Abstract
Nonalcoholic fatty liver disease (NAFLD), a chronic liver condition affects a large number of people around the world with a frequency of 25% of all the chronic liver disease worldwide. Several targets viz. anti-inflammatory, anti-apoptotic and, anti-fibrotic factors, anti-oxidant and insulin-sensitizing pathways, metabolic regulators as well as repurposing traditional medications have been studied for the pharmacologic therapy of NAFLD. Newer pharmacotherapies like caspases blockade, agonists of PPAR and farnesoid X receptor agonists are currently being investigated in treating human NAFLD. However, NAFLD has no FDA-approved pharmacological therapy, therefore there is a considerable unmet therapy need. Apart from the conventional treatment regime, the current approaches to treating NAFLD include lifestyle interventions including healthy diet with adequate nutrition and physical activity. Fruits are known to play a key role in the well-being of human health. Fruits are loaded with a repertoire of bioactive phytoconstituents like catechins, phytosterols, proanthocyanidin, genestin, daidzen, resveratrol, magiferin found in fruits like pear, apricot, strawberries, oranges, apples, bananas, grapes, kiwi, pineapple, watermelon, peach, grape seed and skin, mango, currants, raisins, dried dates, passion fruit and many more. These bioactive phytoconstituents are reported to demonstrate promising pharmacological efficacy like reduction in fatty acid deposition, increased lipid metabolism, modulation of insulin signaling pathway, gut microbiota and hepatic inflammation, inhibition of histone acetyltransferase enzymatic activity to name a few. Not only fruits, but their derivatives like oils, pulp, peel, or their preparations are also found to be equally beneficial in various liver diseases like NAFLD, NASH. Although most of the fruits contains potent bioactive phytoconstituents, however, the presence of sugar in fruits put a question mark on the ameliorative property of the fruits and there has been contrasting reports on the glycemic control post fruit consumption in type 2 diabetic patients. This review is an attempt to summarize the beneficial effects of fruit phytoconstituents on NAFLD based on epidemiological, clinical and experimental evidence, focusing especially on their mechanisms of action.
Keywords: NAFLD, Fruits, Phytoconstituents, Catechins, Resveratrol, Gut microbiota
Graphical abstract
1. Introduction
Nonalcoholic fatty liver disease (NAFLD) is a type of chronic liver disease with a high global prevalence. Globally, it is now the leading cause of chronic liver disease with a prevalence of ∼25% [1,2]. Based on epidemiological studies, NAFLD is common in the general population in India between 9% and 32%, with higher prevalence in people with overweight or obesity, who have diabetes or prediabetes [3]. The term NAFLD describes liver diseases characterized by excessive macro-vesicular fat (more than 5% of hepatocytes) resulting from an alteration in the homeostatic mechanisms that control the synthesis and utilization of fat in the liver [4,5]. NAFLD is classified into two forms: nonalcoholic fatty liver disease, corresponding to no less than 5% triglyceride content in the liver, and nonalcoholic steatohepatitis (NASH), corresponding to inflammation, steatosis, fibrosis, and ultimately cirrhosis [6]. NAFLD pathogenesis is believed to involve a complex interplay between insulin [7], modifications in gut microbiota [2,8,9], genetic susceptibility variants [[10], [11], [12], [13]] and epigenetic factors [10,14,14,15].
NAFLD has no FDA-approved pharmacological therapies as of 2020, therefore there is a considerable unmet therapy need. The current approaches to treating NAFLD include lifestyle interventions including adequate nutrition and physical activity, and repurposing traditional medications that might have anti-NAFLD properties when they treat aspects of the metabolic syndrome [16]. Several targets viz. anti-inflammatory, anti-apoptotic, and, anti-fibrotic factors, anti-oxidant, and insulin-sensitizing pathways, metabolic regulators as well as repurposing traditional medications have been studied for the pharmacologic therapy of NAFLD. Targeting the insulin-sensitizing pathway has been an essential part of several investigations on NAFLD therapy. New targeted pharmacotherapies like Caspases blockade [17], Agonists of PPAR [18] and Farnesoid X receptor agonists [19] are currently being investigated in the treatment of human NAFLD.
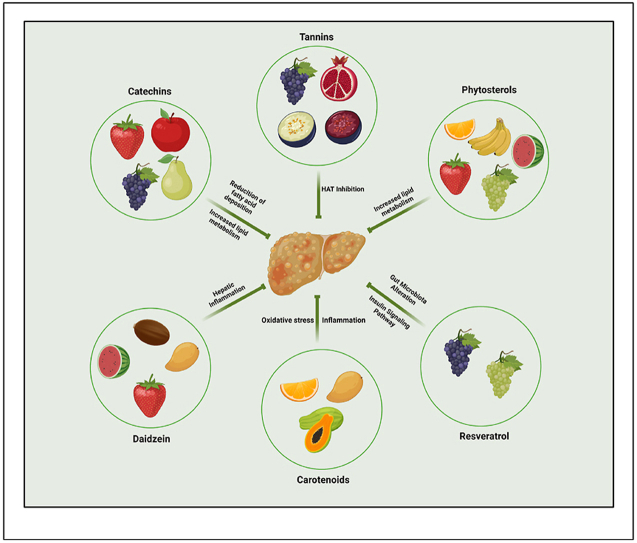
Physical activity together with a healthy diet could lead to weight loss, thereby controlling the progression of NAFLD. From time immemorial, fruits have played an important role in the human well-being healthwise. Fruits are loaded with a repertoire of bioactive phytoconstituents that has been reported to have numerous ameliorative properties in managing human health, especially metabolic diseases. A recent multi-ethnic case-control study reported an inverse trend of fruit consumption and increasing risk of NAFLD [20]. Catechins from fruits like pear, apricot, raspberries, strawberries; phytosterols from fruits like oranges, strawberries, apples, bananas, grapes, kiwi, pear, pineapple, watermelon, peach; proanthocyanidin from grape seed; genestin from currants and raisins; daidzen from dried dates and passion fruit; resveratrol from grape skin and many more bioactive phytoconstituents are reported with significant ameliorative effect in NAFLD. In fruits and vegetables, catechins [[21], [22], [23]], phytosterols [[24], [25], [26]] polyphenolic compounds (Du et al., 2021) [27], carotenoids [[28], [29], [30]] and tannins [31] are well-known physiologically active antioxidants recognized for their potentiality to prevent obesity and obesity-related metabolic syndrome [[32], [33], [34]]. In phase 2 or 3 clinical trials, the sites of action of drugs are based on their primary locus of activity within the liver. Targets include those that regulate lipids [35,36] and glucose [[37], [38], [39]], oxidant stress and hepatocytic mitochondria [[40], [41], [42]], inflammatory signaling pathways on hepatocytes [43,44], and stellate cell activation and fibrogenesis related intracellular targets and inflammatory signals [[45], [46], [47]]. In light of these facts, the manipulation of diet composition and eating patterns may be a sustainable solution to NAFLD treatment.
Given the rapid progress and wide range of unmet needs, this review emphasizes the most recent understanding of NAFLD, providing a comprehensive assessment of its natural history, histopathological features, clinical mediators, and therapeutic approaches. This review is an attempt to summarize the beneficial effects of fruit phytoconstituents on NAFLD based on epidemiological, clinical, and experimental evidence, focusing especially on their mechanisms of action.
2. Pathogenesis of NAFLD
NAFLD has an estimated global prevalence at 24% with the highest rates reported from South America (31%) and the Middle East (32%) [2]. The pathogenesis of NAFLD is multifactorial that comprises of environmental, genetic, extrahepatic as well as intrahepatic episodes. NAFLD develops from noninflammatory isolated steatosis caused by the accumulation of hepatocytic triglyceride (TG), because of an imbalance between synthesis and utilization of TG. This event further leads to NASH, characterized by steatosis, hepatocyte cell ballooning and inflammatory changes with variable levels of liver fibrosis. Progression of NAFLD to steatohepatitis is based on a “two-hit hypothesis” where steatotic oxidative stress and inflammation are the first and second hits respectively. Lipid droplets are lipid-rich organelles and are dynamic in nature. During NAFLD, lipid droplet accumulates in the hepatocytes as well as changes their size, abundance, composition, and organelle interactions in response to metabolic changes [48].
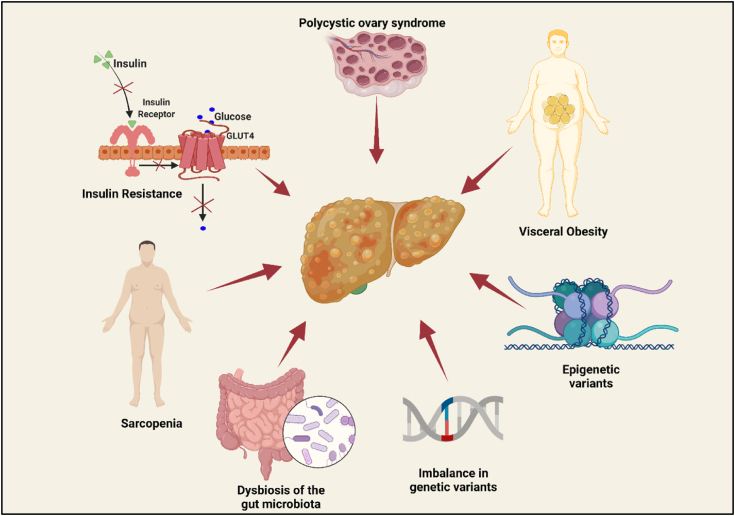
Throughout the years, there has been a lot of development at the cellular and molecular level to decipher the underlying pathogenesis of NAFLD, however, many mechanisms are yet to unfold that may aid in identifying many signaling pathways which will provide thrust in developing newer treatment strategies. This section recapitulates the current information on the pathogenesis of NAFLD and correlates the same presenting novel insights based on the recent literature available. The pathogenesis of NAFLD is thought to involve complex interactions among insulin resistance, alterations in gut microbiota, genetic susceptibility variants, and environmental factors (Fig. 1, Fig. 2).
Fig. 1.
Pathogenesis of non-alcoholic fatty liver disease (Created with www.biorender.com).
Fig. 2.
Precursors and variants involved in the pathogenesis of non-alcoholic fatty liver disease (Created with www.biorender.com).
2.1. Insulin resistance
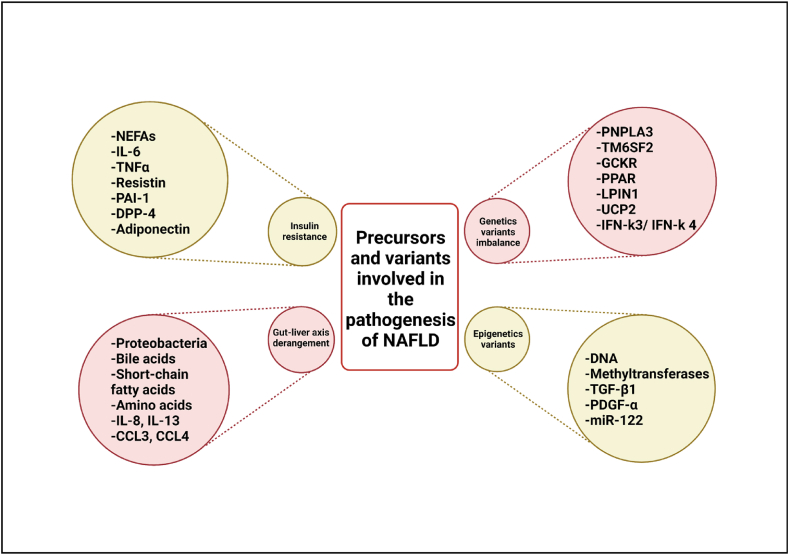
It has long been known that among other metabolic syndromes, T2DM has a clear biological link with NAFLD as 75% of patients with type 2 diabetes (T2DM) have NAFLD. As per evidence, T2DM patients have 80% higher liver fat contents as compared to that non-diabetics, signifying a high risk of fatty liver-associated complications and developing NASH [7]. In T2DM, uninhibited lipolysis in the adipose tissue (AT) due to insulin resistance causes an excess influx of non-esterified fatty acids (NEFAs) to the liver and are taken up by fatty acid transport protein 2 (FATP2), FATP5, and other transport proteins like FA binding protein, cluster of differentiation 36 (CD36), and caveolin-1 [49]. Again, dysregulated lipolysis suppression, adipocyte stress, recruitment, and infiltration by macrophages in the AT are caused by impairment of insulin action in adipocytes further leading to the release of proinflammatory adipocytokines like tumor necrosis factor-alpha (TNFα), interleukin 6 (IL-6), resistin, and plasminogen activator inhibitor-1 (PAI-1), etc. Alternately, adipokines (e.g. adiponectin) that reduce IR are reduced in NAFLD patients. These series of events further promote IR in other insulin-sensitive tissues. Stimulation of dipeptidyl peptidase (DPP)-4, secreted by hepatocytes can be another precursor for metabolic dysregulation in NAFLD, as the former promotes insulin resistance [50]. It is evident that NAFLD and metabolic syndrome (in particular T2DM) are linked bidirectionally. Therefore, an effective treatment regimen can benefit both the comorbidities parallelly.
2.2. Dysbiosis of the gut microbiota
Studies have suggested critical roles for microbiota-host interactions and dysbiosis in the pathophysiology of NAFLD [8]. Dysbiosis is characterized by either the reduction or complete loss of some commensals or the overgrowth of potentially pathogenic commensals, leading to functional alterations of gut microbiota [2]. Both beneficial and harmful gut-metabolites are transported to the liver for energy production and detoxification. Due to dysbiosis of the gut microbiota, gut impermeability or unhealthy dietary habits, the liver becomes overloaded with harmful or proinflammatory molecules negatively affecting liver physiology [2]. Toll-like receptors (TLRs) families such as TLR4, TLR9, TLR2, and TLR5 present in hepatocytes, hepatic stellate cells, and Kupffer cells sense these gut metabolites and this trigger downstream inflammatory reactions followed by cytokine production. Furthermore, the dysfunctional liver cannot effectively control the gut microbiota via bile acids and other microbiome-regulating factors. Escherichia, Bacteroides, Bifidobacterium, Clostridium, and Klebsiella pneumonia are the group of bacteria that are reported to be associated with dysbiosis in NAFLD. The disease progression is caused by increased gram-negative bacteria, mostly Proteobacteria (phylum), and decreased gram-positive bacteria, mostly Firmicutes (phylum). Moreover, there is a shift from beneficial to harmful microbes. Dysbiosis and the loss of functionality of bacterial metabolites such as bile acids, short-chain fatty acids, amino acids, ethanol, and choline have been linked to the severity of NAFLD [9], which in turn causes.
-
➢
dysregulated bile acid synthesis and energy balance
-
➢
regulatory effect on the gut microbiota and inflammatory responses
-
➢
increased gut permeability causing the translocation of endotoxin-producing bacteria into the systemic circulation
-
➢
excessive production of endogenous ethanol in the gut leading to fluctuations in redox potential and increased inflammatory responses
A recent study found a link between the gut microbiota profile and systemic inflammation in patients with NAFLD-related cirrhosis, specifically increased secretion of IL-8, IL-13, CC-chemokine ligand 3 (CCL3), CCL4, and CCL5 [51]. In light of the above-mentioned facts, the alteration of gut microbiota can be considered for therapeutic modalities like antibiotics, prebiotics, probiotics, and symbiotics to control NAFLD.
2.3. Imbalance in genetic variants
NAFLD prevalence has evidence of inter-individual variability attributed to differences in genetic background. A body of evidence reported that between 38% and 100% of NAFLD variability is due to inherited factors and that first-degree relatives of NAFLD patients are at a much higher risk as compared to the general population. NAFLD has a high heritability component, but known risk variants account for only a small portion of heritability [10]. Mutations in genetic variants influence lipid droplet remodeling and the secretion of lipids from hepatocytes [11]. A genome-wide association study (GWAS) has highlighted a few genetic variants contributing to a better understanding of NAFLD pathogenesis. Patatin-like phospholipase domain-containing protein 3 (PNPLA3), a multifunctional enzyme implicated in lipid regulation, is so far the most robust variant associated with NAFLD-mediated steatosis, fibrosis, and hepatocellular carcinoma. PNPLA3 is a key player in intracellular droplet lipid remodeling, which is a common pathway underlying NAFLD progression [52]. Another variant that contributes to the development of NAFLD is transmembrane 6 superfamily member 2 (TM6SF2). TM6SF2 mostly expressed in the liver and small intestine regulates cholesterol synthesis and the secretion of lipoproteins [53]. According to the exome-wide association study, glucokinase regulator (GCKR) and hydroxysteroid 17-dehydrogenase (HSD17B13) have been linked to NAFLD by regulating glucose influx into hepatocytes and lipid droplet-associated retinol dehydrogenase activity. Rather than hepatic fat accumulation, the mechanism linking HSD17B13 to NAFLD involves direct modulation of inflammation and fibrogenesis [52]. A study reported synergistic interaction between obesity and NAFLD variants viz. PNPLA3 I148 M, TM6SF2 E167K, and GCKR P446L to contribute towards gene-environment interactions to NAFLD [12]. Nulcear receptors like peroxisome proliferator-activated nuclear receptors (PPAR), LPIN1, and uncoupling protein 2 (UCP2) are some other genetic variants involved in the regulation of NAFLD progression. ENPP1 and Tribble-1 (TRIB1), modulators of insulin signaling, have been associated with the development and severity of NAFLD by decreasing hepatic insulin signaling. Genetic variation in the interferon (IFN)-k3/IFN-k 4 which regulates innate immunity in the liver is associated with an increased risk of lobular inflammation and severe fibrosis [10]. Another genetic variant membrane-bound o-acyltransferase domain containing 7 (MBOAT7) is associated with increased hepatic fat content and plays a role in liver damage related to NAFLD. Moreover, insulin resistance can synergize with a genetic predisposition to promote more advanced NAFLD. Speliotes and Jacob, have proposed a theory different from that of the classic ‘two-hit hypothesis’ where they call genetic predisposition the first hit and insulin resistance the second hit which when present together lead to advanced liver disease [13]. These findings reflect compelling insights into the biology of NAFLD and provide new impetus for tailoring medical treatments.
2.4. Epigenetic variants
Recent evidence suggests a link between NAFLD and epigenetic modifications i.e., alterations in hereditary gene expression through mitosis and/or meiosis, unrelated to DNA base sequence. Epigenetic modification in NAFLD is caused by exposure to environmental risk factors in previous generations or in the intrauterine environment. Evidence-based studies reported both accelerated fetal growth and intrauterine growth retardation as risk factors for developing NAFLD in adulthood [14]. Moreover, epigenetic changes have been reported to interrelate with inherited risk factors in determining an individual's susceptibility to NAFLD [17]. Epigenetic modification involves DNA methylation, alteration in histone proteins and chromatin remodeling, and RNA-based mechanisms. Although, these are environmentally modulated reversible mechanisms, but can cause severity in pathogenesis.
Histone modification is crucial for the maintenance of chromatin structure and gene expression. It has been established that abnormal histone modification promotes insulin resistance leading to NAFLD. Imbalance in acetylation and deacetylation of histone acetyltransferases and histone deacetylases respectively, influence the phenotypic gene expression in NAFLD, subsequently causing liver damage [13]. DNA methylation is another epigenetic factor that plays a key role in the progression of NAFLD. As per the preclinical research data, the onset of steatosis is accompanied by modifications in the levels of DNA methyltransferases (DNMTs) in the liver which co-relates clinically when DNMT levels were reported to be elevated in patients with steatohepatitis. Moreover, in humans diagnosed with NAFLD that PPARs are hyper-methylated and fibrogenic TGF-β1 and PDGF-α are hypo-methylated in patients with progressive disease [54]. Increased hepatic methylation of mitochondrial DNA encoding for enzymes involved in oxidative phosphorylation has been associated with decreased mitochondrial DNA, insulin resistance, and NAFLD. MicroRNAs, a class of single-chain small non-coding RNA molecules, are deregulated in the pathology of NAFLD and are the most intense topic of discussion as epigenetic factors in NAFLD. miR-122, the most expressed microRNA in the human liver, is inhibited in steatohepatitis. Besides miR-122, other miRNAs viz. miR-192, miR-21, miR-34a, miR-451, miR-1290, miR-27 b-3p, miR-192–5p, miR-301a-3p, miR-34a-5p, miR-375, miR-155 have been identified to be involved in NAFLD [15] progression and characterizing the dysregulated miRNAs in the circulation might aid in exploring key signaling pathways involved in the pathogenesis of NAFLD. Other than the above-discussed factors, other important risk factors include fructose intake and dietary composition, visceral obesity and metabolic syndrome alterations, sarcopenia, polycystic ovary syndrome, and ferritin level. At the later stages of life, possible risk factors for developing NAFLD include age and menopause.
3. Current therapeutic strategy in the treatment of NAFLD
The prevalence of NAFLD has a significant impact on the consumption of healthcare resources [55]. Because of its strong links to obesity, insulin resistance, and dyslipidemia, nonalcoholic fatty liver disease (NAFLD) is the most common cause of liver dysfunction worldwide. Adults with hepatic steatosis have been observed to have a 26% increase in their overall healthcare expenses. It is possible that this is not entirely due to liver disease. There is compelling evidence that patients with comorbid diabetes 2 are at increased risk for cardiovascular disease morbidity and mortality [56]. In today's globalized world, where obesity and diabetes are epidemics, it is expected that the burden of NAFLD would expand considerably in the next years. The increased awareness of the crucial importance of NAFLD has resulted in a spate of studies testing various therapy strategies. Behavioral modifications, pharmaceutical medicines, and surgical intervention are examples of these. NAFLD treatment approaches are discussed in detail in the section below.
3.1. Insulin sensitizers
Targeting the insulin-sensitizing pathway has been an essential part of several investigations on NAFLD therapy. Metformin and thiazolidinediones (TZDs) are well-known insulin sensitizers.
3.1.1. Metformin
Patients with non-diabetic nonalcoholic fatty liver disease (NAFLD) have been studied with Metformin [56]. Transaminase levels and hepatic steatosis have been improved by this treatment. One clinical trial showed fibrosis improvement, although the effect on inflammation was less pronounced. These were primarily fair trials of limited length, and histological follow-up in many of them was insufficient. NAFLD activity score was not associated with improvement in hepatocellular ballooning with metformin (NAS) [57].
3.1.2. Thiazolidinediones
Adiponectin levels, insulin resistance, and hepatocyte fatty acid metabolism have been shown to improve with TZD medications in the treatment of NAFLD. Many studies on TZDs, like those on Metformin, are poor and have discrepancies in their histological and other results. Studies have shown improvements in metabolic endpoints and virtually universal liver disease, such as liver steatosis and transaminase levels [58]. However, there has been no conclusive evidence of fibrosis remission. Overall, TZDs for NAFLD have shown efficacy in clinical trials. However, extended therapy is likely required to produce sustained histological improvement, which may be limited by their adverse effect profile and lack of long-term safety and efficacy in NAFLD patients. TZDs are now designated for second-line treatment in most individuals. Patients with DM2 and NAFLD may benefit from TZD medication [59].
3.2. Statins
Statins are a class of medications that help lower blood levels of low-density lipoprotein (LDL) cholesterol. Although statins are widely used to treat dyslipidemia, their use as a specified treatment for NAFLD is not well established. However, data from the Greek Atorvastatin and Coronary Heart Disease Evaluation study demonstrated a decrease in ALT levels with atorvastatin and the St Francis Heart Study demonstrated a decrease in steatosis radiographically with 20 mg daily of atorvastatin combined with vitamins C and vitamin E [60,61]. However, there is currently no histology evidence to support the use of atorvastatin for NAFLD. Even though histological evidence exists for simvastatin, an RCT of ten patients with biopsy-proven NASH found no statistically significant change in serum liver enzymes, hepatic steatosis, necroinflammatory activity, or fibrosis stage with or without treatment [60]. However, its efficacy in reducing cardiovascular risk in individuals with NAFLD is well established, and there is no indication that patients with NAFLD are at an elevated risk of statin-related liver injury [61].
3.3. Vitamin E
Aside from metabolic syndrome-related medications, liver-specific medicines have been studied in NAFLD. The importance of oxidative stress in disease pathogenesis has prompted research on antioxidants, particularly vitamin E. The most active of the eight tocopherols in vitamin E is a-tocopherol. Its phospholipid bilayer protects cells from nonenzymatic oxidation by free radicals. Vitamin E inhibits profibrotic activity, as are NF-kB-mediated inflammatory pathways in the liver [62]. Vitamin E has been demonstrated to reduce apoptotic and mitochondrial damage in preclinical in vitro and in vivo fibrosis models [63].
3.4. Ursodeoxycholic acid
Ursodeoxycholic acid (UDCA) is a possible cytoprotective drug being explored in NASH due to its ability to inhibit apoptosis and downregulate inflammatory pathways. The significant study to date comparing UDCA and placebo demonstrated an unexpected improvement in the placebo arm, complicating the interpretation of the drug's efficacy. A Cochrane evaluation of four randomized studies utilizing UDCA showed no significant improvement in liver function tests and histopathological data were insufficient. UDCA cannot be suggested for NASH at the moment unless clear histological benefit is demonstrated [64].
3.5. Pentoxifylline
Pentoxifylline (PTX) is a tumor necrosis factor-a agonist that inhibits the generation of oxygen-free radicals. It is used to treat cancer. Animal models have also demonstrated an antifibrotic impact, as well as a significant reduction in NAFLD-associated steatohepatitis in addition to other benefits [65].
3.6. Novel approaches
New targeted pharmacotherapies are currently being investigated in the treatment of human NAFLD (nonalcoholic fatty liver disease). Some of the most important therapies are as follows.
3.6.1. Caspases blockade (GS-9450)
Hepatocyte apoptosis is a characteristic of NASH, and its severity corresponds with disease severity. Uncontrolled placebo-controlled phase-2 trials of selective caspase inhibitors (GS-9450) assigned 124 patients with histologically defined NASH to once-daily placebo or GS-9450 at different doses for 4 weeks. The maximum dose (40 mg) improved both ALT and cytokeratin 18 fragment levels, but only ALT was significant [12].
3.6.2. Agonists of PPAR (GFT-505)
GFT-505 is a dual PPARa/PPARd agonist that has been proven to reduce steatosis, inflammation, and proinflammatory genes in animal models of dietary-induced NASH. GFT-505 has also been shown to have antifibrotic properties independent of metabolic abnormalities. GFT-505 has been shown in human tests to improve liver function, dyslipidaemia and insulin sensitivity in obese, insulin-resistant patients [18].
3.6.3. Farnesoid X receptor agonists
Bile acids are metabolic signaling molecules involved in cholesterol homeostasis, dietary lipid absorption, and enterohepatic triglyceride and glucose metabolism. As a result, they limit hepatic de-novo lipogenesis, gluconeogenesis, and glycogenolysis while improving insulin sensitivity. FXR activation reduces inflammation in animals by blocking NF-kB [66]. In the methionine–choline-deficient model of NASH, there is evidence that FXR agonists protect against liver inflammation and fibrosis. Thus, 23 diabetic patients with NAFLD were examined in a small pilot experiment with obeticholic acid, an FXR agonist [19].
4. Fruits and their bioactive phytoconstituents in the treatment of NAFLD
Drugs obtained from natural origin exhibit a diversified mechanism and the suitable utilization of these drugs depends upon the stage and degree of the non-alcoholic fatty liver disease (NAFLD). Some of the bioactive phytoconstituents derived from different fruits playing a significant role in the treatment of NAFLD along with their pharmacology have been discussed below.
4.1. Reducing the deposition of fatty acid in liver
Mitochondria and peroxisome oxidize the free fatty acids (FFA) that are absorbed by the liver after having a meal. If the uptake of fatty acids by the hepatocytes increases, with that the pools of fatty acid in the liver also increase. As a result of this, the accumulation of acylglycerol in the hepatocytes increases the chance of developing a load in the mitochondria of the hepatic region. Peroxisomes and microsomes subject the unmetabolized fatty acids to either β or ω oxidation. If the process continues and fatty acids keep accumulating in the liver, then oxidative stress is induced and finally, it progresses to NAFLD [21].
4.1.1. Catechins
Catechins are derived from different fruits like apricots, black grapes, strawberries, apples, pears, raspberries, etc. They exhibit potent antioxidant activity that can reduce the oxidative stress generated in the hepatocytes and are found to be effective against NAFLD. Studies have indicated that catechins can obstruct the oxidized lipid formation thereby reducing the fats in the body and the risk of progressing arteriosclerosis [22]. Studies have also indicated that the anti-inflammatory, lipid metabolism, anti-oxidant related biomarkers, and sterol regulatory element-binding proteins (SREBPs) related genes are the crucial mediators of NAFLD [67,68].
4.1.2. (−)-Epicatechin
(−)-Epicatechin (EC) is a natural flavonoid under the flavonol class found in various fruits like black grapes, apricot, pears, etc. [69]. EC is reported to possess a plethora of beneficial effects like anti-diabetic, anti-hypertensive, neuroprotective, cardioprotective, and many others [69]. EC also reported possessing promising hepatoprotective effects in animal models [70]. In a hyperlipidemic rat model, EC demonstrated its protective nature by improving the lipid profile in the blood and significantly decreasing fat accumulation in the liver [71]. Another recent study reported EC to potentially act on nonalcoholic steatohepatitis (NASH) induced in an experimental diet-induced mice model by significantly halting the hepatic and metabolic development. The study demonstrated that EC administration to ice normalized the blood lipid and liver enzyme profile along with decreasing expression of Plin 2 and increasing concentration of adiponectin, CD36, and UCP2 expression [72].
4.2. Increased metabolism of lipids in the liver
Improving the metabolism of fatty acids is considered to be an effective approach to the treatment of NAFLD. However, the effectiveness of bioactive derived from fruit in targeting the metabolism of fatty acid has been examined both in the clinical and preclinical research associated with NAFLD. Studies have indicated that the fruit-derived bioactive can suppress lipogenesis of the hepatic system by lowering the expression of the vital lipogenic enzymes and transcriptional factors like Fatty Acid Synthase (FAS), Peroxisome-Proliferator-Activated Receptor g (PPAR-g), Sterol Regulatory Element Binding Protein 1c (SREBP-1c), Stearoyl-CoA Dwsaturase 1 SCD1 and Acetyl-CoA Carboxylase (ACC) [[73], [74], [75], [76], [77]].
4.2.1. Catechins
Catechins found in various fruits like pear, raspberries, apples, strawberries, etc. were found to promote the metabolism of lipids in the liver. In the case of the experimental obese mouse model C57BL/6 J, the adiposity and body weight were found to be blunted upon the administration of catechin. In the case of the catechin-administered group, an increase in the mRNA expression of a peroxisomal β-oxidizing enzyme named acyl-CoA oxidase (ACO) and a mitochondrial β-oxidizing enzyme named medium-chain acyl-CoA dehydrogenase (MCAD) was observed in the liver of the experimental animals. With an increase in the activity of β-oxidation in the hepatocellular mitochondria, the breakdown of fatty acid is promoted providing a preventive mechanism for NAFLD [23].
4.2.2. Phytosterols
Phytosterols also known as plant sterols obtained from fruits like oranges, strawberries, apples, bananas, grapes, kiwi, pear, pineapple, watermelon, peach, etc. were found to have anti-fibrotic effects in animal models with NAFLD [24]. The phytosterols and the ester form of stanol were found to prevent high-fat diet (HFD) inflammation and lower the plasma lipids in the experimental animals [25]. Sterols namely β-sitosterol and stigmasterol significantly lowered cholesterol and alleviated NAFLD, liver TGs and intestinal bile acid levels [26]. When the esters of phytosterols were combined with docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), then a potentiation of activity was observed and the combination significantly lowered the levels of low-density lipid cholesterol (LDL cholesterol), pro-inflammatory cytokines, triglycerides (TGs) and LDL cholesterol in subjects with NAFLD. High-fructose diet-induced steatohepatitis and macrovesicular steatosis were mitigated upon taking β-sitosterol supplementation for about 12 weeks [78].
4.2.3. Grape seed proanthocyanidins
Grape seed proanthocyanidin (GSP) is basically a composite mixture of oligomeric compounds that exhibit high antioxidant properties along with preventive action from oxidative injury. This bioactive is highly present in grapes and was found to be effective in lowering the level of apolipoproteins and TGs in the liver of experimental rats [79,80].
4.2.4. Genestin
Genestin is categorized under the isoflavone class of bioactive that is rich in fruits currants and raisins. The bioactive is considered to be very potent against NAFLD and the supplementation of genestin for 12 weeks at the dose of 2 g/kg and 4 g/kg diet downregulated peroxisome proliferator-activated receptor- γ (PPAR-γ) and SREBP-1c I mice with NAFLD. Further, at the mentioned dose they were potentially found to lower the liver and serum lipids. However, they also markedly suppressed the expression of two receptors namely the hepatic thromboxane A2 receptor and cyclooxygenase-1 receptor through the thromboxane A2 (TXA2) pathway [[81], [82], [83]].
4.2.5. Rutin
Rutin is categorized as an antioxidant flavonoid glycoside and is obtained from fruits like buckwheat, apricots, cherries, grapes, grapefruit, plums, and oranges [84,85]. Rutin demonstrated potent pharmacological efficacy as anti-inflammatory, anti-diabetic, neuroprotective, hepatoprotective to name a few [86]. Rutin is reported to prevent NAFLD by the inhibition of SREBP-1c and leptin [36,76].
4.3. Amelioration of the hepatic inflammation
The deposition of lipid and their redistribution from adipose tissue to the liver results in hepatic inflammation that further produces inflammatory liver injury. The development of hepatic inflammation accelerates the formation of NAFLD from the state of fibrosis and hepatic steatosis to steatohepatitis [45]. In order to overcome NAFLD, amelioration of the inflammation in the hepatic region is of utmost importance. Research into the field has indicated that the extract of bioactive derived from fruits exerts preventive and protective action against the development of hepatic steatosis to steatohepatitis. Some of the bioactive were also found to have anti-inflammatory potential that could easily slow the progression of NAFLD [47,[87], [88], [89], [90], [91], [92]].
4.3.1. Daidzein
Studies have indicated that one of the core stimulators of lipogenesis named carbohydrate-responsive element-binding protein (ChREBP) and its associated target gene encoding different lipogenic enzymes like fatty acid synthase, adenosine triphosphate citrate lyase, acetyl-CoA carboxylase b (ACACb), and 1-acylglycerol-3- phosphate O-acyltransferase-2 were downregulated upon the intake of 1.5- fold to 3.4-fold of daidzein supplementation. Further, the supplementation lowered the expression of ChREBP activator and liver X receptor (LXR) to four-fold level. However, the expression of ChREBP inhibitor and protein kinase A (PKA) was augmented to a level of 1.7-fold upon the intake daidzein supplementation [[93], [94], [95], [96], [97]].
4.3.2. Polysaccharides
The polysaccharides found in multiple plants play a vital role in inhibiting the development of NAFLD. Chicory polysaccharides are reported to regulate the expression of genes involved in lipid metabolism. Further, it facilitates the β-oxidation of fatty acids by inhibiting the de novo lipogenesis resulting in an improvement of the steatosis of the liver. Another polysaccharide named astragalus polysaccharide inhibits the progression of the liver in kuffer cells by regulating apoptosis and autophagy [89,90].
4.3.3. Insulin signaling pathway and altered fatty acid metabolism-related gene expression
The insulin receptor substrate-1 (IRS-1) is recruited by the insulin-induced insulin receptor that activates the phosphoinositide 3-kinase (PI3K)/Protein kinase B (AKT) resulting in the activation of glycogen synthase kinase-3β(GSK-3β) and PKA that encourages the synthesis of glycogen and promotes lipogenesis [37,[98], [99], [100], [101]].
4.3.4. Resveratrol
Resveratrol (RSV) was found to be effective in repairing the damaged insulin signaling pathway by affecting the phosphorylated protein level and overall protein level molecules in the pathway but it cannot affect the phosphorylation ratio. Further, some of the genes related to lipogeneses like Pparg, Gpat1, and Mogat were also inhibited upon treatment with RSV. In the case of animals in the HFD group, an increase in the expression of genes related to the oxidation of fatty acids like Acox1, and Cpt1 was suppressed and abolished after the treatment of RSV [27].
4.3.5. Phloretin
Phloretin is a dihydrochalcone and is categorized under natural phenol. Phloretin is reported to ameliorate NAFLD by stimulating the AMPK signaling pathway. Further, in the 3T3-L1 adipocytes, it prevents the deposition of oil droplets that produces inhibition of adipogenesis and FAS-related transcription parameters. It can also enhance lipolysis by accelerating the enzyme lipase and phosphorylating AMPK in the 3T3-L1 cells [37].
4.4. Gut microbiota alteration in NAFLD
Patients with gut dysbiosis were found to have a different mode of NAFLD development. The association between availability of Bacteroides in the gut microbiota and the presence of NAFLD were inversely associated indicating their importance in the progression of NAFLD. Hence, the alteration of microbiota in the intestine region has become an important strategy for the treatment of NAFLD. The gut microbiota can help contribute towards NAFLD by regulating energy homeostasis through the fermentation of short-chain fatty acids (SCFAs) that further induces lipogenesis in the liver [102].
4.4.1. Resveratrol
Research and experiments indicated that RSV markedly increased the Enterorhabdus genera, Actinobacteria phylum and Olsenella in the gut microbiota of the experimental models. Studies have indicated that Olsenella can ameliorate inflamed and fatty liver. The presence of Flavonifractor, Anaerotruncus, Oscillibacter and Clostridium IV genus increased the severity of the disease, and treatment with RSV in the experimental rats reduced the concentration of these genera. Hence, studies concluded that RSV has an obvious therapeutic efficacy on NAFLD and can easily alter the microbiota composition of the gut region [27].
4.4.2. Anthocyanins
Anthocyanins are secondary metabolites obtained from berries, currants, and grapefruits. This bioactive can overcome NAFLD by ameliorating dyslipidemia and microbiome dysbiosis of the gut that stimulates the fermentation of the short fatty acid chains. Further, lipogenesis induction occurs in the liver [103].
4.4.3. Myricitrin
Myricitrin found in nuts and berries produces an anti-NAFLD effect by modulating the composition of gut microbiota. Myricitrin lowers the inflammation and synthesis of lipids by altering the gut microbiota related to fecal butyric acid and protecting the barrier function of the gut [103].
4.4.4. Gallic acid
Gallic acid found in blueberries, blackberries, strawberries, plums, grapes, mangoes, cashew nuts, hazelnuts, walnuts, etc. produces hepatoprotective activity and helps overcome NAFLD through the metabolism of choline, glucose, lipid, and gut microbiota [103].
4.5. Oxidative stress and inflammatory process in NAFLD
Highly reactive ROS may interact with proteins, nucleic acids and cellular membranes [102,104]. Hepatic overloading of FFA with mitochondrial β-oxidation or microsomal enzymes like cytochrome P4502E1 (CYP2E1), may result in oxidative stress. Moreover, increased CYP-P450 activity has been observed in both human and rodent obesity and NAFLD [40]. Enhanced NADPH oxidase activity caused by increased mitochondrial fatty acid oxidation and CYP2E1 results in endogenous and exogenous substrates mediated redox cycling production of the reactive oxygen species (ROS) superoxide and hydrogen peroxide [41,105]. Moreover, the overproduction of ROS causes cellular damage, further inducing lipid peroxidation and mitochondrial dysfunction and contributing to hepatocellular damage. In response to the process of oxidative stress, glutathione (GSH) peroxidase, superoxide dismutase, and catalase activities are enhanced in NAFLD [106]. Again, ROS production also has an indirect hepatotoxic effect mediated by mitochondrial β-oxidation. Storage and metabolism of fatty acid are reported to be regulated by peroxisome proliferator-activated receptors (PPARs). Furthermore, FFA-induced PPAR-up-regulates and boosts the expression of carnitine palmitoyltransferase-1 (CPT-1) and oxidation of mitochondrial, regulating fatty acid uptake and clearance [43,44,107].
4.5.1. Carotenoids
Carotenoids, the most studied dietary compounds are sourced mostly from fruits and vegetables. Lycopene and β-carotene are the most studied carotenoids. Multiple mechanisms, including antioxidant and anti-inflammatory effects of carotenoids, have been reported to be involved in protective effects for NAFLD [28,29]. A clinical study investigating the association of non-alcoholic fatty liver (NAFL) and serum levels of carotenoids, reported that serum β-carotene was decreased with fat accumulation in the liver, without alteration of lycopene, β-cryptoxanthin, α-carotene, and lutein levels. Another study in patients with non-alcoholic steatohepatitis (NASH) reported that plasma levels of carotenoids viz. lutein, β-cryptoxanthin, zeaxanthin, lycopene, β-carotene and α-carotene were significantly decreased as compared to control subjects [30,108].
4.5.2. Kaemferol
Kaempferol found in cherries, cranberries and mangoes play a vital role in preventing the development of NAFLD by hindering the transcriptional activity of NF-ᴋB at the nuclear level. Hence, it can be used for the prevention and development of NAFLD as a dietary supplement or functional food (Aubert et al., 2011) [40].
4.6. Inhibiting histone acetyltransferase (HAT) enzymatic activity
Studies have indicated that by decreasing the rate of acetylation at the lysine amino acid residues 9 and 36 of the H3 histone protein, the symptoms related to NAFLD were improved. Further, lowering the mRNA expressions of genes associated with lipid accumulation and lipogenesis also ameliorated the features of NAFLD including a serum lipid profile, liver mass, body weight, and fat mass [31].
4.6.1. Tannins
Experimental studies have demonstrated the anti-lipogenic effects of tannins in HepG2 cells for evaluating if tannins block effectively the accumulation of hepatic lipids. The OPA-induced lipid accumulation is reduced by tannins. Studies demonstrated the cell viability assay for excluding the likelihood of effect due to cytotoxicity of tannin. It was reported that tannins have not shown cytotoxicity even at the maximum concentration used. OPA remarkably improved the mRNA expression of the genes. But tannins averted the increment and also overruled their expression of the protein. For elucidating the relationship between HAT activity and mRNA expression of the lipogenic genes. Collectively, data was found from a study that tannin enhances the accumulation of lipids by obstructing lipogenic genes via anti-HAT activity [31].
4.7. NLRP3 inflammasome and NAFLD
Pyroptosis is triggered by the release of inflammatory particles by an active NLRP3 inflammasome and is strongly linked to NAFLD. Additionally, active NLRP3 inflammasomes also trigger the activation of caspase-1 [[109], [110], [111]]. Pro-IL 18 and pro-IL-1β are converted to IL-18 and IL-1β by the activated caspase-1. Due to the persistence of this process, significant amounts of IL-18 and IL-1 are produced, which triggers pyroptosis and aids in the emergence of inflammatory illnesses. These occurrences increase and promote NAFLD and the inflammatory response of the liver, respectively, by spreading the inflammatory process extracellularly and releasing a large number of inflammasome particles [112]. On the other hand, the activation of caspase-1 by the inflammasome is necessary for pyroptosis. Plasma membrane pores are created as a result of the pyroptosis medium Gasdermin D's (GSDMD) insertion, which also causes intracellular protein release, water influx, ion decompensation, and cell enlargement. GSDMD's cleavage, which is aided by active caspase-1, allows it to bind to the cell membrane's phosphatidylinositol and phosphatidylserine and create membrane holes that signal the start of cell death. Many DAMPs and active inflammatory cytokines can enter the extracellular environment through these pores, where they are recognized by inflammatory cells and result in pyroptosis [113]. This study also reported that in the NAFLD/NASH liver tissues, there was increased expression of GSDMD and their pyrogenic-induced fragment GSDMD-N protein. Additionally, research has linked the liver GSDMD-N protein to increased NAFLD fibrosis and a score that supports the link between the expression of the NLRP3 inflammasome-GSDMD pyroptosis triplet and the development of NASH [114].
4.7.1. Naringenin
A flavonoid under the subclass flavanones, naringenin (Nar), is found in many citrus fruits, bergamot, as well as in other fruits like tomatoes and cherries [115]. As a herbal remedy, Nar is reported to possess significant antioxidant, anti-cancer, anti-inflammatory, neuroprotective, as well as hepatoprotective effects [116]. According to reports, naringin is reported to play a crucial role in the amelioration of NAFLD. By using several pathways, Nar regulates several NAFLD-related biological functions, such as oxidative stress, energy balance, metabolism of lipids and glucose, and inflammation [117]. In a recent study conducted on tissue-engineered fatty liver, Nar demonstrated a potential NAFLD ameliorative property by decreasing fatty acid absorption and de novo lipogenesis and enhancing fatty acid oxidation [118]. Recently, Nar is reported to inhibit the NLRP3/NF-κB pathway in a methionine-choline deficient (MCD) model of mice as well as in HepG2 cells, primary hepatocytes, and Kupffer cells (KCs) [119]. In the study, potent Nar exhibited its inhibitory role in the activation of the NLRP3/NF-κB pathway demonstrated in the liver cells when stimulated by oleic acid and lipopolysaccharides. Interestingly, it was observed that the anti-lipid deposition effects of Nar were not effective in NLRP3−/− hepatocytes, however, the same was restored in NLRP3−/− hepatocytes when re-expressed by adenovirus [119].
4.7.2. Apigenin
Apigenin (Api) is a flavone type of flavonoid found in fruits like oranges and grapes [120], bilimbi fruit, guava, wolfberry leaves, kadok, etc. [121]. Api presents a myriad of pharmacological effects that includes anti-oxidation, anti-inflammatory, anti-depressant, anti-tumor, anti-microbial, anti-toxicant, nephroprotective, cardioprotective, and neuroprotective as well as regulates glucose and lipid metabolism [122,123]. Api has been reported to possess potent hepatoprotective efficacy in both the in-vitro and in-vivo setups. A recent report demonstrated the protective role of Api where the former ameliorated liver injury by inhibiting oxidative stress and inflammation in hydrogen peroxide (H2O2)-induced oxidative stress in HepG2 cells and carbon tetrachloride (CCl4)-induced acute liver injury in mice [124]. Api was recently discovered to protect against high-fat diet (HFD)-induced NAFLD by modulating the xanthone oxidase/NLRP3 pathways. The study demonstrated that gavage administration of Api to HFD-fed obese mice protects them from liver injury by inhibiting the pro-inflammatory cytokines like IL-1β and IL-18 and reactive oxygen inhibitor enzyme xanthine oxidase thereby reversing the activation of NLRP3 inflammasome [125].
4.7.3. Cyanidin-3-O-glucoside
Cyanidin-3-O-glucoside (C3G) is a flavonoid under the sub-class anthocyanin and is found mainly in red to blue fruits like raspberries, strawberries, Chinese bayberry fruit, and mulberry that gives the characteristics red hue to fruits [[126], [127], [128]]. C3G is reported to have a potent anti-cancer [129], anti-obesity, anti-diabetic [127] and protects gut microbiota [130], and diabetes-induced nephropathy [131]. C3G when tested against NAFLD, demonstrated promising results in a mice model as well as in cell lines like AML-12 and hepatocytes. The study reported that C3G treatment cleared off the damaged mitochondria through PINK1-initiated mitophagy by elevating the expression of PINK1/Parkin and localizing mitochondria [132]. Not only NAFLD, but C3G has also demonstrated promising results against alcoholic liver (ALD) disease too. C3G was also reported to act against NLRP3 inflammasomes in a pre-clinical model. Recently in an experimental mice model of alcoholic steatohepatitis, N3G was reported to demonstrate inhibitory potential against the activation of NLRP3 inflammasome via the SirT1/NF-κB signaling pathway [133].
4.7.4. Mangiferin
Mangiferin (Man) is a polyphenol under the xanthone class, primarily found in the pulp, fruit skin, bark, and root of mangoes as well as in honeybush tea [134] and other medicinal plants. Man has been reported to have promising anti-inflammatory, anti-oxidant, anti-diabetic, immunomodulatory, cardioprotective, and anti-cancer activities [135,136]. In the liver, Man has been reported to exert an ameliorative effect on a mice model of ALD by suppressing the inflammation-mediated adipose hyperlipolysis through activation of AMPK/TBK1 signaling, stabilization of phosphodiesterase 3 B protein and suppression of the activation of NF-κB [137]. Man has been reported to showcase potent pharmacological intervention against NAFLD in a high-fat diet-induced obese mice model. In the study, Man ameliorated obesity-associated NAFLD by suppressing the pyroptosis and activation of NLRP3 inflammasome whereas upregulated the AMPK expression, In the same study, Man administration boosted glucose consumption and decreased the free fatty acid-induced buildup of intracellular triglycerides in the HepG2 cells [138].
4.8. Effect of fruit derivatives on NAFLD
Various studies have found fruit derivatives like oils, pulp, peel, or their preparations equally beneficial. Oils like olive oil, raspberry seed oil, as well as flaxseed seed oil, have demonstrated remarkable health benefits in different areas including liver diseases like NAFLD, NASH, ALD, etc. Some therapeutical benefits of the fruit-derived oil as well as their peel/pulp are discussed below.
4.8.1. Fruit-derived essential oil and its bioactive constituents
Several studies have confirmed the beneficial role of oil extracted from fruits in the onset and progression of NAFLD. The presence of bioactive compounds such as phytosterols, phenolic compounds, carotenoids, and tocopherols was confirmed in the oils extracted from the seeds of apple, guava, kumquat, citron, grape, mangaba, melon, strawberry, mango, orange, passion fruit, papaya, pumpkin, soursop, and tomato. Furthermore, the fatty acid profile revealed that some oils may be excellent sources of essential fatty acids [139]. Considering the high presence of polyphenols, olive oil may prove beneficial in reducing the progression of NAFLD. A randomized clinical trial reported a significant decrease in the liver ALT and AST enzymes in patients with NAFLD on a hypocaloric olive oil diet versus a hypocaloric normal fat diet. The results of another multicenter clinical study reported that consumption of olive oil showed positive effects on oxidized LDL, hydroxy fatty acids, conjugated dienes as well as reduced LDL versus HDL ratio [140]. In another trial, 12 weeks of olive oil consumption alleviated fatty liver severity in patients with NAFLD. Hendawy et al. discovered that raspberry seed oil can help prevent NAFLD caused by a high-fat diet in a pre-clinical experimental model. The study suggested that the high content of polyunsaturated fatty acids, tocopherols, and phenolic compounds led to reduced insulin resistance and glucose levels, improved lipid parameters, and suppressed inflammation via inhibition of NF-κB and modulation of leptin, adiponectin, and PPARc levels [141]. A randomized controlled trial found that flaxseed oil may benefit patients with NAFLD by improving weight, fatty liver grade, and IL-6 levels in the context of moderate physical activities and a low-energy diet, implying that flaxseed has a direct protective role on fat accumulation in the liver [142].
4.8.2. Fruit peel/pulp and their bioactive constituents
Citrus fruit peel (CPP) is a valuable source of essential oils, polyphenols, and pectin. Limonene, pectin, and narirutin are the important components of CPP which have antioxidant and anticholesterolemic properties that further help in preventing lipid formation and inhibiting the production of pro-inflammatory cytokines [143]. A recent study has reported the preventive effect of orange and pomelo peel powder on NAFLD in mice by reducing HFD-induced dyslipidemia and hepatic triglyceride accumulation with a positive effect on liver inflammation [143]. Administration of pomegranate peel extract reduced body weight, improved liver enzymes, ameliorated hepatic morphology, and inhibited lipogenesis in HFD-induced NAFLD rats [144]. Pomegranate fruit pulp has recently been shown to reduce HFD-induced obesity, hepatic steatosis, and insulin resistance in mice, possibly due to changes in the gut microbiota [145]. The administration of lychee pulp altered the cellular dynamics of adipose tissue, reducing adipocyte hypertrophy and counteracting fat accumulation in the liver of NAFLD-induced rats [146].
5. Conclusion and future perspective
The key to preventing and treating non-alcoholic fatty liver disease (NAFLD) is to take a holistic approach to lifestyle changes, one which emphasizes unprocessed foods, exercise, and balanced drinking and smoking habits. Fruits have the potential to influence metabolism-related risk factors such as diabetes, dyslipidemia, and overweight/obesity, all of which are major pathological processes in NAFLD. Studies examining the association between fruit consumption and NAFLD pathogenesis have found similar outcomes in regard to the protective effects of fruits and the presence of bioactive phytoconstituents in them. Further, most experimental studies also supported the protective properties of several bioactive phytoconstituents like catechins, phytosterols, proanthocyanidins, genestin, and, daidzein present in like apricots, black grapes, strawberries, apples, pears, and, raspberries. Further research should be conducted to investigate the protective effects of unexplored fruits on NAFLD and identify and isolate their bioactive components and a deeper understanding of the mechanisms of action is warranted. There have been contrasting reports on the ameliorative property of fruits due to the presence of sugar in them. The presence of sugar in fruits may alter the glycemic control in type 2 diabetic patients and may worsen the diabetic condition. So, caution should be maintained while consuming fruits rich in sugar by patients having metabolic disturbance. It is necessary to conduct well-designed clinical trials to determine the optimal dose of phytoconstituents for achieving specific therapeutic or preventive effects. The seasonality of fruits further makes the development of innovative functional foods and dietary supplements essential to promoting the use of bioactive compounds from fruits in daily diets. Identifying the exact molecular pathways underlying NAFLD development will enable the development of molecular therapeutic agents that target specific receptors and signaling pathways.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
No data was used for the research described in the article.
Declaration of interest's statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
No additional information is available for this paper.
Author contribution
All authors have participated sufficiently in the work and take responsibility for the content, including conceptualization, writing, editing, or revision of the manuscript.
Authors MPP and KP conceptualized and prepared the manuscript outline.
Authors MPP, KP, RS, UG, and PP drafted the manuscript.
Authors MPP, KP, RS, UG, and PP contributed to the interpretation of data for the tables and designing of figures; edited the manuscript.
Authors MPP, AP, PC, PP analyzed and interpreted the reports.
Acknowledgment
The authors are thankful to Assam down town University, Guwahati, Assam, India, for providing all necessary facilities and support to carry out this work.
References
- 1.Younossi Z.M., Koenig A.B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 2.Hrncir T., Hrncirova L., Kverka M., Hromadka R., Machova V., Trckova E., Kostovcikova K., Kralickova P., Krejsek J., Tlaskalova-Hogenova H. Gut microbiota and NAFLD: pathogenetic mechanisms, microbiota signatures, and therapeutic interventions. Microorganisms. 2021;9:957. doi: 10.3390/microorganisms9050957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duseja A. Nonalcoholic fatty liver disease in India – a lot done, yet more required. Indian J. Gastroenterol. 2010;29:217–225. doi: 10.1007/s12664-010-0069-1. [DOI] [PubMed] [Google Scholar]
- 4.Cohen J.C., Horton J.D., Hobbs H.H. Human fatty liver disease: old questions and new insights. Science. 2011;332:1519–1523. doi: 10.1126/science.1204265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diehl A.M., Day C. Cause, pathogenesis, and treatment of nonalcoholic steatohepatitis. N. Engl. J. Med. 2017;377:2063–2072. doi: 10.1056/nejmra1503519. [DOI] [PubMed] [Google Scholar]
- 6.Chalasani N., Younossi Z., Lavine J.E., Charlton M., Cusi K., Rinella M., Harrison S.A., Brunt E.M., Sanyal A.J. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2017;67:328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 7.Benedict M., Zhang X. Non-alcoholic fatty liver disease: an expanded review. World J. Hepatol. 2017;9:715. doi: 10.4254/wjh.v9.i16.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang X., Zheng J., Zhang S., Wang B., Wu C., Guo X. Advances in the involvement of gut microbiota in pathophysiology of NAFLD. Front. Med. 2020;7 doi: 10.3389/fmed.2020.00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen J., Vitetta L. Gut microbiota metabolites in NAFLD pathogenesis and therapeutic implications. Int. J. Mol. Sci. 2020;21:5214. doi: 10.3390/ijms21155214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eslam M., Valenti L., Romeo S. Genetics and epigenetics of NAFLD and NASH: clinical impact. J. Hepatol. 2018;68:268–279. doi: 10.1016/j.jhep.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Valenti L., Romeo S. Destined to develop NAFLD? The predictors of fatty liver from birth to adulthood. J. Hepatol. 2016;65:668–670. doi: 10.1016/j.jhep.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 12.Stender S., Kozlitina J., Nordestgaard B.G., Tybjærg-Hansen A., Hobbs H.H., Cohen J.C. Adiposity amplifies the genetic risk of fatty liver disease conferred by multiple loci. Nat. Genet. 2017;49:842–847. doi: 10.1038/ng.3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Speliotes E.K., George J. Metabolic and genetic contributions to NAFLD: really distinct and homogeneous? J. Hepatol. 2022;76:498–500. doi: 10.1016/j.jhep.2021.12.024. [DOI] [PubMed] [Google Scholar]
- 14.Suomela E., Oikonen M., Pitkänen N., Ahola-Olli A., Virtanen J., Parkkola R., Jokinen E., Laitinen T., Hutri-Kähönen N., Kähönen M., et al. Childhood predictors of adult fatty liver. The cardiovascular risk in young Finns study. J. Hepatol. 2016;65:784–790. doi: 10.1016/j.jhep.2016.05.020. [DOI] [PubMed] [Google Scholar]
- 15.Blaya D., Aguilar-Bravo B., Hao F., Casacuberta-Serra S., Coll M., Perea L., Vallverdú J., Graupera I., Pose E., Llovet L., et al. Expression of microRNA-155 in inflammatory cells modulates liver injury. Hepatology. 2018;68:691–706. doi: 10.1002/hep.29833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campbell P., Symonds A., Barritt A.S. Therapy for nonalcoholic fatty liver disease: current options and future directions. Clin. Therapeut. 2021;43:500–517. doi: 10.1016/j.clinthera.2021.01.021. [DOI] [PubMed] [Google Scholar]
- 17.Ratziu V., Sheikh M.Y., Sanyal A.J., Lim J.K., Conjeevaram H., Chalasani N., Abdelmalek M., Bakken A., Renou C., Palmer M., et al. A phase 2, randomized, double-blind, placebo-controlled study of GS-9450 in subjects with nonalcoholic steatohepatitis. Hepatology. 2011;55:419–428. doi: 10.1002/hep.24747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Staels B., Rubenstrunk A., Noel B., Rigou G., Delataille P., Millatt L.J., Baron M., Lucas A., Tailleux A., Hum D.W., et al. Hepatoprotective effects of the dual peroxisome proliferator-activated receptor alpha/delta agonist, GFT505, in rodent models of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatology. 2013;58:1941–1952. doi: 10.1002/hep.26461. [DOI] [PubMed] [Google Scholar]
- 19.Zhang S., Wang J., Liu Q., Harnish D.C. Farnesoid X receptor agonist WAY-362450 attenuates liver inflammation and fibrosis in murine model of non-alcoholic steatohepatitis. J. Hepatol. 2009;51:380–388. doi: 10.1016/j.jhep.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 20.Kim S.-A., Shin S. Fruit and vegetable consumption and non-alcoholic fatty liver disease among Korean adults: a prospective cohort study. J. Epidemiol. Community Health. 2020 doi: 10.1136/jech-2020-214568. jech-2020. [DOI] [PubMed] [Google Scholar]
- 21.Kersten S., Seydoux J., Peters J.M., Gonzalez F.J., Desvergne B., Wahli W. Peroxisome proliferator-activated receptor α mediates the adaptive response to fasting. J. Clin. Invest. 1999;103:1489–1498. doi: 10.1172/jci6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagao T., Komine Y., Soga S., Meguro S., Hase T., Tanaka Y., Tokimitsu I. Ingestion of a tea rich in catechins leads to a reduction in body fat and malondialdehyde-modified LDL in men. Am. J. Clin. Nutr. 2005;81:122–129. doi: 10.1093/ajcn/81.1.122. [DOI] [PubMed] [Google Scholar]
- 23.Murase T., Nagasawa A., Suzuki J., Hase T., Tokimitsu I. Beneficial effects of tea catechins on diet-induced obesity: stimulation of lipid catabolism in the liver. Int. J. Obes. 2002;26:1459–1464. doi: 10.1038/sj.ijo.0802141. [DOI] [PubMed] [Google Scholar]
- 24.Plat J., Hendrikx T., Bieghs V., Jeurissen M.L.J., Walenbergh S.M.A., van Gorp P.J., De Smet E., Konings M., Vreugdenhil A.C.E., Guichot Y.D., et al. Protective role of plant sterol and stanol esters in liver inflammation: insights from mice and humans. PLoS One. 2014;9 doi: 10.1371/journal.pone.0110758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng S., Dai Z., Liu A.B., Huang J., Narsipur N., Guo G., Kong B., Reuhl K., Lu W., Luo Z., Yang C.S. Intake of stigmasterol and β-sitosterol alters lipid metabolism and alleviates NAFLD in mice fed a high-fat western-style diet. Biochim. Biophys. Acta, Mol. Cell Biol. Lipids. 2018;1863:1274–1284. doi: 10.1016/j.bbalip.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song L., li Y., Qu D., Ouyang P., Ding X., Wu P., Guan Q., Yang L. The regulatory effects of phytosterol esters (PSEs) on gut flora and faecal metabolites in rats with NAFLD. Food Funct. 2020;11:977–991. doi: 10.1039/c9fo01570a. [DOI] [PubMed] [Google Scholar]
- 27.Du F., Huang R., Lin D., Wang Y., Yang X., Huang X., Zheng B., Chen Z., Huang Y., Wang X., et al. Resveratrol improves liver steatosis and insulin resistance in non-alcoholic fatty liver disease in association with the gut microbiota. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.611323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaulmann A., Bohn T. Carotenoids, inflammation, and oxidative stress-implications of cellular signaling pathways and relation to chronic disease prevention. Nutr. Res. 2014;34:907–929. doi: 10.1016/j.nutres.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 29.Rao A., Rao L. Carotenoids and human health. Pharmacol. Res. 2007;55:207–216. doi: 10.1016/j.phrs.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 30.Park S., Lee H., Lee D., Lee S., Chun B., Kim S., Lee H., Son H., Kim S.-H. Associations of non alcoholic fatty liver with the metabolic syndrome and serum carotenoids. J. Prev. Med. Public Health. 2008;41:39. doi: 10.3961/jpmph.2008.41.1.39. [DOI] [PubMed] [Google Scholar]
- 31.Chung M.-Y., Song J.-H., Lee J., Shin E.J., Park J.H., Lee S.-H., Hwang J.-T., Choi H.-K. Tannic acid, a novel histone acetyltransferase inhibitor, prevents non-alcoholic fatty liver disease both in vivo and in vitro model. Mol. Metabol. 2019;19:34–48. doi: 10.1016/j.molmet.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anhê F.F., Nachbar R.T., Varin T.V., Vilela V., Dudonné S., Pilon G., Fournier M., Lecours M.-A., Desjardins Y., Roy D. A polyphenol-rich cranberry extract reverses insulin resistance and hepatic steatosis independently of body weight loss. Mol. Metabol. 2017;6:1563–1573. doi: 10.1016/j.molmet.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vivarelli F., Canistro D., Sapone A., De Nicola G.R., Babot Marquillas C., Iori R., Antonazzo I.C., Gentilini F., Paolini M. Raphanus sativus cv. Sango sprout juice decreases diet-induced obesity in sprague dawley rats and ameliorates related disorders. PLoS One. 2016;11 doi: 10.1371/journal.pone.0150913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song H., Lai J., Tang Q., Zheng X. Mulberry ethanol extract attenuates hepatic steatosis and insulin resistance in high-fat diet-fed mice. Nutr. Res. 2016;36:710–718. doi: 10.1016/j.nutres.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 35.Park M., Yoo J.-H., Lee Y.-S., Lee H.-J. Lonicera caerulea extract attenuates non-alcoholic fatty liver disease in free fatty acid-induced HepG2 hepatocytes and in high fat diet-fed mice. Nutrients. 2019;11:494. doi: 10.3390/nu11030494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Han H.Y., Lee S.K., Choi B.K., Lee D.R., Lee H.J., Kim T.W. Preventive effect of citrus aurantium peel extract on high-fat diet-induced non-alcoholic fatty liver in mice. Biol. Pharm. Bull. 2019;42:255–260. doi: 10.1248/bpb.b18-00702. [DOI] [PubMed] [Google Scholar]
- 37.Kwon E.Y., Shin S.K., Choi M.S. Ursolic acid attenuates hepatic steatosis, fibrosis, and insulin resistance by modulating the circadian rhythm pathway in diet-induced obese mice. Nutrients. 2018;10:1719. doi: 10.3390/nu10111719. http://doi: 10.3390/nu10111719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mulvihill E.E., Allister E.M., Sutherland B.G., Telford D.E., Sawyez C.G., Edwards J.Y., Markle J.M., Hegele R.A., Huff M.W., et al. Naringenin prevents dyslipidemia, apolipoprotein B overproduction, and hyperinsulinemia in LDL receptor-null mice with diet-induced insulin resistance. Diabetes. 2009;58:2198–2210. doi: 10.2337/db09-0634. 10.2337/db09- 0634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yuk T., Kim Y., Yang J., Sung J., Jeong H.S., Lee J. Nobiletin inhibits hepatic lipogenesis via activation of AMP-activated protein kinase. Evid. Based Complementary Altern. Med. 2018;2018:1–8. doi: 10.1155/2018/7420265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aubert K. Begriche, Knockaert L., Robin M.A., Fromenty B. Increased expression of cytochrome P450 2E1 in nonalcoholic fatty liver disease: mechanisms and pathophysiological role. Clin. Res. Hepatol. Gastroenterol. 2011;35:630–637. doi: 10.1016/j.clinre.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 41.Schattenberg J.M., Wang Y., Singh R., Rigoli R.M., Czaja M.J. Hepatocyte CYP2E1 overexpression and steatohepatitis lead to impaired hepatic insulin signaling. J. Biol. Chem. 2005;280:9887–9894. doi: 10.1074/jbc.m410310200. [DOI] [PubMed] [Google Scholar]
- 42.Ekström G., Ingelman-Sundberg M. Rat liver microsomal NADPH-supported oxidase activity and lipid peroxidation dependent on ethanol-inducible cytochrome P-450 (P-450IIE1) Biochem. Pharmacol. 1989;38:1313–1319. doi: 10.1016/0006-2952(89)90338-9. [DOI] [PubMed] [Google Scholar]
- 43.Hassan K. Nonalcoholic fatty liver disease: a comprehensive review of a growing epidemic. World J. Gastroenterol. 2014;20 doi: 10.3748/wjg.v20.i34.12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reddy J.K., Hashimoto T. Peroxisomal beta-oxidation and peroxisome proliferator-activated receptor alpha: an adaptive metabolic system. Annu. Rev. Nutr. 2001;21:193–230. doi: 10.1146/annurev.nutr.21.1.193. [DOI] [PubMed] [Google Scholar]
- 45.Marra F., Lotersztajn S. Pathophysiology of NASH: perspectives for a targeted treatment. Curr. Pharmaceut. Des. 2013;19:5250–5269. doi: 10.2174/13816128113199990344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Q. Effect of Sinai San decoction on the development of non-alcoholic steatohepatitis in rats. World J. Gastroenterol. 2005;11:1392. doi: 10.3748/wjg.v11.i9.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tang W., Zeng L., Yin J., Yao Y., Feng L., Yao X., et al. Hugan qingzhi exerts anti-inflammatory effects in a rat model of nonalcoholic fatty liver disease. Evidence-Based Complementary Altern. Med. 2015 doi: 10.1155/2015/810369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seebacher F., Zeigerer A., Kory N., Krahmer N. Hepatic lipid droplet homeostasis and fatty liver disease. Semin. Cell Dev. Biol. 2020;108:72–81. doi: 10.1016/j.semcdb.2020.04.011. [DOI] [PubMed] [Google Scholar]
- 49.Arab J.P., Arrese M., Trauner M. Recent insights into the pathogenesis of nonalcoholic fatty liver disease. Annu. Rev. Pathol. 2018;13:321–350. doi: 10.1146/annurev-pathol-020117-043617. [DOI] [PubMed] [Google Scholar]
- 50.Friedman S.L., Neuschwander-Tetri B.A., Rinella M., Sanyal A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 2018;24:908–922. doi: 10.1038/s41591-018-0104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Byrne C.D., Targher G. What's new in NAFLD pathogenesis, biomarkers and treatment? Nat. Rev. Gastroenterol. Hepatol. 2019;17:70–71. doi: 10.1038/s41575-019-0239-2. [DOI] [PubMed] [Google Scholar]
- 52.Trépo E., Valenti L. Update on NAFLD genetics: from new variants to the clinic. H Hepatol. 2020;72:1196–1209. doi: 10.1016/j.jhep.2020.02.020. [DOI] [PubMed] [Google Scholar]
- 53.Eslam M., George J. Genetic contributions to NAFLD: leveraging shared genetics to uncover systems biology. Nat. Rev. Gastroenterol. Hepatol. 2020;17:40–52. doi: 10.1038/s41575-019-0212-0. [DOI] [PubMed] [Google Scholar]
- 54.Del Campo J.A., Gallego-Durán R., Gallego P., Grande L. Genetic and epigenetic regulation in nonalcoholic fatty liver disease (NAFLD) Int. J. Mol. Sci. 2018;19:911. doi: 10.3390/ijms19030911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Anstee Q.M., Targher G., Day C.P. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat. Rev. Gastroenterol. Hepatol. 2013;10:330–344. doi: 10.1038/nrgastro.2013.41. [DOI] [PubMed] [Google Scholar]
- 56.Ekstedt M., Franzén L.E., Mathiesen U.L., Thorelius L., Holmqvist M., Bodemar G., Kechagias S. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865–873. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 57.Haukeland J.W., Konopski Z., Eggesbo H.B., von Volkmann H.L., Raschpichler G., Bjøro K., Haaland T., Løberg E.M., Birkeland K. Metformin in patients with nonalcoholic fatty liver disease: a randomized, controlled trial. Scand. J. Gastroenterol. 2009;44:853–860. doi: 10.1080/00365520902845268. [DOI] [PubMed] [Google Scholar]
- 58.Galli A., Crabb D.W., Ceni E., Salzano R., Mello T., Svegliati-Baroni G., Ridolfi F., Trozzi L., Surrenti C., Casini A. Antidiabetic thiazolidinediones inhibit collagen synthesis and hepatic stellate cell activation in vivo and in vitro. Gastroenterology. 2002;122:1924–1940. doi: 10.1053/gast.2002.33666. [DOI] [PubMed] [Google Scholar]
- 59.Sears I.B., MacGinnitie M.A., Kovacs L.G., Graves R.A. Differentiation-dependent expression of the brown adipocyte uncoupling protein gene: regulation by peroxisome proliferator-activated receptor gamma. Mol. Cell Biol. 1996;16:3410–3419. doi: 10.1128/mcb.16.7.3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Foster T., Budoff M.J., Saab S., Ahmadi N., Gordon C., Guerci A.D. Atorvastatin and antioxidants for the treatment of nonalcoholic fatty liver disease: the St Francis Heart Study randomized clinical trial. Am. J. Gastroenterol. 2011;106:71–77. doi: 10.1038/ajg.2010.299. [DOI] [PubMed] [Google Scholar]
- 61.Athyros V.G., Tziomalos K., Gossios T.D., Griva T., Anagnostis P., Kargiotis K., Pagourelias E.D., Theocharidou E., Karagiannis A., Mikhailidis D.P. Safety and efficacy of long-term statin treatment for cardiovascular events in patients with coronary heart disease and abnormal liver tests in the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) Study: a post-hoc analysis. Lancet. 2010;376:1916–1922. doi: 10.1016/s0140-6736(10)61272-x. [DOI] [PubMed] [Google Scholar]
- 62.Lavine J.E. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents. JAMA. 2011;305:1659. doi: 10.1001/jama.2011.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sanyal A.J., Chalasani N., Kowdley K.V., et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N. Engl. J. Med. 2010;362:1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lindor K.D., Kowdley K.V., Heathcote E.J., Harrison M.E., Jorgensen R., Angulo P., Lymp J.F., Burgart L., Colin P. Ursodeoxycholic acid for treatment of nonalcoholic steatohepatitis: results of a randomized trial. Hepatology. 2004;39:770–778. doi: 10.1002/hep.20092. [DOI] [PubMed] [Google Scholar]
- 65.Sanyal A.J., Abdelmalek M.F., Suzuki A., Cummings O.W., Chojkier M. No significant effects of ethyl-eicosapentanoic acid on histologic features of nonalcoholic steatohepatitis in a phase 2 trial. Gastroenterology. 2014;147:377–384.e1. doi: 10.1053/j.gastro.2014.04.046. [DOI] [PubMed] [Google Scholar]
- 66.Wagner M., Zollner G., Trauner M. Nuclear bile acid receptor farnesoid X receptor meets nuclear factor-kappaB: new insights into hepatic inflammation. Hepatology. 2008;48:1383–1386. doi: 10.1002/hep.22668. [DOI] [PubMed] [Google Scholar]
- 67.Sakata R., Nakamura T., Torimura T., Ueno T., Sata M. Green tea with high-density catechins improves liver function and fat infiltration in non-alcoholic fatty liver disease (NAFLD) patients: a double-blind placebo-controlled study. Int. J. Mol. Med. 2013;32:989–994. doi: 10.3892/ijmm.2013.1503. [DOI] [PubMed] [Google Scholar]
- 68.Chen C., Liu Q., Liu L., Hu Y., Feng Q. Potential biological effects of (−)-Epigallocatechin-3-gallate on the treatment of nonalcoholic fatty liver disease. Mol. Nutr. Food Res. 2018;62 doi: 10.1002/mnfr.201700483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tsanova-Savova S., Ribarova F., Gerova M. (+)-Catechin and (−)-epicatechin in Bulgarian fruits. J. Food Compos. Anal. 2005;18:691–698. doi: 10.1016/j.jfca.2004.06.008. [DOI] [Google Scholar]
- 70.Hidalgo I., Ortiz-Flores M., Villarreal F., Fonseca-Coronado S., Ceballos G., Meaney E., Nájera N. Is it possible to treat nonalcoholic liver disease using a flavanol-based nutraceutical approach? Basic and clinical data. J. Basic Clin. Physiol. Pharmacol. 2022;33:703–714. doi: 10.1515/jbcpp-2021-0285. [DOI] [PubMed] [Google Scholar]
- 71.Cheng H., Xu N., Zhao W., Su J., Liang M., Xie Z., Wu X., Li Q. ‐Epicatechin regulates blood lipids and attenuates hepatic steatosis in rats fed high‐fat diet. Mol. Nutr. Food Res. 2017;61 doi: 10.1002/mnfr.201700303. [DOI] [PubMed] [Google Scholar]
- 72.Hidalgo I., Nájera N., Meaney E., Pérez-Durán J., Valdespino-Vazquez Y., Villarreal F., Ceballos G. Effects of (−)-epicatechin on the time course of the expression of perilipins in a diet-induced model of nonalcoholic steatohepatitis. J. Nutr. Biochem. 2020;77 doi: 10.1016/j.jnutbio.2019.108296. [DOI] [PubMed] [Google Scholar]
- 73.Li H., Ying H., Hu A., Hu Y., Li D. Therapeutic effect of gypenosides on nonalcoholic steatohepatitis via regulating hepatic lipogenesis and fatty acid oxidation. Biol. Pharm. Bull. 2017;40:650–657. doi: 10.1248/bpb.b16-00942. [DOI] [PubMed] [Google Scholar]
- 74.Lin Z., Wu Z.F., Jiang C.H., Zhang Q.W., Ouyang S., Che C.T., et al. The chloroform extract of Cyclocarya paliurus attenuates high-fat diet induced non-alcoholic hepatic steatosis in Sprague Dawley rats. Phytomedicine. 2016;23:1475–1483. doi: 10.1016/j.phymed.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 75.Li Y., Zhao J., Zheng H., Zhong X., Zhou J., Hong Z. Treatment of nonalcoholic fatty liver disease with total alkaloids in rubus aleaefolius poir through regulation of fat metabolism. Evid. Based. Complement. Alternat. Med. 2014 doi: 10.1155/2014/768540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Radhakrishnan A., Goldstein J.L., McDonald J.G., Brown M.S. Switch-like control of SREBP-2 transport triggered by small changes in ER cholesterol: a delicate balance. Cell Metabol. 2008;8:512–521. doi: 10.1016/j.cmet.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Song L., Zhao X.G., Ouyang P.L., Guan Q., Yang L., Peng F., et al. Combined effect of n-3 fatty acids and phytosterol esters on alleviating hepatic steatosis in non-alcoholic fatty liver disease subjects: a double-blind placebo-controlled clinical trial. Br. J. Nutr. 2020;123:1148–1158. doi: 10.1017/S0007114520000495. [DOI] [PubMed] [Google Scholar]
- 78.Gumede N.M., Lembede B.W., Brooksbank R.L., Erlwanger K.H., Chivandi E. β-Sitosterol shows potential to protect against the development of high-fructose diet-induced metabolic dysfunction in female rats. J. Med. Food. 2020;23:367–374. doi: 10.1089/jmf.2019.0120. [DOI] [PubMed] [Google Scholar]
- 79.Sanchez-Moreno C., Cao G., Ou B., Prior R.L. Antho-cyanin and proanthocyanidin content in selected white and red wines. Oxygen radical absorbance capacity comparison with nontraditional wines obtained from highbush blueberry. J. Agric. Food Chem. 2003;51:4889–4896. doi: 10.1021/jf030081t. [DOI] [PubMed] [Google Scholar]
- 80.Del Bas J.M., Fernandez-Larrea J., Blay M., Ardèvol A., Josepa Salvadó M., Arola L., Bladé C. Grape seed procyanidins improve atherosclerotic risk index and induce liver CYP7A1 and SHP expression in healthy rats. FASEB (Fed. Am. Soc. Exp. Biol.) J. 2005;19:479–481. doi: 10.1096/fj.04-3095fje. [DOI] [PubMed] [Google Scholar]
- 81.Kim M.H., Kang K.S. Isoflavones as a smart curer for non-alcoholic fatty liver disease and pathological adiposity via ChREBP and Wnt signaling. Prev. Med. 2012;54:S57–S63. doi: 10.1016/j.ypmed.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 82.Eui S.S., Hyoung H.L., Si Y.C., Hyun W.P., Sang J.L., Tae R.L. Genistein downregulates SREBP-1 regulated gene expression by inhibiting site-1 protease expression in HepG2 cells. J. Nutr. 2007;137:1127–1131. doi: 10.1093/jn/137.5.1127. [DOI] [PubMed] [Google Scholar]
- 83.Wang W., Chen J., Mao J., Li H., Wang M., Zhang H H., Li H., Chen W. Genistein ameliorates non-alcoholic fatty liver disease by targeting the thromboxane A2 pathway. J. Agric. Food Chem. 2018;66:5853–5859. doi: 10.1021/acs.jafc.8b01691. [DOI] [PubMed] [Google Scholar]
- 84.Atanassova M., Bagdassarian V. Rutin content in plant products. J. Chem. Technol. Metall. 2009;44:201–203. [Google Scholar]
- 85.Memon A.F., Solangi A.R., Memon S.Q., Mallah A., Memon N., Memon A.A. Simultaneous determination of quercetin, rutin, naringin, and naringenin in different fruits by capillary zone electrophoresis. Food Anal. Methods. 2017;10:83–91. doi: 10.1007/s12161-016-0552-0. [DOI] [Google Scholar]
- 86.Ganeshpurkar A., Saluja A.K. The pharmacological potential of rutin. Saudi Pharmaceut. J. 2017;25:149–164. doi: 10.1016/j.jsps.2016.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim K.D., Jung H.-Y., Ryu H.G., Kim B., Jeon J., Yoo H.Y., Park C.H., Choi B.-H., Hyun C.-K., Kim K.-T., Fang S., Yang S.H., Kim J.-B. Betulinic acid inhibits high-fat diet-induced obesity and improves energy balance by activating AMPK. Nutr. Metabol. Cardiovasc. Dis. 2019;29:409–420. doi: 10.1016/j.numecd.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 88.Choi E., Jang E., Lee J.H. Pharmacological activities of alisma orientale against nonalcoholic fatty liver disease and metabolic syndrome: literature review, evid. Based complement. Alternative Med. 2019 doi: 10.1155/2019/2943162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ahmad O., Wang B., Ma K., Deng Y., Li M., Yang L., Yang Y., Zhao J., Cheng L., Zhou Q., Shang J. Lipid modulating anti-oxidant stress activity of gastrodin on nonalcoholic fatty liver disease larval zebrafish model. Int. J. Mol. Sci. 2019;20:1984. doi: 10.3390/ijms20081984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yang L., Ren S., Xu F., Ma Z., Liu X., Wang L. Recent advances in the pharmacological activities of dioscin. BioMed Res. Int. 2019 doi: 10.1155/2019/5763602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang J., Zhang H., Deng X., Zhang N., Liu B., Xin S., Li M.G., Xu K. Baicalin attenuates non-alcoholic steatohepatitis by suppressing key regulators of lipid metabolism, inflammation and fibrosis in mice. Life Sci. 2018;192:46–54. doi: 10.1016/j.lfs.2017.11.027. [DOI] [PubMed] [Google Scholar]
- 92.Hong M., Li S., Wang N., Tan H.Y., Cheung F., Feng Y. A biomedical investigation of the hepatoprotective effect of radix salviae miltiorrhizae and network pharmacology-based prediction of the active compounds and molecular targets. Int. J. Mol. Sci. 2017;18:620. doi: 10.3390/ijms18030620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen G., Liang G., Ou J., Goldstein J.L., Brown M.S. Central role for liver X receptor in insulin-mediated activation of Srebp-1c transcription and stimulation of fatty acid synthesis in liver. Proc. Natl. Acad. Sci. USA. 2004;101:11245–11250. doi: 10.1073/pnas.0404297101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kawaguchi T., Takenoshita M., Kabashima T., Uyeda K. Glucose and cAMP regulate the L-type pyruvate kinase gene by phosphorylation/dephosphorylation of the carbohydrate response element binding protein. Proc. Natl. Acad. Sci. U.S.A. 2001;98:13710–13715. doi: 10.1073/pnas.231370798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dentin R., Benhamed F., Hainault I., Fauveau V., Foufelle F., Dyck J., Girard J., Postic C. Liver-specific inhibition of ChREBP improves hepatic steatosis and insulin resistance in ob/ob. Mice Diabetes. 2006;55:2159–2170. doi: 10.2337/db06-0200. [DOI] [PubMed] [Google Scholar]
- 96.Chen H.W., Yang J.J., Tsai C.W., Wu J.J., Sheen L.Y., Ou C.C., Lii C.K. Dietary fat and garlic oil independently regulate hepatic cytochrome p(450) 2B1 and the placental form of glutathione S-transferase expression in rats. J. Nutr. 2001;131:1438–1443. doi: 10.1093/jn/131.5.1438. [DOI] [PubMed] [Google Scholar]
- 97.Mulvihill E.E., Allister E.M., Sutherland B.G., Telford D.E., Sawyez C.G., Edwards J.Y., Markle J.M., Hegele R.A., Huff M.W. Naringenin prevents dyslipidemia, apolipoprotein B overproduction, and hyperinsulinemia in LDL receptor-null mice with diet-induced insulin resistance. Diabetes. 2009;58:2198–2210. doi: 10.2337/db09-0634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yu X., Ye L., Zhang H., Zhao J., Wang G., Guo C., Shang W. Ginsenoside Rb1 ameliorates liver fat accumulation by upregulating perilipin expression in adipose tissue of db/db obese mice. J. Ginseng Res. 2015;39:199–205. doi: 10.1016/j.jgr.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lee M.R., Park K.I., Ma J.Y. Leonurus japonicus houtt attenuates nonalcoholic fatty liver disease in free fatty acid-induced HepG2 cells and mice fed a high-fat diet. Nutrients. 2017;10:20. doi: 10.3390/nu10010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kim Y.J., Choi M.S., Woo J.T., Jeong M.J., Kim S.R., Jung U.J. Long-term dietary supplementation with low-dose nobiletin ameliorates hepatic steatosis, insulin resistance, and inflammation without altering fat mass in dietinduced obesity. Mol. Nutr. Food Res. 2017;61 doi: 10.1002/mnfr.201600889. [DOI] [PubMed] [Google Scholar]
- 101.Lim D.W., Kim H., Lee S.J., Yu G.R., Kim J.E., Park W.H. Jwa kum whan attenuates nonalcoholic fatty liver disease by modulating glucose metabolism and the insulin signaling pathway. Evid. Based Complement Alternat. Med. 2019 doi: 10.1155/2019/4589810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rao A.V., Ray M.R., Lycopene L.G. Rao. Adv. Food Nutr. Res. 2006;51:99–164. doi: 10.1016/S1043-4526(06)51002-2. [DOI] [PubMed] [Google Scholar]
- 103.Mouzaki M., Comelli E.M., Arendt B.M., Bonengel J., Fung S.K., Fischer S.E., et al. Intestinal microbiota in patients with nonalcoholic fatty liver disease. Hepatology. 2013;58:120–127. doi: 10.1002/hep.26319. [DOI] [PubMed] [Google Scholar]
- 104.Chew B.P., Park J.S. Carotenoid action on the immune response. J. Nutr. 2004;134:257S. doi: 10.1093/jn/134.1.257S. 61S. [DOI] [PubMed] [Google Scholar]
- 105.Madan K., Bhardwaj P., Thareja S., Gupta S.D., Saraya A. Oxidant stress and antioxidant status among patients with nonalcoholic fatty liver disease (NAFLD) J. Clin. Gastroenterol. 2006;40:930–935. doi: 10.1097/01.mcg.0000212608.59090.08. [DOI] [PubMed] [Google Scholar]
- 106.Perlemuter G., Davit-Spraul A., Cosson C., Conti M., Bigorgne A., Paradis V., Corre M.-P., Prat L., Kuoch V., Basdevant A., Pelletier G., Oppert J.-M., Buffet C. Increase in liver antioxidant enzyme activities in non-alcoholic fatty liver disease. Liver Int. 2005;25:946–953. doi: 10.1111/j.1478-3231.2005.01126.x. [DOI] [PubMed] [Google Scholar]
- 107.Rogue A., Renaud M.P., Claude N., Guillouzo A., Spire C. Comparative gene expression profiles induced by PPARγ and PPARα/γ agonists in rat hepatocytes. Toxicol. Appl. Pharmacol. 2011;254:18–31. doi: 10.1016/j.taap.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 108.Erhardt A., Stahl W., Sies H., Lirussi F., Donner A., Häussinger D. Plasma levels of vitamin e and carotenoids are decreased in patients with nonalcoholic steatohepatitis (nash) Eur. J. Med. Res. 2011;16:76. doi: 10.1186/2047-783x-16-2-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Qiu T., Pei P., Yao X., Jiang L., Wei S., Wang Z., Bai J., Yang G., Gao N., Yang L., Qi S., Yan R., Liu X., Sun X. Taurine attenuates arsenic-induced pyroptosis and nonalcoholic steatohepatitis by inhibiting the autophagic-inflammasomal pathway. Cell Death Dis. 2018;9 doi: 10.1038/s41419-018-1004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yu X., Hao M., Liu Y., Ma X., Lin W., Xu Q., Zhou H., Shao N., Kuang H. Liraglutide ameliorates non-alcoholic steatohepatitis by inhibiting NLRP3 inflammasome and pyroptosis activation via mitophagy. Eur. J. Pharmacol. 2019;864 doi: 10.1016/j.ejphar.2019.172715. [DOI] [PubMed] [Google Scholar]
- 111.Shi H., Zhang Y., Xing J., Liu L., Qiao F., Li J., Chen Y. Baicalin attenuates hepatic injury in non-alcoholic steatohepatitis cell model by suppressing inflammasome-dependent GSDMD-mediated cell pyroptosis. Int. Immunopharm. 2020;81 doi: 10.1016/j.intimp.2020.106195. [DOI] [PubMed] [Google Scholar]
- 112.Henao-Mejia J., Elinav E., Jin C., Hao L., Mehal W.Z., Strowig T., Thaiss C.A., Kau A.L., Eisenbarth S.C., Jurczak M.J., et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482:179–185. doi: 10.1038/nature10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Swanson K.V., Deng M., Ting J.P.-Y. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019;19:477–489. doi: 10.1038/s41577-019-0165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Xu B., Jiang M., Chu Y., Wang W., Chen D., Li X., Zhang Z., Zhang D., Fan D., Nie Y., et al. Gasdermin D plays a key role as a pyroptosis executor of non-alcoholic steatohepatitis in humans and mice. J. Hepatol. 2018;68:773–782. doi: 10.1016/j.jhep.2017.11.040. [DOI] [PubMed] [Google Scholar]
- 115.Salehi B., Fokou P., Sharifi-Rad M., Zucca P., Pezzani R., Martins N., Sharifi-Rad J. The therapeutic potential of naringenin: a review of clinical trials. Pharmaceuticals. 2019;12:11. doi: 10.3390/ph12010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Motallebi M., Bhia M., Rajani H.F., Bhia I., Tabarraei H., Mohammadkhani N., Pereira-Silva M., Kasaii M.S., Nouri-Majd S., Mueller A.-L., et al. Naringenin: a potential flavonoid phytochemical for cancer therapy. Life Sci. 2022;305 doi: 10.1016/j.lfs.2022.120752. [DOI] [PubMed] [Google Scholar]
- 117.Naeini F., Namkhah Z., Ostadrahimi A., Tutunchi H., Hosseinzadeh-Attar M.J. A comprehensive systematic review of the effects of naringenin, a citrus-derived flavonoid, on risk factors for nonalcoholic fatty liver disease. Adv. Nutr. 2021;12:413–428. doi: 10.1093/advances/nmaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang X., Zhang Y., Gao W., Guo Z., Wang K., Liu S., Duan Z., Chen Y. Naringin improves lipid metabolism in a tissue-engineered liver model of NAFLD and the underlying mechanisms. Life Sci. 2021;277 doi: 10.1016/j.lfs.2021.119487. [DOI] [PubMed] [Google Scholar]
- 119.Wang Q., Ou Y., Hu G., Wen C., Yue S., Chen C., Xu L., Xie J., Dai H., Xiao H., Zhang Y., Qi R. Naringenin attenuates non‐alcoholic fatty liver disease by down‐regulating the NLRP3/NF‐κB pathway in mice. Br. J. Pharmacol. 2020;177:1806–1821. doi: 10.1111/bph.14938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shukla S., Gupta S. Apigenin: a promising molecule for cancer prevention. Pharm. Res. (N. Y.) 2010;27:962–978. doi: 10.1007/s11095-010-0089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Miean K.H., Mohamed S. Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. J. Agric. Food Chem. 2001;49:3106–3112. doi: 10.1021/jf000892m. [DOI] [PubMed] [Google Scholar]
- 122.Ali F., Rahul, Naz F., Jyoti S., Siddique Y.H. Health functionality of apigenin: a review. Int. J. Food Prop. 2016;20:1197–1238. doi: 10.1080/10942912.2016.1207188. [DOI] [Google Scholar]
- 123.Li Z., Zhou J., Ji L., Liang Y., Xie S. Recent advances in the pharmacological actions of apigenin, its complexes, and its derivatives. Food Rev. Int. 2022:1–34. doi: 10.1080/87559129.2022.2122989. [DOI] [Google Scholar]
- 124.Yue S., Xue N., Li H., Huang B., Chen Z., Wang X. Hepatoprotective effect of apigenin against liver injury via the non-canonical NF-κB pathway in vivo and in vitro. Inflammation. 2020;43:1634–1648. doi: 10.1007/s10753-020-01238-5. [DOI] [PubMed] [Google Scholar]
- 125.Lv Y., Gao X., Luo Y., Fan W., Shen T., Ding C., Yao M., Song S., Yan L. Apigenin ameliorates HFD-induced NAFLD through regulation of the XO/NLRP3 pathways. J. Nutr. Biochem. 2019;71:110–121. doi: 10.1016/j.jnutbio.2019.05.015. [DOI] [PubMed] [Google Scholar]
- 126.Olivas-Aguirre F., Rodrigo-García J., Martínez-Ruiz N., Cárdenas-Robles A., Mendoza-Díaz S., Álvarez-Parrilla E., González-Aguilar G., de la Rosa L., Ramos-Jiménez A., Wall-Medrano A. Cyanidin-3-O-glucoside: physical-chemistry, foodomics and health effects. Molecules. 2016;21:1264. doi: 10.3390/molecules21091264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sun C.-D., Zhang B., Zhang J.-K., Xu C.-J., Wu Y.-L., Li X., Chen K.-S. Cyanidin-3-Glucoside-Rich extract from Chinese bayberry fruit protects pancreatic β cells and ameliorates hyperglycemia in streptozotocin-induced diabetic mice. J. Med. Food. 2012;15:288–298. doi: 10.1089/jmf.2011.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lee J.S., Kim Y.R., Song I.G., Ha S.-J., Kim Y.E., Baek N.-I., Hong E.K. Cyanidin-3-glucoside isolated from mulberry fruit protects pancreatic β-cells against oxidative stress-induced apoptosis. Int. J. Mol. Med. 2014;35:405–412. doi: 10.3892/ijmm.2014.2013. [DOI] [PubMed] [Google Scholar]
- 129.Wu C.-F., Wu C.-Y., Lin C.-F., Liu Y.-W., Lin T.-C., Liao H.-J., Chang G.-R. The anticancer effects of cyanidin 3-O-glucoside combined with 5-fluorouracil on lung large-cell carcinoma in nude mice. Biomed. Pharmacother. 2022;151 doi: 10.1016/j.biopha.2022.113128. [DOI] [PubMed] [Google Scholar]
- 130.Chen W., Tu P., Ye X., Tang Q., Yu T., Zheng X. Cyanidin-3-O-glucoside impacts fecal discharge of polystyrene microplastics in mice: potential role of microbiota-derived metabolites. Toxicol. Appl. Pharmacol. 2022;453 doi: 10.1016/j.taap.2022.116212. [DOI] [PubMed] [Google Scholar]
- 131.Wang S., Huang Y., Luo G., Yang X., Huang W. Cyanidin-3-O-glucoside attenuates high glucose–induced podocyte dysfunction by inhibiting apoptosis and promoting autophagy via activation of SIRT1/AMPK pathway. Can. J. Physiol. Pharmacol. 2021;99:589–598. doi: 10.1139/cjpp-2020-0341. [DOI] [PubMed] [Google Scholar]
- 132.Li X., Shi Z., Zhu Y., Shen T., Wang H., Shui G., Loor J.J., Fang Z., Chen M., Wang X., Peng Z., Song Y., Wang Z., Du X., Liu G. Cyanidin‐3‐ O ‐glucoside improves non‐alcoholic fatty liver disease by promoting PINK1‐mediated mitophagy in mice. Br. J. Pharmacol. 2020;177:3591–3607. doi: 10.1111/bph.15083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhou Y., Wang S., Wan T., Huang Y., Pang N., Jiang X., Gu Y., Zhang Z., Luo J., Yang L. Cyanidin-3-O-β-glucoside inactivates NLRP3 inflammasome and alleviates alcoholic steatohepatitis via SirT1/NF-κB signaling pathway. Free Radic. Biol. Med. 2020;160:334–341. doi: 10.1016/j.freeradbiomed.2020.08.006. [DOI] [PubMed] [Google Scholar]
- 134.Gold-Smith F., Fernandez A., Bishop K. Mangiferin and cancer: mechanisms of action. Nutrients. 2016;8:396. doi: 10.3390/nu8070396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Vyas A., Syeda K., Ahmad A., Padhye S., Sarkar F.H. Perspectives on medicinal properties of mangiferin. Mini-Rev. Med. Chem. 2012;12:412–425. doi: 10.2174/138955712800493870. [DOI] [PubMed] [Google Scholar]
- 136.Miura T., Ichiki H., Hashimoto I., Iwamoto N., Kao M., Kubo M., Ishihara E., Komatsu Y., Okada M., Ishida T., Tanigawa K. Antidiabetic activity of a xanthone compound, mangiferin. Phytomedicine. 2001;8:85–87. doi: 10.1078/0944-7113-00009. [DOI] [PubMed] [Google Scholar]
- 137.Dong M., Li L., Li G., Song J., Liu B., Liu X., Wang M. Mangiferin protects against alcoholic liver injury via suppression of inflammation-induced adipose hyperlipolysis. Food Funct. 2020;11:8837–8851. doi: 10.1039/d0fo01436b. [DOI] [PubMed] [Google Scholar]
- 138.Yong Z., Ruiqi W., Hongji Y., Ning M., Chenzuo J., Yu Z., Zhixuan X., Qiang L., Qibing L., Weiying L., Xiaopo Z. Mangiferin ameliorates HFD-induced NAFLD through regulation of the AMPK and NLRP3 inflammasome signal pathways. J. Immunol Res. 2021;2021:1–17. doi: 10.1155/2021/4084566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.da Silva A.C., Jorge N. Bioactive compounds of oils extracted from fruits seeds obtained from agroindustrial waste. Eur. J. Lipid Sci. Technol. 2016;119 doi: 10.1002/ejlt.201600024. [DOI] [Google Scholar]
- 140.Abenavoli L., Milanović M., Milić N., Luzza F., Giuffrè A.M. Olive oil antioxidants and non-alcoholic fatty liver disease. Expet Rev. Gastroenterol. Hepatol. 2019;13:739–749. doi: 10.1080/17474124.2019.1634544. [DOI] [PubMed] [Google Scholar]
- 141.Hendawy O., Gomaa H.A.M., Hussein S., Alzarea S.I., Qasim S., Abdel Rahman F.E.-Z.S., Ali A.T., Ahmed S.R. Cold-pressed raspberry seeds oil ameliorates high-fat diet triggered non-alcoholic fatty liver disease. Saudi Pharmaceut. J. 2021;29:1303–1313. doi: 10.1016/j.jsps.2021.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rezaei S., Sasani M.R., Akhlaghi M., Kohanmoo A. Flaxseed oil in the context of a weight loss programme ameliorates fatty liver grade in patients with non-alcoholic fatty liver disease: a randomised double-blind controlled trial. Br. J. Nutr. 2020;123:994–1002. doi: 10.1017/s0007114520000318. [DOI] [PubMed] [Google Scholar]
- 143.Hu M., Zhang L., Ruan Z., Han P., Yu Y. The regulatory effects of citrus peel powder on liver metabolites and gut flora in mice with non-alcoholic fatty liver disease (NAFLD) Foods. 2021;10:3022. doi: 10.3390/foods10123022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Al-Shaaibi S.N.K., Waly M.I., Al-Subhi L., Tageldin M.H., Al-Balushi N.M., Rahman M.S. Ameliorative effects of pomegranate peel extract against dietary-induced nonalcoholic fatty liver in rats. Prev. Nutr. Food Sci. 2016;21:14–23. doi: 10.3746/pnf.2016.21.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Song H., Shen X., Chu Q., Zheng X. Pomegranate fruit pulp polyphenols reduce diet‐induced obesity with modulation of gut microbiota in mice. J. Sci. Food Agric. 2021;102:1968–1977. doi: 10.1002/jsfa.11535. [DOI] [PubMed] [Google Scholar]
- 146.Alexander-Aguilera A., Aguirre-Maldonado I., Antolín J.R., Toledo L.N., Rodríguez I.S., Sánchez Otero M.G. Effect of Litchi chinensis on adipose and hepatic tissues in rats with obesity and non-alcoholic fatty liver disease (NAFLD) J. Saudi Soc. Agric. Sci. 2019;18:235–240. doi: 10.1016/j.jssas.2017.06.002. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.