Abstract
Cancer treatment is one of the main challenges of global health. For decades, researchers have been trying to find anti-cancer compounds with minimal side effects. In recent years, flavonoids, as a group of polyphenolic compounds, have attracted the attention of researchers due to their beneficial effects on health. Xanthomicrol is one of the flavonoids that has the ability to inhibit growth, proliferation, survival and cell invasion and ultimately tumor progression. Xanthomicrol, as active anti-cancer compounds, can be effective in the prevention and treatment of cancer. Therefore, the use of flavonoids can be suggested as a treatment along with other medicinal agents. It is obvious that additional investigations in cellular levels and animal models are still needed. In this review article, the effects of xanthomicrol on various cancers have been reviewed.
Keywords: Xanthomicrol, Cancer, Treatment, Apoptosis
Graphical Abstract
Highlights
-
•
Xanthomicrol is a flavonoids.
-
•
Xanthomicrol has the ability to inhibit the growth, proliferation, survival of cancer cells.
-
•
Xanthomicrol is cell invasion and ultimately tumor progression inhibitor.
1. Introduction
Cancer, as one of the most incurable diseases, has affected human society and the health system in different countries [1]. Researchers have encountered many problems in the treatment of cancer, and so far no 100% cure has been discovered that can completely eradicate the disease in patients [2]. Cancer treatment is very difficult due to cancer cell immortality, invasion and metastasis, as well as angiogenesis [3]. Various methods such as chemotherapy, radiotherapy, etc. have been suggested for cancer, but because the side effects of these cases are very high for the patient and also have a high cost, researchers are trying to use the size of anti-cancer agents to treat patients [4]. In recent years, adjuvant therapy has been studied and researched. In this treatment, along with the main chemotherapy drug or radiotherapy, this other substance is used as an adjunct drug [5]. The results of such research have been promising, and some plant-based materials have shown positive effects in this regard [6]. Because of synergic effects with chemotherapeutic regimens and also cancer prevention, phytochemicals have been extensively studied recently [7]. Phytochemicals have a unique capacity to affect pathological processes including tumor angiogenesis and vascularization[8]. Flavonoids are among the important phytochemical, with preventive and suppressive characters in tumor biology which, have received considerable attention nowadays and naturally can be found in a variety of fruits and vegetables [9]. Rapid metabolism and low bioavailability are two important features of flavonoids [10]. Nonetheless, low bioavailability can be increased with methylation of flavonoids' hydroxyl groups, which also affects the metabolization time, robust transport rate and leads to increased absorption rates in target tissues [11]. So, in comparison with non-methylated flavonoids, methoxy-flavones with higher bioavailability are more beneficial and helpful. Different mechanisms apply to flavonoids effects on target tissues, such as heightening of mitochondrial membrane permeability, MAPK pathway modification, and apoptosis stimulation [12]. Xanthomicrol, as a plant substance, has recently been considered by scientists. This herbal agent has shown positive effects in preventing and reducing the symptoms of various diseases such as inflammation, and fungal diseases, but in the case of cancer [13]. In this review article, we try to examine the positive effects of xanthomicrol on preventing and reducing the symptoms of cancer and to collect the work that has been done in this field.
2. Properties of xanthomicrol
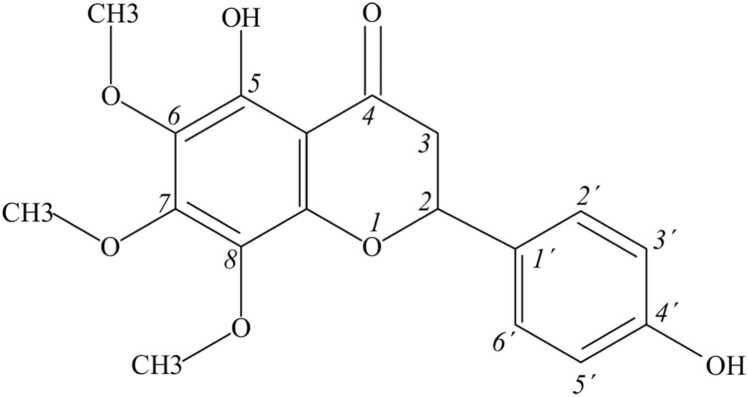
Trimethoxyflavone named xanthomicrol is a flavone, with substituted methoxy groups at positions 6, 7 and 8 and hydroxy groups at positions 5 and 4′ (Fig. 1). It has a role as an antineoplastic agent and a plant metabolite [14]. It is trimethoxyflavone and a dihydroxyflavone. It derives from a flavone. Xanthomicrol is a natural product found in Siparuna, Clinopodium douglasii, and other organisms with data available [15]. 4′, 5-dihydroxy-6, 7, 8-trimethox-y flavone the IUPAC name of Xanthomicrol is the important active element in Dra-cocephalum kotschyi Boiss leaf extract which also known as Spinal-Z [18], in Iranian traditional herbal remedy [16].
Fig. 1.
Chemical structure of xanthomicrol.
3. Xanthomicrol effects on cell proliferation and apoptosis
In the vast majority of multicellular organisms, apoptosis plays a crucial role in the establishment and maintenance of homeostasis. The apoptotic process involves biological and structural changes to the mitochondria, including mitochondrial dysfunction, disruption of the outer membrane of the mitochondria, and the release of cytochrome c, which may cause Apaf1 and Caspase-9 to connect, activating Caspase-9. Then, Caspase-9 activates Caspase-3, which causes cellular and morphological abnormalities and ultimately leads to cellular death [17], [18], [19]. Apoptosis evasion is among the important characteristics of cancer cells which can be a target in the therapeutic process in the fight against cancer. Flavonoids showed anti-apoptotic and anti-proliferation in a variety of cancer, include breast cancer. For example, sideritoflavone suppresses not only the proliferation of non-CSC but also CSC subpopulations which are responsible for tumor relapse, chemo-resistance and VMs. In the molecular view, sideritoflavone led to cell cycle arrest in the G2 phase, the Wnt, Myc/Max, transforming growth factor-β (NFK- β) pathways activation and increase p65/ nuclear factor kappa-light-chain-enhancer of activated Β cells [20]. Xanthomicrol as a flavonoid is non-toxic to vital organs, despite its high bioavailability. 50 and 100 μM are reported via documents as IC50 of xanthomicrol between breast cancer cell lines [21]. Xanthomicrol inhibitory effects on 4T1 breast cancer cell viability were reported previously with no harmful effect on the normal fibroblast cells, which makes it a suitable compound to be considered for cancer treatment. Also xanthomicrol, expressively heightened the percentage of the apoptotic cells in 4T1 cells.
Moreover, xanthomicrol induced cell cycle arrest at the G1 phase and significantly decreased Ki67 expression as a proliferation marker[13]. In vivo experiments showed that due to biphasic effects of flavonoids, higher concentration of xanthomicrol seemed to lose its inhibitory effect on tumor growth compared to the lower concentration [13], [22]. Another important point is the hydrophobic nature of flavonoids, which leads to increased their attachment to blood proteins, which in turn delays their delivery to the tumor site, and so lowers their efficacy [23]. However, two weeks of treatment with low doses in animal models dramatically reduced the tumor proliferation rate.
In the molecular view, flavonoids like xanthomicrol through anti-inflammatory effects have inhibitory effect on pro-inflammatory cytokines such as TNFα and so inducing apoptosis effects [24]. TNFα as one of the molecular targets for inducing apoptosis in cancer cells associated with malignant breast neoplasm [25].
Another important factor in cancer biology and the design of anti-cancer drugs are miRNAs, which play a very important role in tumor initiation and tumor progression through inhibitory and induction effects [26]. miR21, miR27a, and miR125b as apoptosis inhibitors are among xanthomicrol target molecules [27], [28]. Induction of apoptosis and inhibition of tumor growth through activation of caspase9, caspase3 and intrinsic pathway of apoptosis have been reported via miR21 suppression. More over the xanthomicrol possess the capacity to up-regulate of the Bcl2 family, Bax protein, through miR27suppression. Several pro-apoptotic transcripts, including p53 and Bak1, are among miR125b targets. Therefore, repression of this miR125b could sensitize the tumor cells to the therapeutic reagents[13]. Moreover, xanthomicrol treatment led to up-regulation of miR34 as a tumor suppressor which down-regulated in glioma [29], breast and bladder [30].
Moreover, recently Mariella Nieddu et al. showed evidence that xanthomicrol induced cytotoxicity, apoptosis, and cell cycle arrest, affecting lipid profile in cancer HeLa cells [31].
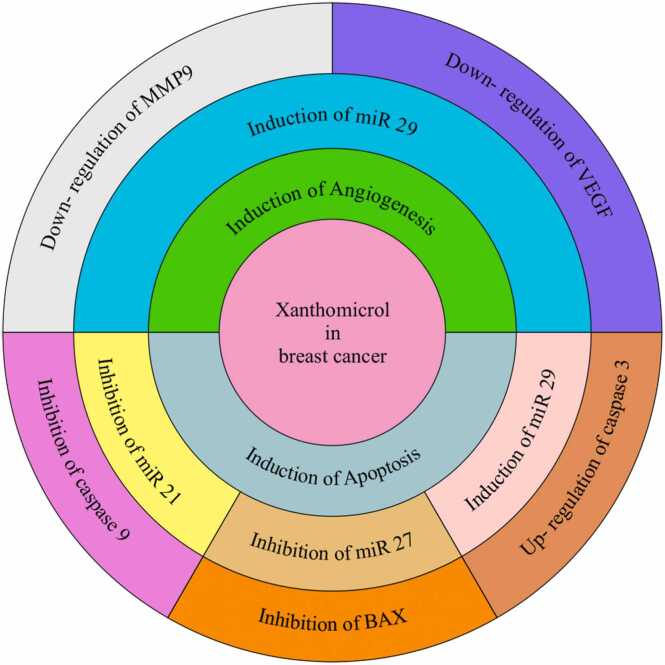
In summary, it can be conclude that xanthomicrol has the ability to inhibit the growth of cancer cells, especially breast cancer cells, through the effect on various molecules, including the molecules involved in apoptosis, such as caspase 9, Bax, and miRNAs (Fig. 2).
Fig. 2.
Effects of xanthomicrol on breast cancer apoptosis and angiogenesis.
4. Xanthomicrol effects on angiogenesis and metastasis
Anti-angiogenic therapy in tumors consists of inhibiting the formation of new vessels or targeting the vessels that have already formed [32]. The anti-angiogenic therapy method has led to the design and development of compounds that inhibits the tumor growth by blocking the ability to form feeding vessels or by targeting the existing vessels, the basis factor in drug design for angiogenesis is based on the fact that the vessels inside the tumor are phenotypically are different with the vessels in the natural organs, so they can be selectively identified by antibodies and ligands [33]. Based on this, over the past two decades, agents have been developed to target tumor angiogenesis. Most of these agents act indirectly and remove angiogenic growth factors from the blood circulation or interfere with the receptors and angiogenesis signaling pathways [34]. Inhibitors can inhibit angiogenesis in the stages of proliferation of endothelial cells, attachment to the extracellular matrix and their migration. Extracellular matrix is required for invasion, morphogenesis and differentiation and stability of endothelial cells, so anti-angiogenic agents can also prevent metastasis, which is an important issue in the treatment of malignant cancers. However, the progress of this promising method, due to the lack of sufficient knowledge about the action of the effective compounds is in early stages [35], [36]. Some important molecules play important functions in angiogenesis. Some growth factors such as VEGF are very specific for endothelial cells, while others such as bFGF (basic fibroblast growth factor) and MMPs (matrix metalloproteinase) have a wider functional range in angiogenesis [37]. Activating factors can be secreted by tumor cells, tumor micro-environment, or by macrophages and fibroblasts in the tumor tissue [38]. Xanthomicrol can act as a vessel formation inhibitor. In breast cancer(in vivo) and melanoma (in vitro), the xanthomicrol treatment led to decreased expression of VEGF, HIF-α and MMP9 [13], [39]. In melanoma, xanthomicrol treatment leads to inhibition of PI3K/Akt signaling pathway activity through reducing Akt phosphorylation levels. The PI3K/Akt signaling pathway has a key role in progression of cancer through induction of HIF-1α and VEGF expression [39]. In hepato-cellular carcinoma xanthomicrol treatment led to Huh7 cells viability reduction, migration and invasion suppression through down-regulation the expression level of MMP-2 and MMP-9 proteins, blocking EMT via up-regulating the level of E-cadherin and down-regulating the protein expression levels of N-cadherin, Vimentin and Snail [40]. Moreover, there is an indirect relation between anti-angiogenic and anti-apoptotic effects of xanthomicrol, because angiogenesis inhibitors could initiate apoptosis in the tumor cells. So, xanthomicrol may be a potential anti-tumor agent through antiangiogenic and apoptosis induction effects.
5. Adjuvant therapy with Xanthomicrol
Adjuvant therapy applied subsequently after surgery and removal of all obvious tumor mass to newly diagnosed cancer patients [41] in regard to optimizing treatment process and targeting any residual or micro- metastatic tumors and leading to increased disease-free and overall survival [42]. It has been found that flavonoids have the ability to act as adjuvants in the treatment processes of cancer patients. On the other hand, flavonoids can be used together with other chemotherapy drugs and reduce the side effects of these drugs. For example, delphinidin is a type of anthocyanin primarily found in the maqui berry has protective effect on diabetes-driven cancer and also increase the efficiency of chemotherapy via autophagy induction. Moreover, Silibinin is a flavonoid compound extracted from Silybum marianum L. Gaertn seeds also have anticancer effects via autophagy induction. Morin is another flavonoids that combined with several chemotherapeutic drugs, include cisplatin, paclitaxel, 5-fluorouracil (5-FU), uranofin, bortezomib, imatenib, doxorubicin, incristine and exert potent synergistic anticancer effects via increase apoptosis, down-regulating the expression of anti-apoptotic protein galectin-3, targeting miR-155/GATA3 axis, suppresses STAT3 activity, up-regulating the expression of tumor suppressor gene PTEN, inhibiting the P-glycoproteinand [12]. Taken together, these findings show that the combination of flavonoids with chemotherapeutic drugs can be figured out as a promising approach for cancer therapy. Two important factors related to reduction of chemotherapy and radiotherapy agents’ efficacy are VEGF and VEGFR. Xanthomicrol has the potential to modified VEGF/VEGFR signaling, so it can be used as chemotherapy and radiotherapy adjuvant which may increase the efficiency of the cancer treatment approaches [43]. Although few studies have been conducted on the anti-cancer effects of xanthomicrol and the use of xanthomicrol as an adjunctive treatment has not been studied, and it is suggested that studies to investigate the effects of xanthomicrol as an adjunctive treatment and along with other treatments present in different cancers and in laboratory and animal models.
6. Xanthomicrol effects on vascular mimicry
Various methods are used by the tumor mass to obtain nutrients and oxygen necessary for growth and metastasis that angiogenesis, vasculogenic mimicry, bone marrow-derived vasculogenesis, vasculogenesis by cancer stem cells(CSCs), vascular intussusception, and myeloid cell-driven angiogenesis are among important mechanisms [44]. Until 1999, angiogenesis was thought to be the only way to feed the cancer cells. Therefore, scientists have tried to discover drugs that inhibit angiogenesis. The results of angiogenesis treatments were not satisfactory in most cases, and in most cases the recurrence of the disease and even the death of the patient were observed. Vascular mimicry (VM) is one of the mechanisms applied by cancer cells to form vessels like structures to overcome anti-angiogenic treatments [45]. Firstly, VM was introduced in metastatic melanoma [46]. Investigations reveal the important relation between poor prognosis, recurrences of tumor, resistance to anti-angiogenesis drugs and VM structures [47]. For example, it is confirmed that VM formation led to resistance to anti-VEGF antibodies (Avastin and bevacizumab) and tyrosine kinase activity of VEGF receptor inhibitors(sorafenib and sunitinib) [48] Moreover, VM formation, decrease Endostatin and bevacizumab efficacy in cancer treatment[49]. So, the new agents with VM inhibitory effects needed in cancer treatment procedures. Myricetin is a natural flavonoid compound abundant in various fruits and vegetables with anti-inflammatory, anti-oxidative and antitumor activities. Recently, Ming Wang etal. showed myricetin reverses epithelial–endothelial transition (EMT) and suppresses vasculogenic mimicry and angiogenesis of hepatocellular carcinoma by directly targeting PAR1 [50]. Luteolin shows the ability to suppress VM formation via Notch1/VEGF in gastric cancer [51]. Moreover, curcumin is a flavonoid polyphenol that is the active ingredient in the spice turmeric, which can inhibit VM formation in hepatocellular carcinoma via suppression EphA2, PI3K, MMPs, STAT3, and PI3K/AKT [52]. Therefore, flavonoids have the potential to prevent the formation of VMs. According to the anti-tumor effects of xanthomicrol, it is possible that it has the ability to inhibit the formation of VMs, although this is a hypothesis and laboratory and animal studies should be carried out for confirmation.
7. Xanthomicrol and chemoresistance
Chemotherapy is one of the most effective treatments for metastatic tumors. Simultaneous resistance of cancer cells to different drugs or multi-drug resistance (MDR) is still one of the main obstacles in the way to a successful chemotherapy. The most common reason for drug resistance against a wide range of anticancer drugs is the high expression of ATP-dependent transporters (ABC) in the cell, which are responsible for the drug's exit from the cell. The most famous ATP-dependent transporter is a phosphoglycoprotein called glycoprotein P, which is the product of the MDR1 or ABCB1 gene [53]. In human, forty-eight ABC proteins in seven subfamilies (A-G) have been identified. The generation of MDR mostly due to three transporter-subfamilies including, ABC B member 1 (ABCB1/MDR1/P-glycoprotein, P-gp), subfamily C member 1 (ABCC1/ MRP1), and subfamily G member 2 (ABCG2/BCRP) [54]. Various studies have shown that P-glycoprotein is highly expressed in many human cancers, including gastrointestinal cancer, blood cancers, and neuroblastoma cancers. Transfer of a variety of chemotherapy agents includes anthracyclines and epipodophyllotoxins (type II topoisomerase inhibitors), vinca alkaloids(anti-mitotic and microtubule disturbution function), taxanes (a drug with interference with microtubule)performed via ABCB1 led to reduction drug efficiency [55]. Moreover, ABCG2 is involved in efflux of type II topoisomerase inhibitors (Mitoxantrone and anthracyclines), inhibitors of kinases, DNA topoisomerase, and intercalative binding to DNA (indolocarbazole) [56]. quercetin, kaempferol, or morin demonstrated anti- chemo-resistance effects. For example quercetin overcome to docetaxel resistance in a prostate cancer xenograft model via reversing the up-regulation of P-gp, activation of androgen receptor and PI3K/Akt signaling pathways. So Xanthomicrol may have potential inhibitory effect on chemo-resistance [57].
8. Conclusion and future perspective
New cancer treatment methods focus on inhibiting the message transmission pathways related to growth, survival, angiogenesis, and metastasis in cancer cells. Xanthomicrol has been considered as chemo-protective agents affecting these pathways. In general, the review of studies confirms the role of flavonoids in slowing the progression of cancer. In general, considering that the consumption of flavonoids, especially xanthomicrol is non-toxic and non-allergic, the use of these natural compounds is of great importance to increase the efficiency of cancer treatment. According to the results of the studies that indicate the positive and beneficial effects of xanthomicrol in inhibiting the progression of cancer (Table 1), therefore, the use of xanthomicrol can be suggested as a treatment along with other medicinal agents, but the definitive recommendation requires additional studies at the cellular level and animal model.
Table 1.
Approved anti-tumor of xanthomicrol.
| Cancer type | Effect | Mechanisms | References |
|---|---|---|---|
| Breast cancer | Inhibitory effects on 4T1 breast cancer cell viability Apoptosis induction |
|
21 |
| Breast cancer | Anti- proliferative effect |
|
13 |
| Glioma, Breast and Bladder cancer | Anti- proliferative effect |
|
29–30 |
| Breast cancer and Melanoma | Anti-angiogenesis |
|
13,38 |
| Melanoma | Anti-angiogenesis |
|
38 |
| Hepato-cellular carcinoma | Cells viability reduction, migration and invasion suppression blocking EMT |
|
40 |
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Handling Editor: Dr. Lawrence Lash
Contributor Information
Mohsen Rashidi, Email: dr.mohsenrashidi@yahoo.com.
Nazila Fathi Maroufi, Email: n.fathi6788@gmail.com.
Data Availability
No data was used for the research described in the article.
References
- 1.Pourmohammad P., et al. Potential therapeutic effects of melatonin mediate via miRNAs in cancer. Biochem. Genet. 2021;60:1–23. doi: 10.1007/s10528-021-10104-4. [DOI] [PubMed] [Google Scholar]
- 2.Hajipour H., et al. Improved anticancer effects of epigallocatechin gallate using RGD-containing nanostructured lipid carriers. Artif. Cells Nanomed., Biotechnol. 2018;46(sup1):283–292. doi: 10.1080/21691401.2017.1423493. [DOI] [PubMed] [Google Scholar]
- 3.Maroufi N.F., et al. The apatinib inhibits breast cancer cell line MDA-MB-231 in vitro by inducing apoptosis, cell cycle arrest, and regulating nuclear factor-κB (NF-κB) and mitogen-activated protein kinase (MAPK) signaling pathways. Breast Cancer. 2020;27(4):613–620. doi: 10.1007/s12282-020-01055-6. [DOI] [PubMed] [Google Scholar]
- 4.Mohammadian J., et al. Formulation of Stattic as STAT3 inhibitor in nanostructured lipid carriers (NLCs) enhances efficacy of doxorubicin in melanoma cancer cells. Naunyn-Schmiede 'S. Arch. Pharmacol. 2020;393(12):2315–2323. doi: 10.1007/s00210-020-01942-x. [DOI] [PubMed] [Google Scholar]
- 5.Talib W.H., et al. Melatonin in cancer treatment: current knowledge and future opportunities. Molecules. 2021;26(9):2506. doi: 10.3390/molecules26092506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van den Heerik A.S.V., et al. Adjuvant therapy for endometrial cancer in the era of molecular classification: radiotherapy, chemoradiation and novel targets for therapy. Int. J. Gynecol. Cancer. 2021;31:4. doi: 10.1136/ijgc-2020-001822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haiaty S., et al. Targeting vasculogenic mimicry by phytochemicals: a potential opportunity for cancer therapy. IUBMB life. 2020;72(5):825–841. doi: 10.1002/iub.2233. [DOI] [PubMed] [Google Scholar]
- 8.Yuan M., et al. The role of bioactive compounds in natural products extracted from plants in cancer treatment and their mechanisms related to anticancer effects. Oxid. Med. Cell. Longev. 2022;2022:1–19. doi: 10.1155/2022/1429869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amin A.R., et al. Perspectives for cancer prevention with natural compounds. J. Clin. Oncol. 2009;27(16):2712. doi: 10.1200/JCO.2008.20.6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wen X., Walle T. Methylated flavonoids have greatly improved intestinal absorption and metabolic stability. Drug Metab. Dispos. 2006;34(10):1786–1792. doi: 10.1124/dmd.106.011122. [DOI] [PubMed] [Google Scholar]
- 11.Koirala N., et al. Methylation of flavonoids: chemical structures, bioactivities, progress and perspectives for biotechnological production. Enzym. Microb. Technol. 2016;86:103–116. doi: 10.1016/j.enzmictec.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 12.Mottaghi S., Abbaszadeh H. The anticarcinogenic and anticancer effects of the dietary flavonoid, morin: Current status, challenges, and future perspectives. Phytother. Res. 2021;35(12):6843–6861. doi: 10.1002/ptr.7270. [DOI] [PubMed] [Google Scholar]
- 13.Attari F., et al. Inhibitory effect of flavonoid xanthomicrol on triple‐negative breast tumor via regulation of cancer‐associated microRNAs. Phytother. Res. 2021;35(4):1967–1982. doi: 10.1002/ptr.6940. [DOI] [PubMed] [Google Scholar]
- 14.Fattahi M., et al. Xanthomicrol: a comprehensive review of its chemistry, distribution, biosynthesis and pharmacological activity. Mini Rev. Med. Chem. 2014;14(9):725–733. doi: 10.2174/1389557514666140820122818. [DOI] [PubMed] [Google Scholar]
- 15.Jalezadeh A., et al. Investigation of structural, electronic, and antioxidant properties of calycopetrin and xanthomicrol as two polymethoxylated flavones using DFT calculations. Struct. Chem. 2022:1–10. [Google Scholar]
- 16.Soureshjani E.H., Babaheydari A.K., Saberi E. DNA Methyltransferases directed anti-cancerous plant medicine (Xanthomicrol and Galloyl) based molecular docking and dynamics simulation. Comput. Mol. Biosci. 2015;5(02):13. [Google Scholar]
- 17.Varışlı B., et al. Hesperidin attenuates oxidative stress, inflammation, apoptosis, and cardiac dysfunction in sodium fluoride‐Induced cardiotoxicity in rats. Cardiovasc. Toxicol. 2022;22(8):727–735. doi: 10.1007/s12012-022-09751-9. [DOI] [PubMed] [Google Scholar]
- 18.Varışlı B., et al. Chrysin mitigates diclofenac-induced hepatotoxicity by modulating oxidative stress, apoptosis, autophagy and endoplasmic reticulum stress in rats. Mol. Biol. Rep. 2022:1–10. doi: 10.1007/s11033-022-07928-7. [DOI] [PubMed] [Google Scholar]
- 19.Caglayan C., et al. Neuroprotective effects of 18β-glycyrrhetinic acid against bisphenol A-induced neurotoxicity in rats: involvement of neuronal apoptosis, endoplasmic reticulum stress and JAK1/STAT1 signaling pathway. Metab. brain Dis. 2022;37(6):1931–1940. doi: 10.1007/s11011-022-01027-z. [DOI] [PubMed] [Google Scholar]
- 20.Zamani S.-S., et al. Pharmacokinetics of calycopterin and xanthmicrol, two polymethoxylated hydroxyflavones with anti-angiogenic activities from Dracocephalum kotschyi Bioss. DARU. J. Pharm. Sci. 2016;24(1):1–10. doi: 10.1186/s40199-016-0161-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sotillo W.S., et al. Breast cancer cell line toxicity of a flavonoid isolated from Baccharis densiflora. BMC Complement. Med. Ther. 2021;21(1):1–11. doi: 10.1186/s12906-021-03349-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jodynis-Liebert J., Kujawska M. Biphasic dose-response induced by phytochemicals: experimental evidence. J. Clin. Med. 2020;9(3):718. doi: 10.3390/jcm9030718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Croom E. Metabolism of xenobiotics of human environments. Prog. Mol. Biol. Transl. Sci. 2012;112:31–88. doi: 10.1016/B978-0-12-415813-9.00003-9. [DOI] [PubMed] [Google Scholar]
- 24.Nair M.P., et al. The flavonoid quercetin inhibits proinflammatory cytokine (tumor necrosis factor alpha) gene expression in normal peripheral blood mononuclear cells via modulation of the NF-κβ system. Clin. Vaccin. Immunol. 2006;13(3):319–328. doi: 10.1128/CVI.13.3.319-328.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu M., et al. Targeting transmembrane TNF-α suppresses breast cancer growthanti-tmTNF-α antibody with antitumor activities. Cancer Res. 2013;73(13):4061–4074. doi: 10.1158/0008-5472.CAN-12-3946. [DOI] [PubMed] [Google Scholar]
- 26.Soheilyfar S., et al. In vivo and in vitro impact of miR-31 and miR-143 on the suppression of metastasis and invasion in breast cancer. J. BUON. 2018;23(5):1290–1296. [PubMed] [Google Scholar]
- 27.Ma Y., et al. miR-27a regulates the growth, colony formation and migration of pancreatic cancer cells by targeting Sprouty2. Cancer Lett. 2010;298(2):150–158. doi: 10.1016/j.canlet.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 28.Yin H., et al. Progress on the relationship between miR-125 family and tumorigenesis. Exp. Cell Res. 2015;339(2):252–260. doi: 10.1016/j.yexcr.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Z., et al. Upregulation of p72 enhances malignant migration and invasion of glioma cells by repressing Beclin1 expression. Biochem. (Mosc. ) 2016;81(6):574–582. doi: 10.1134/S0006297916060031. [DOI] [PubMed] [Google Scholar]
- 30.Wang R., et al. Functional role of miR-34 family in human cancer. Curr. Drug Targets. 2013;14(10):1185–1191. doi: 10.2174/13894501113149990191. [DOI] [PubMed] [Google Scholar]
- 31.Nieddu M., et al. Xanthomicrol activity in cancer HeLa cells: comparison with other natural methoxylated flavones. Molecules. 2023;28(2):558. doi: 10.3390/molecules28020558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bergers G., Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat. Rev. Cancer. 2008;8(8):592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maroufi N.F., et al. Therapeutic potentials of Apatinib in cancer treatment: Possible mechanisms and clinical relevance. Life Sci. 2020;241 doi: 10.1016/j.lfs.2019.117106. [DOI] [PubMed] [Google Scholar]
- 34.Sagar S., Yance D., Wong R. Natural health products that inhibit angiogenesis: a potential source for investigational new agents to treat cancer—Part 1. Curr. Oncol. 2006;13(1):14–26. doi: 10.3747/co.v13i1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kikuchi S., et al. Involvement of extracellular vesicles in vascular-related functions in cancer progression and metastasis. Int. J. Mol. Sci. 2019;20(10):2584. doi: 10.3390/ijms20102584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahmadi M., Rezaie J. Tumor cells derived-exosomes as angiogenenic agents: possible therapeutic implications. J. Transl. Med. 2020;18(1):1–17. doi: 10.1186/s12967-020-02426-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Melincovici C.S., et al. Vascular endothelial growth factor (VEGF)-key factor in normal and pathological angiogenesis. Rom. J. Morphol. Embryol. 2018;59(2):455–467. [PubMed] [Google Scholar]
- 38.Quintero-Fabián S., et al. Role of matrix metalloproteinases in angiogenesis and cancer. Front. Oncol. 2019;9:1370. doi: 10.3389/fonc.2019.01370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ghazizadeh F., et al. Xanthomicrol exerts antiangiogenic and antitumor effects in a mouse melanoma (B16F10) allograft model. Evid. -Based Complement. Altern. Med. 2020:2020. doi: 10.1155/2020/8543872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin Z.-Z., et al. Xanthomicrol suppresses human hepatocellular carcinoma cells migration and invasion ability via Μu-opioid receptor. J. Pharm. Pharmacol. 2022;74(1):139–146. doi: 10.1093/jpp/rgab104. [DOI] [PubMed] [Google Scholar]
- 41.Patel S. Emerging adjuvant therapy for cancer: propolis and its constituents. J. Diet. Suppl. 2016;13(3):245–268. doi: 10.3109/19390211.2015.1008614. [DOI] [PubMed] [Google Scholar]
- 42.Agha A., Tarhini A.A. Adjuvant therapy for melanoma. Curr. Oncol. Rep. 2017;19(5):1–9. doi: 10.1007/s11912-017-0594-5. [DOI] [PubMed] [Google Scholar]
- 43.Bagri A., et al. Use of anti-VEGF adjuvant therapy in cancer: challenges and rationale. Trends Mol. Med. 2010;16(3):122–132. doi: 10.1016/j.molmed.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 44.Plate K.H., Scholz A., Dumont D.J. Tumor angiogenesis and anti-angiogenic therapy in malignant gliomas revisited. Acta Neuropathol. 2012;124(6):763–775. doi: 10.1007/s00401-012-1066-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hendrix M.J., et al. Tumor cell vascular mimicry: novel targeting opportunity in melanoma. Pharmacol. Ther. 2016;159:83–92. doi: 10.1016/j.pharmthera.2016.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maniotis A.J., et al. Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am. J. Pathol. 1999;155(3):739–752. doi: 10.1016/S0002-9440(10)65173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu Y., et al. Short-term anti-vascular endothelial growth factor treatment elicits vasculogenic mimicry formation of tumors to accelerate metastasis. J. Exp. Clin. Cancer Res. 2012;31(1):1–7. doi: 10.1186/1756-9966-31-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fathi Maroufi N., et al. Vascular mimicry: changing the therapeutic paradigms in cancer. Mol. Biol. Rep. 2020;47(6):4749–4765. doi: 10.1007/s11033-020-05515-2. [DOI] [PubMed] [Google Scholar]
- 49.Hayes D.F. Bevacizumab treatment for solid tumors: boon or bust? Jama. 2011;305(5):506–508. doi: 10.1001/jama.2011.57. [DOI] [PubMed] [Google Scholar]
- 50.Wang M., et al. Myricetin reverses epithelial–endothelial transition and inhibits vasculogenic mimicry and angiogenesis of hepatocellular carcinoma by directly targeting PAR1. Phytother. Res. 2022;36(4):1807–1821. doi: 10.1002/ptr.7427. [DOI] [PubMed] [Google Scholar]
- 51.Zang M., et al. Luteolin suppresses angiogenesis and vasculogenic mimicry formation through inhibiting Notch1-VEGF signaling in gastric cancer. Biochem. Biophys. Res. Commun. 2017;490(3):913–919. doi: 10.1016/j.bbrc.2017.06.140. [DOI] [PubMed] [Google Scholar]
- 52.Chiablaem K., et al. Curcumin suppresses vasculogenic mimicry capacity of hepatocellular carcinoma cells through STAT3 and PI3K/AKT inhibition. Anticancer Res. 2014;34(4):1857–1864. [PubMed] [Google Scholar]
- 53.Wu Q., et al. Multi-drug resistance in cancer chemotherapeutics: mechanisms and lab approaches. Cancer Lett. 2014;347(2):159–166. doi: 10.1016/j.canlet.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 54.Avner B.S., Fialho A.M., Chakrabarty A.M. Overcoming drug resistance in multi-drug resistant cancers and microorganisms: a conceptual framework. Bioengineered. 2012;3(5):262–270. doi: 10.4161/bioe.21130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bugde P., et al. The therapeutic potential of targeting ABC transporters to combat multi-drug resistance. Expert Opin. Ther. Targets. 2017;21(5):511–530. doi: 10.1080/14728222.2017.1310841. [DOI] [PubMed] [Google Scholar]
- 56.Niu S., et al. A novel chitosan-based nanomedicine for multi-drug resistant breast cancer therapy. Chem. Eng. J. 2019;369:134–149. [Google Scholar]
- 57.Liskova A., et al. Flavonoids as an effective sensitizer for anti-cancer therapy: insights into multi-faceted mechanisms and applicability towards individualized patient profiles. EPMA J. 2021;12(2):155–176. doi: 10.1007/s13167-021-00242-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.