Abstract
Background
We examined the number of lung cancers diagnosed, the quality of care and the socio-economic and clinical characteristics among patients with lung cancer during the COVID-19 pandemic compared to previous years.
Methods
We included all patients ≥ 18 years old diagnosed with lung cancer from 01 January 2018 to 31 August 2021 as registered in the Danish Lung Cancer Registry. Using a generalised linear model, we estimated prevalence ratios (PR) and 95% confidence intervals (CI) of the associations between the pandemic and socioeconomic and clinical factors, and indicators of quality.
Results
We included 18,113 patients with lung cancer (82.0% non-small cell lung cancer (NSCLC)), which was similar to the preceding years, although a decline in NSCLC cases occurred during the first lockdown period in 2020. No difference in distribution of income or educational level was observed. No difference was observed in the quality of treatment – as measured by curative intent, proportion of patients resected or who died within 90 days of diagnosis.
Conclusion
Using nationwide population-based data, our study reassuringly shows no adverse effects of the COVID-19 pandemic on the diagnosis, socio-economic characteristics nor quality of treatment of lung cancer, as compared to the preceding years.
Keywords: Lung cancer; COVID-19 pandemic; Quality of care, Socio-economic factors, Epidemiology
1. Introduction
The COVID-19 pandemic is a global health crisis, which has caused major disruptions to society and healthcare systems worldwide. In Denmark, in the effort to mitigate the impact of the COVID-19 pandemic on the healthcare system and to minimise the spread of the infection, large sections of society were closed down for extended periods. These population-wide restrictions (“lockdowns”), combined with postponed elective procedures at hospitals may have caused unforeseen consequences in regard to the diagnosis and treatment of lung cancer, even though cancer diagnosis and treatment remained open in Denmark throughout the pandemic. Overall, these efforts to mitigate the impact of the COOVID-19 pandemic may have resulted in fewer, albeit more advanced lung cancers being diagnosed during the pandemic.
Studies have indicated that the number of diagnosed lung cancer cases declined during the early phase of the COVID-19 pandemic [1], [2], [3], [4], [5], [6]. A Danish study showed a 10% reduction in lung cancer cases from April to May 2020 and a 4% reduction from March to December 2020 [2]. However, reports from the Danish Lung Cancer Registry and the Danish Cancer Registry did not find any marked decline in lung cancer cases in 2020 [7], [8]. However, if diagnoses were delayed, the risk of being diagnosed in more advanced tumour stage could be increased, which in turn could lead to more extensive treatment and a worse prognosis [9], [10]. However, there is scarce evidence to support an increase in advanced stage lung cancer during the COVID-19 pandemic in Denmark [2].
The quality of treatment, e.g., surgical treatment, curative treatment, and the survival of lung cancer patients may have been impacted during the pandemic, as a result of the restrictions imposed, the relocation of healthcare personnel, and not least the risk of an increase in advanced stage lung cancer, as described above. However, this hypothesis still stands unchallenged at a population level.
Generally, lung cancer disproportionally affects lower socio-economic groups [11] and a concern is that this disproportionality may have been exacerbated during the pandemic, as was the case in relation to the screening for breast, cervical and colorectal cancer [12], [13], [14]. Patients of lower socio-economic status may have more difficulties in navigating the healthcare system, both in general and during the pandemic, and access to healthcare among these groups may have worsened during the pandemic.
We examined the diagnosis and quality of treatment of lung cancer in Denmark during the COVID-19 pandemic in comparison with the preceding years. Moreover, we characterised patients diagnosed with lung cancer in terms of socio-demographic factors and clinical characteristics (i.e., underlying disease and performance status) during the pandemic in comparison with the previous years.
2. Methods
2.1. Setting
The study was set in Denmark, which has a population of approximately 5.8 million inhabitants [15]. The five Danish administrative regions run a tax-funded health care system that provides free healthcare for all citizens in Denmark. Since 2008, lung cancer diagnostics and treatment has been organised as a standardised cancer patient pathway (CPP) for lung cancer [16]. In Denmark, nationwide population-based registries record extensive administrative and medical data for the whole population, which can be linked using the unique personal identifier that is assigned to all residents at birth or immigration [17], [18].
2.2. The COVID-19 pandemic in Denmark
In Denmark, there have been three COVID-19 pandemic waves: in the spring of 2020, in the winter of 2020/2021 and again in the winter of 2021/2022 [19]. The pandemic response comprised population-wide restrictions (“lockdown”), COVID-19 testing and vaccination. Extensive COVID-19 testing has been provided free-of-charge to all inhabitants in Denmark since May 2020 [20]. COVID-19 vaccination began in December 2020 and, by March 2022, approximately 81% of the population had received two doses and more than 61% had received three doses of the vaccine [21]. The vaccination strategy was to first vaccinate individuals living in nursing homes, thereafter individuals ≥ 85 years, then healthcare personnel, thereafter individuals with underlying health conditions and their relatives and, finally, COVID-19 vaccination was offered by decreasing age (75–79 years, 65–74 years, 60–64 years, etc.) [22].
2.3. Study population
The study population comprised all individuals aged ≥ 18 years diagnosed with lung cancer (International Classification of Diseases 10th edition (ICD-10) codes C33.0-C34.9) from 1 January 2018 to 31 August 2021, as registered in the Danish Lung Cancer Registry [23].
We excluded 69 patients who had either emigrated within 90 days of diagnosis (n = 19), had unknown region of residence (n = 38) or were diagnosed with carcinoma in situ (n = 12).
2.4. Defining the COVID-19 pandemic periods
We considered the COVID-19 pandemic as the exposure of interest, which was operationalised in two ways.
First, the exposure was dichotomised into "before the pandemic" (1 January 2018 – 31 January 2020) and "during the pandemic" (1 February 2020 – 31 August 2021).
Secondly, the exposure was split into the different phases of the pandemic in Denmark, in accordance with the government responses to the COVID-19 pandemic, as follows:
-
•
Pre-pandemic period: 1 January 2018 to 31 January 2020
-
•
Pre-lockdown period: 1st February to 10 March 2020
-
•
1st lockdown: 11 March to 15 April 2020
-
•
1st re-opening: 16 April to 15 December 2020
-
•
2nd lockdown: 16 December 2020 to 27 February 2021
-
•
2nd re-opening: 28 February 2021 to 31 August 2021 (end of inclusion period)
2.5. Defining the variables of interest
The main variables of interest fall into four groups: a) number and stage distribution of lung cancer (non-small cell (NSCLC) and small cell lung cancer (SCLC)), b) sociodemographic characteristics, c) clinical characteristics and d) indicators of clinical quality.
Sociodemographic and clinical characteristics included age, sex, ethnicity, cohabitation status, educational level, and disposable income. Age was defined at the date of diagnosis, as registered in the Danish Lung Cancer Registry [23]. From Statistics Denmark [15], we obtained information on ethnicity, cohabitation status, educational level and level of income. Ethnicity was categorised as Danish descent (i.e. born in Denmark) and immigrants (Western immigrant, Non-western immigrant and descendants of immigrants). Cohabitation status was categorised as living alone and married/cohabiting (i.e. married, co-habiting or co-living). Education level was defined in accordance with the International Standard Classification of Education (ISCED) of the United Nations Education, Scientific and Cultural Organization (UNESCO) into short (ISCED level 1–2), medium (ISCED level 3–5) and long (ISCED level 6–8) [15]. Income was defined as official yearly disposable income depreciated to 2015 level and categorised into five quintiles.
Clinical characteristics included comorbidity, body mass index (BMI), performance status, and lung function. The Charlson Comorbidity Index (CCI) was used to account for comorbidity. A CCI score was calculated on the basis of diagnoses registered in the Danish National Patient Register in the 10-year-period prior to the patient's lung cancer diagnosis. We computed the total CCI score for each patient and grouped levels of comorbidity into “none” (CCI score = 0), “low” (CCI score = 1), “moderate” (CCI score = 2), and “high” (CCI score ≥ 3). Body Mass index (BMI) was categorised according to the official WHO nomenclature, except that all three obesity classes were combined into one "obesity" category. Smoking was defined as either smoker (defined as current or previous smokers) or non-smoker, based on whether or not any use of tobacco was registered in DLCR. Performance status was measured using the Eastern Cooperative Oncology Group (ECOG) performance status scale [24]. Lung function was expressed by the Tiffeneau-Pinelli index (Forced Expired Volume in the first second (FEV1) / Forced Vital Capacity (FVC)).
Indicators of treatment quality included resection of lung tissue in patients with NSCLC, whether treatment (surgical or oncological) had a curative aim (overall and for NSCLC only) as registered by the treating doctor and the proportion of patients who died within 90 days of diagnosis. Indication of clinical quality was operationalised as a yes/no variable. Resection was defined as 'yes' when the patient had undergone any resection (wedge, segment, lobectomy, bi-lobectomy or pneumonectomy).
2.6. Statistical analyses
Using descriptive statistics, we examined the number of patients within each variable of interest (except for continuous variables, in which case we examined the mean) before and during the pandemic phases.
For the categorical outcomes, we used a generalised linear model (GLM) with log link for the Poisson family with robust standard errors (SE) to estimate prevalence ratios (PR) and 95% confidence intervals (CI) of each variable of interest during the different phases of the pandemic. For continuous outcomes, we used quantile regression to estimate the mean difference in medians, applying a bootstrapping procedure to establish the 95% CIs.
We present the results for the adjusted analyses; adjusted for month of diagnosis to allow for seasonality, and for year of diagnosis to take account of the annual trend in the number of lung cancers diagnosed, and also for sex and age to take account of the effect of sex and age on the other variables.
All analyses were conducted using STATA version 17.0.
2.7. Ethical considerations
The study is registered at the Central Denmark Region's register of research projects (journal number 1–16–02–381–20). In accordance with Danish law, register-based studies are not required to be reported to the National Committee on Health Research Ethics. Furthermore, Danish law does not require patient consent for register-based studies.
3. Results
3.1. Descriptive characteristics of the study population
Altogether, 18,113 patients (51.3% women; median age at diagnosis 72 years (interquartile interval (IQI)= 65–78)) were diagnosed with lung cancer during the study period. The majority of lung cancers were NSCLC (82.0%). The majority of lung cancers were stage IV at the time of diagnosis (43.3%). In total, 94.9% of lung cancer patients were of Danish descent, 39.7% of patients were living alone, 94.8% of patients were current or previous smokers, 40.2% of patients had no underlying comorbidity, 7.6% were underweight and 41.1% of patients had performance status 0 ( Table 1).
Table 1.
Characteristics of lung cancer patients diagnosed in Denmark from 01 January 2018–31 August 2021; shown for before and during the COVID-19 pandemic in Denmark.
| Before pandemic (1 January 2018 – 31 January 2020) |
Pandemic (1 February 2020 −31 August 2021) |
Total 1 January 2018 – 31 August 2021) |
|
|---|---|---|---|
| Total | 10368 (57.2) | 7745 (42.8) | 18113 (100.0) |
| Non-Small Cell Lung Cancer (NSCLC) | |||
| No | 1338 (12.9) | 974 (12.6) | 2312 (12.8) |
| Yes | 8509 (82.1) | 6351 (82.0) | 14860 (82.0) |
| Unknown | 521 (5.0) | 420 (5.4) | 941 (5.2) |
| Sex | |||
| Women | 5313 (51.2) | 3979 (51.4) | 9292 (51.3) |
| Men | 5055 (48.8) | 3766 (48.6) | 8821 (48.7) |
| Age, median (IQI) | 72.0 (65.0; 77.0) | 72.0 (66.0; 78.0) | 72.0 (65.0; 78.0) |
| Age groups (years) | |||
| 18–59 | 1162 (11.2) | 840 (10.8) | 2002 (11.1) |
| 60–69 | 3043 (29.3) | 2091 (27.0) | 5134 (28.3) |
| 70–79 | 4309 (41.6) | 3394 (43.8) | 7703 (42.5) |
| ≥ 80 | 1854 (17.9) | 1420 (18.3) | 3274 (18.1) |
| Ethnicity | |||
| Danish descent | 9836 (94.9) | 7360 (95.0) | 17196 (94.9) |
| Immigrant | 532 (5.1) | 385 (5.0) | 917 (5.1) |
| Cohabitation status | |||
| Single/living alone | 4129 (39.9) | 3022 (39.0) | 7151 (39.6) |
| Married/co-living | 6207 (60.1) | 4718 (61.0) | 10925 (60.4) |
| Educational level (ISCED) | |||
| 1–2 | 4193 (40.4) | 3109 (40.1) | 7302 (40.3) |
| 3–5 | 4540 (43.8) | 3411 (44.0) | 7951 (43.9) |
| 6 or more | 1635 (15.8) | 1225 (15.8) | 2860 (15.8) |
| Disposable income (quintiles) | |||
| First (lowest) | 2066 (19.9) | 1342 (17.3) | 3408 (18.8) |
| Second | 2158 (20.8) | 1539 (19.9) | 3697 (20.4) |
| Third | 2187 (21.1) | 1716 (22.2) | 3903 (21.6) |
| Fourth | 2056 (19.8) | 1667 (21.5) | 3723 (20.6) |
| Fifth (highest) | 1893 (18.3) | 1480 (19.1) | 3373 (18.6) |
| Comorbidity (CCI score) | |||
| None (0) | 4137 (39.9) | 3148 (40.6) | 7285 (40.2) |
| Low (1) | 2170 (20.9) | 1549 (20.0) | 3719 (20.5) |
| Moderate (2) | 1706 (16.5) | 1307 (16.9) | 3013 (16.6) |
| High (≥3) | 2355 (22.7) | 1741 (22.5) | 4096 (22.6) |
| Smoker / previous smoker | 8366 (94.0) | 5986 (95.9) | 14352 (94.8) |
| Performance status (ECOG) | |||
| 0 (zero) | 3975 (42.0) | 2732 (39.8) | 6707 (41.1) |
| 1 | 2879 (30.4) | 2131 (31.1) | 5010 (30.7) |
| 2 | 1487 (15.7) | 1162 (16.9) | 2649 (16.2) |
| 3 + 4 | 1124 (11.9) | 835 (12.2) | 1959 (12.0) |
| Forced Expired Volume in the first second (L/min), median (IQI) | 1.8 (1.3; 2.3) | 1.8 (1.3; 2.3) | 1.8 (1.3; 2.3) |
| UICC stage | |||
| Stage I | 2162 (25.4) | 1742 (27.4) | 3904 (26.3) |
| Stage II | 836 (9.8) | 549 (8.6) | 1385 (9.3) |
| Stage III | 1625 (19.1) | 1144 (18.0) | 2769 (18.6) |
| Stage IV | 3702 (43.5) | 2726 (42.9) | 6428 (43.3) |
| Unknown | 184 (2.2) | 190 (3.0) | 374 (2.5) |
| Stage by curability (UICC stage) | |||
| Possibly curable (IA-IIIA) | 3821 (44.9) | 2871 (45.2) | 6692 (45.0) |
| May be curable (IIIB) | 548 (6.4) | 414 (6.5) | 962 (6.5) |
| Not curable (IIIC-IVB) | 3956 (46.5) | 2876 (45.3) | 6832 (46.0) |
| Unknown | 184 (2.2) | 190 (3.0) | 374 (2.5) |
| Resection - NSCLC | 2404 (28.3) | 1761 (27.7) | 4165 (28.0) |
| Curative aim | 2535 (24.5) | 1949 (25.2) | 4484 (24.8) |
| Curative aim (NSCLC only) | 2477 (29.1) | 1892 (29.8) | 4369 (29.4) |
| Died within 90 days of diagnosis | 1906 (18.4) | 1457 (18.8) | 3363 (18.6) |
SCLC: small cell lung cancer; NSCLC: non-small cell lung cancer; ISCED: International Standard Classification of Education; ECOG: Eastern Cooperative Oncology Group performance status scale; FEV1: Forced Expired Volume in the first second; FVC: Forced Vital Capacity. IQI=Inter Quartile Interval
3.2. Diagnosing and treatment of lung cancer during the COVID-19 pandemic
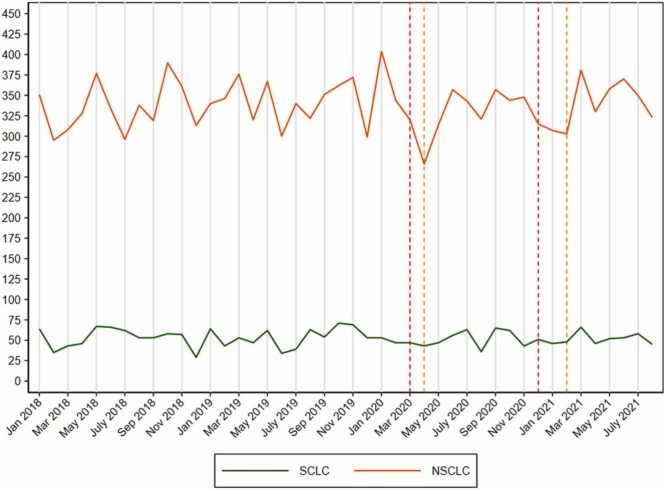
The overall number of new lung cancer cases was 4912 in 2020, which is only 0.6% (N = 30) fewer cases than the average number of lung cancers in 2018–2019 ( Fig. 1). From January to August 2021, 0.8% (n = 26) fewer lung cancers were diagnosed than the average number of lung cancers from January to August in 2018–2019. However, a drop in the number of NSCLC was seen during the 1st lockdown (Fig. 1).
Fig. 1.
Number of patients diagnosed with lung cancer in Denmark from 1 January 2018 to 31 August 2021 (small cell lung cancer (SCLC) in green and non-small cell lung cancer (NSCLC) in orange). The dotted vertical red lines indicate the month of the start of the national lockdowns and the yellow vertical dotted lines indicate the month of the start of the re-opening of the society in Denmark.
A higher proportion of patients were diagnosed with stage I lung cancer (27.4 vs 25.4%; PR=1.08; 95% CI: 1.02–1.14), while fewer patients were diagnosed with stage II (8.6 vs 9.8%; PR=0.89; 95% CI: 0.80–0.99). However, no difference in the stage distribution by curable stage was found ( Tables 1 and 2), nor when we accounted for the known increase in stage I tumours from 2015 to 2019 (PR=1.00; 95%CI: 0.89–1.11).
Table 2.
Estimated risk (expressed as prevalence ratios (PRs)) for socio-demographics and socioeconomic factors in patients with lung cancer during the pandemic overall (second column of estimates) and by pandemic period (light grey columns) compared to before the pandemic (first column) (i.e. period from 01 Jan 2018–31 Jan 2020); adjusted for seasonal variation (month), age and sex.
| Before Pandemic (01Jan2018-31Jan2020) |
Pandemic overall (01Feb2020-31Aug2021) |
Pre-lockdown (01Feb2020-10Mar2020) |
1st lock-down (11Mar2020-15Apr2020) |
1st re-opening (16Apr2020-15Dec2020) |
2nd lock-down (16Dec2020-27Feb2021) |
2nd re-opening (28Feb2021-31Aug2021) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PR | [95%CI] | PR | [95%CI] | PR | [95%CI] | PR | [95%CI] | PR | [95%CI] | PR | [95%CI] | PR | [95%CI] | ||
| Sex | |||||||||||||||
| Women | 1.00 | reference | 1.00 | [0.97; 1.03] | 0.94 | [0.85; 1.03] | 0.93 | [0.84; 1.04] | 1.03 | [0.99; 1.07] | 0.96 | [0.89; 1.03] | 1.01 | [0.96; 1.05] | |
| Men | 1.00 | Reference | 1.00 | [0.97; 1.03] | 1.07 | [0.97; 1.18] | 1.07 | [0.96; 1.20] | 0.97 | [0.93; 1.01] | 1.04 | [0.97; 1.12] | 0.99 | [0.95; 1.04] | |
| Age groups (years) | |||||||||||||||
| 18–59 | 1.00 | Reference | 0.98 | [0.85; 1.12] | 1.33 | [0.96; 1.83] | 1.18 | [0.93; 1.50] | 0.85 | [0.69; 1.05] | 1.14 | [0.87; 1.50] | 1.04 | [0.86; 1.26] | |
| 60–69 | 1.00 | Reference | 0.93 | [0.89; 0.98] | 1.04 | [0.90; 1.20] | 0.86 | [0.71; 1.04] | 0.92 | [0.85; 0.98] | 0.91 | [0.80; 1.03] | 0.97 | [0.90; 1.04] | |
| 70–79 | 1.00 | Reference | 1.04 | [1.00; 1.07] | 0.96 | [0.86; 1.08] | 1.03 | [0.91; 1.17] | 1.05 | [1.00; 1.10] | 1.06 | [0.98; 1.16] | 1.02 | [0.97; 1.07] | |
| ≥ 80 | 1.00 | Reference | 0.99 | [0.93; 1.05] | 1.06 | [0.90; 1.24] | 1.12 | [0.91; 1.38] | 0.94 | [0.86; 1.02] | 1.04 | [0.91; 1.19] | 1.01 | [0.92; 1.11] | |
| Ethnicity | |||||||||||||||
| Danish descent | 1.00 | Reference | 1.00 | [0.99; 1.01] | 1.00 | [0.99; 1.01] | 1.00 | [0.97; 1.02] | 1.02 | [1.00; 1.04] | 1.00 | [0.99; 1.02] | 1.01 | [0.99; 1.03] | |
| Immigrant | 1.00 | Reference | 1.00 | [0.87; 1.13] | 0.94 | [0.77; 1.15] | 1.03 | [0.65; 1.63] | 0.64 | [0.35; 1.16] | 0.94 | [0.74; 1.18] | 0.87 | [0.59; 1.26] | |
| Cohabitation status | |||||||||||||||
| Single/living alone | 1.00 | Reference | 0.97 | [0.93; 1.00] | 0.96 | [0.85; 1.08] | 1.06 | [0.93; 1.20] | 0.93 | [0.88; 0.98] | 1.03 | [0.94; 1.12] | 1.02 | [0.93; 1.12] | |
| Married/co-living | 1.00 | Reference | 1.02 | [1.00; 1.05] | 1.03 | [0.95; 1.11] | 0.96 | [0.88; 1.06] | 1.05 | [1.02; 1.08] | 0.98 | [0.92; 1.04] | 1.03 | [0.99; 1.07] | |
| Educational level (ISCED) | |||||||||||||||
| 1–2 | 1.00 | Reference | 0.98 | [0.95; 1.02] | 1.01 | [0.90; 1.14] | 0.93 | [0.81; 1.07] | 0.98 | [0.94; 1.03] | 1.02 | [0.94; 1.12] | 1.02 | [0.94; 1.12] | |
| 3–5 | 1.00 | Reference | 1.02 | [0.98; 1.05] | 1.01 | [0.90; 1.13] | 1.09 | [0.97; 1.23] | 1.01 | [0.96; 1.06] | 0.97 | [0.89; 1.06] | 1.04 | [0.99; 1.10] | |
| 6 or more | 1.00 | Reference | 1.00 | [0.93; 1.07] | 0.94 | [0.76; 1.18] | 0.93 | [0.71; 1.21] | 1.02 | [0.92; 1.12] | 1.02 | [0.86; 1.20] | 0.99 | [0.89; 1.11] | |
| Disposable income (quintiles) | |||||||||||||||
| First (lowest) | 1.00 | Reference | 0.87 | [0.82; 0.93] | 0.89 | [0.72; 1.09] | 0.90 | [0.71; 1.13] | 0.92 | [0.85; 1.00] | 0.83 | [0.71; 0.97] | 0.81 | [0.73; 0.90] | |
| Second | 1.00 | Reference | 0.95 | [0.90; 1.01] | 1.07 | [0.88; 1.30] | 0.93 | [0.73; 1.17] | 0.91 | [0.84; 0.99] | 1.14 | [0.99; 1.32] | 0.91 | [0.83; 1.00] | |
| Third | 1.00 | Reference | 1.04 | [0.98; 1.10] | 1.04 | [0.86; 1.25] | 1.35 | [1.12; 1.62] | 0.99 | [0.92; 1.07] | 1.02 | [0.88; 1.17] | 1.07 | [0.98; 1.16] | |
| Fourth | 1.00 | Reference | 1.09 | [1.03; 1.16] | 1.08 | [0.91; 1.30] | 0.79 | [0.62; 1.01] | 1.13 | [1.05; 1.23] | 0.97 | [0.84; 1.13] | 1.13 | [1.03; 1.24] | |
| Fifth (highest) | 1.00 | Reference | 1.05 | [0.99; 1.12] | 0.92 | [0.76; 1.12] | 1.02 | [0.82; 1.28] | 1.05 | [0.97; 1.14] | 1.05 | [0.91; 1.21] | 1.09 | [0.99; 1.20] | |
| Comorbidity (CCI score) | |||||||||||||||
| None (0) | 1.00 | Reference | 1.04 | [1.00; 1.08] | 0.93 | [0.82; 1.06] | 0.95 | [0.82; 1.09] | 1.07 | [1.02; 1.12] | 1.00 | [0.92; 1.09] | 1.05 | [0.99; 1.11] | |
| Low (1) | 1.00 | Reference | 0.95 | [0.90; 1.01] | 0.97 | [0.81; 1.16] | 0.94 | [0.76; 1.17] | 0.91 | [0.84; 0.99] | 1.03 | [0.89; 1.18] | 0.97 | [0.88; 1.06] | |
| Moderate (2) | 1.00 | Reference | 1.01 | [0.95; 1.08] | 0.99 | [0.80; 1.23] | 1.10 | [0.87; 1.40] | 0.98 | [0.90; 1.08] | 0.98 | [0.83; 1.16] | 1.07 | [0.96; 1.18] | |
| High (≥3) | 1.00 | Reference | 0.97 | [0.92; 1.03] | 1.15 | [0.98; 1.36] | 1.08 | [0.89; 1.31] | 0.97 | [0.90; 1.05] | 0.99 | [0.87; 1.14] | 0.90 | [0.83; 0.99] | |
| Performance status (ECOG) | |||||||||||||||
| 0 (zero) | 1.00 | Reference | 0.96 | [0.92; 1.00] | 0.98 | [0.87; 1.10] | 1.10 | [0.96; 1.25] | 0.97 | [0.92; 1.02] | 0.93 | [0.85; 1.02] | 0.93 | [0.87; 0.99] | |
| 1 | 1.00 | Reference | 1.02 | [0.97; 1.07] | 1.04 | [0.89; 1.21] | 1.09 | [0.92; 1.29] | 0.98 | [0.92; 1.05] | 1.08 | [0.97; 1.22] | 1.04 | [0.96; 1.12] | |
| 2 | 1.00 | Reference | 1.06 | [0.99; 1.14] | 0.96 | [0.76; 1.22] | 0.81 | [0.61; 1.07] | 1.12 | [1.02; 1.23] | 1.08 | [0.90; 1.29] | 1.02 | [0.91; 1.14] | |
| 3 + 4 | 1.00 | Reference | 1.00 | [0.92; 1.09] | 1.05 | [0.81; 1.35] | 0.75 | [0.53; 1.05] | 0.98 | [0.87; 1.10] | 0.94 | [0.76; 1.17] | 1.11 | [0.98; 1.26] | |
PR: Prevalence Ratio; 95%CI: 95% confidence interval; ISCED: International Standard Classification of Education; ECOG: Eastern Cooperative Oncology Group performance status scale.
No changes in performance status was seen during the pandemic; e.g. ECOG level 2: 16.9% before vs. 15.7% during the pandemic (PR=1.01; 0.96–1.06) (Table 1 & 2).
3.3. Socio-demographic and clinical characteristics
A lower proportion of the patients diagnosed with lung cancer during the pandemic were aged 60–69 (26.8 vs 29.4%; PR=0.93; 95% CI: 0.88–0.97) as compared to the years preceding the pandemic, while a higher proportion of patients were aged 70–79 (44.5 vs 41.5%; PR=1.04; 1.01–1.08); nonetheless, no change was observed in the median age at diagnosis. The proportion of lung cancer patients in the lowest income quintile decreased (17.3 vs 19.8%; PR=0.87; 95% CI: 0.82–0.93) across the time of the pandemic. However, when the underlying annual increase in disposable income was accounted for, no difference in income was observed (PR=0.98; 95% CI: 0.86–1.11). Neither was any difference in educational level found.
Additionally, a higher proportion of lung cancer patients had no underlying comorbidity during the pandemic (41.0 vs 39.7%; PR=1.05; 95% CI: 1.01–1.08) ( Table 1, Table 3).
Table 3.
Estimated risk (expressed as prevalence ratios (PRs)) for various clinical quality factors among patients with lung cancer during the pandemic overall (second column of estimates) and by pandemic period (light grey columns)), compared to before the pandemic (first column) (i.e. period from 01 Jan 2018–31 Jan 2020); adjusted for seasonal variation (month), age and sex.
| Before Pandemic (01Jan2018-31Jan2020 |
Pandemic overall (01Feb2020-31Aug2021) |
Pre-lockdown (01Feb2020-10Mar2020) |
1st lock-down (11Mar2020-15Apr2020) |
1st re-opening (16Apr2020-15Dec2020) |
2nd lock-down (16Dec2020-27Feb2021) |
2nd re-opening (28Feb2021-31Aug2021) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PR | [95%CI] | PR | [95%CI] | PR | [95%CI] | PR | [95%CI] | PR | [95%CI] | PR | [95%CI] | PR | [95%CI] | ||
| UICC stage | |||||||||||||||
| Stage I | 1.00 | reference | 1.08 | [1.02; 1.14] | 1.03 | [0.86; 1.24] | 1.20 | [0.98; 1.46] | 1.07 | [0.99; 1.16] | 1.13 | [0.99; 1.30] | 1.16 | [1.06; 1.27] | |
| Stage II | 1.00 | Reference | 0.89 | [0.80; 0.99] | 0.96 | [0.70; 1.32] | 0.60 | [0.37; 0.98] | 0.94 | [0.82; 1.08] | 0.57 | [0.43; 0.76] | 0.95 | [0.81; 1.11] | |
| Stage III | 1.00 | reference | 0.94 | [0.87; 1.00] | 1.11 | [0.90; 1.37] | 0.79 | [0.60; 1.04] | 0.94 | [0.86; 1.04] | 1.00 | [0.85; 1.19] | 0.89 | [0.80; 0.99] | |
| Stage IV | 1.00 | Reference | 0.99 | [0.95; 1.02] | 0.93 | [0.81; 1.07] | 1.16 | [1.01; 1.33] | 1.01 | [0.96; 1.07] | 0.97 | [0.88; 1.08] | 0.95 | [0.89; 1.01] | |
| Unknown | 1.00 | Reference | 1.41 | [1.15; 1.72] | 1.02 | [0.84; 1.24] | 0.86 | [0.68; 1.10] | 0.97 | [0.89; 1.06] | 1.13 | [0.98; 1.30] | 1.05 | [0.95; 1.15] | |
| Stage by curability | |||||||||||||||
| Possible curable (IA-IIIA) | 1.00 | reference | 1.01 | [0.97; 1.05] | 1.07 | [0.95; 1.21] | 1.00 | [0.86; 1.15] | 1.01 | [0.97; 1.07] | 0.97 | [0.88; 1.07] | 1.05 | [0.99; 1.12] | |
| Maybe curable (IIIB) | 1.00 | Reference | 1.01 | [0.89; 1.14] | 0.95 | [0.62; 1.45] | 0.80 | [0.49; 1.32] | 0.96 | [0.80; 1.13] | 0.90 | [0.66; 1.24] | 1.01 | [0.84; 1.22] | |
| Not curable (IIIC-IVB) | 1.00 | reference | 0.97 | [0.94; 1.01] | 0.92 | [0.81; 1.05] | 1.10 | [0.97; 1.26] | 1.00 | [0.95; 1.06] | 0.98 | [0.89; 1.08] | 0.93 | [0.87; 0.99] | |
| Unknown | 1.00 | Reference | 1.41 | [1.15; 1.72] | 1.02 | [0.84; 1.24] | 0.86 | [0.68; 1.10] | 0.98 | [0.89; 1.06] | 1.13 | [0.98; 1.30] | 1.05 | [0.95; 1.15] | |
| Resection - NSCLC | 1.00 | Reference | 1.00 | [0.94; 1.05] | 0.92 | [0.78; 1.09] | 1.03 | [0.85; 1.24] | 1.00 | [0.93; 1.08] | 0.99 | [0.87; 1.12] | 1.01 | [0.93; 1.10] | |
| Curative aim | 1.00 | Reference | 1.05 | [0.99; 1.10] | 0.90 | [0.76; 1.06] | 1.07 | [0.88; 1.29] | 1.03 | [0.95; 1.10] | 1.02 | [0.91; 1.16] | 1.12 | [1.03; 1.22] | |
| Curative aim (NSCLC only) | 1.00 | Reference | 1.04 | [0.99; 1.09] | 0.90 | [0.76; 1.06] | 1.07 | [0.88; 1.29] | 1.03 | [0.95; 1.10] | 1.02 | [0.91; 1.16] | 1.12 | [1.03; 1.22] | |
| Died within 90 days of diagnosis | 1.00 | Reference | 1.02 | [0.96; 1.08] | 1.03 | [0.84; 1.26] | 1.09 | [0.86; 1.37] | 1.05 | [0.96; 1.14] | 0.91 | [0.78; 1.06] | 1.01 | [0.92; 1.12] | |
PR: Prevalence Ratio; 95%CI: 95% confidence interval; UICC: Union for International Cancer Control; NSCLC: Non-Small Cell Lung Cancer.
3.4. Quality of treatment
During the pandemic, there was no tendency for a higher proportion of patients to be treated with curative intent than before the pandemic, neither overall (25.2 vs 24.5%; PR=1.05; 95% CI: 0.99–1.10) nor in NSCLC only (29.8 vs 29.1%; PR=1.00; 95% CI: 0.94–1.05). There was no difference in the proportion of patients with NSCLC that were resected (27.7 vs 28.3%; PR=1.00; 95% CI: 0.94–1.05). Among all patients with lung cancer, there was no difference in the proportion who died within 90 days of diagnosis (18.8 vs 18.4%; PR=1.02; 95% CI: 0.96–1.08) during the pandemic, as compared with the preceding years (Table 1, Table 2).
These results were similar across the pandemic phases; although with less precise estimates, as some of the phases constituted low numbers. For all remaining variables, no overall changes were observed from before to during the pandemic or across individual pandemic periods (Table 2 & 3).
4. Discussion
4.1. Main findings
Altogether, 18,113 patients were diagnosed with lung cancer (82.0% NSCLC) during the study period. A decline in the number of NSCLC was seen during the 1st lockdown; however, the overall number of lung cancers in 2020 and 2021 was similar to the previous years. A higher proportion of patients were diagnosed with stage I lung cancer during the pandemic which was in line with a trend already seen in the years before the pandemic; however, no difference in the stage distribution by curability was found. Furthermore, no difference in distribution of income or educational level was observed among lung cancer patients during the pandemic. During the pandemic, a higher proportion of patients were treated with a curative aim, due to the increase in stage I cancers. No difference in the proportion of patients resected or dying within 90 days of diagnosis was found.
4.2. Comparison with previous studies and explanation of findings
Our finding – of a similar number of lung cancer patients in 2020 to the preceding years – contrasts with findings from another Danish study, by Skovlund et al., who reported a 10% reduction in the number of lung cancers diagnosed from March to May 2020 and an overall 4% reduction from March to December 2020 [2]. However, in line with our findings, the annual reports of the Danish Lung Cancer Registry and Danish Cancer Registry did not find any marked decline in lung cancer cases in 2020, as compared with the previous years [7], [8]. The decline in the number of lung cancers, as reported by Skovlund et al., [2] may reflect their use of the Danish National Patient Registry as a data source, as registration of cancer diagnoses in the Danish National Patient Registry is known to be delayed for months, for at least 5% of lung cancer diagnoses [25]. Compared to Sweden and the UK, our findings of constant numbers of lung cancer cases is noteworthy, as both Sweden and the UK experienced a large decline in the spring of 2020, which did not compensate later in the year [5], [6].
Our findings of equal number of patients with lung cancer and with similar socio-economic disparities during the COVID-19 pandemic as compared to before speaks against that the fear that the COVID-19 pandemic would impact socioeconomic groups differently in accessing healthcare when experiencing symptoms that might be cancer [2], [26], [27]. There may be several reasons for this. One possible explanation may be that the majority of lung cancer are diagnosed at stage IV (43.3%) at the time of diagnosis and when patients with lung cancer do seek help, their symptoms (e.g., haemoptysis) are so severe that they will seek help in all circumstances. However, as many symptoms of lung cancer (e.g., coughing) resemble symptoms of COVID-19, patients may have been re-directed for a COVID-19 test before lung cancer diagnostics were performed, which could have caused a delay in diagnosis. However, given that testing for COVID-19 in Denmark is performed free of charge, and either instantly or with minimal waiting time, referral to a test for COVID-19 would not have a detrimental effect on prognosis.
The organisation of the diagnosis and treatment of lung cancer, as a standardised cancer patient pathway (CPP) with well-defined criteria for who should be referred to CPP, in Denmark together with well-defined procedures with maximum time frames, most likely helped to ensure high quality and less variation in diagnosis and treatment that was seen in our study. The proportion of patients with lung cancer that underwent the lung CPP course within the maximum time frame was 71% in 2020, which was 7% points more than in 2018 [28]. Indeed, the CPP for lung cancer has been linked to improvements in both speed of diagnostics and tumour stage at diagnosis [29], [30]. Furthermore, Danish general practices were open for acute and necessary medical assistance during the COVID-19 pandemic. Also, based only on a telephone consultation, Danish general practitioners can refer patients with suspected lung cancer directly for a diagnostic thoracic-abdominal CT scan. This could have minimised the possible effect of lockdowns on the help-seeking and referral of patients with lung cancer symptoms.
4.3. Strengths and limitations
The main strength of the study is the use of prospectively collected data from a nationwide quality database that has complete coverage of patients with lung cancer linked to nationwide socio-economic data, with only minor exclusions. Limitations of the study include that, we could not identify the patients who were not diagnosed with lung cancer during the study period, or identify any delay in registration. However, as the number of diagnoses are almost identical to previous years, this possible under-ascertainment seems very unlikely. Lastly, unmeasured confounding could have affected our results; e.g., we did not have information on COVID-19 infection or vaccination status, which could affect health-seeking behaviour or the route of diagnosis and lung cancer treatment. However, again, the unaltered number of diagnoses speaks against this to bias our results.
4.4. Implications of findings
Our study indicates that no major differences in number of diagnoses, quality of care or socio-economic position of the patient could be observed during the COVID-19 pandemic. It thus seems important, in case of a similar pandemic, that the diagnosis and treatment of cancer in Denmark should be prioritized and that the CPPs remain operational.
Although the COVID-19 pandemic was not associated with the diagnosis and treatment of lung cancer in Denmark, the inferior survival rate of lung cancer in Denmark compared to other countries implies that there still is room for a general improvement of the diagnosis and treatment of lung cancer in Denmark [31]. It is therefore important to ensure that people in risk of developing lung cancer are encouraged to seek medical assistance before severe symptoms arise.
4.5. Conclusion
This nation-wide study of all patients with lung cancer in Denmark from 1 January 2018 to 31 August 2021 showed that, during the COVID-19 pandemic, the number of patients diagnosed with and treated for lung cancer equalled that before the pandemic. Reassuringly, the study showed no exacerbation in the quality of treatment or in the socio-economic position of patients with lung cancer. Thus, the care of patients with lung cancer appears to be resilient to the indirect effects of the pandemic response to COVID-19 in Denmark.
Ethical considerations
The study is registered at the Central Denmark Region's register of research projects (journal number 1–16–02–381–20). In accordance with Danish law, register-based studies are not required to be reported to the National Committee on Health Research Ethics. Furthermore, Danish law does not require patient consent for register-based studies.
Funding
The study was funded by the Danish Cancer Society Scientific Committee (grant number R321-A17417) and the Danish regions.
CRediT authorship contribution statement
TBO, TRR, EJ, HE, OL, HM, JWJ and HJ designed the study. TBO and HJ acquired the data. HJ analysed the data. All authors contributed to the interpretation of the data. TBO and HJ drafted the article. All authors revised the article critically for important intellectual content. All authors approved the final version of the article to be published. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Declaration of Competing Interest
The authors report that there are no conflicts of interest.
Data Availability
The datasets generated and analysed during this project are not publicly available. The data are stored and maintained electronically at Statistics Denmark, where it only can be accessed by pre-approved researchers using a secure VPN remote access.
References
- 1.Serra Mitjà P., Àvila M., García-Olivé I. Impact of the COVID-19 pandemic on lung cancer diagnosis and treatment. Med Clin. (Engl. Ed. ) 2022;158(3):138–139. doi: 10.1016/j.medcle.2021.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skovlund C.W., Friis S., Christensen J., Nilbert M.C., Mørch L.S. Drop in cancer diagnosis during the COVID-19 pandemic in Denmark: assessment of impact during 2020. Acta Oncol. 2022:1–4. doi: 10.1080/0284186X.2021.2024879. [DOI] [PubMed] [Google Scholar]
- 3.van Breeschoten J., Ismail R., Smit H., Schuurbiers O., Schramel F. An invisible group of COVID-19 victims; impact on Dutch lung cancer care. Lung Cancer. 2021;159:177–178. doi: 10.1016/j.lungcan.2021.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dinmohamed A.G., Visser O., Verhoeven R.H.A., Louwman M.W.J., van Nederveen F.H., Willems S.M., et al. Fewer cancer diagnoses during the COVID-19 epidemic in the Netherlands. Lancet Oncol. 2020;21(6):750–751. doi: 10.1016/S1470-2045(20)30265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gysling S., Morgan H., Ifesemen O., West D., Conibear J., Navani N., et al. The Impact of COVID-19 on Lung Cancer Incidence in England: Analysis of the National Lung Cancer Audit 2019 and 2020 Rapid Cancer Registration Datasets. Chest. 2023 doi: 10.1016/j.chest.2023.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johansson A.L.V., Larønningen S., Skovlund C.W., Kristiansen M.F., Mørch L.S., Friis S., et al. The impact of the COVID-19 pandemic on cancer diagnosis based on pathology notifications: a comparison across the Nordic countries during 2020. Int. J. Cancer. 2022;151(3):381–395. doi: 10.1002/ijc.34029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Regionernes Kliniske Kvalitetsudviklingsprogram (RKKP), 2021. Dansk Lungecancer Register. Årsrapport 2020. [In Danish] 2021.
- 8.Sundhedsdatastyrelsen. Analyser om nye kræfttilfælde. [In Danish] 2022.
- 9.Tørring M.L., Falborg A.Z., Jensen H., Neal R.D., Weller D., Reguilon I., et al. Advanced-stage cancer and time to diagnosis: An International Cancer Benchmarking Partnership (ICBP) cross-sectional study. Eur. J. Cancer Care (Engl. ) 2019;28(5) doi: 10.1111/ecc.13100. [DOI] [PubMed] [Google Scholar]
- 10.Tørring M.L., Frydenberg M., Hansen R.P., Olesen F., Vedsted P. Evidence of increasing mortality with longer diagnostic intervals for five common cancers: a cohort study in primary care. Eur. J. Cancer. 2013;49(9):2187–2198. doi: 10.1016/j.ejca.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 11.Dalton S.O., Frederiksen B.L., Jacobsen E., Steding-Jessen M., Østerlind K., Schüz J., et al. Socioeconomic position, stage of lung cancer and time between referral and diagnosis in Denmark, 2001-2008. Br. J. Cancer. 2011;105(7):1042–1048. doi: 10.1038/bjc.2011.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olesen T.B., Jensen H., Møller H., Jensen J.W., Andersen B., Vejborg I., et al., 2022. Nation-wide mammography screening participation in Denmark during the COVID-19 pandemic: An observational study. UNDER REVIEW (pre-print at medRxix). 2022.
- 13.Olesen T.B., Jensen H., Møller H., Jensen J.W., Waldstrøm M., Andersen B. Participation in the nation-wide cervical cancer screening programme in Denmark during the COVID-19 pandemic: An observational study. eLife. 2023;12 doi: 10.7554/eLife.81522. e81522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olesen T.B., Jensen H., Møller H., Jensen J.W., Andersen B., Rasmussen M. ation-wide participation in FIT-based colorectal cancer screening in Denmark during the COVID-19 pandemic: An observational study. eLife. 2023;2 doi: 10.7554/eLife.81808. 81808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Statistics Denmark [15/01/2021]. Available from: 〈https://www.dst.dk/en#〉.
- 16.Probst H.B., Hussain Z.B., Andersen O. Cancer patient pathways in Denmark as a joint effort between bureaucrats, health professionals and politicians--a national Danish project. Health Policy. 2012;105(1):65–70. doi: 10.1016/j.healthpol.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt M., Pedersen L., Sørensen H.T. The Danish Civil Registration System as a tool in epidemiology. Eur. J. Epidemiol. 2014;29(8):541–549. doi: 10.1007/s10654-014-9930-3. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt M., Schmidt S.A.J., Adelborg K., Sundbøll J., Laugesen K., Ehrenstein V., et al. The Danish health care system and epidemiological research: from health care contacts to database records. Clin. Epidemiol. 2019;11:563–591. doi: 10.2147/CLEP.S179083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Statens Serum Institut. Overvågningdata for covid-19 i Danmark og Europa [17/01/2021]. Available from: 〈https://covid19.ssi.dk/overvagningsdata〉.
- 20.Pottegård A., Kristensen K.B., Reilev M., Lund L.C., Ernst M.T., Hallas J., et al. Existing Data Sources in Clinical Epidemiology: The Danish COVID-19 Cohort. Clin. Epidemiol. 2020;12:875–881. doi: 10.2147/CLEP.S257519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Statens Serum Institut. Vaccination mod covid-19 [17/01/2021]. Available from: 〈https://covid19.ssi.dk/vaccination〉.
- 22.Sundhedsstyrelsen. Vaccinationskalender 2021 [Available from: 〈https://www.sst.dk/da/udgivelser/2021/vaccinationskalender〉.
- 23.Jakobsen E., Rasmussen T.R. The Danish Lung Cancer Registry. Clin. Epidemiol. 2016;8:537–541. doi: 10.2147/CLEP.S99458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oken M.M., Creech R.H., Tormey D.C., Horton J., Davis T.E., McFadden E.T., et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982;5(6):649–655. [PubMed] [Google Scholar]
- 25.Larsen M.B., Jensen H., Hansen R.P., Olesen F., Vedsted P. Identification of patients with incident cancers using administrative registry data. Dan. Med. J. 2014;61(2):A4777. [PubMed] [Google Scholar]
- 26.Khanijahani A., Iezadi S., Gholipour K., Azami-Aghdash S., Naghibi D. A systematic review of racial/ethnic and socioeconomic disparities in COVID-19. Int. J. Equity Health. 2021;20(1):248. doi: 10.1186/s12939-021-01582-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ip A., Black G., Vindrola-Padros C., Taylor C., Otter S., Hewish M., et al. Socioeconomic differences in help seeking for colorectal cancer symptoms during COVID-19: a UK-wide qualitative interview study. Br. J. Gen. Pr. 2022;72(720):e472–e482. doi: 10.3399/BJGP.2021.0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sundhedsstyrelsen [the Danish National Borad of Health]. Monitorering af forløbstider på kræftområdet [Monitoring durations of cancer courses] Copenhagen S; 2021.
- 29.Jensen H., Tørring M.L., Olesen F., Overgaard J., Fenger-Grøn M., Vedsted P. Diagnostic intervals before and after implementation of cancer patient pathways - a GP survey and registry based comparison of three cohorts of cancer patients. BMC Cancer. 2015;15:308. doi: 10.1186/s12885-015-1317-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jensen H., Tørring M.L., Fenger-Grøn M., Olesen F., Overgaard J., Vedsted P. Tumour stage and implementation of standardised cancer patient pathways: a comparative cohort study. Br. J. Gen. Pr. 2016;66(647):e434–e443. doi: 10.3399/bjgp16X684805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arnold M., Rutherford M.J., Bardot A., Ferlay J., Andersson T.M., Myklebust T., et al. Progress in cancer survival, mortality, and incidence in seven high-income countries 1995-2014 (ICBP SURVMARK-2): a population-based study. Lancet Oncol. 2019;20(11):1493–1505. doi: 10.1016/S1470-2045(19)30456-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analysed during this project are not publicly available. The data are stored and maintained electronically at Statistics Denmark, where it only can be accessed by pre-approved researchers using a secure VPN remote access.