Abstract
Transplantation-associated thrombotic microangiopathy (TA-TMA) is a complication of allogeneic hematopoietic cell transplantation (HCT) that often occurs following the development of acute graft-versus-host disease (aGVHD). In this study, we aimed to identify early TMA biomarkers among patients with aGVHD. We performed a nested-case-control study from a prospective cohort of allogeneic HCT recipients, matching on the timing and severity of antecedent aGVHD. We identified 13 TMA cases and 25 non-TMA controls from 208 patients in the cohort. Using multivariable conditional logistic regression, the odds ratio for TMA compared with non-TMA was 2.65 (95% confidence interval [CI], 1.00 to 7.04) for every 100 ng/mL increase in terminal complement complex sC5b9 and 2.62 (95% CI, 1.56 to 4.38) for every 1000 pg/mL increase in angiopoietin-2 (ANG2) at the onset of aGVHD. ADAMTS13 and von Willebrand factor (VWF) antigens were not appreciably associated with TMA. Using a Cox regression model incorporating sC5b9 >300 ng/mL and ANG2 >3000 pg/mL at the onset of aGVHD, the adjusted hazard ratio for mortality was 5.33 (95% CI, 1.57 to 18.03) for the high-risk group (both elevated) and 4.40 (95% CI, 1.60 to 12.07) for the intermediate-risk group (one elevated) compared with the low-risk group (neither elevated). In conclusion, we found that elevated sC5b9 and ANG2 levels at the onset of aGVHD were associated with the development of TMA and possibly mortality after accounting for the timing and severity of aGVHD. The results suggest important roles of complement activation and endothelial dysfunction in the pathogenesis of TMA. Measurement of these biomarkers at the onset of aGVHD may inform prognostic enrichment for preventive trials and improve clinical care.
Keywords: Bone Marrow Transplantation, Thrombotic Microangiopathies, TMA, TA-TMA, Complement Membrane Attack, Complex, Angiopoietin-2
INTRODUCTION
Transplant-associated thrombotic microangiopathy (TA-TMA) is a hematologic complication that can occur following allogeneic hematopoietic cell transplantation (HCT). It presents clinically with microangiopathic hemolytic anemia (MAHA), consumptive thrombocytopenia, and microvascular thrombosis and is associated with acute kidney injury and increased mortality [1]. Over the past decade, several large epidemiologic studies from high-volume allogeneic HCT centers have identified key clinical risk factors for TMA. Among these, high grade acute graft-versus-host disease (aGVHD) has emerged as a strong and consistent predisposing factor [2–5].
Despite the strong association between the 2 diseases, only a subset of patients with aGVHD develop TMA. The identification of prognostic biomarkers could potentially identify those aGVHD patients who may benefit from earlier TMA-directed therapy. Previous biomarker studies have focused on distinct pathways in unselected post-transplant patients: ADAMTS13 cleavage and von Willebrand Factor (VWF) hyperreactivity [6,7]; complement activation fragments, especially terminal complement complex sC5b9 [8–11]; and endothelial activation/dysfunction [12]. Nonetheless, these results have been limited by discrepancies in clinical diagnostic criteria between centers, potential sampling bias, small sample size, and, most importantly, time-varying confounding by aGVHD. Furthermore, clinical studies have postulated that patients with concurrent aGVHD and TMA have higher mortality compared with patients with aGVHD alone [4,12]. However, because severe and steroid-refractory aGVHD is highly associated with both TMA and mortality as a confounder, assessing unconfounded associations has proven challenging.
Here, using existing biospecimens from a cohort of 208 adult allogeneic HCT recipients, we performed a nested case-control study to assess the association between levels of representative biomarkers in the coagulation, complement, and endothelial systems, measured at the time of aGVHD incidence, on the development of TMA. Each TMA case was matched to 2 non-TMA controls by the presence and severity of aGVHD. Specifically, we examined plasma concentrations of ADAMTS13, VWF, sC5b9, and angiopoetin-2 (ANG2). We also examined the associations of these biomarkers at the time of aGVHD diagnosis and overall mortality.
METHODS
Study Design and Population
We performed a nested case-control study using a prospective cohort of patients that underwent allogeneic HCT from 2006 to 2013 at the Fred Hutchinson Cancer Research Center (FHCRC) in Seattle, Washington. This study was approved by the FHCRC Institutional Review Board. The parent cohort study enrolled patients undergoing their first HCT at FHCRC. Because this was not an interventional design, the study was not registered on ClinicalTrials.gov.
Case and Control Sampling
For the current nested case-control study, TMA cases were defined as persistent MAHA without coagulopathy related to disseminated intravascular coagulation. The case ascertainment and validation algorithm were described in our previous study [2]. In brief, we screened all patients from the prospective cohort using a set of laboratory criteria to identify consecutive MAHA using daily laboratory features captured in our Gateway database (Table 1). From this list of candidate patients, we then individually reviewed the chart of each to exclude patients with other well-known causes or mimics of TMA. To obtain unbiased estimates of relative risk, controls were selected using the incidence density sampling method (Figure 1). Specifically, at the onset of every TMA case occurrence, 2 non-TMA controls (1:2) were chosen at random from the HCT population at risk. These controls were specifically matched for the onset and clinical grading (severity) of antecedent aGVHD to mitigate the influence of aGVHD treatment on the biomarker analysis.
Table 1.
Study Definition for TA-TMA
| Criteria 1: Must have 2 or more laboratory criteria for MAHA within 7 days or single MAHA with a haptoglobin checked within 7 days (suspicion for hemolysis) | |
| Red cell fragmentation and | Slight, moderate, or marked schistocytes (>2/HPF) |
| Lactate dehydrogenase and | ≧2 times the upper limit of normal |
| Thrombocytopenia and | Platelets ≦50k or ≦100k if transfused or ≧50% drop |
| Anemia and | Hemoglobin or hematocrit ≦lower limit of normal |
| Timing | All 4 laboratory values must occur concurrently within 24 hours |
| Criteria 2. Does not have other obvious causes for TMA through clinical chart review | |
| Not isolated MAHA and | must be persistent more than twice |
| Not immune hemolysis and | negative direct Coombs test (DAT) if checked |
| Not severe coagulopathy and | does not meet ISTH criteria for DIC |
| Not disease relapse | no documented pretransplantation disease relapse |
Figure 1.

Incidence density sampling for the nested case-control study. This diagram shows the schema of case and control selection. At the onset of every TA-TMA case, 2 non-TMA controls were chosen randomly after matching on the timing and severity of previous aGVHD. Among 208 adult allogeneic HCT recipients enrolled in the prospective cohort, 13 TA-TMA cases were identified, and 25 matched non-TMA aGVHD controls were sampled.
Exposure (Biomarker) Testing
Morning blood samples were collected in a citrated tube before initiation of the conditioning regimen (baseline) and then weekly through day 100 post-transplantation. Samples were placed on ice immediately and delivered to the lab within 1 hour. Blood was centrifuged at 2500 rpm at 4 °C for 15 minutes, and plasma was aspirated and frozen (−80 °C) in 2-mL aliquots no more than 2 hours after collection. For this study, samples were thawed slowly in a room temperature water bath without vigorous shaking or vortex to avoid complement activation [13]. All analyses were performed in samples that had undergone no more than 2 freeze-thaw cycles. For the primary analysis, biomarker testing was performed using samples drawn at the onset of aGVHD (pre-TMA). For exploratory longitudinal analysis, additional samples were included in 2-week intervals pre-transplant and post-transplant for up to 100 days.
Laboratory Assays
ADAMTS13 antigen was measured by enzyme-linked immunosorbent assay (ELISA). VWF antigen was measured by ELISA using a polyclonal VWF antibody as the capture antibody, and the bound VWF was detected by a horseradish peroxidase-conjugated polyclonal VWF antibody (DAKO North America, Carpinteria, CA). The multimer patterns of the samples with low VWF antigens were examined on 1.7% agarose-sodium dodecyl sulfate gel, followed by Western blotting, to ensure the quality of VWF in the samples. The samples were excluded from the VWF and ADAMTS13 analysis if VWF was lost. The ADAMTS13 and VWF antigens were expressed as percentage of fold-pooled normal plasma from healthy volunteer samples (Cryocheck Normal Reference Plasma, Precision Biologic, Dartmouth, NS, Canada). ADAMTS13 antigen expression was also recorded as the ratio of ADAMTS/VWF. The terminal complement pathway sC5b9 was measured by ELISA (MicroVue; Quidel, San Diego, CA) and expressed as ng/mL. Finally, angiopoietin-2 (ANG2) was measured via electrochemiluminescent immunoassays (Meso Scale Discovery, Rockville, MD) and expressed as pg/mL. All samples were tested in duplicates and blinded to participant characteristics, as described previously [14].
Clinical Parameters
Demographic information and pretransplant data were uniformly collected on all participating patients. The following clinical parameters were recorded in the study: age, sex, and race for both donor and recipient, and for the patient, HCT Comorbidity Index [15,16], disease type, donor match, graft source, conditioning regimen, total body irradiation, and aGVHD prophylaxis regimen. Post-transplantation complications recorded included the onset and severity of aGVHD, diffuse alveolar hemorrhage, venous thromboembolism, and various infections, such as bacteremia, aspergillosis, cytomegalovirus reactivation, BK viremia, adenovirus infection, human herpesvirus 6 infection, or Epstein-Barr virus infection. aGVHD was defined and graded according to established criteria [17,18]. Post-transplant complications were collected from an ongoing surveillance database. Routine laboratory testing was done daily on an inpatient basis or 3 times weekly on an outpatient basis. Patients were routinely contacted post-transplant for accurate vital status determination.
Statistical Analysis
For patient demographics within each outcome group (TMA versus non-TMA), descriptive statistics for categorical variables, including sample size, frequency count, and percentage, were compared using Fisher’s exact test. Descriptive statistics for continuous variables included sample size, median, and interquartile range (IQR), and they were compared using the Wilcoxon rank-sum test. For comparison of cases and controls at the onset of aGVHD before TMA, biomarker levels were shown as mean and 95% CI. Univariable and multivariable conditional logistic regression models (conditioned on matched groups) were built. Because no clinically validated cutoff values exist for research laboratory assays, the exposure values were modeled as incremental increases in mean values of each biomarker. The Youden Index, which maximizes sensitivity and specificity, was used to determine optimal cutoff thresholds. Unadjusted Kaplan-Meier curves were used to depict the overall survival after the onset of aGVHD. Because the study design incorporated both incidence density sampling and matching on the onset of aGVHD, immortal time bias would not be present if the survival analysis were restricted to matched groups; therefore, we used Cox regression adjusted by the matching factors to estimate the hazard ratio associated with mortality after aGVHD. For exploratory longitudinal analysis, we reported mean values and 95% CIs using pooled values at 4-week intervals to examine potential trends. All data analyses were performed using Stata 16 (Stata Corp, College Station, TX).
RESULTS
Patient Characteristics
Between 2006 and 2013, 208 adult patients undergoing their first allogeneic HCT were enrolled in the prospective cohort and provided longitudinal samples. Among these patients, we identified 13 TMA cases (6.3%), a percentage consistent with that observed in previous adult allogeneic HCT studies [2,4,5,19]. We then identified 25 non-TMA controls matched on the timing and severity of antecedent aGVHD (Figure 1). One patient with delayed TMA could be matched to only one appropriate control.
Patient characteristics are shown in Table 2. Within the case group, the median onset of TMA was 37 days (IQR, 23 to 80 days), and 6 out of 13 patients developed disease within 1 month of transplantation. At the time of HCT, the median patient age was 50 years (IQR, 45 to 54 years), and 38% were women. The indication for allogenic HCT was myeloid malignancy in 62% of the patients, lymphoid malignancy in 23%, and nonmalignant conditions in 15%. A mismatched donor was used in 54% of the transplantations. Approximately 77% of the patients received a myeloablative conditioning regimen, and all patients received calcineurin inhibitors for aGVHD prophylaxis (62% tacrolimus; 38% cyclosporine). All TMA patients in this study had previous episodes of grade II or higher aGVHD (31% grade III-IV, 69% grade II), with a median onset of 21 days (IQR, 11 to 34 days). All patients received systemic glucocorticoids for aGVHD, and 31% of patients received additional treatment for refractory disease.
Table 2.
Patient Characteristics for TMA Cases versus Non-TMA Matched Controls
| Characteristic | TA-TMA Cases (N = 13) | Non-TMA Matched Controls (N = 25) | P Value |
|---|---|---|---|
| Age, yr, median (IQR) | 50 (45–53) | 50 (40–58) | .89 |
| Female sex, % (n) | 38 (5) | 48 (12) | .73 |
| Disease, % (n) | |||
| Myeloid | 62 (8) | 76 (19) | .18 |
| Lymphoid | 23 (3) | 23 (6) | |
| Nonmalignant | 15 (2) | 0 (0) | |
| Donor, % (n) | |||
| Mismatched | 54 (7) | 40 (10) | .50 |
| Regimen, % (n) | |||
| Myeloablative | 77 (10) | 80 (20) | 1.00 |
| Previous autologous HCT, % (n) | 0 (0) | 0 (0) | 1.00 |
| GVHD prophylaxis, % (n) | |||
| Tacrolimus | 62 (8) | 60 (15) | .87 |
| Cyclosporine | 38 (5) | 32 (8) | |
| Cyclophosphamide | 0 (0) | 8 (2) | |
| Sirolimus + CNI | 0 (0) | 0 (0) | |
| aGVHD onset, d, median (IQR)* | 21 (11–34) | 21 (16–31) | .71 |
| aGVHD grade, % (n)* | |||
| II | 69 (9) | 76 (19) | .85 |
| III | 8 (1) | 8 (2) | |
| IV | 23 (3) | 16 (4) | |
| aGVHD type, % (n)† | |||
| Gut | 77 (10) | 84 (21) | .67 |
| Skin | 62 (8) | 64 (16) | 1.00 |
| Liver | 23 (3) | 24 (6) | 1.00 |
| aGVHD treatment, % (n) | |||
| Systemic corticosteroid | 100 (13) | 96 (24) | |
| Second-line (steroid-refractory)‡ | 31 (4) | 16 (4) | .41 |
| TMA onset, d, median (IQR) | 37 (23–80) | N/A | N/A |
N/A indicates not applicable.
These are the matching variables of the study.
aGVHD types are not mutually exclusive.
Second-line treatment included antithymocyte globulin, infliximab, alemtuzumab, or mycophenolate.
None of the 25 control patients developed TMA during follow-up. Age, sex, indication for HCT, donor matching, conditioning regimen, and aGVHD prophylaxis regimen were similar between cases and controls (Table 2). All 25 controls had grade II or higher aGVHD (24% grade III-IV, 76% grade II), with a median onset of 21 days (IQR 16 to 31 days) from the time of HCT. Ninety-six percent of the controls received systemic glucocorticoids, and 16% received secondary therapy for refractory aGVHD. A detailed breakdown of the types of aGVHD (patients often had more than 1 type) is shown in Table 2. There did not appear to be appreciable differences between cases and controls in the type of aGVHD or systemic treatment.
Association of Biomarkers with TMA at Discrete Time Points
Owing to the matching design of the study, the primary focus on our analysis was on determining whether biomarker exposures at the aGVHD incidence were associated with future development of TMA. Among the biomarkers measured, mean sC5b9 levels (Figure 2A) were considerably higher in the TMA cases than in the non-TMA controls at the onset of aGVHD (352 ± 142 ng/mL versus 243 ± 72 ng/mL; odds ratio [OR], 2.97; 95% CI, 1.29 to 6.85 for every 100 ng/mL increase) (Table 3). Mean ANG2 levels (Figure 2B) were also higher in the TMA cases than in the non-TMA controls at the onset of aGVHD (5845 ± 2431 pg/mL 2431] vs. 3387 ± 1735 pg/mL; OR, 2.29; 95% CI, 1.12 to 4.65 for every 1000 pg/ml increase) (Table 3). Among the TMA cases, these biomarker levels at the onset of aGVHD were not appreciably higher in those patients who had more severe disease as defined by renal or neurologic involvement compared with patients without organ damage (data not shown).
Figure 2.

Comparison of biomarkers at time points before the onset of TMA. The mean values and 95% CIs of biomarkers are shown for 13 TA-TMA cases (dark bar) versus 25 non-TMA controls (light bar) at each discrete time point (pretransplantation and onset of aGVHD). Significance differences (*P < .05) were detected for sC5b9 and ANG2 at the onset of aGVHD.
Table 3.
Association between Biomarkers Measured at the Onset of aGVHD and the Development of TMA
| Biomarker | OR for TMA (95% CI) |
|---|---|
| Univariable models | |
| sC5b9 Ag Δ 100 ng/mL ↑ at aGVHD | 2.97 (1.29–6.85) |
| ANG2 Ag Δ 1000 pg/mL ↑ at aGVHD | 2.29 (1.12–4.65) |
| ADAMTS13 Ag Δ 10% ↑ at aGVHD | 0.99 (0.83–1.19) |
| VWF Ag Δ 10% ↑ at aGVHD | 1.01 (0.96–1.08) |
| Multivariable model | |
| sC5b9 Δ 100 ng/mL ↑ and | 2.65 (1.00–7.04) |
| ANG2 Δ 1000 pg/mL ↑ at aGVHD | 2.62 (1.56–4.38) |
Conditional logistic regressions were used to assess the association between TA-TMA and biomarker expression. ORs and 95% CIs are reported. The biomarkers were modeled as incremental increases in mean values. A multivariable model was created from the incorporation of SC5b9 and ANG2.
Mean antigen values of ADAMTS13 (Figure 2C), VWF (Figure 2D), and ADAMTS13/VWF ratio (Figure 2E) were not appreciably different at the onset of aGVHD (Table 3). There were no differences pretransplant for any of the biomarkers (Figure 2 A–E). To assess whether sC5b9 and ANG2 were independent predictors of TMA at the onset of aGVHD, we tested both biomarkers in a multivariable conditional logistic regression analysis. The OR for TMA was 2.65 (95% CI, 1.00 to 7.04) for every 100 ng/mL increase in sC5b9 and 2.62 (95% CI, 1.56 to 4.38) for every 1000 pg/mL increase in ANG2 (Table 3).
Association of Biomarkers with TMA over Time
As an exploratory analysis, we plotted the longitudinal biomarker levels over time. The average number of longitudinal samples tested from cases and controls were 6 and 4, respectively. Among the controls, there were no significant variations in sC5b9 and ANG2 levels over time. In contrast, the mean levels of sC5b9 and ANG2 were initially elevated approximately 2 weeks post-transplantation in TMA cases and remained elevated over time (Figure 3A and B). When the biomarkers were examined with aGVHD as the anchoring event, both sC5b9 and ANG2 appeared elevated at the onset of aGVHD in TMA cases (not observed in controls); however, sC5b9 remained persistently elevated until the development of TMA, whereas ANG2 returned closer to the baseline level. ADAMTS13 followed the same trend in the cases and controls, whereas VWF had a steeper rise in both cases and controls (Figure 3C and D). There was no significant difference in ADAMTS13/VWF ratio between cases and controls (Figure 3E).
Figure 3.
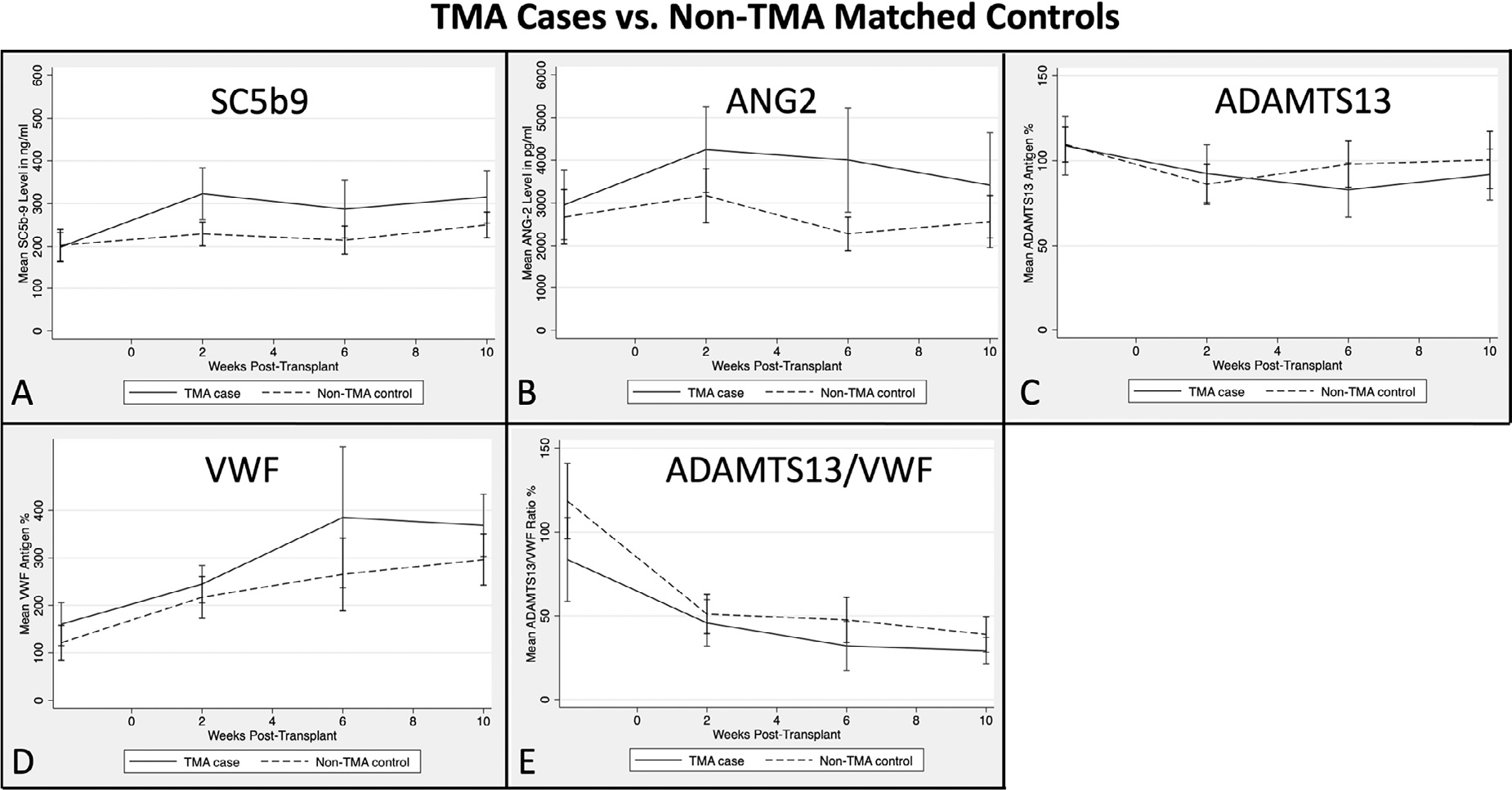
Longitudinal assessment of biomarkers over time pre- and post-HCT. The mean values and 95% CIs of biomarkers are shown longitudinally for 13 TA-TMA cases (solid line) and 25 non-TMA matched controls (dash line). There was a significant difference between cases and controls for sC5b9 and ANG2 from 2 to 4 weeks.
Association between Biomarkers and Infection
We examined the association between biomarker values and systemic infections. Since complement and endothelial activation could be a component of innate immunity and an ancient defense mechanism against invading pathogens, we explored the values of these biomarkers in any samples drawn within 1 week (−3 to +7 days) from the onset of the documented active systemic infections and compared with post-transplant samples without documented infection. Of note, this is an exploratory analysis that does not take the matching design into consideration. Overall, 36 post-transplant samples from 26 patients met the infection definition (some had more than 1 infection), and 161 post-transplant samples from 55 patients did not. As shown in Table 4, there was heterogeneity in biomarker expressions depending on the type of infection. Patients with certain infections, such as gram-negative bacteremia, BK viremia, and human herpesvirus 6 infection, had numerically higher average levels of sC5b9 and ANG2 compared with their noninfectious counterparts.
Table 4.
Mean Biomarker Values in Samples Drawn During Active Infections
| Biomarker | sC5b9, ng/mL | ANG2, pg/mL | ADAMTS13, % | VWF, % | Samples, n |
|---|---|---|---|---|---|
| No documented infection | 251 | 3452 | 93 | 267 | 161 |
| Any documented infection | 296 | 3752 | 85 | 275 | 36 |
| Gram-negative bacteremia | 357 | 3722 | 73 | 226 | 5 |
| Invasive aspergillosis | 307 | 3456 | 73 | 241 | 9 |
| BK viremia | 390 | 3975 | 63 | 343 | 5 |
| Human herpesvirus 6 | 395 | 4197 | 88 | 251 | 7 |
| Adenovirus | 303 | 3987 | 57 | 280 | 3 |
| Epstein-Barr virus | 313 | 3542 | 97 | 315 | 5 |
| Cytomegalovirus reactivation | 282 | 3984 | 85 | 280 | 19 |
The mean values of biomarkers from samples drawn within −3 to +7 days from the onset of the systemic infection are shown. There were 36 samples from 26 patients who had at least 1 infection and 161 samples from 55 patients without infections. There was heterogeneity of biomarker expression depending on the type of infection.
Association of Biomarkers with Mortality
Finally, we assessed the association between biomarkers and overall survival among patients with aGVHD. We reclassified patients into 3 categories using a combination of sC5b9 and ANG2 values obtained from samples drawn at the onset of aGVHD before the occurrence of TMA and included both cases and controls. Using the Youden Index to identify optimal threshold values for survival, patients were assigned to the low-risk group if they had both sC5b9 ≤300 ng/mL and ANG2 ≤3000 pg/mL, to the intermediate-risk group if they had either sC5b9 >300 ng/mL or ANG2 >3000 pg/mL, and to the highrisk group if they had both sC5b9 >300 ng/mL and ANG2 >3000 pg/mL. Two patients did not have available samples drawn in this time frame, so only 36 of the 38 were included in this analysis. In the analysis, death occurred in 11 of 12 patients in the high-risk group, in 9 of 12 patients in the intermediate-risk group, and in 4 of 12 patients in the low-risk group. In a Cox regression analysis adjusted by aGVHD severity/grouping, the hazard ratio was 5.33 (95% CI, 1.57 to 18.03) for the high-risk group and 4.40 (95% CI 1.60–12.07) for the intermediate-risk group compared with the low-risk group. This result suggests that this combination of biomarkers associated with the development of TMA also may be associated with mortality in patients with aGVHD; however, this requires validation in an unselected cohort of patients.
DISCUSSION
In the current nested case-control study from a prospective cohort of allogeneic HCT recipients, we found that a distinctive combination of biomarkers in complement and endothelial activation pathways was independently associated with the development of TMA after carefully controlling for the timing and severity of antecedent aGVHD. Specifically, we observed that elevated levels of sC5b9 and ANG2 at the initial onset of aGVHD in the early post-transplant period were associated with the development of TMA and possibly higher mortality. The findings from this study, if validated in other prospective cohorts, could lead to a preemptive surveillance strategy among high-risk patients for early identification of TMA before overt disease manifestation. Measurement of these biomarkers at the onset of aGVHD may inform prognostic enrichment for preventive clinical trials and may improve clinical care.
Our case-control study purposefully matched cases and controls on the timing and severity of aGVHD. We chose this study design to help mitigate the strong confounding effect of the timing, severity, and treatment of aGVHD on the development of TMA to assess biomarkers specific to TMA. The strong association between higher clinical grades of aGVHD and TMA development has been well demonstrated in several recent epidemiologic studies using both laboratory and clinician-reported TMA definitions [2,3]. In addition to clinical grading of aGVHD, TMA also has been reported at higher frequency in patients with steroid-refractory aGVHD compared with all other patients (45% versus 2%) [20] or compared with patients with steroid-sensitive aGVHD (79% versus 42%) [12]. Our study was designed to answer the question “what biomarkers are associated with subsequent development of TMA among similar patients with grade II-IV aGVHD?” In this regard, we found that elevated sC5b9 and ANG2 levels were observed in patients with aGVHD and TMA rather than in those with aGVHD alone.
The association between TMA and the terminal complement complex biomarker sC5b9 has been reported in several other studies. At the time of TMA diagnosis, higher median sC5b9 levels were reported in TMA patients compared with non-TMA patients. The reported median values were 333 ng/mL (IQR, 274 to 445 ng/mL) and 201 ng/mL (IQR, 172 to 274 ng/mL), respectively, in one study [8] and 1025 ng/mL (IQR, 907 to 1158 ng/mL) and 621 ng/mL (IQR, 550 to 770 ng/mL) in another [10]. Furthermore, Horváth et al. [9] reported higher sC5b9 levels at day 28 in 10 TMA patients compared with 23 controls (median, 411 ng/mL [IQR, 337 to 471 ng/mL] versus 201 ng/mL [IQR, 185 to 290 ng/mL), although this study was not controlled for aGVHD, and many patients would have developed TMA by day 28. To the contrary, Sartain et al. [11] did not detect increases in sC5b9 at any time point at the onset or after the diagnosis of disease in 7 patients with TMA [11]. A variety of specimen processing and assay-related factors may have contributed to the discrepancies in the published results. Similar to some of these previous studies, we found elevated sC5b9 levels at the time of TMA diagnosis compared with matched controls (data not shown). The novel aspect of the present study, however, is the discovery that elevated sC5b9 at the initial onset of aGVHD was also prognostic for later development of TMA. Therefore, sC5b9 might serve a role not only as a diagnostic biomarker, but also as an early prognostic biomarker, in high-risk aGVHD patients.
In contrast to the complement pathway, the role of ANG2 has not been well studied in TMA patients. ANG2 is a TIE2-binding antagonist that disrupts ANG1-mediated vascular stabilization and leads to impaired endothelial function and increased microvascular permeability [21]. As an endothelial biomarker, ANG2 has been mostly studied in patients with aGVHD, and high levels of ANG2/VEGF have been associated with steroid-refractory aGVHD [22]. Given our finding of the greatest ANG2 elevation at the onset of aGVHD, it is plausible that ANG2 elevation results from vascular damage related to severe refractory aGVHD, which in turn could lead to uncontrolled complement activation. As not every patient would have elevated sC5b9, additional prognostic biomarkers such as ANG2 could serve complementary and confirmatory roles in the clinical setting.
Finally, although we found no difference in VWF levels between TMA cases and matched aGVHD controls, VWF antigens were consistently higher in the patients with TMA, while their ADAMTS13 levels were only marginally lower. Cytokine storm during aGVHD can activate endothelial cells to release large amounts of ultra-large VWF without the corresponding increase in ADAMTS13 expression. As a result, there may be an imbalance between the substrate VWF and the VWF-cleaving metalloprotease, thereby reducing the rate of VWF cleavage and potentially changing the adhesive activity of VWF multimers [23–25]. We are currently testing the hypothesis that the VWF multimers found in these patients are hyperadhesive and adherent to platelet and endothelial cells. Furthermore, VWF can be oxidized to further resist cleavage or become laterally associated to form hyperadhesive fibrils [26,27]. It is possible that elevated VWF concentration and reactivity in plasma are necessary but not sufficient for the development of TMA. A “second hit” from either complement activation may promote multimerization of excessive VWF, leading to microvascular thrombosis [28].
This study has several strengths and limitations. We chose a nested case-control study with incidence sampling to allow for unbiased relative risk assessment. The matching on aGVHD onset and severity helped remove aGVHD as a strong confounder, as it is challenging to otherwise control for aGVHD severity or treatment. Nonetheless, we chose to not match on other pretransplantation characteristics to avoid overmatching; as such, the observed differences still could be attributable to other unadjusted characteristics. For instance, there were 2 patients with TMA who had nonmalignant disease as the indication for transplantation, whereas no such patients existed in the control set. The number of patients with mismatched donors (54% versus 40%) and those receiving second-line aGVHD treatments (31% versus 16%) were numerically higher in the TMA group compared with the control group. The differences in disease pathophysiology and treatments might partially explain our observed differences in complement and endothelial pathways. We measured longitudinal samples from patients at close follow-up intervals to be able to measure biomarkers before the onset of clinically relevant events. Finally, we performed concurrent testing of plausible biomarkers from multiple pathways. As for limitations, most of the assays used in the study are not yet available in routine commercial laboratories, and as such, our findings would be difficult to replicate in the clinical setting. The definition for TMA was clinical rather than biopsy-proven, as most of our patients did not undergo renal biopsy or autopsy. Fortunately, we had previously shown that our clinical definition had good consistency with relevant TMA case definitions [2]. Finally, although we matched on aGVHD to remove confounding, our current sample size was too small for adequate adjustment of potential confounding from infections or other post-transplantation complications. It should be noted that complement and endothelial biomarkers were frequently elevated in samples drawn at the times of active infection, especially in those with gram-negative bacteremia, BK viremia, and human herpesvirus 6 infection. Therefore, the interpretation needs to be considered in the context of the appropriate clinical scenario.
We realize that our present findings are associative rather than mechanistic; nonetheless, they can be useful for future experimental designs. TMA resulting from aGVHD is multifactorial and likely involves multiple pathways. First, we should assess whether aGVHD-related severity biomarkers (such as sST-2 and REG3-alpha) are overexpressed or underexpressed in patients with both aGVHD and TMA compared with those with aGVHD alone. Second, although we examined ADAMTS13 and VWF antigen expression in this study, we need to further assess the endogenous VWF proteolytic activity through mass spectrometry cleavage assays. Third, we should confirm in a prospective cohort study that elevated levels of sC5b9 and ANG2 at the onset of aGVHD are associated with TMA and mortality in an unselected patient population; such a cohort also would allow us to estimate the positive and negative predictive values of biomarker performance in preemptive surveillance. Ultimately, our goal is the prevention of TMA and its associated morbidity and mortality through the development of a personalized and validated biomarker panel among highrisk patients.
CONCLUSIONS
In conclusion, we found that elevated sC5b9 and ANG2 levels at the onset of aGVHD were associated with the development of TMA and possibly mortality, after accounting for the timing and severity of aGVHD. Our study suggests that early biomarker panel screening at the onset of aGVHD may help predict which individuals will later develop overt TMA.
ACKNOWLEDGMENTS
Financial disclosure:
The research reported in this article was supported by the Texas Cancer Prevention and Research Institute of Texas (RR190104), the Hemostasis and Thrombosis Research Society (Mentored Research Award) supported by an independent medical educational grant from Shire, National Hemophilia Foundation Shire Clinical Fellowship Award (to A. L.), and National Institutes of Health Grants R01 HL137991 (to D.W.C.) and R35 HL145262 ( to J.A.L.). Kypha provided the commercial sC5b9 ELISA kits.
Footnotes
Conflict of interest statement: W.C.L. is an inventor on 2 patents related to ANG2: “Biomarkers for early determination of a critical or life-threatening response to illness and/or treatment response” (US14/916,758); and 2) “Biomarkers and uses thereof for selecting immunotherapy intervention” (US16/484,788). J.A.L. and J.C. are inventors on “Biomarkers and uses thereof for selecting immunotherapy intervention” (US16/484,788).
REFERENCES
- 1.Laskin BL, Goebel J, Davies SM, Jodele S. Small vessels, big trouble in the kidneys and beyond: hematopoietic stem cell transplantation-associated thrombotic microangiopathy. Blood. 2011;118:1452–1462. [DOI] [PubMed] [Google Scholar]
- 2.Li A, Wu Q, Davis C, et al. Transplant-associated thrombotic microangiopathy is a multifactorial disease unresponsive to immunosuppressant withdrawal. Biol Blood Marrow Transplant. 2019;25:570–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Epperla N, Li A, Logan B, et al. Incidence, risk factors for and outcomes of transplant-associated thrombotic microangiopathy. Br J Haematol. 2020;189:1171–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Postalcioglu M, Kim HT, Obut F, et al. Impact of thrombotic microangiopathy on renal outcomes and survival after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2018;24:2344–2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cho BS, Yahng SA, Lee SE, et al. Validation of recently proposed consensus criteria for thrombotic microangiopathy after allogeneic hematopoietic stem-cell transplantation. Transplantation. 2010;90:918–926. [DOI] [PubMed] [Google Scholar]
- 6.Zeigler ZR, Rosenfeld CS, Andrews DF 3rd, et al. Plasma von Willebrand factor antigen (vWF:AG) and thrombomodulin (TM) levels in adult thrombotic thrombocytopenic purpura/hemolytic uremic syndromes (TTP/HUS) and bone marrow transplant-associated thrombotic microangiopathy (BMT-TM). Am J Hematol. 1996;53:213–220. [DOI] [PubMed] [Google Scholar]
- 7.Arai S, Allan C, Streiff M, Hutchins GM, Vogelsang GB, Tsai HM. Von Willebrand factor-cleaving protease activity and proteolysis of von Willebrand factor in bone marrow transplant-associated thrombotic microangiopathy. Hematol J. 2001;2:292–299. [DOI] [PubMed] [Google Scholar]
- 8.Jodele S, Davies SM, Lane A, et al. Diagnostic and risk criteria for HSCT-associated thrombotic microangiopathy: a study in children and young adults. Blood. 2014;124:645–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horváth Kállay K, Csuka D, et al. Early increase in complement terminal pathway activation marker sC5b-9 is predictive for the development of thrombotic microangiopathy after stem cell transplantation. Biol Blood Marrow Transplant. 2018;24:989–996. [DOI] [PubMed] [Google Scholar]
- 10.Qi J, Wang J, Chen J, et al. Plasma levels of complement activation fragments C3b and sC5b-9 significantly increased in patients with thrombotic microangiopathy after allogeneic stem cell transplantation. Ann Hematol. 2017;96:1849–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sartain S, Shubert S, Wu MF, Wang T, Martinez C. The alternative complement pathway activation product Ba as a marker for transplant-associated thrombotic microangiopathy. Pediatr Blood Cancer. 2020;67:e28070. [DOI] [PubMed] [Google Scholar]
- 12.Wall SA, Zhao Q, Yearsley M, et al. Complement-mediated thrombotic microangiopathy as a link between endothelial damage and steroidrefractory GVHD. Blood Adv. 2018;2:2619–2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang S, McGookey M, Wang Y, Cataland SR, Wu HM. Effect of blood sampling, processing, and storage on the measurement of complement activation biomarkers. Am J Clin Pathol. 2015;143:558–565. [DOI] [PubMed] [Google Scholar]
- 14.Bhatraju PK, Cohen M, Nagao RJ, et al. Genetic variation implicates plasma angiopoietin-2 in the development of acute kidney injury sub-phenotypes. BMC Nephrol. 2020;21:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sorror ML. How I assess comorbidities before hematopoietic cell transplantation. Blood. 2013;121:2854–2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 18.Thomas E, Storb R, Clift RA, et al. Bone-marrow transplantation (first of two parts). N Engl J Med. 1975;292:832–843. [DOI] [PubMed] [Google Scholar]
- 19.Oran B, Donato M, Aleman A, et al. Transplant-associated microangiopathy in patients receiving tacrolimus following allogeneic stem cell transplantation: risk factors and response to treatment. Biol Blood Marrow Transplant. 2007;13:469–477. [DOI] [PubMed] [Google Scholar]
- 20.Zeisbrich M, Becker N, Benner A, et al. Transplant-associated thrombotic microangiopathy is an endothelial complication associated with refractoriness of acute GvHD. Bone Marrow Transplant. 2017;52:1399–1405. [DOI] [PubMed] [Google Scholar]
- 21.Yuan HT, Khankin EV, Karumanchi SA, Parikh SM. Angiopoietin 2 is a partial agonist/antagonist of Tie2 signaling in the endothelium. Mol Cell Biol. 2009;29:2011–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luft T, Dietrich S, Falk C, et al. Steroid-refractory GVHD: T-cell attack within a vulnerable endothelial system. Blood. 2011;118:1685–1692. [DOI] [PubMed] [Google Scholar]
- 23.Bockmeyer CL, Claus RA, Budde U, et al. Inflammation-associated ADAMTS13 deficiency promotes formation of ultra-large von Willebrand factor. Haematologica. 2008;93:137–140. [DOI] [PubMed] [Google Scholar]
- 24.Claus RA, Bockmeyer CL, Budde U, et al. Variations in the ratio between von Willebrand factor and its cleaving protease during systemic inflammation and association with severity and prognosis of organ failure. Thromb Haemost. 2009;101:239–247. [PubMed] [Google Scholar]
- 25.Claus RA, Bockmeyer CL, Sossdorf M, Lo€sche W. The balance between von-Willebrand factor and its cleaving protease ADAMTS13: biomarker in systemic inflammation and development of organ failure? Curr Mol Med. 2010;10:236–248. [DOI] [PubMed] [Google Scholar]
- 26.Choi H, Aboulfatova K, Pownall HJ, Cook R, Dong JF. Shear-induced disulfide bond formation regulates adhesion activity of von Willebrand factor. J Biol Chem. 2007;282:35604–35611. [DOI] [PubMed] [Google Scholar]
- 27.Chen J, Fu X, Wang Y, et al. Oxidative modification of von Willebrand factor by neutrophil oxidants inhibits its cleavage by ADAMTS13. Blood. 2010;115:706–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng L, Zhang D, Cao W, Song WC, Zheng XL. Synergistic effects of ADAMTS13 deficiency and complement activation in pathogenesis of thrombotic microangiopathy. Blood. 2019;134:1095–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]


