Abstract
Background
Description of cerebral and retinal infarction in patients with bicuspid aortic valve (BAV) is limited to case reports. We aimed to characterize cerebral and retinal infarction and examine outcomes in patients with BAV.
Methods and Results
Consecutive patients from 1975 to 2015 with BAV (n=5401) were retrospectively identified from the institutional database; those with confirmed cerebral or retinal infarction were analyzed. Infarction occurring after aortic valve replacement was not included. Patients were grouped according to infarction pathogenesis: embolism from a degenerative calcific BAV (BAVi); non‐BAV, large artery atherosclerotic or lacunar infarction (LAi); and non‐BAV, non‐large artery embolic infarction (nLAi). There were 83/5401 (1.5%) patients, mean age 54±12 years and 28% female, with confirmed cerebral or retinal infarction (LAi 23/83 [28%]; nLAi 30/83 [36%]; BAVi 26/83 [31%]; other 4/83 [5%]). Infarction was embolic in 72/83 (87%), and 35/72 (49%) were cardioembolic. CHA2DS2‐VASc score was 1.4±1.2 in BAVi (P=0.188 versus nLAi) and 2.3±1.2 in LAi (P=0.005). Recurrent infarction occurred in 41% overall (50% BAVi, P=0.164 and 0.803 versus LAi and nLAi). BAVi was more commonly retinal (39% BAVi versus 13% LAi, P=0.044 versus 0% nLAi, P=0.002). Patients with BAVi and LAi were more likely to have moderate‐to‐severe aortic stenosis and undergo aortic valve replacement compared with patients with nLAi.
Conclusions
Cardioembolism, often from degenerative calcification of the aortic valve, is a predominant cause of cerebral and retinal infarction in patients with BAV and is frequently recurrent. Cerebral and retinal infarction should be regarded as a complication of BAV.
Keywords: aortic valve calcification, bicuspid aortic valve, cerebral infarction, embolism, retinal infarction, stroke
Subject Categories: Valvular Heart Disease, Ischemic Stroke
Abnormal bicuspid aortic valve (BAV) geometry predisposes the cusps to high mechanical and functional stress that may result in degenerative calcification and subsequent aortic stenosis (AS) or regurgitation. Previous case reports and necropsy series have reported peripheral and/or cerebral calcific embolus in the setting of calcific AS, 1 although reports of calcific BAV and cerebrovascular events (CVE), from calcific embolism or rarely spontaneous thrombus, are limited to single case reports or small case series. 2 , 3 In larger cohorts of patients with calcific AS and stroke, underlying valve morphology was not assessed. 1 , 4 , 5 , 6 , 7 , 8 , 9 While aortic valve calcification (AVC) is considered a minor‐risk potential embolic source in patients with embolic stroke of undetermined source (ESUS), 10 other studies show no association between calcific AS and stroke. 4 , 6 , 8 , 11 The purpose of the current study was to (1) characterize cerebral and retinal infarction among patients with BAV, and (2) describe clinical and echocardiographic characteristics and examine outcomes of patients with BAV and cerebral or retinal infarction.
Methods
The study was approved by the Institutional Review Board, and informed consent was obtained for all subjects. The data that support the findings of this study are available from the corresponding author upon reasonable request. We retrospectively identified 5401 adult patients (aged 18 years or older) with an International Classification of Diseases Ninth Revision or Tenth Revision (ICD‐9; ICD‐10) diagnosis of BAV at Mayo Clinic, Rochester from January 1, 1975, to December 31, 2015, and patients with CVE equivalent International Classification of Diseases diagnoses including transient ischemic attacks, cerebral infarction, retinal infarction, and hemorrhagic stroke, were identified among these patients. Each patient with a CVE equivalent diagnosis underwent detailed medical record review. Neurologist documentation was used to characterize the CVE. CVE was not included if the neurologist documentation suggested an alternative diagnosis for the symptoms in question (for example, migraine or seizure) or if the CVE occurred after aortic valve replacement (AVR). The analysis was limited to ischemic cerebral and retinal infarction, and patients with transient ischemic attacks and hemorrhagic stroke were excluded. CVE type (cerebral infarction, retinal infarction, transient ischemic attack, or hemorrhagic stroke), cerebral infarction or retinal infarction pathogenesis (large artery atherosclerosis, cardioembolic, lacunar, other, or unknown), ischemia localization (supratentorial, infratentorial, or retinal), and cerebral or retinal infarction recurrence before AVR were determined based on documented clinical assessment and available diagnostic and imaging studies.
Cerebrovascular Event Definitions
Cerebral infarction was defined as rapid onset of a focal neurologic deficit persisting for >24 hours presumed secondary to altered perfusion of a known cerebral, cerebellar, or brainstem territory with or without diagnostic imaging (computed tomography or magnetic resonance imaging). Diagnostic imaging with radiographic signs of infarction compatible with the clinical signs and symptoms was available in 93% of the patients with cerebral infarction.
Retinal circulation infarction, caused by central or branch retinal artery occlusion, was defined based on a visual field defect consistent with retinal ischemia persisting for >24 hours, often corroborated with funduscopic examination by an ophthalmologist, and when imaging with computed tomography or magnetic resonance imaging was performed, no other pathogenesis for the vision loss was defined. Funduscopic examination, computed tomography, or magnetic resonance imaging was available in 77% of the patients with retinal infarction.
Undefined CVE was a recorded history of CVE without supporting documentation in the medical record.
Cerebral and Retinal Infarction Characterization
Cerebral and retinal infarction characterization was based on expert neurologist opinion and their documentation in the medical record.
To examine clinical, echocardiographic, and outcome differences between patients with different cerebral or retinal infarction pathogeneses, patients were placed into 3 subgroups for comparison: (1) patients with large artery atherosclerotic or lacunar infarction (LAi); (2) patients with non‐valve‐related, non‐large artery embolic infarction (nLAi); and (3) patients with infarction from an embolism from a degenerative calcific BAV (BAVi).
The nLAi cohort included patients with embolic infarction that was cardioembolic, such as atrial fibrillation, or deep venous thrombosis with patent foramen ovale or atrial septal defect, or embolic from unknown source. Infarction pathogenesis was considered unknown if the neurologist documentation did not give a clear opinion. In some patients with unknown infarction pathogenesis, the neurologists suspected the mechanism was embolic, although the embolic source was unknown. These patients with embolic infarction with unknown embolic sources were further divided into those with potential embolic sources or embolic stroke of undetermined source (ie, ESUS). Patients were labeled as ESUS based on the following criteria: a nonlacunar stroke, absence of ≥50% atherosclerotic luminal stenosis in the arteries supplying the area of ischemia, no other specific pathogenesis identified, and no major‐risk cardioembolic risk factors (including atrial fibrillation, intracardiac thrombus, cardiac tumor, mitral stenosis, recent myocardial infarction, left ventricular systolic dysfunction, or valvular vegetations). 10
The BAVi cohort included patients with cerebral or retinal infarction diagnosed by the neurologist as valve‐related with characteristic calcific aortic valve degeneration on echocardiogram and no other more likely sources of embolism.
Cerebral or retinal infarction was determined to be recurrent based on documentation by neurologist in the medical record of a new neurological deficit fitting criteria for infarction as outlined above, separated in time from the index infarction event.
Clinical, Echocardiographic, and Outcome Data
Clinical data were as obtained from the electronic medical record. The CHA2DS2‐VASc score was calculated. Carotid artery stenosis was defined as an internal carotid artery stenosis of ≥50%. Data from the echocardiographic assessment closest to the infarction diagnosis were retrieved from the medical record. Outcomes were incident AVR and overall mortality during follow‐up.
Statistical Analysis
Categorical variables were expressed as numbers (percentage), and means were compared with χ2 or Fisher exact test. Continuous variables were reported as mean±SD or median (interquartile range) and were compared with Student t test. Survival after infarction was estimated using Kaplan–Meier methods. BlueSky Statistics (Chicago, IL) software was used for all data analyses.
Results
Of 5401 patients with BAV diagnosis, there were 194 patients (3.6%) with a diagnosis of CVE. Figure 1 shows a flow chart of CVE characterization. There were sufficient data to characterize the CVE in 112/194 patients (58%); CVE was ischemic in 108/112 patients (96%) (70 cerebral infarctions, 13 retinal infarctions, and 25 transient ischemic attacks) and hemorrhagic in 4/112 (4%).
Figure 1. Cerebrovascular event characterization flow chart.
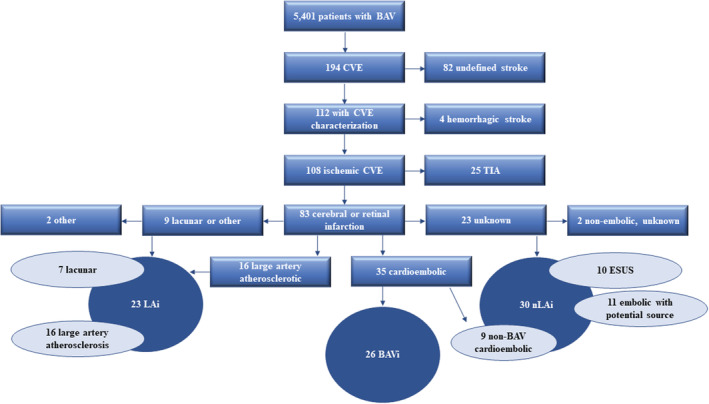
BAV indicates bicuspid aortic valve; BAVi, cerebral or retinal infarction caused by calcific BAV thromboembolism; CVE, cerebrovascular event; ESUS, embolic stroke of undetermined source; LAi, non‐BAV, large artery atherosclerotic or lacunar cerebral or retinal infarction; nLAi, non‐BAV, non‐large artery embolic cerebral or retinal infarction; and TIA, transient ischemic attack.
The focus of the analyses and outcomes presented below is on the 83 patients with confirmed cerebral or retinal infarction. The mean age of the 83 patients was 54±12 years, and 28% were female (Table).
Table 1.
Infarction, Clinical, and Echocardiographic Characteristics and Outcomes
| Total (N=83) | BAVi (N=26) | LAi (N=23) | P value versus BAVi | nLAi (N=30) | P value versus BAVi | |
|---|---|---|---|---|---|---|
| Embolic infarction, N (%) | 72 (87) | 26 (100) | 16 (70) | 0.002† | 30 (100) | 1.0 |
| Recurrent infarction, N (%) | 34 (41) | 13 (50) | 7 (30) | 0.164 | 14 (47) | 0.803 |
| Cerebral and retinal infarction pathogenesis | ||||||
| Large artery atherosclerosis, N (%) | 16 (19) | 16 (70) | ||||
| Cardioembolic, N (%) | 35 (42) | 26 (100) | 9 (30) | |||
| BAVi, N (%) | 26 (31) | |||||
| Lacunar, N (%) | 7 (8) | 7 (30) | ||||
| Other, N (%) | 2 (2) | |||||
| Unknown, N (%) | 23 (28) | 21 (70) | ||||
| ESUS, N (%) | 10 (12) | 10 (33) | ||||
| Cerebral and retinal infarction localization | ||||||
| Supratentorial, N (%) | 56 (68) | 15 (58) | 15 (65) | 0.590 | 23 (77) | 0.129 |
| Retinal, N (%) | 13 (16) | 10 (39) | 3 (13) | 0.044† | 0 | 0.002† |
| Infratentorial, N (%) | 9 (11) | 1 (4) | 2 (9) | 0.480 | 5 (17) | 0.122 |
| Unknown, N (%) | 5 (6) | 0 | 3 (13) | 0.057 | 2 (7) | 0.180 |
| Clinical characteristics | ||||||
| Age at infarction, y, mean±SD | 54±12 | 52±17 | 58±9 | 0.128 | 54±9 | 0.587 |
| Female, N (%) | 23 (28) | 8 (31) | 5 (22) | 0.475 | 8 (27) | 0.735 |
| Diabetes, N (%) | 14 (17) | 2 (8) | 6 (26) | 0.082 | 6 (20) | 0.189 |
| Hyperlipidemia, N (%) | 43 (52) | 10 (39) | 17 (74) | 0.013† | 13 (43) | 0.712 |
| Peripheral vascular disease, N (%) | 27 (33) | 4 (15) | 12 (52) | 0.006† | 10 (33) | 0.122 |
| Heart failure, N (%) | 5 (6) | 0 | 0 | 1.0 | 5 (17) | 0.029† |
| Atrial fibrillation or atrial flutter, N (%) | 11 (13) | 2 (8) | 0 | 0.174 | 9 (30) | 0.036† |
| CHA2DS2‐VASc score, mean±SD | 1.8±1.2 | 1.4±1.2 | 2.3±1.2 | 0.005† | 1.8±1.2 | 0.188 |
| Carotid stenosis, N (%) | 16 (19) | 0 | 14 (61) | <0.001† | 2 (7) | 0.180 |
| ASD or PFO, N (%) | 6 (7) | 0 | 0 | 1.0 | 6 (20) | 0.016† |
| DVT history, N (%) | 7 (8) | 0 | 1 (4) | 0.283 | 6 (20) | 0.016† |
| On antiplatelet, N (%) | 34 (44) | 8 (32) | 13 (57) | 0.087 | 13 (50) | 0.192 |
| On anticoagulation, N (%) | 5 (6) | 0 | 1 (4) | 0.292 | 4 (14) | 0.049† |
| Echocardiographic characteristics* | ||||||
| Aortic valve calcification, N (%) | 37 (59) | 21 (100) | 5 (31) | <0.001† | 12 (52) | 0.001† |
| Moderate to severe AS, N (%) | 25 (39) | 13 (62) | 7 (44) | 0.272 | 4 (16) | 0.003 |
| LV ejection fraction, mean±SD | 62±8 | 63±6 | 63±5 | 0.649 | 59±11 | 0.163 |
| Aortic valve velocity, m/s, mean±SD | 3.0±1.0 | 3.5±0.9 | 3.2±0.9 | 0.348 | 2.5±1.0 | 0.004† |
| Aortic valve mean gradient, mm Hg, mean±SD | 27±17 | 34±18 | 27±14 | 0.175 | 21±18 | 0.028† |
| Aortic valve area, cm2, mean±SD | 1.7±0.8 | 1.6±0.8 | 1.5±0.6 | 0.534 | 2.0±0.9 | 0.113 |
| Atrial septal aneurysm, N (%) | 2 (3) | 0 | 0 | 1.0 | 2 (9) | 0.167 |
| Outcomes | ||||||
| Aortic valve replacement, N (%) | 51 (61) | 22 (85) | 15 (65) | 0.115 | 13 (43) | 0.001† |
| Days between infarction and AVR, median (IQR) | 821 (39–2791) | 52 (14–945) | 851 (649–3112) | 0.014† | 2701 (1628–6145) | <0.001† |
| Indication for AVR is infarction, N (%) | 25 (49) | 20 (91) | 4 (27) | <0.001† | 1 (8) | <0.001† |
AS indicates aortic stenosis; ASD, atrial septal defect; AVR, aortic valve replacement; BAV, bicuspid aortic valve; BAVi, cerebral or retinal infarction caused by calcific BAV thromboembolism; DVT, deep vein thrombosis; ESUS, embolic stroke of undetermined source; IQR, interquartile range; LAi, non‐BAV, large artery atherosclerotic or lacunar cerebral or retinal infarction; LV, left ventricle; nLAi, non‐BAV, non‐large artery embolic cerebral or retinal infarction; and PFO, patent foramen ovale.
Complete echocardiographic data were available overall in 64/83 patients (77%); these data were available in 21/26 (81%) patients with BAVi, 16/23 (70%) patients with LAi, and 25/30 (83%) patients with nLAi. Missing data results in the sum of the columns not equal to the total N.
P < 0.05.
Cerebral and Retinal Infarction Characteristics
The pathogenesis and location of cerebral or retinal infarction are shown in the Table. The most common pathogenesis was cardioembolic, in 35/83 (42%). The most common infarction location overall was supratentorial in 56/83 (68%). Overall, LAi occurred in 23/83 (28%), nLAi in 30/83 (36%), and BAVi in 26/83 (31%) (Figure 1).
The mechanism of infarction was embolic in 72/83 patients (87%) and of those, 59 were cerebral infarctions and 13 were retinal infarctions; retinal infarction was more common in BAVi. Figure 2 shows the embolic sources, which was most commonly a calcific BAV in 26/72 (36%). The embolic source of all instances of retinal infarction was known, but the embolic source of cerebral infarction was unknown in 21/59 patients (36%); of these, there were 11/21 (52%) patients with potential embolic sources (4/11 atrial fibrillation, 3/11 large artery atherosclerosis, 3/11 deep venous thrombosis, and 6/11 abnormal hypercoagulable state) and 10/21 (48%) patients who lacked any potential embolic source (other than a degenerative calcific BAV) and met criteria for ESUS.
Figure 2. Cerebral and retinal infarction in patients with bicuspid aortic valve.
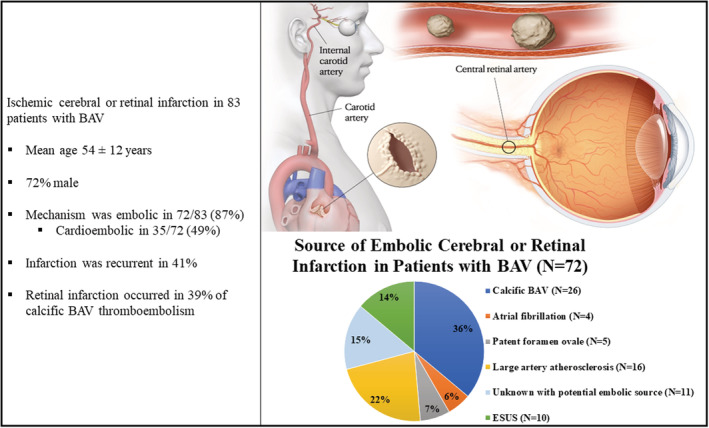
Of 5401 patients with BAV diagnosis, there were 83 patients with confirmed ischemic cerebral or retinal infarction. The mechanism of infarction was embolic in 72/83 (87%). Greater than one‐third of embolic infarction was diagnosed as caused by a calcific BAV, while 35% was from a non‐BAV source, and 29% was from an unknown embolic source. Patients with a calcific BAV embolism commonly had a retinal infarction. BAV indicates bicuspid aortic valve; and ESUS, embolic stroke of undetermined source.
Recurrent cerebral or retinal infarction before AVR occurred in 41% (34/83) of patients with infarction overall, and recurrent infarction occurred in 50% (13/26) of patients with BAVi.
Patient Clinical Characteristics
Noteworthy clinical characteristics of patients overall and stratified by BAVi, LAi, and nLAi are shown in the Table. Patients with BAVi, compared with patients with LAi, had lower prevalence of hypertension, hyperlipidemia, peripheral vascular disease, carotid stenosis, and a lower mean CHA2DS2‐VASc score. Patients with BAVi, compared with patients with nLAi, had less heart failure, atrial fibrillation or atrial flutter, atrial septal defect or patent foramen ovale, and history of deep venous thrombosis.
Echocardiographic Characteristics
Echocardiographic data are shown in the Table. Other than more prevalent AVC, there were no echocardiographic differences between patients with BAVi and LAi, including moderate‐to‐severe underlying AS. However, patients with BAVi, compared with patients with nLAi, were more likely to have AVC and moderate‐to‐severe AS with higher aortic valve velocities and aortic valve mean gradients.
Outcomes
There were 51/83 patients (61%) who underwent AVR during follow‐up, as shown in the Table. The rate of AVR was not different in patients with BAVi compared with patients with LAi. However, patients with BAVi were more likely to undergo AVR compared with patients with nLAi. Patients with BAVi underwent AVR sooner after infarction compared with both LAi and nLAi.
Overall, 42/83 patients (51%) died during follow‐up, on average 15 years after the diagnosis of cerebral or retinal infarction. There was no difference in mortality between patients with BAVi compared with patients with LAi (P=0.495) nor patients with nLAi (P=0.473). The cause of death was known in 27/42 patients (64%), and 15/27 (56%) were cardiac deaths. There was no difference in rates of cardiac death based on infarction pathogenesis.
Discussion
This study analyzed the characteristics of CVE in >5000 patients with a diagnosis of native BAV at Mayo Clinic, Rochester over 40 years. The main findings were that in patents with a BAV: (1) the most common mechanism of confirmed cerebral or retinal infarction was cardioembolism, frequently from a calcific BAV (BAVi); (2) recurrent infarction was common; and (3) patients with a BAVi had similarly severe AS compared with patients with large artery atherosclerotic or lacunar infarction (LAi) but more severe AS compared with patients with other cardioembolic or unknown embolic sources of infarction (nLAi).
Previous studies on the association between AVC or AS and risk of stroke have yielded mixed results. 4 , 5 , 6 , 7 , 8 However, these studies did not specify valve morphology or attempt to identify source of stroke, 4 , 5 , 6 , 7 , 8 and most did not report severity of AS. 4 , 5 , 8 The current study provides comprehensive data on both valve and non‐valve‐related cerebral and retinal infarctions in the context of an underlying BAV in a large cohort of relatively young patients.
It is thought that valve disease is a marker of other atherosclerotic disease that increases risk of CVE. 6 , 11 , 12 However, the most common pathogenesis of cerebral or retinal infarction in our cohort was cardioembolism from a degenerative calcific BAV (BAVi). The BAV is unique in that the abnormal valve geometry leads to progressive valve degeneration and AVC early in life, and the risk of calcific material or thrombi dislodgement is increased by asymmetric systolic opening, high shear stress, abnormal systolic flow patterns, and mixed stenosis and regurgitation. 13 Accordingly, patients with BAVi in this cohort had low CHA2DS2‐VASc scores and lower prevalence of other traditional risk factors for cerebral or retinal infarctions, which provides further evidence that the origin of infarction was likely from a valve‐related embolism in these patients. In addition, the reduced cardiovascular risk factors in patients with BAVi is interesting in light of the findings in the Danish retrospective cohort study in which the relative risk of ischemic stroke among patients with a CHA2DS2‐VASc of 0 with AS compared with age‐ and sex‐matched controls without AS was increased at 2.37 (95% CI, 2.04–2.75), 5 highlighting the increased risk of stroke among patients with AS even in the absence of other cardiovascular risk factors.
The source of embolic cerebral infarction was unknown in 21 patients. These patients had moderate AS, over two‐thirds had degenerative AVC, and about half lacked a potential nonvalvular source of embolism and met criteria for ESUS. Cardiac valve disease is considered one of the potential embolic sources in ESUS, 10 and incident stroke from a calcific aortic valve has previously been thought to be underestimated. 1 , 9 Therefore, we may be underestimating the true prevalence of BAVi in this study. Unfortunately, the source of cerebral infarction in these patients could not be reliably confirmed even after extensive evaluation. Interestingly, infarction recurrence was similarly common in patients where the source of embolic infarction was unknown (52%) as in those with BAVi (50%).
Patients with BAVi were likely to have moderate‐to‐severe AS and underwent AVR soon after infarction. In half of these patients, there was recurrent infarction before AVR. Embolic cerebral or retinal infarction is not currently factored into decision‐making regarding AVR for AS, but AVR has been shown to reduce rates of stroke in patients with AS. 5 In clinical practice, an argument for AVR could be made in certain patients with less than severe AS, but with incident unexplained embolic infarction and valvular calcification, especially in patients with low surgical risk. 1 Alternatively, anticoagulation could be considered when thrombus is suspected. 2 However, the use of anticoagulation therapy to prevent infarction in moderate valvular heart disease in the absence of another indication (such as atrial fibrillation) has not been studied, and trials on the use of anticoagulation in ESUS have not demonstrated efficacy. 10
Lastly, infarction from a calcific BAV was more commonly located in the retinal circulation compared with infarction from an alternate source. Interestingly, the retinal arteries are an often‐cited destination of embolism in the case reports and case series describing calcific embolism from an aortic valve. 1 , 9 In a large series of slightly older patients than in our cohort with retinal artery occlusion, embolism was the most common cause. 14 The most common source of embolism was carotid artery plaque followed by cardioembolism, and there was often overlap of abnormal carotid imaging and aortic valve calcification on echocardiogram in these patients. 14 Examination of cardiovascular disease in younger patients with retinal arterial occlusion has also shown aortic valve stenosis to be the most frequent underlying valve lesion. 15
Limitations
This is an observational, retrospective study from a single tertiary care center spanning a long period. Of the 194 patients with CVE, 82/194 patients (42%) were self‐reported and there was not sufficient data (clinical history, neurologic imaging, or echocardiographic data) to characterize the CVE or to compare them to the 112/194 patients (58%) for whom we could characterize the CVE. Given the small sample size of 83 patients, the statistical power to identify differences was limited. Importantly, the true prevalence of BAVi may be underestimated even in these patients we could characterize, because patients with BAVi may have been labeled as non‐BAVi because of the presence of common comorbidities associated with CVE. Because of the retrospective design, we could not evaluate whether AVR reduced infarction recurrence. Finally, this study is not a comparative study of CVE in patients with underlying BAV versus tricuspid aortic valve, but the study does provide additional insights for clinical practice when evaluating patients with CVE and underlying BAV.
Conclusions
In this cohort of patients with BAV, the most common type of cerebral or retinal infarction was cardioembolic, frequently because of embolism related to degenerative calcification of the aortic valve. These patients commonly had recurrent cerebral or retinal ischemic events and moderate‐to‐severe valve disease, which prompted AVR soon after infarction. Cardioembolism from a calcific BAV should be recognized as an important potential complication in the natural history of BAV.
Sources of Funding
None.
Disclosures
None.
For Sources of Funding and Disclosures, see page 7.
REFERENCES
- 1. Khetarpal V, Mahajan N, Madhavan R, Batra S, Mopala P, Sagar A, Rapolu P, Nangia S, Afonso L. Calcific aortic valve and spontaneous embolic stroke: a review of literature. J Neurol Sci. 2009;287:32–35. doi: 10.1016/j.jns.2009.07.018 [DOI] [PubMed] [Google Scholar]
- 2. Shabtaie SVV, Michelena HI. Spontaneous and simultaneous bicuspid aortic valve and aorta thrombosis. J Heart Valve Dis. 2018;27:173–177. [Google Scholar]
- 3. Mahajan N, Khetarpal V, Afonso L. Stroke secondary to calcific bicuspid aortic valve: case report and literature review. J Cardiol. 2009;54:158–161. doi: 10.1016/j.jjcc.2008.10.011 [DOI] [PubMed] [Google Scholar]
- 4. Fashanu OE, Bizanti A, Al‐Abdouh A, Zhao D, Budoff MJ, Thomas IC, Longstreth WT Jr, Michos ED. Progression of valvular calcification and risk of incident stroke: the Multi‐Ethnic Study of Atherosclerosis (MESA). Atherosclerosis. 2020;307:32–38. doi: 10.1016/j.atherosclerosis.2020.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Andreasen C, Gislason GH, Kober L, Abdulla J, Martinsson A, Smith JG, Torp‐Pedersen C, Andersson C. Incidence of ischemic stroke in individuals with and without aortic valve stenosis: a Danish retrospective cohort study. Stroke. 2020;51:1364–1371. doi: 10.1161/STROKEAHA.119.028389 [DOI] [PubMed] [Google Scholar]
- 6. Boon A, Lodder J, Cheriex E, Kessels F. Risk of stroke in a cohort of 815 patients with calcification of the aortic valve with or without stenosis. Stroke. 1996;27:847–851. doi: 10.1161/01.STR.27.5.847 [DOI] [PubMed] [Google Scholar]
- 7. Petty GW, Khandheria BK, Whisnant JP, Sicks JD, O'Fallon WM, Wiebers DO. Predictors of cerebrovascular events and death among patients with valvular heart disease: a population‐based study. Stroke. 2000;31:2628–2635. doi: 10.1161/01.STR.31.11.2628 [DOI] [PubMed] [Google Scholar]
- 8. Bos D, Bozorgpourniazi A, Mutlu U, Kavousi M, Vernooij MW, Moelker A, Franco OH, Koudstaal PJ, Arfan Ikram M, van der Lugt A. Aortic valve calcification and risk of stroke: the Rotterdam Study. Stroke. 2016;47:2859–2861. doi: 10.1161/STROKEAHA.116.015200 [DOI] [PubMed] [Google Scholar]
- 9. Holley KE, Bahn RC, McGoon DC, Mankin HT. Spontaneous calcific embolization associated with calcific aortic stenosis. Circulation. 1963;27:197–202. doi: 10.1161/01.CIR.27.2.197 [DOI] [PubMed] [Google Scholar]
- 10. Ntaios G. Embolic stroke of undetermined source: JACC review topic of the week. J Am Coll Cardiol. 2020;75:333–340. doi: 10.1016/j.jacc.2019.11.024 [DOI] [PubMed] [Google Scholar]
- 11. Greve AM, Dalsgaard M, Bang CN, Egstrup K, Ray S, Boman K, Rossebø AB, Gohlke‐Baerwolf C, Devereux RB, Køber L, et al. Stroke in patients with aortic stenosis: the simvastatin and ezetimibe in aortic stenosis study. Stroke. 2014;45:1939–1946. doi: 10.1161/STROKEAHA.114.005296 [DOI] [PubMed] [Google Scholar]
- 12. Adler Y, Levinger U, Koren A, Tanne D, Fink N, Vaturi M, Iakobishvili Z, Battler A, Zelikovski A, Sagie A. Relation of nonobstructive aortic valve calcium to carotid arterial atherosclerosis. Am J Cardiol. 2000;86:1102–1105. doi: 10.1016/S0002-9149(00)01167-X [DOI] [PubMed] [Google Scholar]
- 13. Katayama S, Umetani N, Hisada T, Sugiura S. Bicuspid aortic valves undergo excessive strain during opening: a simulation study. J Thorac Cardiovasc Surg. 2013;145:1570–1576. doi: 10.1016/j.jtcvs.2012.05.032 [DOI] [PubMed] [Google Scholar]
- 14. Hayreh SS, Podhajsky PA, Zimmerman MB. Retinal artery occlusion: associated systemic and ophthalmic abnormalities. Ophthalmology. 2009;116:1928–1936. doi: 10.1016/j.ophtha.2009.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wilson LA, Warlow CP, Russell RW. Cardiovascular disease in patients with retinal arterial occlusion. Lancet. 1979;1:292–294. doi: 10.1016/S0140-6736(79)90704-9 [DOI] [PubMed] [Google Scholar]


