Abstract
Background
Various hemodynamic changes occur following transcatheter aortic valve implantation (TAVI) that may impact therapeutic decisions. NICaS is a noninvasive bioimpedance monitoring system aimed at hemodynamic assessment. We used the NICaS system in patients with severe aortic stenosis (AS) to evaluate short‐term hemodynamic changes after TAVI.
Methods and Results
We performed hemodynamic analysis using NICaS on 97 patients with severe AS who underwent TAVI using either self‐expandable (68%) or balloon‐expandable (32%) valves. Patients were more often women (54%) and had multiple comorbidities including hypertension (83%), coronary artery disease (46%), and diabetes (37%). NICaS was performed at several time points—before TAVI, soon after TAVI, at hospital discharge, and during follow‐up. Compared with baseline NICaS measurements, we observed a significant increase in systolic blood pressure and total peripheral resistance (systolic blood pressure 132±21 mm Hg at baseline versus 147±23 mm Hg after TAVI, P<0.001; total peripheral resistance 1751±512 versus 2084±762 dynes*s/cm5, respectively, P<0.001) concurrent with a decrease in cardiac output and stroke volume (cardiac output 4.2±1.5 versus 3.9±1.3 L/min, P=0.037; stroke volume 61.4±14.8 versus 56.2±15.9 mL, P=0.001) in the immediate post‐TAVI period. At follow‐up (median 59 days [interquartile range, 40.5–91]) these measurements returned to values that were not different from the baseline. A significant improvement in echocardiography‐based left ventricular ejection fraction was observed from baseline to follow‐up (55.6%±11.6% to 59.4%±9.4%, P<0.001).
Conclusions
Unique short‐term adaptive hemodynamic changes were observed using NICaS in patients with AS soon after TAVI. Noninvasive hemodynamic evaluation immediately following TAVI may contribute to the understanding of complex hemodynamic changes and merits favorable consideration.
Keywords: aortic stenosis, hemodynamics, monitoring, non‐invasive, TAVI
Subject Categories: Valvular Heart Disease, Catheter-Based Coronary and Valvular Interventions
Nonstandard Abbreviations and Acronyms
- AS
aortic stenosis
- CO
cardiac output
- SV
stroke volume
- TAVI
transcatheter aortic valve replacement
- TPR
total systemic peripheral resistance
Clinical Perspective.
What Is New?
There are few experiences with noninvasive hemodynamic monitoring following transcatheter aortic valve implantation.
Using a noninvasive cardiac system in patients with severe aortic stenosis following transcatheter aortic valve implantation, major acute hemodynamic alterations were noted shortly after the procedure and reverted to near baseline values at follow‐up.
What Are the Clinical Implications?
Noninvasive hemodynamic monitoring may provide insights into the complex physiologic changes occurring during the periprocedural time in patients with transcatheter aortic valve implantation and thereby promotes a more precise treatment strategy.
Transcatheter aortic valve implantation (TAVI) for the treatment of severe aortic stenosis (AS) is accompanied by various short‐ and long‐term hemodynamic and physiologic effects. 1 , 2 , 3 , 4 , 5 , 6 Among these are improved aortic valve area, decreased mean transvalvular gradients, and reverse cardiac remodeling characterized by reduced left ventricular (LV) mass index, augmented systolic and diastolic LV function, and lower natriuretic peptide levels. 1 , 2 , 3 , 4 , 5 , 6 In addition, shortly after the aortic valve gradient reduction during the TAVI procedure, augmented blood pressure (BP) response has been frequently reported 7 , 8 accompanied by higher stroke volume (SV) and cardiac output (CO). The elevated BP is thought to be associated with a decrease in muscle sympathetic nerve activity and an increase in systemic arterial baroreflex response. 9 These hemodynamic changes may have a significant impact on patient management and/or clinical outcomes following TAVI interventions. 7 , 8
NICaS (NI Medical Ltd, Ra'anana, Israel), a noninvasive cardiac system, is a bedside monitoring system designed for a comprehensive assessment of the body's hemodynamics. 10 It is based on bioimpedance changes measured throughout the peripheral tissues' vasculature during systole and diastole, using 2 surface limb leads. 10 , 11 Making available near‐instantaneous results, NICaS is capable of estimating total body water, CO and cardiac index, SV, and total systemic peripheral resistance (TPR). Thus, it can guide treatment goals such as the need for antihypertensive drugs or vasopressors, fluid volume, or diuretics. 12 This method has been validated against invasive means of hemodynamic assessment, such as thermodilution, 13 , 14 , 15 and has proven accurate in estimating CO, SV, and other measurements for a range of cardiac and other clinical settings. 11 , 16 , 17 , 18 , 19 The NICaS system also demonstrated a good correlation with Doppler echocardiography 16 , 20 and cardiac magnetic resonance 21 imaging‐derived CO and SV. There is, however, a paucity of data on the practice of NICaS for the assessment of hemodynamic changes following TAVI among patients with severe AS. 22 , 23 , 24 In this setting, peri‐procedural NICaS may be of use in detecting deviations from expected physiologic adaptations, thereby guiding tailored management.
Thus, in the current clinical investigation, we aimed to evaluate the acute and short‐term hemodynamic changes after TAVI among patients with severe AS using the NICaS system.
METHODS
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Population
We performed a single‐center prospective cohort study in the cardiac intensive care unit of a tertiary hospital in Israel, where a substantial number of TAVI procedures are performed each year. The study included 183 consecutive patients with severe AS who underwent TAVI between October 2019 and December 2020. We excluded patients referred to TAVI for combined severe stenosis and regurgitation or any additional severe valvular disease. Other excluded patients were patients who underwent the procedure with general anesthesia, patients with unstable hemodynamics attributable to cardiogenic or hemorrhagic shock who required inotropes, vasopressors, or mechanical circulatory support, and those who died during the periprocedural period.
All recruited patients signed an informed consent form following the approval of the institutional review board ethics committee in compliance with the Declaration of Helsinki.
Study Procedures
Baseline patient characteristics were collected including patient demographics, comorbidities, clinical status, and background medical treatment.
NICaS Study
NICaS (NI Medical Ltd, Ra'anana, Israel) is a US Food and Drug Administration and European conformity‐mark‐approved completely noninvasive hemodynamic monitoring tool. It is based on bioimpedance changes in peripheral tissues when transmitting a small electrical current through the body by using 2 surface limb leads in a wrist‐to‐ankle configuration. With each heartbeat, the volume of blood in the arterial system changes, and this results in a change in the body's electrical resistance—NICaS measures this change. Also, 1 lead electrocardiography monitor is recorded. CO as well as other hemodynamic and respiratory parameters are calculated by a proprietary algorithm (Figure S1 and Table S1). Upon measuring bioimpedance changes throughout the cardiac cycle, and dependent on additional data (such as blood hematocrit and sodium), SV calculations and other hemodynamic measures can be made. Accordingly, patients' weight, systolic and diastolic BP, blood hematocrit, sodium, and peripheral oxygen saturation data were taken separately and given as input for NICaS at each analysis. All measurements were performed in a supine position after 5 minutes at rest. At least 3 measurements were performed at each analysis, and the recorded measurement was an average of all 3 readings to ensure analysis validity. Each analysis was performed by an independent investigator trained in the study operation and blinded to prior and follow‐up results.
Before assessing the study population, we performed an internal validation of NICaS measurements against invasive hemodynamic measurements in a cohort of 15 stable elective patients with heart failure (Figure S2).
Patients were assessed by NICaS at the following time points—before valve implantation (baseline), within 6 hours after the procedure (ie, soon after TAVI), before hospital discharge, and during the first clinical follow‐up. The hemodynamic parameters measured were SV, SV index (SVi), CO, cardiac index, TPR, and total body water. The NICaS software enabled collected data to be transferred directly to an XLS file, a feature that minimized possible errors. We excluded or skipped NICaS measurements if the patient's condition was significantly altered during the periprocedural period (for example because of arrhythmias, significant anemia, high fever, sepsis) to avoid including possible confounders that might significantly affect the hemodynamic results.
All patients underwent transthoracic echocardiography assessment parallel to NICaS evaluation at baseline, during the procedure, before discharge, and at follow‐up. Echocardiographic variables were defined by standard European definitions according to the European Society of Cardiology guidelines available during the study period, and included among others, left ventricular ejection fraction (LVEF), transvalvular aortic gradients, and presence and severity of aortic regurgitation following TAVI (paravalvular leak).
TAVI Procedure
Patients underwent implantation of either the self‐expandable Medtronic Evolut R/Evolut Pro (Medtronic, Minneapolis, MN) and SYMETIS ACURATE Neo (Boston Scientific, MA) or the balloon‐expandable Edwards Sapien 3 valve (Edwards LifeSciences, Irvine, CA, USA). Transfemoral access was the default approach. Characteristics of the TAVI procedure and periprocedural complications were collected and described. Further patient data, such as medical treatment at discharge, was collected as well.
The study's primary endpoint was to assess hemodynamic changes before and following TAVI at various time points using the NICaS system.
Statistical Analysis
Patient characteristics were presented as n (%) for categorical variables, and as mean ± SD or median (interquartile range [IQR]), as appropriate, with few missing values and the valid percent reported. NICaS parameters at 2 time points were compared using paired‐sample t‐test, and at 3 or 4 time points using the general linear model repeated measures ANOVA and presented with box plots. Echocardiographic characteristics over time were compared by the general linear model repeated measures ANOVA in the case of symmetrically distributed continuous variables, by Friedman nonparametric test in the case of asymmetrically distributed continuous or ordinal variables, and by Cochran test in the case of binomial (dichotomous) variables. To compare every 2 paired ordinal variables, Wilcoxon signed‐rank test was used. Correlation between NICaS and invasive catheterization‐derived hemodynamic measures was conducted by Pearson correlation and agreement between the methods was examined using the Bland–Altman plot and the intraclass correlation coefficient. For all comparisons, the exact number of patients (n) was specified with no missing measurements for the patients included. All tests were conducted at a 2‐sided alpha level of 0.05, which was considered statistically significant. All statistical analyses were performed using IBM SPSS Statistics for Windows, Version 27.0 (Armonk, NY: IBM Corp, 2020).
RESULTS
Out of 183 patients admitted for TAVI during the study period, 99 patients were eventually enrolled (64 patients were excluded because of mixed valvular pathology, other significant valvular pathology, or hemodynamic instability, and 20 patients were unattainable or unwilling to sign an informed consent). Two additional patients were excluded because of periprocedural fatality. Thus, the final study population included 97 patients. Baseline characteristics of patients are presented in Table 1. There were slightly more women than men and the median age was 82 (IQR, 77–86) years. The study cohort had multiple comorbidities, of which hypertension (83%), dyslipidemia (79%), and coronary artery disease (46%) were the most prevalent. At baseline, the Doppler‐based median aortic valve area was 0.7 cm2 (0.6–0.8 cm2), and median valve pressure gradients were 67/44 mm Hg (59/38–91/60 mm Hg, max/mean gradient). Thirty‐two patients (34%) had low‐flow, low‐gradient severe AS, of which 27 patients had low gradients in the presence of preserved LV function (“paradoxical AS”), and 5 patients had low gradient in the presence of reduced LV function. The median transvalvular peak‐to‐peak pressure gradient was 50 mm Hg (40–65 mm Hg) during TAVI and before valve implantation. Most patients had a good systolic LV function with a median ejection fraction (EF) of 60% (50%–65%), while 22 patients (23%) had a reduced EF (<50%). Significant diastolic dysfunction (≥grade II) was present in 12% of patients (Table 1). Significant LV hypertrophy (>mild) occurred in 18% of patients. TAVI was performed predominantly via the transfemoral route, using either the Medtronic Evolut (54%), Edwards Sapien 3 (32%), or SYMETIS ACURATE Neo (14%). Immediately following the aortic valve implantation, the echocardiographic median gradients declined from previous values to 10/5 mm Hg (7/3–15/8 max/mean gradient mm Hg). Postprocedural complications are presented in Table 2, with 21 (22%) requiring a permanent pacemaker and 7% experiencing vascular complications. Repeat echocardiography results before hospital discharge are presented in Table 3.
Table 1.
Demographics and Baseline Characteristics of Patients
| All cohort (n=97) | |
|---|---|
| Sex, men | 45 (46.4) |
| Age, y | 82 [77–86] |
| BMI, kg/m2 | 27.9±4.6 |
| BSA, m2 | 1.8±0.2 |
| Hypertension | 80 (83.3) |
| Dyslipidemia | 76 (79.2) |
| Diabetes | 35 (37.2) |
| Peripheral vascular disease | 14 (14.6) |
| Atrial fibrillation, persistent | 6 (6.3) |
| Atrial fibrillation, paroxysmal | 16 (16.7) |
| Permanent pacemaker | 8 (8.3) |
| CAD | 44 (45.8) |
| CKD | 28 (29.5) |
| Chronic anemia | 26 (27.7) |
| Baseline echocardiographic parameters | |
| Ejection fraction | 60 [50–65] |
| AVA, cm2 | 0.7 [0.6–0.8] |
| Peak pressure gradient, mm Hg | 67 [59, 91] |
| Mean pressure gradient, mm Hg | 44 [38–60] |
| Stroke volume, mL | 70.2±17.6 |
| Diastolic dysfunction | 62 (75.6) |
| Grade 1 | 52 (63.4) |
| Grade 2 | 9 (11) |
| Grade 3 | 1 (1.2) |
| Systolic pulmonary artery pressure, mm Hg | 37.5±12.8 |
| Aortic regurgitation | 47 (52.2) |
| Mild | 35 (38.9) |
| Moderate | 12 (13.3) |
| Mitral regurgitation | 60 (65.9) |
| Mild | 39 (42.9) |
| Moderate | 19 (20.9) |
| Tricuspid regurgitation | 46 (51.1) |
| Mild | 34 (37.8) |
| Moderate | 11 (12.2) |
| Mitral stenosis, up to moderate | 18 (19.8) |
| RV failure | 5 (5.5) |
| LVH | 68 (74.7) |
| Baseline medical treatment | |
| Beta blockers | 61 (63.5) |
| Furosemide | 44 (45.8) |
| Spironolactone | 12 (12.5) |
| ACEi/ARBs | 48 (50.5) |
| CCB | 19 (30.6) |
| Other vasodilators | 22 (23.2) |
ACEi indicates angiotensin‐converting enzyme inhibitor; ARBs, angiotensin receptor blocker; AVA, aortic valve area; BMI, body mass index; BSA, body surface area; CAD, coronary artery disease; CCB, calcium channel blocker; CKD, chronic kidney disease; LVH, left ventricular hypertrophy; and RV, right ventricular.
All values are presented as n (%), mean±SD, or median [interquartile range].
Table 2.
Characteristics of the TAVI Procedure and Complications
| All cohort (n=97) | |
|---|---|
| Peak‐to‐peak pressure gradient, mm Hg | 50 [40–65] |
| Valve type | |
| Evolute R/Pro | 52 (54.2) |
| Sapien 3 | 31 (32.3) |
| SYMETIS/ACURATE neo | 13 (13.5) |
| Valve size, mm | 26 [26–29] |
| Access | |
| Transfemoral | 94 (97.9) |
| Axillary | 1 (1) |
| Valve in valve | 9 (9.4) |
| Peak pressure gradient post‐TAVI, mm Hg* | 10 [7–15] |
| Mean pressure gradient post‐TAVI, mm Hg* | 5 [3–8] |
| PVL per TTE—mild* | 29 (31.2) |
| PVL per TTE—moderate* | 9 (9.7) |
| PVL per angiography* | 18 (18.8) |
| Need for permanent pacemaker | 21 (21.9) |
| CVA | 1 (1) |
| Bleeding | 6 (6.3) |
| Vascular complications | 7 (7.2) |
| Pericardial effusion | 4 (4.2) |
CVA indicates cerebrovascular accident; PVL, paravalvular leak; and TTE, transthoracic echocardiography.
*Peak and mean pressure gradients and the presence of paravalvular leak by angiography and transthoracic echocardiography were estimated immediately following TAVI.
All values are presented as n (%), or median [interquartile range].
Table 3.
Echocardiographic Characteristics and Medications at Discharge
| All cohort (n=97) | |
|---|---|
| Ejection fraction | 60 [57.5–65] |
| Peak pressure gradient, mm Hg | 15.5 [12–25] |
| Mean pressure gradient, mm Hg | 8 [6–14] |
| PVL, mild | 42 (45.2) |
| PVL, moderate | 7 (7.5) |
| Pulmonary hypertension, mm Hg | 36 [29.5–44.5] |
| Mitral regurgitation, ≥mild | 50 (54.3) |
| Tricuspid regurgitation, ≥mild | 52 (56.5) |
| Mitral stenosis, ≥moderate | 13 (14.1) |
| RV failure | 4 (4.3) |
| Beta blockers | 52 (56.5) |
| Furosemide | 41 (44.6) |
| Spironolactone | 8 (8.7) |
| ACEi/ARB | 50 (54.3) |
| Vasodilators | 28 (30.4) |
ACEi indicates angiotensin‐converting enzyme inhibitors; ARB, angiotensin receptor blockers; PVL, paravalvular leak; and RV, right ventricular.
Values are presented as n (%) or median [interquartile range].
NICaS Results
First, we compared NICaS hemodynamic measurements between baseline (within 24 hours before TAVI) and the first hours after TAVI among the entire cohort and found significant differences with respect to all hemodynamic parameters evaluated by NICaS: a mean increase of 15±21 mm Hg in systolic BP (132±21 to 147±23 mm Hg, P<0.001), parallel with a decrease in SV and CO (61.4±14.8 to 56.2±15.9 mL, P=0.001, and 4.2±1.5 to 3.9±1.3 L/min, P=0.037, respectively), and an increase of 333±828 dynes×s/cm5 in TPR (1751±512 to 2084±762, P<0.001) and in total body water (38.8±8.2 to 43.6±10.7%, P<0.001). Subsequently, we compared the NICaS measurements of patients (n=76) between the 3 time points—before TAVI, within the first hours post‐TAVI, and at a median follow‐up of 59 days (40.5–91 days)—and observed a reciprocal significant decrease in systolic BP, TPR, and total body water, with an increase in SV and CO from early post‐TAVI to values at follow‐up not statistically different from the baseline (Table 4, Figure 1). CO, SV, SVi, and cardiac index showed minor and nonsignificant improvements from baseline to follow‐up (Table 4). A comparison of NICaS measurements between all 4 time points (n=67), including hospital discharge, was performed as well, and demonstrated nonsignificant differences in hemodynamic measurements between discharge and follow‐up values (Table S2).
Table 4.
NICaS Parameters Over Time (n=76)
| Baseline NICaS (1) | NICaS shortly Following TAVI (2) | Follow‐up NICaS (3) | P value | Significant difference between time points | |
|---|---|---|---|---|---|
| Systolic BP, mm Hg | 132.5±22.3 | 148.4±23.9 | 137.2±17.2 | <0.001 | 1–2 (<0.001), 2–3 (<0.001) |
| Diastolic BP, mm Hg | 65±12 | 61.5±11.8 | 68.8±10.5 | <0.001 | only 2–3 (<0.001) |
| MAP, mm Hg | 87.1±11.9 | 90.1±13.1 | 91.3±10.1 | 0.024 | only 1–3 (0.02) |
| HR, bpm | 69.6±12.6 | 69.5±13.5 | 70.2±11.8 | 0.9 | |
| SV, mL | 62.1±15 | 56±15.8 | 62.6±17.9 | 0.002 | 1–2 (0.003), 2–3 (0.013) |
| SVi, mL/m2 | 34.6±7.6 | 31±7.7 | 34.8±8.8 | 0.001 | 1–2 (0.001), 2–3 (0.009) |
| CO, L/min | 4.3±1.2 | 3.9±1.2 | 4.4±1.3 | 0.013 | only 2–3 (0.03) |
| Cardiac index, L/min/m2 | 2.38±0.61 | 2.14±0.61 | 2.43±0.68 | 0.006 | 1–2 (0.047), 2–3 (0.019) |
| TPR, dynes×s/cm5 | 1754±520 | 2094±752 | 1808±548 | <0.001 | 1–2 (0.002), 2–3 (0.011) |
| TBW, % | 38.9±8.4 | 44.1±11.5 | 39.5±8.2 | <0.001 | 1–2 (<0.001), 2–3 (0.008) |
BP indicates blood pressure; CO, cardiac output; HR, heart rate; MAP, mean arterial pressure; RR, respiratory rate; SV, stroke volume; SVi, stroke volume index; TBW, total body water; and TPR, total systemic peripheral resistance.
Figure 1. Comparison of NICaS parameters over time.
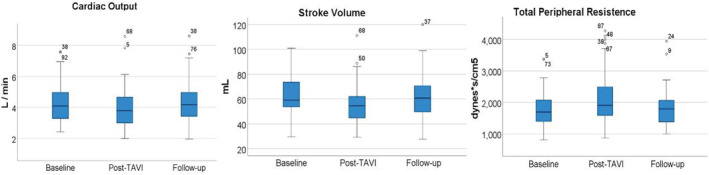
A total of 76 patients with available data for the 3 time points: baseline, post‐transcatheter aortic valve replacement, and follow‐up; 95% CI for cardiac output (L/m): baseline (3.98–4.52), post‐transcatheter aortic valve replacement (3.58–4.15), follow‐up (4–4.6); 95% CI for stroke volume (mL): baseline (58.6–65.5), post‐transcatheter aortic valve replacement (52.4–59.6), follow‐up (58.5–66.7); and 95% CI for total peripheral resistance (dynes ×s/cm5): baseline (1685–1873), post‐transcatheter aortic valve replacement (1922–2266), follow‐up (1683–1933). CO indicates cardiac output; SV, stroke volume; TAVI, transcatheter aortic valve replacement; and TPR, total peripheral resistance.
The stratification of the cohort by high‐gradient AS versus low‐flow, low‐gradient AS (mean gradient ≥40 mm Hg or <40 mm Hg, respectively) revealed a similar trend in SV, CO, and TPR over time after TAVI (Figure 2A); yet those with a low mean gradient (n=23) had higher values of TPR and lower values of SV and CO. However, the interaction with the mean gradient was statistically insignificant (P=0.47 for SV, P=0.18 for CO, and P=0.09 for TPR).
Figure 2. Comparison of NICaS parameters over time according to mean gradient and ejection fraction.
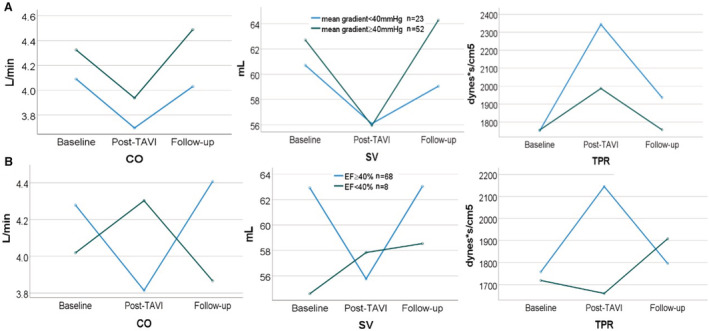
A, NICaS parameters over time stratified by a low transvalvular mean gradient. A low mean gradient was defined as <40 mm Hg. B, NICaS parameters over time stratified by a reduced ejection fraction. Reduced ejection fraction was defined as ejection fraction <40%. CO indicates cardiac output; EF, ejection fraction; SV, stroke volume; TAVI, transcatheter aortic valve replacement; and TPR, total peripheral resistance.
Comparing NICaS measurements at the 3 time points stratified by the presence of a significant LV dysfunction (defined as EF≤40%), we observed conflicting temporal trends in the subgroup of patients with a reduced EF (n=8); TPR following TAVI decreased compared with baseline measurements, while SV and CO increased (Figure 2B). However, this interaction with a significantly reduced EF was not statistically significant (P=0.46 for SV, P=0.76 for CO, and P=0.39 for TPR).
Concerning the comparison between echocardiographic measurements over time, LVEF improved modestly from baseline to follow‐up values, from 55.6%±11.6% to 59.4%±9.4% (P<0.001; n=63 with available data). Echocardiographic parameters compared over time are presented in Table 5.
Table 5.
Echocardiographic Parameters Compared Over Time
| Baseline echocardiography (1) | Echocardiography at procedure (2) | Echocardiography before discharge (3) | Follow‐up echocardiography (4) | P value | |
|---|---|---|---|---|---|
| EF, mean±SD | 55.6±11.6 | … | 58.3±11.1 | 59.4±9.4 | <0.001 |
| SPAP, mean±SD | 39.1±12.8 | … | 41±15.4 | 36.3±15.1 | 0.17 |
| Peak gradient, median, [IQR] | 68 [59–87] | 10 [7–15] | 15 [12–24] | 14 [10–20] | <0.001 |
| Mean gradient, median, [IQR] | 44 [37–57] | 5 [3–8] | 8 [6–13] | 8 [5–10] | <0.001 |
| PVL≥mild, n (%) | 35 (42.1) | 46 (55.4) | 48 (57.8) | 0.23 | |
| Diastolic dysfunction≥grade 1, n (%) | 53 (75.7) | … | … | 59 (84.3) | 1 |
| MR≥mild, n (%) | 52 (64.2) | … | 44 (54.3) | 53 (65.4) | 0.023 |
| TR≥mild, n (%) | 42 (52.5) | … | 45 (56.3) | 48 (60) | 0.23 |
| Mitral stenosis, n (%) | 16 (19.8) | … | 11 (13.6) | 19 (23.5) | 0.14 |
| RV failure, n (%) | 4 (4.9) | … | 3 (3.7) | 2 (2.5) | 0.37 |
EF indicates ejection fraction; IQR, interquartile range; LVH, left ventricular hypertrophy; MR, mitral regurgitation; PVL, paravalvular leak; RV, right ventricular; SPAP, systolic pulmonary artery pressure; and TR, tricuspid regurgitation.
DISCUSSION
The present study represents a single‐center experience with the NICaS system as a peri‐procedural noninvasive hemodynamic monitoring system in patients who undergo TAVI for severe AS. Several findings were demonstrated; first, the immediate hemodynamic response to TAVI in our cohort showed a significant increase in systolic BP and TPR and declining CO and SV. However, all hemodynamic measurements returned to baseline (ie, pre‐TAVI levels) with only minor, nonsignificant improvement in CO, SV, SVi, and cardiac index during follow‐up. Notably, the early period after TAVI was characterized by the most prominent hemodynamic fluctuations which may have a bearing on clinical status and require tailored monitoring and reaction.
The progressive severe AS process is associated with a gradual increase in LV afterload, elevated LV end‐diastolic pressure, outflow tract obstruction, and stiffening of the systemic arterial system. 25 , 26 The latter is an additive factor to the stenosis of the valve that maintains increased LV afterload, although, unlike the valve, the arterial stiffness does not immediately reverse with TAVI. The elevated LV afterload is key to the characteristic increased LV mass in severe AS, which evolves into a stiff hypertrophic ventricle. 27 Furthermore, the valvular stenosis may conceal intraventricular obstruction induced by the small‐cavity hypertrophied ventricle. 28 , 29 , 30 However, the abrupt elimination of the valvular stenosis unmasks and even augments the dynamic obstruction with hemodynamic collapse known as “suicide ventricle” in its extreme manifestation. 31 , 32 The latter is a representative example to support the need for careful hemodynamic monitoring in the early period after TAVI.
Several studies investigated the unique hemodynamic changes occurring following TAVI 4 , 7 , 9 ; a hypertensive response immediately following TAVI was reported and was thought to result from myocardial contractile reserve and a relative improvement in cardiac function 4 , 7 or be related to the sympathetic nerve activity and arterial baroreflex response. 9 This phenomenon was in fact associated with improved patient outcomes. 7 Additionally, the prompt elimination of the LV to aortic pressure gradient was associated with augmented SV and CO. 7
Our study showed a similar pattern and magnitude of blood pressure augmentation after TAVI as reported in previous studies. 4 , 7 , 9 However, we observed a contradicting trend of decreased SV and CO immediately after TAVI as assessed by the NICaS system. A possible explanation to this observation may be the combination of a compromised LV contractile reserve (that does not recover as quickly as the eliminated pressure gradient) in our cohort and a relatively shallow reduction in LV afterload because of an increase in mean BP. Hence, CO did not increase as expected despite the elimination of valvular stenosis, and TPR (being roughly the mean arterial pressure divided by CO) increased accordingly. On follow‐up examination, CO and SV have modestly recovered, probably reflecting delayed adaptation of the LV to the new hemodynamic state. Another explanation lies in the hypertrophic myocardium, which becomes hyperdynamic following the elimination of the aortic valve pressure gradient. The hyperdynamic contraction of the hypertrophied ventricle generates a narrow functional LV cavity which results in low SV and CO. This phenomenon may also be further augmented by a relatively hypovolemic state because of long‐standing diuretic treatment, fasting before the procedure, and, rarely, bleeding complications. This explanation may also settle the inconsistency between the higher values of LVEF measured by echo‐Doppler (hyperdynamic contractility) and the lower SV and CO (compared with baseline) measured by NICaS shortly after the TAVI procedure.
Our findings are supported by the study of Yotti et al, who explored the interaction between valvular and vascular functions in patients with AS after TAVI by measuring aortic pressure and flow simultaneously. They demonstrated stiffer vascular behavior with a hypertensive response in half of the patients post‐TAVI and a decrease in SVi and cardiac index that correlated with indices of increased arterial load (elevated SVR and impedance and reduced arterial compliance) that limited the procedure's acute afterload relief. 33 Our findings are also supported by the study of Seppelt et al, who revealed impaired systolic and diastolic functions in the early phase after TAVI using an invasive pressure‐volume loop analysis but nonetheless found indications for early improvement of global cardiovascular energy efficiency. 34
Noninvasive hemodynamic evaluation following TAVI using NICaS was previously performed by Markus and colleagues. 22 They found an increment in CO and cardiac index, and a decline in TPR when comparing baseline to discharge values, yet no significant changes in SV and SVi were observed. When 6 to 8 hours post‐TAVI measurements were compared with baseline, no significant changes in CO, cardiac index, and TPR were observed. These findings are clearly different, in part even inverse, from our study findings (Table S2). It may be partially explained by the distinct timing at when measurements were taken (a median of 6–8 hours compared with 2–4 hours in our study for the early‐after‐TAVI measurements, and at a mean of 6.2±1.1 days compared with a median of 24 hours for the prior‐to‐discharge measurements). The notably lower mean LVEF and the different statistical methods used compared with our study may have also contributed to the differing results. However, in agreement with our results, SV and the Granov‐Goor‐Index, 35 a surrogate for LV systolic function, diminished immediately after TAVI. Given the unchanged heart rate and the reduced SV found early after TAVI in the study by Markus et al, a diminished rather than unchanged CO would have been expected. This may support the validity of our study findings.
Implementing the NICaS system in harmony with transthoracic echocardiography enables more comprehensive and accessible monitoring of the patient's hemodynamic state and fluctuations after TAVI. Furthermore, NICaS may be helpful to overcome pitfalls in echocardiography assessments such as poor imaging or Doppler biases because of inaccurate flow sampling. It has been previously shown that transthoracic echocardiography has significant limitations in critical patients under certain conditions which may interfere with the acoustic window and alignment of the doppler beam while sampling the left ventricular outflow tract for SV calculations. 36
Our findings are useful for identifying a unique and intriguing acute hemodynamic response to TAVI and have potential implications for the acute medical management of this cohort. Our study may serve as a starting point for further studies to be conducted aiming to improve the management of patients following TAVI by better understanding the complex hemodynamic changes occurring during the periprocedural time and thereby promoting a more precise treatment strategy.
Our prospective study is one of few reports depicting the experience with the NICaS system in this population. However, the study is limited because it is relatively small in size, conducted in a single‐center, and observational. Furthermore, follow‐up data are incomplete because of the COVID‐19 pandemic restrictions and the “stay‐home” policy that prevented patients from arriving at the hospital. Specifically, 21 measurements during the third time point were missing or excluded because of altered patient conditions and concern for introducing a potential bias in the hemodynamic evaluation. Finally, the hemodynamic data of both echocardiography and NICaS cannot be challenged by direct measurements as in invasive monitoring. Nevertheless, before study initiation, we performed an internal validation of NICaS measurements against invasive hemodynamic measurements in a cohort of stable elective patients and demonstrated good correlation and agreement between the 2 methods (Figure S2). Another internal validation was performed in 46 patients of the study population for SV calculated by NICaS compared with echocardiography the day after the procedure, also demonstrating good correlation (correlation coefficient index=0.58, P<0.001, Figure S3).
CONCLUSIONS
In the present study, a unique pattern of adaptive hemodynamic changes following TAVI was demonstrated using the NICaS system in patients with AS. This pattern was characterized primarily by major transient hemodynamic alterations shortly after the procedure, which reverted to near baseline values at follow‐up while LVEF increased. Hence, the implementation of this diagnostic modality in patients with TAVI merits favorable consideration and may provide new insights into physiologic changes occurring after TAVI.
Sources of Funding
None.
Disclosures
None.
Supporting information
Tables S1–S2
Figures S1–S3
Acknowledgments
We thank Yuval Lewental, head of clinical affairs in New NI Medical Limited, and Petah Tikva, Israel, for his expertise and assistance throughout the study providing support on the use of the NICaS system and addressing technical issues.
This work was presented at the ESC Acute CardioVascular Care congress, March 18–19, 2022, and at the Israel Heart Society Conference, May 25–26, 2022.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.028479
For Sources of Funding and Disclosures, see page 8.
REFERENCES
- 1. da Silva C, Sahlen A, Winter R, Bäck M, Rück A, Settergren M, Manouras A, Shahgaldi K. Hemodynamic outcomes of transcatheter aortic valve implantation with the corevalve system: an early assessment. Clin Physiol Funct Imaging. 2015;35:216–222. doi: 10.1111/cpf.12153 [DOI] [PubMed] [Google Scholar]
- 2. Clavel MA, Webb JG, Pibarot P, Altwegg L, Dumont E, Thompson C, De Larochellière R, Doyle D, Masson JB, Bergeron S, et al. Comparison of the hemodynamic performance of percutaneous and surgical bioprostheses for the treatment of severe aortic stenosis. J Am Coll Cardiol. 2009;53:1883–1891. doi: 10.1016/j.jacc.2009.01.060 [DOI] [PubMed] [Google Scholar]
- 3. Sherif MA, Abdel‐Wahab M, Awad O, Geist V, El‐Shahed G, Semmler R, Tawfik M, Khattab AA, Richardt D, Richardt G, et al. Early hemodynamic and neurohormonal response after transcatheter aortic valve implantation. Am Heart J. 2010;160:862–869. doi: 10.1016/j.ahj.2010.07.017 [DOI] [PubMed] [Google Scholar]
- 4. Gotzmann M, Lindstaedt M, Bojara W, Mügge A, Germing A. Hemodynamic results and changes in myocardial function after transcatheter aortic valve implantation. Am Heart J. 2010;159:926–932. doi: 10.1016/j.ahj.2010.02.030 [DOI] [PubMed] [Google Scholar]
- 5. Vizzardi E, D'aloia A, Fiorina C, Bugatti S, Parrinello G, De Carlo M, Giannini C, Di Bello V, Petronio AS, Curello S, et al. Early regression of left ventricular mass associated with diastolic improvement after transcatheter aortic valve implantation. J Am Soc Echocardiogr. 2012;25:1091–1098. doi: 10.1016/j.echo.2012.06.010 [DOI] [PubMed] [Google Scholar]
- 6. Alenezi F, Fudim M, Rymer J, Dunning A, Chiswell K, Swaminathan M, Bottiger B, Velagapudi P, Nicoara A, Kisslo J, et al. Predictors and changes in cardiac hemodynamics and geometry with transcatheter aortic valve implantation. Am J Cardiol. 2019;123:813–819. doi: 10.1016/j.amjcard.2018.11.038 [DOI] [PubMed] [Google Scholar]
- 7. Perlman GY, Loncar S, Pollak A, Gilon D, Alcalai R, Planer D, Lotan C, Danenberg HD. Post‐procedural hypertension following transcatheter aortic valve implantation: incidence and clinical significance. JACC Cardiovasc Interv. 2013;6:472–478. doi: 10.1016/j.jcin.2012.12.124 [DOI] [PubMed] [Google Scholar]
- 8. Reinthaler M, Stähli BE, Gopalamurugan AB, Xiu PY, Aggarwal SK, Fröhlich G, Delahunty N, Mullen MJ. Post‐procedural arterial hypertension: implications for clinical outcome after transcatheter aortic valve implantation. J Heart Valve Dis. 2014;23:675–682. [PubMed] [Google Scholar]
- 9. Dumonteil N, Vaccaro A, Despas F, Labrunee M, Marcheix B, Lambert E, Esler M, Carrie D, Senard JM, Galinier M, et al. Transcatheter aortic valve implantation reduces sympathetic activity and normalizes arterial spontaneous baroreflex in patients with aortic stenosis. JACC Cardiovasc Interv. 2013;6:1195–1202. doi: 10.1016/j.jcin.2013.06.012 [DOI] [PubMed] [Google Scholar]
- 10. Cotter G, Schachner A, Sasson L, Dekel H, Moshkovitz Y. Impedance cardiography revisited. Physiol Meas. 2006;27:817–827. doi: 10.1088/0967-3334/27/9/005 [DOI] [PubMed] [Google Scholar]
- 11. Cotter G, Moshkovitz Y, Kaluski E, Cohen AJ, Miller H, Goor D, Vered Z. Accurate, noninvasive continuous monitoring of cardiac output by whole‐body electrical bioimpedance. Chest. 2004;125:1431–1440. doi: 10.1378/chest.125.4.1431 [DOI] [PubMed] [Google Scholar]
- 12. Greco B, Chait Y, Nathanson BH, Germain MJ. A novel hypertension management algorithm guided by hemodynamic data. Kidney Int Rep. 2022;7:330–333. doi: 10.1016/j.ekir.2021.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cohen AJ, Arnaudov D, Zabeeda D, Schultheis L, Lashinger J, Schachner A. Non‐invasive measurement of cardiac output during coronary artery bypass grafting. Eur J Cardiothorac Surg. 1998;14:64–69. doi: 10.1016/S1010-7940(98)00135-3 [DOI] [PubMed] [Google Scholar]
- 14. Torre‐Amione G, Milo O, Kaluski E, Vered Z, Cotter G. Whole‐body electrical bio‐impendance is accurate in non invasive determination of cardiac output: a thermodilution controlled, prospective, double blind evaluation. J Card Fail. 2004;10:S38–S39. doi: 10.1016/j.cardfail.2004.06.072 [DOI] [Google Scholar]
- 15. Paredes OL, Shite J, Shinke T, Watanabe S, Otake H, Matsumoto D, Imuro Y, Ogasawara D, Sawada T, Yokoyama M. Impedance cardiography for cardiac output estimation ‐ reliability of wrist‐to‐ankle electrode configuration. Circ J. 2006;70:1164–1168. doi: 10.1253/circj.70.1164 [DOI] [PubMed] [Google Scholar]
- 16. Tanino Y, Shite J, Paredes OL, Shinke T, Ogasawara D, Sawada T, Kawamori H, Miyoshi N, Kato H, Yoshino N, et al. Whole body bioimpedance monitoring for outpatient chronic heart failure follow up. Circ J. 2009;73:1074–1079. doi: 10.1253/circj.CJ-08-0847 [DOI] [PubMed] [Google Scholar]
- 17. Matsuda Y, Kawate H, Shimada S, Matsuzaki C, Nagata H, Adachi M, Ohnaka K, Nomura M, Takayanagi R. Perioperative sequential monitoring of hemodynamic parameters in patients with pheochromocytoma using the non‐invasive cardiac system (NICaS). Endocr J. 2014;61:571–575. doi: 10.1507/endocrj.EJ13-0471 [DOI] [PubMed] [Google Scholar]
- 18. Epstein D, Guinzburg A, Sharon S, Kiso S, Glick Y, Marcusohn E, Glass YD, Miller A, Minha S, Furer A. A noninvasive stroke volume monitoring for early detection of minimal blood loss: a pilot study. Shock. 2021;55:230–235. doi: 10.1097/SHK.0000000000001621 [DOI] [PubMed] [Google Scholar]
- 19. Germain MJ, Joubert J, O'Grady D, Nathanson BH, Chait Y, Levin NW. Comparison of stroke volume measurements during hemodialysis using bioimpedance cardiography and echocardiography. Hemodial Int. 2018;22:201–208. doi: 10.1111/hdi.12589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Leitman M, Sucher E, Kaluski E, Wolf R, Peleg E, Moshkovitz Y, Milo‐Cotter O, Vered Z, Cotter G. Non‐invasive measurement of cardiac output by whole‐body bio‐impedance during dobutamine stress echocardiography: clinical implications in patients with left ventricular dysfunction and ischaemia. Eur J Heart Fail. 2006;8:136–140. doi: 10.1016/j.ejheart.2005.06.006 [DOI] [PubMed] [Google Scholar]
- 21. Hassan‐Tash P, Ismail U, Kirkpatrick IDC, Ravandi A, Jassal DS, Hiebert B, Kass M, Krasuski RA, Shah AH. Correlation of impedance cardiography‐derived and cardiac magnetic resonance‐derived stroke volumes. Curr Probl Cardiol 2023;48:101457. doi: 10.1016/j.cpcardiol.2022.101457 [DOI] [PubMed] [Google Scholar]
- 22. Markus B, Karatolios K, Wulle C, Pethig D, Rastan A. Peri‐procedural, non‐invasive hemodynamic monitoring In TAVI‐patients: potential impact on patient selection and outcome prediction. Arch Med. 2019;11:298. doi: 10.36648/1989-5216.11.2.298 [DOI] [Google Scholar]
- 23. Markus B, Ahrens H, Heinicke C, Pethig D, Schnurbus M, Chatzis G, Schieffer B, Divchev D. Non‐invasive hemodynamic monitoring in TAVI‐patients reveals more pronounced early in‐hospital circulatory recovery for low‐gradient aortic stenosis. Arch Med. 2021;13:36. [Google Scholar]
- 24. Fung CR, Williams R, Tyrrell B. Measurement of cardiac output by non‐invasive continuous whole‐body bioimpedance cardiography in patients with aortic stenosis. Can J Cardiol. 2014;30:S226. doi: 10.1016/j.cjca.2014.07.380 [DOI] [Google Scholar]
- 25. London GM, Marchais SJ, Guerin AP, Pannier B. Arterial stiffness: pathophysiology and clinical impact. Clin Exp Hypertens. 2004;26:689–699. doi: 10.1081/CEH-200031982 [DOI] [PubMed] [Google Scholar]
- 26. Roşca M, Magne J, Cǎlin A, Popescu BA, Piérard LA, Lancellotti P. Impact of aortic stiffness on left ventricular function and B‐type natriuretic peptide release in severe aortic stenosis. Eur J Echocardiogr. 2011;12:850–856. doi: 10.1093/ejechocard/jer120 [DOI] [PubMed] [Google Scholar]
- 27. Kupari M, Turto H, Lommi J. Left ventricular hypertrophy in aortic valve stenosis: preventive or promotive of systolic dysfunction and heart failure? Eur Heart J. 2005;26:1790–1796. doi: 10.1093/eurheartj/ehi290 [DOI] [PubMed] [Google Scholar]
- 28. Tsuruta H, Hayashida K, Yashima F, Yanagisawa R, Tanaka M, Arai T, Minakata Y, Itabashi Y, Murata M, Kohsaka S, et al. Incidence, predictors, and midterm clinical outcomes of left ventricular obstruction after transcatheter aortic valve implantation. Catheter Cardiovasc Interv. 2018;92:E288–E298. doi: 10.1002/ccd.27508 [DOI] [PubMed] [Google Scholar]
- 29. Wiseth R, Skjaerpe T, Hatle L. Rapid systolic intraventricular velocities after valve replacement for aortic stenosis. Am J Cardiol. 1993;71:944–948. doi: 10.1016/0002-9149(93)90911-U [DOI] [PubMed] [Google Scholar]
- 30. Bartunek J, Sys SU, Rodrigues AC, Van Schuerbeeck E, Mortier L, De Bruyne B. Abnormal systolic intraventricular flow velocities after valve replacement for aortic stenosis. Circulation. 1996;93:712–719. doi: 10.1161/01.CIR.93.4.712 [DOI] [PubMed] [Google Scholar]
- 31. Alfonso F, Domínguez L, Rivero F, Benedicto A, Trillo R. Severe intraventricular dynamic gradient following transcatheter aortic valve implantation: suicide ventricle? EuroIntervention. 2015;11:e1. doi: 10.4244/EIJV11I1A16 [DOI] [PubMed] [Google Scholar]
- 32. Endo N, Otsuki H, Domoto S, Yamaguchi J. Haemodynamic collapse immediately after transcatheter aortic valve implantation due to dynamic intraventricular gradient: a case report and review of the literature. Eur Heart J Case Rep. 2021;5:5. doi: 10.1093/EHJCR/YTAA565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yotti R, Bermejo J, Gutiérrez‐Ibañes E, Pérez Del Villar C, Mombiela T, Elízaga J, Benito Y, González‐Mansilla A, Barrio A, Rodríguez‐Pérez D, et al. Systemic vascular load in calcific degenerative aortic valve stenosis: insight from percutaneous valve replacement. J Am Coll Cardiol. 2015;65:423–433. doi: 10.1016/j.jacc.2014.10.067 [DOI] [PubMed] [Google Scholar]
- 34. Seppelt PC, De Rosa R, Mas‐Peiro S, Zeiher AM, Vasa‐Nicotera M. Early hemodynamic changes after transcatheter aortic valve implantation in patients with severe aortic stenosis measured by invasive pressure volume loop analysis. Cardiovasc Interv Ther. 2022;37:191–201. doi: 10.1007/s12928-020-00737-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rozenman Y, Rotzak R, Patterson RP. Detection of left ventricular systolic dysfunction using a newly developed, laptop based, impedance cardiographic index. Int J Cardiol. 2011;149:248–250. doi: 10.1016/j.ijcard.2011.02.029 [DOI] [PubMed] [Google Scholar]
- 36. Blancas R, Martínez‐González Ó, Ballesteros D, Núñez A, Luján J, Rodríguez‐Serrano D, Hernández A, Martínez‐Díaz C, Parra CM, Matamala BL, Alonso MA, Chana M. Lack of correlation between left ventricular outflow tract velocity time integral and stroke volume index in mechanically ventilated patients. Med Intensiva (Engl Ed). 2019;43:73–78. doi: 10.1016/j.medin.2017.11.012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S2
Figures S1–S3
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


