Abstract
OBJECTIVE:
To estimate the incidence of gestational trophoblastic neoplasia following complete and partial molar pregnancy after reaching normal human chorionic gonadotropin (hCG) levels to guide evidence-based follow-up recommendations.
DATA SOURCES:
MEDLINE, EMBASE, Web of Science, POPLINE, Cochrane, and ClinicalTrials.gov were searched from inception to November 2018, using the intersection of “gestational trophoblastic disease,” “molar pregnancy,” and “human chorionic gonadotropin” themes.
METHODS OF STUDY SELECTION:
Search results were screened to identify cohort studies of molar pregnancy reporting gestational trophoblastic neoplasia development, with at least 6 months of intended normal hCG follow-up.
TABULATION, INTEGRATION, AND RESULTS:
Two reviewers independently identified articles for inclusion. Data were extracted using a standardized form. For meta-analysis, cumulative incidence of gestational trophoblastic neoplasia, with CIs by the Agresti-Coull method, and pooled risk ratios (RRs) comparing complete and partial mole were calculated. Among the 19 eligible studies that reported adequate data for inclusion in the primary meta-analysis, we found low incidence of gestational trophoblastic neoplasia after normal hCG level following both complete mole (64/18,357, 0.35%, 95% CI 0.27–0.45%), and partial mole (5/14,864, 0.03%, 95% CI 0.01–0.08%). There was a significantly higher risk of gestational trophoblastic neoplasia after complete compared with partial molar pregnancy (RR 4.72, 95% CI 1.81–12.3, P = .002). Among gestational trophoblastic neoplasia cases after normal hCG level following complete mole, 89.6% occurred when the time from evacuation to normalization was 56 days or longer, and 60.7% were diagnosed beyond the commonly recommended 6-month surveillance interval. Sensitivity analyses, including those limiting to studies at low risk of bias, did not significantly affect results. We found an overall incidence of gestational trophoblastic neoplasia of 15.7% for complete mole (1,354/8,611, 95% CI 15.0–16.5%) and 3.95% for partial mole (221/5,593, 95% CI 3.47–4.50%).
CONCLUSION:
Gestational trophoblastic neoplasia development after normal hCG level following molar pregnancy is rare. Recommendations for frequency and duration of hCG follow-up can be minimized to lessen burden on patients and informed by the type of molar pregnancy and time interval from uterine evacuation to hCG normalization.
SYSTEMATIC REVIEW REGISTRATION:
PROSPERO, CRD42019116414.
Hydatidiform mole, or molar pregnancy, is an uncommon, genetically abnormal pregnancy that occurs in approximately 1 in 700 (partial mole) to 1 in 2,000 (complete mole) pregnancies.1 Complete and partial molar pregnancies are distinct pathologic entities with unique genetic and risk profiles. Both pathologies put the woman at risk of developing gestational trophoblastic neoplasia, a form of locally invasive or metastatic malignancy arising from the abnormal products of conception.
The recommended treatment of any suspected molar pregnancy, usually identified on the basis of ultrasound findings, is with uterine evacuation. Post-procedural care includes monitoring the β-hCG level in the blood or urine for evidence of residual or progressing disease.2,3 Clinical recommendations for follow-up surveillance after evacuation of molar pregnancy are variable. The American College of Obstetricians and Gynecologists previously recommended testing serum human chorionic gonadotropin (hCG) levels every 1–2 weeks until normal (less than international units/L), then at 1–2-month intervals for 6–12 months.4 The American College of Obstetricians and Gynecologists now defers to International Federation of Gynecology and Obstetrics (FIGO) recommendations for two consecutive undetectable hCG levels separated by 1 month after partial mole and six consecutive undetectable hCG levels at monthly intervals after complete mole.5 The Royal College of Obstetricians and Gynaecologists recommends 6 months of hCG follow-up after either uterine evacuation or first normal hCG level, with no specified frequency.6 Only the FIGO guidelines distinguish between molar pregnancy types, despite the substantial difference in risk of gestational trophoblastic neoplasia development of 15% for complete moles compared with 5% for partial moles.2,3
Practically, the prolonged follow-up with frequent health system contacts is difficult for patients. A study conducted at Cook County Hospital in Chicago found only 18% of its urban, primarily low-income patient population were able to complete follow-up surveillance as recommended.7 Furthermore, the duration of follow-up is not without harm and may have psychological and physical effects on patients, including depression and anxiety during the follow-up period regarding cancer risk, delayed childbearing, and concerns for poor future pregnancy outcomes.8–10
Thus, our primary objective was to compare the cumulative incidence and pattern of gestational trophoblastic neoplasia after reaching normal hCG levels following complete and partial molar pregnancy, to guide evidence-based follow-up recommendations that are not excessively burdensome to patients. Secondarily, we sought to compile a robust estimate of overall incidence of gestational trophoblastic neoplasia following molar pregnancy.
SOURCES
A standard methodology was used to perform our search and analyses and report our findings, following the published MOOSE (Meta-analysis of Observational Studies in Epidemiology) guidelines.11 Reviewers all are practicing or in-training gynecologists, including fellowship-trained experts in family planning (S.S., and C.A.S.) and gynecologic oncology (E.M.K.). A biomedical librarian was consulted for development of the search strategy. The MEDLINE (via Ovid), EMBASE, Web of Science, POPLINE, Cochrane (CDSR and CENTRAL), and ClinicalTrials.gov databases were queried from inception through November 12, 2018. MeSH terms and keywords were used to populate thematic sets related to “Gestational Trophoblastic Disease,” “Molar Pregnancy,” and “human chorionic gonadotropin,” then the Boolean term “AND” was used to find the intersection. See Appendix 1, available online at http://links.lww.com/AOG/B656, for details of included MeSH headings and keywords. Limits or restrictions on time or language were not used during the initial search process. The original protocol was registered and published in the PROSPERO international prospective register of systematic reviews (2019 CRD42019116414).12
STUDY SELECTION
We required that studies meet the following eligibility criteria: 1) the study represented a retrospective or prospective cohort study (study type); 2) the cohort consisted of patients with complete or partial molar pregnancy diagnosed on ultrasound examination or with histology or genetics (study population); 3) the study reported development of gestational trophoblastic neoplasia by following patients with serial serum hCG measurement (study data); 4) the study followed patients, with intended follow-up of at least 6 months after first undetectable hCG level (study outcome); 5) the study was published in manuscript format, was available in English, and did not represent duplicative data with other included studies. In incidences of overlapping data, the study with more recent or complete data was retained. References from included studies were manually assessed for additional unique eligible studies.
After removing duplicate records from the initial search, title and abstracts were independently manually reviewed and clearly irrelevant studies excluded by two reviewers (B.B.A. and J.S.). Full text was then independently reviewed for all potentially relevant studies by the same investigators to determine final eligibility, with disagreements resolved through consultation with a third reviewer (S.S.). Data extraction, including methodologic quality assessment, was completed using a standardized form by a combination of three reviewers (B.B.A., J.S., and S.S.), with any disagreements or uncertainties resolved by consensus discussion among the authors. Study authors were not contacted to obtain additional unpublished data. The quality of included studies was assessed using the Cochrane Tool to Assess Risk of Bias in Cohort Studies, which uses predefined questions to assess eight different domains.13,14 For the category of “Was follow-up adequate?” studies were considered to have inadequate follow-up if more than 25% of total patients were lost to follow-up before 6 months of normal hCG levels.
The primary outcome was cumulative incidence of gestational trophoblastic neoplasia after normal hCG level. The definition of normal hCG level varied by study depending on the sensitivity of the particular hCG assay in use at that time and place, and whether it was a serum or urine assay. Patients who had at least one normal hCG level but were lost to follow-up before completion of 6 months of normal hCG surveillance were included in cohort totals based on the assumption that these patients would have represented to the referral centers trying to follow them if they became symptomatic with disease. Those lost to follow-up before documented normal hCG level were excluded from all analyses.
Additionally, we assessed cumulative incidence of gestational trophoblastic neoplasia within varying time intervals from the first normal hCG level to diagnosis of disease (less than 1, 1–6, less than 6, more than 6 months), for studies reporting in that detail. We also classified cases of gestational trophoblastic neoplasia by time from uterine evacuation to hCG normalization (less than 56, 56 days or more) when reported. This timeframe was based on the 2010 Royal College of Obstetricians and Gynaecologists’ guidelines on management of gestational trophoblastic neoplasia, which recommend a longer length of surveillance for patients with 56 days (8 weeks) or longer from evacuation to first normal hCG level.6 Secondary outcomes included the overall incidence of gestational trophoblastic neoplasia (including cases identified before normal hCG level), presenting symptoms of gestational trophoblastic neoplasia beyond 6 months of hCG surveillance, and morbidity or mortality from development of gestational trophoblastic neoplasia after normal hCG level.
For binomial proportions, the Agresti-Coull method was used to calculate 95% CIs in STATA 15.1.15 For outcomes that were reported with adequate detail for statistical pooling, RevMan 5.316 was used to create summary estimates and calculate pooled risk ratios (RRs) between complete and partial molar pregnancy. Pooled RRs were calculated from the sum of complete and partial molar pregnancy cases across all studies, including studies that reported only on either partial or complete molar pregnancy, and, thus, had no internal RR calculated. Owing to the more conservative assumptions about the similarity of included studies and expected variation in type, length, and completeness of follow-up across studies, we used random effects modeling to minimize the risk of type 1 error.13 Heterogeneity between studies was assessed with Higgins I2. Publication bias was assessed graphically using funnel plots.
Sensitivity analyses were performed to assess the consistency and reliability of results. Sensitivity of results to risk of bias was assessed with analyses excluding studies considered to be high risk of bias for relevant categories. For studies that did not clearly differentiate type of antecedent molar pregnancy (complete vs partial), sensitivity analyses were conducted assuming that antecedent pregnancies were either all complete mole, or equally divided between complete and partial moles, to assess for the range of possible results. Lastly, sensitivity analyses were conducted excluding studies with nonstandard upfront treatment (ie, chemotherapy), and studies using urinary hCG or a cutoff greater than 5 international units/L. Studies not reporting a specific cutoff for normal hCG level were assumed to have sensitivity to 5 international units/L if included years were 2000 or after, given that this test was widely available by 1995.17
RESULTS
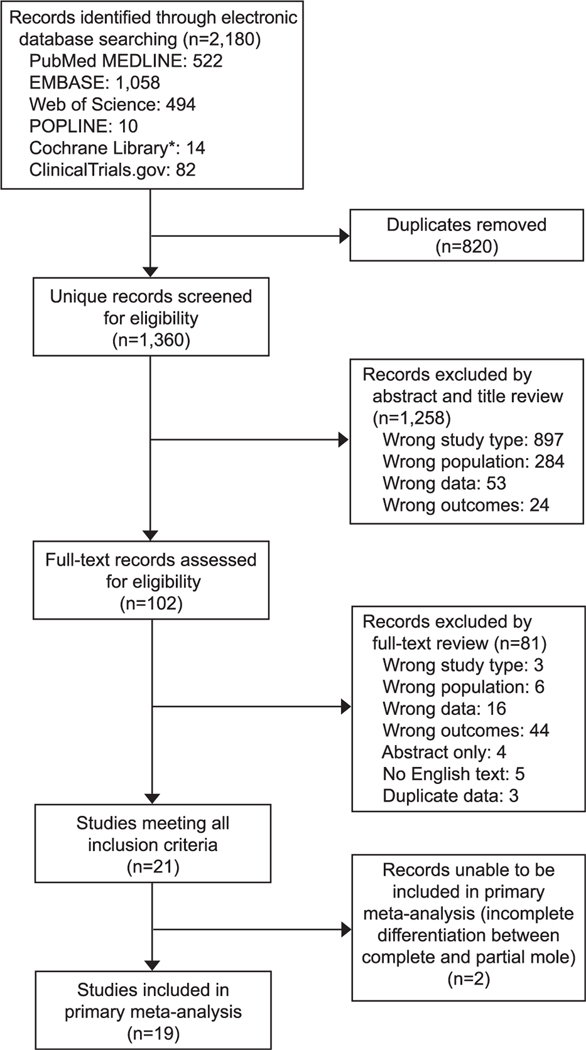
A total of 2,180 records of potential interest were identified from the search of electronic databases described above (Fig. 1). After eliminating duplicate records, 1,360 unique records were screened for eligibility. A total of 1,258 records were excluded by abstract and title review, with the majority found to be the wrong study type (review papers, case-control studies, randomized studies of interventions, and case reports), or wrong population, with many records representing cohort studies of patients with gestational trophoblastic neoplasia rather than molar pregnancy.
Fig. 1.
Study selection flow diagram. *Cochrane Library includes the following databases: CENTRAL (Cochrane Central Register of Controlled Trials) and CDSR (Cochrane Database of Systematic Reviews).
A total of 102 records required assessment of the full text for eligibility determination. The majority of exclusions at this stage were because the study did not report on hCG follow-up (wrong data) or did not follow the hCG level with intended follow-up of 6 months beyond the first normal value (wrong outcome). Finally, a total of 12 studies were excluded for being published only in abstract format because there was no available English version of the full text, or because the study included duplicate data (data from the same patient population or trophoblastic disease center, overlapping in time).
A total of 21 studies met all inclusion criteria (Table 1).17–38 Two of these studies were unable to be included in the primary meta-analysis owing to incomplete information differentiating the type of antecedent molar pregnancy (partial vs complete) in cases of gestational trophoblastic neoplasia.30,33 No additional relevant studies representing unique data were identified for inclusion from reference review.
Table 1.
Characteristics of Included Studies
| Study ID | Location | Years | Molar Pregnancy Diagnosis | Molar Pregnancy Treatment | Normal hCG Definition | Intended Follow-up |
|---|---|---|---|---|---|---|
| Complete and partial molar pregnancy | ||||||
| Alazzam et al, 201119 | United Kingdom | 1989–2008 | Local, otherwise ND, GTN cases central confirmed | Uterine evacuation | Urine less than 50 international units/L | 2 y hCG (CM) 1 year hCG (PM) |
| Batorfi et al, 200420 | Hungary | 1998–2001 | Central, but otherwise ND | Uterine evacuation | Serum “undetectable” | 6 mo hCG (CM) 3–6 mo hCG (PM) |
| Braga et al, 201521 | Brazil | 2002–2013 | Histologic (local), GTN cases central confirmed |
Uterine evacuation | Serum less than 5 international units/L | 6 mo hCG more than 2 y clinical |
| Coyle et al, 201722 | United Kingdom | 1980–2009 | Histologic (central) | Uterine evacuation | Serum “normal” | 6 mo hCG Ongoing clinical |
| Gueye et al, 201425 | Senegal | 2006–2012 | ND | Uterine evacuation vs hysterectomy with or without CT by risk* | Serum less than 5 international units/L | 2 y clinical or hCG |
| Nirmala et al, 201331 | Malaysia | 2005–2010 | Central, otherwise ND | Uterine evacuation | Serum “undetectable” | 6 mo hCG |
| Riadh et al, 200932 | Tunisia | 1991–2007 | Histologic (central) | Uterine evacuation | Serum or urine “Nondetectable” | 1 y hCG |
| Schmitt et al, 201334 | France | 2000–2010 | Histologic (90% central confirmed) | Uterine evacuation | Serum “normal” | 1 y hCG (CM) 6 mo hCG(PM) |
| Usui et al, 201835 | Japan | 2007–2017 | Histologic (central) | Uterine evacuation (98%) Hysterectomy (2%) | Serum less than 1 international units/L | 6 mo hCG |
| Wiesma et al, 200636,37 | Australia | 1992–2001 | Histologic (central) | Uterine evacuation | Urine less than 2.5 international units/L or Serum less than 2 international units/L | 1 y hCG |
| Wolfberg et al, 200417, 200638 | United States | 1973–2003 | Histologic (local) | Uterine evacuation | Serum less than 10 international units/L (before 1993) Serum 5 international units/L (1993 or after) | 6 mo hCG |
| Complete molar pregnancy only | ||||||
| Al-Talib, 201618 | Saudi Arabia | 2005–2014 | Histologic (central) | Uterine evacuation | Serum less than 5 international units/L | 6 mo hCG |
| Fatima et al, 201123 | Pakistan | 1994–1996 | Histologic (central) | Curettage (86%) Hysterectomy (14%), with or without CT by risk* | Serum “normal” | More than 2 y clinical |
| Hoppenot et al, 201626 | United States | 2003–2013 | Histologic + p57 (central) | Uterine evacuation | Serum less than 5 international units/L | 1 y hCG |
| Horn et al, 200627 | Germany | 1985–1996 | Histologic (central) | D&C (95%), hysterectomy (5%) | Serum “normal” | Ongoing clinical |
| Kerkmeijer et al, 200728 | Netherlands | 1994–2004 | ND | Uterine evacuation | Serum less than 2.5 international units/L | 6 mo hCG Ongoing clinical |
| Partial molar pregnancy only | ||||||
| Lavie et al, 200529 | United States | 1983–2003 | Histologic (central) | Uterine evacuation | Serum less than 5 international units/L | 6 mo hCG |
| Undifferentiated complete vs partial molar pregnancy | ||||||
| Matsui et al, 200130 | Japan | 1981–1999 | Histologic (central) | Uterine evacuation | Serum less than 1 international units/L | 2 y clinical |
| Schlaerth et al, 198133 | United States | 1976–1978 | Histologic (central) | Uterine evacuation | Serum “remission” | 6–12 mo hCG |
ND, not described; GTN, gestational trophoblastic neoplasia; hCG, human chorionic gonadotropin; CM, complete mole; PM, partial mole; CT, chemotherapy; D&C, dilation and curettage.
Certain patients given upfront chemotherapy for being “high risk,” see individual references for details.
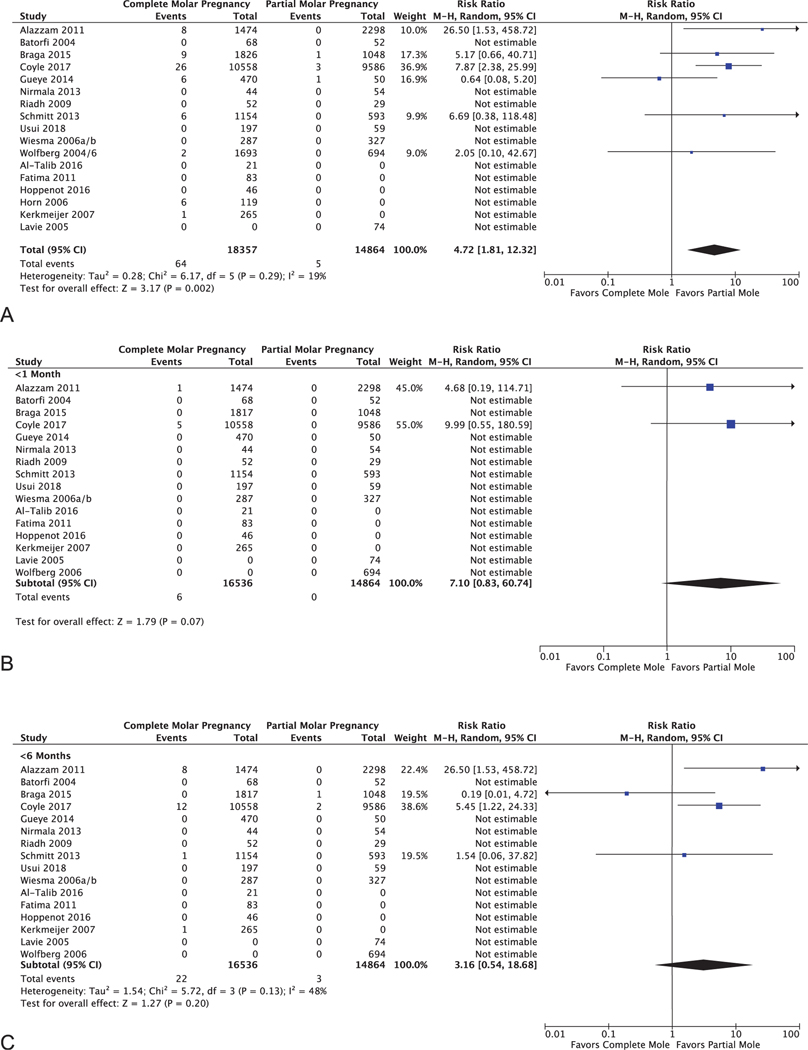
Among the 19 included studies in the primary analysis, nine reported on both complete and partial molar pregnancy,19–22,25,31,32,34,35 five reported only on complete molar pregnancy,18,23,26–28 one reported only on partial molar pregnancy,29 and four studies represented two pairs of reports from the same cohort, over the same time period, on complete and partial molar pregnancy.17,36–38 Among complete molar pregnancy, there were 64 cases of gestational trophoblastic neoplasia after reaching normal hCG level among 18,357 patients (0.35%, 95% CI 0.27–0.45%); among partial molar pregnancy, there were five cases among 14,864 patients (0.03%; 95% CI 0.01–0.08%). Complete molar pregnancies were significantly more likely to develop gestational trophoblastic neoplasia after normal hCG level than partial molar pregnancies (RR 4.72, 95% CI 1.81–12.32, P = .002, I2 = 0.19; Fig. 2A).
Fig. 2.
Forest plot for primary outcome: cumulative incidence of gestational trophoblastic neoplasia following complete vs partial molar pregnancyafter normal hCG level at any point (A), within 1 month of first normal (B), and within 6 months of first normal (C). M-H, Mantel-Haenszel.
The rare cases of gestational trophoblastic neoplasia development after normal hCG level occurred outside of the commonly recommended 6-month follow-up window in 60.7% of cases following complete mole and 40% of cases following partial mole. For complete molar pregnancy, the cumulative incidence of gestational trophoblastic neoplasia diagnosis in the first month after normal hCG level was 6 of 16,545 (0.036%), and in the first 6 months after hCG normalization (during the currently recommended surveillance interval) was 22 of 16,536 (0.13%; 95% CI 0.09–0.20%). Likewise, for partial molar pregnancy, the incidence of gestational trophoblastic neoplasia diagnosis in the first month after normal hCG level was 0 of 14,864 (0%), and in the first 6 months after hCG normalization was 3 of 14,864 (0.02%; 95% CI 0.004–0.06%), respectively (Fig. 2B and C, Tables 2 and 3). Thus, surveillance to 6 months captures 41.0% of cases. Extrapolating further, surveillance to 1 year would capture 59% of cases, surveillance to 18 months would capture 69% of cases, and surveillance to 2 years would capture 77% of cases.
Table 2.
Summary of Cases of Gestational Trophoblastic Neoplasia After Normal Human Chorionic Gonadotropin Level
| Time From First Normal to GTN |
Time to First Normal hCG |
||||||
|---|---|---|---|---|---|---|---|
| Study ID | Cumulative Incidence | Less Than 1 mo | 1–6 mo | Less Than 6 mo | Less Than 56 d | 56 d or more | Morbidity and Mortality |
| Complete mole | |||||||
| Alazzam et al, 201119 | 8/1,474 | 1 | 7 | 0 | NR | NR | NR |
| Braga et al, 201521 | 9/1,826 | 0 | 0 | 9 | 0 | 9 | Death ×1, hysterectomy ×3, lung lobectomy ×1 |
| Coyle et al, 201722 | 26/10,558 | 5 | 7 | 14 | 3 | 23 | NR |
| Gueye et al, 201425 | 6/470 | 0 | 0 | 6 | 0 | 6 | Death ×1 |
| Horn et al, 200627 | 6/119 | NR | NR | NR | NR | NR | NR |
| Kerkmeijer 200728 | 1/265 | 0 | 1 | 0 | 0 | 1 | None |
| Schmitt et al, 201334 | 6/1,154 | 0 | 1 | 5 | 2 | 4 | Progression or exenteration ×1, hysterectomy ×1 |
| Wolfberg et al, 200417 | 2/1,693 | NR | NR | NR | NR | NR | NR |
| Total | 6/56 | 16/56 | 34/56 | 5/48 | 43/48 | ||
| Percent | 10.7 | 28.6 | 60.7 | 10.4 | 89.6 | ||
| Partial mole | |||||||
| Braga et al, 201521 | 1/1,048 | 0 | 1 | 0 | 1 | 0 | None |
| Coyle et al, 201722 | 3/9,586 | 0 | 2 | 1 | 1 | 2 | NR |
| Gueye et al, 201425 | 1/50 | 0 | 0 | 1 | 0 | 1 | Death ×1 |
| Total | 0/5 | 3/5 | 2/5 | 2/5 | 3/5 | ||
| Percent | 0 | 60 | 40 | 40 | 60 | ||
GTN, gestational trophoblastic neoplasia; hCG, human chorionic gonadotropin; NR, not reported.
Data are n/N or n unless otherwise specified.
Studies with no documented cases of GTN after normal hCG level not listed.
Table 3.
Summary of Results of Secondary Outcomes and Sensitivity Analyses for Cumulative Incidence of Gestational Trophoblastic Neoplasia After Complete Compared With Partial Molar Pregnancy
| Outcome | Included Studies | Complete Mole | Partial Mole | RR (95% CI) | Heterogeneity (I2) (%) |
|---|---|---|---|---|---|
| GTN after normal hCG level | |||||
| Overall | 19 | 64/18,357 (0.35) | 5/14,864 (0.03) | 4.72 (1.81–12.3) | 19 |
| By time from normal to GTN | |||||
| Less than 1 mo | 17 | 6/16,545 (0.04) | 0/14,864 (0) | 7.10 (0.83–60.7) | 0 |
| Less than 6 mo | 17 | 22/16,536 (0.13) | 3/14,864 (0.02) | 3.16 (0.54–18.7) | 48 |
| More than 6 mo | 10 | 34/16,194 (0.21) | 2/13,928 (0.01) | 4.36 (0.90–21.1) | 41 |
| Sensitivity to risk of bias* | |||||
| Adequate follow-up | 12 | 61/15,885 (0.38) | 5/13,464 (0.04) | 5.10 (1.70–15.3) | 31 |
| Similar co-interventions | 12 | 51/16,951 (0.30) | 4/14,215 (0.03) | 6.87 (2.85–16.6) | 0 |
| Sensitivity to treatment or assay | |||||
| Standard upfront treatment | 17 | 58/17,402 (0.33) | 4/14,289 (0.03) | 6.87 (2.85–16.6) | 0 |
| Sensitive serum hCG assay | 11 | 22/3,703 (0.59) | 2/1,727 (0.12) | 2.53 (0.53–12.2) | 29 |
| Standard treatment+sensitive serum hCG | 10 | 16/3,233 (0.49) | 1/1,603 (0.06) | 5.78 (1.08–30.9) | 0 |
| Sensitivity to undifferentiated† | |||||
| Assumed all CM | 21 | 74/19,287 (0.38) | 5/14,864 (0.03) | 4.72 (1.81–12.3) | 19 |
| Assumed half CM, half PM | 21 | 69/18,847 (0.37) | 10/15,304 (0.07) | 3.25 (1.17–9.01) | 47 |
| GTN overall | 16 | 1,354/8,611 (15.7) | 221/5,593 (3.95) | 4.64 (3.01–7.13) | 78 |
RR, relative risk; GTN, gestational trophoblastic neoplasia; hCG, human chorionic gonadotropin; CM, complete mole; PM, partial mole.
Data are n or n/N (%) unless otherwise specified.
Standard upfront treatment=uterine evacuation or hysterectomy in select cases.
Sensitive serum hCG assay considered normal at less than 5 international units/L, assumed for serum assays after the year 2000.
Excluding studies at high risk of bias for the indicated categories.
Among 11 studies reporting on gestational trophoblastic neoplasia cases after normal hCG level, eight reported details on time from uterine evacuation to hCG normalization for these cases. We found that the majority of gestational trophoblastic neoplasia diagnoses (43/48 cases [89.6%] for complete mole, and 3/5 cases [60%] for partial mole) were made in cases in which time from evacuation to first normal hCG level was longer than 56 days (8 weeks). See Table 2 for details.
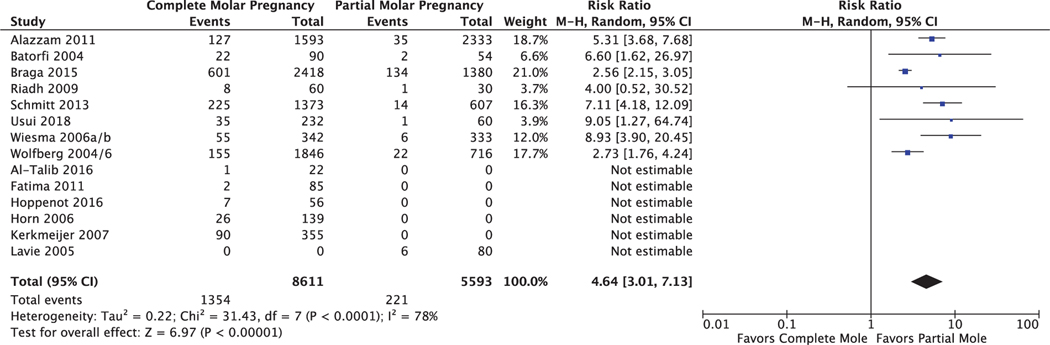
Because this review included the collection of molar pregnancy cohorts with the most complete follow-up (those attempting to follow patients with serial hCG levels for at least 6 months after the first normal value), it represents a robust cohort from which to estimate overall gestational trophoblastic neoplasia incidences following molar pregnancy, including cases in patients without normal hCG levels. Of the 19 studies in the primary analysis, 16 reported numbers of gestational trophoblastic neoplasia cases before normal hCG level with breakdown by molar pregnancy type; the other three studies did not differentiate between complete and partial mole and were excluded from this analysis (Fig. 3). Among included studies, there were 1,354 cases of gestational trophoblastic neoplasia among 8,611 cases of complete molar pregnancy (15.7%; 95% CI 15.0–16.5%) and 221 cases of gestational trophoblastic neoplasia among 5,593 cases of partial molar pregnancy (3.95%; 95% CI 3.47–4.50%). Patients with complete mole were at higher relative risk of gestational trophoblastic neoplasia development (RR 4.64, 95% CI 3.01–7.13, P<.001, I2=78%).
Fig. 3.
Forest plot for secondary outcome: overall cumulative incidence of gestational trophoblastic neoplasia following complete vs partial molar pregnancy. M-H, Mantel-Haenszel.
Only two studies21,34 reported details of the clinical presentation for 14 cases of gestational trophoblastic neoplasia diagnosed beyond the commonly recommended 6 months of hCG surveillance. These included amenorrhea (n = 7), abnormal bleeding (n = 4), hemoptysis (n = 1), dyspnea (n = 1), and nausea (n = 1). Cases of gestational trophoblastic neoplasia after normal hCG level tended to be treatable in most instances. Among 24 patients with reported follow-up on outcomes, there were three deaths, one patient with progression requiring exenteration, and one patient with metastasis requiring lung lobectomy, with no other significant morbidity reported (Table 2).
Studies were assessed across eight different categories for risk of bias (see Appendix 2, available online at http://links.lww.com/AOG/B656). Owing to the simplicity of the outcome and the importance of the raw incidences of gestational trophoblastic neoplasia after normal hCG level following complete and partial molar pregnancy, over the relative risk between the two, there was a low overall risk of bias in the results. Follow-up was considered particularly important for detection of these rare cases. Seven studies were considered high risk of bias for inadequate follow-up, with more than 25% of the total patients lost to follow-up, or with conception of a new pregnancy before 6 months of normal hCG levels.17,28,29,31,36–38 The two studies that did not differentiate between complete and partial mole were considered high risk in exposure assessment and prognostic factor assessment.30,33 These studies were included in the primary meta-analysis and excluded in various sensitivity analyses discussed further below. Lastly, the funnel plot for risk of publication bias shows the expected distribution, but cannot account for studies that include only partial or complete molar pregnancy (see Appendix 3, available online at http://links.lww.com/AOG/B656).
To assess the robustness of results, sensitivity analyses were performed across three domains: 1) sensitivity to risk of bias, excluding studies at high risk of bias owing to loss to follow-up or owing to differential inclusion or intervention between complete and partial mole; 2) sensitivity to upfront treatment and hCG assay, excluding studies including upfront chemotherapy and urine or low sensitivity hCG assays; and 3) sensitivity to varying assumptions for the two studies not differentiating between complete and partial mole. Overall, there was only minor variation in estimated incidences, and results were overall consistent with the primary analysis. The estimates for cumulative incidence of gestational trophoblastic neoplasia after normal hCG level following complete mole ranged from 0.30–0.59%; the respective estimates for partial mole ranged from 0.03–0.12% (Table 3).
DISCUSSION
For our primary outcome, among 19 studies included in the meta-analysis, the development of gestational trophoblastic neoplasia after reaching normal hCG level following molar pregnancy was found to be rare, with a cumulative incidence of 0.35% following complete mole, and 0.03% following partial mole. Furthermore, 59% of cases after normal hCG levels were identified beyond the currently recommended 6-month surveillance window (60.7% for complete mole, 40% for partial mole). Patients with longer time from uterine evacuation to hCG normalization may be at elevated risk, with 87% of cases developing after a time interval of 56 days (8 weeks or about 2 months) or longer (89.6% for complete mole, 60% for partial mole). Thus, the majority of cases of gestational trophoblastic neoplasia after normal hCG level occur when time from evacuation to hCG normalization takes longer than 8 weeks, and most are diagnosed beyond the currently recommended 6 months of hCG follow-up.
The findings presented here represent a compilation of molar pregnancy follow-up data from a combination of regional or national trophoblastic disease centers, and single institution cohorts. This systematic review is also novel in its quantitative global estimate of overall incidence of gestational trophoblastic neoplasia following molar pregnancy. Among 16 included studies, our estimate for overall incidence of gestational trophoblastic neoplasia is overall consistent with commonly referenced values,2,3 with gestational trophoblastic neoplasia diagnosed in 15.7% of complete moles and 3.95% of partial moles. This estimate is not inclusive of all available literature; it omits studies that did not follow moles to at least 6 months of normal hCG follow-up. However, this stringent follow-up requirement means that our estimate represents the subset of studies with the most robust and complete patient follow-up.
We can compute from primary analysis results that 752 cases of complete mole, or 4,955 cases of partial mole, with normal hCG level, would have to be followed for 6 months to identify one case of gestational trophoblastic neoplasia. Even with such surveillance, nearly 60% of cases of gestational trophoblastic neoplasia after normal hCG level would be missed because they occur beyond the 6-month window. Furthermore, these rare cases of gestational trophoblastic neoplasia after normal hCG level tended to be highly treatable, with only three deaths reported across all included studies. The numerous recommended phlebotomy visits, along with the requirement to avoid pregnancy during the follow-up window, represents a significant burden for women.
Many of the identified studies, and the centers they came from, have discussed changing follow-up surveillance recommendations. The authors from the Charring Cross registry in the United Kingdom recommend urine hCG follow-up for 6 months of normal hCG levels after complete mole and a single confirmatory urine hCG level at 1 month after partial mole.22 This group also trialed prolonging follow-up to 2 years, but they found it identified only one additional case of gestational trophoblastic neoplasia among 6,701 women, and recommended against prolonged surveillance.39 The experts at the New England Trophoblastic Disease Center now recommend surveillance of moles with 3 weekly normal hCG levels, followed by 3 months of normal hCG levels for partial moles and 6 months for complete moles.40 Authors from the French Trophoblastic Disease Reference Center recommend following partial moles to a single normal hCG level, and complete moles for 6 months of normal values.34 The recent 2018 FIGO guidelines cut back on recommended follow-up to just 1 month of normal hCG levels for partial moles, and retained 6 months of surveillance for all complete moles.5
Based on the data presented here, we would make the following suggestions for future surveillance guidelines. Because only five cases of gestational trophoblastic neoplasia after normal hCG level were diagnosed among nearly 15,000 included cases of partial mole, we suggest that these patients could safely exit surveillance after a single confirmatory normal hCG level. Additionally, given the low overall risk of gestational trophoblastic neoplasia after normal hCG level following complete mole of 0.35%, and that nearly 90% of these cases were diagnosed when time from evacuation to hCG normalization was 56 days (8 weeks) or longer, we similarly suggest that patients with complete mole with hCG normalization time less than 56 days could also safely exit surveillance after a single confirmatory normal hCG level. For patients with complete mole with hCG normalization time of 56 days or longer, extended hCG surveillance is likely warranted. The low mortality from these late cases of gestational trophoblastic neoplasia suggests that the frequency of surveillance could be lessened from monthly to every third month. In determining the length of surveillance, a longer interval balances improved sensitivity to detect gestational trophoblastic neoplasia (40% at 6 months, 59% at 1 year, 69% at 18 months, and 77% at 2 years), with greater patient burden. Extending surveillance to 1-year total at 3-month intervals would improve sensitivity from 40 to 59% and still have fewer overall hCG checks.
It should also be noted that hCG surveillance recommendations may to be tailored to the targeted population, balancing local risk of disease with the costs and burdens of follow-up, which may be greater in more rural or more impoverished areas. Regardless of hCG surveillance recommendations, patients with both partial and complete molar pregnancy should be counseled to continue regular gynecologic care and to seek evaluation expediently if new symptoms arise, including amenorrhea, abnormal bleeding, abdominal bloating, nausea, pain, hemoptysis, or dyspnea.
The strength of our study is in its comprehensiveness, including all major databases to capture studies globally without any restrictions, as well as its robustness, as we limited to studies written in manuscript form with at least 6 months of intended hCG follow-up. Only a few studies had to be excluded for unavailability of English full text; it is unclear whether they would have met criteria for inclusion, and they would be unlikely to significantly affect the results presented. Additionally, two reviewers performed the identification of studies for inclusion independently in duplicate. Owing to the simplicity of the relevant data, aside from loss to follow-up, there was generally low risk of bias across studies.
Our study has limitations. Most of the identified studies are retrospective, increasing risk of selection bias. The primary outcome is rare, requires large cohorts for identification of many cases, and subanalyses are limited by the completeness and congruency of reported data across studies. Some studies did not differentiate clearly between complete and partial mole, others had less complete follow-up, and some included atypical molar pregnancy treatment or hCG surveillance. All of these issues were addressed with sensitivity analyses. In regard to follow-up, there is always the possibility that there were additional cases and the presented incidences represent underestimates. However, many of these studies represent large referral centers, and we would expect women with disease to be more likely to re-present to these centers or be referred back after development of disease, as compared with those women who are disease free. Lastly, some of these cases remote from pregnancy could have been related to interval pregnancies, recognized or unrecognized, as most studies did not compare the disease genetics with those of the index case of molar pregnancy.
In conclusion, gestational trophoblastic neoplasia following molar pregnancy after normal hCG level is rare. We believe that this data can be used to guide changes to surveillance recommendations to minimize unnecessary burden on patients and improve efficiency of care. In future research, we hope to assess the cost effectiveness of alternative follow-up recommendations. The worldwide obstetrics and gynecology community should continue to gather data on molar pregnancy and trophoblastic neoplasia with patient registries and referral centers, to determine more precise region-specific estimates of gestational trophoblastic neoplasia development.
Supplementary Material
Financial Disclosure
Sarita Sonalkar reports receiving money paid to their institution from the National Institutes of Health and World Health Organization, and individual payment from PCORI.
Courtney Schreiber is supported in part by R01 HD071920-01, and a Society of Family Planning Mid-career Award.
The authors thank Melanie Cedrone, MS, a biomedical librarian who assisted in the development of the search strategy.
Footnotes
Each author has confirmed compliance with the journal’s requirements for authorship.
The other authors did not report any potential conflicts of interest.
REFERENCES
- 1.Berkowitz RS, Goldstein DP. Current management of gestational trophoblastic diseases. Gynecol Oncol 2009;112:654–62. [DOI] [PubMed] [Google Scholar]
- 2.Berkowitz RS, Goldstein DP. Molar pregnancy. N Engl J Med2009;360:1639–45. [DOI] [PubMed] [Google Scholar]
- 3.Lurain JR. Gestational trophoblastic disease I: epidemiology, pathology, clinical presentation and diagnosis of gestational trophoblastic disease, and management of hydatidiform mole. Am J Obstet Gynecol 2010;203:531–9. [DOI] [PubMed] [Google Scholar]
- 4.Diagnosis and treatment of gestational trophoblastic disease. ACOG Practice Bulletin No. 53. American College of Obstetricians and Gynecologists. Obstetrics Gynecol 2004;103:1365–77. [DOI] [PubMed] [Google Scholar]
- 5.Ngan HYS, Seckl MJ, Berkowitz RS, Xiang Y, Golfier F, Sekharan PK, et al. Update on the diagnosis and management of gestational trophoblastic disease. Int J Gynecol Obstet 2018; 143:79–85. [DOI] [PubMed] [Google Scholar]
- 6.Royal College of Obstetricians and Gynaecologists. Gestational trophoblastic Disease. Green-top guideline No. 38. London, United Kingdom: RCOG; 2010. [Google Scholar]
- 7.Massad LS, Abu-Rustum NR, Lee SS, Renta V. Poor compliance with postmolar surveillance and treatment protocols by indigent women. Obstet Gynecol 2000;96:940–4. [DOI] [PubMed] [Google Scholar]
- 8.Petersen RW, Ung K, Holland C, Quinlivan JA. The impact of molar pregnancy on psychological symptomatology, sexual function, and quality of life. Gynecol Oncol 2005;97:535–42. [DOI] [PubMed] [Google Scholar]
- 9.Stafford L, Judd F. What do women with gestational trophoblastic disease understand about the condition? Int J Gynecol Cancer 2011;21:161. [DOI] [PubMed] [Google Scholar]
- 10.Lok CA, Donker M, Calff MM, Massuger LF, Ansink AC. Psychologic impact of follow-up after low-risk gestational trophoblastic disease. J Reprod Med 2011;56:47–52. [PubMed] [Google Scholar]
- 11.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA 2000;283: 2008–12. [DOI] [PubMed] [Google Scholar]
- 12.PROSPERO: International Prospective Register of Systematic Reviews. The rates of gestational trophoblastic disease following complete versus partial molar pregnancy after normalization of human chorionic gonadotropin (HCG): a systematic review and meta-analysis. Available at: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID5116414. Retrieved January 10, 2019.
- 13.Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions. Hoboken, NJ: Wiley Online Library; 2008. [Google Scholar]
- 14.Cochrane Library. Tool to assess risk of risk of bias in cohort studies. Copenhagen, Denmark. The Nordic Cochrane Centre, The Cochrane Collaboration. Available at: https://methods.cochrane.org/sites/methods.cochrane.org.bias/files/public/uploads/Tool%20to%20Assess%20Risk%20of%20Bias%20in%20Cohort%20Studies.pdf. Retrieved November 1, 2018. [Google Scholar]
- 15.Brown LD, Cai T, Dasgupta A. Interval estimation for a binomial proportion. Stat Sci 2001;16:101–33. [Google Scholar]
- 16.Cochrane Community. Review manager (RevMan) version 5.3. Copenhagen, Denmark: The Nordic Cochrane Centre, The Cochrane Collaboration; 2014. [Google Scholar]
- 17.Wolfberg AJ, Feltmate C, Goldstein DP, Berkowitz RS, Lieberman E. Low risk of relapse after achieving undetectable HCG levels in women with complete molar pregnancy. Obstet Gynecol 2004;104:551–4. [DOI] [PubMed] [Google Scholar]
- 18.Al-Talib AA. Clinical presentation and treatment outcome of molar pregnancy: ten years experience at a tertiary care hospital in Dammam, Saudi Arabia. J Family Community Med 2016; 23:161–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alazzam M, Young T, Coleman R, Hancock B, Drew D, Wilson P, et al. Predicting gestational trophoblastic neoplasia (GTN): is urine hCG the answer? Gynecol Oncol 2011;122: 595–9. [DOI] [PubMed] [Google Scholar]
- 20.Batorfi J, Vegh G, Szepesi J, Szigetvari I, Doszpod J, Fulop V. How long should patients be followed after molar pregnancy? Analysis of serum hCG follow-up data. Eur J Obstet Gynecol Reprod Biol 2004;112:95–7. [DOI] [PubMed] [Google Scholar]
- 21.Braga A, Maestá I, Matos M, Elias KM, Rizzo J, Viggiano MG. Gestational trophoblastic neoplasia after spontaneous human chorionic gonadotropin normalization following molar pregnancy evacuation. Gynecol Oncol 2015;139:283–7. [DOI] [PubMed] [Google Scholar]
- 22.Coyle C, Short D, Jackson L, Sebire NJ, Kaur B, Harvey R, et al. What is the optimal duration of human chorionic gonadotrophin surveillance following evacuation of a molar pregnancy? A retrospective analysis on over 20,000 consecutive patients. Gynecol Oncol 2018;148:254–257. [DOI] [PubMed] [Google Scholar]
- 23.Fatima M, Kasi PM, Baloch SN, Kassi M, Marri SM, Kassi M. Incidence, management, and outcome of molar pregnancies at a tertiary care hospital in Quetta, Pakistan. ISRN Obstet Gynecol 2011;2011:925316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feltmate CM, Batorfi J, Fulop V, Goldstein DP, Doszpod J, Berkowitz RS. Human chorionic gonadotropin follow-up in patients with molar pregnancy: a time for reevaluation. Obstet Gynecol 2003;101:732–6. [DOI] [PubMed] [Google Scholar]
- 25.Gueye M, Kane-Gueye SM, Ndiaye-Gueye MD, Mbaye M, Diouf AA, Niang MM, et al. Gestational trophoblastic neoplasia after achieving a nondetectable serum human chorionic gonadotrophin level. BJOG 2014;121:1415–9. [DOI] [PubMed] [Google Scholar]
- 26.Hoppenot C, Zimmerman L, Arlandson M, Lurain JR, Patel A. Follow-up after molar pregnancy evacuation: feasibility of using semi-quantitative urine pregnancy tests. J Reprod Med 2016;61: 192–6. [PubMed] [Google Scholar]
- 27.Horn LC, Kowalzik J, Bilek K, Richter CE, Einenkel J. Clinicopathologic characteristics and subsequent pregnancy outcome in 139 complete hydatidiform moles. Eur J Obstet Gynecol Reprod Biol 2006;128:10–4. [DOI] [PubMed] [Google Scholar]
- 28.Kerkmeijer LG, Wielsma S, Massuger LF, Sweep FC, Thomas CM. Recurrent gestational trophoblastic disease after hCG normalization following hydatidiform mole in The Netherlands. Gynecol Oncol 2007;106:142–6. [DOI] [PubMed] [Google Scholar]
- 29.Lavie I, Rao GG, Castrillon DH, Miller DS, Schorge JO. Duration of human chorionic gonadotropin surveillance for partial hydatidiform moles. Am J Obstet Gynecol 2005;192:1362–4. [DOI] [PubMed] [Google Scholar]
- 30.Matsui H, Iitsuka Y, Suzuka K, Seki K, Sekiya S. Subsequent pregnancy outcome in patients with spontaneous resolution of HCG after evacuation of hydatidiform mole: comparison between complete and partial mole. Hum Reprod 2001;16: 1274–7. [DOI] [PubMed] [Google Scholar]
- 31.Nirmala CK, Azlin MIN, Harry SR, Lim PS, Shafiee MN, Azurah AGN, et al. Outcome of molar pregnancies in Malaysia: a tertiary centre experience. J Obstet Gynaecol 2013;33:191–3. [DOI] [PubMed] [Google Scholar]
- 32.Riadh BT, Abdellatif C, Wissal H, Leila A, Taher M, Abdelhamid K. Clinical analysis and management of gestational trophoblastic diseases: a 90 cases study. Int J Biomed Sci 2009;5:321–5. [PMC free article] [PubMed] [Google Scholar]
- 33.Schlaerth JB, Morrow CP, Kletzky OA, Nalick RH, D’Ablaing GA. Prognostic characteristics of serum human chorionic gonadotropin titer regression following molar pregnancy. Obstet Gynecol 1981;58:478–82. [PubMed] [Google Scholar]
- 34.Schmitt C, Doret M, Massardier J, Hajri T, Schott AM, Raudrant D, et al. Risk of gestational trophoblastic neoplasia after hCG normalization according to hydatidiform mole type. Gynecol Oncol 2013;130:86–9. [DOI] [PubMed] [Google Scholar]
- 35.Usui H, Qu J, Sato A, Pan Z, Mitsuhashi A, Matsui H, et al. Gestational trophoblastic neoplasia from genetically confirmed hydatidiform moles: prospective observational cohort study. Int J Gynecol Cancer 2018;28:1772–80. [DOI] [PubMed] [Google Scholar]
- 36.Wiesma S, Kerkmeijer L, Bekkers R, Pyman J, Tan J, Quinn M. Persistent trophoblast disease following partial molar pregnancy. Aust N Z J Obstet Gynaecol 2006;46:119–23. [DOI] [PubMed] [Google Scholar]
- 37.Wiesma S, Kerkmeijer L, Bekkers R, Pyman J, Tan J, Quinn M. Guidelines following hydatidiform mole: a reappraisal. Aust N Z J Obstet Gynaecol 2006;46:112–8. [DOI] [PubMed] [Google Scholar]
- 38.Wolfberg AJ, Growdon WB, Feltmate CM, Goldstein DP, Genest DR, Chinchilla ME, et al. Low risk of relapse after achieving undetectable HCG levels in women with partial molar pregnancy. Obstet Gynecol 2006;108:393–6. [DOI] [PubMed] [Google Scholar]
- 39.Sebire NJ, Foskett M, Short D, Savage P, Stewart W, Thomson M, et al. Shortened duration of human chorionic gonadotrophin surveillance following complete or partial hydatidiform mole: evidence for revised protocol of a UK regional trophoblastic disease unit. BJOG 2007;114:760–2. [DOI] [PubMed] [Google Scholar]
- 40.Berkowitz RS, Goldstein DP, Horowitz NS. Hydatidiform mole: treatment and follow-up. UpToDate; 2017. Available at: https://www.uptodate.com/contents/hydatidiform-mole-treatment-and-follow-up. Retrieved November 1, 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.