Abstract
Since the coronavirus disease 2019 (COVID-19) pandemic epidemic, the excessive usage of chlorinated disinfectants raised the substantial risks of disinfection by-products (DBPs) exposure. While several technologies may remove the typical carcinogenic DBPs, trichloroacetic acid (TCAA), their application for continuous treatment is limited due to their complexity and expensive or hazardous inputs. In this study, degradation and dechlorination of TCAA induced by an in situ 222 nm KrCl* excimer radiation as well as role of oxygen in the reaction pathway were investigated. Quantum chemical calculation methods were used to help predict the reaction mechanism. Experimental results showed that UV irradiance increased with increasing input power and decreased when the input power exceeded 60 W. Decomposition and dechlorination were simultaneously achieved, where around 78% of TCAA (0.62 mM) can be eliminated and 78% dechlorination within 200 min. Dissolved oxygen showed little effect on the TCAA degradation but greatly boosted the dechlorination as it can additionally generate hydroxyl radical (•OH) in the reaction process. Computational results showed that under 222 nm irradiation, TCAA was excited from S0 to S1 state and then decayed by internal crossing process to T1 state, and a reaction without potential energy barrier followed, resulting in the breaking of C–Cl bond and finally returning to S0 state. Subsequent C–Cl bond cleavage occurred by a barrierless •OH insertion and HCl elimination (27.9 kcal/mol). Finally, the •OH attacked (14.6 kcal/mol) the intermediate byproducts, leading to complete dechlorination and decomposition. The KrCl* excimer radiation has obvious advantages in terms of energy efficiency compared to other competitive methods. These results provide insight into the mechanisms of TCAA dechlorination and decomposition under KrCl* excimer radiation, as well as important information for guiding research toward direct and indirect photolysis of halogenated DBPs.
Keywords: KrCl* excimer radiation, Trichloroacetic acid, Degradation mechanism, Theoretical calculations
Graphical abstract
1. Introduction
Chlorination performs a critical role in preventing infectious illnesses from spreading among people through drinking water (Gilca et al., 2020). However, noxious chlorinated disinfection by-products (DBPs) were usually generated by the reaction of free chlorine (HOCl and OCl−) with natural organic matter (Liang et al., 2022). The use of chlorine for disinfection has grown since the COVID-19 pandemic epidemic, which is raising the hazards of chlorinated DBPs exposure, especially in raw water (Wang et al., 2020b; Cai et al., 2021). Due to widespread distribution, substantial quantities and considerable toxicity, trichloroacetic acid (TCAA) as one of the most common chlorinated DBPs, had drawn the public's attention (Humans et al., 2004; Hamidin et al., 2008; Varshney et al., 2014). Numerous studies have demonstrated the potential carcinogenicity of TCAA for human (Humans et al., 2004; Mazhar et al., 2020). World Health Organization, U.S., China, Japan, European Union, Australia and Canada have each published guidance to limit TCAA maximum concentration levels. Therefore, TCAA contaminated water must be effectively treated at the point-of-use (POU) before drinking (Sobsey et al., 2008).
To date, various removal methods for TCAA via POU systems have been reported, including heating/boiling (Chen et al., 2021), activated carbon (AC) adsorption (Wu et al., 2017) and membrane filtration (Pérez-Vidal et al., 2016), as well as electrolysis (Hill et al., 2022), ultraviolet (UV) based (Hejazi and Taghipour, 2022), and UV radiation (Ao et al., 2021) emerging technology. Boiling decreased poor levels of TCAA while increased the formation of dichloroacetic acid (DCAA), resulting in unchanged average levels of total risk (Levesque et al., 2006; Pan et al., 2014). TCAA removal via AC adsorption was relatively low due to its high hydrophilicity, and water quality may be deteriorated over time (Wang et al., 2018). AC cartridges also requires frequent replenishment or replacement (Xiao et al., 2014). High energy consumption and low water recovery limited the widespread applications of membrane filtration in large-scale engineering processes (Drioli et al., 2011). The addition of medication and oxygen, as well as material preparation and recycling, considerably raises the risk and cost of the UV/H2O2, UV/TiO2, UV/Fenton (Liu et al., 2016) and electrolysis. UV rays can sanitize the water for POU without additional chemicals and has the potential to eliminate TCAA (Lui et al., 2014). Due to poor absorption capability in the UV domain (<240 nm), traditional mercury lamp (254 nm) and UV light-emitting diodes (UV-LEDs) (i.e., 255, 265, 280, and 310 nm) are difficult to degrade TCAA. Although vacuum UV (VUV) can radiate 185 nm and 254 nm light simultaneously, it suffers low specific energy and potential release of highly toxic mercury into the environment (Silva et al., 2021). Excimer molecules (rare gas, halogen) and exciplex molecules (rare gas halide RgX*) can be selected to provide narrow band radiation at different wavelengths. Low wavelength UV at 222 nm generated by a KrCl* excimer operated by a dielectric barrier discharge (DBD) has drawn interest as a novel POUs strategy (Oh et al., 2020). Its obvious advantages include instant-on capability, very quick warm-up time, flat geometry, dimmability (Raeiszadeh and Taghipour, 2019), environmental friendliness (mercury-free) (Hejazi and Taghipour, 2021) and without adding additional oxygen during usage. Recent studies have shown that irradiation with 222 nm UV might be an optimum choice for effective disinfection that is biologically safe for human cells (Narita et al., 2018; Bhardwaj et al., 2021). Vibrationally relaxed excimer molecules of KrCl* can decay through radiative processes via the following reactions (Eqs. (1), (2), (3), (4), (5), (6), (7), (8)) and generate UV radiation of 222 nm (Belasri et al., 2013).
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
| (6) |
| (7) |
| (8) |
There have been few mechanistic investigations on the use of KrCl* excimer radiation for both dechlorination and decomposition of halogenated organic pollutants, to the best of our knowledge. The purpose of this study was to assess the feasibility and analyze the reaction mechanisms of KrCl* excimer radiation for TCAA degradation and dechlorination. A series of operating variables and environmental conditions, such as input power (P in), oxygen effect, UV irradiance value (I UV), on the degradation and dechlorination were investigated. Quantum chemical calculation method was also utilized to help explore the reaction mechanisms, pathways, products, and driving forces (i.e., direct and indirect photolysis) potentially responsible for the photochemical process because some intermediate products are non-detectable (Yeung et al., 2021).
2. Materials and methods
2.1. Chemicals and materials
AnalaR grade TCAA, 5, 5-dimethyl-1-pyrroline N-oxide (DMPO) and methyl-tert-butyl-ether (MTBE) were purchased from the Aladdin Industrial Corporation, Shanghai, China. Chlorine and krypton were supplied from Hong Kong Specialty Gases Co., LTD. All other analytical-grade chemicals were obtained from National Medicines Corporation Ltd, China.
2.2. Experiment setup
Experimental device was composed of a power supply, a KrCl* excimer discharge tube, a reaction vessel and a series of analytical instruments, which were shown in Fig. 1 (a). The reaction vessel was a cylindrical quartz beaker (diameter: 4 cm, height: 42 cm) where the KrCl* excimer discharge tube was vertically positioned in the center. The KrCl* excimer discharge tube consisted of an inner discharge electrode and outer quartz tube, where the gap between the inner electrode and the inside surface of the outer tube was approximately 0.5 cm. The gap was filled with Kr and Cl2 (V Kr:V Cl2 ≈ 200: 1) at a total pressure of about 200 Torr. At each run, 570 mL of TCAA solution was poured into the reaction vessel for treatment. The solution was earthed and as the outer electrode. DBD was initiated by switching on the AC high-voltage power supply (CORONA LAB. CTP-2000K). Light emission from the discharge tube was analyzed by using a spectrometer (Maya, 2000PRO, Ocean Optics, Dunedin, FL, USA). Voltage and current waveforms of the discharge were monitored using a digital oscilloscope (TBS 1102 B, Tektronix Technology Co., Ltd. USA). Typical emission spectra of the KrCl* excimer radiation and the molar absorption coefficients of TCAA were demonstrated in Fig. 1 (b). I UV was determined according to the iodie/dodate chemical actinometry (S1). During deoxygenation experiments, dissolved oxygen (DO) levels were kept below 1.0 mg/L by purging the solutions with argon gas for 30 min, then maintaining a flow rate of 0.2 mL/min throughout the reaction procedure.
Fig. 1.
(a) Schematic of the experimental setup; (b) Molar absorption coefficient of TCAA and typical emission spectrum of the KrCl* excimer radiation; (c) equivalent circuit diagram of the KrCl* excimer discharge tube; (d) typical voltage and current waveforms of the discharge.
2.3. Analysis of TCAA and its degradation products
Concentrations of TCAA ([TCAA]0 and [TCAA]) and chloride ion ([Cl−]0 and [Cl−]) at the beginning and time t of the reaction in the solution were determined by Ion-chromatography (ICS-1100, Thermo Fisher, USA). Percentages of remaining (Eq. (9)) and dechlorination (Eq. (10)) were chosen as TCAA removal performance metrics in the photoreactor.
| (9) |
| (10) |
A liquid-liquid microextraction-acidic methanol derivatization together gas chromatography/mass spectrometry (GC/MS, Shimadzu GC-2010/QP2010, Japan) detection methods were developed for determining intermediate chloroacetic acids (CAAs) in solution (S2).Carbon dioxide (CO2) was determined by the titrimetric method for free CO2 (APHA, 1995). Amount of Total Organic Carbon (TOC) was measured by the high-temperature combustion method via a TOC analyzer (TOC-VCPH, Shimadzu, Japan). Short-lived species such as hydroxyl radicals (•OH) were trapped by DMPO and then were analyzed by Electron Paramagnetic Resonance spectrometer (EPR, Bruker E500, Germany) (Li et al., 2022b).
2.4. Analysis of discharge indices
The P in was directly displayed from the power supply. The Lissajous figure assumed that the KrCl* excimer discharge tube was regarded as a capacitor C t, which constituted by the equivalent capacitance of the quartz dielectric layer (C d) and the discharge gap (C g) in series relationship (Wang et al., 2020a). The equivalent circuit model is shown in Fig. 1 (c). The quantitative connection between the three capacitors is given in Eq. (11). Under low-frequency sinusoidal voltage driving, the voltage across the discharge tube (U KrCl*) and charge quantity (Q KrCl*) in the equivalent capacitance will appear on the digital oscilloscope as an approximate parallelogram figure (Fang et al., 2008).
The Q KrCl* was measured using a capacitor (Cx) of 0.47 μF in series with the KrCl* excimer discharge tube, where U x is the voltage across the Cx at time t. When the charges deposited on the dielectric surfaces are zero, the gas gap voltage (U gap) in Eq. (12) equals the applied voltage (U a and U b), which is the distance between the two points on the parallelogram intersects the x-axis of the Lissajous figure. The average power consumption () of KrCl* excimer discharge tube could be calculated in Eqs. (13), (14), (15), where area inside the closed Lissajous curve (S lisa) divided by the AC cycle period was equal to (Wang et al., 2021b).
| (11) |
| (12) |
| (13) |
| (14) |
| (15) |
The voltage and current waveforms in Fig. 1 (d) had a sinusoidal shape at 19 kHz. The current waveform featured a sequence of high-amplitude spikes caused by each micro-discharge, resulting in a significant electrical impedance fluctuation within the gas chamber (Li et al., 2020).
2.5. Quantum calculation
Quantum mechanical calculations of TCAA were performed using Gaussian 16 (Frisch et al., 2016). Geometry optimizations and vibrational frequencies of TCAA were calculated using the density functional theory (DFT) at M06–2x/6-311 + G** basis set. Their vertical excitation properties were computed by time-dependent density functional theory (TDDFT) calculations with the function mentioned above. No imaginary frequency modes were observed at the stationary states of the optimized structures shown in this study. To mimic the water environment, solvent effect was an integral equation formalism polarizable continuum model (IEF-PCM) (Klamt et al., 2015). Besides, potential energy curves were obtained from relaxed scans. Electrophilic and nucleophilic interactions were predicted from the Fukui function (Yang and Parr, 1985) calculations.
3. Results and discussion
3.1. Effect of input power on the KrCl* excimer radiation
Functionality of the designed KrCl* excimer radiation was validated by analyzing the Lissajous figures in Fig. 2 (a). The curves were standard parallelograms (Rodrigues et al., 2018) with the same slopes and intercepts at different P in, C g and U gap with the P in increasing were similar values calculated by Eqs. (11), (12) in Table S1, it demonstrated discharge uniformity and stable transition of the discharge mode between positive and negative half-cycles. The possible reason was that P in only affects the reaction of the gas in the discharge gap, as the insulating medium layer (Zhang et al., 2017), but the materials, configurations of high-voltage pole (Li et al., 2019) and the distance of the discharge gap (Uytdenhouwen et al., 2018) determined the uniformity and stability of the discharge.
Fig. 2.
(a) Lissajous-figures of the KrCl* excimer radiation at different Pin; (b) and IUV of the KrCl* excimer radiation under different Pin. ([TCAA]0 = 0.62 mM; DO = 9.62 mg/L; initial solution pH 7.0).
Both and I UV were measured to obtain the optimal P in, as I UV plays a vital role in TCAA removal. Fig. 2 (b) shows values of and I UV under different P in, which were calculated by Eqs. (13), (14), (15). It can be observed that both and I UV increased with increasing P in from 30 W to 60 W. However, when P in exceeded 60 W, P in dropped while increased as usual. This may be explained by the fact that when the reaction rate increases, the dominating mechanism for exciplex production shifts from harpooning to three-body recombination, resulting in enhanced radiant power of the major bands (Eq. (6)) (Zhuang et al., 2010) with increasing . But when P in further increased from 60 W to 70 W, I UV decreased from 0.12 mW/cm2 to 0.08 mW/cm2, because the dissociation and quenching processes become more dramatic at higher P in, owing to the frequent collision of excited molecules with other particles as a result of the high concentration in the following reaction (Eq. (8)) (M. and I., 2004), making I UV dramatically reduces. Therefore, 60 W was selected as the optimum P in in this study.
3.2. Degradation and dechlorination of TCAA
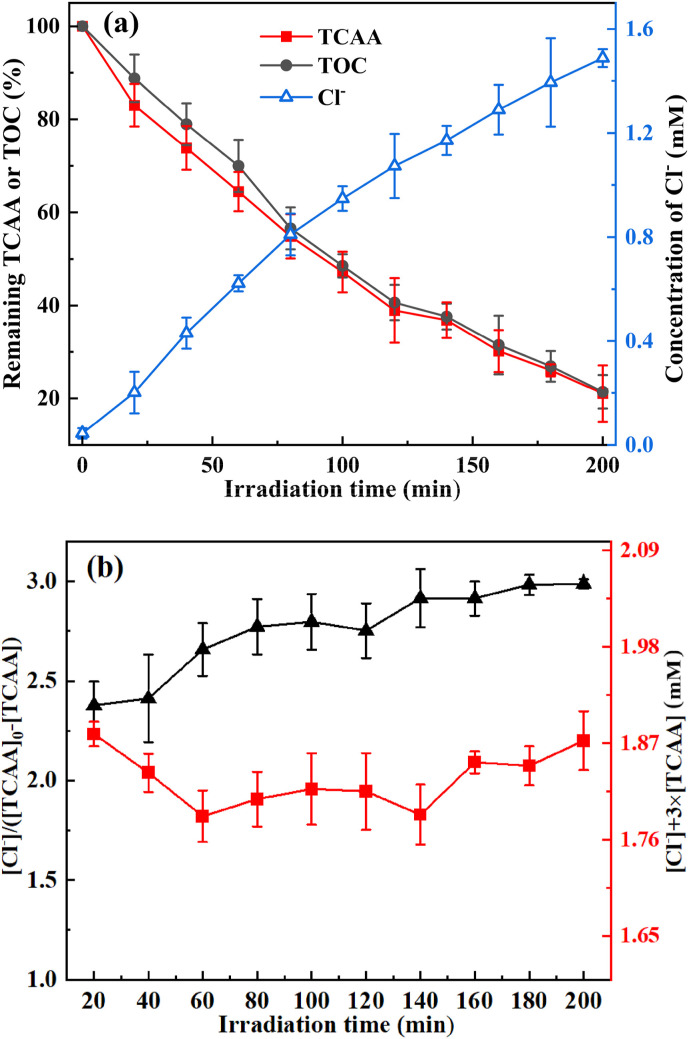
The trend of TOC, TCAA, and Cl− during the irradiation treatment is depicted in Fig. 3 (a). As demonstrated in Fig. 3 (a), the remaining TCAA concentrations and TOC declined while the concentration of Cl− rose with increasing irradiation period. Approximately 78% of the TOC was eliminated, 79% of the TCAA was removed, and 1.48 mM Cl− was produced after 200 min treatment. Additionally, the elimination of TOC was extremely close to that of TCAA, implying that litter organic intermediates generated during the irradiation. The bulk of the carbon atoms in TCAA were clearly mineralized into inorganic carbon, specifically carbon dioxide.
Fig. 3.
(a) Degradation of TCAA and generation of Cl− in the photoreactor as a function of irradiation time; (b) Variations of [Cl−]/([TCAA]0-[TCAA]) and ([Cl−] + 3 × [TCAA]) during UV irradiation treatment of TCAA ([TCAA]0 = 0.62 mM; Pin = 60 W; DO = 9.62 mg/L; initial solution pH 7.0).
The variation of [Cl−]/([TCAA]0-[TCAA]) with irradiation time estimated from Fig. 3 (a) was displayed in Fig. 3 (b) to clarify the chlorine balance. As demonstrated in Fig. 3 (b), the ratio between the amount of Cl− produced and the quantity of TCAA removed increased with irradiation time and finally to ca. 3:1 (2.4 ∼ 2.9:1 in the figure). This means that during irradiation, the majority of the chlorine atoms of TCAA were converted to Cl−, progressively to total dechlorination. The possible reason is that TCAA undergoes efficient C–Cl bond cleavage from a repulsive surface (Saha et al., 2013). Sections 3.4 and 3.5 will go into detail on the formation of intermediate byproducts and the dechlorination procedure.
3.3. Effects of dissolved oxygen on decomposition and dechlorination
Oxygen often played important roles in water treatment (Cao et al., 2023). Degradation and dechlorination of TCAA in oxygenated and deoxygenated water samples were shown in Fig. 4 (a) and (b), respectively. It can be seen that degradation of TCAA in deoxygenated water was close to, but dechlorination was much lower than those in oxygenated solution. This finding means that oxygen directly or indirectly participates in the dechlorination of TCAA. A prior study discovered •OH was generated under 254 nm UV irradiation of halogoacetic acids due to oxygen bubbling (Wang et al., 2021a). We employed DMPO as a trapping reagent to further explore the mechanism of the oxygen effect. In the absence of irradiation (control), no signals were captured, but a four-line signal with a 1:2:2:1 intensity ratio DMPO-•OH symbol was observed in the irradiated water (Fig. 4 (c)). The signal strength increased as oxygen content rose. This suggests that O2 was a necessary element in the formation of •OH. In general, water molecules only generated •OH under VUV (<200 nm) conditions (Li et al., 2021; Wang et al., 2022) or •OH was generated by an excited state halogoacetic acids electron transfer to O2. However, O2 could be decomposed in the UV at a wavelength less than 240 nm (Farooq et al., 2014). According to the results, a possible pathway for •OH production was proposed in this study as follows (Eqs. (17), (18), (19), (20), (21)) (Xu et al., 2020).
| (17) |
| (18) |
| (19) |
| (20) |
| (21) |
Fig. 4.
Degradation (a) and dechlorination (b) of TCAA as a function of irradiation time in oxygenated (DO = 9.62 mg/L) and deoxygenated (DO < 1 mg/L) water; (c) EPR spectra of DMPO spin adducts recorded under different conditions: blue line: oxygenated water without irradiation; red line: oxygenated water with 2 min irradiation; black line: deoxygenated water with 2 min irradiation. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Moreover, the intensity of DMPO-•OH spin was reduced compared to water with the same oxygen concentration when TCAA was present in the solution, further indicating that dissolved oxygen can additionally generate •OH in the irradiation process. The roles of •OH in the degradation and dechlorination will be discussed in section 3.5.
3.4. Theoretical prediction of the reaction mechanism
The TCAA potential energy curves of the S0, S1, and T1 (Lee et al., 2021) states were explored to further investigate the mechanism of dechlorination in the irradiation process and understand the optical characteristics. The curves were drawn by increasing the bond length with fixed step sizes as well as revealed stable structures and reactive potential barriers. Firstly, the potential energy curve of the C–Cl cleavage in the S0 state was investigated. As illustrated in Fig. 5 (black), barrier-free C–Cl recombination will result in the production of TCAA. Even where the species are 4.0 Å apart, there is no barrier to recombination, implying that TCAA dechlorination was hard to carry out, even when passing an energy barrier of around 55 kcal/mol.
Fig. 5.
TCAA potential energy curves: the black, red, and blue lines depict energies versus C–Cl bond lengths in the S0, S1, and T1 states, respectively. The minimal energy crossover point (MECP) and its associated structure have been shown. The numerical value on the graph represents the reaction's energy barrier. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Secondly, we studied the potential energy curves in the S1 state. The TDDFT vertical transition and oscillator strength for TCAA was summarized in Table S2. The oscillator strength of TCAA in the S1 state is 0.0035, implying that TCAA in the S1 state exists for a short time. Therefore, The potential energy curves of the T1 state were further calculated which had a longer lifetime than S1 (Lower and El-Sayed, 1966). Fig. 5 demonstrated that the energy level difference between S1 and T1 was small enough. The energy level difference between the S1 and T1 states was just 0.43 eV, implying that the intersystem crossing (ISC) mechanism would take place quickly (Li et al., 2022a). In addition, as the bond length of C–Cl increased, the energy decreased rapidly, and the C–Cl bond length was increased to 2.9 Å, minimizing the energy, indicating that the bond breaking occurred spontaneously in the T1 state. The TCAA structure was then thought to undergo a non-radiative transition from the T1 to the S0 states. As a result, herein searching for the MECP has become a major task for us. In this investigation, the sobMECP suite (Lu, 2016) was utilized, and the MECP structure is depicted in Fig. 5. The C–Cl bond length was around 2.9 Å. The ISC process may be the dominant channel in the T1 → S0 process at this stage. Based on the foregoing, the photodissociation could be obtained from the radiation less decay process, •CCl2COOH and •Cl were the intermediated products.
3.5. Subsequent •OH attack reaction
To understand the roles of •OH on the degradation and dechlorination, Fukui function was calculated to predict the electrophilic and nucleophilic sites for a molecule, which was shown in Fig. 6 (a). The dark blue indicated that it was easily attacked by free radicals, including oxygen, C–C bonds, and Cl of •CCl2COOH. The potential energy curves of the radical attack were further calculated, and the radical recombination between •CCl2COOH and •OH occurs barrierless forming the intermediate CCl2OHCOOH (Li et al., 2006).
Fig. 6.
Fukui function descriptor surfaces (a) and (c). Free radical parts are marked in dark blue. Optimized geometries for CCl2OHCOOH, TS1, TS2, , and schematic potential energy surface produced by the M06–2x/6-311 + G** calculations for (b) Second step dechlorination and (d) the third step dechlorination. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
The second step dechlorination as demonstrated in Fig. 6 (b), the process of HCl-elimination requires the energy of about 27.9 kcal/mol, which means CCl2OHCOOH can be decomposed in water and subsequently release HCl molecules. As shown in Fig. 6 (c), the C–C bond of was easily attacked. The free energy profile for the product continues to react with the •OH as shown in Fig. 6 (d), which represent the third step dechlorination. We proposed that the optimum structures of this process are a one-step reaction in which the C–Cl bond broken and is followed by the •OH insertion. The energy barrier reaches 14.6 kcal/mol, suggesting that it could carry out reactions at 222 nm. Finally, the overall decomposition pathway (Fig. S1) for TCAA would lead to HCl and CO2 effectively.
3.6. Energy efficiency
When comparing different techniques and assessing its potential applications, energy efficiency is a crucial ingredient to consider. In this study, the energy efficiency of TCAA degradation (J TCAA) is defined as the ratio of decomposing half of the initial TCAA molecules to the necessary energy. J TCAA can be formulated as follows (Eq. (22)):
| (22) |
where Vol is the volume of the TCAA solution in L, represents half of the initial TCAA concentration (mM), W denotes the treatment technology's overall power consumption in W, and t 1/2 represents the time necessary to decompose half of the initial TCAA molecules (s). The current work's energy efficiencies as well as some common competitive techniques are shown in Table 1 .
Table 1.
Energy efficiency of KrCl* excimer radiation compared to other competing TCAA removal technologies.
| [TCAA]0 (mM) | Method | JTCAA (10−9 mmol/J) | References |
|---|---|---|---|
| 0.62 | KrCl* excimer radiation, 60 W, DO 9.62 mg/L, pH0 7.0 |
613.54 | This work |
| 1.00 | Glow discharge plasma, 50 W, pH0 6.5 | 162.76 | Wang et al. (2014) |
| 0.13 | UV high pressure mercury lamp, 250 W, DO 5 mg/L, pH0 7.0 | 8.67 | Li et al. (2018) |
| 0.62 | Ultrasonic/UV lamps, 98 W, pH0 7.0, TiO2 62.5 μg/mL | 11.72 | Hu et al. (2014) |
| 1.00 | Pd/Ni electrode glow discharge electrolysis, 35 W | 198.4 | Zhao et al. (2019) |
As can be shown, the energy efficiency of TCAA degradation by the KrCl* excimer radiation is not only than those of photocatalysis and electrolysis, but also only requires electric energy to drive, without the use of additional substances, oxygen or materials. Besides, it should be emphasized that the only byproducts of the TCAA decomposition are HCl and CO2 that are neither poisonous or detrimental. These results show that KrCl* excimer radiation is a viable and dependable technique to eliminate TCAA.
4. Conclusions
In this work, our study focused on the applicability of in situ KrCl* excimer radiation for TCAA degradation and dichlorination. as well as role of oxygen in the pathway. The following conclusions can be drawn.
-
i)
In general, and I UV increased with increasing P in. However, when P in exceeded 60 W, quenching and dissociation processes resulted in a rapid fall in I UV. Therefore, 60 W was the optimum P in in this study. The energy efficiency of KrCl* excimer radiation has obvious energy consumption advantages compared to other methods.
-
ii)
TCAA can be efficiently decomposed and dechlorinated under the KrCl* excimer irradiation. The majority of carbon and chlorine atoms were mineralized to inorganic carbon and chloride ion, respectively.
-
iii)
Dissolved oxygen shows little effect on the degradation but greatly enhances the dechlorination because it can additionally generate •OH in the irradiation process.
-
iV)
Under 222 nm irradiation, TCAA was excited from S0 state to S1 state and then by ICS process to T1 state, and a reaction without potential energy barrier occurs, resulting in the breaking of C–Cl bond and finally returning to S0 state. •OH reacts with the resulting •CCl2COOH, forming the intermediate product CCl2OHCOOH. Subsequently, C–Cl bond cleavage resulted in the formation of and the release of HCl. •OH attacked on C–Cl, causing the breakage of the C–Cl bond as well as disintegration of , producing and •Cl. Finally, the was completely decomposed to the final product CO2.
Credit author statement
Jiaming Gan and Lei Wang conceived the ideas and designed the methodology; Jiaming Gan, Yizhan Zhang, Ting Li, and Min Zhao carried out the experiments and analyzed the data; Ting Zhu, Dailin Li, Jiaming Gan, and ZengXia Zhao provided guidance for model calculations; Jiaming Gan, Ting Zhu, and Lei Wang contributed to the revision of this manuscript; Dailin Li and Lei Wang supervised the project, provided critical feedback, and helped shape the research and manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This research was supported Natural Science Foundation of Fujian province (Grant No. 2020J01259).
Handling Editor: Junfeng Niu
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.chemosphere.2023.138753.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Ao X.-w., Eloranta J., Huang C.-H., Santoro D., Sun W.-j., Lu Z.-d., Li C. Peracetic acid-based advanced oxidation processes for decontamination and disinfection of water: a review. Water Res. 2021;188 doi: 10.1016/j.watres.2020.116479. [DOI] [PubMed] [Google Scholar]
- APHA, A.J.A.P.H.A.A.W.W.A.W.E.F., Washington DC, USA . 1995. WPCF, Standard Methods for the Examination of Water and Wastewater. [Google Scholar]
- M B.A., I Y.S. Simulation of KrCl (222 nm) and XeCl (308 nm) excimer lamps with Kr/HCl(Cl2 and Ne/Kr/Cl2) and Xe/HCl(Cl2) binary ternary mixtures excited by glow discharge. Laser Phys. 2004;14:1–14. [Google Scholar]
- Belasri A., Bachir N.L.D., Harrache Z. Plasma chemical and electrical modeling of a dielectric barrier discharge in Kr–Cl2 gas mixtures. Plasma Chem Plasma. 2013;33:131–146. doi: 10.1007/s11090-012-9416-6. [DOI] [Google Scholar]
- Bhardwaj S.K., Singh H., Deep A., Khatri M., Bhaumik J., Kim K.-H., Bhardwaj N. UVC-based photoinactivation as an efficient tool to control the transmission of coronaviruses. Sci. Total Environ. 2021;792 doi: 10.1016/j.scitotenv.2021.148548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y., Long X., Luo Y.-H., Zhou C., Rittmann B.E. Stable dechlorination of Trichloroacetic Acid (TCAA) to acetic acid catalyzed by palladium nanoparticles deposited on H2-transfer membranes. Water Res. 2021;192 doi: 10.1016/j.watres.2021.116841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Li J., Zhao Y., Zhao Y., Qiu W., Pang S., Jiang J. Degradation of metoprolol by UV/sulfite as an advanced oxidation or reduction process: the significant role of oxygen. J. Environ. Sci. 2023;128:107–116. doi: 10.1016/j.jes.2022.07.008. https://10.1016/j.jes.2022.07.008 [DOI] [PubMed] [Google Scholar]
- Chen B., Jiang J., Yang X., Zhang X., Westerhoffd P. Roles and knowledge gaps of point-of-use technologies for mitigating health risks from disinfection byproducts in tap water: a critical review. Water Res. 2021;200 doi: 10.1016/j.watres.2021.117265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drioli E., Stankiewicz A.I., Macedonio F. Membrane engineering in process intensification—an overview. J. Membr. Sci. 2011;380:1–8. doi: 10.1016/j.memsci.2011.06.043. [DOI] [Google Scholar]
- Fang Z., Qiu Y., Sun Y., Wang H., Edmund K. Experimental study on discharge characteristics and ozone generation of dielectric barrier discharge in a cylinder–cylinder reactor and a wire–cylinder reactor. J. Electrost. 2008;66:421–426. doi: 10.1016/j.elstat.2008.04.007. [DOI] [Google Scholar]
- Farooq Z., Chestakov A.D., Yan B., Groenenboom G.C., Zande W.J.v.d., Parker D.H. Photodissociation of singlet oxygen in the UV region. Phys. Chem. Chem. Phys. 2014;16:3305–3316. doi: 10.1039/c3cp54696a. [DOI] [PubMed] [Google Scholar]
- Frisch M.J., Trucks G.W., Schlegel H.B., Scuseria G.E., Robb M.A., Cheeseman J.R., Scalmani G., Barone V., Petersson G.A., Nakatsuji H., Li X., Caricato M., Marenich A.V., Bloino J., Janesko B.G., Gomperts R., Mennucci B., Hratchian H.P., Ortiz J.V., Izmaylov A.F., Sonnenberg J.L., Williams Ding F., Lipparini F., Egidi F., Goings J., Peng B., Petrone A., Henderson T., Ranasinghe D., Zakrzewski V.G., Gao J., Rega N., Zheng G., Liang W., Hada M., Ehara M., Toyota K., Fukuda R., Hasegawa J., Ishida M., Nakajima T., Honda Y., Kitao O., Nakai H., Vreven T., Throssell K., Montgomery J.A., Jr., Peralta J.E., Ogliaro F., Bearpark M.J., Heyd J.J., Brothers E.N., Kudin K.N., Staroverov V.N., Keith T.A., Kobayashi R., Normand J., Raghavachari K., Rendell A.P., Burant J.C., Iyengar S.S., Tomasi J., Cossi M., Millam J.M., Klene M., Adamo C., Cammi R., Ochterski J.W., Martin R.L., Morokuma K., Farkas O., Foresman J.B., Fox D.J. 2016. Gaussian 16 Rev. C.01. [Google Scholar]
- Gilca A.F., Teodosiu C., Fiore S., Musteret C.P. Emerging disinfection byproducts: a review on their occurrence and control in drinking water treatment processes. Chemosphere. 2020;259 doi: 10.1016/j.chemosphere.2020.127476. [DOI] [Google Scholar]
- Hamidin N., Yu Q.J., Connell D.W. Human health risk assessment of chlorinated disinfection by-products in drinking water using a probabilistic approach. Water Res. 2008;42:3263–3274. doi: 10.1016/j.watres.2008.02.029. [DOI] [PubMed] [Google Scholar]
- Hejazi S.A., Taghipour F. Microplasma ultraviolet radiation integrated with electrochemical in situ generation of hydrogen peroxide for degradation of organic pollutants in water. J. Clean. Prod. 2021;314 doi: 10.1016/j.jclepro.2021.127923. [DOI] [Google Scholar]
- Hejazi S.A., Taghipour F. A novel UV-LED hydrogen peroxide electrochemical photoreactor for point-of-use organic contaminant degradation. Chemosphere. 2022;292 doi: 10.1016/j.chemosphere.2021.133353. [DOI] [PubMed] [Google Scholar]
- Hill C.L., Harris J.D., Turner S.S., Wason K.L., Gaylord A.P., Hatley M.G., Hardcastle L.T., Roberts I.T., You J.Y., Renneker K.O., Edokpayi J.N., Smith J.A. Field and laboratory assessment of a new electrolytic point-of-use water treatment technology. Water. 2022;14:1077. doi: 10.3390/w14071077. [DOI] [Google Scholar]
- Hu B., Wu C., Zhang Z., Wang L. Sonophotocatalytic degradation of trichloroacetic acid in aqueous solution. Ceram. Int. 2014;40:7015–7021. https://10.1016/j.ceramint.2013.12.029 [Google Scholar]
- Humans, I.W.G.o.t.E.o.C.R.t. Organization W.H., Cancer I.A.f.R.o. IARC; 2004. Some Drinking-Water Disinfectants and Contaminants, Including Arsenic. [PMC free article] [PubMed] [Google Scholar]
- Klamt A., Moya C., Palomar J. A comprehensive comparison of the IEFPCM and SS(V)PE continuum solvation methods with the COSMO approach. J. Chem. Theor. Comput. 2015;11:4220–4225. doi: 10.1021/acs.jctc.5b00601. [DOI] [PubMed] [Google Scholar]
- Lee Y.H., Shin Y.-S., Lee T., Jung J., Lee J.-H., Lee M.H. Managing local triplet excited states of boron-based TADF emitters for fast spin-flip process: toward highly efficient TADF-OLEDs with low efficiency roll-off. Chem. Eng. J. 2021;423 doi: 10.1016/j.cej.2021.130224. [DOI] [Google Scholar]
- Levesque S., Rodriguez M.J., Serodes J., Beaulieu C., Proulx F.-o. Effects of indoor drinking water handling on trihalomethanes and haloacetic acids. Water Res. 2006;40:2921–2930. doi: 10.1016/j.watres.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Li Y., Xie Y., Peng S., Lu G., Li S. Photocatalytic hydrogen generation in the presence of chloroacetic acids over Pt/TiO2. Chemosphere. 2006;63:1312–1318. doi: 10.1016/j.chemosphere.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Li T., Zhang Q., Li C., Ai W., Zhang L. Efficient photolytic degradation of disinfection by-products by using a high photon flux UV system: monochloroacetic acid, dichloroacetic acid and trichloroacetic acid. Water Supply. 2018;18:2063–2070. https://10.2166/ws.2018.029 [Google Scholar]
- Li S., Yu X., Dang X., Guo H., Liu P., Qin C. Using non-thermal plasma for decomposition of toluene adsorbed on γAl2O3 and ZSM-5: configuration and optimization of a double dielectric barrier discharge reactor. Chem. Eng. J. 2019;375 doi: 10.1016/j.cej.2019.122027. [DOI] [Google Scholar]
- Li S., Dang X., Yu X., Abbas G., Zhang Q., Cao L. The application of dielectric barrier discharge non-thermal plasma in VOCs abatement: a review. Chem. Eng. J. 2020;388 doi: 10.1016/j.cej.2020.124275. [DOI] [Google Scholar]
- Li J., Zhang Q., Chen B., Wang L., Zhu R., Yang J. Hydrogen peroxide formation in water during the VUV/UV irradiation process: impacts and mechanisms of selected anions. Environ. Res. 2021;195 doi: 10.1016/j.envres.2021.110751. [DOI] [PubMed] [Google Scholar]
- Li Q., Wan Y., Zhou Q., Li Y., Li B., Zhu L., Wan Y., Yin H., Shi Y. Exploring the effect of nitrile substituent position on fluorescence quantum yield of ESIPT-based oxazoline derivatives: a TDDFT investigation. Spectrochim. Acta. 2022;272 doi: 10.1016/j.saa.2022.120953. [DOI] [PubMed] [Google Scholar]
- Li X., Zhao H., Qu B., Tian Y. Photoformation of environmentally persistent free radicals on particulate organic matter in aqueous solution: role of anthracene and formation mechanism. Chemosphere. 2022;291 doi: 10.1016/j.chemosphere.2021.132815. [DOI] [PubMed] [Google Scholar]
- Liang J.-K., Lu Y., Song Z.-M., Ye B., Wu Q.-Y., Hu H.-Y. Effects of chlorine dose on the composition and characteristics of chlorinated disinfection byproducts in reclaimed water. Sci. Total Environ. 2022;824 doi: 10.1016/j.scitotenv.2022.153739. [DOI] [PubMed] [Google Scholar]
- Liu X., Zhong J., Fang L., Wang L., Ye M., Shao Y., Li J., Zhang T. Trichloroacetic acid reduction by an advanced reduction process based on carboxyl anion radical. Chem. Eng. J. 2016;303:56–63. doi: 10.1016/j.cej.2016.05.130. [DOI] [Google Scholar]
- Lower S.K., El-Sayed M.A. The triplet state and molecular electronic processes in organic molecules. Chem. Rev. 1966;66:199–241. [Google Scholar]
- Lu T. The sobMECP Program. 2016. http://sobereva.com/286
- Lui G.Y., Roser D., Corkish R., Ashbolt N., Jagals P., Stuetz R. Photovoltaic powered ultraviolet and visible light-emitting diodes for sustainable point-of-use disinfection of drinking waters. Sci. Total Environ. 2014;493:185–196. doi: 10.1016/j.scitotenv.2014.05.104. [DOI] [PubMed] [Google Scholar]
- Mazhar M.A., Khan N.A., Ahmed S., Khan A.H., Hussain A., Rahisuddin Changani F., Yousefi M., Ahmadi S., Vambol V. Chlorination disinfection by-products in municipal drinking water – a review. J. Clean. Prod. 2020;273 doi: 10.1016/j.jclepro.2020.123159. [DOI] [Google Scholar]
- Narita K., Asano K., Morimoto Y., Igarashi T., Hamblin M.R., Dai T., Nakane A. Disinfection and healing effects of 222-nm UVC light on methicillin-resistant Staphylococcus aureus infection in mouse wounds. J. Photochem. Photobiol., B. 2018;178:10–18. doi: 10.1016/j.jphotobiol.2017.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh C., Sun P.P., Araud E., Nguyen T.H. Mechanism and efficacy of virus inactivation by a microplasma UV lamp generating monochromatic UV irradiation at 222 nm. Water Res. 2020;186 doi: 10.1016/j.watres.2020.116386. [DOI] [PubMed] [Google Scholar]
- Pan Y., Zhang X., Wagner E.D., Osiol J., Plewa M.J. Boiling of simulated tap water: effect on polar brominated disinfection byproducts, halogen speciation, and cytotoxicity. Environ. Sci. Technol. 2014;48:149–156. doi: 10.1021/es403775v. [DOI] [PubMed] [Google Scholar]
- Pérez-Vidal A., Diaz-Gómez J., Castellanos-Rozo J., Usaquen-Perilla O.L. Long-term evaluation of the performance of four point-of-use water filters. Water Res. 2016;98:176–182. doi: 10.1016/j.watres.2016.04.016. [DOI] [PubMed] [Google Scholar]
- Raeiszadeh M., Taghipour F. Microplasma UV lamp as a new source for UV-induced water treatment: protocols for characterization and kinetic study. Water Res. 2019;164 doi: 10.1016/j.watres.2019.114959. [DOI] [PubMed] [Google Scholar]
- Rodrigues F., Pascoa J., Trancossi M. Heat generation mechanisms of DBD plasma actuators. Exp. Therm. Fluid Sci. 2018;90:55–65. doi: 10.1016/j.expthermflusci.2017.09.005. [DOI] [Google Scholar]
- Saha A., Kawade M.N., Upadhyaya H.P., Kumar A., Naik P.D. Photoexcitation of 2-bromo-2-chloro-1,1,1-trifluoroethane (halothane) to repulsive surface nσ*(C–Br) at 234 nm: dynamics of C–Br and C–Cl bond rupture. Chem. Phys. 2013;416:1–10. doi: 10.1016/j.chemphys.2013.02.017. [DOI] [Google Scholar]
- Silva D.B., Buttiglieri G., Babić T., Ćurković L., Babić S. Impact of UV-LED photoreactor design on the degradation of contaminants of emerging concern. Process Saf Environ. 2021;153:94–106. doi: 10.1016/j.psep.2021.07.015. [DOI] [Google Scholar]
- Sobsey M.D., Stauber C.E., Casanova L.M., Brown J.M., Elliott M.A. Point of use household drinking water filtration: a practical, effective solution for providing sustained access to safe drinking water in the developing world. Environ. Sci. Technol. 2008;42:4261–4267. doi: 10.1021/es702746n. [DOI] [PubMed] [Google Scholar]
- Uytdenhouwen Y., Alphen S.V., Michielsen I., Meynen V., Cool P., Bogaerts A. A packed-bed DBD micro plasma reactor for CO2 dissociation: does size matter? Chem. Eng. J. 2018;557–568 doi: 10.1016/j.cej.2018.04.210. [DOI] [Google Scholar]
- Varshney M., Chandra A., Chauhan L.K.S., Goel S.K. In vitro cytogenetic assessment of trichloroacetic acid in human peripheral blood lymphocytes. Environ. Sci. Pollut. Res. Int. 2014;21:843–850. doi: 10.1007/s11356-013-1949-6. [DOI] [PubMed] [Google Scholar]
- Wang L., Zeng H., Yu X. Dechlorination and decomposition of trichloroacetic acid by glow discharge plasma in aqueous solution. Electrochim. Acta. 2014;115:332–336. https://10.1016/j.electacta.2013.10.160 [Google Scholar]
- Wang L., Chen B., Zhang T. Predicting hydrolysis kinetics for multiple types of halogenated disinfection byproducts via QSAR models. Chem. Eng. J. 2018;342:372–385. doi: 10.1016/j.cej.2018.02.106. [DOI] [Google Scholar]
- Wang B., Wang X., Su H. Influence of electrode interval and barrier thickness in the segmented electrode micro-plasma DBD reactor on CO2 decomposition. Plasma Chem. Plasma Process. 2020;40:1189–1206. doi: 10.1007/s11090-020-10091-1. [DOI] [Google Scholar]
- Wang J., Shen J., Ye D., Yan X., Zhang Y., Yang W., Li X., Wang J., Zhang L., Pan L. Disinfection technology of hospital wastes and wastewater: suggestions for disinfection strategy during coronavirus Disease 2019 (COVID-19) pandemic in China. Environ. Pollut. 2020;262 doi: 10.1016/j.envpol.2020.114665. http://10.1016/j.envpol.2020.114665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Chen Y., Chen B., Yang J. Generation of hydroxyl radicals during photodegradation of chloroacetic acids by 254 nm ultraviolet: a special degradation process revealed by a holistic radical determination methodology. J. Hazard Mater. 2021;404 doi: 10.1016/j.jhazmat.2020.124040. [DOI] [PubMed] [Google Scholar]
- Wang R., Member I.E.E.E., Yang Y., Chen S., Jiang H., Martin P. Power calculation of pulse power-driven DBD plasma. Ieee T Plasma Sci. 2021;49:2210–2216. doi: 10.1109/tps.2021.3084601. [DOI] [Google Scholar]
- Wang C., Du J., Liang Z., Liang J., Zhao Z., Cui F., Shi W. High-efficiency oxidation of fluoroquinolones by the synergistic activation of peroxymonosulfate via vacuum ultraviolet and ferrous iron. J. Hazard Mater. 2022;422 doi: 10.1016/j.jhazmat.2021.126884. [DOI] [PubMed] [Google Scholar]
- Wu C.-C., Ghosh S., Martin K.J., Pinto A.J., Denef V.J., Olson T.M., Love N.G. The microbial colonization of activated carbon block point-of-use (PoU) filters with and without chlorinated phenol disinfection by-products. Environ Sci-wat Res. 2017;3:830–843. doi: 10.1039/c7ew00134g. [DOI] [Google Scholar]
- Xiao J., Yue Q., Gao B., Sun Y., Kong J., Gao Y., Li Q., Wang Y. Performance of activated carbon/nanoscale zero-valent iron for removal of trihalomethanes (THMs) at infinitesimal concentration in drinking water. Chem. Eng. J. 2014;253:63–72. doi: 10.1016/j.cej.2014.05.030. [DOI] [Google Scholar]
- Xu S., Jirasek V., Lukes P. Molecular dynamics simulations of singlet oxygen atoms reactions with water leading to hydrogen peroxide. J. Phys. D Appl. Phys. 2020;53 doi: 10.1088/1361-6463/ab8321. [DOI] [Google Scholar]
- Yang W., Parr, R.G.J.P.o.t.N.A.o.S. vol. 82. 1985. Hardness, Softness, and the Fukui Function in the Electronic Theory of Metals and Catalysis; pp. 6723–6726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung C.S., Tse H.-Y., Lau C.Y., Guan J., Huang J., Phillips D.L., Leu S.-Y. Insights into unexpected photoisomerization from photooxidation of tribromoacetic acid in aqueous environment using ultrafast spectroscopy. J. Hazard Mater. 2021;418 doi: 10.1016/j.jhazmat.2021.126214. [DOI] [PubMed] [Google Scholar]
- Zhang X., Li Y., Xiao S., Tang J., Tian S., Deng Z. Decomposition mechanism of C5F10O: an environmentally friendly insulation medium. Environ. Sci. Technol. 2017;51:10127–10136. doi: 10.1021/acs.est.7b02419. [DOI] [PubMed] [Google Scholar]
- Zhao C., Yang H., Ju M., Zhao X., Li L., Wang S., An B. Simultaneous degradation of aqueous trichloroacetic acid by the combined action of anodic contact glow discharge electrolysis and normal electrolytic processes at the cathode. Plasma Chem. Plasma Process. 2019;39:751–767. https://10.1007/s11090-019-09984-7 [Google Scholar]
- Zhuang X., Han Q., Zhang H., Feng X., Roth M., Rosier O., Zhu S., Zhang S. The efficiency of coaxial KrCl* excilamps. J. Phys. D Appl. Phys. 2010;43 doi: 10.1088/0022-3727/43/20/205202. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.