Graphical abstract
Keywords: Phosphatidylethanol (PEth), Ethyl glucuronide (EtG), Alcohol use, Self-reported alcohol use, Alcohol use biomarkers, Pregnancy
Highlights
-
•
Depending on the cutoff, PEth identified 47%–70% as alcohol-consuming.
-
•
Self-report from participants identified 65% to 75% as alcohol-consuming.
-
•
Lower PEth cutoffs had high sensitivity and accuracy when compared to self-report.
-
•
For human research purposes, PEth > 2 and > 4 ng/ml are most sensitive and accurate.
-
•
For non-forensic purposes, PEth > 20 ng/ml may be too strict a threshold.
Abstract
In the literature on alcohol use biomarkers, there has been debate as to what a valid and/or utilitarian cut off level should be for various research applications. In this manuscript, we assessed the sensitivity and specificity of multiple cutoff values for phosphatidylethanol (PEth) from bloodspots relative to self-report, the Alcohol Use Disorder Identification Test (AUDIT) scores, and another alcohol use biomarker ethyl glucuronide (EtG) from fingernails in a sample of 222 pregnant women in the Western Cape Province of South Africa. Receiver operating characteristic (ROC) curves were used to assess the area under the curve (AUC) and assess PEth cutoff values of ≥2, ≥4, ≥8, ≥14, and ≥20 nanograms per milliliter (ng/ml). The highest AUC value was attained when PEth was compared to an AUDIT score of 1 or more. Depending on the cutoff used to determine alcohol consumption, PEth identified 47%–70% of the individuals as alcohol-consuming while 62.6%–75.2% were identified by self-reported measures, and 35.6% were identified by EtG. In this sample, sensitivity and accuracy were highest at less stringent PEth cutoffs when compared to self-report, AUDIT score of 1 or more, 5 or more, 8 or more, and EtG ≥ 8 picograms per milligram (pg/mg). For research purposes, less stringent cutoffs, such as PEth ≥ 8 ng/ml, may be considered a valid, positive cutoff for identifying women who consume alcohol during pregnancy in this population. A cutoff of PEth ≥ 20 ng/ml may miss individuals who reported consuming alcohol (false negatives).
Introduction
In order to accurately make a diagnosis within the continuum of fetal alcohol spectrum disorders (FASD), accurate determination of the presence or absence of prenatal alcohol exposure to a fetus is essential. The most recent revised Institute of Medicine (IOM) diagnostic guidelines (Hoyme et al., 2016) defined alcohol exposure to include: a) 6 or more drinks for 2 or more weeks during pregnancy or b) 3 or more drinks per occasion on 2 or more occasions during pregnancy (Hoyme et al., 2016). Other diagnostic guidelines have their own criteria for defining what is sufficient for prenatal alcohol exposure to qualify for an FASD diagnosis (Cook et al., 2016, Bower and Elliott, 2016). A challenge for diagnosis continues to be access to reliable and accurate alcohol exposure information.
Pregnant women are often believed to be less than accurate when providing alcohol-use information, especially when the studies seek actual levels of exposure (dosage) and when they are carried out in prenatal clinic settings in the United States and Europe (Wetterling et al., 1998, Ernhart et al., 1988, Siegfried, 2001). Underreporting of the extent of alcohol consumption has been suspected when self-report is the sole assessment method. When self-reported prevalence has been compared to the results from one or more alcohol-specific biomarkers, in a variety of biological specimens, underreporting is often verified (Bakhireva et al., 2017, Lange et al., 2014, Gareri et al., 2008, Papas et al., 2016, Wurst et al., 2008). However, there is also ample evidence that individuals in many populations report accurately if proper interviewing techniques are used, rapport is built, and multiple measures of alcohol use over time are used, especially in research studies carried out separately from clinical/medical activities (Baldwin et al., 2015, Czarnecki et al., 1990, Fortin et al., 2017, Hannigan et al., 2010, Howlett et al., 2017, Jacobson et al., 2002). Therefore, self-report may or may not be sufficient to determine prenatal alcohol exposure.
Alcohol use biomarkers
Alcohol use biomarkers are increasingly being relied upon in research to indicate if alcohol consumption has occurred. Two direct alcohol biomarkers, phosphatidylethanol (PEth) and ethyl glucuronide (EtG), have been used in several biological matrices (e.g., blood, urine, fingernails, placenta) in multiple prenatal studies to assess alcohol consumption.
PEth is an abnormal phospholipid produced following alcohol consumption, and it is considered to be specific to alcohol exposure (Bakhireva et al., 2014, Isaksson et al., 2011). Age, sex, and disease state have been shown to not affect the formation of PEth (Stewart et al., 2009, Wurst et al., 2010). PEth can accumulate over time, and with frequent drinking occasions, PEth has a half-life of approximately 6 days (Helander et al., 2019). PEth can be detected in blood 3–12 days after a single dose of alcohol (Schröck et al., 2017) or 2–4 weeks depending on the individual’s quantity and frequency of alcohol consumption and the individual’s metabolic efficiency (Isaksson et al., 2011, Schröck et al., 2017, Ulwelling and Smith, 2018). For clinical and forensic testing, a cutoff of ≥ 20 nanograms per milliliter (ng/ml) for PEth has been adopted, by consensus among the four accredited United States laboratories that perform PEth analysis (Ulwelling and Smith, 2018). The 20 ng/ml cutoff was selected to differentiate between alcoholic beverage consumption and abstinence or incidental exposures, although at least one laboratory has used a lower threshold of 8 ng/ml for research, or non-clinical or non-forensic, testing (Fleming et al., 2017, Baldwin et al., 2020). Internationally, cutoff for PEth of 15 ng/ml, 20 ng/ml, and 35 ng/ml have been adopted as positive indicators of alcohol consumption (Ulwelling and Smith, 2018, Hill-Kapturczak et al., 2018, Schröck et al., 2016). However, currently there is not a clear link between a minimum drinking quantity or blood alcohol concentration (BAC) necessary to achieve a PEth at or above 20 ng/ml or any specific PEth cutoff (Stoth et al., 2023, Schröck et al., 2017). None of the thresholds are supported by overwhelming evidence as to what the definitive threshold for determining alcohol consumption vs. abstinence should be. Each threshold represents differences in the need for sensitivity, specificity, and accuracy of the measure (Reisfield et al., 2020). Thus, for research studies, the question remains: which cutoff should be used for PEth?
EtG is also a metabolite that is produced only in the presence of alcohol (Berger et al., 2014, Substance Abuse and Mental Health Services Administration SAMHSA, 2012). EtG can be detected in a variety of biological matrices. In nails, EtG becomes embedded into the keratin fibers along the entire fingernail and toenail. EtG has a window of detection for moderate to heavy drinking of approximately 3 months in fingernails after alcohol cessation (Berger et al., 2014). Previous work has demonstrated that an EtG value of ≥ 8 picogram per milligram (pg/mg) as an indication of alcohol exposure detected all high-risk drinkers and over 80% of moderate-risk drinkers (Berger et al., 2014). A value of ≥ 8 pg/mg for EtG in fingernails has been considered a positive value to validate alcohol consumption by some researchers and experienced laboratory practitioners (Ulwelling and Smith, 2018, Reisfield et al., 2020, Berger et al., 2014, Reisfield et al., 2020).
Previous studies using PEth in South Africa
A previous study in the Western Cape Province of South Africa compared the accuracy of self-reported alcohol use during pregnancy to positive results for PEth and EtG biomarkers combined. The study concluded that women in this population reported similar alcohol exposures, in a binary sense (yes/no), when compared to the combined results from these two biomarkers with a cutoff of ≥ 8 ng/ml for PEth and ≥ 8 pg/mg for EtG (May et al., 2018). Since there was no significant difference in the percent of positive cases from combined positive results from one and/or both of the two biomarkers and the self-reported values, it was concluded that reporting was accurate and quite valid in this South African population.
Study purpose
Because there remains debate in the literature about the appropriate cutoff of PEth to denote alcohol consumption for research studies, the purpose of this study was to investigate the sensitivity, specificity, and accuracy of various PEth cutoffs relative to a) self-reported alcohol consumption and b) EtG among pregnant women in South Africa.
Methods
Human subjects/ethics protocols and consent forms were approved by the University of North Carolina and the Ethics Committee of Stellenbosch University, Faculty of Medicine and Health Sciences. All participants who were interviewed about maternal risk factors and provided biological samples for the study provided written consent.
Sample
Women were recruited from prenatal clinics which serve the study communities in the Western Cape Province of South Africa. The racial composition of these predominately agricultural and light industrial communities was approximately 70% of mixed-race (“Coloured”) ancestry, 16% Black, 12% White, and 1% Indian/Asian descent (Statistics South Africa and Census, 2011). Inclusion criteria were pregnant women who were between 5 and 36 weeks gestation and who reported: a) alcohol use during the past seven days; or b) alcohol use during the past 30 to 90 days; or c) abstention over the past 90 days.
Two-hundred and twenty-two (2 2 2) pregnant women completed in-depth maternal interviews during pregnancy, and each consented individual provided both blood and fingernail samples for biomarker analyses: PEth was analyzed in bloodspots and EtG was analyzed in fingernails. Data from 193 of the women in this study were analyzed and presented in a previous paper as a utilitarian test of using these markers in a binary fashion (yes/no) for an objective and accurate detection of alcohol use during pregnancy (May et al., 2018).
Because most drinking occurs on weekends in this population, blood specimens for the PEth biomarker analyses were collected on a Monday or Tuesday for accurate estimates of drinking prevalence. Many fingernail samples were collected at first contact (at the same time as the blood samples). Alternatively, because many of these women work with their hands in agricultural vocations, some required a return visit once the nails had grown sufficiently to provide clippings (3 mm per nail) for adequate sampling. Approximately 30–40% of participants returned within three weeks of the interview to provide nail sample that was adequate for analysis.
Self-report measures and cutoffs
Self-reported alcohol measures were the Alcohol Use Disorders Identification Test (AUDIT) (Babor et al., 2001) and whether a woman reported drinking any alcohol during pregnancy when asked a number of direct questions about her quantity, frequency, and timing of alcohol use during pregnancy. The AUDIT is a 10-item instrument which screens for excessive drinking and harmful drinking patterns in the previous 12 months. Scores can range from 0 to 40 with a score of 8 or more defined as high-risk for alcohol-related health problems for healthy, non-pregnant individuals (Babor et al., 2001), while lower AUDIT scores may indicate risky alcohol consumption during pregnancy. Given the known risk of consuming alcohol while pregnant, this study used three different thresholds of the AUDIT to denote alcohol consumption. These were AUDIT scores of: 1 or more, 5 or more, and 8 or more.
A second self-reported measure was a binary (yes/no) variable that was derived from 7-day drinking recall logs completed during pregnancy. If a woman indicated alcohol consumption in any of her drinking logs, the self-report measure was coded ‘yes’. If no alcohol consumption was reported, the self-report measure was coded ‘no’.
Data analysis
Spearman correlations were undertaken to examine the association between PEth, EtG, and the quantity of drinks per drinking day. Receiver operating characteristic (ROC) curves assessed the overall classification performance of various PEth cutoff points by comparing to three AUDIT cutoffs (1 or more, 5 or more, and 8 or more), self-report (yes/no), and EtG. Because the consensus-established threshold for positive indication of alcohol consumption in clinical and forensic testing in the United States is set at ≥ 20 ng/ml for PEth, the sensitivity and specificity of PEth ≥ 2 ng/ml through ≥ 20 ng/ml were the focus of this manuscript. All data were analyzed in SPSS, version 28.
Results
Table 1 – Sample Characteristics
Table 1.
Entire Sample Characteristics for PEth, EtG, AUDIT, and Drinks per Drinking Day (n = 222).
| Mean | (SD) | Min | Max | |
|---|---|---|---|---|
| PEth Value (ng/ml)A | 77.6 | (144.3) | 0.0 | 914.0 |
| PEth Value (n = 156)1 | 110.5 | (161.4) | 2.0 | 914.0 |
| EtG Value (pg/mg)B | 29.5 | (66.1) | 0.0 | 522.0 |
| EtG Value (n = 92)1 | 71.2 | (87.2) | 4.0 | 522.0 |
| AUDIT Score | 11.4 | (9.2) | 0.0 | 33.0 |
| AUDIT Score (n = 167)1 | 15.2 | (7.5) | 1.0 | 33.0 |
| Average Drinks per Drinking Day (DDD) in 1st trimester | 4.0 | (4.3) | 0.0 | 24.5 |
| Average Drinks per Drinking Day (DDD) in 1st trimester – among those who drank (n = 143)1 | 6.3 | (3.9) | 0.4 | 24.5 |
| Average Drinks per Drinking Day (DDD) in previous 7 days | 1.0 | (2.2) | 0.0 | 11.7 |
| Average Drinks per Drinking Day (DDD) in previous 7 days – among those who drank (n = 55)1 | 4.0 | (2.6) | 0.2 | 11.7 |
APEth (phosphatidylethanol) values are measured in nanograms per milliliter (ng/ml).
BEtG (ethyl glucuronide) values are measured in picograms per milligram (pg/mg).
1Excluded individuals who had a score of 0.
AUDIT: Alcohol Use Disorder Identification Test.
Two hundred and twenty-two (2 2 2) women were included in this sample. The mean PEth value of the entire sample of women was 77.6 ng/ml (SD = 114.3) with a range from 0.0 to 914.0 ng/ml. Among women who had a non-zero value for PEth, the mean was 110.5 ng/ml (SD = 161.4). The entire sample mean EtG level was 29.5 pg/mg (SD = 66.1) with a range from 0.0 to 522.0 pg/mg. The AUDIT score ranged from 0 to 33 with a sample mean of 11.4 (SD = 9.2) and a mean of 15.2 for drinkers only. This was a heavy drinking sample. First trimester drinking was reported by 143 women (64.4%) with the mean drinks per drinking day (DDD) reported to be 6.3. Fifty-five women (24.8%) reported consuming alcohol in the previous 7 days, and among those who drank, the reported mean was 4.0 DDD (SD = 2.6).
Correlation of PEth and EtG with self-report
PEth level was significantly and strongly correlated with the AUDIT score (r = 0.522), average DDD in 1st trimester (r = 0.501), average DDD in the previous 7 days (r = 0.536), and self-reported alcohol use during pregnancy (r = 0.524) (see Table 2). PEth and EtG scores had the strongest correlation at 0.560. In Table 3, EtG also had significant correlations with the AUDIT score (r = 0.358), average DDD in 1st trimester (r = 0.315), average DDD in the previous 7 days (r = 0.354), and reported alcohol use during pregnancy (r = 0.337). But these correlations were not as strong as the correlation coefficients between PEth and the AUDIT and quantity of DDD.
Table 2.
Spearman’s Rho Correlation Coefficient Comparing PEth Scores to EtG Scores and Select Self-Report Measures.
| EtG | AUDIT | Average DDD – 1st trimester | Average DDD – previous 7 days | Self-reported alcohol consumption during pregnancy (no/yes) | |
|---|---|---|---|---|---|
| r | 0.560 | 0.522 | 0.501 | 0.536 | 0.524 |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| N | 222 | 222 | 222 | 222 | 222 |
AUDIT: Alcohol Use Disorder Identification Test; DDD: drinks per drinking day; EtG: ethyl glucuronide; PEth: phosphatidylethanol.
Table 3.
Spearman’s Rho Correlation Coefficient Comparing EtG to PEth and Select Self-Report Measures.
| PEth | AUDIT | Average DDD – 1st trimester | Average DDD – previous 7 days | Self-reported alcohol consumption during pregnancy (no/yes) | |
|---|---|---|---|---|---|
| r | 0.560 | 0.358 | 0.315 | 0.354 | 0.337 |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| N | 222 | 222 | 222 | 222 | 222 |
AUDIT: Alcohol Use Disorder Identification Test; DDD: drinks per drinking day; EtG: ethyl glucuronide; PEth: phosphatidylethanol
Comparing PEth Levels: 2 vs. 4 vs. 8 vs. 14. vs. 20 ng/ml
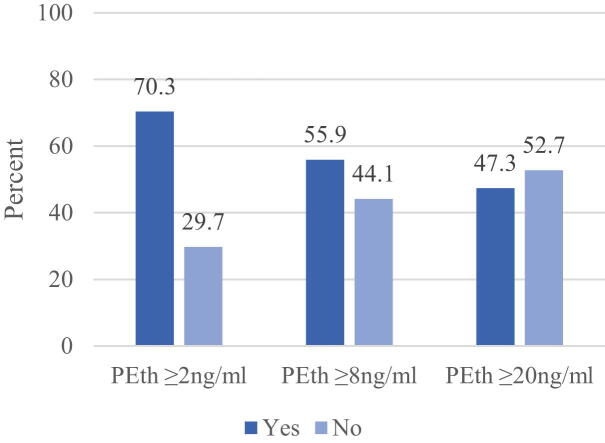
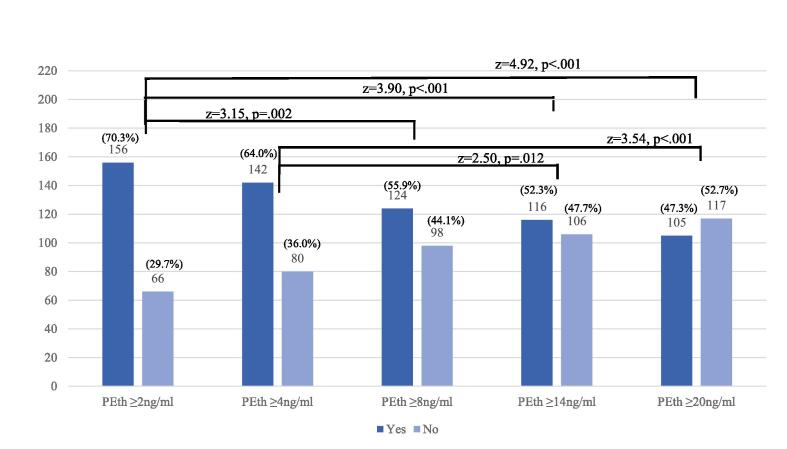
Fig. 1 presents the number and percent of participants identified as alcohol-consuming by various PEth cutoffs. PEth ≥ 2 ng/ml identified 156 women (70.3%) as alcohol consuming, ≥ 4 ng/ml identified 142 (64.0%), ≥ 8 ng/ml identified 124 (55.9%), ≥ 14 ng/ml identified 116 (52.3%), and PEth ≥ 20 ng/ml identified 105 (47.3%). Z-score test of proportions in Fig. 1 indicated that PEth ≥ 2 ng/ml identified significantly more women as alcohol consuming than ≥ 8, ≥ 14, and ≥ 20 ng/ml. PEth ≥ 4 ng/ml also identified significantly more than either PEth ≥ 14 and ≥ 20 ng/ml. There were no significant differences in the percent identified as alcohol consuming between PEth ≥ 8, ≥ 14, and ≥ 20 ng/ml, although the difference between PEth 8 vs 20 ng/ml approached significance (z = 1.80, p = .071).
Fig. 1.
Count and Percent Identified by Select Cutoffs of PEth. Two trends towards significance were: PEth ≥ 4 ng/ml vs PEth ≥ 8 ng/ml (z = 1.73, p =.081) and PEth ≥ 8 ng/ml vs PEth ≥ 20 ng/ml (z = 1.80, p =.071). No other comparisons were significantly different from one another.
Sensitivity and specificity
Table 4 shows the number and percent identified as positive and negative for the two biomarkers PEth (at various levels), EtG ≥ 8 pg/mg, self-report, and an AUDIT score or 1 or more, 5 or more, and 8 or more. The highest number and percent positive was identified by an AUDIT score of 1 or more (75.2%), followed by PEth ≥ 2 ng/ml (70.3%), an AUDIT score of 5 or more (69.4%), self-report (65.3%), PEth ≥ 4 ng/ml (64.0%), and an AUDIT score of 8 or more (62.6%). PEth ≥ 8, ≥ 14, ≥ 20 ng/ml and EtG ≥ 8 pg/mg identified less than 60% as alcohol-consuming.
Table 4.
Percent positive and negative by various measures and cutoffs.
| Yes |
No |
|||
|---|---|---|---|---|
| N | % | N | % | |
| Biomarkers | ||||
| PEth ≥ 2 ng/ml | 156 | 70.3 | 66 | 29.7 |
| PEth ≥ 4 ng/ml | 142 | 64.0 | 80 | 36.0 |
| PEth ≥ 8 ng/ml | 124 | 55.9 | 98 | 44.1 |
| PEth ≥ 14 ng/ml | 116 | 52.3 | 106 | 47.7 |
| PEth ≥ 20 ng/ml | 105 | 47.3 | 117 | 52.7 |
| EtG ≥ 8 pg/mg | 79 | 35.6 | 143 | 64.4 |
| Self-report measures | ||||
| AUDIT score of 1 or more | 167 | 75.2 | 55 | 24.8 |
| AUDIT score of 5 or more | 154 | 69.4 | 68 | 30.6 |
| AUDIT score of 8 or more | 139 | 62.6 | 83 | 37.4 |
| Binary self-report (yes/no) | 145 | 65.3 | 77 | 34.7 |
To determine how well PEth performed, ROC curves were utilized to depict the sensitivity and 1-specificity of PEth when using self-report, the AUDIT, and EtG as the references. In other words, if we assumed self-report, the AUDIT, and EtG were correct, the ROC demonstrates how well PEth did to identify true positives (sensitivity), true negatives (specificity), and overall correctly classify individuals (accuracy). Because the AUDIT is a continuous measure, and ROC curves necessitate binary measures, three AUDIT cutoffs (1 or more, 5 or more, and 8 or more) were examined.
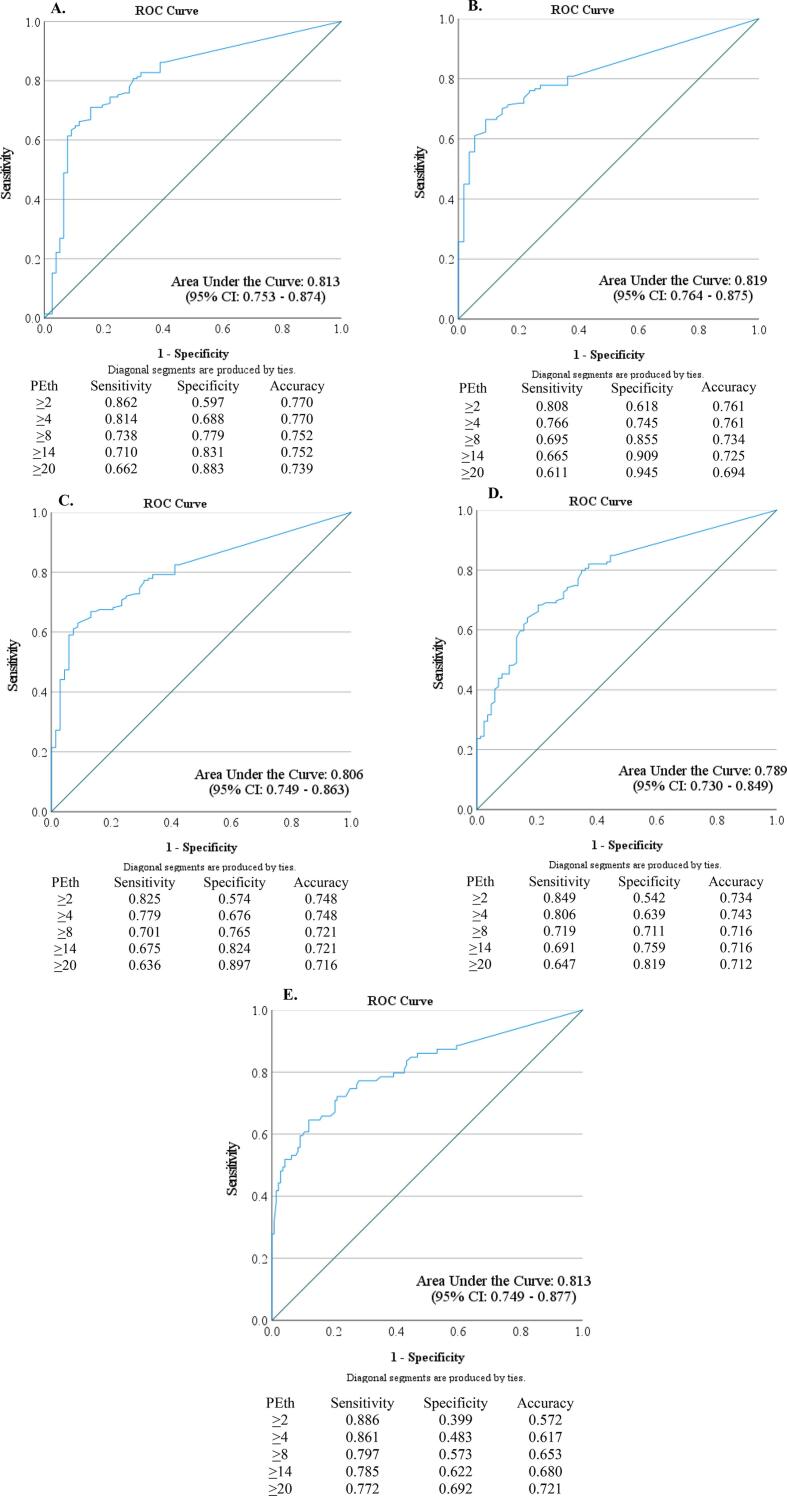
As demonstrated in ROC curves in Fig. 2, PEth was informative in determining whether a woman consumed alcohol or not. The area under the curve (AUC) was highest when PEth was compared to an AUDIT score of 1 or more (AUC = 0.819; Fig. 2B) and lowest when PEth was compared to an AUDIT score of 8 or more (AUC = 0.789; Fig. 2D). Except for PEth vs. AUDIT score of 8 or more, all AUC were in the 0.8 to 0.9 range which can be considered excellent (Hosmer et al., 2013). To further examine the sensitivity, sensitivity, and accuracy of PEth, select coordinates are explicitly shown in Fig. 2A–E.
Fig. 2.
Receiver Operating Characteristic (ROC) curves for: A) PEth Relative to Using Self-Reported Alcohol Consumption (yes/no) During Pregnancy as Reference; B) PEth Relative to Using Self-Reported AUDIT score of 1 or more During Pregnancy as Reference; C) PEth Relative to Using Self-Reported AUDIT score of 5 or more as Reference; D) PEth Relative to Using Self-Reported AUDIT score of 8 or more as Reference E) PEth Relative to Using EtG ≥ 8 pg/mg as Reference.
Using the binary (yes/no) self-report variable as the reference (Fig. 2A), PEth sensitivity ranged from 0.862 when the PEth cutoff was ≥ 2, to 0.0662 when the PEth cutoff was ≥ 20. Specificity was greatest when PEth was ≥ 20. Accuracy was greatest at 0.770 when the cutoff of PEth was ≥ 2 or ≥ 4 and accuracy was lowest at 0.739 when the cutoff of PEth ≥ 20 was used.
Compared to an AUDIT score of 1 or more, PEth sensitivity ranged from 0.808 to 0.611 when PEth was ≥ 2 and ≥ 20, respectively (Fig. 2B). Accuracy was again highest when PEth ≥ 2 or ≥ 4 (accuracy = 0.761). When an AUDIT score of 5 or more (Fig. 2C) and AUDIT score of 8 or more (Fig. 2D) were used as the reference, sensitivity ranged from 0.825 to 0.636 and 0.849 to 0.647, respectively. Relative to an AUDIT score of 5, the accuracy was greatest when PEth ≥ 2 or ≥ 4 and relative to an AUDIT score of 8, accuracy was highest at PEth ≥ 4.
The sensitivity of PEth ranged from 0.886 when PEth was ≥ 2 to 0.772 when PEth was ≥ 20 when compared to EtG (Fig. 2E). Relative to EtG, the PEth accuracy was lowest when PEth ≥ 2 was used and the greatest when PEth ≥ 20 was used.
As shown overall in Fig. 2, the ability to identify true positives (sensitivity) was highest when the lowest PEth cutoff (≥2) was used with various reference variables. This was true when PEth ≥ 2 was compared to self-report, AUDIT score of 1 or more, 5 or more, 8 or more, and EtG ≥ 8 pg/mg. The ability to identify true negatives (specificity) was greatest when the highest PEth cutoff (≥20) was used. When compared to self-report and the AUDIT, the ability of PEth to correctly classify individuals (accuracy) was greatest when the lowest PEth cutoff (≥2) or low PEth cutoff (≥4) was used. When compared to EtG, the accuracy of PEth was greatest when the highest PEth (≥20) cutoff was used; but overall EtG identified the fewest women as alcohol consuming. Therefore, a more stringent PEth was needed to be comparable to EtG and maximize accuracy.
Discussion
This study indicates that: 1) this expanded sample from a South African small town and rural population of the Western Cape Province once again supports that it is an accurate population for reporting alcohol consumption (Schröck et al., 2016), and 2) relative to self-report and the AUDIT, the accuracy of PEth was highest when less stringent cutoffs were used.
Accurate reporting in this South African population
In this sample overall, self-reported information identified > 65% of the sample as alcohol consuming. This reinforces the belief that women of mixed-race (‘Cape Coloured’) ancestry in the Western Cape Province report their alcohol consumption candidly and accurately. This accuracy of self-reporting may be attributable, in part, to two factors. First, self-reported information obtained through in-person interviewers via non-clinical setting with well-trained, experienced, and empathic research staff using sensitively-worded questions and sequenced prompts, may allow for greater self-disclosure of alcohol use information (Jacobson et al., 2002, Fortin et al., 2017) than if carried out in a prenatal or family practice clinical setting. Second, there is little stigma around drinking in general, even for women during pregnancy, in certain subgroups in the Western Cape. Several other populations have also demonstrated accuracy in their reporting of alcohol consumption (in the binary sense) in the prenatal period when carried out in similar research studies (Fortin et al., 2017, Jacobson et al., 2002, Petersen-Williams et al., 2014).
Peth can accurately identify individuals as alcohol consuming
Despite the different assessment windows (e.g., the AUDIT in the past 12 months, 1st trimester drinking, and drinking in the previous 7 days), PEth correlated strongly with each assessment even though PEth has a window of detection of approximately 1–4 weeks. Others have also noted a similar correlation with PEth and self-reported assessments with different assessment windows (Röhricht et al., 2020). The high correlation demonstrated in this study is consistent with the stable drinking pattern observed in some women in the Western Cape Province. Regular weekend drinking is common among women of childbearing age as a form of recreation and stress management (Fletcher et al., 2018), and alcohol cessation may not occur prior to or during pregnancy. The overall high correlation between PEth with the AUDIT score, drinks per drinking day (DDD) in 1st trimester, and DDD in the previous 7 days supports the utility of using PEth for identifying pregnancies with alcohol exposure.
Depending on the cutoff used to determine alcohol consumption, PEth identified 47%–70% of the individuals as alcohol-consuming while 62.6% to 75.2% were identified by self-report measures and 35.6% were identified by EtG. Depending on the cutoff of PEth utilized, PEth may under-identify individuals as alcohol consuming. The quantity of PEth can vary due to considerable individual variation in both the formation and elimination of PEth (Baldwin et al., 2015, Schröck et al., 2017, Reisfield et al., 2020) with shorter PEth half-life being noted among studies of heavier drinkers (>5 drinks on a regular basis) (Isaksson et al., 2011). Therefore, PEth may not identify all women who consume alcohol, especially if the quantity and/or frequency of alcohol consumption is light or infrequent. Therefore, in a population which generally reports accurately, self-report may outperform PEth. But when less stringent cutoffs of PEth are used to determine alcohol exposure, PEth and self-report may perform very similarly.
In this study we demonstrated that the PEth ≥ 2 ng/ml and PEth ≥ 4 ng/ml cutoffs identified significantly more women as alcohol-consuming than did PEth ≥ 14 ng/ml and PEth ≥ 20 ng/ml. This indicates ≥ 2 ng/ml and PEth ≥ 4 ng/ml had fewer false negatives than did the higher PEth cutoffs. In terms of identifying pregnancies and children at risk for having FASD, it may be more advantageous to reduce the number of false negatives as much as possible. Henderson et al. (2022) have also demonstrated that a lower PEth cutoff (≥ 8 ng/ml) in infants had greater sensitivity than PEth ≥ 20 ng/ml at identifying women who self-reported modest, late pregnancy alcohol consumption (Henderson et al., 2022). Moreover, Stoth et al. (2023) have shown that blood alcohol concentrations ranging from 0.10 to 0.63 can be achieved without reaching PEth ≥ 20 ng/ml threshold (Stoth et al., 2023). In populations where alcohol information is unknown, using a less restrictive PEth cutoff, will likely be more sensitive and therefore identify more individuals as alcohol-consuming and may be a useful biomarker to aid in the diagnosis of alcohol misuse and identify at-risk pregnancies for children with FASD. This may be particularly important in research studies where the primary focus is on the identification and/or prevention of alcohol-exposed pregnancies. In a clinical setting or for forensic purposes, the ≥ 20 ng/ml level provides more specificity and therefore may be appropriate to use to minimize false positives.
Implications for other populations
This South African population differs from many populations in the United States and Europe where there has been documentation of significant underreporting of alcohol consumption when compared to various biomarkers obtained during pregnancy, at birth (via umbilical samples), or in meconium (e.g., fatty acid ethyl esters, PEth, and EtG) (Bakhireva et al., 2017, Gareri et al., 2008, Wurst et al., 2008, Garcia-Algar et al., 2008, Pichini et al., 2012, Sanvisens et al., 2016). In clinical settings, indirect measures of alcohol consumption such as carbohydrate deficient transferrin, gamma-glutamyl-transferase, and mean corpuscular volume have also been shown to be poorer indicators of alcohol consumption when compared to the AUDIT (Neumann et al., 2009, Coulton et al., 2006). A previous study in the large metropolis of Cape Town concluded that the AUDIT was a sensitive tool, and it did not miss individuals with an alcohol problem when compared to either FAEE or EtG (Kader et al., 2012). Similar to that study, our findings in small town and rural areas of South Africa support the use of the AUDIT as one tool for identifying women at risk for hazardous drinking (both long-term and recent). However, in some populations where alcohol information is unavailable or the validity of the information is suspect, using less stringent PEth cutoffs may better identify individuals who consume alcohol, particularly at moderate or greater levels and recently. Our data have further shown PEth to be more sensitive and accurate for recent drinking at low to moderate levels than the biomarker EtG extracted from fingernails.
Strengths and limitations
This study had several strengths. First, this is one of few studies to utilize the combined results of two biomarkers to assess alcohol consumption: one biomarker which can detect recent alcohol consumption and a second biomarker which can detect heavy or significant moderate alcohol consumption in the past three months when collected and analyzed from fingernails. EtG from nails captured a more complete window of moderate to heavy exposure across the pregnancy. Second, two different self-reported measures were collected and utilized to compare with two biomarkers. Third, this is a population that has been shown to report alcohol consumption candidly overall and in pregnancy. This allowed for comparing the accuracy of the biomarkers to identify women as alcohol consuming or not. This type of comparison may not be possible or valid in other populations due to underreporting or denial of alcohol consumption during pregnancy.
There were also limitations to this study. First, this is a somewhat unique population in terms of their regular alcohol consumption patterns, for drinking frequently at binge levels (4 or more drinks per occasion) is consistently practiced by many individuals on a majority of weekends. Similar studies may be needed in populations where different drinking patterns exist. Second, PEth and EtG were collected only once during the pregnancy. Multiple collections of blood spots and fingernail samples over the duration of pregnancy may better identify women who consume alcohol during pregnancy, and would help identify critical periods of exposure that are associated with greater or less influence on the exposed fetus and particular outcome traits (e.g., physical features and/or brain development).
Conclusion
In these South African communities, self-report may be sufficient in identifying the majority of individuals who consume alcohol. Yet even in these communities, using less stringent PEth cutoffs may have accurately identified more individuals who have recently consumed alcohol. Therefore, in non-forensic, research studies in certain populations, relying on self-report and/or less stringent PEth cutoffs may be necessary to identify the totality of alcohol-exposed pregnancies and individuals who are at-risk for teratogenic alcohol exposure and for having child with FASD.
CRediT authorship contribution statement
Julie M. Hasken: Conceptualization, Formal analysis, Writing – original draft. Anna-Susan Marais: Project administration, Data curation. Marlene M. de Vries: Project administration, Data curation. Wendy O. Kalberg: Conceptualization, Project administration. David Buckley: Project administration. Charles D.H. Parry: Project administration. Soraya Seedat: Project administration. Philip A. May: Conceptualization, Funding acquisition, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements:
This study was funded by a grant from the National Institute on Alcohol Abuse and Alcoholism, R01 AA015134. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We are grateful to the participants of this study for their willingness to provide the biological samples and the time for thoughtful responses to the interview process. The following, highly experienced, research team members dedicated many hours, days, and months to the diligent and accurate collection of data and biological specimens for the biomarker study. Recruitment and transportation of clients was performed by Theresa Alexaander, Florette Kamfer, and Fredeline Philander. Maternal interviews were conducted by Nerina Bester, Isobel Botha, Marise Cloete, Natalie Cronje, Belinda Joubert, Cecile Kriel, Suzelle Kruger, Karen Martin, Sumien Roux, Carisa Siemens, and Helene van Rensberg. The collection and processing of specimens were performed by Sophie Hopp and Sanet van Rooyen. Preliminary data processing was performed by Andrea Englebrecht, Suzelle Kruger, and Shumaya Uithaler.
Data availability
Deidentified data that supports the results may be available following reasonable requests.
References
- Babor T.F., Higgins-Biddle J.C., Saunders J.B., Monteiro M.G. AUDIT: The Alcohol Use Disorders Identification Test: Guidelines for use in primary care. World Health Organization; Geneva, Switzerland: 2001. [Google Scholar]
- Bakhireva L.N., Leeman L., Savich R.D., Cano S., Gutierrez H., Savage D.D., Rayburn W.F. The Validity of Phosphatidylethanol in Dried Blood Spots of Newborns for the Identification of Prenatal Alcohol Exposure. Alcohol. Clin. Exp. Res. 2014;38:1078–1085. doi: 10.1111/acer.12349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakhireva L.N., Sharkis J., Shrestha S., Miranda-Sohrabji T.J., Williams S., Miranda R.C. Prevalence of Prenatal Alcohol Exposure in the State of Texas as Assessed by Phosphatidylethanol in Newborn Dried Blood Spot Specimens. Alcohol. Clin. Exp. Res. 2017;41:1004–1011. doi: 10.1111/acer.13375. [DOI] [PubMed] [Google Scholar]
- Baldwin A.E., Jones J., Jones M., Plate C., Lewis D. Retrospective assessment of prenatal alcohol exposure by detection of phosphatidylethanol in stored dried blood spot cards: An objective method for determining prevalence rates of alcohol consumption during pregnancy. Int. J. Alcohol Drug Res. 2015;4:131–137. doi: 10.7895/ijadr.v4i2.209. [DOI] [Google Scholar]
- Baldwin A.E., Hayes N., Ostrander E., Magri R., Sass N., dos Anjos Mesquita M., Martínez M., Juliani M.C., Cabral P., Fleming M. Phosphatidylethanol Levels in Postpartum Women and Their Newborns in Uruguay and Brazil. Alcohol. Clin. Exp. Res. 2020;44:1292–1299. doi: 10.1111/acer.14339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger L., Fendrich M., Jones J., Fuhrmann D., Plate C., Lewis D. Ethyl glucuronide in hair and fingernails as a long-term alcohol biomarker. Addiction. 2014;109:425–431. doi: 10.1111/add.12402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower, C., Elliott, E.J., 2016. Report to the Australian Government Department of Health: Australian guide to the diagnosis of Fetal Alcohol Spectrum Disorder (FASD). Retrieved from: www.fasdhub.org.au/siteassets/pdfs/australian-guide-to-diagnosis-of-fasd_all-appendices.pdf.
- Cook J.L., Green C.R., Lilley C.M., Anderson S.M., Baldwin M.E., Chudley A.E., Conry J.L., LeBlanc N., Loock C.A., Lutke J., Mallon B.F., McFarlane A.A., Temple V.K., Rosales T. Fetal Alcohol Spectrum Disorder: A Guidelines for Diagnosis across the Lifespan. CMAJ. 2016;188:191–197. doi: 10.1503/cmaj.141593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulton S., Drummond C., James D., Godfrey C., Bland J.M., Parrott S., Peters T. Opportunistic screening for alcohol use disorders in primary care: Comparative study. Br. Med. J. 2006;332:511–517. doi: 10.1136/bmj.38743.421574.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czarnecki D.M., Russell M., Cooper M.L., Salter D. Five-year reliability of self-reported alcohol consumption. J. Stud. Alcohol. 1990;51:68–76. doi: 10.15288/jsa.1990.51.68. [DOI] [PubMed] [Google Scholar]
- Ernhart C.B., Morrow-Tlucak M., Sokol R.J., Martier S. Underreporting of Alcohol Use in Pregnancy. Alcohol. Clin. Exp. Res. 1988;12:506–511. doi: 10.1111/j.1530-0277.1988.tb00233.x. [DOI] [PubMed] [Google Scholar]
- Fleming M.F., Smith M.J., Oslakovic E., Lucey M.R., Vue J.X., Al-Saden P., Levitsky J. Phosphatidylethanol Detects Moderate-to-Heavy Alcohol Use in Liver Transplant Recipients. Alcohol. Clin. Exp. Res. 2017;41:857–862. doi: 10.1111/acer.13353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher O.V., May P.A., Seedat S., Sikkema K.J., Watt M.H. Attitudes Toward Alcohol Use During Pregnancy Among Women Recruited from Alcohol-Serving Venues in Cape Town, South Africa: A Mixed-Methods Study. Soc. Sci. Med. 2018;215:98–106. doi: 10.1016/j.socscimed.2018.09.008. [DOI] [PubMed] [Google Scholar]
- Fortin M., Muckle G., Jacobson S.W., Jacobson J.L., Belanger R.E. Alcohol Use Among Inuit Pregnant Women: Validity of Alcohol Ascertainment Measures Over Time. Neurotoxicol. Teratol. 2017;64:73–78. doi: 10.1016/j.ntt.2017.10.007. [DOI] [PubMed] [Google Scholar]
- Garcia-Algar O., Kulaga V., Gareri J., Koren G., Vall O., Zuccaro P., Pacifici R., Pichini S. Alarming prevalence of fetal alcohol exposure in a Mediterranean city. Ther. Drug Monit. 2008;30:249–254. doi: 10.1097/FTD.0b013e31816a8657. [DOI] [PubMed] [Google Scholar]
- Gareri J., Lynn H., Handley M., Rao C., Koren G. Prevalence of fetal ethanol exposure in a regional population-based sample by meconium analysis of fatty acid ethyl esters. Ther. Drug Monit. 2008 doi: 10.1097/FTD.0b013e318167cfe5. [DOI] [PubMed] [Google Scholar]
- Hannigan J.H., Chiodo L.M., Sokol R.J., Janisse J., Ager J.W., Greenwald M.K., Delaney-Black V. A 14-year retrospective maternal report of alcohol consumption in pregnancy predicts pregnancy and teen outcomes. Alcohol. 2010;44:583–594. doi: 10.1016/j.alcohol.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helander A., Böttcher M., Dahmen N., Beck O. Elimination Characteristics of the Alcohol Biomarker Phosphatidylethanol (PEth) in Blood during Alcohol Detoxification. Alcohol Alcohol. 2019;54:251–257. doi: 10.1093/alcalc/agz027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson E.M.A., Tappin D., Young D., Favretto D., Mactier H. Assessing maternal alcohol consumption in pregnancy: does phosphatidylethanol measured from day 5 newborn blood spot cards have any value? An observational, population- – based study. Arch. Dis. Child. 2022;108:1–6. doi: 10.1136/archdischild-2022-324394. [DOI] [PubMed] [Google Scholar]
- Hill-Kapturczak N., Dougherty D.M., Roache J.D., Karns-Wright T.E., Javors M.A. Differences in the Synthesis and Elimination of Phosphatidylethanol 16:0/18:1 and 16:0/18:2 After Acute Doses of Alcohol. Alcohol. Clin. Exp. Res. 2018;42:851–860. doi: 10.1111/acer.13620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosmer D.W., Jr., Lemeshow S., Studivant R.X. Applied Logistic Regression. Third Edition. John Wiley & Sons, Inc; Hobboken, New Jersey: 2013. [DOI] [Google Scholar]
- Howlett H., Abernethy S., Brown N.W., Rankin J., Gray W.K. How strong is the evidence for using blood biomarkers alone to screen for alcohol consumption during pregnancy? A systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017;213:45–52. doi: 10.1016/j.ejogrb.2017.04.005. [DOI] [PubMed] [Google Scholar]
- Hoyme H.E., Kalberg W.O., Elliott A.J., Blankenship J., Buckley D., Marais A.-S., Manning M.A., Robinson L.K., Adam M.P., Abdul-Rahman O., Jewett T., Coles C.D., Chambers C., Jones K.L., Adnams C.M., Shah P.E., Riley E.P., Charness M.E., Warren K.R., May P.A. Updated Clinical Guidelines for Diagnosing Fetal Alcohol Spectrum Disorders. Pediatrics. 2016;138:e20154256. doi: 10.1542/peds.2015-4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaksson A., Walther L., Hansson T., Andersson A., Alling C. Phosphatidylethanol in blood (B-PEth): a marker for alcohol use and abuse. Drug Test. Anal. 2011;3:195–200. doi: 10.1002/dta.278. [DOI] [PubMed] [Google Scholar]
- Jacobson S.W., Chiodo L.M., Sokol R.J., Jacobson J.L. Validity of maternal report of prenatal alcohol, cocaine, and smoking in relation to neurobehavioral outcome. Pediatrics. 2002;109:815–825. doi: 10.1542/peds.109.5.815. [DOI] [PubMed] [Google Scholar]
- Kader R., Seedat S., Koch J.R., Parry C.D. A preliminary investigation of the AUDIT and DUDIT in comparison to biomarkers for alcohol and drug use among HIV-infected clinic attendees in Cape Town, South Africa. African J. Psychiatry. 2012;15:346–351. doi: 10.4314/ajpsy.v15i5.43. [DOI] [PubMed] [Google Scholar]
- Lange S., Shield K., Koren G., Rehm J., Popova S. A comparison of the prevalence of prenatal alcohol exposure obtained via maternal self-reports versus meconium testing: A systematic literature review and meta-analysis. BMC Pregnancy Childbirth. 2014;14:127. doi: 10.1186/1471-2393-14-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May P.A., Hasken J.M., De Vries M.M., Marais A.S., Stegall J.M., Marsden D., Parry C.D.H., Seedat S., Tabachnick B. A Utilitarian Comparison of Two Alcohol Use Biomarkers With Self-Reported Drinking History Collected in Antenatal Clinics. Reprod. Toxicol. 2018;77:25–32. doi: 10.1016/j.reprotox.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann T., Gentilello L.M., Neuner B., Weiß-Gerlach E., Schürmann H., Schröder T., Müller C., Haas N.P., Spies C.D. Screening trauma patients with the alcohol use disorders identification test and biomarkers of alcohol use. Alcohol. Clin. Exp. Res. 2009;33:970–976. doi: 10.1111/j.1530-0277.2009.00917.x. [DOI] [PubMed] [Google Scholar]
- Papas R.K., Gakinya B.N., Mwaniki M.M., Keter A.K., Lee H., Loxley M.P., Klein D.A., Sidle J.E., Martino S., Baliddawa J.B., Schlaudt K.L., Maisto S.A. Associations Between the Phosphatidylethanol Alcohol Biomarker and Self-Reported Alcohol Use in a Sample of HIV-Infected Outpatient Drinkers in Western Kenya. Alcohol. Clin. Exp. Res. 2016;40:1779–1787. doi: 10.1111/acer.13132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen-Williams P., Jordaan E., Mathews C., Lombard C., Parry C.D.H. Alcohol and Other Drug Use during Pregnancy among Women Attending Midwife Obstetric Units in the Cape Metropole, South Africa. Adv. Prev. Med. 2014;2014:871427. doi: 10.1155/2014/871427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichini S., Marchei E., Vagnarelli F., Tarani L., Raimondi F., Maffucci R., Sacher B., Bisceglia M., Rapisardi G., Elicio M.R., Biban P., Zuccaro P., Pacifici R., Pierantozzi A., Morini L. Assessment of prenatal exposure to ethanol by meconium analysis: results of an Italian multicenter study. Alcohol. Clin. Exp. Res. 2012;36:417–424. doi: 10.1111/j.1530-0277.2011.01647.x. [DOI] [PubMed] [Google Scholar]
- Reisfield G.M., Teitelbaum S.A., Opie S., Jones J., Morrison D.G., Lewis B. The roles of phosphatidylethanol, ethyl glucuronide, and ethyl sulfate in identifying alcohol consumption among participants in professionals health programs. Drug Test. Anal. 2020;12:1102–1108. doi: 10.1002/dta.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisfield G.M., Teitelbaum S.A., Jones J.T., Mason D., Bleiweis M., Lewis B. Blood Phosphatidylethanol Concentrations Following Regular Exposure to an Alcohol-Based Mouthwash. J. Anal. Toxicol. 2020;147:1–7. doi: 10.1093/jat/bkaa147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röhricht M., Paschke K., Sack P.M., Weinmann W., Thomasius R., Wurst F.M. Phosphatidylethanol Reliably and Objectively Quantifies Alcohol Consumption in Adolescents and Young Adults. Alcohol. Clin. Exp. Res. 2020;44:2177–2186. doi: 10.1111/acer.14464. [DOI] [PubMed] [Google Scholar]
- Sanvisens A., Robert N., Hernández J., Zuluaga P., Farré M., Coroleu W., Serra M., Tor J., Muga R. Alcohol consumption during pregnancy: Analysis of two direct metabolites of ethanol in meconium. Int. J. Mol. Sci. 2016;17:417. doi: 10.3390/ijms17030417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröck A., Pfäffli M., König S., Weinmann W. Application of phosphatidylethanol (PEth) in whole blood in comparison to ethyl glucuronide in hair (hEtG) in driving aptitude assessment (DAA) Int. J. Leg. Med. 2016;130:1527–1533. doi: 10.1007/s00414-016-1394-4. [DOI] [PubMed] [Google Scholar]
- Schröck A., Wurst F.M., Thon N., Weinmann W. Assessing phosphatidylethanol (PEth) levels reflecting different drinking habits in comparison to the alcohol use disorders identification test – C (AUDIT-C) Drug Alcohol Depend. 2017;178:80–86. doi: 10.1016/j.drugalcdep.2017.04.026. [DOI] [PubMed] [Google Scholar]
- Schröck A., Thierauf-Emberger A., Schürch S., Weinmann W. Phosphatidylethanol (PEth) detected in blood for 3 to 12 days after single consumption of alcohol—a drinking study with 16 volunteers. Int. J. Leg. Med. 2017;131:153–160. doi: 10.1007/s00414-016-1445-x. [DOI] [PubMed] [Google Scholar]
- Siegfried N. Profile of drinking behaviour and comparison of self-report with the CAGE questionnaire and carbohydrate-deficient transferrin in a rural Lesotho community. Alcohol Alcohol. 2001;36:243–248. doi: 10.1093/alcalc/36.3.243. [DOI] [PubMed] [Google Scholar]
- Statistics South Africa, South African National Census, 2011. http://www.statssa.gov.za/publications/P03014/P030142011.pdf.
- Stewart S.H., Reuben A., Brzezinski W.A., Koch D.G., Basile J., Randall P.K., Miller P.M. Preliminary evaluation of phosphatidylethanol and alcohol consumption in patients with liver disease and hypertension. Alcohol Alcohol. 2009;44:464–467. doi: 10.1093/alcalc/agp039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoth F., Kotzerke E., Thierauf-Emberger A., Weinmann W., Schuldis D. Can PEth be Detected with a Cutoff of 20 ng/mL after Single Alcohol Consumption? J. Anal. Toxicol. 2023;46:e232–e238. doi: 10.1093/jat/bkac069. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA)., 2012. The Role of Biomarkers in the Treatment of Alcohol Use Disorders, 2012 Revision, SAMHSA Advis. 11.
- Ulwelling W., Smith K. The PEth Blood Test in the Security Environment: What it is; Why it is Important; and Interpretative Guidelines. J. Forensic Sci. 2018;63:1634–1640. doi: 10.1111/1556-4029.13874. [DOI] [PubMed] [Google Scholar]
- Wetterling T., Kanitz R.D., Rumpf H.J., Hapke U., Fischer D. Comparison of CAGE and MAST with the alcohol markers CDT, γ-GT, ALAT, ASAT and MCV. Alcohol Alcohol. 1998;33:424–430. doi: 10.1093/oxfordjournals.alcalc.a008414. [DOI] [PubMed] [Google Scholar]
- Wurst F.M., Kelso E., Weinmann W., Pragst F., Yegles M., Poromaa I.G. Measurement of direct ethanol metabolites suggests higher rate of alcohol use among pregnant women than found with the AUDIT-a pilot study in a population-based sample of Swedish women. Am. J. Obstet. Gynecol. 2008;198:407.e1-5. doi: 10.1016/j.ajog.2007.10.801. [DOI] [PubMed] [Google Scholar]
- Wurst F.M., Thon N., Aradottir S., Hartmann S., Wiesbeck G.A., Lesch O., Skala K., Wolfersdorf M., Weinmann W., Alling C. Phosphatidylethanol: Normalization during detoxification, gender aspects and correlation with other biomarkers and self-reports. Addict. Biol. 2010;15:88–95. doi: 10.1111/j.1369-1600.2009.00185.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Deidentified data that supports the results may be available following reasonable requests.