Abstract
Cytoskeletal proteins are associated with actin in the microfilaments and have a major role in microfilament assembly and function. The expression of some of these proteins has been implicated in cell growth and transformation. Specifically, the 3′-untranslated regions (3′-UTRs) of tropomyosin, troponin and cardiac actin can induce muscle cell differentiation and appear to function as tumor suppressors. These RNA sequences are predicted to fold to form secondary structures with extended stretches of duplex. We show that the 3′-UTRs of the cytoskeletal mRNAs interact with the RNA-binding domain of the RNA-activated protein kinase PKR. Correspondingly, these RNAs activate PKR in vitro and inhibit globin translation in the rabbit reticulocyte lysate translation system. These data are consistent with a mechanism whereby PKR mediates the differentiation- and tumor-related actions of the cytoskeletal 3′-UTR sequences.
INTRODUCTION
Cell transformation is associated with cytoskeletal reorganization resulting from alterations in the expression of cytoskeletal protein components such as α-actinin, vinculin and α-tropomyosin (TM) (1). These proteins are associated with actin in microfilaments and have a major role in microfilament assembly and function. The levels of these structural proteins are decreased in transformed cells relative to normal cells (2–9). Suppression of α-actinin and TM synthesis induces the transformed phenotype in fibroblasts indicating that these cytoskeletal proteins play a tumor suppressor role (1). Several lines of evidence corroborate this notion. Loss of expression of TM isoforms is seen in human breast cancer cells (4,6). In differentiating muscle cells, cytoskeletal proteins such as TM, troponin (TP) and α-cardiac actin (AC) are expressed at high levels (10). Complementation studies have demonstrated that the 3′-untranslated regions (3′-UTRs) of the mRNAs encoding these proteins can induce differentiation in a differentiation-incompetent murine muscle cell line (10). In addition, stable expression of the TM 3′-UTR sequence alone blocked the ability of these cells to form tumors in nude mice, indicating that it can function as a tumor suppressor (11). Similar results were obtained in non-muscle cells. The TM 3′-UTR sequence also reversed the transformed phenotype of v-raf-transformed rat kidney cells (12,13).
The 3′-UTRs of TM, TP and AC are predicted to fold into stable secondary structures (Fig. 1). The RNA-dependent protein kinase PKR is activated by double-stranded (ds) and highly structured RNAs (14) and has been proposed to act as a tumor suppressor (15–17). Originally characterized as an inhibitor of protein synthesis that is important in the interferon-mediated antiviral response, a growing body of evidence indicates that PKR also plays a role in the regulation of cellular processes such as cell death, growth arrest, differentiation, proliferation and signal transduction. Consequently, it was suggested that the differentiation-inducing and tumor-suppressing activities of the cytoskeletal mRNA 3′-UTR sequences might operate through the activation of PKR (18).
Figure 1.
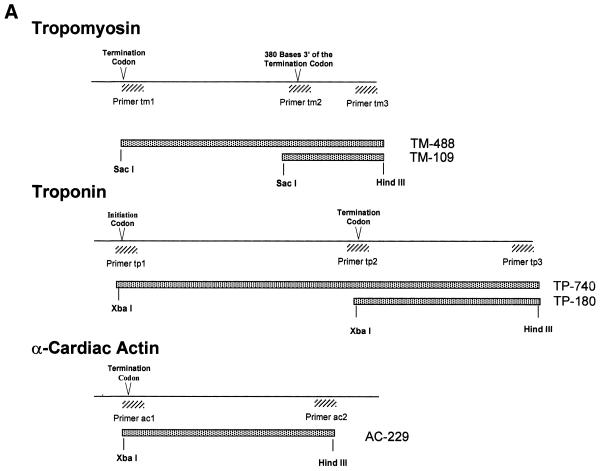
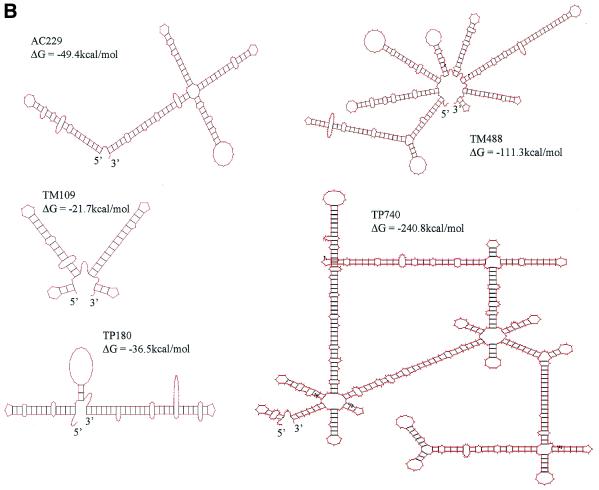
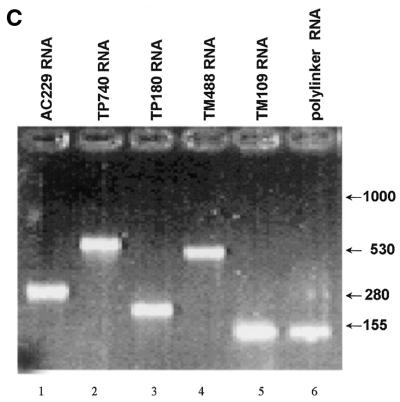
(Opposite and above) Cytoskeletal RNAs. (A) Schematic representation of portions of the TM, TP and AC genes. Transcripts generated in this study are represented by stippled boxes. Hatched boxes mark the PCR primers used in amplifying the sequences from a cDNA library. (B) Secondary structures of the transcripts predicted by MFold (GCG) web-based sequence analysis program. (C) Integrity of the TM, TP, AC and polylinker RNAs. The RNAs were synthesized in vitro using T7 RNA polymerase, purified, and 200 ng of each was loaded onto a 1% agarose gel. Positions of RNA markers (Gibco BRL) are indicated.
PKR is activated by low concentrations of perfectly duplexed dsRNA in a process that entails intermolecular autophosphorylation. Activation of PKR by dsRNA is sequence independent but dependent on the length of the RNA duplex. At least 30 bp are minimally required and the efficiency of activation increases up to ~85 bp (19–22). Imperfect duplexes are able to activate PKR as long as they have sufficient ds character, but short duplexes of 11 bp or less generally inhibit PKR activation. It is thought that two PKR molecules must be able to bind to the RNA at the same time for activation to take place, whereas the binding of only one PKR molecule to a small RNA inhibits the autophosphorylation event (23 and references therein). Once activated, PKR phosphorylates its substrates, including the translation initiation factor eIF2 as well as several proteins in the transcriptional pathway such as NF90 (24–26), RNA helicase A (26) and HIV-1 Tat (27,28). The best-characterized effect of PKR activation is phosphorylation of eIF2α, which results in the sequestration of another initiation factor, eIF2B, thus leading to inhibition of protein synthesis (14).
In pursuit of the hypothesis that the cytoskeletal mRNA 3′-UTRs might act via PKR, Davis and Watson (18) studied the effect of the TM 3′-UTR on PKR in vitro. They found that this RNA binds to and activates PKR, and consequently inhibits translation in vitro. We have extended their study by examining the activities of full-length and truncated 3′-UTR sequences of the three genes TM, TP and AC, which were shown to have tumor suppressor activity in murine muscle cells. The results showed that all of the 3′-UTRs bound to PKR with similar efficiency, although they activated it to varying degrees. At high concentrations, they inhibited PKR, which is reminiscent of the bell-shaped activation curve given by dsRNA. We also tested the effect of these 3′-UTR RNAs on cell-free translation. As expected, the RNAs that activated PKR also inhibited translation in a concentration-dependent manner. These results suggest that the function of these 3′-UTR sequences in differentiation and tumor suppression may involve the activation of PKR.
MATERIALS AND METHODS
Plasmids
The 3′-UTR RNA-expressing plasmids for in vitro RNA synthesis were generously provided by Dr Julia Watson (RiboGene, Inc.). Construction of TM UTR expression plasmid has been described previously (18). The 3′-UTR sequences from TM, TP and AC were amplified using PCR from a human skeletal muscle cDNA library in λgt10 (Clontech) using appropriate primers. PCR products were cloned into the multiple-cloning region of Bluescript II KS plasmid (Fig. 1). TM488 and TM109 contain nucleotides 923–1410 and 1302–1440, respectively, from the human TM gene (GenBank accession no. AL050179). TP740 contains the entire coding region and the 3′-UTR region of human slow-twitch skeletal troponin I (TNN1) cDNA (29), while TP180 contains a region between nucleotides 637 and 816. AC277 contains the 3′ flanking region (374–602 bp) of human AC (30,31). In the plasmids TP740, TP180 and AC229, the PCR products were cloned between HindIII and SacI sites in the multiple-cloning region in Bluescript II KS plasmid, while TM515 and TM133 were cloned into HindIII and XbaI sites. Therefore the TP784, TP233 and AC277 RNA products have 28 extra bases at the 3′ end that the TM488 and TM109 transcripts do not possess.
In vitro synthesis and purification of RNA
Expression vectors were cut with HindIII in preparation for run-off transcription. Radiolabeled RNAs used in the gel shift assays were synthesized using T7 RNA polymerase as described (32). Unlabeled RNAs for kinase and translation reactions were synthesized in the presence of only a small amount of [α-32P]UTP so as to be able to track the RNA. To eliminate ds contaminants, the RNAs were double gel purified (32). pT7VA (33) was used for the production of VAI RNA.
Gel mobility shift assays
Uniformly labeled RNA (∼20 000 c.p.m.) was incubated with varying concentration of p20 protein [purified as described previously (34) in gel shift buffer (15 mM HEPES pH 7.4; 5 mM MgCl2; 1 mM DTT; 1 µg/ml of each of the protease inhibitors pepstatin A, leupeptin, and aprotinin; 10 µM PMSF; 10 µg/ml calf liver tRNA; 1 U RNasin) in a final volume of 10 µl on ice. After 20 min, 4 µl 50% glycerol containing xylene cyanol, and bromphenol blue was added and samples were loaded onto a 5% non-denaturing polyacrylamide gel and run at 150 V.
PKR activation assay
PKR activation potential of RNAs was tested in PKR kinase reactions performed as described previously (35) in a final volume of 10 µl containing 0.1 mM ATP, 15 mM HEPES-KOH pH 7.4, 5 mM MgCl2, 1 µg/ml pepstatin A, 1 µg/ml leupeptin, 1 µg/ml aprotinin, 1 mM DTT and 2 µM PMSF, 2.5 µCi [γ-32P]ATP (ICN Biochemicals), 0.5 µl of purified PKR [purified to the Mono S stage (36), ∼5 ng], with or without the test RNA. Reovirus dsRNA was used as a positive control. Reactions were incubated at 30°C for 20 min and analyzed by SDS–10% PAGE and visualized by autoradiography.
In vitro translation assays
Translation inhibition reactions were carried out using the PROMEGA Flexi rabbit reticulocyte lysate reagents as per the manufacturer’s protocol, with the following modifications. Purified test RNAs were preincubated with 7 µl of reticulocyte lysate at 30°C for 15 min. The preincubated reticulocyte lysate was then tested for globin mRNA translation in a final reaction volume of 12.5 µl containing 20 µM amino acids (minus methionine), 1 mM Mg acetate, 0.1 mM KCl, 1.9 mM DTT, 6 U RNasin, 0.5 µl 35S-Translabel and 4 µg/ml globin mRNA. Translation reaction was incubated at 30°C for 30 min and then a 5 µl aliquot was analyzed by SDS–PAGE and autoradiography.
RESULTS
Work from Blau’s laboratory showed that the 3′-UTR sequences of the mRNAs encoding the muscle-specific proteins TM, TP and AC exert tumor suppressor activity in muscle cells (11). Consistent with the suggestion that the tumor suppressor activity results from the activation of PKR, the 3′-UTR of TM mRNA interacted with and activated PKR in vitro (18). To assess the generality of this finding we have extended the investigation to the 3′-UTRs of the cytoskeletal genes TP and AC.
PKR binds to 3′-UTR sequences of cytoskeletal mRNAs
The 3′-UTRs of TM, TP and AC are 488, 180 and 229 nt long, respectively (Fig. 1A). Using appropriate primers, DNA sequences including the 3′-UTR (stippled bars in Fig. 1A) were amplified from a human muscle cDNA library and cloned into a plasmid vector so as to enable the synthesis of the 3′-UTRs of TM, TP and AC mRNAs (TM488, TP180 and AC229, respectively) from the T7 promoter. In addition, constructs were generated to produce the 3′ terminal 109 nt of TM mRNA (TM109) and nearly full-length TP mRNA (TP740, containing the coding sequence as well as the 5′- and 3′-UTR). RNAs were synthesized using T7 RNA polymerase and extensively purified to remove possible dsRNA contaminants (37). The integrity of the five RNA species was verified by gel electrophoresis (Fig. 1C).
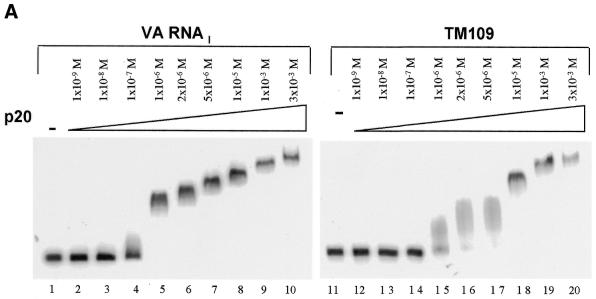
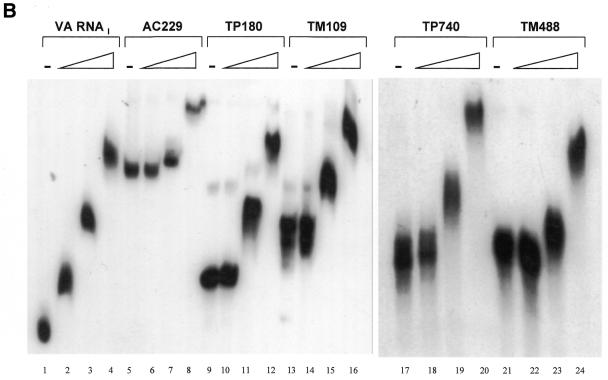
We first examined the ability of the RNAs to bind to p20, the N-terminal domain of PKR that contains its two dsRNA-binding motifs (dsRBMs), in an electrophoretic mobility shift assay. Radiolabeled TM109 RNA and adenovirus VA RNAI, which is known to interact with p20 (34), were incubated with increasing concentrations of p20 (1 × 10–9 to 3 × 10–3 M) and analyzed by polyacrylamide gel electrophoresis under native conditions (Fig. 2A). VA RNAI began to shift at ~1 × 10–7 M (lane 4) and gave a clear shift at 1 × 10–6 M p20 (lanes 5–10). Increasing the concentration of p20 above 1 × 10–6 M progressively shifted the RNA higher up in the gel. A similar laddering effect has been observed before and attributed to a concentration-dependent protein oligomerization that is independent of RNA concentration (34). TM109 RNA began to shift at 1 × 10–6 M p20 (lane 15) and gave a sharp band at 1 × 10–5 M (lane 18) with a laddering effect at higher concentrations. Thus, TM109 binds p20 but with an affinity ∼10-fold less than that for VA RNAI.
Figure 2.
Electrophoretic mobility shift assays with cytoskeletal RNAs. (A) Comparison of the binding of p20, at the molarities shown, to adenovirus VA RNAI (lanes 1–10) or TM109 (lanes 11–20). (B) Assays of p20 binding to the cytoskeletal RNAs AC229 (lanes 5–8), TP180 (lanes 9–12), TM109 (lanes 13–16), TP740 (lanes 17–20) or TM488 (lanes 21–24). VA RNAI was used as a control (lanes 1–4). Ascending p20 concentrations were 0, 1 × 10–6, 1 × 10–5 and 1 × 10–4 M.
Next we performed gel mobility shift assays with the five cytoskeletal RNAs over a narrower p20 concentration range (1.3 × 10–6 to 1.3 × 10–4 M). Figure 2B shows that p20 bound to all of these RNAs with similar efficiencies. They showed mobility shift only at the two higher concentrations tested (1.3 × 10–5 M and 1.3 × 10–4 M), indicating that they all bind p20 ∼10-fold less efficiently than VA RNAI regardless of their sequence or length. The laddering effect was also observed with all the cytoskeletal RNAs.
Cytoskeletal RNAs activate PKR
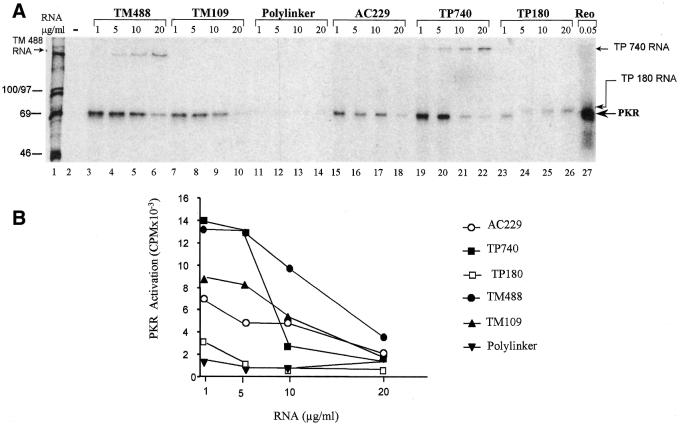
Highly structured RNAs are known that can either activate PKR (e.g. extended CUG repeats) or prevent its activation (e.g. VA RNAI). To assess the effect of the cytoskeletal mRNA 3′-UTRs on PKR’s enzyme activity we conducted in vitro kinase assays. The RNAs were incubated with purified PKR in the presence of [γ-32P]ATP and analyzed by SDS–PAGE followed by autoradiography (Fig. 3). Reovirus dsRNA, used as a positive control, readily activated PKR (lane 27). In contrast, a plasmid polylinker sequence (151 nt), which was transcribed and purified in the same way as the cytoskeletal RNAs as a negative control, failed to activate PKR (lanes 10–13).
Figure 3.
Cytoskeletal RNAs activate PKR. Purified PKR was incubated with [γ-32P]ATP and the indicated concentrations of cytoskeletal RNAs, then analyzed by gel electrophoresis and autoradiography. (A) Reactions lacking RNA (lane 2), and RNA transcribed from a plasmid polylinker (lanes 11–14) were negative controls. Reovirus dsRNA was a positive control (lane 27). Positions of autophosphorylated PKR, radiolabeled RNAs and molecular weight standards (lane 1) are indicated. (B) Quantitation of PKR phosphorylation data from (A).
The 3′-UTR RNAs were tested at four different concentrations (1, 5, 10 and 20 µg/ml). They were mildly radioactive and are therefore visible in some lanes, together with the autophosphorylated PKR. The 3′-UTR RNAs all activated PKR to varying extents (quantified in Fig. 3B). TM109 (lanes 7–10), which represents the 109 nt from the extreme 3′-terminal portion of the TM 3′-UTR, activated PKR ∼60% as well as the full-length TM 3′-UTR (TM488; lanes 3–6). This suggests that the 3′-terminal part of the UTR contains the majority of the sequence necessary for PKR activation by the TM mRNA. The full-length TP mRNA (TP740; lanes 19–22) also activated PKR efficiently. However, TP180 (lanes 23–26), which contains the entire 3′-UTR sequence of the TP mRNA, activated PKR only 15% as efficiently as the full-length form, suggesting that the PKR activating property is unlikely to reside in the 3′-UTR of this mRNA. (Note that the band visible in lanes 24–26 is labeled TP180 RNA, running slightly slower than the activated PKR band visible in the adjacent lanes 23 and 27.) The 3′-UTR of AC mRNA (AC229; lanes 15–18) was ∼45% as efficient in PKR activation as the full-length TP mRNA or 3′-UTR of TM mRNA.
Maximum activation with all the cytoskeletal RNAs was achieved at 1–5 µg/ml of RNA. Activation fell off sharply at lower concentrations (data not shown) and declined gradually at higher concentrations (Fig. 3B) to give a bell-shaped curve. In this regard the response of PKR to the cytoskeletal RNAs resembles that of authentic dsRNA. However, the bell-shaped curve seen with the cytoskeletal RNAs is shifted relative to that with reovirus dsRNA, which reaches a maximum at 0.1 µg/ml and decreases to zero at 1 µg/ml.
Inhibition of translation in rabbit reticulocyte lysate
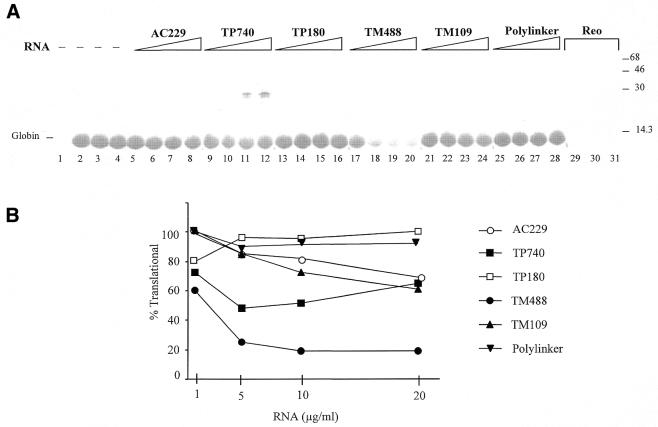
Agents that activate PKR generally inhibit protein synthesis, although hepatitis delta virus (HDV) RNA is an exception to this rule. HDV RNA activates purified PKR in vitro but fails to block translation in the rabbit reticulocyte lysate (38). To determine whether the cytoskeletal RNAs function as translational inhibitors in this cell-free system, given their ability to activate PKR, the reticulocyte lysate was first preincubated with the RNAs so as to facilitate their interaction with PKR and then used for translation of globin mRNA (Fig. 4A). As expected, the polylinker RNA that did not activate PKR had no effect on translation (lanes 25–28), while reovirus dsRNA reduced translation by ∼91% at 50 ng/ml (lanes 29–31).
Figure 4.
Inhibition of translation by cytoskeletal RNAs. (A) Translation assays were performed by preincubating rabbit reticulocyte lysate with no RNA (lanes 2–4), with 50 ng/ml reovirus dsRNA (lanes 29–31), or with 1, 5, 10 or 20 µg/ml of AC229 (lanes 5–8), TP740 (lanes 9–12), TP180 (lanes 13–16), TM488 (lanes 17–20), TM109 (lanes 21–24) or polylinker RNA (lanes 25–28). Globin mRNA and [35S]methionine were then added (except for lane 1, from which globin mRNA was omitted) and incubation continued. Reactions were analyzed by gel electrophoresis and autoradiography. The positions of the globin and TP translation products and of molecular weight standards are indicated. (B) Quantitation of globin synthesis normalized to control reactions (lanes 2–4).
All of the cytoskeletal RNAs inhibited translation to some extent. As in the PKR activation assay, these RNAs were less effective than reovirus dsRNA both in terms of the degree of inhibition and the concentrations of 3′-UTR RNAs required (Fig. 4B). The full-length RNAs, TM488 and TP740, were more efficient at inhibiting translation than their truncated versions, TM109 and TP180, correlating with their PKR activation capability. TM488 gave a maximum inhibition of ∼80%, while TP740 reached 51%. It should be noted that TP740 contains the entire TP open reading frame and accordingly generated a product of 30 kDa. As in the kinase assay, TM109 retained a substantial fraction of the inhibitory activity seen with TM488 (peaking at 38%), while TP180 elicited a very modest inhibition of translation, which was seen only at the lowest concentration tested in the two assays. AC229 3′-UTR RNA inhibited translation to an intermediate degree, maximally ∼30%. Thus the translational effects largely mirror the results of kinase assays.
DISCUSSION
We find that the 3′-UTRs of three cytoskeletal protein mRNAs, encoding TM, TP and AC, are able to bind and activate PKR. These findings are in keeping with the hypothesis that such sequences play regulatory roles in vivo (10,11) and with previous results with the TM 3′-UTR in vitro (18). Our data (Fig. 2) indicate that PKR binds in vitro to all of these cytoskeletal muscle 3′-UTRs with an affinity that is about 10 times less than that of VA RNAI (3.5 × 10–7) (34), i.e. their Kd is ∼3.5 × 10–6 M. In contrast, Davis and Watson (18) reported that TM 3′-UTR bound to PKR 12 times better than VA RNAI by co-immunoprecipitation using PKR antibody-bound protein A–Sepharose. This difference might imply that the intact protein is more efficient than the isolated dsRBMs in binding TM488 although the discrepancy could also be due to differences between the assays used.
Even though all of the RNAs bind PKR, they activate PKR with varying efficiencies (Fig. 3), which appear to reflect their double-stranded character. Although direct examination is needed to elucidate the nature, number and length of the duplexed regions that are presented to PKR, the predicted secondary structures of the RNAs (Fig. 1B) correlate with their relative activities. Thus, the species with the most stable and longest uninterrupted stretches of duplex (TM488 and TP740) are the best activators of PKR; species in which the duplexes have more extensive and frequent interruptions are weaker activators (AC229 and TM109); and TP180, with limited regions of base pairing, has very little activity. PKR activation involves the dimerization of PKR on RNA and requires a more extended duplexed RNA structure than binding alone (19). This explains why the less highly structured RNAs in our series were only weak activators despite their evident ability to bind p20. Comparison of the activities of the longer and shorter versions of the TM RNA suggests that the duplexed regions in this RNA are primarily located in the 3′-UTR. On the other hand, sequences outside the 3′-UTR are apparently required in TP RNA.
The few cellular transcripts that are known to date as PKR activators include examples of both coding and non-coding RNAs. In the latter category, R-RNA (regulatory RNA) is synthesized in differentiating cells and is thought to be responsible for the activation of PKR during cell differentiation (39,40). The Alu RNAs are RNA polymerase III transcripts generated from short repetitive sequences interspersed throughout the primate genome. They have been reported to inhibit PKR activation induced by viral infection and other types of cell stress (41,42). Among mRNAs, that encoding PKR itself has long untranslated regions and is reported to autoregulate its own translation, probably via PKR activation. Another example is found in myotonic dystrophy (DM), which is characterized by large expansions of CTG repeats within the 3′-UTR of the DM protein kinase gene. In normal individuals, the number of CTG repeats at this locus is 5–37, whereas individuals with DM have more than 50 to as many as 3000 repeats. These longer CUG repeat sequences activate PKR in vitro (43), suggesting a possible mechanism for DM pathogenesis. Similarly, in Huntington’s disease, mutant mRNA containing the CAG repeats that lead to poly-glutamine expansions in the protein are reported to bind and activate PKR in the diseased tissue of patients (44).
The ability of the cytoskeletal RNAs to activate PKR roughly parallels their translational inhibition potency in the reticulocyte lysate system (Fig. 4). As expected, the strongest activators of PKR (TM488 and TP740) were the most effective inhibitors of translation while the weak PKR activators (AC229, TM109 and especially TP180) inhibited translation weakly. In this regard, the cytoskeletal mRNA sequences behave more predictably than hepatitis delta RNA, which fails to inhibit translation even though it binds to and activates PKR (38). This anomaly has been tentatively attributed to interactions of the viral RNA with other proteins in the reticulocyte lysate, leading to conformational changes in the RNA. Similar phenomena may account for discrepancies between the concentration curves for PKR activation and translational inhibition in the data presented here. Even with the same RNA preparations, we consistently observed that the reticulocyte assay was more refractory to inhibition than expected from PKR activation results. In the case of TP740, which includes the TP coding region, structural alterations brought about by translating ribosomes or translation factors are likely contributory factors. Possibly for the same reason, appending the 3′-UTRs to a reporter construct did not reduce gene expression in transfection experiments (data not shown).
Our findings support the hypothesis that the 3′-UTRs of cytoskeletal gene mRNAs represent a class of cellular regulators of PKR activity. These cytoskeletal genes have been strongly implicated in cell growth control and transformation. Their interaction with PKR may contribute to the mechanism of tumor suppression induced by these RNA sequences. In view of evidence that PKR can act as a tumor suppressor in 3T3 cells (15–17), a straightforward scenario would be through activation of the kinase. However, the mechanism may be more complicated. Recent evidence suggests that PKR has a tumor promoting activity in some cancer cells (45) and is also important for signal transduction pathways that induce cell proliferation via platelet-derived growth factor (46,47). In breast cancer cells, both the level and activity of PKR are several-fold higher than in non-transformed breast epithelial cells. Taken together, these results establish PKR as an upstream regulator of Stat3 activation and as a mediator of both growth-promoting and growth-inhibitory signals.
Breast carcinoma cells have developed a mechanism to neutralize the potent inhibitory effect of PKR on protein synthesis by overproducing eIF2B, which permits initiation to occur in the presence of phosphorylated eIF2α. This allows them to exploit the positive action of PKR on cell growth and proliferation pathways without simultaneously incurring a translational block. Furthermore, TM, TP and AC are expressed at high levels in differentiating muscle cells (10). In addition, a loss of expression of TM isoforms is seen in human breast cancer cells (4,6), which contain higher activity and levels of PKR than non-transformed breast epithelial cells (45). In light of our observation that 3′-UTRs at high concentrations inactivate PKR, it is tempting to propose that these RNA molecules function as tumor suppressors by blocking the PKR activity that is required for the proliferative property of cancer cells.
Acknowledgments
ACKNOWLEDGEMENTS
We thank Dr Julia C. Watson for the 3′-UTR constructs. This work was supported by grant AI34552 from the National Institutes of Health.
REFERENCES
- 1.Ben-Ze’ev A. (1997) Cytoskeletal and adhesion proteins as tumor suppressors. Curr. Opin. Cell Biol., 9, 99–108. [DOI] [PubMed] [Google Scholar]
- 2.Gluck U. and Ben-Ze’ev,A. (1994) Modulation of alpha-actinin levels affects cell motility and confers tumorigenicity on 3T3 cells. J. Cell Sci., 107, 1773–1782. [DOI] [PubMed] [Google Scholar]
- 3.Volberg T., Geiger,B., Kam,Z., Pankov,R., Simcha,I., Sabanay,H., Coll,J.L., Adamson,E. and Ben-Ze’ev,A. (1995) Focal adhesion formation by F9 embryonal carcinoma cells after vinculin gene disruption. J. Cell Sci., 108, 2253–2260. [DOI] [PubMed] [Google Scholar]
- 4.Franzen B., Linder,S., Alaiya,A.A., Eriksson,E., Fujioka,K., Bergman,A.C., Jornvall,H. and Auer,G. (1997) Analysis of polypeptide expression in benign and malignant human breast lesions. Electrophoresis, 18, 582–587. [DOI] [PubMed] [Google Scholar]
- 5.Ljungdahl S., Linder,S., Franzen,B., Binetruy,B., Auer,G. and Shoshan,M.C. (1998) Down-regulation of tropomyosin-2 expression in c-Jun-transformed rat fibroblasts involves induction of a MEK1-dependent autocrine loop. Cell Growth Differ., 9, 565–573. [PubMed] [Google Scholar]
- 6.Bhattacharya B., Prasad,G.L., Valverius,E.M., Salomon,D.S. and Cooper,H.L. (1990) Tropomyosins of human mammary epithelial cells: consistent defects of expression in mammary carcinoma cell lines. Cancer Res., 50, 2105–2112. [PubMed] [Google Scholar]
- 7.Prasad G.L., Fuldner,R.A. and Cooper,H.L. (1993) Expression of transduced tropomyosin 1 cDNA suppresses neoplastic growth of cells transformed by the ras oncogene. Proc. Natl Acad. Sci. USA, 90, 7039–7043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prasad G.L., Fuldner,R.A., Braverman,R., McDuffie,E. and Cooper,H.L. (1994) Expression, cytoskeletal utilization and dimer formation of tropomyosin derived from retroviral-mediated cDNA transfer. Metabolism of tropomyosin from transduced cDNA. Eur. J. Biochem., 224, 1–10. [DOI] [PubMed] [Google Scholar]
- 9.Shah V., Braverman,R. and Prasad,G.L. (1998) Suppression of neoplastic transformation and regulation of cytoskeleton by tropomyosins. Somat. Cell Mol. Genet., 24, 273–280. [DOI] [PubMed] [Google Scholar]
- 10.Rastinejad F. and Blau,H.M. (1993) Genetic complementation reveals a novel regulatory role for 3′ untranslated regions in growth and differentiation. Cell, 72, 903–917. [DOI] [PubMed] [Google Scholar]
- 11.Rastinejad F., Conboy,M.J., Rando,T.A. and Blau,H.M. (1993) Tumor suppression by RNA from the 3′ untranslated region of alpha-tropomyosin. Cell, 75, 1107–1117. [DOI] [PubMed] [Google Scholar]
- 12.Masuda A., Takenaga,K., Kondoh,F., Fukami,H., Utsumi,K. and Okayama,H. (1996) Role of a signal transduction pathway which controls disassembly of microfilament bundles and suppression of high-molecular-weight tropomyosin expression in oncogenic transformation of NRK cells. Oncogene, 12, 2081–2088. [PubMed] [Google Scholar]
- 13.Takenaga K. and Masuda,A. (1994) Restoration of microfilament bundle organization in v-raf-transformed NRK cells after transduction with tropomyosin 2 cDNA. Cancer Lett., 87, 47–53. [DOI] [PubMed] [Google Scholar]
- 14.Kaufman R.J. (2000) The double-stranded RNA-activated protein kinase PKR. In Sonenberg,N., Hershey,J.W.B. and Mathews,M.B. (eds), Translational Control of Gene Expression. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp. 503–528.
- 15.Koromilas A.E., Roy,S., Barber,G.N., Katze,M.G. and Sonenberg,N. (1992) Malignant transformation by a mutant of the IFN-inducible dsRNA-dependent protein kinase. Science, 257, 1685–1689. [DOI] [PubMed] [Google Scholar]
- 16.Meurs E.F., Galabru,J., Barber,G.N., Katze,M.G. and Hovanessian,A.G. (1993) Tumor suppressor function of the interferon-induced double-stranded RNA-activated protein kinase. Proc. Natl Acad. Sci. USA, 90, 232–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Romano P.R., Garcia-Barrio,M.T., Zhang,X., Wang,Q., Taylor,D.R., Zhang,F., Herring,C., Mathews,M.B., Qin,J. and Hinnebusch,A.G. (1998) Autophosphorylation in the activation loop is required for full kinase activity in vivo of human and yeast eukaryotic initiation factor 2 alpha kinases PKR and GCN2. Mol. Cell. Biol., 18, 2282–2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davis S. and Watson,J.C. (1996) In vitro activation of the interferon-induced, double-stranded RNA-dependent protein kinase PKR by RNA from the 3′ untranslated regions of human alpha-tropomyosin. Proc. Natl Acad. Sci. USA, 93, 508–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manche L., Green,S.R., Schmedt,C. and Mathews,M.B. (1992) Interactions between double-stranded RNA regulators and the protein kinase DAI. Mol. Cell. Biol., 12, 5238–5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bevilacqua P.C. and Cech,T.R. (1996) Minor-groove recognition of double-stranded RNA by the double-stranded RNA-binding domain from the RNA-activated protein kinase PKR. Biochemistry, 35, 9983–9994. [DOI] [PubMed] [Google Scholar]
- 21.Bevilacqua P.C., George,C.X., Samuel,C.E. and Cech,T.R. (1998) Binding of the protein kinase PKR to RNAs with secondary structure defects: role of the tandem A-G mismatch and noncontiguous helixes. Biochemistry, 37, 6303–6316. [DOI] [PubMed] [Google Scholar]
- 22.Zheng X. and Bevilacqua,P.C. (2000) Straightening of bulged RNA by the double-stranded RNA-binding domain from the protein kinase PKR. Proc. Natl Acad. Sci. USA, 97, 14162–14167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pe’ery T. and Mathews,M.B. (2000) Viral translation strategies and host defense mechanisms. In Sonenberg,N., Hershey,J.W.B. and Mathews,M.B. (eds), Translational Control of Gene Expression. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp. 371–424. [Google Scholar]
- 24.Langland J.O., Kao,P.N. and Jacobs,B.L. (1999) Nuclear factor-90 of activated T-cells: A double-stranded RNA-binding protein and substrate for the double-stranded RNA-dependent protein kinase, PKR. Biochemistry, 38, 6361–6368. [DOI] [PubMed] [Google Scholar]
- 25.Patel R.C., Vestal,D.J., Xu,Z., Bandyopadhyay,S., Guo,W., Erme,S.M., Williams,B.R. and Sen,G.C. (1999) DRBP76, a double-stranded RNA-binding nuclear protein, is phosphorylated by the interferon-induced protein kinase, PKR. J. Biol. Chem., 274, 20432–20437. [DOI] [PubMed] [Google Scholar]
- 26.Parker L.M., Fierro-Monti,I. and Mathews,M.B. (2001) Nuclear factor 90 is a substrate and regulator of the eukaryotic initiation factor 2 kinase double-stranded RNA-activated protein kinase. J. Biol. Chem., 276, 32522–32530. [DOI] [PubMed] [Google Scholar]
- 27.Brand S.R., Kobayashi,R. and Mathews,M.B. (1997) The Tat protein of human immunodeficiency virus type 1 is a substrate and inhibitor of the interferon-induced, virally activated protein kinase, PKR. J. Biol. Chem., 272, 8388–8395. [DOI] [PubMed] [Google Scholar]
- 28.McMillan N.A., Chun,R.F., Siderovski,D.P., Galabru,J., Toone,W.M., Samuel,C.E., Mak,T.W., Hovanessian,A.G., Jeang,K.T. and Williams,B.R. (1995) HIV-1 Tat directly interacts with the interferon-induced, double-stranded RNA-dependent kinase, PKR. Virology, 213, 413–424. [DOI] [PubMed] [Google Scholar]
- 29.Wade R., Eddy,R., Shows,T.B. and Kedes,L. (1990) cDNA sequence, tissue-specific expression and chromosomal mapping of the human slow-twitch skeletal muscle isoform of troponin I. Genomics, 7, 346–357. [DOI] [PubMed] [Google Scholar]
- 30.Hamada H., Petrino,M.G. and Kakunaga,T. (1982) Molecular structure and evolutionary origin of human cardiac muscle actin gene. Proc. Natl Acad. Sci. USA, 79, 5901–5905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gunning P., Ponte,P., Blau,H. and Kedes,L. (1983) alpha-skeletal and alpha-cardiac actin genes are coexpressed in adult human skeletal muscle and heart. Mol. Cell. Biol., 3, 1985–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pe’ery T. and Mathews,M.B. (1997) Synthesis and purification of single-stranded RNA for use in experiments with PKR and in cell-free translation systems. Methods, 11, 371–381. [DOI] [PubMed] [Google Scholar]
- 33.Mellits K.H. and Mathews,M.B. (1988) Effects of mutations in stem and loop regions on the structure and function of adenovirus VA RNAI. EMBO J., 7, 2849–2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmedt C., Taylor,D.R., Manche,L., Green,S.R., Ma,Y. and Mathews,M.B. (1995) Functional characterization of the RNA binding domain and motif of the double-stranded RNA-dependent protein kinase DAI. J. Mol. Biol., 249, 29–44. [DOI] [PubMed] [Google Scholar]
- 35.Mellits K.H., Kostura,M. and Mathews,M.B. (1990) Interaction of adenovirus VA RNAI with the protein kinase DAI: non-equivalence of binding and function. Cell, 61, 843–852. [DOI] [PubMed] [Google Scholar]
- 36.Kostura M. and Mathews,M.B. (1989) Purification and activation of the double-stranded RNA-dependent eIF-2 kinase DAI. Mol. Cell. Biol., 9, 1576–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mellits K.H., Pe’ery,T., Manche,L., Robertson,H.D. and Mathews,M.B. (1990) Removal of double-stranded contaminants from RNA transcripts: synthesis of adenovirus VA RNAI from a T7 vector. Nucleic Acids Res., 18, 5401–5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robertson H.D., Manche,L. and Mathews,M.B. (1996) Paradoxical interactions between human delta hepatitis agent RNA and the cellular protein kinase PKR. J. Virol., 70, 5611–5617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li J. and Petryshyn,R.A. (1991) Activation of the double stranded RNA-dependent eIF-2a kinase by cellular RNA from 3T3-F442A cells. Eur. J. Biochem., 195, 41–48. [DOI] [PubMed] [Google Scholar]
- 40.Petryshyn R.A., Ferrenz,A.G. and Li,J. (1997) Characterization and mapping of the double-stranded regions involved in activation of PKR within a cellular RNA from 3T3-F442A cells. Nucleic Acids Res., 25, 2672–2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chu W.-M., Ballard,R., Carpick,B.W., Williams,B.R.G. and Schmid,C.W. (1998) Potential Alu function: Regulation of the activity of double stranded RNA-activated kinase PKR. Mol. Cell. Biol., 18, 58–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmid C.W. (1998) Does SINE evolution preclude Alu function? Nucleic Acids Res., 26, 4541–4550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tian B., White,R.J., Xia,T., Welle,S., Turner,D.H., Mathews,M.B. and Thornton,C.A. (2000) Expanded CUG repeat RNAs form hairpins that activate the double-stranded RNA-dependent protein kinase PKR. RNA, 6, 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peel A.L., Rao,R.V., Cottrell,B.A., Hayden,M.R., Ellerby,L.M. and Bredesen,D.E. (2001) Double-stranded RNA-dependent protein kinase, PKR, binds preferentially to Huntington’s disease (HD) transcripts and is activated in HD tissue. Hum. Mol. Genet., 10, 1531–1538. [DOI] [PubMed] [Google Scholar]
- 45.Kim S.H., Forman,A.P., Mathews,M.B. and Gunnery,S. (2000) Human breast cancer cells contain elevated levels and activity of the protein kinase, PKR. Oncogene, 19, 3086–3094. [DOI] [PubMed] [Google Scholar]
- 46.Mundschau L.J. and Faller,D.V. (1995) Platelet-derived growth factor signal transduction through the interferon-inducible kinase PKR. Immediate early gene induction. J. Biol. Chem., 270, 3100–3106. [DOI] [PubMed] [Google Scholar]
- 47.Deb A., Zamanian-Daryoush,M., Xu,Z., Kadereit,S. and Williams,B.R. (2001) Protein kinase PKR is required for platelet-derived growth factor signaling of c-fos gene expression via Erks and Stat3. EMBO J., 20, 2487–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]