Abstract
Background:
Each year, infertility affects 15% of couples worldwide, with 50% of cases attributed to men. Globozoospermia is an uncommon cause of male factor infertility, characterized by defects in sperm acrosome formation, leading to round-headed spermatozoa.
Objective:
We generated phosducin-like 2 (PDCL2) knockout (−/−) mice to investigate the essential roles of PDCL2 in mammalian reproduction.
Materials and Methods:
We used RT-PCR to demonstrate that PDCL2 was expressed exclusively in the male reproductive tract in mice and humans. We created Pdcl2−/− mice using the CRISPR-Cas9 system and analyzed their fertility. Pdcl2 null spermatozoa underwent further evaluation using computer-assisted sperm analysis (CASA), light microscopy, and ultrastructural microscopy. We used immunoblot analysis and immunofluorescence to elucidate relationships between PDCL2 and other acrosomal proteins.
Results:
The PDC family is highly conserved in eukaryotes. Mouse and human PDCL2 are testis-enriched and localized to the testicular endoplasmic reticulum. Loss of the protein causes sterility due to abnormal acrosome biogenesis during spermiogenesis and immotility. Furthermore, Pdcl2 null spermatozoa have rounded heads, similar to globozoospermia in humans. Observation of the knockout testis shows a lack of acrosomal cap formation, aberrant localization of mitochondria in the sperm head, and misshapen nuclei.
Conclusion:
PDCL2 is essential for sperm acrosome development and male fertility in mice and is a putative contraceptive target in men
Keywords: Acrosome formation, CRISPR-Cas9, Globozoospermia, Infertility, Spermatogenesis
INTRODUCTION
According to the World Health Organization, 15% of reproductive-aged couples are affected by infertility worldwide, but much is still unknown about this global health issue (https://icd.who.int/en). Male fertility problems account for 50% of infertility cases1. Abnormal sperm morphology can affect fertility. Globozoospermia, or round-headed sperm, is a rare category of sperm defects, usually characterized by improper acrosomal membrane formation during spermiogenesis, the final stage of spermatogenesis where spermatids mature into spermatozoa2. Often, individuals with globozoospermia are infertile, unable to conceive without the help of assisted reproductive technologies. Acrosomal membrane formation is a process that has yet to be fully understood3. There are four distinct stages: Golgi, cap, acrosome, and maturation phases. During the Golgi stage, the trans-Golgi network begins producing proacrosomal vesicles needed to create the mature acrosome. These vesicles fuse to form the acrosomal granule. In the cap phase, the acrosomal granule begins to enlarge and flatten around the nuclear envelope to form a cap. In the acrosomal phase, the acrosome moves over the ventral surface of the spermatid nucleus as it elongates, and concurrently condenses4. Finally, in the maturation phase, the nucleus changes its morphology and the acrosomal granule finishes spreading over the entire acrosomal membrane. Proteins essential for proper acrosome development include DPY19L2, FAM209, FAM71F1, FAM71F2, GBA2, GOPC, PICK1, SPACA1, SSMEM1, and ZPBP15–13. Many of these proteins, such as DPY19L2, FAM209, GBA2, GOPC, PICK1, SPACA1, SSMEM1, and ZPBP1, cause globozoospermia when mutated.
In this paper, we focused on phosducin-like 2 (PDCL2), a protein that shares extensive homology with phosphoducin14. According to UniProt, PDCL2 is a modulator of heterotrimeric G proteins and has been hypothesized to bind with G protein beta-gamma subunits (https://www.ebi.ac.uk/interpro/entry/InterPro/IPR024253/). According to Treefam, the PDC family is highly conserved across multiple species including invertebrates (http://www.treefam.org/family/TF315179). We show that mouse PDCL2 is a testicular germ cell-specific endoplasmic reticulum (ER)-resident protein expressed during the early stages of spermiogenesis, especially acrosomal development. We characterized a deletion of mouse Pdcl2 generated via the CRISPR-Cas9 system and suggest that PDCL2 is essential for acrosome formation.
METHODS
Animals.
All animal experiments were approved by the Animal Care and Use Committees of the National Cerebral and Cardiovascular Center Research Institute and the Research Institute for Microbial Diseases, Osaka University. Human tissues were collected as nonhuman subject research by the Human Tissue Acquisition & Pathology Core at Baylor College of Medicine under the institutional review board-approved Protocol H-14435. Mice were maintained under a 12-hr light/dark cycle. Wild-type and W/Wv mutant mice were purchased from Japan SLC (Shizuoka, Japan). In this study, we used transgenic mouse line (B6D2-Tg(CAG/Su9-DsRed2,Acr3-EGFP)RBGS002Osb)15 and generated genetically modified mouse line, Pdcl2 mutant mice (B6D2-Pdcl2<em1Ncvc>). This line will be deposited to the RIKEN BioResource Research Center (http://mus.brc.riken.jp/en/) and the Center for Animal Resources and Development (CARD), Kumamoto University (http://card.medic.kumamoto-u.ac.jp/card/english/).
RT-PCR analysis.
Briefly, using TRIzol reagent (Invitrogen, USA), total RNA was isolated from multiple adult mouse and human tissues obtained from the animal facility and the Human Tissue Acquisition & Pathology (HTAP) Core at Baylor College of Medicine, respectively. Informed consent of these human tissues was obtained. Mouse and Human cDNA were prepared using SuperScript III Reverse Transcriptase (Invitrogen, USA) following the manufacturer’s instruction. The primers used were: 5’-AGATCAAGTGTCCCAATGTGC-3’ and 5’-CTCTGTATCGCTCCAACTTCTG-3’ for mouse Pdcl2; 5’-TGGATATGCCCTTGACTATAATGAG-3’ and 5’-TGGCAACATCAACAGGACTC-3’ for mouse Hprt; 5’-TGACTCTTGCACAGCTAAAGG-3’ and 5’-CACATTGGGATGCTTGATCTG-3’ for human PDCL2; 5’-AATCCCATCACCATCTTCCAG-3’ and 5’-ATGACCCTTTTGGCTCCC-3’ for human GAPDH.
Antibodies.
The polyclonal antibodies against mouse CALMEGIN and SPACA1 were described previously6,16. The IZUMO1 monoclonal antibody was described previously17. Other antibodies were purchased from Atlas Antibodies (HPA071264 for DPY19L2 and HPA024018 for GOPC), Santa Cruz Biotechnology (sc-46700 for BASIGIN), Cell Signaling Technology (#2118 for GAPDH), and Proteintech (17407–1-AP for PDCL2). Dilutions were 1:200 to 1:300 for immunostaining and 1:500 to 1:1000 for immunoblot analysis.
Immunoblot analysis.
Immunoblot analysis was performed as described previously18. Briefly, sperm samples were collected from the cauda epididymis. These samples were homogenized in lysis buffer containing 1% Triton X-100 and 1% protease inhibitor (Nacalai Tesque, Kyoto, Japan) and then were centrifuged (10,000g for 20 min at 4°C), and the supernatants were collected. Protein lysates were separated by SDS/PAGE under reducing conditions and transferred to PVDF membranes (Bio-Rad Laboratories, CA, USA). After blocking, blots were incubated with primary antibodies overnight at 4°C and then incubated with secondary antibodies conjugated with horseradish-peroxidase. Detection was performed using Chemi-Lumi One Ultra (Nacalai Tesque, Kyoto, Japan).
Immunostaining.
Immunostaining analysis was performed as described previously19. Hoechst 33342 (Nacalai Tesque, Kyoto, Japan) was used for nuclear staining. Testicular germ cells (TGCs) and spermatozoa were observed under a phase-contrast microscope (BZ-X800, Keyence, Osaka, Japan; IX81, Olympus, Tokyo, Japan).
Generation of Pdcl2−/− mice with CRISPR-Cas9.
Pdcl2−/− mice were generated by introducing gRNA/CAS9 protein solution into fertilized eggs (B6D2 background) with an electroporator (NEPA21, Nepagene, Chiba, Japan). A search for gRNA and off-target sequences was performed using CRISPRdirect software (http://crispr.dbcls.jp/). Screening of the obtained mutant mice was performed by direct sequencing following PCR. The gRNAs and primers used were: 5’-AAAAGATGAAATTGAAGAGA-3’ for the second exon of Pdcl2 and 5’-ATTTATGACAGCGATAGCTC-3’ for the sixth exon of Pdcl2. The genotyping primers used were: 5’-GCCCCATTCTTGGTGGGAGAAGTAGG-3’ for Pr.1, 5’-GCATGTGCCTTTCAACATACACATCGGC-3’ for Pr.2, and 5’-GAGGTCAATTCATTTGTAGCTAAAGTCAGCAGG-3’ for Pr.3.
Male fertility test.
Sexually mature mutant male mice were caged with 2-month-old B6D2F1 or mutant females for several months, and the number of pups in each cage was counted within a week of birth. Average litter sizes (pups per delivery) are presented as the number of total pups born divided by the number of deliveries for each genotype.
Testicular histology and morphology.
After breeding studies, males were sacrificed by cervical dislocation following anesthesia. Bodies and testes were weighed individually, then testes fixed overnight in Bouin’s solution and were processed for paraffin embedding. Paraffin sections were cut at 5 μm, stained with periodic acid-Schiff, and then counterstained with Mayer’s hematoxylin solution (Wako, Osaka, Japan). The cauda epididymal spermatozoa were dispersed in phosphate-buffered saline (PBS), and then sperm morphology was observed under a phase-contrast microscope (BX53, Olympus, Tokyo, Japan).
Sperm motility analysis.
Sperm motility analysis was performed as previously described20. Briefly, cauda epididymal spermatozoa were dispersed in a 100 μL drop of TYH medium and incubated at 37°C under 5% CO2. After an incubation period of 10 and 120 min, the percentage of motile spermatozoa was examined as using the CEROS II sperm analysis system (software version 1.5; Hamilton Thorne Biosciences)
Electron microscope observation.
For transmission electron microscopy (TEM) analysis, testis and epididymis were fixed in phosphate buffered 2% glutaraldehyde-2% paraformaldehyde, and subsequently post-fixed in 2% osmium tetra-oxide for 3 hours in an ice bath. Then, the specimens were dehydrated in a graded ethanol series and embedded in epoxy resin. Ultrathin sections were obtained by using an ultramicrotome. Ultrathin sections stained with uranyl acetate for 10 minutes and lead staining solution for 5 minutes were submitted to TEM observation (HITACHI H-7600). For scanning electron microscopy (SEM) analysis, epididymides were fixed in phosphate buffered 2% glutaraldehyde, and subsequently post-fixed in 2% osmium tetra-oxide for 2 hours in an ice bath. Then, the specimens were dehydrated in a graded ethanol series and dried by t-Butyl alcohol freeze-drying. Dried specimens were coated by an osmium plasma ion coater and were submitted to SEM observation (JSM-7500F at 5 kV). Observations were performed at the Hanaichi Ultrastructure Research Institute (Aichi, Japan).
Statistical analysis.
Statistical analyses were performed using Student’s t test inserted into Microsoft Excel after the data were tested for normality of distribution. Differences were considered significant at **P<0.01 and ***P<0.001.
RESULTS
Mouse Pdcl2 is a conserved and testis-specific gene
Mouse Pdcl2 is a testis-specific gene located on chromosome 5 that encodes a 240 amino acid protein. Human and mouse PDCL2 show 87.5% similarity in protein sequence (Figure S1A). RT-PCR analysis in mouse tissues showed strong expression of Pdcl2 in the adult testis and epididymis (Figure 1A). Further analysis showed that expression was detectable at postnatal day 10 in the testis (Figure 1B), indicating that the gene is expressed earlier than when haploid expression occurs in the testis. When RT-PCR was conducted using human samples, we found specific expression in the testis (Figure 1C). To confirm the specificity of PDCL2 expression in the testis, we did immunoblotting experiments using W/Wv mutant mice, which have been bred to lack germ cells. PDCL2 was expressed normally in wild-type testis and purified germ cells, but disappeared in W/Wv male mice testis (Figure 1D). These results indicated that PDCL2 is a TGC-specific protein. Expression in the mouse epididymis is secondary to the continued presence of Pdcl2 mRNA in mature sperm.
Figure 1: Characterization of PDCL2 and immunostaining of PDCL2, CALMEGIN, GOPC, and IZUMO1 in testicular germ cells.
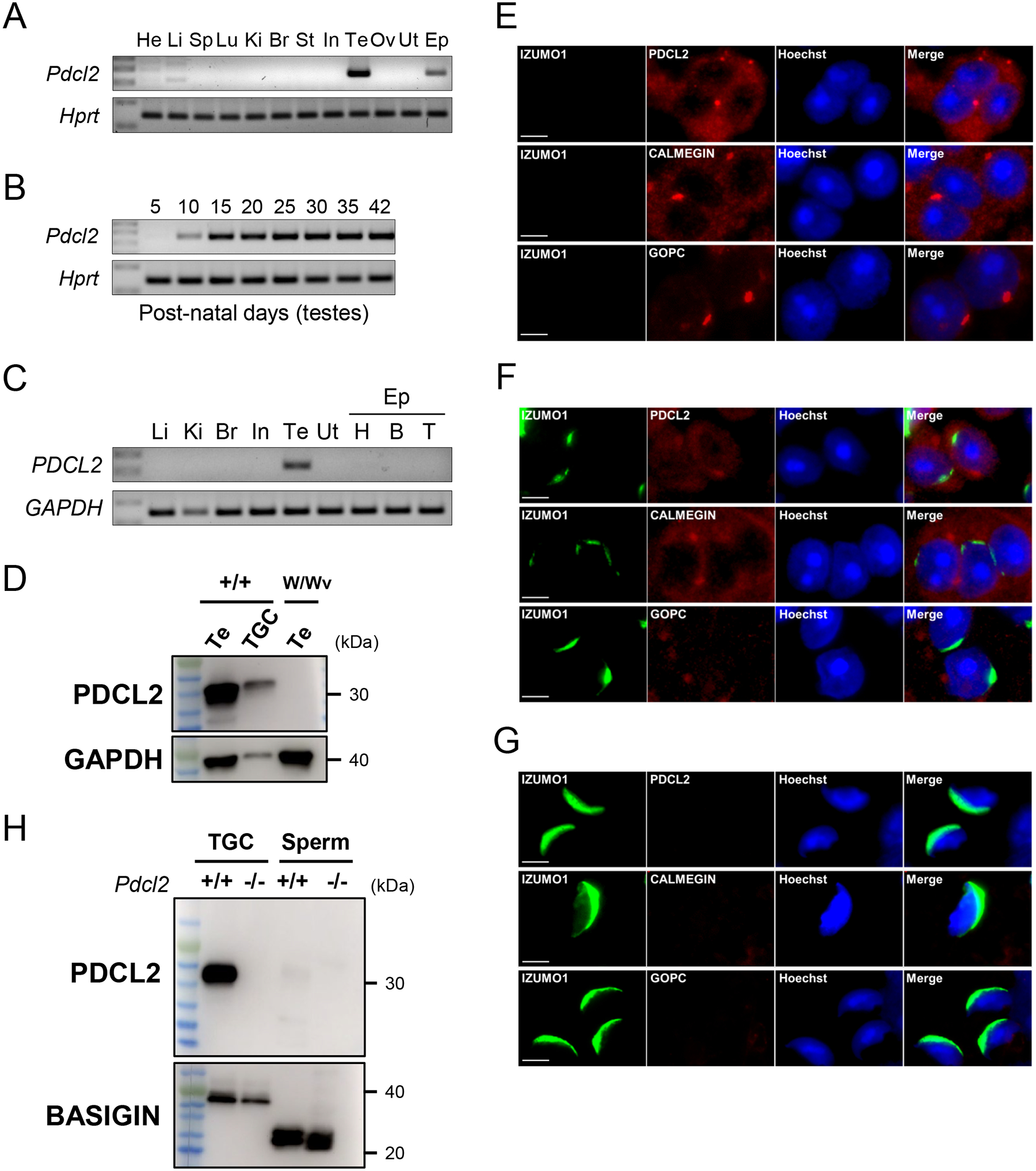
(A) The expression of mouse Pdcl2 in various organs examined by RT-PCR. Pdcl2 is testis- and epididymis-enriched. Hprt was used as an expression control. He, heart; Li, liver; Sp, spleen; Lu, lung; Ki, kidney; Br, brain; St, stomach; In, intestine; Te, testis; Ov, ovary; Ut, uterus; Ep, epididymis.
(B) The expression of mouse Pdcl2 on indicated postnatal days in the testis was examined by RT-PCR. Pdcl2 begins expression at postnatal day 10. Hprt was used as an expression control.
(C) The expression of human PDCL2 in various organs examined by RT-PCR. PDCL2 is testis-specific. GAPDH was used as an expression control. Li, liver; Ki, kidney; Br, brain; In, intestine; Ut, uterus; Ep, epididymis; H, head; B, body; T, tail.
(D) PDCL2 expression in wild-type testis (Te), purified testicular germ cells (TGC), and germ cell depleted male mice (W/Wv) was examined by immunoblot analysis. PDCL2 is not expressed in W/Wv mutant testis. GAPDH was used as an expression control.
(E) Immunostaining of PDCL2 in wild-type spermatids. Isomorphic to CALMEGIN, PDCL2 is localized in the ER. CALMEGIN is a TGC-specific marker for the ER. GOPC is a marker for the Golgi. Nuclei were stained with Hoechst 33342 (blue). Scale bars: 5 μm.
(F) Round spermatids were observed at step 5–6. Isomorphic to CALMEGIN, PDCL2 is localized in the ER. PDCL2, CALMEGIN, and GOPC signals were detected (red) at step 5–6 round spermatids. CALMEGIN is a TGC-specific marker for the ER. GOPC is a marker for the Golgi. Nuclei were stained with Hoechst 33342 (blue). Scale bars: 5 μm.
(G) Testicular spermatozoa were observed at step 15–16. PDCL2, CALMEGIN, and GOPC signals disappeared at step 15–16 spermatozoa in mouse testis. CALMEGIN is a TGC-specific marker for the ER. GOPC is a marker for the Golgi. Nuclei were stained with Hoechst 33342 (blue). Scale bars: 5 μm.
(H) Protein expression of PDCL2 in wild-type and Pdcl2−/− testicular germ cells and cauda epididymal spermatozoa. BASIGIN was used as a loading control.
To begin to understand the role of PDCL2, we performed immunostaining of PDCL2 in wild-type testis to examine its location. We used a rabbit polyclonal antibody developed for PDCL2 to stain for the protein in TGCs and found that it was localized to the ER (Figure 1E and 1F). Although PDCL2, CALMEGIN, and GOPC were expressed during acrosome biogenesis, they disappeared in spermatozoa (Figure 1G).
To elucidate the role of PDCL2 in fertility, we used the CRISPR-Cas9 system to excise a large portion of the gene between exon 2 and 6 (Figure S1B). We obtained a mutant mouse with a 12,565 bp deletion within the Pdcl2 gene. Homozygous mice (Pdcl2−/−) did not have any overt abnormalities. This deletion was confirmed via PCR using primer 1 and 2 to detect the wild-type allele and primer 1 and 3 (in the 3’ UTR of exon 6) to detect the mutant allele (Figure S1C) and by sequence analysis (Figure S1D). We predicted that 205 amino acids were deleted (Figure S1E). To confirm that the PDCL2 protein was deleted in Pdcl2−/− mice, we performed western blot analysis using the PDCL2 antibody. A strong band at ~30 kDa was present in the wild-type TGCs but disappeared in the Pdcl2−/− mutants (Figure 1H). PDCL2 was not detected in sperm because of its probable localization to the ER, as indicated by immunofluorescence (Figure 1E and 1F).
PDCL2 is required for male fertility
To test the fertility of male mice, Pdcl2−/− males and wild-type controls were housed with females for two months. While the control mice had 22 deliveries with an average of 8 pups per delivery, the four Pdcl2−/− males were sterile despite the formation of copulatory plugs (Figure 2A) [average litter size: 8.0±2.4 (wild-type males); 0 (Pdcl2−/− males)].
Figure 2: Fertilizing ability and phenotypic analysis of Pdcl2−/− male mice.
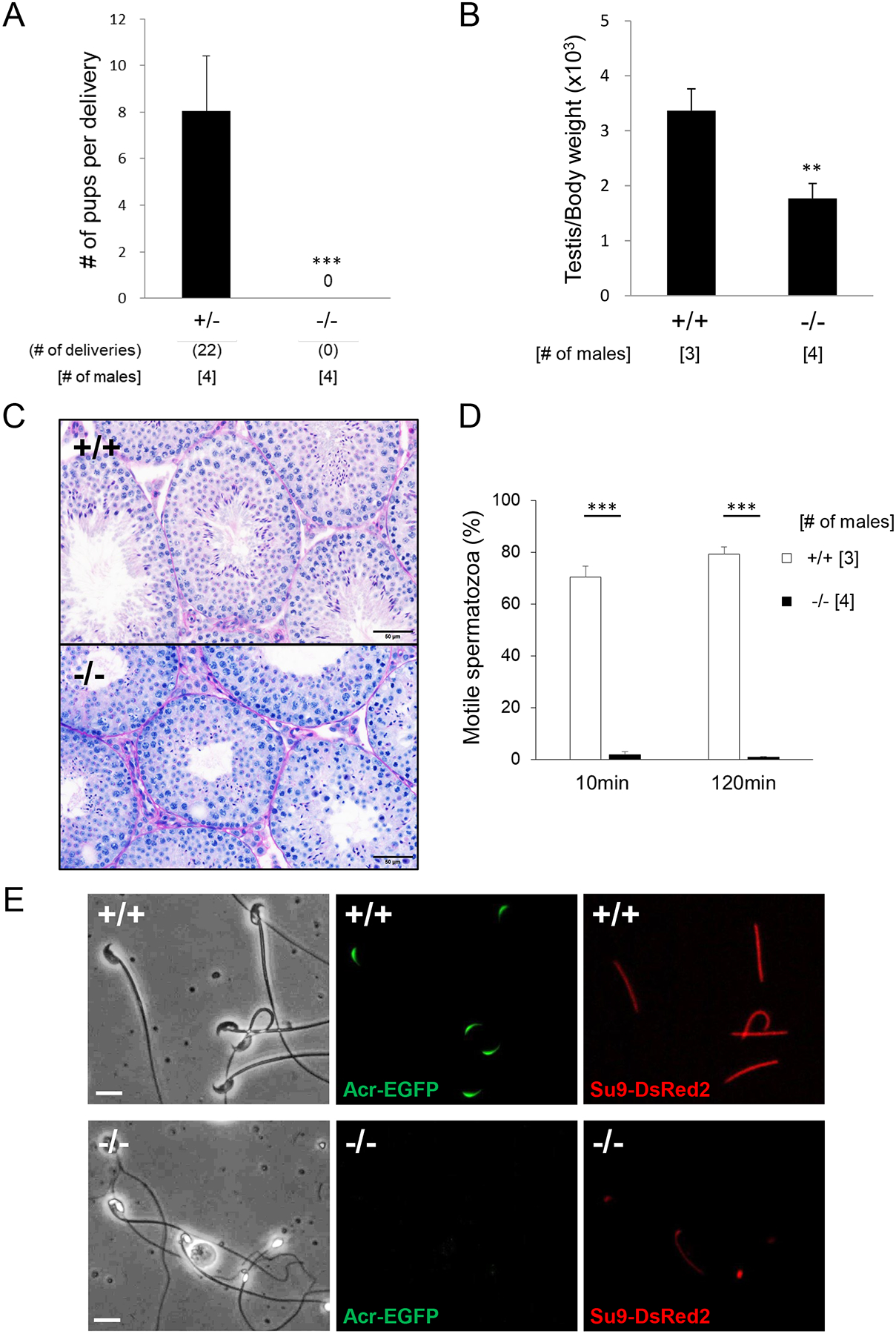
(A) Average number of pups born per delivery. N=4 males each for Pdcl2+/− and Pdcl2−/−, mated with two females per male. ***P<0.001, Student’s t-test.
(B) Testis to body weight ratio. N=3 males for wild-type and N=4 Pdcl2−/−. **P<0.01, Student’s t-test.
(C) PAS and hematoxylin staining of testicular sections from wild-type and Pdcl2−/− mice. Scale bars: 50 μm.
(D) Sperm motility results using computer-assisted sperm analysis (CASA). ***P<0.001, Student’s t-test.
(E) Observation of the acrosome and flagella using EGFP expressed under the Acrosin promoter and mitochondria-targeted DsRed2 in the principal piece in wild-type and Pdcl2−/− sperm. Scale bars: 10 μm.
To probe cause of the fertility defects of the Pdcl2−/− males, we observed the morphology of the testis and cauda epididymal spermatozoa. The testis to body weight ratio was significantly reduced in Pdcl2−/− males (Figure 2B) [3.4±0.40 (wild-type males); 1.8±0.27 (Pdcl2−/− males)]. Periodic acid-Schiff (PAS) and hematoxylin staining of testis sections showed that the lumen of the Pdcl2−/− seminiferous tubules were on average smaller than the wild-type tubules (Figure 2C). Wild-type tubules showed normal, elongated spermatids with flagella. In contrast, the mutant tubules were impaired production of spermatids compared to the control. Nuclear condensation was also impaired, as round, abnormal head shapes were observed in the tissue surrounding the lumen. Because in vivo fertility is severely impacted by lack of PDCL2, we wanted to understand if the spermatozoa had difficulties fertilizing oocytes in vitro as well. However, Pdcl2 null spermatozoa were immotile. Accordingly, we used a computer-assisted sperm analyzer (CASA) to decipher Pdcl2−/− mouse motility issues. After incubation in a capacitating medium for 10 and 120 minutes, the Pdcl2 null spermatozoa had a severe decrease in overall motility [Pdcl2 null = 1.73 ± 1.18%, wild-type = 70.43 ± 4.24% at 10 min (P<0.001); Pdcl2 null = 0.90 ± 0.14%, wild-type = 79.30 ± 2.79% at 120 min (P<0.001)] (Figure 2D). Further investigation using light microscopy confirmed the presence of globozoospermia in Pdcl2 null spermatozoa (Figure S2; 100% of the Pdcl2 null spermatozoa had abnormal head shapes (757 spermatozoa from 4 Pdcl2−/− males were counted)). To examine the integrity of the acrosome and flagellar mitochondria of spermatozoa, transgenic mice expressing both acrosome-targeted EGFP and mitochondria-targeted DsRed2 were created and used for fluorescent microscopy (Figure 2E). In wild-type sperm, the acrosome is clearly marked along the hook of the sperm head. However, severe defects in localization are seen in the Pdcl2 null spermatozoa, with no signals found. Interestingly, mitochondria signal is also impaired, as showcased using Su9-DsRed2. While control spermatozoa have clear and continuous signaling throughout the entire length of the flagellar midpiece, only a few of the Pdcl2 null sperm flagella have the same signaling pattern, suggesting that there may be an issue with flagella formation during spermatogenesis and likely secondary to defects in acrosome biogenesis.
Ultrastructural analysis of Pdcl2 null spermatozoa
Further morphological exploration via electron microscopy showed abnormalities in the acrosome, flagella, and the sperm head (Figure 3A–C). There are four phases of acrosome biogenesis, referred to as the Golgi, cap, acrosome, and maturation phases3. Transmission electron microscopy (TEM) revealed abnormal dissociation of the acrosome and the granule during cap biogenesis in the testis, around step 7 (Figure 3A). To see how this affected spermiogenesis, we created sections from the cauda epididymis to observe head and tail structure of mature spermatozoa (Figure 3B). In wild-type sperm, the nucleus is the electron dense portion and the cap at the rostral end is the acrosome. However, Pdcl2 null sperm heads have abnormal blebbing around the misshapen nucleus and there is no acrosomal cap. Furthermore, mature spermatozoa show disorganized mitochondria sequestered in cytoplasmic droplets. The Pdcl2 null sperm flagella also show deformities. Typically, wild-type spermatozoa have an axoneme consisting of a 9+2 microtubule pattern, with nine doublets forming a ring around a pair of microtubules, as well as a mitochondrial sheath in the midpiece. Pdcl2 null spermatozoa were found to have flagella with disrupted axonemes and incomplete midpieces (Figure 3B). These results suggest that these disruptions are indicative of why the spermatozoa are immotile. Scanning electron microscopy (SEM) was conducted to observe the structure of the sperm head. Instead of the normal, hook-like shape that wild-type spermatozoa form during spermiogenesis, the Pdcl2 null spermatozoa have round, granular heads, classically found in the human condition globozoospermia (Figure 3C). These results indicate that PDCL2 is required for proper sperm acrosome formation.
Figure 3: Impaired sperm acrosome formation in Pdcl2−/− male mice.
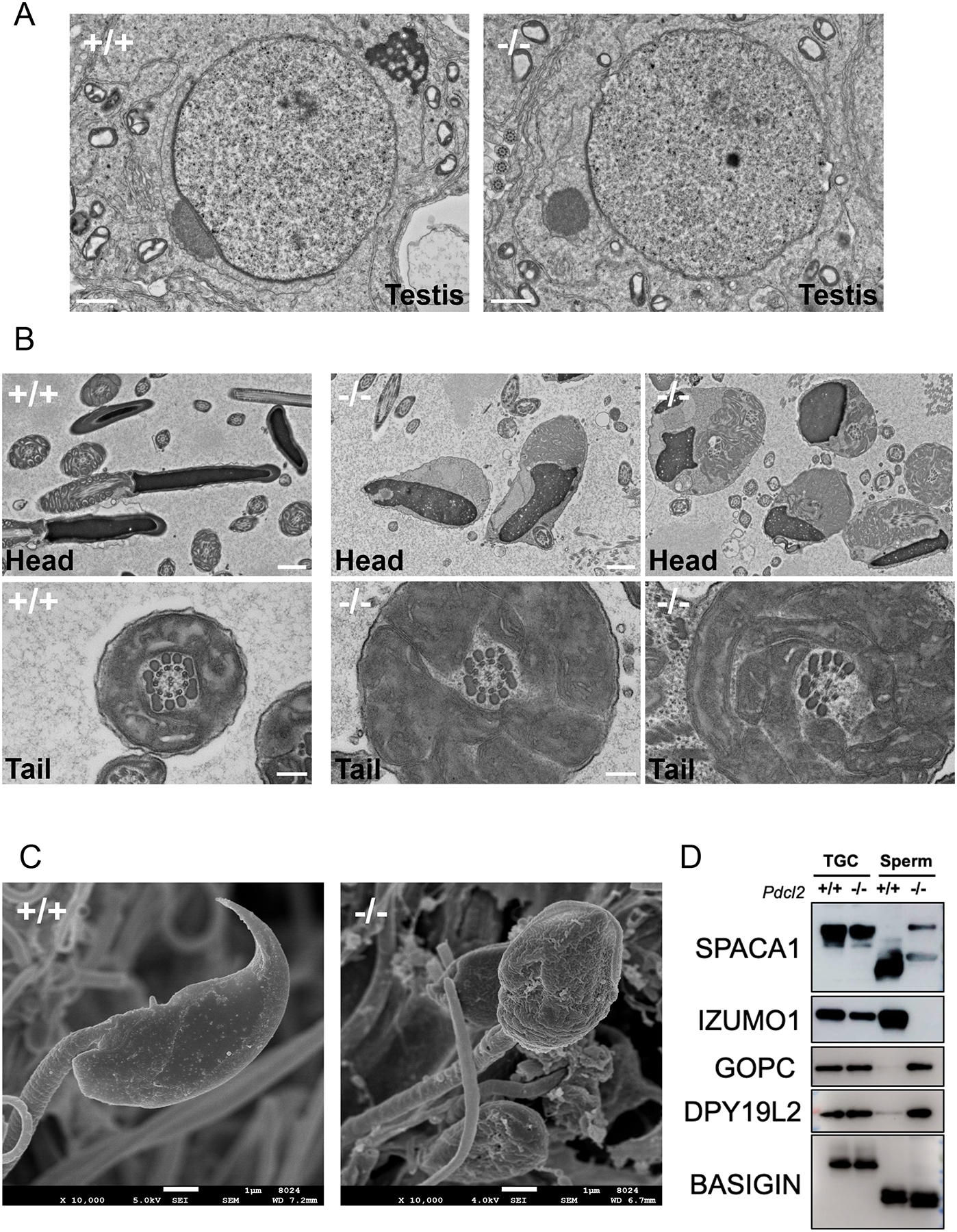
(A) TEM observation of round spermatids. Abnormal dissociation of the acrosome and the granule during cap biogenesis was observed in step 7 of round spermatid. Scale bars: 1 μm.
(B) TEM observation of mature spermatozoa in the cauda epididymis. Pdcl2 null sperm heads have misshapen nuclei and disorganized mitochondria in cytoplasmic droplets. There was no evidence of an acrosome cap. Pdcl2 null spermatozoa have disrupted axonemes and incomplete midpieces. Scale bars: 1 μm.
(C) SEM observation of mature spermatozoa extracted from the cauda epididymis. Scale bars: 1 μm.
(D) Immunoblot analysis of proteins related to acrosome biogenesis in wild-type and Pdcl2−/− testicular germ cells and cauda epididymal spermatozoa: SPACA1, IZUMO1, GOPC, DPY19L2. BASIGIN was used as a loading control.
Relationship between PDCL2 and other globozoospermia-related proteins
To evaluate why the acrosome in Pdcl2 null spermatozoa is malformed, we investigated other proteins important for acrosome formation and fertility (Figure 3D). SPACA1, a protein required for acrosome biogenesis, was found to cause globozoospermia due to the disruption of the nuclear plate when knocked out in mice6. There are two forms of SPACA1: testicular and epididymal. During spermatogenesis, the testicular form is cleaved and results in the epididymal form, as showcased in the wild-type immunoblot results. However, in Pdcl2−/− mice, the testicular form of SPACA1 in round spermatids are aberrantly retained, indicating that sperm maturation is abnormal in the Pdcl2 null spermatozoa.
GOPC, a Golgi protein localized to round spermatid and also important for acrosome formation, and DPY19L2, a protein implicated in globozoospermia and localized to the acrosomal inner nuclear envelope, are retained in Pdcl2 null spermatozoa10,12,21. Similar to SPACA1, both GOPC and DPY19L2 are shed after spermatogenesis, but the Pdcl2 null spermatozoa continue to express the two proteins, indicative of aberrant retention as well.
Further investigation of the interaction of PDCL2 and other acrosome proteins showed similar issues. IZUMO1, a protein required for sperm-egg fusion and found throughout the acrosome membrane, is depleted in Pdcl2 null spermatozoa (Figure 3D)22,23. Thus, while IZUMO1 is produced normally, it is not sequestered into mature spermatozoa because the acrosome is not localized to the sperm head.
DISCUSSION
We have presented that Pdcl2 is an evolutionarily conserved, male germ cell-enriched gene encoding a phosducin domain. Pdcl2 RNA expression began at postnatal day 10, which is when only Sertoli cells and spermatogonia are present in the seminiferous tubules24. Mouse PDCL2 shares homology with other phosducin proteins, which are expressed in various tissues, but only PDCL2 is expressed in the testicular germ cells.
To determine the function of PDCL2, we created Pdcl2 mutant mice using the CRISPR-Cas9 system. We generated a large gene deletion of 12,565 base pairs, resulting in a nonfunctional protein. When we examined the effect of excising Pdcl2 in spermatogenesis, we found severe defects in spermatogenesis. Electron microscopy showed dramatic abnormalities in acrosome formation and proper sperm head and tail development (e.g., acrosome loss and mid-piece size expansion, etc.). Breeding studies confirmed that homozygous null males are sterile, mainly due to immotile spermatozoa.
In PDCL2-mutant testis and spermatozoa, abnormal SPACA1, GOPC, and DPY19L2 bands were observed by immunoblot analysis (Figure 3D). If PDCL2 functions at an earlier step in acrosome formation, abnormal processing of downstream proteins might be a secondary effect due to abnormal acrosome formation. For example, SPACA1 is known to have a testicular form that is then cleaved into an epididymal form6. However, Pdcl2−/− mice had both the SPACA1 testicular and epididymal form present, indicating that acrosome formation is impaired. Furthermore, PDCL2 depletion may cause a functional abnormality in the Golgi apparatus, as showcased by the aberrant retention of GOPC in spermatozoa, further indicating that PDCL2 activity is upstream to GOPC12.
To examine the mechanism of action, we found that PDCL2 was localized to the endoplasmic reticulum. Because of this, there may be a relationship between PDCL2 and GBA2, a ubiquitously expressed resident enzyme in the ER13. Gba2−/− mice have a similar phenotype: round headed sperm, abnormal acrosomes, and defective motility. Compared to other known proteins associated with globozoospermia, Pdcl2 null spermatozoa had an exceptionally drastic phenotype. While Spaca1 KO mice exhibited malformed sperm heads, their flagella were normal. This has been attributed to the fact that SPACA1 is located in the inner acrosomal membrane and is considered as a late stage protein. GOPC, an earlier stage protein localized to the Golgi, also has globozoospermia and impaired motility, but studies showed a 7% motility rate in contrast with PDCL2’s 1% motility rate. In contrast, Gba2−/− mice had a 33% motility rate, thus PDCL2 may be more upstream or influencing more proteins required for acrosome biogenesis compared to GBA2.
CONCLUSIONS
In this study, we found that PDCL2 is essential for male fertility and acrosome formation. As a highly conserved gene, Pdcl2 may be linked to globozoospermia in humans. Further analysis of its function and its relationship with GBA2 and other acrosomal proteins may help us further understand the mechanisms behind acrosome formation and globozoospermia in humans.
Supplementary Material
ACKNOWLEDGMENTS
We thank Naoko Nagasawa, Saori Haga, Yoshie Hamaguchi, and Yukiko Ohno for technical assistance, and Julio M. Castaneda for critical reading of this mansucript. This work was supported by Ministry of Education, Culture, Sports, Science and Technology (MEXT)/Japan Society for the Promotion of Science (JSPS) KAKENHI grants (JP20KK0155, JP21K19198, and JP21H02397 to Y.F., JP19H05750 and JP21H05033 to M.I.); Japan Agency for Medical Research and Development (AMED) grant JP18gm5010001 to M.I.; Takeda Science Foundation grants to Y.F. and M.I.; the Senri Life Science Foundation grant to Y.F.; the Mochida Memorial Foundation for Medical and Pharmaceutical Research grant to Y.F.; the Sumitomo Foundation Grant for Basic Science Research Projects to Y.F.; Intramural Research Fund (21-2-6, 22-A-3, 30-2-5, and 31-6-3) for Cardiovascular Diseases of National Cerebral and Cardiovascular Center to Y.F.; the Eunice Kennedy Shriver National Institute of Child Health and Human Development grants (R01HD088412 and P01HD087157 to M.M.M. and M.I.); and the Bill & Melinda Gates Foundation grant (INV-001902 to M.M.M. and M.I.). The Human Tissue Acquisition & Pathology Core is funded through the P30 Cancer Center Support Grant (NCI CA125123).
Footnotes
CONFLICT of INTEREST STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Agarwal A, Mulgund A, Hamada A, Chyatte MR. A unique view on male infertility around the globe [Research Support, Non-U.S. Gov’t]. Reprod Biol Endocrinol. Apr 26 2015;13:37. 10.1186/s12958-015-0032-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dam AH, Feenstra I, Westphal JR, Ramos L, van Golde RJ, Kremer JA. Globozoospermia revisited [Review]. Hum Reprod Update. Jan-Feb 2007;13(1):63–75. 10.1093/humupd/dml047. [DOI] [PubMed] [Google Scholar]
- 3.Khawar MB, Gao H, Li W. Mechanism of Acrosome Biogenesis in Mammals [Review]. Front Cell Dev Biol. 2019;7:195. 10.3389/fcell.2019.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hess RA, Renato de Franca L. Spermatogenesis and cycle of the seminiferous epithelium [Review]. Adv Exp Med Biol. 2008;636:1–15. 10.1007/978-0-387-09597-4_1. [DOI] [PubMed] [Google Scholar]
- 5.Castaneda JM, Shimada K, Satouh Y, et al. FAM209 associates with DPY19L2, and is required for sperm acrosome biogenesis and fertility in mice [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t]. J Cell Sci. Nov 1 2021;134(21). 10.1242/jcs.259206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fujihara Y, Satouh Y, Inoue N, Isotani A, Ikawa M, Okabe M. SPACA1-deficient male mice are infertile with abnormally shaped sperm heads reminiscent of globozoospermia [Research Support, Non-U.S. Gov’t]. Development. Oct 2012;139(19):3583–9. 10.1242/dev.081778. [DOI] [PubMed] [Google Scholar]
- 7.Lin YN, Roy A, Yan W, Burns KH, Matzuk MM. Loss of zona pellucida binding proteins in the acrosomal matrix disrupts acrosome biogenesis and sperm morphogenesis [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t]. Mol Cell Biol. Oct 2007;27(19):6794–805. 10.1128/MCB.01029-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morohoshi A, Miyata H, Oyama Y, Oura S, Noda T, Ikawa M. FAM71F1 binds to RAB2A and RAB2B and is essential for acrosome formation and male fertility in mice [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t]. Development. Nov 1 2021;148(21). 10.1242/dev.199644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nozawa K, Zhang Q, Miyata H, et al. Knockout of serine-rich single-pass membrane protein 1 (Ssmem1) causes globozoospermia and sterility in male micedagger. Biol Reprod. Aug 4 2020;103(2):244–253. 10.1093/biolre/ioaa040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pierre V, Martinez G, Coutton C, et al. Absence of Dpy19l2, a new inner nuclear membrane protein, causes globozoospermia in mice by preventing the anchoring of the acrosome to the nucleus [Research Support, Non-U.S. Gov’t]. Development. Aug 2012;139(16):2955–65. 10.1242/dev.077982. [DOI] [PubMed] [Google Scholar]
- 11.Xiao N, Kam C, Shen C, et al. PICK1 deficiency causes male infertility in mice by disrupting acrosome formation [Research Support, Non-U.S. Gov’t]. J Clin Invest. Apr 2009;119(4):802–12. 10.1172/JCI36230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yao R, Ito C, Natsume Y, et al. Lack of acrosome formation in mice lacking a Golgi protein, GOPC [Research Support, Non-U.S. Gov’t]. Proc Natl Acad Sci U S A. Aug 20 2002;99(17):11211–6. 10.1073/pnas.162027899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yildiz Y, Matern H, Thompson B, et al. Mutation of beta-glucosidase 2 causes glycolipid storage disease and impaired male fertility [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t]. J Clin Invest. Nov 2006;116(11):2985–94. 10.1172/JCI29224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lou X, Bao R, Zhou CZ, Chen Y. Structure of the thioredoxin-fold domain of human phosducin-like protein 2 [Comparative Study Research Support, Non-U.S. Gov’t]. Acta Crystallogr Sect F Struct Biol Cryst Commun. Feb 1 2009;65(Pt 2):67–70. 10.1107/S1744309108037342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hasuwa H, Muro Y, Ikawa M, Kato N, Tsujimoto Y, Okabe M. Transgenic mouse sperm that have green acrosome and red mitochondria allow visualization of sperm and their acrosome reaction in vivo. Exp Anim. 2010;59(1):105–7. 10.1538/expanim.59.105. [DOI] [PubMed] [Google Scholar]
- 16.Ikawa M, Nakanishi T, Yamada S, et al. Calmegin is required for fertilin alpha/beta heterodimerization and sperm fertility [Research Support, Non-U.S. Gov’t]. Dev Biol. Dec 1 2001;240(1):254–61. 10.1006/dbio.2001.0462. [DOI] [PubMed] [Google Scholar]
- 17.Ikawa M, Tokuhiro K, Yamaguchi R, et al. Calsperin is a testis-specific chaperone required for sperm fertility [Research Support, Non-U.S. Gov’t]. J Biol Chem. Feb 18 2011;286(7):5639–46. 10.1074/jbc.M110.140152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujihara Y, Oji A, Larasati T, Kojima-Kita K, Ikawa M. Human Globozoospermia-Related Gene Spata16 Is Required for Sperm Formation Revealed by CRISPR/Cas9-Mediated Mouse Models. Int J Mol Sci. Oct 21 2017;18(10). 10.3390/ijms18102208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kotaja N, Kimmins S, Brancorsini S, et al. Preparation, isolation and characterization of stage-specific spermatogenic cells for cellular and molecular analysis [Research Support, Non-U.S. Gov’t]. Nat Methods. Dec 2004;1(3):249–54. 10.1038/nmeth1204-249. [DOI] [PubMed] [Google Scholar]
- 20.Miyata H, Satouh Y, Mashiko D, et al. Sperm calcineurin inhibition prevents mouse fertility with implications for male contraceptive [Research Support, Non-U.S. Gov’t]. Science. Oct 23 2015;350(6259):442–5. 10.1126/science.aad0836. [DOI] [PubMed] [Google Scholar]
- 21.Koscinski I, Elinati E, Fossard C, et al. DPY19L2 deletion as a major cause of globozoospermia [Research Support, Non-U.S. Gov’t]. Am J Hum Genet. Mar 11 2011;88(3):344–50. 10.1016/j.ajhg.2011.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inoue N, Ikawa M, Isotani A, Okabe M. The immunoglobulin superfamily protein Izumo is required for sperm to fuse with eggs [Research Support, Non-U.S. Gov’t]. Nature. Mar 10 2005;434(7030):234–8. 10.1038/nature03362. [DOI] [PubMed] [Google Scholar]
- 23.Satouh Y, Inoue N, Ikawa M, Okabe M. Visualization of the moment of mouse sperm-egg fusion and dynamic localization of IZUMO1 [Research Support, Non-U.S. Gov’t]. J Cell Sci. Nov 1 2012;125(Pt 21):4985–90. 10.1242/jcs.100867. [DOI] [PubMed] [Google Scholar]
- 24.Montoto LG, Arregui L, Sanchez NM, Gomendio M, Roldan ER. Postnatal testicular development in mouse species with different levels of sperm competition [Comparative Study Research Support, Non-U.S. Gov’t]. Reproduction. Mar 2012;143(3):333–46. 10.1530/REP-11-0245. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


