Abstract
Increased thermogenesis in brown adipose tissue might have an obesity-reducing effect in humans. In transgenic mice, depletion of genes involved in creatine metabolism results in disrupted thermogenic capacity and altered effects of high-fat feeding on body weight. Data analyses of a sex-stratified genome-wide association study (GWAS) for body mass index (BMI) within the genomic regions of genes of this pathway (CKB, CKMT1B, and GATM) revealed one sex-dimorphic BMI-associated SNP in CKB (rs1136165). The effect size was larger in females than in males. A mutation screen of the coding regions of these three candidate genes in a screening group (192 children and adolescents with severe obesity, 192 female patients with anorexia nervosa, and 192 healthy-lean controls) identified five variants in each, CKB and GATM, and nine variants in the coding sequence of CKMT1B. Non-synonymous variants identified in CKB and CKMT1B were genotyped in an independent confirmation study group (781 families with severe obesity (trios), 320 children and adolescents with severe obesity, and 253 healthy-lean controls). In silico tools predicted mainly benign yet protein-destabilizing potentials. A transmission disequilibrium test in trios with severe obesity indicated an obesity-protective effect of the infrequent allele at rs149544188 located in CKMT1B. Subsequent correlation analyses in 1,479 individuals of the Leipzig Obesity BioBank revealed distinct correlations of CKB with the other two genes in omental visceral adipose tissue (VAT) and abdominal subcutaneous adipose tissue (SAT). Furthermore, between-subject comparisons of gene expression levels showed generally higher expressions of all three genes of interest in VAT than in SAT. Future in vitro analyses are needed to assess the functional implications of these findings.
Keywords: GWAS, creatine metabolism, in silico, BAT, TDT
1 Introduction
Globally, the prevalence of obesity is rising continuously (Williams et al., 2015; Schienkiewitz et al., 2017; Janssen et al., 2020; Hinney et al., 2022). By 2030, approximately 50% of the global population is projected to exhibit a body mass index (BMI) greater than 30 kg/m2 and will thus be considered obese (World Health Organisation, 2000; Finkelstein et al., 2012; González-Muniesa et al., 2017; Ward et al., 2019). The global prevalence of severe obesity in children is approximately 2%–6% (Hinney et al., 2022).
A proposed mechanism to counteract obesity is to increase energy expenditure via enhanced activity of the brown adipose tissue (BAT), commonly referred to as adaptive thermogenesis (Chouchani et al., 2019; Pan et al., 2020). In addition to the known uncoupling protein 1 (UCP1)-dependent pathway, an alternative creatine-dependent mechanism has been shown to affect thermogenesis (Chouchani et al., 2019). A reduction in creatine levels through the inhibition of its biosynthesis diminished thermogenesis and energy expenditure, leading to a weight gain (Kazak et al., 2015; Kazak et al., 2017). Upon high-fat feeding, mice in which the first and thus rate-restricting enzyme of the creatine biosynthesis, glycine amidinotransferase (GATM), was fat-selectively deprived, showed an increased body weight (Kazak et al., 2017). GATM transports a guanido group from arginine to glycine resulting in guanidinoacetic acid. The latter is a direct creatine precursor (Morris, 2004).
Throughout creatine biosynthesis, creatine kinases catalyze the reversible transfer of a phosphate from adenosine triphosphate (ATP) to creatine, resulting in phosphocreatine (PCr; Hettling and van Beek, 2011). Mice in which either creatine kinase B (Ckb) or creatine kinase mitochondrial 1 (Ckmt1) was knocked out (KO) showed a clinically mild phenotype. These mice were fertile but had behavioral abnormalities, such as reduced spatial learning abilities (Jost et al., 2002; Streijger et al., 2004). It has been suspected that the respective other kinase compensates for the KO, and thus, a double-KO mouse model was studied (Streijger et al., 2005). In these double-KO mice, lacking both creatine kinases (CK−/−), an 18%–20% lower body weight as well as a 7% reduction of BAT was found (Streijger et al., 2005; Streijger et al., 2009). In addition, these mice exhibited problems maintaining their body temperature, which was proposed to be due to a communication loss of thermogenesis-associated pathways in the hypothalamus induced by reduced levels of creatine kinases (Streijger et al., 2009). However, brown adipocyte-selective inhibition of Ckb in mice predisposes to obesity due to a reduction in thermogenic capacity (Rahbani et al., 2021). Similarly, upon depletion of the creatine transporter (CrT) in fat depots, mice gained more weight than their wild-type littermates when fed a high-fat diet. This CrT KO led to reduced creatine and phosphocreatine levels in adipocytes and diminished energy expenditure. Accordingly, in purified human subcutaneous adipocytes, the expression of CrT correlated with a decreased BMI (Kazak et al., 2019).
Previous studies have reported differences in the regulation of BAT activity and body weight between males and females (e.g., Shi and Clegg, 2009; Kaikaew et al., 2021). Animal models have demonstrated that sex hormones might mediate the sexually dimorphic regulation. Accordingly, androgens inhibited BAT activity, whereas estrogens showed a stimulatory effect. Generally, female rodents were found to have a higher prevalence of active BAT than males (Kaikaew et al., 2021). In human BAT, conclusive evidence is still lacking. Several studies reported that BAT activity and mass are higher in females than males, while others were unable to detect any differences between the sexes (Kaikaew et al., 2021).
In contrast, sex differences at the genetic level of body weight regulation have been detected in a genome-wide association study (GWAS) for waist-to-hip ratio adjusted for BMI (Pulit et al., 2019). Higher heritability and larger effects of genetic variants were found in women compared to men, while approximately 1/3 of all GWAS signals generally displayed a sexually dimorphic effect (Pulit et al., 2019). Additionally, several studies have provided evidence for genetic overlaps between BMI and anorexia nervosa (AN; Bulik-Sullivan et al., 2015; Hinney et al., 2017; Watson et al., 2019; Zheng et al., 2022). AN is a severe psychiatric disorder marked by an extremely reduced body weight and a distorted body image (Hübel et al., 2019; Mitchell and Peterson, 2020). It mainly occurs in females and exhibits one of the highest mortality rates in psychiatric disorders (e.g., Arcelus et al., 2011). As alleles predisposing to AN are also associated with a lower BMI (Hinney et al., 2017), genetic analyses in both extremes of the BMI range may help to improve the general understanding of body weight regulation.
Consequently, we aimed to analyze sex-stratified effects of single nucleotide polymorphisms (SNPs) previously identified in GWAS for BMI (Pulit et al., 2019) or AN (Watson et al., 2019) and located in the genomic regions of CKB, CKMT1B, and GATM. Subsequently, we sequenced the coding regions of these genes in children and adolescents with severe obesity and healthy-lean controls. Given the results of our sex-stratified analysis, the coding sequence (CDS) of CKB was further screened in female patients with AN. Non-synonymous variants identified in CKB and CKMT1B were genotyped in additional independent samples. In silico analyses were conducted to explore putative functional implications. Next, we conducted correlation analyses of the genes of interest with several metabolic and anthropometric parameters, such as BMI, body weight, height and serum leptin concentrations, as well as between-subject comparisons based on gene expression levels, in omental visceral adipose tissue (VAT) and abdominal subcutaneous adipose tissue (SAT) of 1,479 individuals of the Leipzig Obesity BioBank (LOBB).
2 Materials and methods
2.1 GWAS-related sex-specific analyses
To evaluate sex-specific effects of genetic variants within the genes of interest (CKB, CKMT1B, and GATM), data from a sex-stratified meta-analysis of GWAS for BMI (Pulit et al., 2019) were utilized (https://zenodo.org/record/1251813#.YbsPznyZNaR; downloaded on 8 June 2022). A total of 434,794 female and 374,756 male samples as well as 27, 381, 302 SNPs were included (Pulit et al., 2019). Information regarding the genomic position, rsID, minor allele frequency (MAF), standard error (SE), beta and p-values (p) of the SNPs located within the genes and a 500 kb region adjacent to the genes were extracted from the datasets. Given the spatial proximity of GATM and CKMT1B, these genes were analyzed collectively within one region (see Table 1). After assessing whether the effect alleles for males and females were homogeneously assigned, palindromic SNPs with a MAF between 0.48 and 0.52 were excluded. The SNPs’ effect directions were subsequently harmonized to eliminate strand issues by inverting the beta values of SNPs tested for their major allele. Next, z-scores were calculated for each SNP (see Eq. 1; Khramtsova et al., 2019; Zheng et al., 2022). Furthermore, additional data from a GWAS for AN (Watson et al., 2019) were obtained (https://www.med.unc.edu/pgc/download-results/; downloaded on 21 October 2021). However, as no information on MAF was available in this dataset, effect size harmonization was omitted. SNPs were considered trait-associated if they exhibited a GWAS-derived p < 5*10–8.
| (1) |
TABLE 1.
Genomic positions investigated in the sex-stratified analyses. Since CKMT1B and GATM are located in close proximity, these two genes were investigated collectively within one region (*). The analyzed regions refer to GRCh37, as all GWAS data were exclusively available for this genome version.
| Gene | Chr | Position | ±500 kb region | Investigated region |
|---|---|---|---|---|
| CKB | 14 | 103,986,004–103,989,167 | 103,486,004–104,489,167 | 103,486,004–104,489,167 |
| CKMT1B* | 15 | 43,885,055–43,891,604 | 43,385,055–44,391,604 | 43,385,055–46,194,425* |
| GATM* | 15 | 45,653,322–45,694,425 | 45,153,322–46,194,425 | 43,385,055–46,194,425* |
SNPs were defined to have a sexually dimorphic effect if they exhibited an absolute z-score of at least 3 (|z-score| ≥ 3), as described previously (Khramtsova et al., 2019).
2.2 Screening and confirmation study group for the mutation screen
We sequenced the DNA of a screening group consisting of 192 children and adolescents with severe obesity (age: 13.88 ± 2.50 years; BMI: 32.36 ± 6.04 kg/m2; all had a BMI percentile >90th; 182 had a BMI >97th percentile; see Table 2; Hinney et al., 2007; Scherag et al., 2010), 192 patients with anorexia nervosa (acute or recovered; age: 21.85 ± 10.60 years; BMI: 16.38 ± 3.12 kg/m2; see Table 2; Boraska et al., 2012; Watson et al., 2019) and 192 healthy-lean controls (age: 25.55 ± 3.91 years; BMI: 18.38 ± 1.10 kg/m2; see Table 2; Hinney et al., 2007). Additional genotyping was performed in a confirmation study group of 781 families comprising a child or adolescent with severe obesity and both biological parents (referred to as trios; age of offspring: 13.45 ± 3.11 years; BMI of offspring: 32.15 ± 5.92 kg/m2; see Table 2) from a family-based obesity GWAS, 320 children and adolescents with severe obesity (age: 14.60 ± 4.24 years; BMI: 33.77 ± 7.00 kg/m2, see Table 2) and 253 healthy-lean controls (age: 26.40 ± 6.84 years; BMI: 17.85 ± 1.11 kg/m2; see Table 2) from a case-control GWAS for obesity (Hinney et al., 2007; Scherag et al., 2010). The 781 trios were composed of a child or adolescent with severe obesity (BMI percentile 90th) and both biological parents. Over 90% of the offspring (723; 92.57%) had a BMI at or above the 97th BMI percentile. The mean BMI of the 1,562 parents was 30.40 kg/m2 (standard deviation: 6.3 kg/m2). Of these, 724 (46.35%) had a BMI above 30 kg/m2, while 822 (52.62%) showed a BMI lower or equal 30 kg/m2. For 16 parents, no BMI data was available. Written informed consent was given by all participants of the screening and confirmation study groups and, in the case of minors, by their parents. This study was further approved by the Ethics committees of the respective universities and institutions and was performed in accordance with the Declaration of Helsinki.
TABLE 2.
Phenotypic data of the screening and confirmation study groups. The initial screening samples subjected to the mutation screen consisted of 192 children and adolescents with severe obesity or AN (acute or recovered) and 192 healthy-lean controls (Hinney et al., 2007; Scherag et al., 2010; Boraska et al., 2012; Watson et al., 2019). Additional genotyping was performed in an independent confirmation study group based on family-based samples consisting of the child or adolescent with severe obesity and both biological parents (trios) as well as additional individuals with severe obesity or normal weight previously recruited for a case-control GWAS for obesity (Hinney et al., 2007; Scherag et al., 2010). Phenotypic data regarding BMI and age were missing for 16 parents. n: number of samples. SD: standard deviation.
| Sample | Status | N | % Female | Age in years: Mean (SD) | BMI in kg/m2: Mean (SD) | BMI-SDSLMS: Mean (SD) | |
|---|---|---|---|---|---|---|---|
| Screening samples | Children and adolescents with severe obesity | 192 | 56 | 13.88 (2.50) | 32.36 (6.04) | 2.60 (0.54) | |
| Patients with AN | total | 192 | 100 | 21.85 (10.60) | 16.38 (3.12) | −2.61 (1.86) | |
| acute | 141 | 100 | 18.44 (8.70) | 14.90 (1.85) | −3.20 (1.79) | ||
| recovered | 51 | 100 | 31.28 (9.68) | 20.47 (2.09) | −0.98 (0.79) | ||
| Controls | 192 | 45 | 25.55 (3.91) | 18.38 (1.10) | −2.21 (0.46) | ||
| Confirmation samples | Family-based samples | Children and adolescents with obesity | 781 | 55 | 13.45 (3.11) | 32.15 (5.92) | 2.65 (0.53) |
| Parents | 1,562 | 50 | 42.60 (6.00) | 30.38 (6.29) | 0.77 (1.12) | ||
| Samples from an obesity GWAS | Children and adolescents with obesity | 320 | 58 | 14.60 (4.24) | 33.77 (7.00) | 2.69 (0.56) | |
| Controls | 253 | 74 | 26.40 (6.84) | 17.85 (1.11) | −2.32 (0.66) | ||
Furthermore, the European, non-Finnish study group extracted from the Genome Aggregation Database (gnomAD; v2.1.1; GRCh37; Karczewski et al., 2020; https://gnomad.broadinstitute.org/; see Supplementary Table S3) was used as an additional control population. Data from either sex were considered as controls for the study group with severe obesity, while data from females served as controls for the screening group of exclusively female patients with AN. Phenotypic data regarding the gnomAD controls and genotype data pertaining to specific variants were unavailable. Additionally, the number of samples varied for each variant.
2.3 Study group for gene expression and correlation analyses
For gene expression and correlation analyses (based on RNA-sequencing data), paired omental visceral and abdominal subcutaneous adipose tissues were collected from 1,479 individuals of the Leipzig Obesity BioBank (LOBB). Individuals were either non-obese (n = 31; 51.6% female; age: 55.8 ± 13.4 years old; BMI: 25.7 ± 2.7 kg/m2) or had obesity (n = 1,448; 71.2% female; age: 46.9 ± 11.7 years old; BMI: 49.2 ± 8.3 kg/m2). Tissue samples were collected during elective laparoscopic abdominal surgery as described before (Langhardt et al., 2018). Laboratory measurements of body composition and metabolic parameters were obtained as detailed previously (Kloting et al., 2010). The study was approved by the Ethics Committee of the University of Leipzig (approval no: 159-12–21052012) and performed in accordance with the Declaration of Helsinki. All individuals gave written informed consent before participating in the study.
2.4 Mutation screen
The coding region of the genes CKB (chr14: 103,519,667–103,552,833; GRCh38; ENST00000348956.7), CKMT1B (chr15: 43,593,054–43,604,901; GRCh38; ENST00000441322.6), and GATM (chr15: 45,361,124–45,402,327; GRCh38; ENST00000396659.8) were Sanger sequenced in 192 children and adolescents with severe obesity and 192 healthy-lean controls (see Table 2). The CDS of CKB was further analyzed in 192 female patients with AN (see Table 2). The respective genomic fragments were amplified by performing polymerase chain reactions (PCR; Veriti96-well Thermal Cycler, Applied Biosystems, Foster City, CA, United States) with coding region-specific primers (see Supplementary Table S1). For the amplification of the CDS of CKMT1B, a nested PCR approach was conducted (see Supplementary Table S1). Confirmation of the desired fragments was achieved by 2.5% agarose gel electrophoresis. Subsequent Sanger sequencing was performed by MicroSynth Seqlab GmbH in Göttingen, Germany. Sequence analysis and genotype assignment were conducted independently by at least two experienced scientists using the SeqMan Pro software (version: 11.0.0; DNAstar, Inc., Madison, WI, United States). Discrepancies were solved by either reaching consensus or by re-sequencing.
2.5 Genotyping in a larger confirmation study group
The CKB- and CKMT1B-located non-synonymous variants identified in patients with severe obesity were additionally genotyped in a larger independent confirmation study group, either by a TaqMan assay (ThermoFisher Scientific, Inc., Waltham, MA, United States) or a restricted fragment length polymorphism (RFLP) analysis (see Figure 1). Therefore, 781 trios with severe obesity as well as 320 children and adolescents with severe obesity and 253 healthy-lean controls from a previous GWAS (Hinney et al., 2007; Scherag et al., 2010) were analyzed (see Table 2). Custom TaqMan assays were designed for the variants rs758572075 (assay ID: ANXGZUF) and rs1230355611 (assay ID: ANYMVED). For the SNP rs146047573, a pre-designed assay was ordered (assay ID: C_162127713_10). All TaqMan assays were performed on the StepOnePlus Real-Time PCR system by Applied Biosystems (Foster City, CA, United States). Genotyping of SNP rs149544188 was performed by subjecting the samples to the restriction enzyme BsaHI (R0556S; New England Biolabs GmbH; Ipswich, MA, United States). Genotypes were assigned by at least two experienced scientists, while discrepancies were solved by either reaching consensus or by repeating the experiments.
FIGURE 1.
Experimental procedure of the genetic study. The genetic study comprised sex-specific analyses for CKB, CKMT1B and GATM as well as a mutation screen in children and adolescents with severe obesity, healthy-lean controls, and patients with anorexia nervosa. In silico analyses were used to predict the functional implications of the detected variants.
2.6 Statistics
To exclude putative genotyping errors, the Hardy-Weinberg-Equilibrium (HWE) was checked. For two variants, a significant deviation of the HWE was detected. Consequently, the respective genotyping was re-checked and repeated. Once genotyping errors were excluded, the corresponding variants remained in the analyses. Furthermore, the transmission disequilibrium test (TDT) was conducted in the 781 trios with severe obesity for one variant analyzed in the confirmation study group (see Table 2; Spielman et al., 1993). Next, putative disease associations with either obesity or AN were assessed by a two-sided chi-square test or a two-sided Fisher’s exact test using IBM SPSS Statistics software (version 28.0.0.0). Associations were considered nominally associated if p < 0.05.
2.7 In silico analyses
All identified variants were subsequently analyzed by various in silico tools regarding their pathogenic implications, conservation (MutationTaster2021; Steinhaus et al., 2021), and potential to alter splice sites (ESEfinder, Cartegni et al., 2003; SpliceMan; Lim and Fairbrother, 2012). The impact of non-synonymous variants on the protein structure was examined with the tool PANTHER-PSEP (position-specific evolutionary preservation; Tang and Thomas, 2016) and the Cologne University Protein Stability Analysis Tool (CUPSAT, Parthiban et al., 2006). The following protein data bank (PDB) reference protein structures were utilized: CKB - 3DRE (Bong et al., 2008), CKMT1B- 1QK1 (Eder et al., 2000), and GATM - 2JDW (Humm et al., 1997).
2.8 Gene expression and correlation analyses
RNA-sequencing (RNA-seq; rRNA-depleted, single-end) data from the LOBB cohort were generated based on a SMARTseq protocol (Picelli et al., 2014). In brief, RNA enrichment and reverse transcription were conducted with oligo (dT) and TSO primers. cDNA amplification was carried out with ISPCR primers, and cDNA was processed with Tn5 using the Nextera DNA Flex kit. RNA libraries were sequenced on a Novaseq 6,000 instrument at the Functional Genomics Center Zurich (FGCZ), Switzerland.
The raw reads were adapter trimmed and checked for sufficient quality by applying Fastp (v0.20.0; Chen et al., 2018), permitting a minimum read length of 18 nts and a quality cut-off of 20. Aligned to the human reference genome (GRCh38.p13), gene level expression quantification (gene model definition from GENCODE release 32) was carried out using Kallisto (v0.46; Bray et al., 2016). Samples with more than 20 million read counts were downsampled to 20 million read counts using the R package ezRun (v3.14.1; https://github.com/uzh/ezRun). Data were homoscedastically normalized with respect to library size using the variance-stabilizing transformation implemented by DESeq2 (v1.32.0; Love et al., 2014). To effectively neutralize the effects of in vitro RNA degradation, normalized counts were calibrated with transcript integrity numbers (TINs; Wang et al., 2016). TINs were estimated using the R package RSeQC (v4.0.0; Wang et al., 2012). Finally, the normalized data were adjusted for the gender and age batches.
Correlation analyses were performed by determining Pearson’s correlation coefficient and a confidence interval of 0.95. Between-subject comparisons for the genes were conducted with a non-parametric one-way Kruskal–Wallis ANOVA and pairwise Dunn’s test utilizing the R package ggstatsplot (v0.9.1; Patil, 2021). p-values were corrected for multiple inference using the Hommel’s method (Hommel, 1988). Adjusted two-sided p-values (padj.) < 0.05 were considered significant. Analyses were performed under R version 4.2.1.
3 Results
3.1 One CKB-located SNP revealed a sex-dimorphic effect on BMI
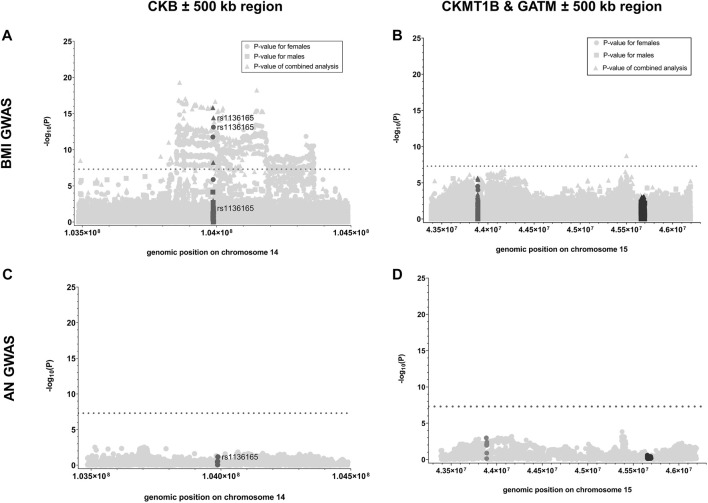
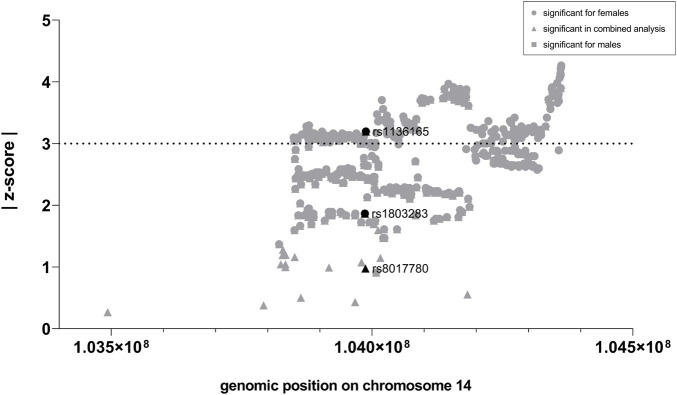
First, we analyzed whether GWAS-identified SNPs for BMI in CKB, CKMT1B, and GATM show sexually dimorphic effects. In the investigated GWAS (Pulit et al., 2019), a total of 24 SNPs were located in the genomic region of CKB, while 35 and 256 were encompassed in the regions of CKMT1B and GATM, respectively (see Supplementary Table S2). Notably, only CKB contained three SNPs (rs1803283, rs8017780, and rs1136165) that were associated with BMI (GWAS-derived p < 5*10–8) when both sexes were analyzed concomitantly. Two of these variants (rs1803283 and rs1136165) showed a significant BMI association in females (see Figure 2A), with their minor alleles predisposing to a lower BMI. No BMI-associated SNPs were detected in the region of CKMT1B and GATM (see Figure 2B; Supplementary Table S2). Calculating the z-scores with harmonized data of BMI-associated SNPs in CKB revealed that one variant (rs1136165; 4.17% of all CKB-located SNPs included in the GWAS) showed a more than 3-fold standard deviation aberration of effect sizes between females and males (absolute z-score = |3.196|; p = 0.0014; see Figure 3; Supplementary Table S2). As the tested allele at rs1136165 was solely associated with BMI in females (GWAS-derived p = 7.51*10–14 for tested allele T; GWAS-derived beta = 0.02 for tested allele T; Pulit et al., 2019) and not in males (GWAS-derived p = 0.013 for tested allele T; GWAS-derived beta = 0.008 for tested allele T; Pulit et al., 2019), this sexual dimorphism indicates a stronger BMI-altering effect in females than males. In the 500 kb region surrounding CKB, 225 SNPs (2.05% of all SNPs in this ±500 kb region) showed a significant sex-dimorphic effect (z-score > |3|). The highest z-score in this adjacent region was detected for rs72712857 (z-score = |4.27|; p = 0.00002; see Supplementary Table S2), which is exclusively BMI-associated in females (GWAS derived p = 1.44*10–9) (Pulit et al., 2019).
FIGURE 2.
p-values of SNPs analyzed in GWAS for BMI or AN located within the genes of interest and their adjacent regions. Here, the negative logarithmic p-values extracted from the sex-stratified BMI GWAS [Pulit et al., 2019; (A, B)] and the GWAS for AN (Watson et al., 2019; (C, D) are plotted against the genomic positions of the SNPs located within CKB (A, C); dark gray), CKMT1B [(B, D); dark gray] and GATM [(B, D); black] as well as their 500 kb adjacent regions (light gray; GRCh37; see Table 1; Supplementary Table S2). For the data regarding the sex-stratified BMI GWAS, the negative logarithmic p-values are presented for the female (dot), male (square), and combined (triangle) analyses. The threshold for genome-wide significance is indicated by the dotted lines (-log10(p) > 7.3).
FIGURE 3.
Z-scores for BMI-associated SNPs located in CKB and the 500 kb adjacent region. Here, the absolute z-scores were plotted against the chromosomal position of each SNP (GRCh37). After the extraction of the sex-stratified BMI GWAS data (Pulit et al., 2019), ambiguous SNPs were removed. Subsequently, the effect directions were harmonized, and the z-scores were calculated for each genome-wide and thus BMI-associated SNP within the region of CKB (black) and its 500 kb surrounding region (gray). A SNP is considered sex-dimorphic if |z-score| ≥ 3.
Given the lack of BMI associations within CKMT1B and GATM and adjacent regions (±500 kb), we solely calculated the z-score for one SNP that was associated with BMI in the sex combined analysis (rs765787; GWAS-derived p = 1.80*10–9) located in the 500 kb region surrounding the two genes of interest (CKMT1B and GATM; see Figure 2B; Supplementary Table S2). However, no sex-specific effect was detected (z-score = |0.38|, p = 0.71). Likewise, in the GWAS data for AN (Watson et al., 2019), we did not find SNPs associated with AN within the gene regions of CKB, CKMT1B, and GATM or in adjacent areas (see Figures 2C,D; Supplementary Table S2). Thus, we did not further analyze putative sex-specific effects within this dataset.
3.2 Mutation screen of CKB
Our sex-stratified analyses suggested a sex-dimorphic effect of the variant rs1136165 (p.Arg152=) located within CKB (see Figures 2, 3; Supplementary Table S2). Considering this GWAS hit and the observation that 1) AN and BMI share certain loci (Bulik-Sullivan et al., 2015; Hinney et al., 2017; Zheng et al., 2022), 2) AN mainly affects women (Hübel et al., 2019; Mitchell and Peterson, 2020), and 3) our GWAS-related sex-specific analyses may have been affected by insufficient power due to the limited sample size of the AN GWAS, we performed a mutation screen of CKB’s CDS in 192 female patients with AN (acute or recovered) as well as in 192 children and adolescents with severe obesity and 192 healthy-lean controls (see Table 2).
In total, we observed five variants (rs762206402, rs1136165, rs200890896, rs146047573, and rs1803283) in the CDS of CKB, one of which is non-synonymous (rs146047573, p.Tyr269Cys; see Table 3). All variants fulfilled the HWE.
TABLE 3.
Identified genetic variants in the investigated study groups. The results include the screening groups of 192 children and adolescents with severe obesity as well as 192 patients with AN and 192 healthy-lean controls. The sample sizes may vary if the variant was genotyped in an additional confirmation group consisting of 781 trios with severe obesity, 320 independent children and adolescents with severe obesity, and 253 healthy-lean control samples (see Table 2). Non-synonymous variants are shown in bold. The represented minor allele is based on the forward strand (*). 11: Homozygous wild type; 12: heterozygous, 22: homozygous alternative allele. MAF: minor allele frequency.
| Gene | Variant | Minor allele* | Amino acid exchange | Study group | Patients with obesity | Patients with AN | Healthy-lean controls | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 11 | 12 | 22 | MAF | 11 | 12 | 22 | MAF | 11 | 12 | 22 | MAF | |||||
| CKB | rs762206402 | C | Pro112= | Screening | 191 | 1 | 0 | 0.003 | 192 | 0 | 0 | 0 | 192 | 0 | 0 | 0 |
| rs1136165 | G | Arg152= | Screening | 31 | 88 | 73 | 0.39 | 28 | 88 | 76 | 0.38 | 29 | 96 | 67 | 0.40 | |
| rs200890896 | G | Ala175= | Screening | 192 | 0 | 0 | 0 | 191 | 1 | 0 | 0.003 | 192 | 0 | 0 | 0 | |
| rs146047573 | C | Tyr269Cys | Screening | 191 | 1 | 0 | 0.003 | 192 | 0 | 0 | 0 | 192 | 0 | 0 | 0 | |
| Confirmation | 1,100 | 1 | 0 | 0.0005 | - | - | - | - | 253 | 0 | 0 | 0 | ||||
| Both | 1,291 | 2 | 0 | 0.0008 | - | - | - | - | 445 | 0 | 0 | 0 | ||||
| rs1803283 | C | Glu364= | Screening | 30 | 84 | 78 | 0.38 | 25 | 91 | 76 | 0.37 | 28 | 92 | 72 | 0.39 | |
| CKMT1B | g.43595061C/T | T | Thr79= | Screening | 190 | 2 | 0 | 0.005 | - | - | - | - | 190 | 2 | 0 | 0.005 |
| g.43595385C/T | T | Asp120= | Screening | 191 | 1 | 0 | 0.003 | - | - | - | - | 192 | 0 | 0 | 0 | |
| g.43595451G/A | A | Thr142= | Screening | 191 | 1 | 0 | 0.003 | - | - | - | - | 192 | 0 | 0 | 0 | |
| rs1230355611 | T | Arg184Stop | Screening | 192 | 0 | 0 | 0 | - | - | - | - | 191 | 1 | 0 | 0.003 | |
| Confirmation | 1,100 | 1 | 0 | 0.0005 | - | - | - | - | 253 | 0 | 0 | 0 | ||||
| Both | 1,292 | 1 | 0 | 0.0004 | - | - | - | - | 444 | 1 | 0 | 0.001 | ||||
| rs758572075 | C | Val191Ala | Screening | 191 | 1 | 0 | 0.003 | - | - | - | - | 190 | 2 | 0 | 0.005 | |
| Confirmation | 1,100 | 1 | 0 | 0.0005 | - | - | - | - | 253 | 0 | 0 | 0 | ||||
| Both | 1,291 | 2 | 0 | 0.0008 | - | - | - | - | 443 | 2 | 0 | 0.002 | ||||
| rs9571 | G | Lys352= | Screening | 188 | 4 | 0 | 0.01 | - | - | - | - | 188 | 4 | 0 | 0.01 | |
| rs13234 | C | Ala362= | Screening | 187 | 5 | 0 | 0.013 | - | - | - | - | 184 | 7 | 1 | 0.02 | |
| rs149544188 | A | Arg399His | Screening | 190 | 2 | 0 | 0.005 | - | - | - | - | 191 | 1 | 0 | 0.003 | |
| Confirmation | 1,090 | 11 | 0 | 0.005 | - | - | - | - | 250 | 3 | 0 | 0.006 | ||||
| Both | 1,280 | 13 | 0 | 0.005 | - | - | - | - | 441 | 4 | 0 | 0.004 | ||||
| rs144820945 | G | Gly403= | Screening | 188 | 4 | 0 | 0.01 | - | - | - | - | 188 | 4 | 0 | 0.01 | |
| GATM | rs1288775 | A | Gln110His | Screening | 99 | 81 | 12 | 0.27 | - | - | - | - | 95 | 83 | 14 | 0.29 |
| rs146057680 | C | Asp234Val | Screening | 192 | 0 | 0 | 0 | - | - | - | - | 191 | 1 | 0 | 0.003 | |
| rs773358289 | A | Pro287Ser | Screening | 192 | 0 | 0 | 0 | - | - | - | - | 191 | 1 | 0 | 0.003 | |
| rs747005297 | C | His292Arg | Screening | 191 | 1 | 0 | 0.003 | - | - | - | - | 192 | 0 | 0 | 0 | |
| rs1145086 | T | Leu418= | Screening | 69 | 93 | 30 | 0.40 | - | - | - | - | 68 | 94 | 30 | 0.40 | |
While the synonymous variant rs1136165 (p.Arg152=) was shown to have a sex-dimorphic effect on BMI (see Figures 2, 3; Supplementary Table S2), similar frequencies were found in our cases with severe obesity or AN as well as in healthy-lean controls (see Table 3). Hence, we were unable to detect associations of the T-allele (based on the forward strand) at rs1136165 with obesity (p = 0.72) or AN (p = 0.63) in our screening group (see Supplementary Table S4 and Supplementary Table S5). Nevertheless, in silico tools predicted a putative splice site-altering effect (see Table 4).
TABLE 4.
In silico predictions of splice site alterations and pathogenicity of the detected variants in CKB, CKMT1B, and GATM. To analyze the putative effects of variants on splicing products, the tools ESEfinder (Cartegni et al., 2003) and Spliceman (Lim and Fairbrother, 2012) were applied. Predictions of pathogenicity and a conservation score were obtained by MutationTaster 2021 (Steinhaus et al., 2021). Non-synonymous variants are shown in bold.
| Gene | Variant | Amino acid exchange | ESEfinder | Spliceman | MutationTaster2021 | |
|---|---|---|---|---|---|---|
| Splice site changes | Splice site—rank (%) | Prediction | PhyloP score | |||
| CKB | rs762206402 | Pro112= | Changed | 55 | Neutral | −0.723 |
| rs1136165 | Arg152= | Changed | 70 | Neutral | −0.515 | |
| rs200890896 | Ala175= | Changed | 73 | Neutral | 0.247 | |
| rs146047573 | Tyr269Cys | Changed | 66 | Neutral | 2.167 | |
| rs1803283 | Glu364= | Changed | 60 | Neutral | 1.733 | |
| CKMT1B | g.43595061C/T | Thr79= | Changed | 58 | Neutral | 1.104 |
| g.43595385C/T | Asp120= | Not changed | 65 | Neutral | −0.388 | |
| g.43595451G/A | Thr142= | Changed | 77 | Neutral | −0.731 | |
| rs1230355611 | Arg184Stop | Changed | 63 | Deleterious | 2.353 | |
| rs758572075 | Val191Ala | Changed | 70 | Neutral | 1.217 | |
| rs9571 | Lys352= | Not changed | 64 | Neutral | 0.774 | |
| rs13234 | Ala362= | Changed | 71 | Neutral | 0.430 | |
| rs149544188 | Arg399= | Changed | 66 | Neutral | 2.638 | |
| rs144820945 | Gly403= | Changed | 57 | Neutral | −0.249 | |
| GATM | rs1288775 | Gln110His | Not changed | 47 | Neutral | 0.902 |
| rs146057680 | Asp234Val | Not changed | 63 | Deleterious | 4.283 | |
| rs773358289 | Pro287Ser | Changed | 68 | Deleterious | 4.949 | |
| rs747005297 | His292Arg | Changed | 58 | Deleterious | 4.099 | |
| rs1145086 | Leu481= | Changed | 64 | Neutral | 1.626 | |
The rare, non-synonymous variant (rs146047573, p. Tyr269Cys) was detected heterozygously in a young girl from our screening group with severe obesity (BMI: 25.53 kg/m2; BMI-SDS: 2.23; 96th BMI percentile; age: 12.54 years) and neither in patients with AN nor in healthy-lean controls (see Table 3). It was predicted to be conserved and might alter splice sites, while the overall pathogenicity was indicated to be benign (see Tables 4 and 5). However, it putatively destabilizes the protein (see Table 5). Consequently, we genotyped rs146047573 in additional independent confirmation samples of 781 families with severe obesity (child or adolescent with severe obesity and both biological parents), 320 children and adolescents with severe obesity, and 253 healthy-lean controls (see Table 2). Thereby, we detected one additional female with severe obesity carrying the mutation heterozygously (BMI: 35.85 kg/m2; BMI-SDS: 2.96; 100th BMI percentile; age: 16.60 years) and no further carriers among the controls (see Table 3). No obesity association for the alternative allele (C) of rs146047573 was detected in our study groups (p = 1; see Supplementary Table S4). However, a nominal association of this variant’s alternative allele with obesity was evident upon comparison of an alternative non-Finnish European control group extracted from gnomAD with the screening group of 192 children or adolescents with severe obesity (p = 0.03) and our larger study group, including our screening and confirmation group (total of 1,293 children or adolescents with severe obesity; p = 0.02; see Table 2; Supplementary Table S4).
TABLE 5.
In silico predictions of putative effects of non-synonymous variants on protein structure. The effect predictions were obtained with the CUPSAT tool (Parthiban et al., 2006) utilizing the protein structures of CKB (PBD ID: 3DRE; Bong et al., 2008), CKMT1B (PBD ID:1QK1; Eder et al., 2000) and GATM (PBD ID: 2JDW; Humm et al., 1997) and the PANTHER-PSEP tool (Tang and Thomas, 2016). As no full-length PBD protein structure was available for CKMT1B, we could not determine the impact of rs149544188 (a). As only non-synonymous variants could be predicted, the non-sense variant rs1230355611 (p. Arg184Stop) in CKMT1B was not analysed.
| Gene | Variant | Amino acid exchange | Cupsat | Panther-PSEP | ||||
|---|---|---|---|---|---|---|---|---|
| Protein stability | Protein torsion | ΔΔG (kcal/mol) | Preservation time | Prediction | Probability of a deleterious effect | |||
| CKB | rs146047573 | Tyr269Cys | Destabilizing | Favorable | −2.93 | 361 | Possibly damaging | 0.5 |
| CKMT1B | rs758572075 | Val191Arg | Stabilizing | Favorable | 0.96 | 324 | Possibly damaging | 0.5 |
| rs149544188a | Arg399His | NA a | NA a | NA a | 361 | Possibly damaging | 0.5 | |
| GATM | rs1288775 | Gln110His | Destabilizing | Favorable | −0.3 | 6 | Probably benign | 0.02 |
| rs146057680 | Asp234Val | Destabilizing | Favorable | −1.21 | 1,368 | Probably damaging | 0.86 | |
| rs773358289 | Pro287Ser | Destabilizing | Favorable | −0.81 | 797 | Probably damaging | 0.74 | |
| rs747005297 | His292Arg | Stabilizing | Unfavorable | 0.06 | 3,806 | Probably damaging | 0.89 | |
The rare synonymous variant rs200890896 (p.Ala175=) was exclusively detected in one heterozygous female with lifetime AN (BMI: 19.53 kg/m2; BMI-SDS: 1.84; age: 45.68 years see Table 3). It was predicted to be benign while still possessing the potential to alter splicing products (see Table 4). Within our study groups, no association with AN could be ascertained (p = 1; see Supplementary Table S5). However, a nominal AN association of the variant’s G allele was found (p = 0.02) when consulting an exclusively female control group extracted from gnomAD (see Supplementary Table S3 and Supplementary Table S5).
An additional rare synonymous variant (rs762206402; p.Pro112=) indicated to exert non-pathogenic effects (see Table 4) was detected in one heterozygous adolescent with severe obesity (BMI: 39.12 kg/m2; BMI-SDS: 3.08; 100th BMI percentile; age: 15.22 years see Table 3) and not in any other group. The infrequent C-allele of rs762206402 was nominally associated with obesity (p = 0.006) when analyzing the alternative gnomAD control group (see Supplementary Tables S3, S4). Additionally, one synonymous variant (rs1803283, p.Glu364 =) was frequently found (MAF ∼40%) in all three investigated screening groups (see Table 3). Non-pathogenic but putatively splice site-altering effects were determined by in silico tools (see Table 4). Previously, a BMI association of rs1803283 was found in females (p = 1.71*10–12) and both sexes combined in a GWAS (p = 1.48*10–16; Pulit et al., 2019).
3.3 Mutation screen of CKMT1B
Nine variants in the protein-coding sequence of CKMT1B were detected in the mutation screen of 192 children and adolescents with severe obesity and 192 lean control individuals (see Table 3). Notably, deviations from the HWE were detected for the novel variant (p.Thr79=) in our screening group with severe obesity and rs13234 (p.Ala362b =) in control individuals. Accordingly, we re-checked and repeated our sequencing in the corresponding samples. The results did not differ from the initial analyses. For instance, for the novel variant p.Thr79=, we detected one heterozygous and one homozygous carrier (see Table 3), leading to deviations from the HWE. Nevertheless, as we were able to exclude genotyping errors, it is feasible that the deviation occurred by chance. Therefore, we retained these variants in the subsequent analyses. All other detected variants fulfilled the HWE.
In total, we observed six synonymous (novel p.Thr79=, novel p.Asp120=, novel p.Thr142=, rs9571, rs13234, and rs144820945), one non-sense (rs1230355611) and two non-synonymous variants (rs758572075 and rs149544188) in the CDS of CKMT1B. The non-synonymous variant rs758572075 leading to a non-conservative amino acid exchange (p.Val191Ala) was found in one individual with obesity (BMI: 35.79 kg/m2; BMI-SDS: 2.96; 100th BMI percentile; age: 16.72 years) and two healthy-lean controls, while the other non-synonymous variant, rs149544188 (p.Arg399His) was identified twice among the children and adolescents with severe obesity and once in the lean control group of the screening samples (see Table 3). Both non-synonymous variants are likely benign but potentially alter splice sites (see Table 4). Furthermore, rs758572075 was predicted to have a stabilizing effect on CKMT1B as well as a favorable impact on its torsion (see Table 5). The non-sense variant rs1230355611 (p.Arg184Stop) was exclusively detected in one lean control individual analyzed in our screening study group (see Table 3). The in silico analyses indicated a deleterious effect of this non-sense mutation (see Table 4).
To evaluate a potential relevance for weight regulation of the protein-altering variants, we genotyped the non-sense (rs1230355611) and both non-synonymous (rs758572075 and rs149544188) variants in additional independent confirmation study groups. Here, within the 781 families with severe obesity, 320 independent children and adolescents with severe obesity, and 253 additional controls (see Table 2), we detected the non-synonymous variant rs758572075 (p.Val191Ala) in one additional boy with obesity (BMI: 32.85 kg/m2; BMI-SDS: 2.57; 100th BMI percentile, age: 14.36 years). The boy was heterozygous for this variant, which the mother who had obesity herself transmitted (BMI: 38.03 kg/m2; BMI-SDS: 1.95, age: 42.94 years), while the lean father (BMI: 22.63 kg/m2; BMI-SDS: 1.54, age: 48.28 years) did not harbor the variant. No association of the C-allele of this SNP with obesity was detected within our screening (p = 1) and confirmation study groups (p = 1; both groups combined: p = 0.27) (see Supplementary Table S4).
Moreover, the non-synonymous variant rs149544188 (p.Arg399His) was detected heterozygously in eleven additional patients with obesity and five healthy-lean controls (see Table 3). Of the eleven cases with obesity, nine were derived from the index cases of the 781 trios with severe obesity. In five cases, the mother transmitted the mutant allele, while the paternal allele was inherited in another four. In an additional 16 families, the child or adolescent with obesity was not a carrier of rs149544188, but one parent harbored the variant heterozygously (not shown). Six fathers and ten mothers were mutation carriers without transmitting the respective allele to their offspring. Consequently, the transmission rate for the mutant A-allele was 36%, indicating a transmission disequilibrium with a reduced transmission rate of the infrequent A-allele (pchi-square = 0.16).
Furthermore, the non-sense variant rs1230355611 (p.Arg184Stop) was observed heterozygously in one girl with severe obesity (BMI: 31.34 kg/m2; BMI-SDS: 2.52; 99.fourth BMI percentile, age: 14.62 years; see Table 3). It was transmitted from the normal-weight mother (BMI: 24.98 kg/m2; BMI-SDS: 0.11; age: 45.12 years) to the offspring with severe obesity. There was no evidence of an obesity-predisposing effect of this variant (screening group: p = 1; confirmation group: p = 1; both groups: p = 0.45; see Supplementary Table S4).
Evidence for further phenotype-related associations was lacking (see Supplementary Table S4). Only when considering an additional control study group extracted from gnomAD (see Supplementary Table S3), the G-allele of the synonymous rs9571 (p.Lys352 =) was nominally associated (p = 0.00001) with obesity (see Supplementary Table S4). This variant was detected with the same frequency in our 192 patients with severe obesity and healthy-lean controls (MAF = 0.01; see Table 3).
3.4 Mutation screen of GATM
Sequencing of the coding region of GATM in 192 children and adolescents with severe obesity and 192 healthy-lean controls revealed one synonymous (rs1145086; p.Leu418=) and four non-synonymous variants (rs1288775, p.Gln110His; rs146057680, p.Asp234Val; rs773358289, p.Pro287Ser; and rs747005297, p.His292Arg; see Table 3). All identified variants fulfilled the HWE and were detected heterozygously. The missense variant rs1288775 and the synonymous SNP rs1145086 were frequently detected in children and adolescents with severe obesity and healthy-lean controls (MAF >20%; see Table 3). Additionally, two rare non-synonymous variants (rs146057680, p.Asp234Val, and rs773358289, p.Pro287Ser) were observed exclusively among controls. One non-synonymous variant, rs747005297 (p.His292Arg), was exclusively identified in a boy with severe obesity from our study group (BMI: 29.55 kg/m2; BMI-SDS: 2.90; 100th BMI percentile; age: 9.92 years). In silico analyses predicted the potential to affect splice sites and exhibit an overall deleterious effect (see Table 4). All non-synonymous SNPs were classified as either protein destabilizing or unfavorable regarding GATM’s torsion (see Table 5). Except for rs1288775 (p.Gln110His), all other non-synonymous variants were predicted to be deleterious (see Table 4). However, we were unable to find evidence for nominal associations of the variants localized in GATM with obesity (see Supplementary Table S4). But, when investigating the additional control cohort of Europeans extracted from gnomAD (see Supplementary Table S3), a nominal obesity association of the alternative C-allele of rs747005297 (p = 0.009) was observed (see Supplementary Table S4).
3.5 Correlations between genes and clinical parameters in visceral and subcutaneous adipose tissue
To complement our results with transcriptional data, we used RNA-seq data from 1,479 individuals of the LOBB. Based on these data, we analyzed correlations between CKB, CKMT1B, and GATM expression levels and specific metabolic and anthropometric parameters in both VAT and SAT of participants with and without obesity.
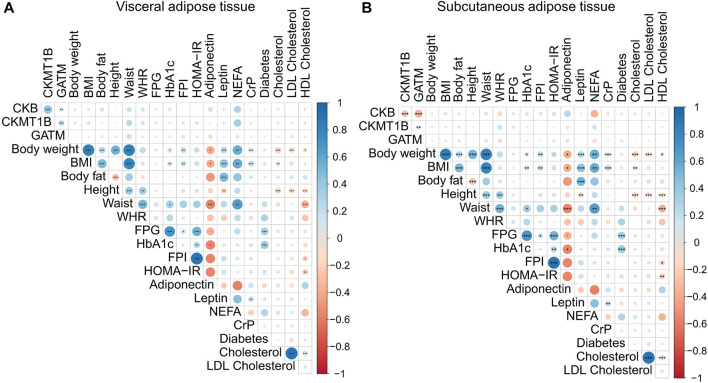
The unadjusted correlation analyses (p < 0.05) suggested relationships between several anthropometric and metabolic parameters and our genes of interest. In VAT, CKB correlated positively with CKMT1B (n = 1,479; ρ = 0.43; p < 0.001) and GATM (n = 1,479; ρ = 0.12; p < 0.001) as well as with body fat (n = 669; ρ = 0.11; p = 0.05), waist (n = 244; ρ = 0.16; p = 0.01), and non-esterified fatty acids (NEFA; n = 44; ρ = 0.30; p = 0.05; see Supplementary Figure S1). In the SAT, inverse correlations were detected. CKB was negatively correlated with CKMT1B (n = 1,479; ρ = −0.26; p < 0.001) and GATM (n = 1,479; ρ = −0.37; p < 0.001) as well as BMI (n = 1,479; ρ = −0.06; p = 0.01), homeostatic model assessment for insulin resistance (HOMA-IR; n = 416; ρ = −0.11; p = 0.02), NEFA (n = 44; ρ = −0.35; p = 0.02), and C-reactive protein (n = 1,435; ρ = −0.07; p = 0.005; see Supplementary Figure S1). CKB correlated negatively with hemoglobin A1C (hbA1C) in VAT (n = 790; ρ = −0.07; p = 0.04) and SAT (n = 790; ρ = −0.08; p = 0.03).
However, after correction for multiple inferences, only the inter-gene correlations were significant in both adipose tissues (see Figure 4 and Supplementary Figures S2, S3). As already seen in the data not corrected for multiple testing, all genes were positively correlated with CKB in VAT (CKMT1B: n = 1,479; ρ = 0.43; padj < 0.001; GATM: n = 1,479; ρ = 0.12; padj < 0.001; see Figure 4A and Supplementary Figure S3), while CKB was negatively correlated with CKMT1B (n = 1,479; ρ = - 0.26; padj. < 0.001) and GATM (n = 1,479; ρ = −0.37; padj. < 0.001) in SAT (see Figure 4B and Supplementary Figure S2). CKMT1B and GATM exhibited positive correlations in both VAT (n = 1,479; ρ = 0.21; padj. < 0.001 see Figure 4 and Supplementary Figure S3) and SAT (n = 1,479; ρ = 0.12; padj. < 0.001; see Figure 4 and Supplementary Figure S2).
FIGURE 4.
Correlations of CKB, CKMT1B, and GATM with clinical parameters. The presented data are based on RNA-sequencing data of omental visceral (A) and abdominal subcutaneous (B) adipose tissues from 1,479 individuals of the LOBB. The correlations were calculated with Pearson’s correlation coefficient. p-values were adjusted for multiple comparisons using Hommel’s method (Hommel, 1988). Positive correlations are shown in blue, while negative correlations are represented in red. The size of the dot refers to the degree of correlation. Significance; padj. < 0.001 (***), padj. < 0.01 (**), and padj. < 0.05 (*). BMI: body mass index. CrP: c-reactive protein. FPI: fasting plasma insulin. FPG: fasting plasma glucose. HbA1C: hemoglobin A1C. HDL: high-density lipoprotein. HOMA-IR: homeostatic model assessment for insulin resistance. LDL: low-density lipoprotein. NEFA: non-esterified fatty acids. WHR: waist-to-hip ratio.
Obesity and body weight regulation are influenced by sex hormones (e.g., Brettle et al., 2022; Vigil et al., 2022). As the mean age of our study groups was around 50 years (non-obese: 55.8 years old; participants with obesity: 46.9 years old) and most women experience their menopause by the age of 49 years (e.g., Davis et al., 2015), we subsequently excluded all participants above the age of 50 years (n = 465) to circumvent a putative menopause-based bias. Despite the removal of putatively post-menopausal women, differences in correlations were marginal (see Supplementary Figure S4). The inter-gene correlations in the visceral adipose tissue remained unchanged (see Supplementary Figure S4) from the analyses with all participants (see Figure 4A). Yet, the positive correlation between CKMT1B and GATM in the subcutaneous adipose tissue was not observed when participants above 50 years of age were excluded (see Supplementary Figure S4).
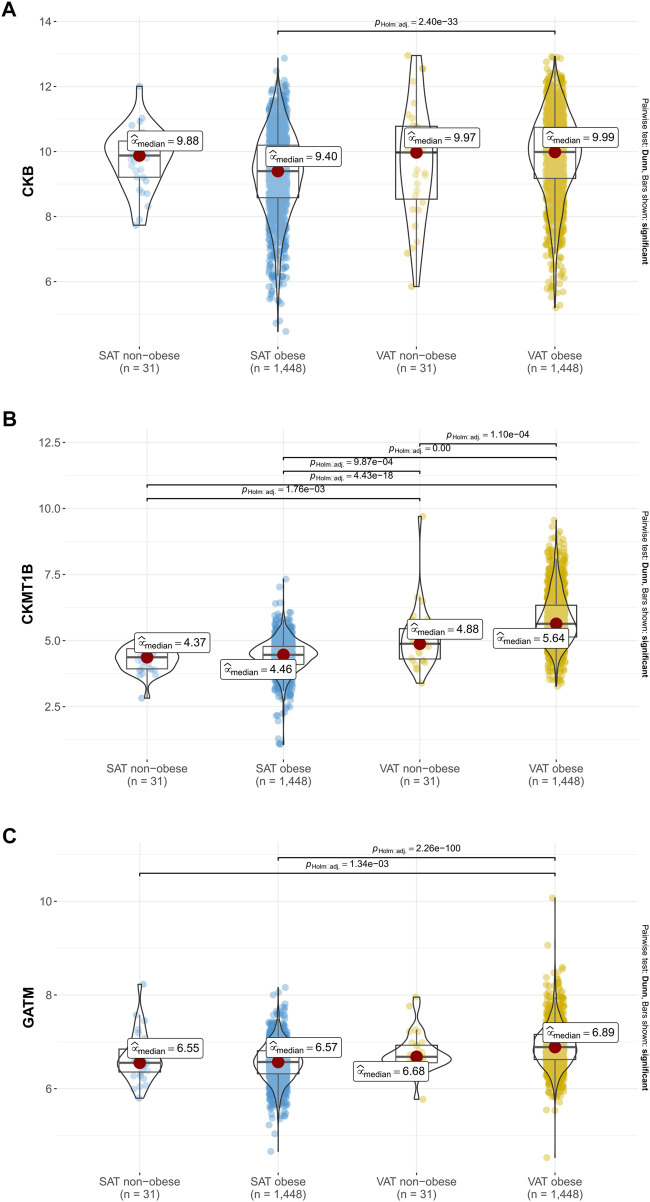
3.6 Gene expression of CKB, CKMT1B and GATM deviates between VAT and SAT
Considering the inverse correlations of CKB with CKMT1B and GATM in visceral and subcutaneous adipose tissue (see Figure 4), we subsequently performed between-subject comparisons of non-obese participants and individuals with obesity from the LOBB for all three genes studied. Increased gene expression levels in the VAT of individuals with obesity in comparison to the SAT of this study group were found for CKB (padj. < 0.001 see Figure 5A), CKMT1B (padj. < 0.001; see Figure 5B), and GATM (padj. < 0.001; see Figure 5C). Deviations between participants with and without obesity in the VAT were exclusively observed for CKMT1B (padj. < 0.001, see Figure 5B).
FIGURE 5.
Gene expression of CKB, CKMT1B and GATM in probands with and without obesity. Expression data of CKB (A), CKMT1B (B) and GATM (C) are based on 1,479 probands with and without obesity from the LOBB. As the data were not normally distributed in all instances, non-parametric Kruskal–Wallis one-way ANOVAs were performed to check whether the location parameters of the distributions were the same in each group. The corresponding effect size was calculated as epsilon square, while pairwise comparisons were performed with Dunn’s test. p-values were adjusted based on Hommel’s method (Hommel, 1988). SAT: subcutaneous adipose tissue. VAT: visceral adipose tissue.
To again prevent bias due to the menopause, we excluded females with an age above 50 years. Again, solely minor differences were detected (see Supplementary Figure S5). Thus, the VAT of participants with and without obesity showed a higher expression of CKB than the SAT of patients with obesity (see Supplementary Figure S5).
4 Discussion
Previous studies have linked the creatine metabolism to BAT thermogenesis with the potential to counteract obesity (Streijger et al., 2005; Streijger et al., 2009; Kazak et al., 2015; Kazak et al., 2017; Pan et al., 2020; Rahbani et al., 2021). Transgenic mice depleted of CKB, CKMT1B, and GATM showed impaired creatine biosynthesis capacity affecting the murine body weight and resulting in a susceptibility to obesity (Streijger et al., 2005; Streijger et al., 2009; Kazak et al., 2017; Rahbani et al., 2021). Moreover, there is evidence of sex-specific effects regarding the regulation of body weight and BAT activity in rodents (Choi et al., 2011) as well as in humans (Hinney et al., 2017; Pulit et al., 2019). Therefore, we aimed to analyze the sex-specific effects of BMI-associated variants in the genes CKB, CKMT1B and GATM. Subsequently, we performed a mutation screen of these genes and in silico analyses. Furthermore, gene expression and correlation analyses based on data from the LOBB were performed.
4.1 Sex-stratified analyses
Considering these findings, we first explored the putative sex-stratified effects of SNPs located within CKB, CKMT1B, and GATM based on data from a BMI GWAS (Pulit et al., 2019). This analysis revealed one common SNP (rs1136165, p.Arg152 =) located in the genomic region of CKB with a sex-dimorphic effect. The BMI-increasing effect (beta-value derived from GWAS = 0.02; Pulit et al., 2019) was shown to be stronger in females than in males. In the 500 kb adjacent region of CKB, more than 4% of the GWAS SNPs revealed a BMI association. Given that enhancers may have a distance of more than 1 million base pairs to the gene’s promoter they affect (Nizovtseva et al., 2017), it is feasible to assume that some of these BMI-associated SNPs in the CKB adjacent region might disrupt enhancer sequences pertaining to the gene and eventually alter its transcription. In contrast, we did not detect evidence for SNPs exerting any effects, including sex-specific effects on CKMT1B and GATM. Moreover, by analyzing data from the latest AN GWAS (Watson et al., 2019), we could not determine variants associated with AN in the regions of interest (CKB, CKMT1B, GATM, and adjacent regions).
4.2 Mutation screen and confirmation studies
The CDSs of CKB, CKMT1B, and GATM were subsequently screened for variants in 192 children and adolescents with severe obesity as well as 192 healthy-lean controls. Furthermore, considering the abovementioned sex-specific GWAS hit, the observation that (1) AN and BMI share certain loci (Bulik-Sullivan et al., 2015; Hinney et al., 2017; Zheng et al., 2022) and 2) AN mainly affects females (Hübel et al., 2019; Mitchell and Peterson, 2020), we additionally screened the coding regions of CKB as the most likely target regarding creatine metabolism in 192 female patients with AN.
We identified five genetic variants in the coding regions of CKB and GATM each, while the CDS of CKMT1B revealed nine variants. The presence of infrequent non-synonymous variants in CKB and CKMT1B (rs146047573, rs758572075, rs1230355611, and rs149544188) was confirmed in independent study groups comprising 781 families with severe obesity, 320 children and adolescents with severe obesity, and 253 healthy lean controls (see Table 2). No evidence for associations of these variants with either obesity or AN was found (see Supplementary Tables S4, S5). Moreover, three novel variants within CKMT1B were detected. As these variants have not previously been described in any other population or regarding other phenotypes, it is not unlikely that, even though no association was detected, these variants might have a putative relevance for obesity. Furthermore, we detected that the variant rs149544188 located in CKMT1B was nominally less frequently transmitted by a heterozygous parent to the child with obesity than expected (transmission rate of 36%, pChi-square = 0.16).
In our study groups, we were unable to determine associations of the detected variants with obesity or AN. Presumably, the comparatively small sample size (see Table 2) as well as the low minor allele frequencies (see Table 3) of the detected variants may account for the lack of associations. Thus, we used larger datasets from gnomAD’s non-Finnish European population as an alternative control group (see Supplementary Tables S3–S5). With this, the infrequent alleles of the variants rs76220640 (p.Pro112 =) and rs146047573 (p.Tyr269Cys) located in CKB, the CKMT1B variant rs9571's allele (p.Lys352 =) as well as the allele of the GATM-located variant rs747005297 (p.His292Arg) were nominally associated with obesity. The infrequent allele at rs200890896 (p.Ala175 =) exhibited a nominal association with AN.
Regarding the interpretation of findings in comparison to the gnomAD control group, it is important to note that no phenotypic data were available regarding the data in this database. The gnomAD database mainly includes genotype information from case‒control studies regarding conditions with an onset in adulthood, such as cardiovascular diseases (Karczewski et al., 2020). Since these diseases are comorbidities of an increased BMI and obesity (Williams et al., 2015; Blüher, 2019), our statistical results using this control cohort might be biased by the recruitment of participants with an elevated BMI. However, as our study group consisted of children and adolescents with severe obesity at the extremes of the BMI distribution in the general population, we can assume that the gnomAD control group included only a few individuals with a similar degree of obesity. Finally, we cannot exclude confounding with respect to ethnic background upon the comparison of our German study groups with the gnomAD control group.
4.3 In silico analyses
Most of the identified non-synonymous variants in the genes of interest were predicted to impact the protein structure. Previously, alterations in the biochemical and biophysical properties of CKB caused by distinct genetic variations leading to amino acid substitutions have been analyzed (Li et al., 2012). Eight non-synonymous variants in CKB were studied. The results demonstrated that half of the variants’ amino acid substitutions affected the catalytic efficiency. For instance, the resultant amino acid exchange of rs13558 (p.Lys267GLu) increases the enzymatic activity by ∼ 30% compared to CKB containing the wild-type lysine (Li et al., 2012). Further amino acid substitution due to other SNP alleles conversely impaired the catalytic efficiency. In mice, some mutated alleles even implied reduced thermal stability below the physiological temperature of 37 °C (Li et al., 2012). Li et al. (2012) suspected that this impairment is related to thermoregulation in the double-KO mouse model (Streijger et al., 2005; Streijger et al., 2009; Li et al., 2012). Our in silico analyses predominantly pointed to benign effects, while the majority of variants exhibited the potential to alter splicing products. Only four non-synonymous variants were predicted to be deleterious overall (see Table 4). One of these variants (rs747005297) located in GATM, which was nominally associated with obesity (based on the alternative control group from gnomAD), was indicated to be protein stabilizing but unfavorable for GATM’s torsion (see Table 5). However, in silico analyses can merely indicate putative consequences of genetic variants and are no substitute for subsequent in vitro studies, which might reveal underlying pathological mechanisms. Particularly considering findings regarding the double-KO mouse model discussed above (Streijger et al., 2005), it is conceivable that polymorphisms in the genes studied here might have an influence on body weight in humans as well. Additionally, as mouse models already suggest compensatory processes between creatine kinases (Streijger et al., 2005), it is possible that mutations in either kinases or even all three genes would have a more severe impact on BMI.
4.4 Gene expression and correlation analyses
By analyzing RNA-seq data of VAT and SAT of 1,479 individuals (1,448 with obesity and 31 non-obese) of the Leipzig Obesity Biobank, we detected that all three genes of interest (CKB, CKMT1B and GATM) showed positive correlations with each other in visceral fat. However, a negative correlation of CKB with CKMT1B and GATM was observed in SAT. Further correlations with anthropometric or metabolic parameters, such as BMI or body fat, were not significant after correction for multiple comparisons. For CKB, CKMT1B and GATM, subsequent analyses revealed gene expression differences between VAT and SAT in 1,448 adipose tissue donors with obesity. We found consistently higher gene expression levels of the three genes in VAT than in SAT. Additionally, VAT CKMT1B expression was higher in patients with obesity in comparison to individuals with a BMI lower than 30 kg/m2.
As expected for genes belonging to the creatine metabolism pathway (Kazak et al., 2015; Kazak et al., 2017), we detected positive correlations between the genes in VAT. Yet, negative correlations among the three genes were found in SAT. Moreover, we found positive correlations between uncorrected gene expression of CKB, CKMT1B, and GATM with body fat in VAT and a negative correlation with BMI in SAT. After correcting for multiple testing, however, these correlations were not significant which indicate small effects that require even larger sample sizes.
CKB was negatively correlated with the other two genes in SAT, while positive correlations were observed in VAT. Differences between the two fat depots are known (e.g., Korsic et al., 2012; Spoto et al., 2014; Ronquillo et al., 2019), and CKB may reflect fat depot specific expression signatures. Expression differences of creatine metabolism pathway genes may therefore reflect functional differences between visceral and subcutaneous fat depots that have been previously reported (Gesta et al., 2006; Spoto et al., 2014; Mittal, 2019; Norreen-Thorsen et al., 2022). Furthermore, global transcriptomic differences in VAT and SAT of individuals with obesity and healthy controls revealed differential expression profiles in a recent study (Ronquillo et al., 2019).
Further, after the removal of 465 female participants older than 50 years, deviations in correlation and gene expression were minimal in comparison to the analysis including all individuals. However, we could depict differences between the inter-gene correlation of CKMT1B and GATM in the subcutaneous adipose tissue when excluding participants above the menopausal age. Based on our data, we can assume that CKB, CKMT1B and GATM are influenced only slightly by sex hormones. Yet, one proteome study in BAT of rats from 2011 revealed 48 proteins exhibiting a sex-specific regulation. Numerous of these proteins affect thermogenesis, such as the creatine kinase, and are more highly expressed in female than male rats (Choi et al., 2011). In order to gain unambiguous evidence whether sex hormones impact the expression of those genes, further and, above all, more targeted analyses are necessary. However, the results of our gene correlation analyses need to be interpreted with caution as the associations were of a small to moderate size (Schober et al., 2018). Nevertheless, we detected adipose tissue-specific expression and correlations of our genes of interest indicating that the alternative creatine-dependent mechanism regulating thermogenesis might be more active in VAT.
5 Conclusion
Taken together, one GWAS-based variant (rs1136165) located in CKB related to creatine metabolism exhibits a sex-stratified BMI effect. In the subsequent mutation screen of the coding regions of CKB, CKTM1B, and GATM and a larger confirmation group, five variants in the CDS of each CKB and GATM and nine variants in the coding region of CKMT1B were detected. No associations of the detected variants with either obesity or AN were found within our study groups. The TDT in 781 samples derived from trios with severe obesity (child or adolescent with severe obesity and both biological parents) suggested a nominal protective effect for obesity of the A-allele at rs149544188 located in CKMT1B. A nominally reduced transmission rate of 36% was determined. Analyses of a larger control cohort based on gnomAD revealed nominal associations of the infrequent alleles of the variants rs762206402 (p.Pro112=), rs146047573 (p.Tyr269Cys), rs9571 (p.Lys352=) and rs747005297 (p.His292Arg) with obesity. The alternative allele at rs200890896 (p.Ala175=) was nominally associated with AN. With our subsequent gene expression and correlation analyses, we were able to show adipose-tissue specific correlations patterns of CKB with CKMT1B and GATM. Furthermore, we detected higher expression levels of all genes in VAT than in SAT. CKMT1B’s expression was even higher in individuals with obesity than non-obese individuals. Hence, our results help to pin down potential targets for subsequent in vitro analyses that could reveal the true impact of the identified variants and explain underlying mechanisms.
Acknowledgments
We thank all participants and their families for their participation. We are further indebted to S. Düerkop for her excellent technical support.
Funding Statement
This study was funded by the Deutsche Forschungsgesellschaft (DFG; HI 865/2-1), the BMBF (01G0820, PALGER 2017-33: 01DH19010) and the Stiftung Unversitätsmedizin Essen. TDM received funding from the German Research Foundation (DFG TRR296, TRR152, SFB1123 and GRK 2816/1), the German Center for Diabetes Research (DZD e.V.) and the European Research Council ERC-CoG Trusted no. 101044445. MB received funding from the DFG (209933838, SFB 1052) and from the German Center for Diabetes Research (Deutsches Zentrum für Diabetesforschung; DZD, 82DZD00601). We further acknowledge support by the Open Access Publication Fund of the University of Duisburg-Essen.
Data availability statement
The datasets for this article are not publicly available due to concerns regarding participant/patient anonymity. Requests to access the datasets should be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics committees of the respective universities and institutions (e.g., Ethics Committee of the University of Leipzig and Ethics Committee of the Medical Faculty of the University of Duisburg-Essen). Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
RH, TDM, MB, LT, and AHi designed and prepared the study. JH, BH-D, JS, MdZ, WH, SE, SZ, KE, RB, MF, and AHi assembled the samples. RH designed the primers. RH, BM-D, and LSR performed the mutation screen in the screening study group. YZ and LSR performed genotyping in the confirmation group and GWAS-related sex-specific analysis. LSR conducted the in silico analysis. TP and LSR performed the statistical analysis. MB and CW contributed the LOBB data. AG, WS, HD, and FN performed RNA-seq. of the LOBB samples. AHo performed bioinformatics analyses of the LOBB sequencing data. LSR has written the manuscript. All authors have read and approved the final version of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2023.1128133/full#supplementary-material
References
- Arcelus J., Mitchell A. J., Wales J., Nielsen S. (2011). Mortality rates in patients with anorexia nervosa and other eating disorders. A meta-analysis of 36 studies. Arch. Gen. Psychiatry 68, 724–731. 10.1001/archgenpsychiatry.2011.74 [DOI] [PubMed] [Google Scholar]
- Blüher M. (2019). Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 15, 288–298. 10.1038/s41574-019-0176-8 [DOI] [PubMed] [Google Scholar]
- Bong S. M., Moon J. H., Nam K. H., Lee K. S., Chi Y. M., Hwang K. Y. (2008). Structural studies of human brain-type creatine kinase complexed with the ADP-Mg2+-NO3- -creatine transition-state analogue complex. FEBS Lett. 582, 3959–3965. 10.1016/j.febslet.2008.10.039 [DOI] [PubMed] [Google Scholar]
- Boraska V., Davis O. S., Cherkas L. F., Helder S. G., Harris J., Krug I., et al. (2012). Genome-wide association analysis of eating disorder-related symptoms, behaviors, and personality traits. Am. J. Med. Genet. B Neuropsychiatr. Genet. 159B, 803–811. 10.1002/ajmg.b.32087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray N. L., Pimentel H., Melsted P., Pachter L. (2016). Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 34, 525–527. 10.1038/nbt.3519 [DOI] [PubMed] [Google Scholar]
- Brettle H., Tran V., Drummond G. R., Franks A. E., Petrovski S., Vinh A., et al. (2022). Sex hormones, intestinal inflammation, and the gut microbiome: Major influencers of the sexual dimorphisms in obesity. Front. Immunol. 13, 971048. 10.3389/fimmu.2022.971048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulik-Sullivan B., Finucane H. K., Anttila V., Gusev A., Day F. R., Loh P. R., Reprogen C., Psychiatric Genomics C., Duncan L., Perry J. R., Patterson N., Robinson E. B., Daly M. J., Price A. L., Neale B. M. C. (2015). An atlas of genetic correlations across human diseases and traits. Nat. Genet. 47, 1236–1241. 10.1038/ng.3406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartegni L., Wang J., Zhu Z., Zhang M. Q., Krainer A. R. (2003). ESEfinder: A web resource to identify exonic splicing enhancers. Nucleic Acids Res. 31, 3568–3571. 10.1093/nar/gkg616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Zhou Y., Chen Y., Gu J. (2018). fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34, i884–i890. 10.1093/bioinformatics/bty560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi D. K., Oh T. S., Choi J. W., Mukherjee R., Wang X., Liu H., et al. (2011). Gender difference in proteome of Brown adipose tissues between male and female rats exposed to a high fat diet. Cell Physiol. Biochem. 28, 933–948. 10.1159/000335807 [DOI] [PubMed] [Google Scholar]
- Chouchani E. T., Kazak L., Spiegelman B. M. (2019). New advances in adaptive thermogenesis: UCP1 and beyond. Cell Metab. 29, 27–37. 10.1016/j.cmet.2018.11.002 [DOI] [PubMed] [Google Scholar]
- Davis S. R., Lambrinoudaki I., Lumsden M., Mishra G. D., Pal L., Rees M., et al. (2015). Menopause. Nat. Rev. Dis. Prim. 1, 15004. 10.1038/nrdp.2015.4 [DOI] [PubMed] [Google Scholar]
- Eder M., Fritz-Wolf K., Kabsch W., Wallimann T., Schlattner U. (2000). Crystal structure of human ubiquitous mitochondrial creatine kinase. Proteins 39, 216–225. [DOI] [PubMed] [Google Scholar]
- Finkelstein E. A., Khavjou O. A., Thompson H., Trogdon J. G., Pan L., Sherry B., et al. (2012). Obesity and severe obesity forecasts through 2030. Am. J. Prev. Med. 42, 563–570. 10.1016/j.amepre.2011.10.026 [DOI] [PubMed] [Google Scholar]
- Gesta S., Bluher M., Yamamoto Y., Norris A. W., Berndt J., Kralisch S., et al. (2006). Evidence for a role of developmental genes in the origin of obesity and body fat distribution. Proc. Natl. Acad. Sci. U. S. A. 103, 6676–6681. 10.1073/pnas.0601752103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Muniesa P., Martínez-González M., Hu F., Després J.-P., Matsuzawa Y., Loos R., et al. (2017). Obesity. Nat. Rev. Dis. Prim. 3, 17034. 10.1038/nrdp.2017.34 [DOI] [PubMed] [Google Scholar]
- Hettling H., Van Beek J. H. (2011). Analyzing the functional properties of the creatine kinase system with multiscale 'sloppy' modeling. PLoS Comput. Biol. 7, e1002130. 10.1371/journal.pcbi.1002130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinney A., Kesselmeier M., Jall S., Volckmar A. L., Focker M., Antel J., et al. (2017). Evidence for three genetic loci involved in both anorexia nervosa risk and variation of body mass index. Mol. Psychiatry 22, 321–322. 10.1038/mp.2016.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinney A., Korner A., Fischer-Posovszky P. (2022). The promise of new anti-obesity therapies arising from knowledge of genetic obesity traits. Nat. Rev. Endocrinol. 18, 623–637. 10.1038/s41574-022-00716-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinney A., Nguyen T. T., Scherag A., Friedel S., Bronner G., Muller T. D., et al. (2007). Genome wide association (GWA) study for early onset extreme obesity supports the role of fat mass and obesity associated gene (FTO) variants. PLoS One 2, e1361. 10.1371/journal.pone.0001361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommel G. (1988). A stagewise rejective multiple test procedure based on a modified Bonferroni test. Biometrika 75, 383–386. 10.1093/biomet/75.2.383 [DOI] [Google Scholar]
- Hübel C., Marzi S. J., Breen G., Bulik C. M. (2019). Epigenetics in eating disorders: A systematic review. Mol. Psychiatry 24, 901–915. 10.1038/s41380-018-0254-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humm A., Fritsche E., Steinbacher S., Huber R. (1997). Crystal structure and mechanism of human L-arginine:glycine amidinotransferase: A mitochondrial enzyme involved in creatine biosynthesis. EMBO J. 16, 3373–3385. 10.1093/emboj/16.12.3373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen F., Bardoutsos A., Vidra N. (2020). Obesity prevalence in the long-term future in 18 European countries and in the USA. Obes. Facts 13, 514–527. 10.1159/000511023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost C. R., Van Der Zee C. E., Zandt T. H. J., Oerlemans F., Verheij M., Streijger F., et al. (2002). Creatine kinase B-driven energy transfer in the brain is important for habituation and spatial learning behaviour, mossy fibre field size and determination of seizure susceptibility. Eur. J. Neurosci. 15, 1692–1706. 10.1046/j.1460-9568.2002.02001.x' [DOI] [PubMed] [Google Scholar]
- Kaikaew K., Grefhorst A., Visser J. A. (2021). Sex differences in Brown adipose tissue function: Sex hormones, glucocorticoids, and their crosstalk. Front. Endocrinol. (Lausanne) 12, 652444. 10.3389/fendo.2021.652444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karczewski K. J., Francioli L. C., Tiao G., Cummings B. B., Alfoldi J., Wang Q., et al. (2020). The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 581, 434–443. 10.1038/s41586-020-2308-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazak L., Chouchani E. T., Jedrychowski M. P., Erickson B. K., Shinoda K., Cohen P., et al. (2015). A creatine-driven substrate cycle enhances energy expenditure and thermogenesis in beige fat. Cell 163, 643–655. 10.1016/j.cell.2015.09.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazak L., Chouchani E. T., Lu G. Z., Jedrychowski M. P., Bare C. J., Mina A. I., et al. (2017). Genetic depletion of adipocyte creatine metabolism inhibits diet-induced thermogenesis and drives obesity. Cell Metab. 26, 693. 10.1016/j.cmet.2017.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazak L., Rahbani J. F., Samborska B., Lu G. Z., Jedrychowski M. P., Lajoie M., et al. (2019). Ablation of adipocyte creatine transport impairs thermogenesis and causes diet-induced obesity. Nat. Metab. 1, 360–370. 10.1038/s42255-019-0035-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khramtsova E. A., Heldman R., Derks E. M., Yu D., Davis L. K., Stranger B. E. (2019). Sex differences in the genetic architecture of obsessive-compulsive disorder, Am. J. Med. Genet. B Neuropsychiatr. Genet. 180, 351–364. TOURETTE SYNDROME/OBSESSIVE-COMPULSIVE DISORDER WORKING GROUP OF THE PSYCHIATRIC GENOMICS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloting N., Fasshauer M., Dietrich A., Kovacs P., Schon M. R., Kern M., et al. (2010). Insulin-sensitive obesity. Am. J. Physiol. Endocrinol. Metab. 299, E506–E515. 10.1152/ajpendo.00586.2009 [DOI] [PubMed] [Google Scholar]
- Korsic M., Gotovac K., Nikolac M., Dusek T., Skegro M., Muck-Seler D., et al. (2012). Gene expression in visceral and subcutaneous adipose tissue in overweight women. Front. Biosci. (Elite Ed. 4, 2734–2744. 10.2741/e587 [DOI] [PubMed] [Google Scholar]
- Langhardt J., Flehmig G., Kloting N., Lehmann S., Ebert T., Kern M., et al. (2018). Effects of weight loss on glutathione peroxidase 3 serum concentrations and adipose tissue expression in human obesity. Obes. Facts 11, 475–490. 10.1159/000494295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Zhang Q., Hu W. J., Mu H., Lin Z., Ma L., et al. (2012). Effect of SNPs on creatine kinase structure and function: Identifying potential molecular mechanisms for possible creatine kinase deficiency diseases. PLoS One 7, e45949. 10.1371/journal.pone.0045949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim K. H., Fairbrother W. G. (2012). Spliceman--a computational web server that predicts sequence variations in pre-mRNA splicing. Bioinformatics 28, 1031–1032. 10.1093/bioinformatics/bts074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love M. I., Huber W., Anders S. (2014). Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550. 10.1186/s13059-014-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell J. E., Peterson C. B. (2020). Anorexia nervosa. N. Engl. J. Med. 382, 1343–1351. 10.1056/NEJMcp1803175 [DOI] [PubMed] [Google Scholar]
- Mittal B. (2019). Subcutaneous adipose tissue and visceral adipose tissue. Indian J. Med. Res. 149, 571–573. 10.4103/ijmr.IJMR_1910_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris S. M., JR. (2004). Enzymes of arginine metabolism. J. Nutr. 134, 2743S–2747S. 10.1093/jn/134.10.2743S [DOI] [PubMed] [Google Scholar]
- Nizovtseva E. V., Todolli S., Olson W. K., Studitsky V. M. (2017). Towards quantitative analysis of gene regulation by enhancers. Epigenomics 9, 1219–1231. 10.2217/epi-2017-0061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norreen-Thorsen M., Struck E. C., Oling S., Zwahlen M., Von Feilitzen K., Odeberg J., et al. (2022). A human adipose tissue cell-type transcriptome atlas. Cell Rep. 40, 111046. 10.1016/j.celrep.2022.111046 [DOI] [PubMed] [Google Scholar]
- Pan R., Zhu X., Maretich P., Chen Y. (2020). Combating obesity with thermogenic fat: Current challenges and advancements. Front. Endocrinol. (Lausanne) 11, 185. 10.3389/fendo.2020.00185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthiban V., Gromiha M. M., Schomburg D. (2006). Cupsat: Prediction of protein stability upon point mutations. Nucleic Acids Res. 34, W239–W242. 10.1093/nar/gkl190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil I. (2021). Visualizations with statistical details: The'ggstatsplot'approach. J. Open Source Softw. 6, 3167. 10.21105/joss.03167 [DOI] [Google Scholar]
- Picelli S., Faridani O. R., Bjorklund A. K., Winberg G., Sagasser S., Sandberg R. (2014). Full-length RNA-seq from single cells using Smart-seq2. Nat. Protoc. 9, 171–181. 10.1038/nprot.2014.006 [DOI] [PubMed] [Google Scholar]
- Pulit S. L., Stoneman C., Morris A. P., Wood A. R., Glastonbury C. A., Tyrrell J., et al. (2019). Meta-analysis of genome-wide association studies for body fat distribution in 694 649 individuals of European ancestry. Hum. Mol. Genet. 28, 166–174. 10.1093/hmg/ddy327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahbani J. F., Roesler A., Hussain M. F., Samborska B., Dykstra C. B., Tsai L., et al. (2021). Creatine kinase B controls futile creatine cycling in thermogenic fat. Nature 590, 480–485. 10.1038/s41586-021-03221-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronquillo M. D., Mellnyk A., Cardenas-Rodriguez N., Martinez E., Comoto D. A., Carmona-Aparicio L., et al. (2019). Different gene expression profiles in subcutaneous and visceral adipose tissues from Mexican patients with obesity. Indian J. Med. Res. 149, 616–626. 10.4103/ijmr.IJMR_1165_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherag A., Dina C., Hinney A., Vatin V., Scherag S., Vogel C. I., et al. (2010). Two new Loci for body-weight regulation identified in a joint analysis of genome-wide association studies for early-onset extreme obesity in French and German study groups. PLoS Genet. 6, e1000916. 10.1371/journal.pgen.1000916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schienkiewitz A., Mensink G., Kuhnert R., Lange C. (2017). Übergewicht und Adipositas bei Erwachsenen in Deutschland. Epidemiologie und Gesundheitsberichterstattung: Robert Koch-Institut. [Google Scholar]
- Schober P., Boer C., Schwarte L. A. (2018). Correlation coefficients: Appropriate use and interpretation. Anesth. Analg. 126, 1763–1768. 10.1213/ANE.0000000000002864 [DOI] [PubMed] [Google Scholar]
- Shi H., Clegg D. J. (2009). Sex differences in the regulation of body weight. Physiol. Behav. 97, 199–204. 10.1016/j.physbeh.2009.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielman R. S., Mcginnis R. E., Ewens W. J. (1993). Transmission test for linkage disequilibrium: The insulin gene region and insulin-dependent diabetes mellitus (IDDM). Am. J. Hum. Genet. 52, 506–516. [PMC free article] [PubMed] [Google Scholar]
- Spoto B., Di Betta E., Mattace-Raso F., Sijbrands E., Vilardi A., Parlongo R. M., et al. (2014). Pro- and anti-inflammatory cytokine gene expression in subcutaneous and visceral fat in severe obesity. Nutr. Metab. Cardiovasc Dis. 24, 1137–1143. 10.1016/j.numecd.2014.04.017 [DOI] [PubMed] [Google Scholar]
- Steinhaus R., Proft S., Schuelke M., Cooper D. N., Schwarz J. M., Seelow D. (2021). MutationTaster2021. Nucleic Acids Res. 49, W446–W451. 10.1093/nar/gkab266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streijger F., Jost C. R., Oerlemans F., Ellenbroek B. A., Cools A. R., Wieringa B., et al. (2004). Mice lacking the UbCKmit isoform of creatine kinase reveal slower spatial learning acquisition, diminished exploration and habituation, and reduced acoustic startle reflex responses. Mol. Cell Biochem. 256-257, 305–318. 10.1023/b:mcbi.0000009877.90129.e3 [DOI] [PubMed] [Google Scholar]
- Streijger F., Oerlemans F., Ellenbroek B. A., Jost C. R., Wieringa B., Van Der Zee C. E. (2005). Structural and behavioural consequences of double deficiency for creatine kinases BCK and UbCKmit. Behav. Brain Res. 157, 219–234. 10.1016/j.bbr.2004.07.002 [DOI] [PubMed] [Google Scholar]
- Streijger F., Pluk H., Oerlemans F., Beckers G., Bianco A. C., Ribeiro M. O., et al. (2009). Mice lacking brain-type creatine kinase activity show defective thermoregulation. Physiol. Behav. 97, 76–86. 10.1016/j.physbeh.2009.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H., Thomas P. D. (2016). PANTHER-PSEP: Predicting disease-causing genetic variants using position-specific evolutionary preservation. Bioinformatics 32, 2230–2232. 10.1093/bioinformatics/btw222 [DOI] [PubMed] [Google Scholar]
- Vigil P., Melendez J., Petkovic G., Del Rio J. P. (2022). The importance of estradiol for body weight regulation in women. Front. Endocrinol. (Lausanne) 13, 951186. 10.3389/fendo.2022.951186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Nie J., Sicotte H., Li Y., Eckel-Passow J. E., Dasari S., et al. (2016). Measure transcript integrity using RNA-seq data. BMC Bioinforma. 17, 58. 10.1186/s12859-016-0922-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Wang S., Li W. (2012). RSeQC: Quality control of RNA-seq experiments. Bioinformatics 28, 2184–2185. 10.1093/bioinformatics/bts356 [DOI] [PubMed] [Google Scholar]
- Ward Z. J., Bleich S. N., Cradock A. L., Barrett J. L., Giles C. M., Flax C., et al. (2019). Projected U.S. State-level prevalence of adult obesity and severe obesity. N. Engl. J. Med. 381, 2440–2450. 10.1056/NEJMsa1909301 [DOI] [PubMed] [Google Scholar]
- Watson H. J., Yilmaz Z., Thornton L. M., Hubel C., Coleman J. R. I., Gaspar H. A., Bryois J., Hinney A., Leppa V. M., Mattheisen M., Medland S. E., Ripke S., Yao S., Giusti-Rodriguez P., Anorexia Nervosa Genetics I., Hanscombe K. B., Purves K. L., Adan R. A. H., Alfredsson L., Ando T., Andreassen O. A., Baker J. H., Berrettini W. H., Boehm I., Boni C., Perica V. B., Buehren K., Burghardt R., Cassina M., Cichon S., Clementi M., Cone R. D., Courtet P., Crow S., Crowley J. J., Danner U. N., Davis O. S. P., De Zwaan M., Dedoussis G., Degortes D., Desocio J. E., Dick D. M., Dikeos D., Dina C., Dmitrzak-Weglarz M., Docampo E., Duncan L. E., Egberts K., Ehrlich S., Escaramis G., Esko T., Estivill X., Farmer A., Favaro A., Fernandez-Aranda F., Fichter M. M., Fischer K., Focker M., Foretova L., Forstner A. J., Forzan M., Franklin C. S., Gallinger S., Giegling I., Giuranna J., Gonidakis F., Gorwood P., Mayora M. G., Guillaume S., Guo Y., Hakonarson H., Hatzikotoulas K., Hauser J., Hebebrand J., Helder S. G., Herms S., Herpertz-Dahlmann B., Herzog W., Huckins L. M., Hudson J. I., Imgart H., Inoko H., Janout V., Jimenez-Murcia S., Julia A., Kalsi G., Kaminska D., Kaprio J., Karhunen L., Karwautz A., Kas M. J. H., Kennedy J. L., Keski-Rahkonen A., Kiezebrink K., Kim Y. R., Klareskog L., Klump K. L., Knudsen G. P. S., La Via M. C., et al. C. (2019). Genome-wide association study identifies eight risk loci and implicates metabo-psychiatric origins for anorexia nervosa. Nat. Genet. 51, 1207–1214. 10.1038/s41588-019-0439-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams E. P., Mesidor M., Winters K., Dubbert P. M., Wyatt S. B. (2015). Overweight and obesity: Prevalence, consequences, and causes of a growing public Health problem. Curr. Obes. Rep. 4, 363–370. 10.1007/s13679-015-0169-4 [DOI] [PubMed] [Google Scholar]
- World Health Organisation W. (2000). Obesity: Preventing and managing the global epidemic. [PubMed] [Google Scholar]
- Zheng Y., Rajcsanyi L. S., Herpertz-Dahlmann B., Seitz J., De Zwaan M., Herzog W., et al. (2022). PTBP2 - a gene with relevance for both Anorexia nervosa and body weight regulation. Transl. Psychiatry 12, 241. 10.1038/s41398-022-02018-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets for this article are not publicly available due to concerns regarding participant/patient anonymity. Requests to access the datasets should be directed to the corresponding author.