Abstract
Ectopic pancreatic tissue can occasionally cause inflammation, hemorrhage, stenosis, and invagination, similar to normal pancreatic tissue; however, tumorigenesis is rare. This case report describes an ectopically observed pancreatic acinar cell carcinoma in the thoracic cavity of a female Fischer (F344/DuCrlCrlj) rat. Histopathologically, polygonal tumor cells with periodic acid-Schiff-positive cytoplasmic eosinophilic granules showed solid proliferation and infrequently formed acinus-like structures. Immunohistochemically, the tumor cells were positive for cytokeratin, trypsin, and human B-cell leukemia/lymphoma 10, which specifically reacted with pancreatic acinar cells, and negative for vimentin and human α-smooth muscle actin. Ectopic pancreas develops in the submucosa of the gastrointestinal tract; however, there are few reports of its development and neoplasia in the thoracic cavity. To the best of our knowledge, this is the first report of ectopic pancreatic acinar cell carcinoma in the thoracic cavity of a rat.
Keywords: acinar cell carcinoma, ectopic tissue, pancreas, thoracic cavity, rats
Ectopic pancreas, a rare incidental finding, is a developmental abnormality of pancreatic tissues and organs other than the normal pancreatic tissues. It is primarily found in the stomach, duodenum, jejunum, mesocolon, and Meckel’s diverticulum and rarely in the anterior mediastinum and esophagus of the thoracic cavity in humans1, 2. Its incidence is between 0.5 to 13.7% at autopsy; however, this may be underestimated, as no clinical symptoms are observed in most cases3. In animals, ectopic pancreas has been reported in the stomach and intestine of dogs, cats, horses, rabbits, and pigs4, 5; however, its occurrence in rats and mice is extremely rare compared to that in humans6, 7, 8. Ectopic pancreatic tissue may cause inflammation, hemorrhage, stenosis, and invagination, similar to normal pancreatic tissue; however, tumorigenesis is rare3. There are a few reports of adenocarcinomas arising from the ectopic pancreas in the upper gastrointestinal tract and anterior mediastinum in humans2, 9, 10, 11. Histopathologically, most cases are ductal adenocarcinomas, with only a few cases of pancreatic acinar cell carcinomas. In rats, no tumor originating from the ectopic pancreas has been reported12. In this study, we report an extremely rare case of ectopic pancreatic acinar cell carcinoma in the thoracic cavity.
The affected animal was a 4-week old female Fischer (F344/DuCrlCrlj) rat purchased from Atsugi Breeding Center, Jackson Laboratories Japan, Inc., formerly known as Charles River Laboratories (Kanagawa, Japan). The rat was assigned to a high-dose group in a two-year feeding carcinogenicity study and housed in a wire-mesh stainless steel cage in a barrier-sustained animal housing room controlled at 22 ± 2°C, humidity 50% ± 20%, ventilation ≥10 times per hour (all-fresh-air basis), and illumination 12 h/day. The rat was provided a commercial diet (MF Mash, Oriental Yeast Co., Ltd., Tokyo, Japan) and tap water ad libitum. This study was conducted in accordance with the Guidelines for Animal Experimentation issued by the Japanese Association for Laboratory Animal Science13 and the Code of Ethics for Animal Experimentation of the Institute of Environmental Toxicology.
The rat exhibited emaciation, decreased spontaneous motor activity, and bradypnea at 100 weeks of age. It was deeply anesthetized with isoflurane and euthanized via exsanguination. At necropsy, a well-demarcated brown mass (35 mm in diameter) attached to the thoracic vertebra was observed in the thoracic cavity, which was located caudal to the lung at the midline and pushed the stomach backward through the diaphragm without continuity with the abdominal organs (Fig. 1). As no other animals in the treatment group had such masses, we considered that this mass was not related to the treatment with the test substance. Additionally, other macroscopic findings in this rat were spontaneous age-related lesions and not related to the mass. The thoracic mass was fixed in 10% neutral-buffered formalin, embedded in paraffin wax, and stained with hematoxylin and eosin. The sections were stained with periodic acid-Schiff (PAS) and immunohistochemically stained with human pan-cytokeratin (clone AE1/AE3, monoclonal, prediluted, Dako, Glostrup, Denmark), mouse vimentin (clone V9, monoclonal, prediluted, Dako), human α-smooth muscle actin (α-SMA, clone1A4, monoclonal, 1:100, Dako), mouse proliferating cell nuclear antigen (PCNA, monoclonal, 1:200, Dako), human protease serine 1 (trypsin1, polyclonal, 1:2000; Atlas Antibodies, Stockholm, Sweden), and human B-cell leukemia/lymphoma 10 (BCL10, monoclonal, 1:50, Santa Cruz Biotechnology Inc., Dallas, TX, USA). BCL10 is an exocrine enzyme highly homologous to carboxyl ester hydrolase, and specifically reacts with pancreatic acinar cells in humans14. Antigen retrieval was performed in 0.1 M citrate buffer (pH 6.0) using an autoclave at 121°C for 5 min for all antibodies. After treatment with 4% Block Ace (Sumitomo Dainippon Pharma Co., Ltd., Osaka, Japan) for 20 min, the sections were incubated with primary antibodies at 4°C overnight, followed by secondary antibody reactions at 37 °C for 30 min using anti-mouse or anti-rabbit EnVision+ System-HRP (Dako). Finally, positive reactions were visualized using 3,3-diaminobenzidine (DAB) solution consisting of one DAB tablet (Wako, Osaka, Japan) in 20 mL of phosphate-buffered saline and 10 μL of 30% hydrogen peroxide, and counterstaining was performed with Gill’s hematoxylin. The normal duodenum was also stained for PAS, cytokeratin, vimentin, α-SMA, and PCNA as positive controls. To compare the immunostaining results for trypsin and BCL10, normal pancreas, parotid gland, and acinar cell carcinoma of the pancreas and parotid gland obtained from male or female Wistar Hannover [BrlHan:WIST@Jcl(GALAS)] rats at 110 weeks of age were immunohistochemically stained.
Fig. 1.
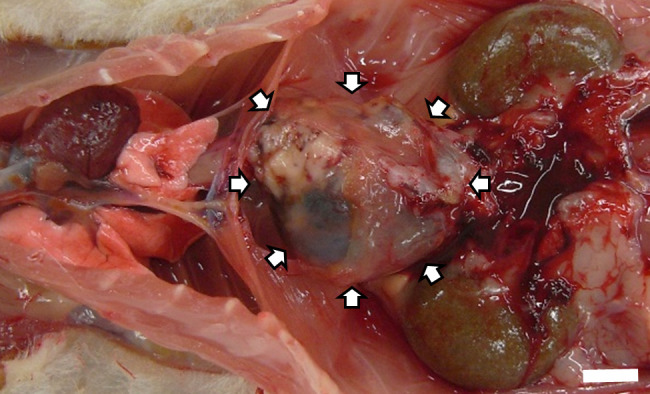
A well-defined brown mass was observed macroscopically on the caudal side of the lung at the midline. Bar=10 mm.
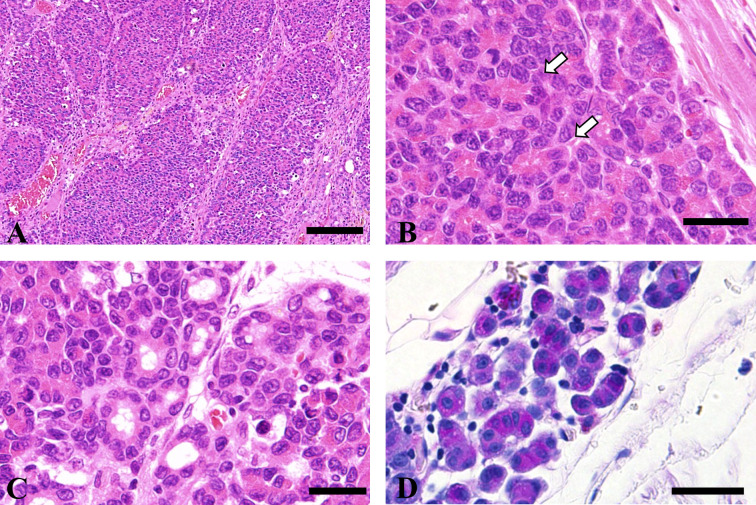
Histologically, the mass was divided into lobules of various sizes by the connective tissue (Fig. 2A). In most lobules, polygonal tumor cells with round nuclei proliferated in a solid or trabecular manner and partially formed acinus-like structures (Fig. 2B). Additionally, some areas showed glandular proliferation (Fig. 2C). Tumor cells comprising the acinus had nuclei on the basal side and PAS-positive eosinophilic granules in the apical portion. Tumor cells infiltrating the surrounding tissue were also PAS positive (Fig. 2D). Nuclear atypia and mitosis were frequently observed. Tumor cells infiltrated the diaphragm; however, no tumor cells were observed in the thoracic and abdominal organs. Immunostaining revealed that the tumor cells were positive for cytokeratin and negative for vimentin and α-SMA. The percentage of PCNA-positive cells in 10 high-power fields was 29.5%.
Fig. 2.
Histopathology of the mass in the thoracic cavity. (A) Large and small alveolar lesions separated by connective tissue. HE stain. Bar=100 μm. (B) Tumor cells form acinar structures (white arrow). HE stain. Bar=20 μm. (C) Some tumor cells form small ducts. HE stain. Bar=20 μm. (D) Cytoplasmic granules of tumor cells composing acinar structures positively stained with periodic acid-Schiff stain. Bar=20 μm.
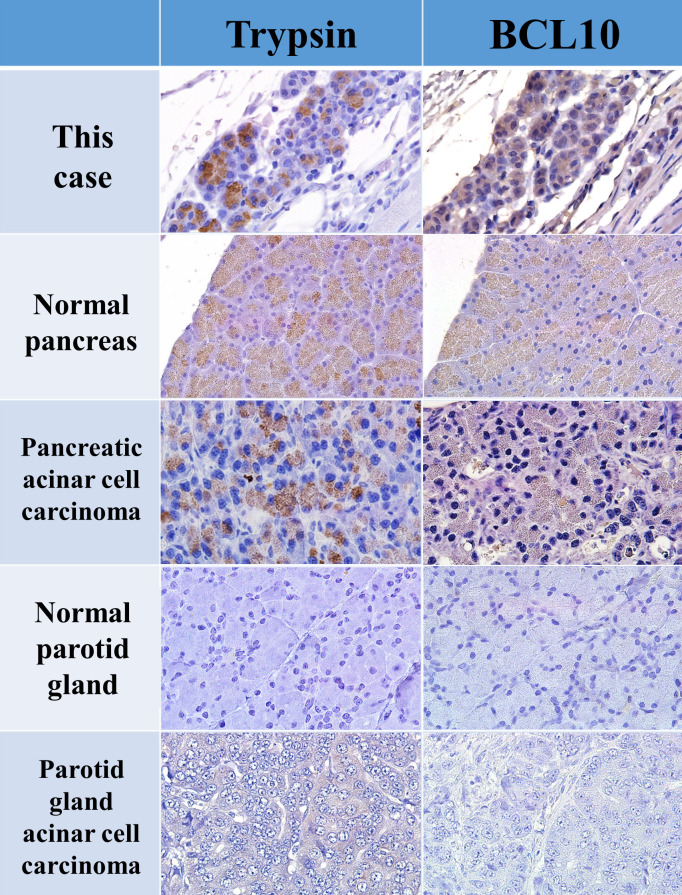
The origin of the acinar cell-like tumor cells with eosinophilic granules was suspected to be a serous gland, such as the pancreas or parotid gland. Typically, masses are identified based on their site of origin. Acinar cell carcinoma of the salivary gland has poorly delineated lobules, composed of acinar cells with secretory granules15. Acinar cell carcinoma of the pancreas is characterized by glandular or solid sheet-like proliferation of the neoplastic acinar cells16. Owing to these histological similarities, differentiation is required for the diagnosis of ectopically identified tumors. In humans, trypsin, chymotrypsin, lipase, and amylase are effective markers for immunostaining pancreatic acinar cell carcinoma, and BCL10 is reportedly valuable for its diagnosis17, 18. BCL10 is more sensitive than trypsin in humans; normal and ectopic pancreas, pancreatic metaplasia, and pancreatic acinar cell carcinoma stain positive for this marker, whereas salivary acinar cell carcinoma stains negative14. However, no study has compared the stainability of these markers in the rat pancreas and parotid glands. Thus, we immunostained the normal pancreas, and parotid glands, and acinar cell carcinoma of rats using trypsin and BCL10 and compared the results with those of the present case. Both normal and tumor tissues in the pancreas stained positive for trypsin but negative in both normal and tumor tissues in the parotid glands. BCL10 was weakly positive in the pancreas but negative in the parotid glands (Fig. 3). In the present case, the cytoplasmic eosinophilic granules in the tumor cells were positive for trypsin and weakly positive for BCL10, suggesting a pancreatic acinar cell origin. To the best of our knowledge, no study has reported BCL10 immunostaining in rat pancreatic tumors. Therefore, this is the first report of BCL10 as a valuable marker in rats as well as humans.
Fig. 3.
Summary of immunohistochemical results for trypsin and BCL10. Trypsin and BCL10 were weakly positive in normal pancreatic tissue and pancreatic acinar cell carcinoma but negative in normal parotid gland tissue and parotid gland acinar cell carcinoma.
Combined with this histopathological and immunological evidence and the fact that the mass was not continuous with any abdominal organs, we diagnosed the present case as ectopic pancreatic acinar cell carcinoma. There are three theories regarding the origin of extra-pancreatic acinar cell carcinoma: heterotopic or metaplastic pancreatic tissue or multipotent stem cells19. Of these, heterotopic pancreatic tissue origin is the most common. The following three criteria are recommended for diagnosing cancer originating from ectopic pancreatic tissues: (1) the tumor is present within or close to the ectopic pancreatic tissue, (2) direct transition is observed between the pancreatic structures and carcinoma, and (3) non-neoplastic pancreatic tissue must comprise at least fully developed acini and ductal structures20. A previous study reported adenocarcinoma arising from ectopic pancreatic tissue in the esophagus and non-neoplastic ectopic pancreatic tissue in the surrounding area21. As our case occurred in the midline of the thoracic cavity, we suspected that it may have originated from ectopic pancreatic tissue in the esophagus. Therefore, specimens were prepared from several other sites in the esophagus; however, a non-neoplastic ectopic pancreas was not observed, and no abnormalities were found in these tissues, including the serous membrane. In this case, we could not determine the origin of the tumor cells. In some cases, no ectopic pancreas was observed in the tumor tissue, suggesting that the ectopic pancreatic tissue may have been destroyed and vanished during tumor progression19.
In conclusion, we identified ectopic pancreatic acinar cell carcinoma in a female F344 rat. This is an extremely rare case that occurred in the thoracic cavity. Additionally, we demonstrated that BCL10 may be a valuable marker of pancreatic tissue in rats and humans.
Disclosure of Potential Conflicts of Interest
The authors declare no potential conflicts of interest regarding the research, authorship, or publication of this article.
Acknowledgments
We thank Ms. Chizuko Tomiyama and Takako Kazami for their assistance with the tissue preparation.
References
- 1.Fayoumi S, Al-Husseini L, Jalil R, and Abbasi S. Ectopic pancreatic tissue in the thoracic cavity: report of two cases. Ann Thorac Surg. 90: e25–e27. 2010. [DOI] [PubMed] [Google Scholar]
- 2.Ulrych J, Fryba V, Skalova H, Krska Z, Krechler T, and Zogala D. Premalignant and malignant lesions of the heterotopic pancreas in the esophagus: a case report and review of the literature. J Gastrointestin Liver Dis. 24: 235–239. 2015. [DOI] [PubMed] [Google Scholar]
- 3.Rezvani M, Menias C, Sandrasegaran K, Olpin JD, Elsayes KM, and Shaaban AM. Heterotopic pancreas: histopathologic features, imaging findings, and complications. Radiographics. 37: 484–499. 2017. [DOI] [PubMed] [Google Scholar]
- 4.Jubb KVV, and Stent AW. Pancreas. In: Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals, 6. MG Maxie (ed). Elsevier, St. Louis. 356. 2016. [Google Scholar]
- 5.Briziarelli G, and Tornaben JA. Ectopic pancreas in the liver of a rat. Vet Pathol. 9: 263–265. 1972. [DOI] [PubMed] [Google Scholar]
- 6.Nolte T, Brander-Weber P, Dangler C, Deschl U, Elwell MR, Greaves P, Hailey R, Leach MW, Pandiri AR, Rogers A, Shackelford CC, Spencer A, Tanaka T, and Ward JM. Nonproliferative and proliferative lesions of the gastrointestinal tract, pancreas and salivary glands of the rat and mouse. J Toxicol Pathol. 29(Suppl): 1S–125S. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kato Y, Hirata A, Kashiwagi-Yamamoto E, Masuno K, Fujisawa K, Matsushima S, and Takasu N. Ectopic tissue consisting of a mixture of glandular gastric, intestinal, and exocrine pancreatic tissue in the forestomach of a rat. J Toxicol Pathol. 27: 87–90. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsushita K, Ishii Y, Kijima A, Takasu S, Kuroda K, Takagi H, Nohmi T, Ogawa K, and Umemura T. Background data of 2-year-old male and female F344 gpt delta rats. J Toxicol Pathol. 34: 23–31. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao H, Zhang Z, Li X, Liu J, Zhang H, Chen G, and Chen J. Resection of anterior mediastinal ectopic pancreas by right thoracoscopy: a case report. Mol Clin Oncol. 11: 147–150. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim KM, Kim CY, Hong SM, and Jang KY. A primary pure pancreatic-type acinar cell carcinoma of the stomach: a case report. Diagn Pathol. 12: 10. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takagi K, Yagi T, Tanaka T, Umeda Y, Yoshida R, Nobuoka D, Kuise T, and Fujiwara T. Primary pancreatic-type acinar cell carcinoma of the jejunum with tumor thrombus extending into the mesenteric venous system: a case report and literature review. BMC Surg. 17: 75. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor I, and Mowat V. Comparison of longevity and common tumor profiles between Sprague-Dawley and Han Wistar rats. J Toxicol Pathol. 33: 189–196. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Japanese Association for Laboratory Animal Science. Guidelines for animal experimentation. Exp Anim. 36: 285–288. 1987. [Google Scholar]
- 14.La Rosa S, Franzi F, Marchet S, Finzi G, Clerici M, Vigetti D, Chiaravalli AM, Sessa F, and Capella C. The monoclonal anti-BCL10 antibody (clone 331.1) is a sensitive and specific marker of pancreatic acinar cell carcinoma and pancreatic metaplasia. Virchows Arch. 454: 133–142. 2009. [DOI] [PubMed] [Google Scholar]
- 15.Suzanne B, and Joel RL. Boorman’s Pathology of the Rat: Reference and Atlas. 2nd ed. Academic Press, an imprint of Elsevier, London, 2018. [Google Scholar]
- 16.Andrew WS. Regis M, and Melissa S. Boorman’s Pathology of the Rat: Reference and Atlas. 2nd ed. Academic Press, an imprint of Elsevier, London, 2018. [Google Scholar]
- 17.Hosoda W, Sasaki E, Murakami Y, Yamao K, Shimizu Y, and Yatabe Y. BCL10 as a useful marker for pancreatic acinar cell carcinoma, especially using endoscopic ultrasound cytology specimens. Pathol Int. 63: 176–182. 2013. [DOI] [PubMed] [Google Scholar]
- 18.Yamane K, Anazawa T, Masui T, Nagai K, Tada S, Inoguchi K, Nakano K, Uchida Y, Yogo A, Takaori K, and Uemoto S. Pancreatic acinar cell carcinoma diagnosed by BCL10 staining: a case report. Suizo. 34: 46–54. 2019. [Google Scholar]
- 19.Yonenaga Y, Kurosawa M, Mise M, Yamagishi M, and Higashide S. Pancreatic-type acinar cell carcinoma of the stomach included in multiple primary carcinomas. Anticancer Res. 36: 2855–2864. 2016. [PubMed] [Google Scholar]
- 20.Minami T, Terada T, Mitsui T, and Nakanuma Y. Adenocarcinoma arising from a heterotopic pancreas in the first portion of the duodenum: a case report. Surg Case Rep. 6: 141. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang YB, Liu YQ, Dai L, Yan WP, Liang Z, and Chen KN. Malignant transformation of heterotopic pancreas as middle esophagus adenocarcinoma—a rare case report and comprehensive literature review. Thorac Cancer. 13: 1083–1087. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]