Abstract
Purpose of Review:
Great progress has been made in understanding the genetic and molecular understanding of pheochromocytoma and paragangliomas (PPGLs). This review highlights the new standards in the diagnosis and management of pediatric PPGLs
Recent findings:
The vast majority of pediatric PPGLs have an associated germline mutation, making genetic studies imperative in the work up of these tumors. Somatostatin receptor based imaging modalities such as 68Ga-DOTATATE and 64Cu-DOTATATE are shown to have the greatest sensitivity in pediatric PPGLs. Peptide receptor radionuclide therapies such as 177Lu-DOTATATE are shown to have efficacy for treating PPGLs.
Summary:
Genetics play an important role in pediatric PPGLs. Advances in somatostatin receptor based technology have led to use of 68Ga-DOTATATE and 64Cu-DOTATATE as preferred imaging modalities. While surgery remains the mainstay for management of PPGLs, peptide receptor radionuclide therapy is emerging as a treatment option for PPGLs
Keywords: Pheochromocytoma, paraganglioma, nuclear medicine
Introduction
Pheochromocytoma and paraganglioma (PPGLs) are tumors arising from chromaffin cells. Chromaffin cell tumors within the adrenal glands are defined as pheochromocytomas, whereas tumors arising from extra-adrenal chromaffin cells are termed paragangliomas. They are often, but not always, biochemically active and can secrete catecholamines including dopamine, epinephrine and norepinephrine [1]. The exact incidence of PPGLs in the pediatric population is unclear, as they are overall rare tumors. It has been estimated that 1.7% of cases of pediatric hypertension are due to PPGLs [2]. The classic triad of symptoms including paroxysmal palpitation, diaphoresis, and headaches can be hard for children to describe and PPGLs can be missed [3]. With increased usage of abdominal imaging, the incidental discovery of PPGLs are becoming more and more common [4].
Unlike other oncologic process, staging of PPGLs does not follow the typical TNM system for classification. Previously the terminologies of “malignant” and “benign” were used to describe PPGLs. There are no histologic systems that can reliable identify the aggressiveness or malignant potential of PPGLs; therefore, all PPGLs are considered to have metastatic potential [5].
Genetics
The association between pediatric PPGLs and germline mutations of underlying cancer syndromes have been well-described. It has been estimated that most pediatric PPGLs are associated with an underlying germline mutations, in fact a recent French retrospective review of national data showed that at least 77% of patients with PPGLs have an associated germline mutation [6,7]. As a result, genetic testing has been recommended for all patients with PPGLs regardless of family history[8].
Genetic studies have led to the classification of PPGL into 3 different clusters based on their molecular defect, these include the pseudohypoxia pathway, Wnt signaling pathway, and kinase signaling pathway [9]. Genes involved in the pseudohypoxia pathway (SDHx, SDHAF2, FH, MDH2, VHL, EGLN1/2, and HIF2A) lead to activation of HIF-related hypoxia signaling pathway. The Wnt signaling pathway includes genes in the PI3K/AKT, MAPK, and mTOR kinase signaling pathway (NF1, KIF1B, MAX, RET, TMEM127, and ATRX). Genes in the Wnt signaling pathway (CSDE1 and MAML3) have also been identified as somatic mutations in PPGLs [10]. Of the above mentioned genetic mutations, genes in the pseudohypoxia pathway especially VHL, SDHB, and SDHD mutations account for almost all genetic abnormalities in children [11,12]. Table 1 include a summary of the common genes implicated in pediatric PPGL.
Table 1.
Common Genes Associated with Pediatric PPGLs
| Cluster | Syndrome | Gene | PPGL type |
|---|---|---|---|
| Pseudohypoxia | Von-Hippel-Lindau | VHL | Pheo > PGL |
| Hereditary Paraganglioma-Pheochromocytoma Syndrome | SDHB | PGL (abdominal) > Pheo | |
| SDHD | PGL (head and neck) | ||
| SDHC | PGL (head and neck) | ||
| SDHA | PGL | ||
| Kinase | Multiple Endocrine Neoplasia Type 2 | RET | Pheo >> PGL |
| Neurofibromatosis Type 1 | NF-1 | Pheo |
The most recent Endocrine Society guidelines suggested targeted gene testing/analysis based on biochemical profile and location of specific PPGLs, however, most commercially available genetic assays include all of the genes described above.
Identifying germline mutations are especially critical in PPGLs as they can be associated with hereditary cancer syndromes such as VHL, MEN2, or hereditary PPGL syndromes such as mutations involving the SDH complexes. Identifying these germline mutations not only aides in follow up surveillance of PPGLs but also determines appropriate active surveillance of other oncologic comorbidities based on the specific tumor/genetic syndromes identified. We therefore recommend all pediatric patients with PPGLs to undergo genetic testing and appropriate genetic counseling.
Biochemical Profile
As described by the most recent societal guidelines, diagnosis of PPGLs are made with biochemical/catecholamine profiles as well as imaging findings[8]. As PPGLs are commonly biochemically active and secrete catecholamines including dopamine, epinephrine, and norepinephrine, direct measurements of catecholamines and their metabolites are commonly measured. Measurements of plasma free metanephrines and urinary fractionated metanephrines are currently considered standard of care [8]. A recent study compared urine and plasma metanephrines by both HPLC and EIA methods and found that all 4 methods are of similar diagnostic utilities, in fact plasma metanephrines by EIA actually had the highest post-test probability in this analysis [13]. In the pediatric population, particular attention must be paid to the logistics of obtaining plasma and urine samples. Venipuncture can be traumatic and technically difficult in children and 24-hour urine collection can be challenging to perform in the home environment. Recent efforts have investigated other novel catecholamine metabolites to improve biochemical diagnostic accuracy of PPGLs. Among them, 3-methoxytyramine, an O-methylated metabolite of dopamine, has emerged as a promising metabolite [14,15]. Eisenhofer et al showed in 2018 that plasma and urinary free metabolites including methoxytyramine offer improved diagnostic accuracy over urinary deconjugated metabolites [16]. Peitzsch et al examined plasma and urinary methoxytyramine and again found that while overnight and daytime urinary methoxytyramine offer similar sensitivities, however, plasma methoxytyramine still has higher sensitivities than urinary studies [17]. While not widely commercially available, reference ranges of plasma and urinary methoxytyramine are in development and may represent the future of biochemical analysis for PPGLs [18,19]. Chromogranin A (CGA) is a common biomarker for neuroendocrine tumors including PPGLs [20]. While CGA is overall less studied in PPGLs compared to other biomarkers, Parisien-La Salle et al in a recent study examined CGA levels in PPGLs [21]. At diagnosis, they found elevated CGA levels in 53 out of 61 patients with PPGLs (87% sensitivity). Of note, CGA was 97.1% sensitive for pheochromocytoma, 85% sensitive for thoracic or abdominal paraganglioma, and 64% sensitive for head and neck paraganglioma. While not part of 2014 Endocrine Society guidelines, CGA is recommended as part of biochemical work up of PPGLs based on European Association of Nuclear Medicine and Society of Nuclear Medicine and Molecular Imaging guidelines [22]. However, CGA can be falsely elevated in many clinical settings including but not limited to cardiovascular disease, GI disease, use of PPI or H2 blockers, etc [23]
Imaging and Nuclear Medicine
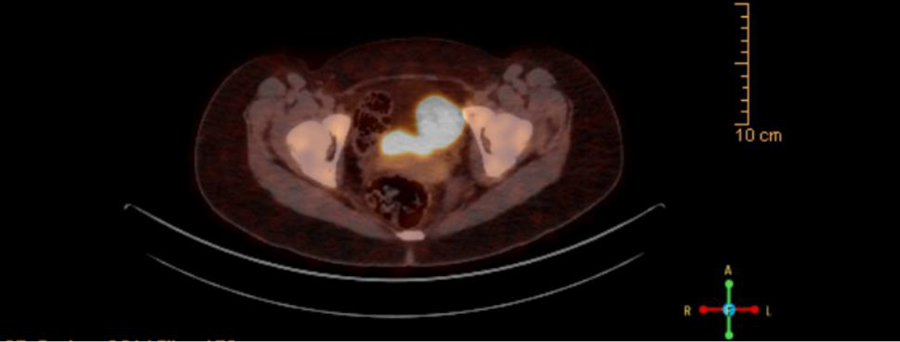
Imaging is integral in identifying the locations of PPGLs for definitive treatment. While PPGLs can be visualized on many different modalities include CT and MRI, they tend to have lower sensitivity compared to molecular imaging [24]. As pediatric PPGLs are more likely to be malignant in children, it is critical to use imaging modalities with high sensitivity in children [25,26]. Functional molecular imaging such as 131I-meta-iodobenzyl-guanidine (131I-MIBG) and 18F-fluorodeoxyglucose PET/CT (18F-FDG PET/CT) are part of the 2014 guideline for the preferred imaging modality [8]. Since then, newer techniques such as somatostatin receptor based PET/CT have been shown to have higher sensitivity in detecting PPGLs. Among the different somatostatin receptor based modalities, 68Ga-DOTA(0)-Tyr(3)-octreotate positron emission tomography–computed tomography (68Ga-DOTATATE PET/CT) has been shown to have improved sensitivity in PPGLs in the adult populations since it was FDA approved in 2016 [22,27–31]. Additionally, 68Ga-DOTATATE was found to have increased sensitivity over 18F-FDG PET/CT for head and neck paragangliomas that are often biochemically silent and difficult to image [32]. While large scale imaging studies for PPGLs are scarce in the pediatric population, Jaiswal et al recently showed that 68Ga-DOTATATE PET/CT has better sensitivity for both primary and metastatic PPGLs compared to both 131I-MIBG and 18F-FDG PET/CT [33]. Figure 1 shows the 68Ga-DOTATATE PET/CT scan for one of our patients with an abdominal PGL. 64Cu-DOTATATE PET/CT is another somatostatin receptor based radiotracer that is approved by the FDA for clinical use in neuroendocrine tumors such as PPGLs. Compared to 68Ga-DOTATATE, 64Cu-DOTATATE has a longer shelf-life, half-life, and thus more user friendly [34]. Early data from Johnbeck et al show that 64Cu-DOTATATE has similar sensitivity to 68Ga-DOTATATE in detecting neuroendocrine tumors but no specific studies for PPGLs or its use in the pediatric population have been published [34].
Figure 1.
68Ga-DOTATATE PET/CT scan of abdominal paraganglioma due to SDHB mutation.
Additionally, the development of DOTATATE imaging modalities have led to novel therapeutic options for PPGLs such as peptide receptor radionuclide therapy (PRRT) with 177Lu-DOTA0,Tyr3-octreotate (177Lu-DOTATATE) or 90Y-DOTA0,Tyr3-octreotide90 (Y-DOTATOC). PRRT takes advantage of radiolabeled somatostatin analogs to deliver radiation therapy to target tissue with somatostatins such as PPGLs [35]. Prior to PRRT, systemic chemotherapy was done through 131I-MIBG. Both 90Y-DOTATOC and 177Lu-DOTATATE have been shown to improve overall survival and progression free survival in patients with metastatic PPGLs [36]. 90Y-DOTATOC is known to be associated with severe nephrotoxicity and recent efforts have attempted personalizing the dose of 90Y-DOTATOC via dosimetry to reduce toxicity with some promise [37,38]. Recently, in a retrospective review of 22 patients with PPGL treated with 177Lu-DOTATATE as first-line therapy, Vyakaranam et al found that 20 patients (90.9%) had stable disease and no patients had significant (grade 3–4) kidney or hematologic toxicity (based on CTCAE v4.0) [39]. Jaiswal et al reported 15 patients with inoperable (metastatic or unresectable) PPGLs who underwent 177Lu-DOTATATE therapy. 12 patients had controlled disease while 3 had progressive disease [40]. Similarly, they reported a favorable side effect profile without any long-term kidney or hematologic toxicities. Comparing those who did and did not respond to therapy, they found that increased enhancement on 68Ga-DOTATATE (SUVmax>21) is predictive of response to 177Lu-DOTATATE. No studies on 177Lu-DOTATATE in pediatric PPGLs have been published so far, but there is an ongoing Clinical Trial that will provide more insights on the role of 177Lu-DOTATATE therapy in the pediatric population in the near future (NCT03923257).
Perioperative blockade
As PPGLs secrete catecholamines (epinephrine, norepinephrine, and dopamine) they have significant and profound cardiovascular effects via alpha and beta adrenergic receptors. While no randomized control studies exist for perioperative blockade versus none, the increase in patient survival and decrease in surgical morbidity and mortality has been largely attributed to the use of alpha blocking agents [41]. Different approaches to blood pressure management exist in the literature but the typical strategy to initially obtain preoperative blood pressure control is through alpha blockade agents [42,43]. Alpha blockade agents can lead to orthostasis and reflex tachycardia, at which point beta blockers are introduced to the antihypertensive regimen. It is important to note that the use beta blockers and other medications without alpha blockade can lead to unopposed stimulation of alpha adrenergic receptors potentially leading to hypertensive crisis [8]. They must be avoided in suspected cases of PPGL or used with caution from expert guidance. Aside from alpha and beta blockers, calcium channel blockers can serve as an adjunct to alpha blockade if goal BP cannot be reached. In cases of severe and refractory hypertension, metyrosine, a tyrosine 3-monooxygenase inhibitor, can be used as well and has been found to be effective in achieving blood pressure control in Japanese children as an add-on to alpha-blockers [44].
Previously there had been limited studies examining the clinical impact of different alpha blockade agents. The PRESCIPT trial published in 2020 is the only multicenter randomized controlled trial comparing alpha blockade agents in PPGLs [45]. PRESCRIPT compared the use of doxazosin and phenoxybenzamine and found similar time in range for blood pressure however, patients treated with phenoxybenzamine group tend to have less hemodynamic instability (requiring less fluids and vasoactive agents by the anesthesiologist). The clinical outcome of this difference in intraoperative instability was unclear as both groups had similar morbidity and mortality.
Guidelines do not exist for pediatric preoperative blood pressure goals in patients with PPGLs. In practice, we use blood pressure parameters as defined by 2017 AAP guidelines on pediatric hypertension as a guide and titrate antihypertensive agents to reach blood pressure measurements below the 90th percentile for height and age [46]. While we prefer using phenoxybenzamine as alpha blocker, however, it is not widely covered by medical insurance.
Surgery
Surgery remains the only curative option for PPGLs. Since the first case of endoscopic removal of pheochromocytoma, minimally invasive approaches are now more and more widely available and is considered the standard of care [47]. For pheochromocytoma, adrenal cortex sparing surgeries are recommended especially in the cases of bilateral pheochromocytoma and those with certain hereditary causes of PPGLs. Data from European-American-Asian-Bilateral-Pheochromocytoma-Registry showed that cortical sparing surgeries can preserve adrenal function in up to 23.5% of patients whereas all individuals who underwent complete bilateral adrenalectomy whether simultaneously or subsequently due to new pheochromocytoma developed adrenal insufficiency [48]. The same study did find that those who underwent adrenal cortical sparing procedures have higher rate of recurrence (13%) compared to those who underwent complete bilateral adrenalectomy (0.7%); however, there is no difference in overall mortality and survival.
As PPGLs are rare diseases in the pediatric population, there is no literature available on the outcomes of adrenal cortical sparing surgery in children. However, as children can have high rate (up to 39%) of tumor recurrence, it is even more important to perform adrenal cortical sparing procedures to preserve adrenal function and prevent morbidity with both glucocorticoid and mineralocorticoid deficiency [49]. Typically, it is believed that 15–30% of residual adrenal tissue may be sufficient to preserve adequate adrenal function [50]. Minimally invasive adrenal cortical sparing surgeries, while considered the gold standard, may not be readily available at pediatric centers. Guidance/assistance from adult endocrine surgeons may be necessary to safely perform the procedure. For paragangliomas, depending on the location, different surgical subspecialties may be involved (ENT, urology, etc). We therefore recommend all pediatric patients with PPGLs to be evaluated and managed by a center with a multidisciplinary team experienced in both medical and surgical management of PPGLs.
Conclusion
PPGLs, though rare, is an important cause of pediatric morbidity and mortality. Recent studies have shown genetic studies are imperative in the diagnosis and work up of PPGLs due to high prevalence of underlying germline mutations in pediatric PPGLs. Identifying underlying germline mutation has great implications in follow up management (screening for associated co-morbidities) as well as current management of PPGL. Somatostatin receptor based nuclear medicine imaging modalities such as 68Ga-DOTATATE and 64Cu-DOTATATE have become the standard of care for imaging of PPGLs. Somatostatin receptor based technology have also made possible novel therapeutic options including PRRT (177Lu-DOTATATE and 90Y-DOTATOC) for metastatic and inoperable PPGLs. Surgery remains the main and definitive treatment for PPGLs. Alpha blockers remain the primary preoperative blockade agent to prevent perioperative catecholamine crisis. Adjuncts to alpha blocker include beta blocker, calcium channel blocker, and/or metyrosine. As described in this review, diagnosis and management of PPGLs is complex and requires multidisciplinary team approach with multiple subspecialties including but not limited to pediatric endocrinology, oncology, genetics, and surgery teams.
Key Points:
Due to high prevalence of germline mutations causing pediatric PPGLs, genetic studies are recommended for all children with PPGLs
Somatostatin receptor based imaging modalities such as 68Ga-DOTATATE and 64Cu-DOTATATE have become the preferred imaging modality for PPGLs
PRRTs (177Lu-DOTATATE and 90Y-DOTATOC) are novel therapies for metastatic and inoperable PPGLs with early promise, however surgery remain the preferred and definitive treatment for PPGLs
Footnotes
Conflicts of interests: none
References:
- 1.Waguespack SG, Ying AK: CHAPTER 14 - Pheochromocytoma and multiple endocrine neoplasia syndromes. In Pediatric Endocrinology (Fourth Edition). Edited by Sperling MA. W.B. Saunders; 2014:533–568.e1. [Google Scholar]
- 2.Wyszyńska T, Cichocka E, Wieteska-Klimczak A, et al. : A single pediatric center experience with 1025 children with hypertension. Acta Paediatr Oslo Nor 1992 1992, 81:244–246. [DOI] [PubMed] [Google Scholar]
- 3.Lo CY, Lam KY, Wat MS, et al. : Adrenal pheochromocytoma remains a frequently overlooked diagnosis. Am J Surg 2000, 179:212–215. [DOI] [PubMed] [Google Scholar]
- 4.Ebbehoj A, Stochholm K, Jacobsen SF, et al. : Incidence and Clinical Presentation of Pheochromocytoma and Sympathetic Paraganglioma: A Population-based Study. J Clin Endocrinol Metab 2021, doi: 10.1210/clinem/dgaa965. [DOI] [PubMed] [Google Scholar]
- 5.Lam AK: Update on Adrenal Tumours in 2017 World Health Organization (WHO) of Endocrine Tumours. Endocr Pathol 2017, 28:213–227. [DOI] [PubMed] [Google Scholar]
- 6. Virgone C, Andreetta M, Avanzini S, et al. : Pheochromocytomas and paragangliomas in children: Data from the Italian Cooperative Study (TREP). Pediatr Blood Cancer 2020, 67:e28332. * Italian cohort of children with PPGL including diagnostic imaging, associated genetic disorder, and surgical and medical therapy.
- 7.Toledo RA, Burnichon N, Cascon A, et al. : Consensus Statement on next-generation-sequencing-based diagnostic testing of hereditary phaeochromocytomas and paragangliomas. Nat Rev Endocrinol 2017, 13:233–247. [DOI] [PubMed] [Google Scholar]
- 8.Lenders JWM, Duh Q-Y, Eisenhofer G, et al. : Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2014, 99:1915–1942. [DOI] [PubMed] [Google Scholar]
- 9.Fishbein L, Leshchiner I, Walter V, et al. : Comprehensive molecular characterization of pheochromocytoma and paraganglioma. Cancer Cell 2017, 31:181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jochmanova I, Pacak K: Genomic Landscape of Pheochromocytoma and Paraganglioma. Trends Cancer 2018, 4:6–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neumann HPH: Pheochromocytoma. In Harrison’s Principles of Internal Medicine. Edited by Jameson JL, Fauci AS, Kasper DL, et al. McGraw-Hill Education; 2018. [Google Scholar]
- 12.Pamporaki C, Hamplova B, Peitzsch M, et al. : Characteristics of Pediatric vs Adult Pheochromocytomas and Paragangliomas. J Clin Endocrinol Metab 2017, 102:1122–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Raber W, Kotal H, Marculescu R, et al. : Measurements of Plasma-Free Metanephrines by Immunoassay Versus Urinary Metanephrines and Catecholamines by Liquid Chromatography with Amperometric Detection for the Diagnosis of Pheochromocytoma/Paraganglioma. J Clin Med 2020, 9. * Retrospective review showing EIA assays of plasma and urine metanephrines have similar positive predictive value to HPLC methods
- 14.Wang K, Gao X, Cong H, et al. : Stability and reference intervals of spot urinary fractionated metanephrines and methoxytyramine by tandem mass spectrometry as a screening method for pheochromocytoma and paraganglioma. Endocrine 2020, 69:188–195. [DOI] [PubMed] [Google Scholar]
- 15.Eisenhofer G, Klink B, Richter S, et al. : Metabologenomics of Phaeochromocytoma and Paraganglioma: An Integrated Approach for Personalised Biochemical and Genetic Testing. Clin Biochem Rev 2017, 38:69–100. [PMC free article] [PubMed] [Google Scholar]
- 16.Eisenhofer G, Prejbisz A, Peitzsch M, et al. : Biochemical Diagnosis of Chromaffin Cell Tumors in Patients at High and Low Risk of Disease: Plasma versus Urinary Free or Deconjugated O-Methylated Catecholamine Metabolites. Clin Chem 2018, 64:1646–1656. [DOI] [PubMed] [Google Scholar]
- 17.Peitzsch M, Kaden D, Pamporaki C, et al. : Overnight/first-morning urine free metanephrines and methoxytyramine for diagnosis of pheochromocytoma and paraganglioma: is this an option? Eur J Endocrinol 2020, 182:499–509. [DOI] [PubMed] [Google Scholar]
- 18.Wang H, Zhang X, Zhen Q, et al. : Detection of spot urinary free metanephrines and 3-methoxytyramine with internal reference correction for the diagnosis of pheochromocytomas and paragangliomas. J Chromatogr B Analyt Technol Biomed Life Sci 2020, 1156:122306. [DOI] [PubMed] [Google Scholar]
- 19.Peitzsch M, Mangelis A, Eisenhofer G, et al. : Age-specific pediatric reference intervals for plasma free normetanephrine, metanephrine, 3-methoxytyramine and 3-O-methyldopa: Particular importance for early infancy. Clin Chim Acta Int J Clin Chem 2019, 494:100–105. [DOI] [PubMed] [Google Scholar]
- 20.Oronsky B, Ma PC, Morgensztern D, et al. : Nothing But NET: A Review of Neuroendocrine Tumors and Carcinomas. Neoplasia N Y N 2017, 19:991–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parisien-La Salle S, Provençal M, Bourdeau I: Chromogranin A in a cohort of pheochromocytomas and paragangliomas: usefulness at diagnosis and as an early biomarker of recurrence. Endocr Pract Off J Am Coll Endocrinol Am Assoc Clin Endocrinol 2021, doi: 10.1016/j.eprac.2020.09.011. [DOI] [PubMed] [Google Scholar]
- 22. Taïeb D, Hicks RJ, Hindié E, et al. : European Association of Nuclear Medicine Practice Guideline/Society of Nuclear Medicine and Molecular Imaging Procedure Standard 2019 for radionuclide imaging of phaeochromocytoma and paraganglioma. Eur J Nucl Med Mol Imaging 2019, 46:2112–2137. ** European guidelines summarizing latest diagnostic criteria for PPGLs including recommendation of 68Ga-DOTATATE PET/CT as imaging modality
- 23.Gut P, Czarnywojtek A, Fischbach J, et al. : Chromogranin A – unspecific neuroendocrine marker. Clinical utility and potential diagnostic pitfalls. Arch Med Sci AMS 2016, 12:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lumachi F, Tregnaghi A, Zucchetta P, et al. : Sensitivity and positive predictive value of CT, MRI and 123I-MIBG scintigraphy in localizing pheochromocytomas: a prospective study. Nucl Med Commun 2006, 27:583–587. [DOI] [PubMed] [Google Scholar]
- 25.Mannelli M, Ianni L, Cilotti A, et al. : Pheochromocytoma in Italy: a multicentric retrospective study. Eur J Endocrinol 1999, 141:619–624. [DOI] [PubMed] [Google Scholar]
- 26.Pham TH, Moir C, Thompson GB, et al. : Pheochromocytoma and paraganglioma in children: a review of medical and surgical management at a tertiary care center. Pediatrics 2006, 118:1109–1117. [DOI] [PubMed] [Google Scholar]
- 27.Jha A, Ling A, Millo C, et al. : Superiority of 68Ga-DOTATATE over 18F-FDG and anatomic imaging in the detection of succinate dehydrogenase mutation (SDHx)-related pheochromocytoma and paraganglioma in the pediatric population. Eur J Nucl Med Mol Imaging 2018, 45:787–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang CA, Pattison DA, Tothill RW, et al. : 68Ga-DOTATATE and 18F-FDG PET/CT in Paraganglioma and Pheochromocytoma: utility, patterns and heterogeneity. Cancer Imaging 2016, 16:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kan Y, Zhang S, Wang W, et al. : 68Ga-somatostatin receptor analogs and 18F-FDG PET/CT in the localization of metastatic pheochromocytomas and paragangliomas with germline mutations: a meta-analysis. Acta Radiol Stockh Swed 1987 2018, 59:1466–1474. [DOI] [PubMed] [Google Scholar]
- 30.Gild ML, Naik N, Hoang J, et al. : Role of DOTATATE-PET/CT in preoperative assessment of phaeochromocytoma and paragangliomas. Clin Endocrinol (Oxf) 2018, 89:139–147. [DOI] [PubMed] [Google Scholar]
- 31.Janssen I, Blanchet EM, Adams K, et al. : Superiority of [68Ga]-DOTATATE PET/CT to Other Functional Imaging Modalities in the Localization of SDHB-Associated Metastatic Pheochromocytoma and Paraganglioma. Clin Cancer Res 2015, 21:3888–3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Janssen I, Taieb D, Patronas NJ, et al. : [68Ga]-DOTATATE PET/CT in the localization of head and neck paragangliomas compared to other functional imaging modalities and CT/MRI. J Nucl Med Off Publ Soc Nucl Med 2016, 57:186–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jaiswal SK, Sarathi V, Malhotra G, et al. : The utility of 68Ga-DOTATATE PET/CT in localizing primary/metastatic pheochromocytoma and paraganglioma in children and adolescents – a single-center experience. J Pediatr Endocrinol Metab 2021, 34:109–119. ** Retrospective cohort of 32 pediatric patients with PPGLs showing highest lesion sensitivity in 68Ga-DOTATATE PET/CT compared to 18F-FDG PET/CT and 131I-MIBG.
- 34.Johnbeck CB, Knigge U, Loft A, et al. : Head-to-Head Comparison of 64Cu-DOTATATE and 68Ga-DOTATOC PET/CT: A Prospective Study of 59 Patients with Neuroendocrine Tumors. J Nucl Med 2017, 58:451–457. [DOI] [PubMed] [Google Scholar]
- 35.Jong M de, Krenning E: New Advances in Peptide Receptor Radionuclide Therapy. J Nucl Med 2002, 43:617–620. [PubMed] [Google Scholar]
- 36.Nastos K, Cheung VTF, Toumpanakis C, et al. : Peptide Receptor Radionuclide Treatment and (131)I-MIBG in the management of patients with metastatic/progressive phaeochromocytomas and paragangliomas. J Surg Oncol 2017, 115:425–434. [DOI] [PubMed] [Google Scholar]
- 37.Menda Y, Madsen MT, O’Dorisio TM, et al. : 90Y-DOTATOC Dosimetry–Based Personalized Peptide Receptor Radionuclide Therapy. J Nucl Med 2018, 59:1692–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vegt E, de Jong M, Wetzels JFM, et al. : Renal toxicity of radiolabeled peptides and antibody fragments: mechanisms, impact on radionuclide therapy, and strategies for prevention. J Nucl Med Off Publ Soc Nucl Med 2010, 51:1049–1058. [DOI] [PubMed] [Google Scholar]
- 39. Vyakaranam AR, Crona J, Norlén O, et al. : Favorable Outcome in Patients with Pheochromocytoma and Paraganglioma Treated with 177Lu-DOTATATE. Cancers 2019, 11. * Retrospective cohort of 22 patients with PPGL who underwent 177Lu-DOTATATE that showed improved overall survival and progression free survivial.
- 40. Kumar Jaiswal S, Sarathi V, Samad Memon S, et al. : 177Lu-DOTATATE therapy in metastatic/inoperable pheochromocytoma-paraganglioma. Endocr Connect 2020, 9:864–873. **177Lu-DOTATATE can lead to disease stability in metastatic or inoperable PPGLs. SUVmax >21 on DOTATE is a predictive factor of response to PRRT.
- 41.Livingstone M, Duttchen K, Thompson J, et al. : Hemodynamic Stability During Pheochromocytoma Resection: Lessons Learned Over the Last Two Decades. Ann Surg Oncol 2015, 22:4175–4180. [DOI] [PubMed] [Google Scholar]
- 42.Berends AMA, Kerstens MN, Lenders JWM, et al. : Approach to the Patient: Perioperative Management of the Patient with Pheochromocytoma or Sympathetic Paraganglioma. J Clin Endocrinol Metab 2020, 105:3088–3102. [DOI] [PubMed] [Google Scholar]
- 43.Pacak K: Preoperative management of the pheochromocytoma patient. J Clin Endocrinol Metab 2007, 92:4069–4079. [DOI] [PubMed] [Google Scholar]
- 44. Naruse M, Satoh F, Tanabe A, et al. : Efficacy and safety of metyrosine in pheochromocytoma/paraganglioma: a multi-center trial in Japan. Endocr J 2018, 65:359–371. * Only randomized control trial of metyrosine in PPGL as adjunct to alpha blockade, which is shown to be effective in outpatient control of blood pressure.
- 45. Buitenwerf E, Osinga TE, Timmers HJLM, et al. : Efficacy of α-Blockers on Hemodynamic Control during Pheochromocytoma Resection: A Randomized Controlled Trial. J Clin Endocrinol Metab 2020, 105:2381–2391. * Randomized control trial comparing phenoxybenzamine and doxazosin. While clinical outcomes are similar for both groups. However, those in the phenoxybenzamine group have less intraop hemodynamic instability.
- 46.Flynn JT, Kaelber DC, Baker-Smith CM, et al. : Clinical Practice Guideline for Screening and Management of High Blood Pressure in Children and Adolescents. Pediatrics 2017, 140. [DOI] [PubMed] [Google Scholar]
- 47.Gagner M, Breton G, Pharand D, et al. : Is laparoscopic adrenalectomy indicated for pheochromocytomas? Surgery 1996, 120:1076–1079; discussion 1079–1080. [DOI] [PubMed] [Google Scholar]
- 48. Neumann HPH, Tsoy U, Bancos I, et al. : Comparison of Pheochromocytoma-Specific Morbidity and Mortality Among Adults With Bilateral Pheochromocytomas Undergoing Total Adrenalectomy vs Cortical-Sparing Adrenalectomy. JAMA Netw Open 2019, 2:e198898. *Multicenter retrospective analysis of total vs cortical-sparing adrenalectomy. 13% of patients with cortical-sparing adrenalectomy developed recurrence but no difference in overall survival was discovered
- 49. de Tersant M, Généré L, Freyçon C, et al. : Pheochromocytoma and Paraganglioma in Children and Adolescents: Experience of the French Society of Pediatric Oncology (SFCE). J Endocr Soc 2020, 4. * Data on children with PPGL from French national registry including genetic studies, treatment received, and 3–5 year survival.
- 50.Brauckhoff M, Gimm O, Thanh PN, et al. : Critical size of residual adrenal tissue and recovery from impaired early postoperative adrenocortical function after subtotal bilateral adrenalectomy. Surgery 2003, 134:1020–1027. [DOI] [PubMed] [Google Scholar]