ultrasound study was performed. Regression coefficients (β) indicate change in the plaque area z score for a 1‐SD increase in the lipid variable. All models were adjusted for sex and adult age. HDL indicates high‐density lipoprotein; and LDL, low‐density lipoprotein.
n=86 for adulthood LDL cholesterol.
n=87 for adulthood non‐HDL and HDL cholesterol.
In addition to the corresponding childhood and adulthood cholesterol, the multivariable model included childhood and adulthood body mass index and systolic blood pressure z scores, smoking status, and family history of coronary heart disease.
DISCUSSION
We found that childhood dyslipidemia, even if resolved by adulthood, predisposes to the development of carotid atherosclerotic plaque in adulthood. Furthermore, childhood lipids (total, LDL, and non‐HDL cholesterol) exhibited stronger correlations with carotid plaque area than adulthood lipids. Our findings reinforce that exposure to elevated childhood lipid levels not only predisposes atherosclerosis observed decades later but also affects the severity of atherosclerosis.
Child cardiovascular risk factors have been shown to predict markers of adult preclinical atherosclerosis such as coronary calcification, carotid plaque, and common carotid IMT independent of contemporary risk factor levels. 3 , 4 , 24 However, data from the i3C (International Childhood Cardiovascular Cohort) Consortium showed that although childhood overweight BMI and obesity is a strong predictor of high common carotid IMT in adulthood, the association tended to be attenuated among those who did not have obesity in adulthood. 6 However, Buscot et al showed, by using long‐term BMI trajectories, that participants who resolved their elevated childhood BMI maintained a residual risk for high common carotid IMT in adulthood. 29 In a separate study from the i3C Consortium, those who were able to resolve their non–HDL cholesterol dyslipidemia by adulthood had a risk of developing high common carotid IMT in adulthood that was not statistically different from those who did not have dyslipidemia in childhood and in adulthood. 8 In addition, the i3C Consortium showed no residual risk for high common carotid IMT in those who resolved their LDL cholesterol dyslipidemia from childhood to adulthood. 30
However, it is unclear how specifically diffuse thickening of the common carotid artery wall, as assessed by IMT, reflects atherosclerosis, as part of such thickening may reflect medial hypertrophy. 9 , 10 At present, IMT is not recommended by the American College of Cardiology/American Heart Association or European Society of Cardiology guidelines for the clinical evaluation of cardiovascular disease risk because the addition of common carotid IMT measurements to the conventional risk algorithms is associated with only small improvements in risk prediction that are unlikely clinically important. 31 , 32 On the other hand, a focal atherosclerotic carotid wall thickening, so called carotid artery plaque, is considered a specific phenotype of active atherosclerosis, 9 , 10 with the association between carotid artery plaque and clinical cardiovascular outcomes well established. 10 , 13 , 14 Our study is the first to demonstrate that resolution of dyslipidemia by adulthood does not negate the association of childhood dyslipidemia to the specific phenotype of atherosclerosis observed in adulthood. This association persisted even after adjusting for other cardiovascular risk factors, such as blood pressure, BMI, smoking, and family history of CHD. These findings reinforce the putative role of childhood dyslipidemia in the lifelong process of atherosclerosis.
Although the association of childhood lipids with adult preclinical atherosclerosis has been demonstrated, our data extend these previous findings to the carotid plaque area, a specific phenotype of active atherosclerosis, which has been shown to improve the predictive utility of cardiovascular diseases. 10 , 11 , 12 Importantly, we show that even among those with carotid plaque, who already have substantially higher lipid levels on average than those without carotid plaque, 4 the higher lipid levels associate with more plaque area. Furthermore, the associations observed for childhood total, LDL, and non‐HDL cholesterol were not solely attributable to the tracking of these risk factors from childhood to adulthood, as the childhood cholesterol levels had a stronger association with adulthood carotid plaque area than did contemporary levels—suggesting a direct effect of childhood lipid levels on adult plaque area.
Would childhood lipid screening help identify those at higher risk for later life atherosclerosis? At present, childhood lipid screening is recommended by the National Heart, Lung, and Blood Institute's Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents. 20 Our findings demonstrate the significant association between childhood lipids and adulthood‐specific phenotype of advanced atherosclerosis. Furthermore, by pooling longitudinal cohort data, the i3C Consortium recently demonstrated that traditional risk factors in childhood, including serum total cholesterol and triglycerides, youth BMI, systolic blood pressure, and smoking were directly and independently associated with adult cardiovascular events. 5 These findings suggest that childhood lipid screening would identify individuals who are at increased risk for future cardiovascular outcomes. However, lipid screening in children is associated with concerns. 33 The US Preventive Services Task Force identified no direct evidence for benefits of childhood lipid screening or treatment on outcomes in adulthood. 34 The current direct interventional evidence of the benefits of childhood screening and treatment is limited. However, such evidence is available from childhood familial hypercholesterolemia. It has been shown that long‐term lowering of LDL cholesterol levels initiated in childhood is associated with a reduced risk of cardiovascular events by mid‐age. 35 However, the requirement of direct evidence in the general population might be unrealistic because organizing intervention studies lasting for several decades to test the hypothesis that screening and interventions initiated in childhood reduce the risk of atherosclerotic events in adulthood would be unlikely. Therefore, the guidelines on screening and early treatment must, for the most part, rely on information provided by observational studies. Extensive evidence from autopsy studies, vascular studies, and cohort studies have shown that elevated childhood lipid levels associate with adult atherosclerosis and cardiovascular events. 1 , 5 Our present findings showing that childhood dyslipidemia, even if resolved by adulthood, is a risk factor for carotid artery plaque emphasize the role of childhood exposure for adult cardiovascular health and suggest the importance of primordial prevention targeted to lipids from an early age. At present, based on the totality of evidence, it could be hypothesized that early identification and management of dyslipidemia would substantially reduce the risk of later life atherosclerosis.
Studies such as this highlight the need for childhood strategies for reducing the risk of cardiovascular diseases. In addition to a medical approach of identifying children with dyslipidemia, our results suggest the importance of public health strategies for maintaining ideal lipoprotein levels in all children. Individuals with lifelong genetic exposure to low levels of LDL cholesterol have a lower risk of cardiovascular disease. 1 In Finland, cholesterol levels and coronary mortality have been exceptionally high in the past, especially in the eastern parts of the country. As a result, a national project was launched in Finland in 1972 (the North Karelian project) focused on lifestyle changes aimed at lowering cholesterol levels, blood pressure, and smoking at the population level. 36 These large‐scale public health efforts in Finland during the past 40 years have led to a decline in the major risk factors and reductions in cardiovascular‐related mortality. 36 Furthermore, recent results from the STRIP (Special Turku Coronary Risk Factor Intervention Project) study showed beneficial effects on risk factors during a period of 26 years after dietary counseling that began in infancy and continued throughout childhood. 37 The positive trends in lipid levels have also been witnessed in the Young Finns Study. 38 In line with these trends, we see in the present analyses that the group with resolving dyslipidemia (group III) had the largest number of participants. Furthermore, the decrease in cholesterol levels from childhood to adulthood seen in group I may be partly attributed to successful public health measures. Parallel with the decrease in serum LDL cholesterol level, we observed an increasing trend in BMI. 39 Therefore, the increase in triglycerides seen in groups II and IV may be associated with the increased adiposity among participants.
This study had limitations. The ultrasound study included only the left carotid artery and did not involve imaging of the internal carotid artery. However, the development of carotid artery plaque typically begins at the carotid bifurcation, 40 and previous studies among age groups comparable with those in our study do not suggest a higher prevalence of carotid plaques. 41 , 42 Still, some misclassifications might have occurred. Because of the relatively small number of participants with carotid plaque, some of our CIs were wide, and our statistically nonsignificant finding of the association between dyslipidemia group II and carotid plaque might be a result of low statistical power. Bias attributed to differential loss to follow‐up is possible in prospective cohort studies. However, the Young Finns Study has a high retention of participants relative to similar cohort studies, and we have previously shown that participants do not differ with nonparticipants except that they are more likely to be female participants and older in age. 17 , 43 Strengths of the study include the large, population‐based cohort of participants who have been repeatedly followed for cardiovascular risk factors and preclinical atherosclerosis from childhood to adulthood.
In summary, we show that childhood dyslipidemia, even if resolved by adulthood, is a risk factor for carotid artery plaque. Furthermore, childhood lipids are independently associated with the level of adulthood atherosclerosis observed decades later. Our data highlight the importance of primordial prevention targeted to lipids from an early age—as subsequent prevention left to adulthood is likely to be too late to limit the risk that has accumulated earlier in life.
Sources of Funding
The Young Finns Study has been financially supported by the Academy of Finland (322098, 286284, 134309 [Eye], 126925, 121584, 124282, 255381, 256474, 283115, 319060, 320297, 314389, 338395, 330809, 104821, 129378 [Salve], 117787 [Gendi], and 41071 [Skidi]), the Social Insurance Institution of Finland, Competitive State Research Financing of the Expert Responsibility Area of Kuopio, Tampere and Turku University Hospitals (X51001), Juho Vainio Foundation, Paavo Nurmi Foundation, Finnish Foundation of Cardiovascular Research, Finnish Cultural Foundation, Tampere Tuberculosis Foundation, The Sigrid Juselius Foundation, Emil Aaltonen Foundation, Yrjö Jahnsson Foundation, Signe and Ane Gyllenberg Foundation, Diabetes Research Foundation of Finnish Diabetes Association, EU Horizon 2020 (755320 for TAXINOMISIS and 848146 for To Aition), European Research Council (742927 for MULTIEPIGEN project), Tampere University Hospital Supporting Foundation, Finnish Society of Clinical Chemistry and the Cancer Foundation Finland, Maud Kuistila Memorial Foundation to Koskinen, Päivikki and Sakari Sohlberg Foundation to Koskinen, Finnish Medical Foundation to Koskinen, Paulon Foundation to Koskinen, Licentiate of Medicine Paavo Ilmari Ahvenainen Foundation to Koskinen, and a National Health and Medical Research Council Investigator Grant (APP1176494) to Magnussen. The contents of the published material are solely the responsibility of the individual authors and do not reflect the views of the National Health and Medical Research Council.
Disclosures
Dr Kytö reports scientific consultancy (AstraZeneca) and congress sponsorship (Bayer). The other authors have no disclosures to report.
Supporting information
Data S1
Table S1
Figure S1
Acknowledgments
Statistical support provided by Johanna Ikonen, MSc, Irina Lisinen, MSc, and Noora Kartiosuo, MSc, from the Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland, is gratefully acknowledged.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.027586
For Sources of Funding and Disclosures, see page 9.
References
- 1. Raitakari O, Pahkala K, Magnussen CG. Prevention of atherosclerosis from childhood. Nat Rev Cardiol. 2022;19:5543–5554. doi: 10.1038/s41569-021-00647-9 [DOI] [PubMed] [Google Scholar]
- 2. Naghavi M, Abajobir AA, Abbafati C, Abbas KM, Abd‐Allah F, Abera SF, Aboyans V, Adetokunboh O, Ärnlöv J, Afshin A, et al. Global, regional, and national age‐sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the global burden of disease study 2016. Lancet. 2017;390:1151–1210. doi: 10.1016/S0140-6736(17)32152-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hartiala O, Magnussen CG, Kajander S, Knuuti J, Ukkonen H, Saraste A, Rinta‐Kiikka I, Kainulainen S, Kähönen M, Hutri‐Kähönen N, et al. Adolescence risk factors are predictive of coronary artery calcification at middle age: the cardiovascular risk in young Finns study. J Am Coll Cardiol. 2012;60:1364–1370. doi: 10.1016/j.jacc.2012.05.045 [DOI] [PubMed] [Google Scholar]
- 4. Koskinen JS, Kytö V, Juonala M, Viikari JSA, Nevalainen J, Kähönen M, Lehtimäki T, Hutri‐Kähönen N, Laitinen T, Tossavainen P, et al. Childhood risk factors and carotid atherosclerotic plaque in adulthood: the Cardiovascular Risk in Young Finns Study. Atherosclerosis. 2020;293:18–25. doi: 10.1016/j.atherosclerosis.2019.11.029 [DOI] [PubMed] [Google Scholar]
- 5. Jacobs DR, Woo JG, Sinaiko AR, Daniels SR, Ikonen J, Juonala M, Kartiosuo N, Lehtimäki T, Magnussen CG, Viikari JSA, et al. Childhood cardiovascular risk factors and adult cardiovascular events. N Engl J Med. 2022;386:1877–1888. doi: 10.1056/NEJMoa2109191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Juonala M, Magnussen CG, Berenson GS, Venn A, Burns TL, Sabin MA, Srinivasan SR, Daniels SR, Davis PH, Chen W, et al. Childhood adiposity, adult adiposity, and cardiovascular risk factors. N Engl J Med. 2011;365:1876–1885. doi: 10.1056/NEJMoa1010112 [DOI] [PubMed] [Google Scholar]
- 7. Magnussen CG, Smith KJ, Juonala M. What the long term cohort studies that began in childhood have taught us about the origins of coronary heart disease. Curr Cardiovasc Risk Rep. 2014;8:373. doi: 10.1007/s12170-014-0373-x [DOI] [Google Scholar]
- 8. Juonala M, Wu F, Sinaiko A, Woo JG, Urbina EM, Jacobs D, Steinberger J, Prineas R, Koskinen J, Sabin MA, et al. Non‐HDL cholesterol levels in childhood and carotid intima‐media thickness in adulthood. Pediatrics. 2020;145:e20192114. doi: 10.1542/peds.2019-2114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Touboul P‐J, Hennerici MG, Meairs S, Adams H, Amarenco P, Bornstein N, Csiba L, Desvarieux M, Ebrahim S, Hernandez Hernandez R, et al. Mannheim carotid intima‐media thickness and plaque consensus (2004–2006–2011). An update on behalf of the advisory board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc Dis. 2012;34:290–296. doi: 10.1159/000343145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Johri AM, Nambi V, Naqvi TZ, Feinstein SB, Kim ESH, Park MM, Becher H, Sillesen H. Recommendations for the assessment of carotid arterial plaque by ultrasound for the characterization of atherosclerosis and evaluation of cardiovascular risk: from the American Society of Echocardiography. J Am Soc Echocardiogr. 2020;33:917–933. doi: 10.1016/j.echo.2020.04.021 [DOI] [PubMed] [Google Scholar]
- 11. Spence JD, Eliasziw M, DiCicco M, Hackam DG, Galil R, Lohmann T. Carotid plaque area: a tool for targeting and evaluating vascular preventive therapy. Stroke. 2002;33:2916–2922. doi: 10.1161/01.STR.0000042207.16156.B9 [DOI] [PubMed] [Google Scholar]
- 12. Mathiesen EB, Johnsen SH, Wilsgaard T, Bønaa KH, Løchen ML, Njølstad I. Carotid plaque area and intima‐media thickness in prediction of first‐ever ischemic stroke: a 10‐year follow‐up of 6584 men and women: the Tromsø Study. Stroke. 2011;42:972–978. doi: 10.1161/STROKEAHA.110.589754 [DOI] [PubMed] [Google Scholar]
- 13. Inaba Y, Chen JA, Bergmann SR. Carotid plaque, compared with carotid intima‐media thickness, more accurately predicts coronary artery disease events: a meta‐analysis. Atherosclerosis. 2012;220:128–133. doi: 10.1016/j.atherosclerosis.2011.06.044 [DOI] [PubMed] [Google Scholar]
- 14. Naqvi TZ, Lee M‐S. Carotid intima‐media thickness and plaque in cardiovascular risk assessment. JACC Cardiovasc Imaging. 2014;7:1025–1038. doi: 10.1016/j.jcmg.2013.11.014 [DOI] [PubMed] [Google Scholar]
- 15. Poredoš P, Cífková R, Maier JAM, Nemcsik J, Šabovič M, Jug B, Ježovnik MK, Schernthaner GH, Antignani PL, Catalano M, et al. Preclinical atherosclerosis and cardiovascular events: do we have a consensus about the role of preclinical atherosclerosis in the prediction of cardiovascular events? Atherosclerosis. 2022;348:25–35. doi: 10.1016/j.atherosclerosis.2022.03.030 [DOI] [PubMed] [Google Scholar]
- 16. Åkerblom HK, Viikari J, Uhari M, Räsänen L, Byckling T, Louhivuori K, Pesonen E, Suoninen P, Pietikäinen M, Lähde PL, et al. Atherosclerosis precursors in Finnish children and adolescents. I. General description of the cross‐sectional study of 1980, and an account of the children's and families' state of health. Acta Paediatr Scand Suppl. 1985;318:49–63. doi: 10.1111/j.1651-2227.1985.tb10082.x [DOI] [PubMed] [Google Scholar]
- 17. Raitakari OT, Juonala M, Rönnemaa T, Keltikangas‐Järvinen L, Räsänen L, Pietikäinen M, Hutri‐Kähönen N, Taittonen L, Jokinen E, Marniemi J, et al. Cohort profile: the cardiovascular risk in Young Finns Study. Int J Epidemiol. 2008;37:1220–1226. doi: 10.1093/ije/dym225 [DOI] [PubMed] [Google Scholar]
- 18. Porkka KV, Raitakari OT, Leino A, Laitinen S, Räsänen L, Rönnemaa T, Marniemi J, Lehtimäki T, Taimela S, Dahl M, et al. Trends in serum lipid levels during 1980‐1992 in children and young adults. The Cardiovascular Risk in Young Finns Study. Am J Epidemiol. 1997;146:64–77. doi: 10.1093/oxfordjournals.aje.a009192 [DOI] [PubMed] [Google Scholar]
- 19. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low‐density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. doi: 10.1093/clinchem/18.6.499 [DOI] [PubMed] [Google Scholar]
- 20. Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents; National Heart, Lung, and Blood Institute . Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics. 2011;128(Suppl 5):S213–S256. doi: 10.1542/peds.2009-2107C [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Welham SJ. Smoothing spline models for longitudinal data. In: Fitzmaurice G, Davidian M, Verbeke G, Molenberghs G, eds. Longitudinal Data Analysis. Handbooks of Modern Statistical Methods. 1st ed. Chapman & Hall/CRC; 2009:253–289. [Google Scholar]
- 22. Lai C‐C, Sun D, Cen R, Wang J, Li S, Fernandez‐Alonso C, Chen W, Srinivasan SR, Berenson GS. Impact of long‐term burden of excessive adiposity and elevated blood pressure from childhood on adulthood left ventricular remodeling patterns: the Bogalusa Heart Study. J Am Coll Cardiol. 2014;64:1580–1587. doi: 10.1016/j.jacc.2014.05.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) . Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. doi: 10.1161/circ.106.25.3143 [DOI] [PubMed] [Google Scholar]
- 24. Raitakari OT, Juonala M, Kähönen M, Taittonen L, Laitinen T, Mäki‐Torkko N, Järvisalo MJ, Uhari M, Jokinen E, Rönnemaa T, et al. Cardiovascular risk factors in childhood and carotid artery intima‐media thickness in adulthood: the Cardiovascular Risk in Young Finns Study. JAMA. 2003;290:2277–2283. doi: 10.1001/jama.290.17.2277 [DOI] [PubMed] [Google Scholar]
- 25. Tonstad S, Joakimsen O, Stensland‐Bugge E, Leren TP, Osa L, Russell D, Bønaa KH. Risk factors related to carotid intima‐media thickness and plaque in children with familial hypercholesterolemia and control subjects. Arterioscler Thromb Vasc Biol. 1996;16:984–991. doi: 10.1161/01.atv.16.8.984 [DOI] [PubMed] [Google Scholar]
- 26. Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090 [DOI] [PubMed] [Google Scholar]
- 27. Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, Hoes AW, Jennings CS, Landmesser U, Pedersen TR, et al. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J. 2016;37:2999–3058. doi: 10.1093/eurheartj/ehw272 [DOI] [PubMed] [Google Scholar]
- 28. Allison PD. Multicollinearity. In: Allison PD, ed. Logistic Regression Using SAS®: Theory and Application. 2nd ed. SAS Institute Inc.; 2012:60–62. [Google Scholar]
- 29. Buscot MJ, Thomson RJ, Juonala M, Sabin MA, Burgner DP, Lehtimäki T, Hutri‐Köhönen N, Viikari JSA, Raitakari OT, Magnussen CG. Distinct child‐to‐adult body mass index trajectories are associated with different levels of adult cardiometabolic risk. Eur Heart J. 2018;39:2263–2270. doi: 10.1093/eurheartj/ehy161 [DOI] [PubMed] [Google Scholar]
- 30. Magnussen CG, Venn A, Thomson R, Juonala M, Srinivasan SR, Viikari JSA, Berenson GS, Dwyer T, Raitakari OT. The association of pediatric low‐ and high‐density lipoprotein cholesterol dyslipidemia classifications and change in dyslipidemia status with carotid intima‐media thickness in adulthood: evidence from the Cardiovascular Risk in Young Finns Study, the Bogalusa Heart Study, and the CDAH (Childhood Determinants of Adult Health) study. J Am Coll Cardiol. 2009;53:860–869. doi: 10.1016/j.jacc.2008.09.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Goff DC, Lloyd‐Jones DM, Bennett G, Coady S, D'Agostino RB Sr, Gibbons R, Greenland P, Lackland DT, Levy D, O'Donnell CJ, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S49–S73. doi: 10.1161/01.cir.0000437741.48606.98 [DOI] [PubMed] [Google Scholar]
- 32. Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Bäck M, Benetos A, Biffi A, Boavida J‐M, Capodanno D, et al. 2021 ESC guidelines on cardiovascular disease prevention in clinical practice: developed by the Task Force for cardiovascular disease prevention in clinical practice with representatives of the European Society of Cardiology and 12 medical societies with the special contribution of the European Association of Preventive Cardiology (EAPC). Eur Heart J. 2021;42:3227–3337. doi: 10.1093/eurheartj/ehab484 [DOI] [PubMed] [Google Scholar]
- 33. Gillman MW, Daniels SR. Is universal pediatric lipid screening justified? JAMA. 2012;307:259–260. doi: 10.1001/jama.2011.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lozano P, Henrikson NB, Morrison CC, Dunn J, Nguyen M, Blasi PR, Whitlock EP. Lipid screening in childhood and adolescence for detection of multifactorial dyslipidemia: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;316:634–644. doi: 10.1001/jama.2016.6423 [DOI] [PubMed] [Google Scholar]
- 35. Luirink IK, Wiegman A, Kusters M, Hof MH, Groothoff JW, de Groot E, Kastelein JJP, Hutten BA. 20‐year follow‐up of statins in children with familial hypercholesterolemia. N Engl J Med. 2019;381:1547–1556. doi: 10.1056/NEJMoa1816454 [DOI] [PubMed] [Google Scholar]
- 36. Puska P, Jaini P. The North Karelia project: prevention of cardiovascular disease in Finland through population‐based lifestyle interventions. Am J Lifestyle Med. 2020;14:495–499. doi: 10.1177/1559827620910981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pahkala K, Laitinen TT, Niinikoski H, Kartiosuo N, Rovio SP, Lagström H, Loo BM, Salo P, Jokinen E, Magnussen CG, et al. Effects of 20‐year infancy‐onset dietary counselling on cardiometabolic risk factors in the Special Turku Coronary Risk Factor Intervention Project (STRIP): 6‐year post‐intervention follow‐up. Lancet Child Adolesc. Health. 2020;4:359–369. doi: 10.1016/S2352-4642(20)30059-6 [DOI] [PubMed] [Google Scholar]
- 38. Raiko JRH, Viikari JSA, Ilmanen A, Hutri‐Kähönen N, Taittonen L, Jokinen E, Pietikäinen M, Jula A, Loo B‐M, Marniemi J, et al. Follow‐ups of the Cardiovascular Risk in Young Finns Study in 2001 and 2007: levels and 6‐year changes in risk factors. J Intern Med. 2010;267:370–384. doi: 10.1111/j.1365-2796.2009.02148.x [DOI] [PubMed] [Google Scholar]
- 39. Viikari JSA, Juonala M, Raitakari OT. Trends in cardiovascular risk factor levels in Finnish children and young adults from the 1970s: the Cardiovascular Risk in Young Finns Study. Exp Clin Cardiol. 2006;11:83–88. [PMC free article] [PubMed] [Google Scholar]
- 40. Solberg LA, Eggen DA. Localization and sequence of development of atherosclerotic lesions in the carotid and vertebral arteries. Circulation. 1971;43:711–724. doi: 10.1161/01.CIR.43.5.711 [DOI] [PubMed] [Google Scholar]
- 41. Prati P, Vanuzzo D, Casaroli M, Di Chiara A, De Biasi F, Feruglio GA, Touboul P‐J. Prevalence and determinants of carotid atherosclerosis in a general population. Stroke. 1992;23:1705–1711. doi: 10.1161/01.str.23.12.1705 [DOI] [PubMed] [Google Scholar]
- 42. Fabris F, Zanocchi M, Bo M, Fonte G, Poli L, Bergoglio I, Ferrario E, Pernigotti L. Carotid plaque, aging, and risk factors. A study of 457 subjects. Stroke. 1994;25:1133–1140. doi: 10.1161/01.STR.25.6.1133 [DOI] [PubMed] [Google Scholar]
- 43. Dwyer T, Sun C, Magnussen CG, Raitakari OT, Schork NJ, Venn A, Burns TL, Juonala M, Steinberger J, Sinaiko AR, et al. Cohort profile: the international childhood cardiovascular cohort (i3c) consortium. Int J Epidemiol. 2013;42:86–96. doi: 10.1093/ije/dys004 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Table S1
Figure S1


